Abstract
Skeletal formation is dependent on timely recruitment of skeletal stem cells and their ensuing synthesis and remodeling of the major fibrillar collagens, type I collagen and type II collagen, in bone and cartilage tissues during development and postnatal growth. Loss of the major collagenolytic activity associated with the membrane-type 1 matrix metalloproteinase (MT1-MMP) results in disrupted skeletal development and growth in both cartilage and bone, where MT1-MMP is required for pericellular collagen dissolution. We show here that reconstitution of MT1-MMP activity in the type II collagen–expressing cells of the skeleton rescues not only diminished chondrocyte proliferation, but surprisingly, also results in amelioration of the severe skeletal dysplasia associated with MT1-MMP deficiency through enhanced bone formation. Consistent with this increased bone formation, type II collagen was identified in bone cells and skeletal stem/progenitor cells of wildtype mice. Moreover, bone marrow stromal cells isolated from mice expressing MT1-MMP under the control of the type II collagen promoter in an MT1-MMP–deficient background showed enhanced bone formation in vitro and in vivo compared with cells derived from nontransgenic MT1-MMP–deficient littermates. These observations show that type II collagen is not stringently confined to the chondrocyte but is expressed in skeletal stem/progenitor cells (able to regenerate bone, cartilage, myelosupportive stroma, marrow adipocytes) and in the chondrogenic and osteogenic lineage progeny where collagenolytic activity is a requisite for proper cell and tissue function.
Key words: membrane-type 1 matrix metalloproteinase, type II collagen, bone cells, cartilage, bone marrow stromal cell, transgenic mouse
INTRODUCTION
Skeletal cells and their progenitors including the self-renewing subset of bone marrow stromal cells (BMSCs) proliferate and differentiate within an extracellular matrix abundant in the major fibrillar collagen types I, II, and III.(1–3) To modify and to enable cell movement within an environment of this composition, the mammalian organism uses two different strategies for the dissolution of collagen, specific either for the mineralized (hard) or the unmineralized (soft) matrices. In the remodeling of mineralized collagen matrix such as bone and calcified cartilage, osteoclasts, specialized cells of the monocyte-macrophage lineage, are used to exert their potent cathepsin-dependent dissolution of mineralized matrix.(4–7) Conversely, soft or unmineralized collagen-rich matrices are degraded and processed by resident connective tissue cells and vascular-associated cells expressing one or more collagenolytic enzymes, including but not limited to, the membrane bound matrix metalloproteinase MT1-MMP.(8–11) Loss of proteolytic activity after selective gene ablation of MT1-MMP leads to severe cellular defects not only in the soft connective tissues associated with mineralized bone but also significantly affects the timely dissolution of the unmineralized or hyaline cartilage anlagen in the skeleton.(12–14) These anatomical structures such as the parietal cartilage(15) normally emerge fully mature in late development and serve in part as repositories for cells with osteogenic potential and phenotype. The direct transformation of these cartilages into bone in early postnatal development through proteolysis has been identified as a separate bone formation mechanism distinct from endochondral ossification and is required for maintenance of unmineralized cartilage and sustained growth later in life. A prominent example of this mechanism in action is the formation of vascular canals in the condylar cartilage, which pave the way for the secondary ossification of long bones.(16,17) Ultimately the disruption of pericellular collagenolytic activity affects both bone and cartilage, yet thus far it remains to be determined to what extent the defects in the cartilaginous portion of the skeleton contribute to the overall skeletal dysplasia associated with disruption of pericellular collagenolysis.(18,19)
In an effort to segregate the effects of disrupted collagenolysis in the bone compartment of the skeleton from that in the cartilage compartment, we used a mouse model of MT1-MMP deficiency.(12) Reconstitution of MT1-MMP expression solely in type II collagen–expressing tissues of this mouse allowed us to evaluate the significance of coordinated collagen turnover in cartilage and its role in the formation of the skeleton as a whole. We report here that expression of MT1-MMP under the control of the type II collagen promoter/enhancer rescues, in part, the proliferation of chondrocytes, but surprisingly also enhances both intramembranous and endochondral bone formation significantly. Accordingly, type II collagen is expressed in a population of bone cells in wildtype mice and in BMSCs—a subset of which is the self-renewing skeletal stem cell.(20) Our results show that type II collagen is found in both the skeletal compartments of bone and cartilage and further that type II collagen and MT1-MMP are associated with bone marrow stromal precursors of bone and cartilage cells. These observations tie the fates and unimpeded functionality of skeletal cells to the expression of a defining extracellular matrix substrate, type II collagen, and its cognate proteolytic counterpart, MT1-MMP, thereby showing that a commitment to type II collagen expression is made early at the progenitor state of skeletal cells and is accompanied by expression of the requisite proteolytic tool for remodeling of this extracellular matrix component.
MATERIALS AND METHODS
MT1-MMP transgene construction
The mouse MT1-MMP cDNA was modified to delete the NotI site in the 3′ untranslated region (UTR) using Quickchange (Stratagene, La Jolla, CA, USA) and subsequently modified to add NotI sites upstream of the initiation methionine codon and at the most 3′ terminus. This NotI cassette was cloned into the pKN185 plasmid (kind gift from Dr. Yoshihiko Yamada, NIDCR) downstream of the collagen (II) α1 promoter/β-globin intron and upstream of the SV40 poly adenylation site and the α1 (II) collagen enhancer (Fig. 1A).
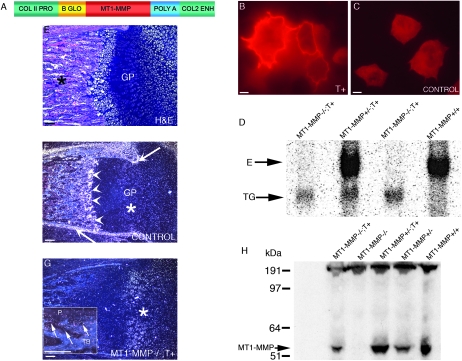
FIG. 1.
