Abstract
The proximal promoter region of ANK, a gene that codes for a protein that regulates the transport of inorganic pyrophosphate, contains two hypoxia responsive elements (HREs); therefore, we studied the expression and function of ANK at different oxygen tensions. ATDC5 and N1511 clonal chondrocytic cells were cultured in either hypoxia (2% O2) or normoxia (21% O2). Transcript and protein levels of ANK were depressed in hypoxic conditions, as were levels of extracellular pyrophosphate (ePPi). To determine whether HIF-1 was involved in the oxemic response, Hif-1α knockdown cells were exposed to varying oxygen conditions and ANK expression was assessed. Knockdown of Hif-1α resulted in low levels of expression of ANK in hypoxia and normoxia. Chromatin immunoprecipitation (ChIP) assays explored the binding of Hif-1α to ANK HREs and showed that Hif-1α is able to bind to the HREs of ANK more avidly in normoxia than in hypoxia. Furthermore, functional studies of Hif-1α activity using luciferase reporter assays of wildtype and mutagenized HREs showed that only HRE-1 binds Hif-1α in normoxia. Expression of ANK in growth plate and articular cartilage was low in hypoxic regions of the tissues, and higher levels of ANK expression were observed in the synovium and meniscus in regions that have a normally higher oxygen tension. The data suggest that ANK expression and function in vitro and in vivo are repressed in hypoxic environments and that the effect is regulated by HIF-1.
Key words: ANK, cartilage biology, hypoxia, HIF-1, gene expression
INTRODUCTION
ANK is a multipass transmembrane protein that regulates the transport of inorganic pyrophosphate (PPi) between the inside and outside of the cell.(1) The fact that PPi is a potent modulator of mineralization implicates ANK as an important player in biomineralization. Genetic studies of abnormal mineralization in joints and bone have identified multiple mutations in ANK that are responsible for autosomal dominant disorders such as familial chondrocalcinosis, characterized by excessive deposition of calcium pyrophosphate dihydrate crystals in affected joints, and cranial metaphyseal dysplasia, Jackson type, whose phenotype includes hyperostosis of craniofacial bones.(2) Although ANK is also expressed in a wide variety of nonskeletal tissues, the dramatic phenotypes displayed in these two diseases has focused attention on the role of ANK in the physiological and pathological mineralization of hard tissues. In the case of articular and growth plate cartilage, ANK expression has been observed in the superficial zone and hypertrophic zone, respectively, and its expression is markedly absent from the mid and deep zones of articular cartilage and from the proliferative zone of the growth plate.(3–5) These patterns of expression suggested to us that ANK expression might be regulated by oxygen, because access to oxygen varies in different levels of articular and growth plate cartilage.(6) Furthermore, we observed that the proximal promoter of ANK contained two hypoxia responsive element (HRE) sequence motifs, potential binding sites for HIF-1.
Hypoxia, a low cellular oxygen tension, is an important regulator of gene expression in many tissues.(7,8) The most global regulator of the hypoxic response is the transcriptional activator hypoxia inducible factor 1 (HIF-1). HIF-1 was cloned in 1995, and since that time, numerous genes that are transcriptionally regulated by HIF-1 have been identified. Active HIF-1 consists of a dimer of two subunits: the hypoxia response factor Hif-1α and the constitutively expressed Hif-1β, also known as ARNT. Under conditions of normoxia, the Hif-1α subunit is hydroxylated by oxygen-dependent prolyl hydroxylases, is bound by the von Hippel-Lindau tumor suppressor protein (VHL), and is eventually targeted for ubiquitination. However, under hypoxic conditions, binding of VHL to Hif-1α is inhibited, resulting in an accumulation of Hif-1α, dimerization with Hif-1β, and binding to the HRE sequence of target genes. The binding of HIF-1 activates increased transcription of these target genes.(7,8) In chondrocytes, low oxygen tensions and HIF-1 are known to be important factors in cartilage homeostasis and in osteoarthritis.(6) Therefore, we studied the effect of oxygen on the expression of ANK in ATDC5 and N1511 chondrocytic cells, as well as in primary human articular chondrocytes, developing articular cartilage, mineralizing osteocytes, and meniscal and synovial tissues. Specifically, we sought to address the following questions. (1) Does oxygen tension affect the expression of ANK at the mRNA and protein levels? (2) Does oxygen affect the PPi transport function of ANK? (3) Is the oxygen regulation of ANK expression likely to involve HIF-1? (4) Does Hif-1α bind to the HREs of the Ank proximal promoter? (5) Which HRE is functionally active in the oxemic regulation of ANK expression?
MATERIALS AND METHODS
Cell culture
ATDC5,(9) N1511,(10) and N1511 Hif-1α knockdown(11) chondropotent cell lines were seeded at low density in DMEM/F12 medium (ATDC5) or αMEM medium (N1511) containing 10% FBS and 1% sodium pyruvate. To differentiate ATDC5 cells to maturity, insulin, transferrin, and selenite were added to the culture medium. At day 14, cells were exposed to normoxic (21% O2) or hypoxic (2% O2) conditions for 24 or 48 h. To differentiate N1511 and N1511 Hif-1α knockdown cells from nonmineralizing hypertrophy, cells were continuously treated with 200 μg/ml BMP-2 and maintained in culture for 6 days.(10) At day 6, cells were exposed to normoxic (21% O2) or hypoxic (2% O2) conditions for 24 or 48 h.
Primary human articular chondrocytes were recovered from an undiseased knee that was obtained from surgical amputation. Chondrocytes were freshly isolated by sequential enzymatic digestion with trypsin and type II collagenase (Sigma). The second collagenase digestion was performed overnight in DMEM medium at 37°C. Released chondrocytes were plated at high density in DMEM (high glucose) containing 10% FBS and antibiotics in 35-mm plates until ∼80% confluent. At this time, cells were submitted to incubation in normoxic or hypoxic conditions. MLO-A5 cells(12) were plated at a density of 1.0–1.5 × 104 cells/mm2 on a substratum of rat tail collagen (BD Biosciences) in medium of αMEM supplemented with 10% FBS and antibiotics. To promote mineralization, cells were treated for 7 days with 10 mM β-glycerophosphate, during which time they were incubated for in either normoxic or hypoxic conditions.(13)
The chondrocytic nature of ATDC5, N1511 and human primary articular chondrocytes was confirmed by monitoring the expression of extracellular matrix and enzymatic markers of chondrocyte maturation as previously described.(14)
RNA expression analyses
RNA from cells was isolated and DNase treated using a commercially available kit (Qiagen). One microgram of total RNA was reverse transcribed using ThermoScript Reverse Transcriptase and subjected to cDNA synthesis (RT-PCR System; Invitrogen). The resultant cDNA was used in real-time PCR to quantitate the relative expression of ANK, β-actin, and vascular endothelial growth factor (VEGF) transcripts using Syber Green detection. Fold changes in steady-state levels of RNA were determined by the equation 2−ddCt, where ddCt = dE − dC; dE = Ctexp −Ctactin, and dC = Ctcontrl − Ctactin. Primers for the amplification of murine ANK have been previously described.(12) For amplification of human ANK, the following primers were used: sense 5′-CTCTGTCACTCACGCTCTGT-3′; antisense 5′-GTCCACTCCGATGATGTCTA-3′ (product size = 75 bp). For amplification of human VEGF, the following primers were used: sense 5′-CCTGCAAAAACACAGACTCG-3′; antisense 5′-TCTGTCGATGGTGATGGTGT-3′ (product size = 202 bp), and for amplification of murine VEGF, the primers were as follows: sense 5′-CCCGACGAGATAGAGTACAT-3′; antisense 5′-ATCTGCTGTGCT GTAGGAAG-3′ (product size = 179 bp).
