Abstract
Parkinson’s Disease (PD) is marked by prominent motor symptoms that reflect striatal dopamine insufficiency. However, non-motor symptoms, including depression, are common in PD. These changes have been suggested to reflect pathological involvement of non-dopaminergic systems. We examined regional changes in serotonin and norepinephrine systems in mice treated with two different 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment paradigms and that survived for 3 or 16 weeks after the last MPTP injection. MPTP caused a decrease in striatal dopamine concentration, the magnitude of which depended on the treatment regimen and survival interval after MPTP treatment. There was significant involvement of other subcortical areas receiving a dopamine innervation, but no consistent changes in serotonin or norepinephrine levels in subcortical sites. In contrast, we observed an enduring decrease in serotonin and norepinephrine concentrations in both the somatosensory and medial prefrontal (PFC) cortex. Immunohistochemical studies also revealed a decrease in the density of PFC norepinephrine and serotonin axons. The decrease in the cortical serotonergic innervation preferentially involved the thick beaded but not smooth fine serotonin axons. Similar changes in the serotonin innervation of postmortem samples of the prefrontal cortex from idiopathic PD cases were seen. Our findings point to a major loss of the serotonin and norepinephrine innervations of the cortex in MPTP-induced parkinsonism, and suggest that loss of the beaded cortical serotonin innervation is associated with a predisposition to the development of depression in PD.
Keywords: depression, dopamine, norepinephrine transporter, prefrontal cortex, serotonin transporter
Parkinson’s disease (PD) is characterized by degeneration of midbrain dopamine neurons and the loss of the striatal dopamine innervation (Hornykiewicz, 1966; Gibb & Lee, 1991). Although most attention has focused on the nigrostriatal system and the motor symptoms of PD (Fahn, 2003), there is also extensive extra-striatal pathology in PD (Braak et al., 2003) with a correspondingly wide range of non-motor symptoms (Nutt and Wooten, 2005; Chaudhuri et al., 2006; Jankovic, 2008; Truong et al., 2008). In particular, there is a high incidence of depression in PD, with the mood changes being responsive to antidepressants, including serotonin- and norepinephrine-selective reuptake inhibitors (Brooks & Doder, 2001; Zesiewicz & Hauser, 2002; Blier, 2006; Miyasaki et al., 2006; Lemke, 2008).
Depression has been linked to changes in cortical norepinephrine (NE) and serotonin (5-HT) (Bunney et al, 1970; Kostic et al, 1987; Mayeux, 1990; Nutt, 2006; Guttman et al., 2007; Michaelson et al., 2007; Lemke, 2008). A substantial body of data points to serotonergic involvement in the depression associated with parkinsonism, including postmortem studies noting Lewy bodies in pontine raphe neurons (Ohama & Ikuta, 1976) and a decreased number of 5-HT neurons in the median raphe (D’Amato et al., 1987; Halliday et al., 1990). In addition, serotonin concentrations in the prefrontal cortex (PFC) are decreased in PD (Scatton et al, 1983). Moreover, imaging studies have found a widespread neocortical decrease in the density of the serotonin transporter (SERT) (D’Amato et al., 1987; Chinaglia et al., 1993; Haapaneimi et al., 2001; Guttman et al., 2007), including a recent study reporting a decrease in SERT in the frontal cortices of patients with early PD who had not received pharmacological treatment (Haapaniemi et al., 2001).
Studies of animal models of parkinsonism have uncovered some changes in forebrain serotonergic and norepinephrine systems. Non-human primates treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) have decreased 5-HT and NE concentrations in the striatum and cortex (Perez-Otano et al., 1991; Pifl et al., 1991; Russ et al., 1991). Changes in 5-HT systems in the mouse have been less consistent. Striatal 5-HT concentrations have been reported to increase, not change, or decrease after MPTP treatment (Hara et al., 1987; Sundstrom et al., 1987; Rozas et al., 1998; Rousselet et al., 2003). The picture in the frontal cortex is similarly muddled (Sundstrom et al., 1987; Andrews and Murphy, 1993; Rousselet et al., 2003). These discrepancies probably reflect different MPTP treatment protocols and survival periods.
We systematically evaluated forebrain 5-HT and NE systems in the MPTP-treated mouse, using two different MPTP treatment regimens and two different survival intervals. Cortical 5-HT and NE concentrations and the density and morphology of the cortical serotonin and norepinephrine innervations were assessed. In a parallel exploration, we examined the cortical serotonin innervation in postmortem samples from persons with idiopathic PD.
Materials and Methods
Animals
Male C57BL/6J mice, 70–77 days of age at the start of experiments, were obtained from Jackson Labs (Bar Harbor, ME). Animals were group housed, with food and water available ad libitum. All studies were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and under the oversight of the Meharry Medical College Animal Care and Use Committee.
MPTP treatment
Two different MPTP treatment regimens were used in order to determine if the cortical serotonin and norepinephrine innvervations of the PFC were compromised. The decision to use the different MPTP treatment protocols was based in part on conflicting results obtained in previous reports using different MPTP treatment protocols and different survival intervals after MPTP treatment. Moreover, the two different protocols we used result in SN dopamine neuron cell death through different mechanisms: the acute treatment regimen causes necrotic cell death (Jackson-Lewis et al., 1995), while the subchronic regimen causes apoptotic loss of nigral dopamine neurons (Tatton and Kish, 1997).
In the acute protocol, mice were injected (ip) with 20 mg/kg MPTP (Sigma-Aldrich; St. Louis, MO) or saline every two hours for a total of four injections, resulting in a cumulative dose of 80 mg/kg. In the subchronic treatment regimen, mice received MPTP injections of 30 mg/kg MPTP (ip) every day for five consecutive days (cumulative dose of 150 mg/kg).
Regional monoamine concentrations
Animals were sacrificed either 3 weeks (acute MPTP protocol MPTP [N=10]; subchronic MPTP [N = 9]; vehicle [N = 8]) or 16 weeks after the last MPTP injection (acute MPTP [N = 10]; subchronic MPTP [N = 9], and vehicle [N = 9]); because some samples were lost because of poor dissections, the final N for each brain site examined in some cases was lower.