Transgene construction and expression of MT1-MMP in skeletal tissues. (A) Linear diagram of the collagen II/MT1-MMP transgene used for expression of MT1-MMP in cartilage tissue. Briefly, the mouse MT1-MMP cDNA (red) was placed under control of the type II collagen promoter/enhancer (green). Also shown is the β-globin intron (yellow) and the SV40 poly A signal sequence (blue). (B) Rat chondrosarcoma cells transfected with the transgene construct show abundant membrane-associated immunoreactivity when reacted with MT1-MMP–specific antibodies. Compare with C, depicting cells transfected with empty vector. (D) Northern blot of total RNA isolated from neonate mice reacted with an MT1-MMP–specific probe detects the endogenous transcript “E” and the transgene derived transcript “TG.” Lane 1, MT1-MMP−/−;T+ mouse; lane 2, MT1-MMP+/−;T+ littermate; lane 3, MT1-MMP−/−;T+ littermate; lane 4, MT1-MMP+/+ littermate. (E) H&E-stained femur from neonate control mouse showing the primary ossification center (asterisk) and the prospective epiphyseal growth plates “GP” of the distal condyle. (F) Dark field in situ hybridization image from the same block reacted with an mt1-mmp–specific antisense probe. Note the signal in periosteum (arrows) and the primary spongiosa (arrowheads). Signal is also detected in the proliferation zone of the prospective growth plate, although to a lesser extent than elsewhere (asterisk). (G) Section from femur of an MT1-MMP−/−;T+ mouse reacted with an antisense mt1-mmp probe. Note that expression is now restricted predominantly to the cartilage tissue (asterisks), whereas less, but still detectable, signal is found in the ossifying tissue (arrows inset in G, showing periosteal “P” and marrow signal between trabecular bone “TB”). (H) Immunoblot of costal chondrocyte extracts from neonate mice detecting MT1-MMP. Lane 1, MT1-MMP−/−;T+ mouse; lane 2, MT1-MMP−/− mouse; lane 3, MT1-MM+/−;T+ littermate; lane 4, MT1-MMP+/− littermate; lane 5, MT1-MMP+/+ littermate. Scale bars: (B and C) 10 μm; (E–G) 100 μm.
Chondrocyte transfection and immunodetection of the MT1-MMP transgene
Rat chondrosarcoma cells (21) were transfected with the transgene vector containing MT1-MMP using Lipofectamine (Invitrogen, Gaithersburg, MD, USA). The cells were fixed and reacted with a mouse anti-human MT1-MMP antibody 1135B7 (Chemicon, Temecula, CA, USA) or with a rabbit anti-mouse MT1-MMP antibody RP1MMP14 (Triple Point Biologics, Forest Grove, OR, USA). Antigen-bound primary antibody was visualized with Alexa Fluor conjugated secondary antibodies (Molecular Probes, Eugene, OR, USA).
Animal use and tissue processing
Animals used in this experiment were housed and handled according to animal study proposals approved by the National Institute of Dental and Craniofacial Research Animal Care and Use Committee.
MT1-MMP–deficient transgenic mice were bred by crosses between transgenic founder mice identified as “T+” and MT1-MMP+/− mice in an FVBN inbred background. Transgenic MT1-MMP–deficient mice were subsequently derived from interbreeding and designated “MT1-MMP−/−;T+.” For histology, tissues were fixed in 4% formaldehyde in PBS overnight, decalcified in 0.25 M EDTA in PBS, processed for paraffin embedding, sectioned at 6 μm, and used for H&E stains, in situ hybridization, or immunohistochemistry.
Alizarin red/Alcian blue staining of skeletons
Whole mount staining of the skeleton was performed as described.(13)
Measurement of the fontanel area
Fontanel width was measured on images of Alizarin red/Alcian blue–stained crania. The edges were traced with the Image J (NIH) free hand tool, and the resulting area was measured using the software. The statistical significance between MT1-MMP–deficient and MT1-MMP−/−;T+ animals was evaluated by two-tailed Student's t-test using Prism software.
μCT and X-ray imaging
μCT analysis of femora was performed using a GE Medical Systems eXplore Locus SP μCT scanner (GE Medical Systems, London, Ontario, Canada) at 8-μm resolution. Tissue mineral density was established on a 1.52-mm-thick cortical slice of the diaphysis using GE Health Care MicroView ABA 2.2 software (GE Medical Systems, London, Ontario, Canada). X-ray imaging was performed using a Faxitron MX-20 X-ray specimen radiography system and LifeRay HM Plus film (Ferrania Tecnologies, Cairo Montenotte, Italy) at 5-s, 20-KeV exposure settings. Bone length was defined as the distance between the mineralized proximal and distal bone collar of the femur (Fig. 3A). Cranial size was established on lateral X-ray exposures by measurement of the distance between the most posterior part of the occipital bone and the most anterior part of the nasal bone, in a straight line, using Image J software.
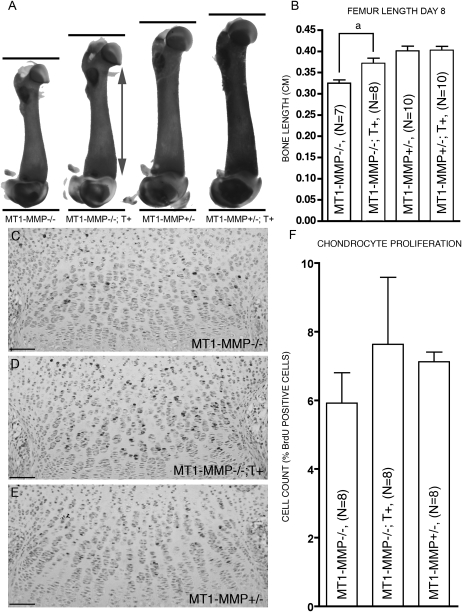
FIG. 3.
Cartilage-specific expression of MT1-MMP is associated with increased chondrocyte proliferation and longitudinal bone growth. (A) Alizarin red/Alcian blue–stained femora from 8-day-old littermates showing the difference in bone growth. Actual length of the femora was established using X-ray analysis (data not shown). (B) MT1-MMP−/−;T+ mice show significantly increased bone length compared with nontransgenic MT1-MMP−/− littermates. (C–F) Increased bone length in the MT1-MMP−/−;T+ mice is correlated with higher BrdU incorporation in chondrocytes of the epiphyseal growth plate (D) compared with MT1-MMP−/− mice (C). (E) MT1-MMP+/− control littermate. (F) Enumeration of proliferating cells from MT1-MMP−/−, MT1-MMP−/−;T+, and MT1-MMP+/− mice. Scale bar: (C–E): 100 μm. aStatistical significance (p < 0.01).
In vivo labeling
Animals were injected with BrdU (200 mg/kg body weight, IP) and killed 1 h later. Paraffin sections were reacted with a Zymed BrdU Staining Kit (Zymed, San Francisco, CA, USA). Proliferating chondrocytes were counted using Image J software, and the difference between MT1-MMP−/− and MT1-MMP−/−;T+ groups was evaluated by Student's t-test.
Immunohistochemistry
Type II collagen was detected with the II-II6B3 monoclonal antibody developed by T. F. Linsenmayer and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA. Sections were deparaffinized, hydrated, and blocked for endogenous peroxidase with 3% H2O2 in methanol, and antigen was retrieved by hyaluronidase treatment (Worthington, Lakewood, NJ, USA). Endogenous mouse immunoglobulin was blocked and primary antibody detected with Histomouse MAX Broad Spectrum (DAB) mouse on mouse kit (Zymed Laboratories, South San Francisco, CA, USA).