Protein expression analyses
Chondrocytic cells at the proliferative phase of their maturation were harvested by scraping into PBS and disrupted by freeze-thawing and sonication in the presence of protease inhibitors. Protein concentrations were determined using the Bradford Coomassie assay (Pierce) and BSA as standard. Proteins (7–10 μg) were resolved on 4–20% SDS polyacrylamide gels and transferred to nitrocellulose by 2D diffusion blotting. One of the blots was stained with Sypro Ruby (Lonza) to determine the efficiency of blotting and the equivalency of loading among samples. The mirror image blot was immunoreacted with a peptide-directed rabbit polyclonal antibody (ANK-218; epitope: DIIPDRSGPEGGD; amino acids positions 218–231(14)) after sensitization with Qentix (Pierce) and blocking in 5% nonfat milk in Tris-saline buffer containing 0.1% Tween 20. After incubation with the primary antibody, blots were thoroughly washed and reacted with horseradish peroxidase–conjugated donkey anti-rabbit IgG (Jackson Immunoresearch) and developed with diaminobenzidine reagent containing NiCl2.
Extracellular PPi assays
PPi assays were performed on chondrocytic cells at the proliferative phase of their maturation. Cell media were cleared of cellular debris, and ePPi levels were evaluated by the enzymatic procedure of Lust and Seegmiller,(15) as modified by Johnson and colleagues,(16) where PPi is determined by differential absorption on activated charcoal of UDP-D-[6-3H]-glucose from the reaction product 6-phospho-[6-3H]-gluconate. All assay results were normalized versus DNA concentration as determined using a Pico Green assay of double-stranded DNA (Molecular Probes).
Chromatin immunoprecipitation assays
Interacting protein and DNA–protein complexes were cross-linked on live N1511 cells grown in normoxic or hypoxic conditions using 0.5%, 1%, or 10% formaldehyde. Cells were lysed, and the crude chromatin extracts were sonicated to shear the DNA. Proteins, together with cross-linked DNA, were immunoprecipitated using an anti-Hif-1α antibody (R&D Systems). Parallel immunoprecipitations were performed in the absence of antibody (negative control). Protein–DNA cross-links in the immunoprecipitates and in the crude chromatin extract (positive control) were reversed in the presence of 5 M NaCl at 65°C, proteins were digested with proteinase K at 45°C, and the DNA fragments were purified and PCR amplified using a primer pair designed to encompass the putative HRE sites in the ANK promoter: sense (−1093 bp with respect to A of the ATG initiation codon = 1), 5′-AGTCTCAGCTCAGCAACTG-3′; antisense (−302 bp with respect to A of the ATG initiation codon = 1), 5′-GACAGCGGCTCCATTATAAG-3′. PCR products were visualized on a 2% agarose gel.
Site-directed mutagenesis was performed using the Quick-Change kit (Stratagene) according to the manufacturer's protocol. For each of the two ANK HREs, a mutagenic oligonucleotide primer pair was designed with the targeted two nucleotide change in the HRE consensus sequence. PCR reactions to generate the mutant HREs were performed using the wildtype ANK proximal promoter (−1093 to −302 bp with respect to the translation initiation) that was cloned into the pGL4.10 luciferase reporter vector as a template. The standard amplification protocol was modified to facilitate PCR amplification of the GC-rich promoter region by addition of 8% dimethyl sulfoxide and by increasing the extension time to 2 min/kb pair. Four microliters of the resultant PCR reactions was used to transform Top10 (Invitrogen) competent cells, and positive colonies were selected and amplified. Plasmid DNA was purified using the EndoFree Plasmid Purification kit (Qiagen).
Renilla luciferase vector and purified wildtype HRE or mutant HRE plasmids were co-transfected into COS-7 cells using the FuGene HD transfection reagent (Roche). Transfected cells were harvested after 24 h, lysed, and subjected to assay for luciferase activity using the Dual Luciferase Reporter Assay kit (Promega). Data were normalized against Renilla luciferase expression, and the protein concentration was determined for each reaction as described above.
Immunohistochemical and immunocytochemical studies
Meniscus was obtained from a mouse knee joint at postnatal day 7. Mouse articular cartilage was obtained from the knee at postnatal day 5. Mouse growth plate tissue was obtained from the tibia at postnatal day 5. Human synovial tissue was obtained from surgical discard material after total knee arthroplasty.
Tissues were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned (6 μm). Paraffin sections were dewaxed, rehydrated, and stained with Harris hematoxylin (Fisher Scientific) and eosin Y (Fisher Scientific) to determine tissue morphology. For immunostaining, rehydrated tissues were treated with a 1:100 dilution of Antigen Unmasking Solution (Vector) two times for 10 min in a microwaved boiling solution, permeabilized with 0.5% Triton X-100 in PBS for 10 min, and blocked with 4% BSA in PBS with 0.1% Tween for 1 h. Sections were incubated overnight at 4°C with primary antibodies recognizing Hif-1α (R&D Systems) used at a dilution of 1:50 or ANK (antibody 218; see above) used at a dilution of 1:250, followed by incubation with an appropriate secondary AlexaFlour-conjugated anti-IgG antibody at a dilution of 1:200 (Invitrogen) in a solution of 1% BSA and 0.1% Tween in PBS for 1 h. As a negative control, the sections were incubated with no primary antibodies. Slides were mounted and microscopically evaluated. Images were acquired with a Retiga EXi digital-cooled CCD camera with RGB electronic filter (QImaging) on a Nikon E800. For immunocytochemical studies of ANK in N1511 and N1511/Hif-1α knockdown cells, harvested cells were suspended in PBS and immediately cytospun onto positively coated glass slides. Cells were immunoreacted with anti-ANK-218 antibody used at a 1:250 dilution and processed as described above, except that treatment with the Antigen Unmasking Solution was performed only one time.