The striatum, nucleus accumbens, prefrontal cortex, somatosensory cortex, substantia nigra, and cerebellum were dissected from 1.0 mm thick coronal slices (Deutch et al., 1985), and samples stored at −80° C until assayed. Regional concentrations of dopamine, serotonin, and norepinephrine (NE) were determined by HPLC-EC as previously described (Deutch & Cameron, 1992), with protein concentrations determined by the method of Lowry et al. (1951).
Immunohistochemistry
Animals subjected to the acute MPTP treatment regimen were deeply anesthetized with isoflurane (MPTP N=5 and vehicle N=3 for both 3 and 16 week survival intervals) and then transcardially perfused, under isoflurane (Baxter; Deerfield, IL) anesthesia with 4% paraformaldehyde in 0.1M phosphate buffer after a brief perfusion with phosphate buffer. The brains were post-fixed in 4% paraformaldehyde overnight, and then cryoprotected in 30% sucrose in phosphate buffer for 1–2 days.
The serotonergic innervation of the prelimbic cortex (area 32) of the medial PFC was examined using a rabbit anti-SERT antibody (1:5,000; Immunostar Inc, Hudson, WI), following our previously described immunohistochemical methods (Bubser et al., 2000). Norepinephrine axons were examined using a rabbit antibody directed against the norepinephrine transporter (NET) (1:2,000; Schroeter et al., 2000). Control procedures included omission of the primary antiserum.
We used a stereological approach to determine the number of serotonergic dorsal (DR) and median (MR) raphe neurons. Sections through the pontine raphe were processed to reveal serotonin using a rabbit anti-serotonin antibody (1:1,200,000; Diasorin; Stillwater, MN). Prior to incubation in the primary antiserum, sections were subjected to an antigen retrieval method involving heating the sections in 10 mM sodium citrate containing 0.05% Tween 20 at 85° C for 20 min.
All immunohistochemical analyses were done by persons unaware of the treatment condition of the animals. SERT- and NET-ir axons in one focal plane were charted in a 100 μm column of cortex running from the pial surface to the white matter of the forceps minor using NeuroLucida (MicroBrightfield, Inc.; Williston, VT). The densities (total immunoreactive axon length/mm2) of SERT- and NET-ir axons were determined in both the superficial (layers II/III) and deep layers (V/VI) of the PFC.
There are two types of serotonin axons in the cortex: axons that have numerous large varicosities (beaded axons), and those with fine-caliber smooth axons (fine axons). In order to assess the relative impact of MPTP treatment on the thick, varicose (beaded) serotonin axons of the cortex, densitometric analysis was performed using NIS-Elements Imaging Software (Nikon Instruments, Inc; Melville, NY). Before analysis, background fluorescence was subtracted from each image. A threshold was implemented to select for the varicosities on beaded SERT-ir axons. Because fine fibers have previously been shown to have varicosities of < 1.0 μm diameter (Kosofsky & Molliver, 1987), the threshold parameters were circularity (0.50 – 1.0, to select for circular structures) and equivalent diameter (1– 9.88 μm) to select for varicosities whose size corresponds to beaded axons. Based on the results of the analysis of the densities of 5-HT and NE axons in the PFC, the number of SERT-ir varicosities were counted in a 233 μm2 area of layers II/III of the PFC.
Stereological assessment of the number of 5-HT neurons in 40 μm thick coronal sections cut through the pontine DR and MR was performed using the Stereologer software package (Stereology Resource Center; Chester, MD). The total number of serotonin-ir neurons was estimated in the dorsal and median raphe, extending rostrally to the anterior portion of the interpeduncular nucleus and posteriorly to the appearance of the rostral locus coeruleus. The two-stage ((Nv × Vref) approach using the optical dissector and Cavalieri method (Sterio, 1984; Gundersen, 1986; West and Gundersen, 1990) was used to calculate the total number of serotonin-ir cells in animals subjected to the acute MPTP-treatment regimen and surviving for 16 weeks.
Postmortem studies of prefrontal cortex in idiopathic Parkinson’s Disease
We used immunohistochemistry to examine the distribution of SERT-ir axons in area 9 of the PFC in three control subjects and two subjects with idiopathic PD. We were unable to obtain satisfactory staining of the PFC using either the NET antibody or an antibody generated against dopamine-beta-hydroxylase in the postmortem tissue, and hence our examination was restricted to assessment of the cortical serotonin innervation. Tissue was obtained from subjects and the experiments conducted under the oversight of the Institutional Review Board of the University of Washington (TJM). All subjects were male, with the three control subjects being 78–87 years of age and the two PD subjects being 57 and 81 years of age, with time since diagnosis being 20 years for the younger patient and 17 years for the older subject. Both patients received dopamine replacement treatment over the course of their illness (including levodopa-carbidopa). Postmortem intervals for all subjects were less than 6.5 hours. Diagnosis of PD was made on the basis of clinical history and pathological examination, with Lewy bodies noted in the SN. No other specific CNS pathology was noted in any of the subjects, with the exception of an infarct in the left dentate nucleus in one of the control cases.
Immunoperoxidase methods as described above were used to reveal SERT-ir axons of the PFC. Using NeuroLucida, SERT-ir axons in a column of cortex running from the pial surface to the white matter of area 9 were charted.
Statistical Analysis
Data on monoamine concentrations (ng amine/mg protein) were analyzed by two-factor ANOVAs (treatment × brain region) for each monoamine at both 3 and 16 week survivals, followed by post-hoc tests when indicated by a significant main effect on the ANOVA; experiment-wise alpha levels were set at 0.05. Data on the density of SERT- and NET-ir axons in layers II/III (superficial layers) and V/VI (deep layers) of the PFC of MPTP-treated and control animals were compared using a two-way ANOVA, with Dunnett’s test as a posthoc analysis when indicated. Students t-test was used to compare the measures of beaded SERT-ir axons in control versus MPTP-treated mice.
Results
MPTP treatment significantly decreased striatal but not PFC dopamine concentrations. MPTP treatment resulted in significant and long-lasting decreases in both the serotonergic and noradrenergic concentrations of the cortex. Decreases in the density of the serotonergic and noradrenergic innervations of the cortex in MPTP-treated mice were also observed. The change in cortical serotonin reflected a preferential loss of the large, beaded serotonin axons. The decrease in the cortical serotonin innervation observed in the mouse was paralleled by similar changes in the density of the serotonin innervation of the PFC samples from Parkinson’s Disease patients.