RNA isolation and RT-PCR
Primary chondrocytes for RNA extraction were cultured for 6 days and extracted with Trizol (Invitrogen, Carlsbad, CA, USA). DNase-treated RNA was reverse-transcribed with Ready-To-Go You-Prime First-Strand Beads (Amersham, Little Chalfont, UK). MT1-MMP was amplified using the oligonucleotide primers KH17 5′-ACAGAGAACTTCGTGTTGCCTGATGAC-3′ and KH19 5′-CCCATTGGGCATCCAGAAGAGAGCT-3′ (GenBank accession no. X83536) specific for exons 7 and 8, respectively. Type II collagen was amplified with the primers col2fw 5′-CCTCATCTTGCCGCATCTGTGT-3′ and col2rev 5′-AGCCTTCTCGTCATACCCTCCA-3′ specific for exon 2 and exon 5, respectively (GenBank accession no. BC051383.1).
Western blot analysis
Primary chondrocytes were cultured for 6 days, lysed in 10 mM phosphate buffer, pH 7.0, 2% SDS, 10 mM phenylmethanesulphonylfluoride (PMSF) 2 mM benzamidine, 10 μg/ml aprotinin, and 4 μg/ml leupeptin, homogenized, boiled, and fractionated on NuPage BisTris gels with 3-(N-morpholino) propanesulfonic acid (MOPS) running buffer (Invitrogen), transferred to nitrocellulose membranes (Whatman, Tewksbury, MA, USA), and incubated with the monoclonal antibody 5F8:H3.(22)
Northern blot analysis
RNA from newborn pups was extracted with Trizol (Invitrogen), and 30–50 μg of RNA was fractionated on formaldehyde agarose gels, transferred to Nytran (Whatman), and hybridized to a [α-32P] dCTP–radiolabeled DNA probe specific for MT1-MMP sequences from exon 2 to exon 4.
In situ hybridization
Sections were hybridized to RNA probes radiolabeled with [α-33P] UTP (Perkin-Elmer NEN, Shelton, CT, USA) as described.(23) MT1-MMP mRNA was detected with a probe corresponding to nucleotides 291–902 of the mouse cDNA (GenBank accession no. X83536). Type II collagen was detected using a collagen (II) α1 chain–specific 1.1-kb cDNA probe transcribed off pKN225 (kindly provided by Dr. Yoshihiko Yamada, NIDCR). This sequence corresponds to part of exon 50-54 (GenBank accession no. BC051383.1).
Isolation of chondrocyte primary cultures
Rib and the sternum cartilages were isolated from 5- to 7-day-old mice. Soft tissue was removed after 90-min incubation at 37°C in DMEM supplemented with 3 mg/ml collagenase type 2 (Worthington) and several washes in PBS. The cleaned cartilage was transferred to 6-well dishes and digested until the cells were fully dispersed. Suspended chondrocytes were aspirated and washed two times with PBS and plated in complete DMEM medium with 10% FBS.
Isolation of BMSCs and in vivo bone formation
BMSCs were isolated from adult wildtype, MT1-MMP–deficient mice or MT1-MMP−/−;T+ mice as described previously.(24) In vitro osteogenic differentiation was performed as described.(25) In vivo osteogenic capacity was assessed in either Gelfoam gelatin scaffold (Pharmacia & Upjohn, Kalamazoo, MI, USA) or hydroxyapatite/tricalcium phosphate ceramic powder (HA/TCP; Zimmer, Warsaw, IN, USA) after transplantation under the skin of Hsd:NIHS-Lysbg Foxn1nu BtkXid, Beige nude Xid (NIH III) mice (Harlan Sprague-Dawley, Indianapolis, IN, USA) and harvesting 10 or 13 wk after transplantation.(24)
RESULTS
To address the role of MT1-MMP in cartilaginous tissues, we constructed an expression vector containing a mouse MT1-MMP cDNA under the control of the collagen (II) α1 promoter/enhancer (Fig. 1A). We first tested the ability of this construct, henceforth denoted T+, to direct expression of immuno-detectable MT1-MMP in cartilage cells after transfection of rat chondrosarcoma cells.(21) Cells transfected with the transgene construct readily showed abundant membrane-associated immunoreactivity (Fig. 1B), whereas cells transfected with an empty vector construct did not show this staining pattern (Fig. 1C).
After pronuclear injection of the transgene, three lines of mice were selected from a pool of transgene positive founder mice based on RT-PCR analysis (data not shown) and analyzed individually. To establish the physiological effects of cartilage-specific expression of MT1-MMP in an otherwise MT1-MMP–deficient background,(12) we next bred T+ mice to MT1-MMP+/− FVB/N mice and subsequently generated MT1-MMP–deficient transgene positive (MT1-MMP−/−; T+) offspring by interbreeding of littermates. Northern blot analysis on total neonate tissues (Fig. 1D) showed that the transgene was expressed and mice carrying the transgene showed no gross adverse effects of the cartilage-specific expression of MT1-MMP nor did histology show any signs of tissue destruction or increased cell demise in cartilage tissues. We further ascertained that the mRNA from the transgene was expressed in cartilage tissues by in situ hybridization of tissue sections from T+ mice. This analysis showed that the mRNA encoded by the transgene was expressed in chondrocytes of all cartilaginous tissues (Fig. 1G) and surprisingly also in noncartilaginous bone lining tissues and bone cells (Fig. 1G, inset). In control mice, MT1-MMP expression is most predominant in bone and bone-associated soft tissues (Figs. 1E and 1F).(13,18)
To confirm that the transgene was expressed as an immuno-reactive protein in mice, we isolated costal chondrocytes derived from neonate MT1-MMP−/−; T+ mice and verified the expression of transgene derived MT1-MMP by immunoblot. As expected from the earlier validation of the transgene function in chondrosarcoma cells and by in situ hybridization on tissue sections, mouse chondrocytes also expressed MT1-MMP protein equivalent to that found in control WT or MT1-MMP+/− mice. We concluded, based on these results, that the transgene was functional, directed expression in the intended location and that the resulting message was translated into protein (Fig. 1H).