Statistical methods
Data are presented as means ± SD, and statistical significance was identified by using the unpaired, two-tailed Student t-test (p values are reported in figure legends), unless otherwise indicated in the figure legend. All assays were performed at least in triplicate; see figure legends for exact number of replicates performed.
RESULTS
Expression of ANK in hypoxic and normoxic conditions in chondrocytic cells
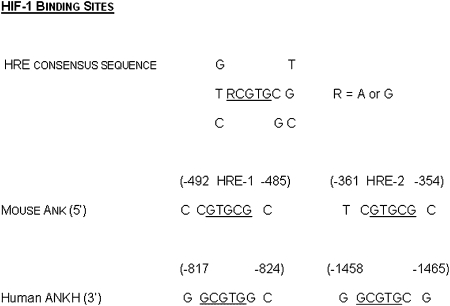
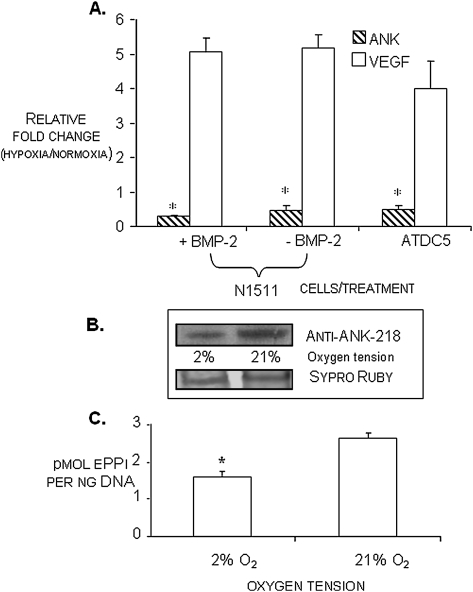
Examination of the 5′ UTR and proximal promoter regions of the murine and human ANK genes showed multiple sequence elements that could be involved in the regulation of gene expression by oxygen (Fig. 1). Therefore, we subjected two different murine chondrogenic cell lines, ATDC5(9) and N1511,(10) to culture in normoxic (21% O2) and hypoxic (2% O2) conditions. We observed that, in both cell lines, ANK mRNA expression was significantly repressed in hypoxia (Fig. 2A). Because of the availability of knockdown reagents prepared in the N1511 cells(11) and the shorter period of time needed for their differentiation,(10) we performed the follow-up studies described below in the N1511 cells and their derivatives. To determine whether the oxemic regulation of ANK expression differed with differentiation status, N1511 cells were subjected to treatment with BMP-2 for a period of 6 days. During this time, the cells progressed to nonmineralizing hypertrophy(10) (and confirmed by detection of type X collagen expression and elevated alkaline phosphatase activity; data not shown). The results depicted in Fig. 2A show that the relative fold change in transcript levels for ANK was <1, indicating that ANK expression is repressed in hypoxia (conversely, expression levels for VEGF are >1, indicating that VEGF is upregulated in hypoxia), and further show that the hypoxic repression of ANK expression was independent of the differentiation status of the cells. We also compared the expression of ANK at constant oxygen tension as a function of differentiation; we observed that levels of expression of ANK did not significantly change between immature cells and nonmineralizing hypertrophic cells when cells were cultured in normoxia (data not shown). The effect of oxygen on ANK expression was also apparent at the level of ANK protein expression (Fig. 2B), with cells cultured in hypoxic conditions showing a dramatic repression of protein expression compared with those cultured in normoxic conditions. Furthermore, we determined that the decrease in ANK protein expression in hypoxia had a significant impact on the elaboration of ePPi (Fig. 2C) and confirmed that the decrease in ePPi was not caused by any effect of hypoxia on the expression and activity of tissue nonspecific alkaline phosphatase or on the expression and activity of the ectoenzyme, nucleotide pyrophosphohydrolase phosphodiesterase (data not shown). Finally, α-amanitin treatment of the cells indicated that the oxemic regulation of ANK expression was not caused by altered stability of the ANK transcript at different oxygen tensions, and treatment of the cells in normoxia and hypoxia with cycloheximide confirmed that de novo protein synthesis was indeed necessary to elicit the regulation of ANK expression by oxygen (data not shown).
FIG. 1.
HIF-1 consensus binding site. Underlined nucleotides represent core consensus element; putative HIF-1 binding sites and their positions in the proximal promoter regions of human and mouse ANKH/Ank genes.
FIG. 2.
(A) Relative expression of ANK and VEGF transcripts in normoxia vs. hypoxia (24-h treatment) as determined by qRT-PCR in ATDC5 and N1511 cells. Transcript levels for ANK are <1, indicating that ANK expression is repressed in hypoxia. Conversely, expression levels for VEGF are >1, indicating that VEGF is upregulated in hypoxia. Relative transcript levels of ANK in N1511 cells at immaturity (−BMP-2) and at nonmineralizing hypertrophy (+BMP-2), as well as in immature ATDC5 cells is shown. *p < 0.05 for fold change for ANK in hypoxia vs. normoxia; n = 9. (B) Western blot analysis of ANK protein expression in mature N1511 cells in hypoxic (2% O2) and normoxic (21% O2) conditions. Sypro Ruby staining of the mirror image blot depicts relative protein loading. (C) Extracellular PPi levels in N1511 cells in normoxia (21% O2) and hypoxia (2% O2) at the proliferative phase of their differentiation. Repression of ANK expression in hypoxia results in statistically significant decrease in the elaboration of ePPi. *p < 0.05, n = 6.