Subcortical monoamine concentrations
ANOVAs to assess changes in regional dopamine concentrations in response to MPTP treatment at the 3 week survival point revealed significant effects of treatment (F2,108 = 126.2, p < .0001) and region (F4,124 = 450.9, p < .0001) as well as a significant interaction (F8,124) = 102.2, p < .0001). Post-hoc assessments found a significant decrease in striatal dopamine concentration in mice treated with either the acute or the subchronic MPTP regimen at the 3 week survival point (see Fig. 1), with the acute treatment regimen resulting in a greater decrease in striatal (including nucleus accumbens) and nigral dopamine concentrations than the subchronic regimen (Fig. 1). ANOVA for the 16 week survival dopamine concentrations also revealed significant effects of treatment (F2,111 = 24.1, p < .0001) and region (F4,111 = 521.8, p < .0001) as well as a significant interaction (F8,111) = 18.2, p < .0001). Significant decreases in striatal dopamine concentration at the 16 weeks survival point were seen in both the acute and subchronic MPTP groups, although there some recovery relative to the three week survival point (see Fig. 1).
Figure 1.
Dopamine concentrations in subcortical regions of MPTP-treated mice. Animals subjected to either acute (4 × 20 mg/kg every two hours) or subchronic (30 mg/kg daily for five days) MPTP treatment regimen. Dopamine concentrations were determined either 3 weeks (A) or 16 weeks (B) after the last MPTP injection. In addition to changes in the striatal complex, a significant decrease in dopamine in the substantia nigra was seen. Abbreviations: CER, cerebellar cortex; NAc, nucleus accumbens; SN, substantia nigra; STR, striatum. Data are expressed as percent of vehicle-injected control mice. Dopamine concentrations (mean ng/mg protein ± SEM) in pooled control mice at 3 weeks and 16 weeks were: STR: 67.9 ± 3.0; NAc: 14.1 ± 0.54; SN: 6.8 ± 0.5; CER: 0.17 ± 0.05.
* p < 0.05, # p < .005; & p < .001 relative to vehicle-injected controls
Analyses of variance for 3 week survival serotonin concentrations uncovered significant effects of treatment (F2,124 = 20.4, p < .0001) and region (F5,124 = 1413, p < .0001), and a significant interaction (F10,124) = 9.9, p < .0001). ANOVAs for serotonin concentration at the 16 week survival interval also revealed significant treatment (F2,131 = 17.6, p < .0001) and region effects (F5,131 = 654.3, p < .0001) but not a significant interaction (F10,131 = 1.55, NS). Post-hoc tests revealed that there was a transient decrease in serotonin concentrations in the SN at 3 but not 16 weeks after MPTP treatment (Fig. 2). There was a trend toward an increase in triatal serotonin concentrations at both time points, but at neither time point were significant changes observed.
Figure 2.
Serotonin concentrations in subcortical sites in MPTP-treated mice. Serotonin concentrations were measured 3 weeks (A) or 16 weeks (B) after the last MPTP treatment. Serotonin concentrations in pooled control mice at 3 weeks and 16 weeks were STR: 1.2 ± 0.07; NAc: 1.7 ± 0.08; SN: 31.8 ± 1.4; CER: 1.8 ± 0.02.
* p < 0.05 relative to vehicle-injected controls
Despite significant treatment (F2,100) = 38.6, p < .0001 and region (F4,100 = 137.9, p < .0001) effects as well as a significant treatment × region interaction (F8,100 = 5.711, p < .0001), posthoc tests did not reveal any significant change in norepinephrine levels in subcortical sites at the 3 week survival point. Similarly, at the 16 week survival point ANOVA uncovered significant treatment (F4,68 = 5.8, p < .0001) and region (F2,68 = 231.5, p < .0001) effects, and a significant interaction (F4,68 = 5.8, p < .0005), but NE concentrations in the subcortical sites examined did not change in response to the MPTP treatments.
Cortical monoamine concentrations in response to MPTP treatment
Post-hoc analyses did not uncover a significant change in dopamine concentrations in the PFC in response to either MPTP regimen at either survival time point. Because there is no specific dopamine innervation of the somatosensory cortex (SSC) of the mouse, we did not analyze dopamine concentrations in this region.
In contrast to the lack of a change in cortical dopamine concentrations, acute but not subchronic MPTP significantly decreased both serotonin and norepinephrine concentrations in the PFC and SSC at both the three and 16 week survival points (see Fig. 3). There was one exception to the statement that only the acute MPTP regimen caused a change in 5-HT or NE: somatosensory cortex norepinephrine concentrations were significantly decreased in mice treated with the subchronic MPTP protocol and sacrificed three weeks later.
Figure 3.
Monoamine concentrations in the medial prefrontal cortex (PFC) and somatosensory cortex (SSC) of MPTP-treated mice, determined either 3 weeks (A) or 16 weeks (B) after the last MPTP injection. No significant change in PFC dopamine concentration was observed. Both serotonin (5-HT) and norepinephrine (NE) concentrations were signficantly decreased in animals subjected to the acute MPTP regimen. Monoamine concentrations (ng/mg protein) in pooled control mice at 3 weeks and 16 weeks were: dopamine: 0.56 ±.05; serotonin: 2.6 ±.16 ng; and norepinephrine: 3.3 ±.45.
* p < 0.05, # p < .01, & p < .001 relative to vehicle-injected controls.
Immunohistochemical localization of cortical 5-HT and NE axons in MPTP-treated mice
Analysis of SERT and NET axon densities (total immunoreactive axon length/mm2) in the PFC were made in animals subjected to the acute MPTP regimen. We observed a significant (24.4%) decrease in the density of SERT-ir axons in the superficial but not deep layers of the PFC in MPTP-treated mice that were sacrificed at 3 weeks after the last MPTP treatment (see Fig. 4). The loss of SERT-ir axons in the cortical superficial layers was still present at the 16 week survival point, although the magnitude of the decrease (11.9%) was somewhat less than that observed in animals examined three weeks after MPTP.
Figure 4.