Expression of transgene-derived cartilage specific MT1-MMP in MT1-MMP–deficient mice improves survival and increases body size
MT1-MMP−/−;T+ mice were born in normal Mendelian ratios and were initially indistinguishable from their MT1-MMP−/− littermates. Despite transgene expression MT1-MMP−/−; T+ mice showed the same characteristic dome shape of the head as MT1-MMP−/− mice.(12) Closer analysis showed that the embryonic cartilages linked to the cranial dysmorphism in MT1-MMP−/− mice also persisted in MT1-MMP−/−;T+ mice despite the transgene expression in the chondrocytes comprising these anatomical structures (data not shown). Analysis for the presence of vascular canal formation in the condyles of long bones showed that transgene expression there did not facilitate the dissolution of cartilage as observed in the wildtype mice. Based on these observations, we concluded that the expression of MT1-MMP in chondrocytes alone was insufficient to facilitate matrix dissolution or that the concomitant expression of type II collagen in cartilage outweighed the proteolytic activity of the MT1-MMP, so no net tissue dissolution was achieved.
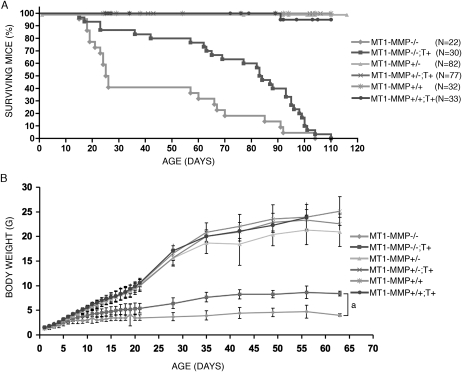
Despite the lack of cartilage dissolution in the head and in the condyles, MT1-MMP−/−;T+ mice survived until weaning in far greater numbers than their MT1-MMP–deficient littermates. Specifically, the median survival time for MT1-MMP−/−;T+ mice was 83.5 days (N = 30), whereas median survival time for MT1-MMP−/− mice was 25.5 days (N = 22). The difference in survival rate could be attributed in part to a complete rescue of the 33% preweaning death observed in MT1-MMP−/− and in part to increased longevity of MT1-MMP−/−; T+ mice compared with the life span of MT1-MMP−/− littermates (Fig. 2A). Additionally, measurement of body weight daily for 21 days after birth and weekly thereafter for a total of 9 wk or to the time of death documented a significant difference in the body weight between MT1-MMP−/− and MT1-MMP−/−;T+ mice (Fig. 2B). This difference was noted starting at day 4 after birth (1.94 ± 0.49 versus 2.20 ± 0.38 g, p < 0.05) and the disparity increased throughout life. We thus concluded that cartilage specific transgene expression promoted not only survival but also the ability of mice to gain weight.
FIG. 2.
Survival and weight gain in mice. (A) Survival of mice. Note the significant reduction in mortality associated with expression of the MT1-MMP transgene (T+) in the MT1-MMP–deficient background (MT1-MMP−/−;T+) compared with MT1-MMP−/− mice. (B) Weight gain in mice after birth measured daily until 21 days of age and then weekly. The expression of the MT1-MMP transgene in MT1-MMP–deficient mice significantly increases weight gain over nontransgenic littermates. aStatistical significance (p < 0.05).
Expression of the MT1-MMP transgene in MT1-MMP–deficient mice results in increased length of the long bones
To study the basis of the increased body size of MT1-MMP−/−;T+ mice in detail, we performed analysis of the skeletal tissues expressing the transgene. First, to address whether there was a significant increase in the bone length in MT1-MMP−/−;T+ animals compared with MT1-MMP−/− animals, we dissected and X-rayed hind limbs and measured the bone length. Transgenic knockout animals had increased lengths of the tibias and femora compared with knockout littermates. This difference was significant starting at day 8 after birth and continued throughout the life of the animals (Figs. 3A, 3B, and 4A)
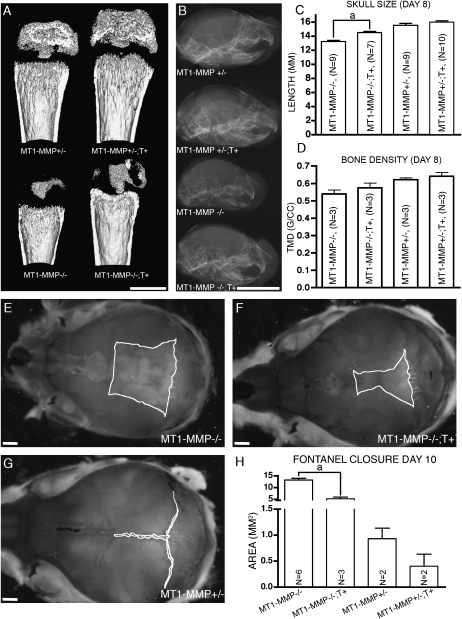
FIG. 4.
Expression of collagen II driven MT1-MMP is associated with increased bone formation. (A) CT images of femora from 15-day-old littermates. Note that presence of the MT1-MMP transgene in MT1-MMP−/−;T+ mice increases the length of bone and the degree of ossification. (B) Crania from 8-day-old mice show increased skull length in MT1-MMP−/−;T+ mice (bottom) compared with nontransgenic MT1-MMP−/− littermates (second from bottom). (C and D) Quantitation of skull length and tissue mineral density (TMD) based on the data shown in A and B. (E–G) Alizarin red/Alcian blue whole mount preparation of crania from 8-day-old mice visualizing the posterior fontanelle. MT1-MMP−/− mice (E) show significantly larger fontanelle sizes compared with the MT1-MMP−/−;T+ littermates (F). However, virtually complete closure as seen in MT1-MMP+/− control is not achieved (G). (H) Measurements of fontanelle areas in 8-day-old mice. Note the expression of the MT1-MMP transgene confers significant reduction of the fontanelle size. Scale bars: (B) 5 mm; (A and E–G): 1 mm. aStatistical significance in C and H (p < 0.001).
Second, to establish whether the increased growth of the long bones in MT1-MMP−/−;T+ animals could be attributed to increased chondrocyte proliferation in the epiphyseal growth plate, we detected proliferating chondrocytes by immunohistochemistry for bromodeoxyuridine (BruU)-labeled cells (Figs. 3C–3F). Enumeration of BrdU+ chondrocytes at day 8 showed that proliferation was higher in MT1-MMP−/−;T+ than in MT1-MMP−/− mice (7.63 ± 1.95% versus 5.91 ± 1.95%, p = 0.44). Based on this observation, we concluded that the increased length of the long bones observed in MT1-MMP−/−; T+ mice compared with nontransgenic MT1-MMP−/− mice likely was caused by an increase in chondrocyte proliferation equivalent to the proliferation rate seen in control MT1-MMP+/− mice (7.12 ± 0.27%).
In the cranium, the expression of the MT1-MMP transgene likewise increased growth significantly in MT1-MMP−/−; T+ mice measuring 14.5 ± 3.99 (N = 7) versus 13.2 ± 5.06 mm (N = 9) recorded for MT1-MMP−/− mice (p < 0.001). Thus, in addition to the increased length of the long bones, MT1-MMP−/−; T+ mice also showed larger heads as a consequence of the transgene expression (Figs. 4B and 4C).