Expression of ANK in Hif-1α knockdown cells
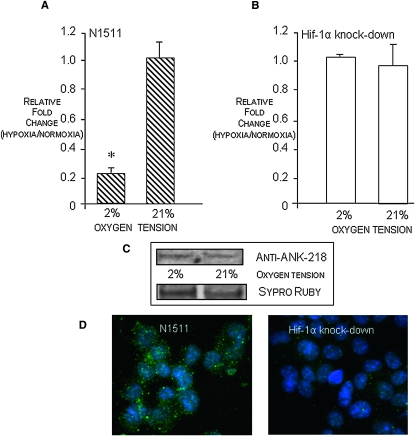
To determine whether the regulation of ANK expression by oxygen was likely to involve HIF-1, we used N1511 cells in which the oxygen-sensitive subunit of HIF-1, Hif-1α, had been knocked down.(11) Figs. 3A and 3B depict the relative mRNA expression levels of ANK in N1511 cells and in Hif-1α knockdown cells when the cells were cultured in either normoxic or hypoxic conditions. In the N1511 cells (Fig. 3A), values <1 indicate that ANK expression was repressed in hypoxic conditions; however, Fig. 3B shows that, in cells in which Hif-1α had been knocked down, the expression levels of ANK in hypoxia were equivalent to the expression of ANK in the Hif-1α knockdown cells at normoxia. Because relative fold changes of ANK expression in hypoxia versus normoxia as detected by real-time PCR do not adequately convey information about the absolute expression of ANK in the Hif-1α knockdown cells in normoxia and hypoxia, we performed Western blot analysis of cell lysates from the cells grown at differing oxygen tensions and immunocytochemical analyses of ANK expression in N1511 and Hif-1α knockdown cells cultured in normoxia. Fig. 3C shows low levels of expression of ANK in the Hif-1α knockdown cells cultured at either 2% or 21% oxygen. To directly compare levels of ANK expression in the N1511 cells and the Hif-1α knockdown cells in normoxia, we performed the immunocytochemical studies depicted in Fig. 3D that further confirm that, when Hif-1α is knocked down, ANK expression is dramatically reduced in normoxia. To delineate the effect of Hif-1α on the oxemic regulation of ANK expression, we next evaluated the binding of an antibody to Hif-1α to the ANK HREs in a chromatin immunoprecipitation (ChIP) assay.
FIG. 3.
(A and B) Real-time PCR of ANK transcript levels in hypoxia vs. normoxia in N1511and Hif-1α knockdown cells. Transcript levels <1 indicate repression of ANK expression in hypoxia; transcript levels >1 indicate stimulation of expression in hypoxia. In contrast to hypoxic repression of ANK expression in N1511 cells, knockdown of Hif-1α results in equivalent expression of ANK in normoxia and hypoxia. (C) Western blot analysis of ANK protein expression in mature Hif-1α knockdown cells in hypoxic (2% O2) and normoxic (21% O2) conditions. Sypro Ruby staining of the mirror image blot depicts relative protein loading. Note low levels of expression of ANK in hypoxia and normoxia, showing relative loss of ANK expression in normoxia (21% O2) in cells in which Hif-1α is knocked down. (D) Immunocytochemical visualization of ANK expression in mature N1511 cells and Hif-1α knockdown cells grown in normoxic conditions showing high expression of ANK in N1511 cells and lack of significant ANK expression in cells in which Hif-1α has been knocked down.
Binding of Hif-1α to ANK HREs
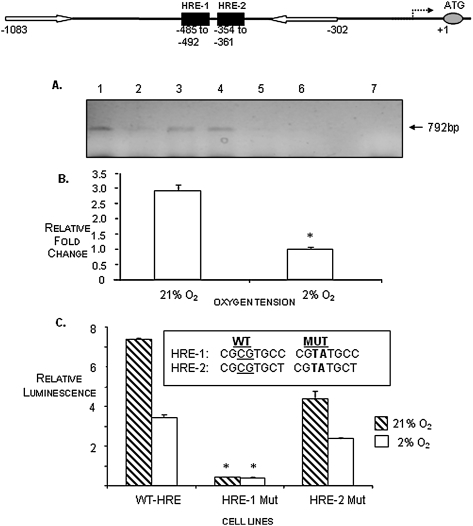
We used ChIP analyses using a Hif-1α–specific antibody and DNA–protein complexes from N1511 cells grown in normoxic or hypoxic conditions to determine whether Hif-1α was able to bind the HREs of the ANK proximal promoter. As shown in Fig. 4, the Hif-1α antibody was capable of binding to the cross-linked DNA–protein complex derived from cells grown in normoxic conditions (Fig. 4A, lane 1), but very little antibody bound to cross-linked DNA–protein complexes derived from cells grown in hypoxic conditions (Fig. 4A, lane 2). The relative amounts of PCR products depicted in the agarose gel of the ChIP assay products were quantitated, and the results indicated that about three times as much Hif-1α antibody was able to bind to the DNA–protein complexes from N1511 cells cultured in normoxia than was able to bind to complexes from cells cultured in hypoxic conditions (Fig. 4B).
FIG. 4.
(A) ChIP assay depicting binding of Hif-1α to the ANK HREs. Hif-1α–immunoprecipitated DNA–protein complexes were disrupted, and the recovered DNA was PCR-amplified with primers (white arrows in depiction of the position of the ANK proximal promoter HREs, HRE-1 and HRE-2, above ChIP assay results) surrounding the HRE elements in the ANK promoter. PCR products (792 bp) were resolved on a 2% agarose gel. Lane 1, IP with anti-Hif-1α antibody in normoxia; lane 2, IP with anti-Hif-1α antibody in hypoxia; lane 3, positive control: input DNA binding to anti-Hif-1α in normoxia; lane 4, positive control: input DNA binding to anti-Hif-1α in hypoxia; lane 5, negative control: no antibody in normoxia; lane 6, negative control: no antibody in hypoxia; lane 7, water control. Note that binding of the Hif-1α antibody to the promoter PCR fragment is repressed in hypoxia (lane 2). (B) qPCR quantitation of the recovery of the Hif-1α–binding PCR fragments in normoxia vs. hypoxia as depicted on the gel. (C) Site-directed mutagenesis studies of the ANK HREs. HRE-1 and HRE-2 were mutagenized as shown, cloned into a luciferase reporter plasmid, and used to transfect COS-7 cells under normoxic conditions. Expression of the ANK promoter harboring wildtype (WT) HRE sequences resulted in relatively high expression of ANK in normoxia compared with hypoxia. When HRE-1 was mutated, the expression of the luciferase reporter construct was dramatically decreased. When the HRE-2 site was mutated, expression of the corresponding luciferase construct was only slightly decreased. These results suggest that HRE-1 functions as the site primarily used for HIF-1 mediation of ANK expression in response to oxygen. *p < 0.05 compared with WT, n = 6.
Impact of mutagenesis of the ANK HREs on the oxemic regulation of ANK expression
To further define the nature of the oxemic regulation of ANK expression, we asked whether mutagenesis of the HREs in the proximal promoter of ANK would affect the ability of Hif-1α to regulate the expression of ANK in varying oxygen tensions. We addressed this question by performing a functional assay of ANK promoter activity using reporter constructs in which the wildtype HREs were preserved or in which one or the other of the HREs was mutated. We hypothesized that mutagenesis of one or more of the ANK HREs would inhibit the binding of Hif-1α to the mutated HRE, thus resulting in a decrease in luciferase activity from the reporter construct that was transfected into COS-7 cells. After determining that COS-7 cells express Hif-1α in normoxic as well as hypoxic conditions (data not shown), we transfected the cells with either a wildtype promoter fragment, in which both HRE sequence elements were undisturbed, or with promoter fragments in which either HRE-1 or HRE-2 was mutated, as shown in Fig. 4C. To monitor for transfection efficiency, a Renilla luciferase vector was co-transfected with the wildtype or mutant ANK reporter constructs. Figure 4C shows that luciferase activity was repressed for cells gown in hypoxia as expected; however, it also shows that HRE-1 is largely responsible for the binding of Hif-1α to regulate the expression of ANK in response to oxygen. Whereas there is also a decrease in the luciferase activity of the HRE-2–mutated construct, it seems to be a minor contributor to the oxemic regulation of ANK expression by Hif-1α.