SERT- and NET-immunoreactive (-ir) axons in the PFC of vehicle- and MPTP-injected (acute paradigm) mice sacrificed at three weeks and sixteen weeks. Panel A. SERT and NET axon densities (total immunoreactive axon length/mm2) in superficial layer (layers II/III) and deep layer (layers V/VI) of the PFC of MPTP-treated and control animals. Decreases in the density of the serotonergic and noradrenergic innervations of the cortex were observed. Panel B. Charting of a 100 μm thick column through the PFC, from the pial surface (left) to the white matter (right), of representative vehicle- and MPTP-treated mice. A marked decrease in SERT-ir axons can be appreciated, particularly in layers II/III.
* p < 0.05 of SERT-ir axons relative to vehicle-injected controls, ** p < 0.05 of NET-ir axons relative to vehicle-injected controls.
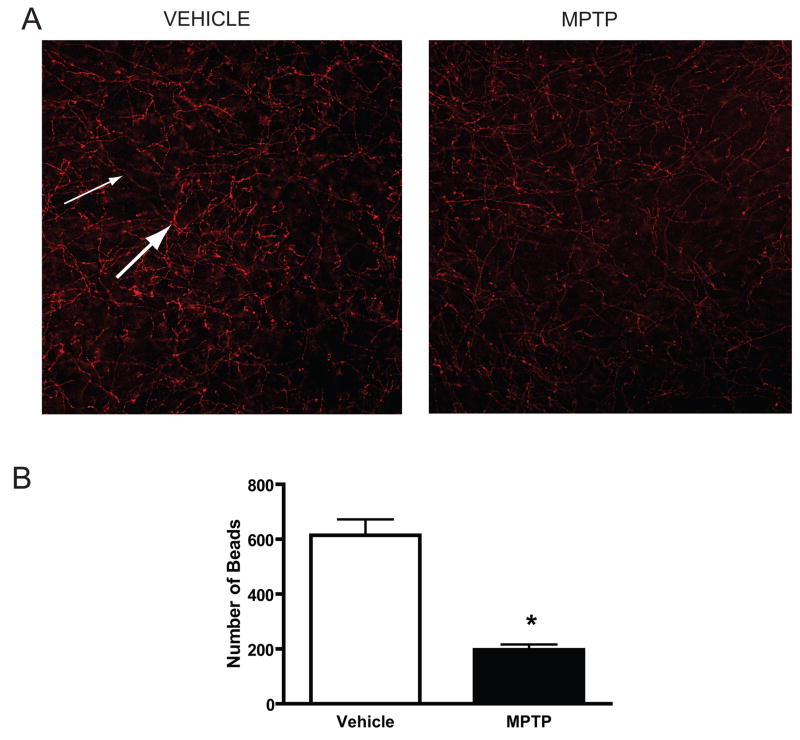
Because there are two distinct types of serotonin axons in the cortex, we quantitatively assessed SERT-ir axon morphology in the PFC of MPTP-treated mice. Acute MPTP treatment resulted in a significant and marked (~68%) decrease in the number of large beaded SERT-ir varicosities in layers I-III (Fig. 5).
Figure 5.
SERT- immunoreactive (-ir) axons in the PFC of vehicle- and MPTP-injected (acute paradigm) mice sacrificed at three weeks. Panel A. Photomicrographs of SERT-ir axons in layers II/III of the PFC from control and MPTP-treated mice. An overall decrease in the density of the serotonin innervation of the PFC can be appreciated. In addition, examination of SERT-ir morphology suggests a preferential decrease in the number of beaded SERT-ir axons (thick arrow) with relative sparing of fine serotonin axons (thin arrow). Panel B. Loss of SERT-ir beaded axons in layers II/III of the PFC of MPTP-treated mice. Analysis of beaded varicosities (≥ 1.0μm in size) revealed a sharp decrease in this type of serotonin axon in the cortex.
* p < 0.0001 relative to vehicle-injected controls
We also observed changes in the density of NET-ir axons in the PFC. In the PFC of mice surviving 3 weeks, there was a 28.4% decrease in NET-ir axon density in the superficial layers and a 24.0% decrease in the deep layers (see Fig. 4). These changes approached but did not reach statistical significance at the three week survival point (e.g., p = .088 in the superficial layers) due to two outlier values. In the 16 week survival group NET-ir axon density was significantly decreased in both the superficial and deep layers.
Numbers of serotonin neurons in the dorsal and median raphe of MPTP-treated mice
There were no significant changes in either the numbers of dorsal or median raphe serotonergic (5-HT-ir) neurons in response to MPTP treatment. Thus, there were 31,550 ± 7,862 DR serotonin neurons in control mice, with 32,140 ± 8,678 serotonergic DR neurons counted in the MPTP-treated group. In the MR we found 9,045 ± 1,165 serotonin-li neurons in the control animals, with 10,025 ± 2,709 serotonin cells in the MR of MPTP-treated mice. Although we found no differences in the numbers of serotonin-li neurons in either the DR or MR, we did subjectively note that the intensity of 5-HT-li staining of raphe neurons in the MPTP treated mice was consistently greater than observed in control mice.
Immunohistochemical localization of PFC serotonin axons in PD
The distribution of SERT-ir axons in area 9 of normal control subjects did not appear to vary markedly across lamina, although layers I and V appeared to have a somewhat greater density of serotonin axons. Discrete SERT-ir axons with large varicosities, as well as tortuous clusters of beaded SERT-ir axons, were present in the control PFC cases. Fine SERT-ir axons were often seen to run for long lengths across different layers of the cortex; fine axons parallel to the pial surface appeared to be somewhat more frequent in the deep layers. As noted above, we could not obtain reliable staining of noradrenergic axons using either NET- or dopamine-beta-hydroxylase-li antibodies in the postmortem samples, and hence our presentation on the anatomical changes in idiopathic PD is restricted to the cortical serotonin innervation.
There was a marked decrease in the density of the serotonin innervation in the PFC in both of the PD cases (see Fig. 6). The decrease in SERT-ir axons appeared to be roughly similar across the different layers. Examination of the material suggested that the fine SERT-ir axons were relatively spared, with a preferential loss of the thick beaded axons (Fig. 6) in both cases. There was a subjective impression that the density of the above-mentioned tortuous clusters of beaded SERT-ir axons was somewhat greater in the sample from the older of the two subjects.
Figure 6.
Prefrontal cortical SERT-ir axons in representative cases of control and PD subjects. The top shows chartings of 200 μm columns of the PFC (area 9) from the pial surface (left) to the white matter (right). There is a marked decrease in the serotonin innervation of the PFC in the PD case. A higher magnification of layer V is seen in the two middle panels, where the PD case (right panel) shows far fewer SERT-ir axons, with a more marked loss of beaded serotonin axons. Bottom panels show representative photomicrographs of control (left) and PD (right) cases.