The cranium of MT1-MMP−/−; T+ mice not only showed increased length, but bone formation also proved to be enhanced compared with MT1-MMP−/− mice when evaluated by measurement of the sagittal fontanel expanse (Figs. 4E–4H). This area is ordinarily diminished in size with the growth of the intramembranous parietal, frontal, and interparietal bones in wildtype mice and generally leads to closure of the fontanels by day 5 postnatally. In MT1-MMP−/− mice, however, the fontanel is conspicuous even past the time of closure in wildtype mice and can be recognized easily in aged animals (Fig. 4E). In contrast, the area of the fontanels in MT1-MMP−/−; T+ mice was consistently smaller compared with MT1-MMP–deficient mice, thus showing increased bone formation in the cranial bones (Fig. 4F). The smaller fontanels in MT1-MMP−/−; T+ animals were grossly evident at birth and became significantly different in size from day 2 postnatally (Fig. 4H).
The apparent correlation between MT1-MMP expression and increased bone formation in the skull led us to ask if this phenomenon also could be observed in long bones. Indeed, long bones from MT1-MMP−/−; T+ mice consistently showed more substantial cortices than MT1-MMP–deficient mice when analyzed by X-ray (data not shown) and μCT analysis (Figs. 4A and 4D).
Type II collagen is expressed in bone cells of wildtype mice
In contrast to the connection between the expression of MT1-MMP in cartilage and increased chondrocyte proliferation, there is no straightforward explanation for the increase in bone formation after cartilage-specific MT1-MMP expression.
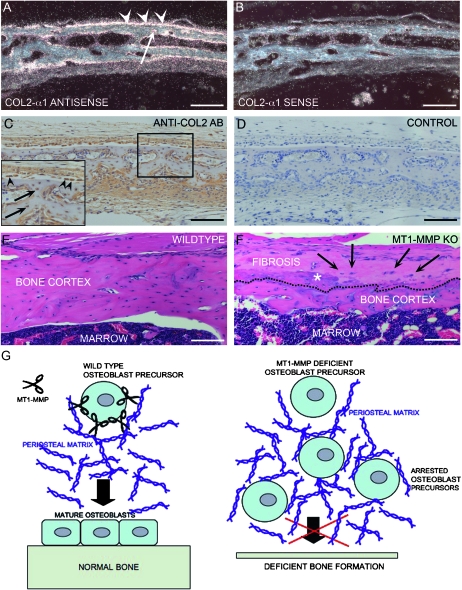
This disconnect led us to question how expression of the transgene could account for the apparent effect on bone cells. Notably, the effect of transgene expression on bone formation was observed in three independent transgenic founder lines and we therefore discounted the notion that ectopic expression by promoter leakage could result in increased bone formation. To reconcile the expression pattern and biological effect, we analyzed wildtype mice for expression of type II collagen in noncartiligenous tissues. Surprisingly, we documented a substantial expression by in situ hybridization in osteogenic tissues, which showed additional bone formation after transgene expression. Accordingly, periosteal tissues of the parietal bone, osteoblasts, and osteocytes showed prominent expression of type II collagen, thus explaining how MT1-MMP expression under a type II collagen promoter/enhancer could be directed to bone-forming tissues in addition to cartilage (Figs. 5A and 5B). The expression was distinctly localized to soft tissue and bone cells proper and clearly discernible from signal derived from cells in cartilage cores of endochondral bone. Because of the rather surprising nature of this observation, we additionally confirmed the presence of type II collagen expression by immunohistochemistry for type II collagen (Figs. 5C and 5D). As expected from the expression pattern shown by in situ hybridization, the identical anatomical structures including osteoblasts and osteocytes also showed immunoreactivity for type II collagen, thereby proving that this protein is in fact expressed in at least a subset of bones and bone-associated cells.
FIG. 5.
Type II collagen in bone cells and function of MT1-MMP. (A) In situ hybridization of the parietal bone from a wildtype mouse. Bone cells (arrowheads) and osteocytes (arrow) react with a collagen (II) α1–specific antisense probe. (B) Section serial to that shown in A reacted with collagen (II) α1 sense control probe. (C and D) Immunohistochemistry for collagen II in bone shows that bone cells and soft connective tissue stain positive for type II collagen. Inset in C depicts high-power magnification of the bone outline in the boxed area with type II collagen–positive osteoblasts (arrowheads) and osteocytes (arrows). (D) Negative control of nonserial section from the same specimen. (E) Sagittal section through the tibial cortex of a 59-day-old wildtype mouse showing abundant cortical bone and associated marrow. (F) Equivalent section of MT1-MMP–deficient littermate showing abundant scarring and fibrosis of the periosteum (above the dashed line) including ectopic calcification (asterisk) and highly aberrant bone matrix below the dashed line. Several osteogenic cells are entrapped in the fibrotic tissue (arrows) (G) Cartoon depicting the result of a cellular deficit in MT1-MMP–dependent collagenolysis and the consequences for remodeling of pericellular collagen. In the absence of MT1-MMP–mediated collagen remodeling, cells become entrapped in collagen as shown in F (arrows), and bone formation and periosteal maintenance are perturbed. Scale bar: (A–F) 100 μm.
MT1-MMP expression in BMSC supports bone, marrow, and adipocyte formation
We previously showed that MT1-MMP is essential for sustained bone cell function where it facilitates the remodeling of pericellular matrices, which enable cells to migrate and dispense with existing collagen-rich tissue in the process of growth as shown in Figs. 5E and 5F and outlined schematically in Fig. 5G.(12,18) A plausible explanation for the observation of more bone matrix after expression of MT1-MMP under the expression of type II collagen could therefore be that type II collagen is expressed not only in cartilage but also in bone cells, where it enables transgene expression and thus rescues MT1-MMP deficiency.