Expression of ANK in skeletal tissues
The studies of the oxemic regulation of ANK expression were performed in chondrogenic cell lines ATDC5 and N1511 that serve as model systems for endochondral ossification.(9,10) Immunohistochemical analyses of ANK expression in the murine growth plate are consistent with the notion that ANK expression is repressed in hypoxic conditions and is relatively higher in regions of the tissue that are exposed to oxygen. Figure 5A shows the staining of the mouse tibial growth plate using anti-ANK antibody; ANK expression is very low in regions of the growth plate that are relatively hypoxic, such as the proliferative and prehypertrophic zones. However, as the depth of the growth plate increases, the expression of ANK increases and is especially prominent in areas of the growth plate where the oxygen-rich vasculature has invaded the calcification zone (Fig. 5A).
FIG. 5.
Immunohistochemical studies of ANK expression in the mouse growth plate and articular cartilage. (A) ANK is primarily expressed in the hypertrophic and calcification zones of the growth plate, and its expression is particularly strong in oxygen-rich areas of the growth plate undergoing vascular invasion. (B) ANK expression in the superficial zone of articular cartilage from 5-day postnatal mice corresponds to relatively high levels of oxygen from synovial fluid compared with the proliferative zone of articular cartilage where severe hypoxia results in very low ANK expression. (C) Immunohistochemical staining of ANK in meniscus shows high levels of expression; also note high levels of ANK in the superficial zone of the femoral condyle and tibial plateau in a mouse knee joint from a P7 animal. Autofluorescing anuclear RBCs in vessel walls emphasize high levels of ANK staining in these areas as well (white arrows). (D) H&E and (E) anti-ANK staining of human synovial tissue, showing high levels of expression of ANK in synoviocytes and in other regions of the tissue (F, fatty globules). (F) qRT-PCR showing very low levels of ANK expression in hypoxia in primary normal human articular chondrocytes. Values >1 indicate a relative increase in expression in hypoxia; values <1 indicate a relative decrease in expression in hypoxia. Data show that the expression of ANK is repressed in hypoxia, whereas the expression of VEGF is upregulated in hypoxia. *p < 0.05 for fold change in ANK expression in hypoxia vs. normoxia; n = 3. (G) qRT-PCR showing the relative expression of ANK in osteocytic MLO-A5 cells in normoxia and hypoxia. Note very high levels of ANK expression in normoxic conditions at times of mineralization (days 5–7) and repression of ANK expression in hypoxia at these same times. Results depict two separate experiments with qRT-PCR analyses performed in triplicate for each experiment.
Because mutations in ANK can cause joint pathology,(2) we also explored the oxemic regulation of ANK expression in articular cartilage. ANK expression was highest in the superficial zone of murine articular cartilage from 5-day-old neonates, a region of articular cartilage that is bathed by oxygen-enriched synovial fluid (Fig. 5B). We also explored the relevance of our observations of the oxemic control of ANK expression in other skeletal cell types, including synovial tissue, meniscus, mineralizing murine osteocytic cells, and freshly isolated normal primary human articular chondrocytes. We performed immunohistochemical analysis of mouse meniscal tissue (Fig. 5C) and observed high expression of ANK in the meniscus, especially in areas of invading vasculature (white arrows on figure). Figure 5D shows H&E staining of human synovial tissue and immunohistochemical staining of the tissue with anti-ANK antibody (Fig. 5E). ANK expression is especially prominent in the synoviocytes at the edge of the tissue where there is contact with the synovial fluid. Figure 5F shows the culture of primary human articular chondrocytes obtained from the knee. The cells were cultured in normoxic versus hypoxic conditions, and, similar to the N1511 murine chondropotent cells, we observed a repression of ANK expression in hypoxia. The chondrocytic nature of the primary human articular chondrocytes was confirmed by evaluation of typical extracellular matrix markers of mature articular chondrocytes, including expression of type II collagen and aggrecan (data not shown). Finally, in Fig. 5G, osteocytic murine cells were cultured over a period of 10 days in mineralizing conditions and exposed to either hypoxic (2% O2) or normoxic (20% O2) conditions. At all times, the expression of ANK was repressed in hypoxia, but this was particularly noteworthy at the late stages of culture when the cells underwent mineralization.