Discussion
MPTP treatment of mice caused an enduring decrease in neocortical serotonin and norepinephrine levels. Both 5-HT and NE concentrations were decreased in the prefrontal and somatosensory cortices, with a corresponding decrease in SERT- and NET-ir axons. The involvement of the cortical serotonergic innervation appeared to be specific to a subpopulation of serotonin axons. Finally, we subjectively noted a decrease in the density of PFC SERT-ir axons in the PFC of PD subjects.
Cortical serotonin and norepinephrine concentrations in MPTP-induced parkinsonism
We examined the effects of two different MPTP treatment protocols, with the “acute” paradigm, which involves MPTP administered in divided doses on a single day, resulting in a somewhat more extensive dopamine loss than seen when MPTP is administered over several days. This observation mirrors earlier reports (Tatton & Kish, 1997; Yang et al., 1998; Dauer et al., 2002).
We found that MPTP decreased cortical 5-HT and NE concentrations only in mice treated with the acute MPTP regimen. Interestingly, the effect of MPTP on serotonin was mainly confined to cortical areas, with no changes in striatal or accumbal serotonin content, suggesting that specific serotonin neurons are differentially vulnerable to the toxin. Consistent with this suggestion were our immunohistochemical finding that serotonin axon density was significantly reduced in the superficial but not deep layers of the PFC, and the finding that the loss of cortical SERT-ir axons preferentially involved the thick beaded but not fine serotonin axons.
PFC and SSC serotonin concentrations did not recover to control levels even at 16 weeks post-treatment. Although striatal dopamine levels partially recover after MPTP treatment, this recovery in mice is largely stabilized by eight weeks post-MPTP treatment (Nishi et al., 1989). If serotonin follows the same temporal pattern, the most parsimonious explanation for our data is that MPTP is toxic to cortical serotonin axons rather than functionally compromising the serotonergic neurons. Consistent with this suggestion is the observation that a different marker of 5-HT neurons, SERT, was also reduced in the cortex of MPTP-treated mice. However, we did not observe a significant decrease in the number of serotonin-ir neurons in the pontine raphe nuclei, suggesting that serotonergic raphe neurons are present and may recover, or alternatively that MPTP treatment results in a slow retrograde axonopathy that has not yet culminated in cell death.
The loss of the NE innervation of the PFC involved both the superficial and deep layers of the cortex, suggesting a more global involvement involvement of this monoamine in MPTP-induced parkinsonism.
Changes in different types of cortical serotonin axons in Parkinson’s Disease
Unless animals are treated with a monoamine oxidase inhibitor, detection of 5-HT in axons by immunohistochemistry underestimates the density of serotonergic fibers because of rapid metabolism of the amine (Nielsen et al., 2006; Deutch and Roth, 2008). We therefore used a SERT rather than a 5-HT antibody for the cortical studies, based on data indicating almost 100% colocalization between SERT- and serotonin-ir axons in the monoamine oxidase-pretreated rat, compared to only 30% colocalization in control rats (Nielsen et al., 2006).
The two types of serotonin axons in the cortex, fine and beaded, originate in different brainstem serotonin neurons. The fine axons are mainly derived from dorsal raphe neurons, while the beaded axons originate in the median raphe (Mamounas and Molliver, 1991). Several neurotoxic amphetamine derivatives, including p-chloroamphetamine and methylenedioxymethylamphetamine (MDMA), have been reported to have neurotoxic effects on cortical serotonin axons, with preferential loss of fine serotonin axons (Mamounas and Molliver, 1988, 1991; Series and Molliver, 1994). In contrast to the effects of substituted amphetamines on fine cortical serotonin axons, we observed a marked decrease (68%) in beaded large serotonergic axons in the PFC of the MPTP-treated mouse. Although we have not developed a quantitative method to analyze specifically the fine serotonin axons, the very large effect on beaded axons, coupled with the impression that fine serotonin axons were largely spared and the fact that the overall decrease in SERT-ir axon density was ~25%, suggests that MPTP preferentially targets the beaded serotonin axons of the cortex. This suggestion is also consistent with the postmortem study of Halliday et al. (1990), who reported a decrease in the number of MR but nor DR serotonin neurons in PD, the former providing the beaded cortical axons.
We also examined the PFC serotonin innervation in idiopathic PD. Although the number of PD cases is too few to make any conclusive statement, the subjective impression was that there is a decrease in the density of SERT-ir axons in the PFC in PD. This is consistent with the recent report of Azmitia and Nixon (2008), who reported a decrease in SERT-ir axons in the prefrontal and entorhinal cortices, and with a number of imaging studies that noted a decrease in cortical SERT binding potential (Chinaglia et al 1993; Haapaneimi et al, 2001; Guttman et al, 2007). And as was seen in the MPTP-treated mouse, we observed a preferential loss of beaded serotonin axons in idiopathic PD.
We are not aware of any studies of the cortical serotonin innervation that have reported preferential loss of beaded cortical serotonin axons in response to any type of manipulation, although none have undertaken a quantitative analysis of this type of axon. The ability of MPTP to preferentially impact the beaded serotonin axons, with amphetamine derivatives targeting fine axons, should provide a useful means by which to untangle the functional consequences of disruption of the beaded cortical 5-HT innervation, possibly including depression, and study the mechanisms of toxic loss and the functional attributes of the two types of cortical serotonin axons.
Comparison with MPTP-induced parkinsonism and idiopathic PD
We observed a non-significant trend toward an increase in striatal serotonin in response to MPTP; NE levels were below detection threshold in our punch-dissected samples of the dorsolateral striatum. As noted earlier, reported effects of MPTP on striatal 5-HT in the mouse have been inconsistent (Hallman et al., 1985; Fukuda et al., 1988; Vukovic et al, 2008), probably because of differences in treatment regimen or survival interval, but perhaps because of changes that are below the threshold for statistical significance.