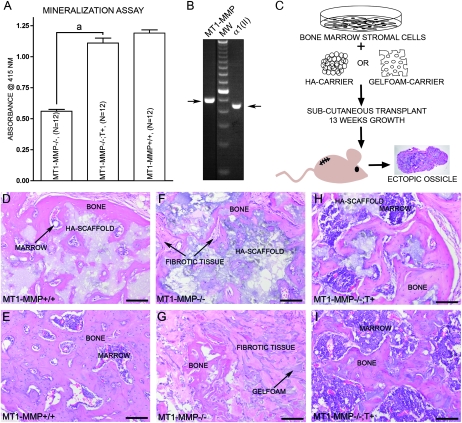
To discover whether there is a functional connection between the observation of type II collagen expression and the apparent ability of bone cells to produce more bone matrix in the presence of transgene-derived MT1-MMP, we isolated BMSCs from MT1-MMP−/− and MT1-MMP−/−; T+ mice. After expansion in culture, we first measured the ability of the cells to support calcium sequestration in vitro by alizarin red staining of cells exposed to osteogenic culture medium in long-term cultures. As expected, cells derived from MT1-MMP−/−;T+ mice accumulated more calcium detectable by alizarin red than cells derived from MT1-MMP−/− littermates (Fig. 6A). When wildtype cells were analyzed for mRNA specific for MT1-MMP and type II collagen, both species were found in detectable quantities by RT-PCR (Fig. 6B). Based on this observation, the promoter of the transgene would therefore be active in null cells, and they would express MT1-MMP. To validate these results in vivo, we next transplanted cells in osteoconductive scaffolds of either hydroxyapatite/tricalcium phosphate or gelatin sponges into immunocompromised mice (Fig. 6C). With both of these scaffolds, cells derived from MT1-MMP−/−;T+ mice generated substantially more bone matrix than the scant amounts observed in transplants made with MT1-MMP−/− cells (Figs. 6D–6I).
FIG. 6.
Expression of type II collagen in BMSCs and analysis of their osteogenic potential. (A) BMSCs derived from either MT1-MMP−/−, MT1-MMP−/−;T+, or MT1-MMP+/− mice show an increasing ability to form in vitro mineralized matrix in osteoconductive medium when measured as alizarin red retention. (B) In accordance with the results observed by in situ hybridization, immunostaining, and in the mineralization assay, BMSCs from wildtype mice show MT1-MMP and type II collagen–specific mRNA by RT-PCR (samples run on the same gel, but not adjacent lanes). This observation explains the expression derived from the collagen II–MT1-MMP transgene and the resulting MT1-MMP expression in BMSCs isolated from MT1-MMP−/−;T+ mice. (C) To test whether the osteogenic potential observed in vitro is biologically relevant, BMSCs on osteoconductive media were implanted in nude mice and allowed to form ectopic ossicles. (D and E) Cells derived from wildtype mice showed the ability to form both bone and marrow in hydroxyapatite (D) or Gelfoam scaffolds (E). Cells derived from MT1-MMP−/− mice failed almost completely to form bone in either hydroxyapatite (F) or Gelfoam (G) osteoinductive carriers nor did they support formation of marrow. Instead, extensive fibrosis was observed (arrows) (F and G). When MT1-MMP−/− cells expressed the collagen II–driven MT1-MMP transgene, the osteogenic potential was restored, and the cells generated abundant bone and marrow with both hydroxyapatite (H) and Gelfoam (I) as scaffolds, thus showing that the type II collagen promoter is active in BMSCs. Scale bar: (D–I) 100 μm. aStatistical significance (p < 0.001).
These experiments thus proved that type II collagen promoter–driven MT1-MMP was expressed by BMSCs and in their later differentiation states. This expression conferred an increased osteogenic potential on the cells in comparison with MT1-MMP–deficient cells as suggested by the observation of increased calcium accumulation in vitro and bone formation in vivo in the MT1-MMP−/−; T+ mice (Figs. 6H and 6I). The most important distinction, however, is that expression of MT1-MMP in BMSCs, in addition to bone, enabled formation of myelosupportive stroma and adipogenesis, properties that characterize skeletal stem cells.
DISCUSSION
To dissect how tissue-specific expression of collagenolytic activity affected cartilage tissues and, in turn, skeletal development, we reconstituted MT1-MMP expression using a transgene driven by a heterologous type II collagen promoter, thereby enabling expression of MT1-MMP in type II collagen–expressing tissues. Using this animal model of selective MT1-MMP expression in an otherwise MT1-MMP–null background, we showed that type II collagen is restricted not only to cells of chondrocytic phenotype but remarkably is also expressed in bone cells and their common mesenchymal progenitor within the bone marrow stroma. Despite this somewhat surprising result, we found that our observations reflect the endogenous type II collagen expression profile in wildtype mice, which previously has been considered to be restricted solely to cartilage. Initial results derived from the MT1-MMP–deficient mouse model have shown that type I and type II collagen remodeling is a prerequisite for functionality of cells associated with the skeleton such as periosteal fibroblastoid cells, tenocytes, chondrocytes, and osteogenic cells proper.(12,14)
Initially, we observed that mice carrying this transgene, despite expression of protein in detectable quantities, did not suffer adverse effects such as dissolution of the articular cartilage or other hyaline cartilages such as tracheal cartilages. This led us to conclude that the mere expression of MT1-MMP even in amounts exceeding the endogenous expression level does not lead to tissue destruction as could be expected after a substantial increase in the protease levels such as that attained with endogenous and transgene-derived MT1-MMP expression in wildtype mice that carry the transgene. Whereas MMP activity is capable of dissolving major components of the cartilage matrix in, for instance, articular cartilage, we deduce from our observations that the loss of cartilage matrix whether coordinated developmentally or associated with pathophysiological processes, is dependent on both a cessation in synthesis of major matrix components and a critical level of MMP expression, as well as inhibitor levels.(18,26,27)
A prominent and revealing observation in our study is the effect of transgene expression on the viability of MT1-MMP–deficient mice. Loss of MT1-MMP is normally associated with a high degree of perinatal and preweaning mortality caused by defects yet to be fully characterized.(12) Surprisingly we observed that selective reconstitution of MT1-MMP expression in only chondrogenic and osteogenic cell types that ordinarily express MT1-MMP is sufficient to rescue all the perinatal and preweaning lethality observed in the null background. We hereby firmly establish that the major functional deficit affecting the viability of MT1-MMP–deficient mice is the loss of MT1-MMP expression in cartilage and a subset of bone cells. This finding specifies skeletal development and growth properties dependent on collagenolysis as essential to survival of mice in early life and its disruption in the absence of MT1-MMP as the major cause of perinatal death. Skeletal development therefore emerges as essential to the overall survival of the mice and highlights the integral role of the skeleton as a vital component of the organism.
We observe that two hallmarks of MT1-MMP deficiency, the persistence of cranial cartilages and defective vascular (cartilage) canal formation, are not rescued by the expression of MT1-MMP under the type II collagen promoter. We have previously described the role of MT1-MMP in these processes and the prominent defects associated with the loss of MT1-MMP. In the cranium, we observed that the MT1-MMP upregulation in chondrocytes coincides with cessation of type II collagen expression and loss of proteoglycan from the matrix.(12,18) Despite systemic deletion of the known endogenous inhibitors of MMPs (TIMPs), the homeostasis of cranial cartilages before MT1-MMP–mediated dissolution is not affected.(28–32) Inhibitor expression thus seems to play a limited role in maintaining homeostasis before degradation. An unresolved question was therefore whether sustained expression of MT1-MMP and the associated pericellular proteolysis could override the accumulation of concomitantly synthesized matrix components. We infer from our observations here that matrix deposition in cranial cartilages equals the loss of matrix to proteolytic degradation thus ensuring maintenance of the collagen scaffold in the cranial cartilages despite expression of MT1-MMP.