DISCUSSION
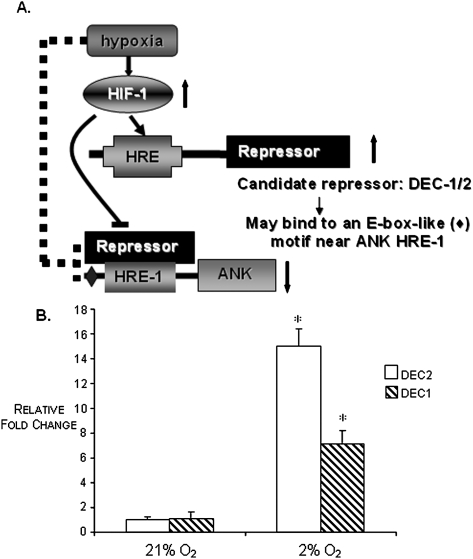
ANK is ubiquitously expressed, and regulation of its expression in skeletal tissues seems to be intimately involved in the normal and pathological mineralization of those tissues. Mutations in ANK are known to affect the PPi transport function of the protein,(1,14,17) presumably as a result of structural perturbations caused by variation in the primary sequence of the protein. However, others have noted that the function of ANK may be affected in a more indirect way; that is, by alterations in levels of expression of the gene product.(1,18) Whereas expression of ANK has been shown to be influenced by several cytokines and growth factors,(3,4,19,20) we showed here for the first time that ANK expression is sensitive to oxygen. Unlike many proteins whose expression is increased in hypoxia, we observed that the expression of ANK is repressed in hypoxia. Specifically, we showed that Hif-1α, the oxygen-sensitive subunit of HIF-1, does not bind to the proximal promoter ANK HREs in hypoxia, although it does bind in normoxia. ANK is not unique in this response to oxygen; other genes whose transcription is downregulated in response to hypoxia have been described. These include α-fetoprotein,(21) the rat α 1a adrenergic receptor gene,(22) the heme 1 oxygenase gene in human cells,(23) and several DNA repair genes including MLH1, RAD51, BRCA1, and MSH2.(24–29) In the case of these genes, it has been hypothesized that the general mechanism of hypoxia repression may involve the elaboration of a negative response element, or a repressor, that mediates transcriptional activation by HIF-1. Our data clearly show that the mRNA and protein expression of ANK are repressed in hypoxia, and we theorize that this effect may likewise be caused by the elaboration of a repressor that interferes with the hypoxic-activating function of HIF-1. This interference may be caused by the binding of an unknown protein to Hif-1α to repress its binding to the HRE(s) of ANK in hypoxia or it may be caused by the binding of an unknown repressor directly to the HRE(s) of ANK in hypoxia. In recently described studies of the hypoxic repression of MLH1, a DNA mismatch repair gene whose expression is downregulated in hypoxia in a number of solid tumor systems, resulting in genomic instability in several cancer cell lines,(30,31) Nakamura et al.(32) showed that DEC 1 and 2, which are transcriptionally activated by HIF-1, were capable of directly binding to an E-box–like element in the proximal promoter of MLH1 to strongly repress the promoter activity of MLH1. DEC1 and 2 have also been reported to regulate the transcriptional repression of other oxygen-sensitive genes, including STAT1 and PPARG, through E-box motifs in their promoters.(33,34) We have detected an E-box–like element, with the same sequence as that reported by Nakamura et al.,(32) in the proximal promoter of murine ANK, ∼480 bp upstream from HRE-1. Furthermore, an examination of the proximal promoter of the human ANKH gene also detected an identical E-box–like element located ∼120 bp upstream from the most proximal HRE. Interestingly, DEC1, a hypoxia inducible gene, was originally identified in chondrogenic studies of cAMP-dependently differentiated chick embryo chondrocytes(35)—a chondrocytic heritage that makes it particularly attractive as a candidate for repression of ANK expression in hypoxia. In ongoing studies in our laboratory, we will seek to determine whether this E-box–like motif is a binding site for DEC1/2, thus potentially enabling the repression of ANK expression in hypoxia. Figure 6A depicts such a scenario and is one of our current working models of the repression of ANK expression in hypoxia. Figure 6B shows that both DEC1 and DEC2 are highly expressed in hypoxia in our N1511 cells.
FIG. 6.
(A) Model depicting the repression of ANK expression in hypoxia. We hypothesize that elevated expression of HIF-1 in hypoxia upregulates the expression of some repressor gene whose protein product is able to repress the expression of ANK in hypoxic conditions. A possible candidate gene(s) for this repressor is DEC1 and/or DEC2. (B) Relative expression of DEC1 and DEC2 in N1511 cells in hypoxia and normoxia showing that these transcripts are highly expressed in hypoxic conditions in the chondrocytic cells used in these studies. n = 3; *p < 0.05 with respect to expression of DEC1 and DEC2 in hypoxia vs. normoxia.
ANK is one of many transporters and channel systems involved in preserving the ionic homeostasis of chondrocytes that are imbedded in a challenging environment of gradations of low oxygen tensions. These transporters regulate the levels of a variety of metabolic substrates, as well as pH and calcium levels.(36) However, until recently, the affects of varying oxygen tensions on the expression and activity of these transporters have not been well characterized.(36) We described the hypoxic repression of ANK expression in a chondrogenic model of endochondral ossification; however, in vitro studies of regulation of gene expression in response to oxygen in cells derived from normally hypoxic environments could be misleading, especially if changes in the oxidative metabolism of the cells have occurred as a result of adaptation to normoxic conditions in vitro. Therefore, we examined tissue sections to supply a more physiologically relevant picture of the expression of ANK in different oxygen tensions that are known to exist in the tissues. We observed that ANK is abundantly expressed in the growth plate, especially in the calcification zone that is destined to undergo endochondral ossification. This area of the growth plate is exposed to oxygen by virtue of the invasion of blood vessels from the endochondral bone, especially compared with the mid and deep zones of the tissue that are markedly hypoxic. In the hypertrophic and calcification zones, ANK may participate in physiological mineralization by providing ePPi that can be acted on by tissue nonspecific alkaline phosphatase to supply some of the inorganic phosphate necessary for ossification. With respect to articular cartilage, ANK is abundantly expressed in developing tissue where normal matrix deposition has not yet been completed and where exposure to oxygen is facilitated by contact with the synovium. At this stage, the availability of ePPi, facilitated by the transport function of ANK, is likely to play an important role in inhibiting unwanted calcification of the developing tissue. As articular cartilage matures, levels of ANK expression decrease and are virtually absent from the mid and deep zones,(4) regions of mature articular cartilage that are markedly hypoxic and where expression of Hif-1α is relatively high.(37) The low expression of ANK in mature articular cartilage suggests that factors other than ePPi (e.g., osteopontin, matrix gla-protein) play a more significant role in the inhibition of pathological crystal formation in disease-free tissue. In contrast, diseased articular cartilage, such as we see in osteoarthritis (OA) exhibits significant expression of ANK in clonal populations of cells that are located in all regions of what is left of the degenerating tissue, and in some cases, ANK expression is observed in regions of the diseased cartilage that are occupied by hypertrophic-looking chondrocytes and in areas of the diseased tissue that express typical molecular markers of chondrocyte hypertrophy.(4,19,38) Here, ANK expression may be influenced not only by oxygen (e.g., as a consequence of greater exposure to oxygen from synovial fluid in fractured cartilage) but also more likely by a variety of growth factors and cytokines that are expressed as part of the osteoarthritic disease process. All of these agents may exert diverse temporal and spatial effects on ANK expression, and the extent to which ANK is expressed is likely to significantly influence the generation of ePPi, and, ultimately, the presence and/or type of crystal that is elaborated by the tissue. Interestingly, recent observations by some of us suggest that Hif-1α may not be the only oxemic regulator of gene expression in cartilage. Bohensky et al.(37) observed that Hif-2α, an isoform of Hif-1α that recognizes the same HRE consensus sequence as Hif-1α,(39) is abundantly expressed in articular cartilage, as well as in the pre-hypertrophic cells of the growth plate. Its expression is decreased, however, with maturation, aging, and the onset of disease. Where Hif-2α expression is decreased, Hif-1α expression is increased. These observations suggest that the two isoforms may act spatially to maintain the functional activity and health of cartilage tissue in the face of varying oxygen tensions across the tissue. The potential role of Hif-2α in the oxemic regulation of ANK expression is currently under study in our laboratory.