Few data are available on the effects of MPTP on cortical serotonin in the mouse. Rousselet et al (2003) did not observe changes in serotonin levels in the frontal cortex of mice treated with an acute MPTP regimen, but both Sundstrom et al (1987) and Fukuda et al (1988) reported transient significant decreases in frontal cortical serotonin concentrations, and more recently Vukovic and colleagues (2008) noted a significant decrease in frontal cortical 5-HT levels in response to MPTP. Moreover, primate studies indicate a loss of the cortical serotonin innervation in response to MPTP (Pifl et al, 1991; Perez-Otano et al., 1991); these changes do not appear to be restricted to one cortical region, consistent with our data.
Our stereological assessment did not uncover a decreased number of DR or MR serotonin-ir neurons after MPTP treatment. This is similar to the conclusion of Gupta et al (1984), who suggested that subchronic MPTP did not result in loss of DR or MR neurons in Swiss-Webster mice; however, this mouse strain is generally quite resistant to the toxic actions of MPTP (Giovanni et al., 1991).
Despite the fact that almost half a century has elapsed since Bernheimer et al. (1961) noted decreased forebrain serotonin levels in parkinsonism, there is a paucity of postmortem data on serotonin in idiopathic PD. In contrast to the minimal effects of MPTP on striatal serotonin in rodents, striatal concentrations of serotonin in PD are decreased (Scatton et al., 1983; Kish et al., 2008), suggesting duration of illness may be an important factor. Most studies of the cortex have suggested a decrease in the serotonergic innervation. Thus, Scatton et al. (1983) observed a decrease in 5-HT concentration in several cortical regions, consistent with recent in vivo imaging studies noting cortical decreases in SERT binding potential (Haapaneimi et al, 2001; Guttman et al, 2007). Moreover, our postmortem data and those of Azmitia and Nixon (2008) are consistent with impression gleaned from biochemical and imaging studies of a decrease in the serotonin innervation of the frontal cortex in PD.
It has long been suggested that there is involvement of the noradrenergic locus coeruleus in idiopathic PD, with cell loss and the presence of Lewy bodies (Forno et al., 1993). However, there are scant quantitative data on cortical changes in NE in the illness. Scatton and colleagues (1983) observed a decrease in frontal cortical NE concentrations in PD. Using immunohistochemical methods, Gaspar et al. (1991) reported a decrease in NE axons in the motor cortex of PD. However, D’Amato et al. (1987) did not find a change in the cortical noradrenergic innervation of the cortex.
Implications for depression in PD
Disturbances in frontal cortical function have been suggested to contribute to depression and cognitive dysfunction in PD (Taylor et al., 1990; Decamp & Schneider, 2004; Chudasama & Robbins, 2006). While the mesocortical dopamine system has figured prominently in discussion of cognition and affect, changes in the cortical serotonin and noradrenergic innervations have been implicated in depression in PD. Thus, Ring et al (1994) reported decreases in regional cerebral blood flow in the PFC and anterior cingulate cortex of depressed subjects and in PD patients who were clinically depressed. Moreover, because depression in PD responds to serotonin- and NE-selective reuptake blockers to roughly the same degree as reported in major depression (Marino et al., 2008; Sheehan et al., 2008; Menza et al., 2009), there has been an assumption that depression in PD is linked to changes in serotonin and norepinephrine systems.
Our data suggest that the beaded serotonin cortical axons may be particularly important to mood changes in PD. There has been the suggestion that chronic use of MDMA, which targets the fine serotonin axons of the cortex, may lead to depression. However, there is usually concurrent use of other drugs with recreational use of MDMA, and recent data have suggested that if there is an increase in depression in persons who use MDMA it is probably associated with concurrent use of other drugs rather than MDMA itself (Guillot & Greenway, 2006; Medina & Shear, 2007; Falck et al., 2008). These considerations and our observations that the beaded cortical serotonin axons are preferentially targeted in MPTP-induced parkinsonism raise the possibility that the loss of the beaded cortical serotonin innervation is associated with a predisposition to the development of depression in Parkinson’s Disease.
Acknowledgments
This work was supported by the National Institutes of Health [U54 NS041071 to TAA, AYD; PO1 NS44282 to AYD; P50 AG05136 to TJM] and the National Parkinson Foundation Center of Excellence [AYD]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disease and Stroke or the National Institute on Aging of the National Institutes of Health or of the National Parkinson Foundation.
We are indebted to the Morphology Core of Meharry Medical College for assistance with the densitometric analyses and stereology, to Dr. Brian Mathur for assistance with rodent dissections and helpful discussions, and to Randy Blakely for contributing the NET antibody.
Abbreviations
- 5-HT
5-hydroxytryptamine, serotonin
- DR
dorsal raphe
- MDMA
methylenedioxymethylamphetamine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MR
median raphe
- NE
norepinephrine
- NET
norepinephrine transporter
- PFC
prefrontal cortex
- SERT
serotonin transporter
- SSC
somatosensory cortex
References
- Andrews AM, Murphy DL. Sustained depletion of cortical and hippocampal serotonin and norepinephrine but not striatal dopamine by 1-methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine (2′-NH2-MPTP): a comparative study with 2′-CH3-MPTP and MPTP. J Neurochem. 1993;60:1167–1170. doi: 10.1111/j.1471-4159.1993.tb03271.x. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Nixon R. Dystrophic serotonergic axons in neurodegenerative diseases. Brain Res. 2008;1217:185–194. doi: 10.1016/j.brainres.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O. Distribution of 5-hydroxytryptamine (serotonin) in the human brain and its behavior in patients with Parkinson’s syndrome. Klin Wochenschr. 1961;39:1056–1059. doi: 10.1007/BF01487648. [DOI] [PubMed] [Google Scholar]