Cartilage vascular canals are considered to facilitate the establishment of the secondary ossification center through the supply of osteogenic cells and osteoclast precursors to the center of the long bone condyle and also to facilitate ossification of the condylar cartilage matrix.(16,33) Moreover, the cartilage canals have been proposed as central in the recruitment of skeletal stem cells for supply of the proliferative activity associated with the prospective epiphyseal growth plate.(17,34) We observed here that, despite reconstitution of the expression of MT1-MMP in chondrocytes, the formation of vascular canals is not rescued in MT1-MMP−/−;T+ mice. If chondrocytes themselves are responsible for the dissolution of the resident matrix, as observed in certain unmineralized cartilage tissues such as the parietal cartilage, we infer that the sustained matrix synthesis prevents rescue of the canal formation despite upregulation of proteolytic degradation.(18) Alternatively, the identity of the cells undertaking the dissolution of the unmineralized part of the condyle in the process of canal formation may be a vascular endothelial cell–type expressing MT1-MMP/MT3-MMP related or identical to a cell type that is responsible for the resorption of transverse unmineralized septae in the primary ossification center of the developing long bone.(35)
Despite failure to efficiently resolve unmineralized cartilage matrices in the skull and in long bone condyles, the expression of the transgene has a significant effect on the proliferation of chondrocytes in the epiphyseal growth plate, with a marked increase in longitudinal growth of long bones as a result. We propose, based on previous observations, that cell proliferation and expression of MT1-MMP in this location is associated with limited dissolution of collagen matrix and the formation of physical space that allows cell division in an otherwise rigid and restraining matrix.(12,13,36)
The most remarkable and unexpected finding of our study was the expression of type II collagen in wildtype bone cells (Fig. 5) and the resulting increase in bone formation in the presence of our transgene construct. This type II collagen promoter–directed MT1-MMP expression in bone cells significantly increased bone formation in all parts of the skeleton, showing that both craniofacial and noncraniofacial bone formation was partially rescued by the collagenolytic activity acquired by osteogenic cells through the expression of transgene encoded MT1-MMP. Our analysis of the expression pattern for type II collagen in wildtype mice showed that the MT1-MMP expression directed by the transgene reflected true type II collagen promoter activity (Fig. 5). Findings of transient type II collagen expression in bone-forming cells at fracture sites have previously been shown, and more recently, sustained Col2α1 expression was convincingly proven in a population of trabecular and cortical bone cells in addition to expression in a subset of BMSCs.(37–39) Here we present further evidence in the form of increased MT1-MMP–dependent bone formation, in vivo, to suggest that type II collagen expression in at least a subset of bone-forming cells is sustained over a longer period of time in normal bone development without histological evidence of cartilage formation. We further showed that skeletal progenitor cells display similar type II collagen expression, which is sufficient to drive ectopic ossicle formation with myelosupportive stroma and adipogenesis, whereas, in the absence of a type II collagen–driven MT1-MMP transgene, such cells are largely defective in their ability to generate bone in vivo because of abrogation of collagenolytic activity. The expression of type II collagen has been reported previously in BMSCs, yet whether this was a feature of in vitro culture was unclear.(40) We show here that type II collagen expression is an in vivo feature of a cell population in which the self-renewing skeletal stem cell can be found.(20) As such, we suggest that type II collagen is expressed in early undifferentiated states of bone marrow stroma in vivo and thus shows the relationship and likely overlapping phenotypes of bone and cartilage as suggested by the apparent expression of late osteogenic markers in “ghost” cartilage rudiments found in the cranium of MT1-MMP–deficient mice.(18) Taken together, our findings point to type II collagen and MT1-MMP as specifying the skeletal stem/progenitor cell population. In this context, it is important to note that the loss of MT1-MMP itself does not disrupt bone formation in the mouse completely but rather results in progressive inability to remodel bone–soft tissue interfaces, which leads to an increasing accumulation of collagen in the pericellular environment that ultimately halts bone formation.(12,13) Based on the criteria that the stem/progenitor cell and its lineage progeny support the cellular remodeling of a dynamic extracellular environment, we therefore propose that MT1-MMP has an essential role in skeletal stem cells.
ACKNOWLEDGMENTS
The authors thank Pamela Gehron Robey, PhD, for comments and critical reading of the manuscript. This study was supported by the DIR, NIDCR of the IRP, NIH, and by European Union contract LSHC-CT-2003-50329 (FP6), by the European Union FP7 program and by grants from the Danish Cancer Society, the Danish Cancer Research Foundation, the Lundbeck Foundation, and the Danish Medical Research Council. S.I. has a personal grant from the University of Copenhagen, the Faculty of Science.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 2.Ricard-Blum S, Ruggiero F. The collagen superfamily: From the extracellular matrix to the cell membrane. Pathol Biol (Paris) 2005;53:430–442. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Goto T, Yamaza T, Tanaka T. Cathepsins in the osteoclast. J Electron Microsc (Tokyo) 2003;52:551–558. doi: 10.1093/jmicro/52.6.551. [DOI] [PubMed] [Google Scholar]
- 5.Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113:377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 6.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 7.Everts V, Korper W, Hoeben KA, Jansen ID, Bromme D, Cleutjens KB, Heeneman S, Peters C, Reinheckel T, Saftig P, Beertsen W. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: Differences between calvaria and long bone. J Bone Miner Res. 2006;21:1399–1408. doi: 10.1359/jbmr.060614. [DOI] [PubMed] [Google Scholar]