In other skeletal cell types, we observed that ANK expression is also sensitive to oxygen. For example, in meniscal tissue, ANK expression is especially high in areas of vascular invasion in the outer, fibroblast-like region of the meniscus—a region of the tissue that Adesida et al.(40) have recently shown is exposed to a relatively high concentration of oxygen. Likewise, in the synovium, which is in contact with oxygen by way of synovial fluid, in situ expression of ANK is high and may protect the tissue from unwanted mineralization. In the osteocytic cell line, MLO-A5, a pre-osteocyte–like cell line that spontaneously mineralizes in culture,(12) ANK expression is again repressed in hypoxia compared with its expression in normoxia. This observation may be relevant to a recent report by Zahm et al.(13) showing that, in hypoxia, there is inhibition of bone formation by these cells—a result that is consistent with low ANK expression and therefore low levels of PPi available for hydrolysis to Pi for bone formation.
The oxemic regulation of ANK expression is a novel observation by our laboratory, and we believe that its importance is relevant not only to the physiological and pathological mineralization of skeletal tissues but to soft tissues and their disease processes as well. For example, in the vasculature-rich cortex of the kidney, ANK expression is high, whereas in the relatively hypoxic regions of the renal papillae, an area prone to the formation of small calcium plaques that are hypothesized to serve as the nidus for stone formation, ANK expression is low and may result in reduced levels of PPi necessary to inhibit calcification (C. Williams, unpublished data, 2008). Likewise, in the oxygen-rich environment of blood vessels, robust expression of ANK enables the transport of PPi to the extracellular spaces, where it may act as a potent inhibitor of unwanted calcification. We have observed high levels of ANK expression in the smooth muscle cells lining blood vessels and extremely low levels of ANK in areas of blood vessels that have accumulated plaque (T. Freeman, unpublished data, 2008). Conversely, it is not difficult to hypothesize that, in the hypoxic centers of solid tumors, low expression of ANK, resulting in low levels of ePPi, might result in the calcification phenotype that so often accompanies solid tumor progression. The involvement of HIF-1 in the oxemic regulation of ANK expression suggests the potential for specific therapeutic modification of ANK gene expression by modulation of the active subunits of HIF-1. Indeed, the pharmacological manipulation of hypoxic pathways may play a significant role in therapeutic modalities for a variety of conditions in the future.
In summary, we established that the mRNA and protein expression of ANK is repressed in hypoxia in two different chondrogenic cell lines, and the repression of ANK expression in hypoxia affects the PPi transport function of the protein. Also, immunohistochemical analyses of ANK expression in the growth plate and in articular cartilage, as well as qRT-PCR analyses of ANK expression in hypoxia versus normoxia in normal human articular chondrocytes and in cultured osteocytic cells, support this finding. Furthermore, the oxemic regulation of ANK expression seems to be regulated by HIF-1. Studies regarding the nature of a putative hypoxic repressor of ANK expression that interferes with the hypoxic-activating function of HIF-1 are currently underway in our laboratory.
ACKNOWLEDGMENTS
This work was supported by NIH/NIAMS R01 AR052619 to C.J.W. The authors thank Dr. Jaime Caro, Cardeza Institute, Thomas Jefferson University, for critical reading of the manuscript and helpful discussions.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 2.Zaka R, Williams CJ. Role of the progressive ankylosis gene in cartilage mineralization. Curr Opin Rheumatol. 2006;18:181–186. doi: 10.1097/01.bor.0000209432.36355.6e. [DOI] [PubMed] [Google Scholar]
- 3.Sohn P, Crowley M, Slattery E, Serra R. Developmental and TGFβ-mediated regulation of ANK mRNA expression in cartilage and bone. Osteoarthrit Cartilage. 2002;10:482–490. doi: 10.1053/joca.2002.0810. [DOI] [PubMed] [Google Scholar]
- 4.Johnson K, Terkeltaub R. Upregulated ank expression in osteoarthritis can promote both chondrocyte MMP-13 expression and calcification via chondrocyte extracellular PPi excess. Osteoarthrit Cartilage. 2004;12:321–335. doi: 10.1016/j.joca.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Xu J, Du B, Kirsch T. Role of the progressive ankylosis gene (ank) in cartilage mineralization. Mol Cell Biol. 2005;25:312–323. doi: 10.1128/MCB.25.1.312-323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfander D, Gelse K. Hypoxia and osteoarthritis: How chondrocytes survive hypoxic environments. Curr Opin Rheumatol. 2007;19:457–462. doi: 10.1097/BOR.0b013e3282ba5693. [DOI] [PubMed] [Google Scholar]
- 7.Kenneth NS, Rocha S. Regulation of gene expression by hypoxia. Biochem J. 2008;414:19–29. doi: 10.1042/BJ20081055. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. Hypoxia inducible factor 1: Oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 9.Atsumi T, Miwa Y, Kimata K, Ikawa Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev. 1990;30:109–116. doi: 10.1016/0922-3371(90)90079-c. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya N, Jikko A, Kimata K, Damsky C, Shimizu K, Watanabe H. Establishment of a novel chondrocytic cell line N1511 derived from p53-null mice. J Bone Miner Res. 2002;17:1832–1842. doi: 10.1359/jbmr.2002.17.10.1832. [DOI] [PubMed] [Google Scholar]
- 11.Terkhorn SP, Bohensky J, Shapiro IM, Koyama E, Srinivas V. Expression of HIF prolyl hydoxylase isozymes in growth plate chondrocytes: Relationship between maturation and apoptotic sensitivity. J Cell Physiol. 2007;210:257–265. doi: 10.1002/jcp.20873. [DOI] [PubMed] [Google Scholar]
- 12.Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald LF. Establishment of an osteoid preosteocyte-like cell MLO-A5 that spontaneously mineralizes in culture. J Bone Miner Res. 2001;16:1622–1633. doi: 10.1359/jbmr.2001.16.9.1622. [DOI] [PubMed] [Google Scholar]