- Blier P. Psychopharmacology for the clinician. Treating depression with selective norepinephrine reuptake inhibitors. J Psychiatry Neurosci. 2006;31:288. [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, De Vos RAI, Jansen Steur ENH, Braak E. Staging brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Doder M. Depression in Parkinson’s disease. Curr Opin Neurol. 2001;14:465–470. doi: 10.1097/00019052-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Bubser M, Scruggs JL, Young CD, Deutch AY. The distribution and origin of the calretinin-containing innervation of the nucleus accumbens of the rat. Eur J Neurosci. 2000;12:1591–1598. doi: 10.1046/j.1460-9568.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Jr, Murphy DL, Goodwin FK, Borge GF. The switch process from depression to mania: relationship to drugs which alter brain amines. Lancet. 1970;1(7655):1022–1027. doi: 10.1016/s0140-6736(70)91151-7. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- Chinaglia G, Landwehrmeyer B, Probst A, Palacios JM. Serotoninergic terminal transporters are differentially affected in Parkinson’s disease and progressive supranuclear palsy: an autoradiographic study with [3H]citalopram. Neuroscience. 1993;54:691–699. doi: 10.1016/0306-4522(93)90240-g. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Zweig RM, Whitehouse PJ, Wenk GL, Singer HS, Mayeux R, Price DL, Snyder SH. Aminergic systems in Alzheimer’s disease and Parkinson’s disease. Ann Neurol. 1987;22:229–236. doi: 10.1002/ana.410220207. [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci USA. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decamp E, Schneider JS. Attention and executive function deficits in chronic low-dose MPTP-treated non-human primates. Eur J Neurosci. 2004;20:1371–1378. doi: 10.1111/j.1460-9568.2004.03586.x. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46:49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. Neurotransmitters. In: Bloom FE, Du Lac S, Ghosh A, Squire LR, Spitzer NC, Berg DK, editors. Fundamental Neuroscience. 3. Elsevier/Academic Press; San Diego: 2008. pp. 133–155. [Google Scholar]
- Deutch AY, Tam SY, Roth RH. Footshock and conditioned stress increase 3,4-dihydroxyphenylacetic acid (DOPAC) in the ventral tegmental area but not substantia nigra. Brain Res. 1985;333:143–146. doi: 10.1016/0006-8993(85)90134-9. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Falck RS, Wang J, Carlson RG. Depressive symptomatology in young adults with a history of MDMA use: a longitudinal analysis. J Psychopharmacol. 2008;22:47–54. doi: 10.1177/0269881107078293. [DOI] [PubMed] [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Langston JW. Similarities and differences between MPTP-induced parkinsonsim and Parkinson’s disease. Neuropathologic considerations. Adv Neurol. 1993;60:600–608. [PubMed] [Google Scholar]
- Fukuda H, Hara K, Nakamura S, Kimura H, Kameyama M. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) enhances tryptophan hydroxylase activity in mouse striatum, but not in the frontal cortex. Brain Res. 1988;449:399–402. doi: 10.1016/0006-8993(88)91063-3. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Duyckaerts C, Alvarez C, Javoy-Agid F, Berger B. Alterations of dopaminergic and noradrenergic innervations in motor cortex in Parkinson’s disease. Ann Neurol. 1991;30:365–374. doi: 10.1002/ana.410300308. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54:388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanni A, Sieber BA, Heikkila RE, Sonsalla PK. Correlation between the neostriatal content of the 1-methyl-4-phenylpyridinium species and dopaminergic neurotoxicity following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration to several strains of mice. J Pharmacol Exp Ther. 1991;257:691–697. [PubMed] [Google Scholar]
- Guillot C, Greenway D. Recreational ecstasy use and depression. J Psychopharmacol. 2006;20:411–416. doi: 10.1177/0269881106063265. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Gupta M, Felten DL, Gash DM. MPTP alters central catecholamine neurons in addition to the nigrostriatal system. Brain Res Bull. 1984;13:737–742. doi: 10.1016/0361-9230(84)90234-x. [DOI] [PubMed] [Google Scholar]
- Guttman M, Boileau I, Warsh J, Saint-Cyr JA, Ginovart N, McCluskey T, Houle S, Wilson A, Mundo E, Rusjan P, Meyer J, Kish SJ. Brain serotonin transporter binding in non-depressed patients with Parkinson’s disease. Eur J Neurol. 2007;14:523–528. doi: 10.1111/j.1468-1331.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- Haapaniemi TH, Ahonen A, Torniainen P, Sotaniemi KA, Myllyla VV. [123I]beta-CIT SPECT demonstrates decreased brain dopamine and serotonin transporter levels in untreated parkinsonian patients. Mov Disord. 2001;16:124–130. doi: 10.1002/1531-8257(200101)16:1<124::aid-mds1007>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Blumbergs PC, Cotton RG, Blessing WW, Geffen LB. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res. 1990;510:104–107. doi: 10.1016/0006-8993(90)90733-r. [DOI] [PubMed] [Google Scholar]
- Hallman H, Lange J, Olson L, Stromberg I, Jonsson G. Neurochemical and histochemical characterization of neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on brain catecholamine neurones in the mouse. J Neurochem. 1985;44:117–127. doi: 10.1111/j.1471-4159.1985.tb07120.x. [DOI] [PubMed] [Google Scholar]
- Hara K, Tohyama I, Kimura H, Fukuda H, Nakamura S, Kameyama M. Reversible serotoninergic neurotoxicity of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mouse striatum studied by neurochemical and immunohistochemical approaches. Brain Res. 1987;410:371–374. doi: 10.1016/0006-8993(87)90341-6. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966;18:925–964. [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, Furukawa Y. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain. 2008;131:120–131. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME. The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- Kostic VS, Djuricic BM, Covickovic-Sternic N, Bumbasirevic L, Nikoloc M, Mrsulja BB. Depression and Parkinson’s disease: possible role of serotonergic mechanisms. J Neurol. 1987;234:94–96. doi: 10.1007/BF00314109. [DOI] [PubMed] [Google Scholar]
- Lemke MR. Depressive symptoms in Parkinson’s disease. Eur J Neurol. 2008;15 (Suppl 1):21–25. doi: 10.1111/j.1468-1331.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- Leroi I, Collins D, Marsh L. Non-dopaminergic treatment of cognitive impairment and dementia in Parkinson’s disease: a review. J Neurol Sci. 2006;248:104–114. doi: 10.1016/j.jns.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mamounas LA, Molliver ME. Evidence for dual serotonergic projections to neocortex: axons from the dorsal and median raphe nuclei are differentially vulnerable to the neurotoxin p-chloroamphetamine (PCA) Exp Neurol. 1988;102:23–36. doi: 10.1016/0014-4886(88)90075-1. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Mullen CA, O’Hearn E, Molliver ME. Dual serotoninergic projections to forebrain in the rat: morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J Comp Neurol. 1991;314:558–586. doi: 10.1002/cne.903140312. [DOI] [PubMed] [Google Scholar]