- 8.Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, Roberts JD, Fay WP, Birkedal-Hansen H, Holmbeck K, Sabeh F, Allen ED, Weiss SJ. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005;202:663–671. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmbeck K, Bianco P, Yamada S, Birkedal-Hansen H. MT1-MMP: A tethered collagenase. J Cell Physiol. 2004;200:11–19. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- 10.Plaisier M, Kapiteijn K, Koolwijk P, Fijten C, Hanemaaijer R, Grimbergen JM, Mulder-Stapel A, Quax PH, Helmerhorst FM, van Hinsbergh VW. Involvement of membrane-type matrix metalloproteinases (MT-MMPs) in capillary tube formation by human endometrial microvascular endothelial cells: Role of MT3-MMP. J Clin Endocrinol Metab. 2004;89:5828–5836. doi: 10.1210/jc.2004-0860. [DOI] [PubMed] [Google Scholar]
- 11.Song F, Wisithphrom K, Zhou J, Windsor LJ. Matrix metalloproteinase dependent and independent collagen degradation. Front Biosci. 2006;11:3100–3120. doi: 10.2741/2036. [DOI] [PubMed] [Google Scholar]
- 12.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Son MY, Yamada S, Szabova L, Kahan S, Chrysovergis K, Wolf L, Surmak A, Holmbeck K. Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev Biol. 2008;313:196–209. doi: 10.1016/j.ydbio.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Ovchinnikov D, Pressman CL, Aulehla A, Lun Y, Johnson RL. Multiple calvarial defects in lmx1b mutant mice. Dev Genet. 1998;22:314–320. doi: 10.1002/(SICI)1520-6408(1998)22:4<314::AID-DVG2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Blumer MJ, Longato S, Schwarzer C, Fritsch H. Bone development in the femoral epiphysis of mice: The role of cartilage canals and the fate of resting chondrocytes. Dev Dyn. 2007;236:2077–2088. doi: 10.1002/dvdy.21228. [DOI] [PubMed] [Google Scholar]
- 17.Lutfi AM. Mode of growth, fate and functions of cartilage canals. J Anat. 1970;106:135–145. [PMC free article] [PubMed] [Google Scholar]
- 18.Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H. MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: A critical process in skeletal growth. J Cell Biol. 2003;163:661–671. doi: 10.1083/jcb.200307061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roach HI, Aigner T, Kouri JB. Chondroptosis: A variant of apoptotic cell death in chondrocytes? Apoptosis. 2004;9:265–277. doi: 10.1023/b:appt.0000025803.17498.26. [DOI] [PubMed] [Google Scholar]
- 20.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Mukhopadhyay K, Lefebvre V, Zhou G, Garofalo S, Kimura JH, de Crombrugghe B. Use of a new rat chondrosarcoma cell line to delineate a 119-base pair chondrocyte-specific enhancer element and to define active promoter segments in the mouse pro-alpha 1(II) collagen gene. J Biol Chem. 1995;270:27711–27719. doi: 10.1074/jbc.270.46.27711. [DOI] [PubMed] [Google Scholar]
- 22.Ingvarsen S, Madsen DH, Hillig T, Lund LR, Holmbeck K, Behrendt N, Engelholm LH. Dimerization of endogenous MT1-MMP is a regulatory step in the activation of the 72 kDa gelatinase, MMP-2, on fibroblasts and fibrosarcoma cells. Biol Chem. 2008;389:943–953. doi: 10.1515/BC.2008.097. [DOI] [PubMed] [Google Scholar]
- 23.Szabova L, Yamada SS, Birkedal-Hansen H, Holmbeck K. Expression pattern of four membrane-type matrix metalloproteinases in the normal and diseased mouse mammary gland. J Cell Physiol. 2005;205:123–132. doi: 10.1002/jcp.20385. [DOI] [PubMed] [Google Scholar]
- 24.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: Comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 25.Bianco P, Kuznetsov SA, Riminucci M, Gehron Robey P. Postnatal skeletal stem cells. Methods Enzymol. 2006;419:117–148. doi: 10.1016/S0076-6879(06)19006-0. [DOI] [PubMed] [Google Scholar]
- 26.Sahebjam S, Khokha R, Mort JS. Increased collagen and aggrecan degradation with age in the joints of Timp3(−/−) mice. Arthritis Rheum. 2007;56:905–909. doi: 10.1002/art.22427. [DOI] [PubMed] [Google Scholar]
- 27.Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107:789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 28.Caterina JJ, Yamada S, Caterina NC, Longenecker G, Holmback K, Shi J, Yermovsky AE, Engler JA, Birkedal-Hansen H. Inactivating mutation of the mouse tissue inhibitor of metalloproteinases-2(Timp-2) gene alters proMMP-2 activation. J Biol Chem. 2000;275:26416–26422. doi: 10.1074/jbc.M001271200. [DOI] [PubMed] [Google Scholar]
- 29.Soloway PD, Alexander CM, Werb Z, Jaenisch R. Targeted mutagenesis of Timp-1 reveals that lung tumor invasion is influenced by Timp-1 genotype of the tumor but not by that of the host. Oncogene. 1996;13:2307–2314. [PubMed] [Google Scholar]
- 30.Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, Mak TW, Khokha R. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3) J Clin Invest. 2001;108:817–829. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.English JL, Kassiri Z, Koskivirta I, Atkinson SJ, Di Grappa M, Soloway PD, Nagase H, Vuorio E, Murphy G, Khokha R. Individual Timp deficiencies differentially impact pro-MMP-2 activation. J Biol Chem. 2006;281:10337–10346. doi: 10.1074/jbc.M512009200. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez J, Costales L, Lopez-Muniz A, Lopez JM. Chondrocytes are released as viable cells during cartilage resorption associated with the formation of intrachondral canals in the rat tibial epiphysis. Cell Tissue Res. 2005;320:501–507. doi: 10.1007/s00441-004-1034-z. [DOI] [PubMed] [Google Scholar]
- 34.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawae Y, Sahara T, Sasaki T. Osteoclast differentiation at growth plate cartilage-trabecular bone junction in newborn rat femur. J Electron Microsc (Tokyo) 2003;52:493–502. doi: 10.1093/jmicro/52.6.493. [DOI] [PubMed] [Google Scholar]
- 36.Wagenaar-Miller RA, Engelholm LH, Gavard J, Yamada SS, Gutkind JS, Behrendt N, Bugge TH, Holmbeck K. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol. 2007;27:6309–6322. doi: 10.1128/MCB.00291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes SS, Hicks DG, O'Keefe RJ, Hurwitz SR, Crabb ID, Krasinskas AM, Loveys L, Puzas JE, Rosier RN. Shared phenotypic expression of osteoblasts and chondrocytes in fracture callus. J Bone Miner Res. 1995;10:533–544. doi: 10.1002/jbmr.5650100405. [DOI] [PubMed] [Google Scholar]
- 38.Nah HD, Pacifici M, Gerstenfeld LC, Adams SL, Kirsch T. Transient chondrogenic phase in the intramembranous pathway during normal skeletal development. J Bone Miner Res. 2000;15:522–533. doi: 10.1359/jbmr.2000.15.3.522. [DOI] [PubMed] [Google Scholar]
- 39.Hilton MJ, Tu X, Long F. Tamoxifen-inducible gene deletion reveals a distinct cell type associated with trabecular bone, and direct regulation of PTHrP expression and chondrocyte morphology by Ihh in growth region cartilage. Dev Biol. 2007;308:93–105. doi: 10.1016/j.ydbio.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satomura K, Krebsbach P, Bianco P, Gehron Robey P. Osteogenic imprinting upstream of marrow stromal cell differentiation. J Cell Biochem. 2000;78:391–403. [PubMed] [Google Scholar]