- 13.Zahm AM, Bucaro MA, Srinivas V, Shapiro IM, Adams CS. Oxygen tension regulates preosteocyte maturation and mineralization. Bone. 2008;43:25–31. doi: 10.1016/j.bone.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaka R, Stokes D, Dion AS, Kusnierz A, Han F, Williams CJ. P5L mutation in ANK results in an increase in extracellular PPi during proliferation and non-mineralizing hypertrophy in stably transduced ATDC5 cells. Arthritis Res Ther. 2006;8:R164. doi: 10.1186/ar2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lust G, Seegmiller JE. A rapid, enzymatic assay for measurement of inorganic pyrophosphate in biological samples. Clin Chim Acta. 1976;66:241–249. doi: 10.1016/0009-8981(76)90061-9. [DOI] [PubMed] [Google Scholar]
- 16.Johnson K, Vaingankar S, Chen Y, Moffa A, Goldring MB, Sano K, Jin-Hua P, Goding J, Terkeltaub R. Differential mechanisms of inorganic pyrophosphate production by plasma cell membrane glycoprotein-1 and B10 in chondrocytes. Arthritis Rheum. 1999;42:1986–1997. doi: 10.1002/1529-0131(199909)42:9<1986::AID-ANR26>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Gurley KA, Reimer RJ, Kingsley DM. Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet. 2006;79:1017–1029. doi: 10.1086/509881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Johnson K, Russell RG, Wordsworth P, Carr AJ, Terkeltaub RA, Brown MA. ANKH mutations cause both familial and sporadic calcium pyrophosphate dihydrate chondrocalcinosis and increase ANKH transcription/translation. Arthritis Rheum. 2005;52:1110–1117. doi: 10.1002/art.20978. [DOI] [PubMed] [Google Scholar]
- 19.Hirose J, Ryan LM, Masuda I. Up-regulated expression of cartilage intermediate layer protein and ANK in articular hyaline cartilage from patients with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 2002;46:3218–3229. doi: 10.1002/art.10632. [DOI] [PubMed] [Google Scholar]
- 20.Cailotto F, Bianchi A, Sebillaud S, Venkatesan N, Moulin D, Jouzeau JY, Netter P. Inorganic pyrophosphate generation by transforming growth factor beta 1 is mainly dependent on ANK induction by Ras/Raf-1/extracellular signal-regulated kinase pathways in chondrocytes. Arthritis Res Ther. 2007;9:R122. doi: 10.1186/ar2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazure NM, Chauvet C, Bvois-Joyeux B, Bernard M-A, Nacer-Cherif H, Adanan J-L. Repression of alpha fetoprotein gene expression under hypoxic conditions in human hepatoma cells: Characterization of a novel negative hypoxia response element that mediates opposite effects of hypoxia indicible factor-1 and c-myc. Cancer Res. 2002;62:1158–1165. [PubMed] [Google Scholar]
- 22.Michelotti GA, Bauman MJ, Smith MP, Schwinn DA. Cloning and characterization of the rat alpha 1a adrenergic receptor gene promoter. Demonstration of cell specificity and regulation by hypoxia. J Biol Chem. 2003;278:8693–8704. doi: 10.1074/jbc.M211986200. [DOI] [PubMed] [Google Scholar]
- 23.Kitamuro T, Takahashi K, Ogawa K, Udono-Fujimori R, Takeda K, Furuyama K, Nakayami M, Sun J, Fujita H, Hida W, Hattori T, Shirato K, Igarashi K, Shibahara S. Bach 1 functions as a hypoxia inducible repressor for the heme oxygenase 1 gene in human cells. J Biol Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 24.Mihaylova VT, Bindra RS, Yuan J, Campisi D, Narayanan L, Jensen R, Giordano F, Johnson RS, Rockwell S, Glazer PM. Decreased expression of the DNA mismatch repair gene MLH1 under hypoxic stress in mammalian cells. Mol Cell Biol. 2003;23:3265–3273. doi: 10.1128/MCB.23.9.3265-3273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bindra RS, Schaffer PJ, Meng A, Woo J, Maseide K, Roth ME, Lizardi P, Hedley DW, Bristow RG, Glazer PM. Down-regulation of Rad51 and deceased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24:8504–8518. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, Bristow RG, Classon MK, Glazer PM. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 27.Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, Huang LE. Hif-1 alpha induces genetic instability by transcriptionally down-regulating MutSa expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Bindra RS, Glazer PM. Repression of RAD51 gene expression by E2F4/p130 complexes in hypoxia. Oncogene. 2006;26:1–10. doi: 10.1038/sj.onc.1210001. [DOI] [PubMed] [Google Scholar]
- 29.Bindra RS, Glazer PM. Co-repression of mismatch repair gene expression by hypoxia in cancer cells: Role of the Myc/Max network. Cancer Lett. 2007;252:93–103. doi: 10.1016/j.canlet.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996;56:5754–5757. [PubMed] [Google Scholar]
- 31.Yuan J, Narayanan L, Rockwell S, Glazer PM. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60:4372–4376. [PubMed] [Google Scholar]
- 32.Nakamura H, Tanimoto K, Hiyama K, Yunokawa M, Kawamoto T, Kato Y, Yoshiga K, Poellinger L, Hiyama E, Nishiyama M. Human mismatch repair gene, MLH1, is transcriptionally repressed by the hypoxia-inducible transcription factors DEC1 and DEC2. Oncogene. 2008;3:1–10. doi: 10.1038/onc.2008.58. [DOI] [PubMed] [Google Scholar]
- 33.Ivanova SV, Salnikov K, Ivaniva AV, Bai L, Lerman MI. Hypoxic repression of STAT1 and its downstream genes by a pVHL/HIH-1 target DEC1/STRA13. Oncogene. 2007;26:802–812. doi: 10.1038/sj.onc.1209842. [DOI] [PubMed] [Google Scholar]
- 34.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF 1-regulated gene DEC1/Stra13: A mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 35.Shen M, Kawamoto T, Yan W, Nakamasu K, Tamagami M, Koyano Y, Noshiro M, Kato Y. Molecular characterization of the novel basic helix-loop-helix protein DEC1 expressed in differentiated human embryo chondrocytes. Biochem Biophys Res Commun. 1997;236:294–298. doi: 10.1006/bbrc.1997.6960. [DOI] [PubMed] [Google Scholar]
- 36.Gibson JS, Milner PI, White R, Fairfax TPA, Wilkins RJ. Oxygen and reactive oxygen species in articular cartilage: Modulators of ionic homeostasis. Eur J Phys. 2008;455:563–573. doi: 10.1007/s00424-007-0310-7. [DOI] [PubMed] [Google Scholar]
- 37.Bohensky J, Terkhorn SP, Freeman TA, Adams CS, Garcia JA, Shapiro IM, Srinivas V. Regulation of autophagy in human and murine cartilage: Hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009;60:1406–1415. doi: 10.1002/art.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uzuki M, Sawai T, Ryan LM, Rosenthal A, Masuda I. Characterization of ANK positive cells in joint tissue from patients with calcium pyrophosphate dihydrate crystal deposition disease (CPPD) Arthritis Rheum. 2005;52:S112. [Google Scholar]
- 39.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 40.Adesida AB, Grady LM, Khan WS, Millward-Sadler SJ, Salter DM, Hardingham TE. Human meniscus cells express hypoxia inducible factor -1a and increased SOX9 in response to low oxygen tension in cell aggregate culture. Arthritis Res Ther. 2007;9:R69. doi: 10.1186/ar2267. [DOI] [PMC free article] [PubMed] [Google Scholar]