- Marino S, Sessa E, Di Lorenzo G, Digangi G, Alagna A, Bramanti P, Di Bella P. Sertraline in the treatment of depressive disorders in patients with Parkinson’s disease. Neurol Sci. 2008;29:391–395. doi: 10.1007/s10072-008-1021-3. [DOI] [PubMed] [Google Scholar]
- Mayeux R. The serotonergic hypothesis’ for depression in Parkinson’s disease. Adv Neurol. 1990;53:163–166. [PubMed] [Google Scholar]
- Medina KL, Shear PK. Anxiety, depression, and behavioral symptoms of executive dysfunction in ecstasy users: contributions of polydrug use. Drug Alcohol Depend. 2007;87:303–311. doi: 10.1016/j.drugalcdep.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menza M, Dobkin RD, Marin H, Mark MH, Gara M, Buyske S, Bienfait K, Dicke A. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurol. 2009;72:886–892. doi: 10.1212/01.wnl.0000336340.89821.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki JM, Shannon K, Voon V, Ravina B, Kleiner-Fisman G, Anderson K, Shulman LM, Gronseth G, Weiner WJ. Practice Parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:996–1002. doi: 10.1212/01.wnl.0000215428.46057.3d. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Brask D, Knudsen GM, Aznar S. Immunodetection of the serotonin transporter protein is a more valid maker for serotonergic fibers than serotonin. Synapse. 2006;59:270–276. doi: 10.1002/syn.20240. [DOI] [PubMed] [Google Scholar]
- Nishi K, Kondo T, Narabayashi H. Difference in recovery patterns of striatal dopamine content, tyrosine hydroxylase activity and total biopterin content after 1-methyl- 4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) administration: a comparison of young and older mice. Brain Res. 1989;489:157–162. doi: 10.1016/0006-8993(89)90018-8. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67 (Suppl 6):3–8. [PubMed] [Google Scholar]
- Nutt JG, Wooten GF. Clinical practice. Diagnosis and initial management of Parkinson’s disease. N Engl J Med. 2005;353:1021–1027. doi: 10.1056/NEJMcp043908. [DOI] [PubMed] [Google Scholar]
- Ohama E, Ikuta F. Parkinson’s disease: distribution of Lewy bodies and monoamine neuron system. Acta Neuropathol (Berl) 1976;34:311–319. doi: 10.1007/BF00696560. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Herrero MT, Oset C, De Ceballos ML, Luquin MR, Obeso JA, Del Rio J. Extensive loss of brain dopamine and serotonin induced by chronic administration of MPTP in the marmoset. Brain Res. 1991;567:127–132. doi: 10.1016/0006-8993(91)91444-6. [DOI] [PubMed] [Google Scholar]
- Pifl C, Schingnitz G, Hornykiewicz O. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience. 1991;44:591–605. doi: 10.1016/0306-4522(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Ring HA, Bench CJ, Trimble MR, Brooks DJ, Frackowiak RS, Dolan RJ. Depression in Parkinson’s disease. A positron emission study. Br J Psychiatry. 1994;165:333–339. doi: 10.1192/bjp.165.3.333. [DOI] [PubMed] [Google Scholar]
- Rousselet E, Joubert C, Callebert J, Parain K, Tremblay L, Orieux G, Launay JM, Cohen-Salmon C, Hirsch EC. Behavioral changes are not directly related to striatal monoamine levels, number of nigral neurons, or dose of parkinsonian toxin MPTP in mice. Neurobiol Dis. 2003;14:218–228. doi: 10.1016/s0969-9961(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Rozas G, Liste I, Guerra MJ, Labandeira-Garcia JL. Sprouting of the serotonergic afferents into striatum after selective lesion of the dopaminergic system by MPTP in adult mice. Neurosci Lett. 1998;245:151–154. doi: 10.1016/s0304-3940(98)00198-0. [DOI] [PubMed] [Google Scholar]
- Russ H, Mihatsch W, Gerlach M, Riederer P, Przuntek H. Neurochemical and behavioural features induced by chronic low dose treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the common marmoset: implications for Parkinson’s disease? Neurosci Lett. 1991;123:115–118. doi: 10.1016/0304-3940(91)90171-o. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Series HG, Molliver ME. Immunocytochemical evidence for serotonergic neurotoxicity of N-ethyl-methylenedioxyamphetamine (MDE) Exp Neurol. 1994;128:50–58. doi: 10.1006/exnr.1994.1112. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Keene MS, Eaddy M, Krulewicz S, Kraus JE, Carpenter DJ. Differences in medication adherence and healthcare resource utilization patterns: older versus newer antidepressant agents in patients with depression and/or anxiety disorders. CNS Drugs. 2008;22:963–973. doi: 10.2165/00023210-200822110-00005. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Preziosi TJ, Berthier ML, Bolduc PL, Mayberg HS, Robinson RG. Depression and cognitive impairment in Parkinson’s disease. Brain. 1989;112 (Pt 5):1141–1153. doi: 10.1093/brain/112.5.1141. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–36. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Sundstrom E, Stromberg I, Tsutsumi T, Olson L, Jonsson G. Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Res. 1987;405:26–38. doi: 10.1016/0006-8993(87)90986-3. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–104. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, Redmond DE., Jr Cognitive and motor deficits in the acquisition of an object retrieval/detour task in MPTP-treated monkeys. Brain. 1990;113 (Pt 3):617–637. doi: 10.1093/brain/113.3.617. [DOI] [PubMed] [Google Scholar]
- Truong DD, Bhidayasiri R, Wolters E. Management of non-motor symptoms in advanced Parkinson disease. J Neurol Sci. 2008;266:216–228. doi: 10.1016/j.jns.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Vukovic MG, Wood RI, Holschneider DP, Abernathy A, Togasaki DM, Smith A, Petzinger GM, Jokowek MW. Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropridine-lesioned mouse model of basal ganglia injury. Neurobiol Dis. 2008;32:319–327. doi: 10.1016/j.nbd.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Yang L, Matthews RT, Schulz JB, Klockgether T, Liao AW, Martinou JC, Penney JB, Jr, Hyman BT, Beal MF. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J Neurosci. 1998;18:8145–8152. doi: 10.1523/JNEUROSCI.18-20-08145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zesiewicz TA, Hauser RA. Depression in Parkinson’s disease. Curr Psychiatry Rep. 2002;4:69–73. doi: 10.1007/s11920-002-0016-7. [DOI] [PubMed] [Google Scholar]