Abstract
Knowledge of blood alcohol levels (BALs) that are achieved following ethanol administration is critical for contemporary efforts to develop animal models of alcoholism. Adolescent and adult male Wistar rats were administered varying doses of ethanol (0.75, 1.5 and 3.0 g/kg) via gavage or intraperitoneal injection and BALs were measured over a two hour period. The results showed that adolescent animals had lower BALs across all time points in comparison to adults following administration of 0.75 g/kg ethanol and that one hour after administration of 1.5 g/kg ethanol, adolescent animals showed an enhanced rate of elimination. The highest dose of ethanol (3.0 g/kg) produced comparable BALs for both adolescents and adults during the two-hour sampling period; however, the BALs for both ages were lower following administration of ethanol by gavage at this dose. Furthermore, an order effects analysis highlights that depending on the route of administration, initial dose size can influence the BALs produced by lower doses of ethanol. The current data identify the importance of measuring the level of alcohol in the blood to confirm that target BALs are achieved for adolescents and equivalent BALs are being reached for both adolescent and adult animals when such comparisons are made.
Keywords: Adolescent, Adult, Ethanol, Blood Alcohol Level, Pharmacokinetics, Gavage, Intraperitoneal
A host of physiological as well as behavioral changes occur during adolescence. From a neurobehavioral standpoint, these changes primarily involve (but are not limited to) the development of cognitive, emotional, hormonal and motivational systems (Chambers et al. 2003;Yurgelun-Todd 2007); components of which are susceptible to the effects of alcohol (Smith 2003;Spear 2000;Witt 1994). In 2004, 17.7% of adolescents (age 12 – 17) were classified as current users of alcohol, with 6% designated as alcohol abusers or alcohol dependent (U.S.Department of Health and Human Services 2005). Taking into consideration evidence suggesting that age of initial alcohol use is predictive of alcohol abuse and dependence in adults (Ehlers et al. 2006;Grant and Dawson 1997), the identification of factors that influence adolescent alcohol use is an important objective. Developing animal models of adolescent alcohol use is of primary importance in order to study the consequences and impact of alcohol exposure during this critical developmental period at the behavioral, neurobiological, molecular and genetic levels. Indeed, numerous researchers are focusing on emerging animal models to evaluate the developmental impact of ethanol exposure during adolescence on the brain and behavior (McBride et al. 2005;Smith 2003;Spear 2000;Walker et al. 2008;Witt 1994).
Dissociations between adolescent and adult responsivity to the effects of alcohol have been identified in a variety of domains. A number of experiments have shown that adolescent rats are either less (Little et al. 1996;Pian et al. 2008;Silveri and Spear 1998;Silveri and Spear 2001;Varlinskaya and Spear 2002;White et al. 2002a;White et al. 2002b) or more (Fernandez-Vidal et al. 2003;Land and Spear 2004;Markwiese et al. 1998;Philpot and Kirstein 2004;Pian et al. 2008;Swartzwelder et al. 1995a;Swartzwelder et al. 1995b) sensitive than adults to the effects of ethanol, depending on the variable being measured. However, the question of whether differences in ethanol pharmacokinetics could subserve differential behavioral outputs has been posited and not completely resolved (Silveri and Spear 2000;Spear 2007). Furthermore, ontogenetic differences in ethanol pharmacokinetics could provide the basis for increases in adolescent ethanol consumption when compared to adults (Brunell and Spear 2005;Doremus et al. 2005;Lancaster et al. 1996;Walker et al. 2008;Yoshimoto et al. 2002).
Although pharmacokinetic differences could be important determinants of differential age-related behaviors, it has been noted that systematic studies comparing adolescent and adult ethanol pharmacokinetics are limited (Hollstedt et al. 1980;Kelly et al. 1987;Silveri and Spear 2000) and have focused on Sprague-Dawley rats. The present study was designed to assess pharmacokinetic responses to ethanol in Wistar rats, another strain of commonly used rats in the alcohol research field. This was addressed by measuring blood alcohol levels (BALs) over a two-hour period in adolescent and adult male Wistar rats following administration of three doses of ethanol via two different routes of administration (either gavage or an intraperitoneal injection). In addition, an order effects analysis was conducted to evaluate whether pre-exposure to ethanol altered subsequent BALs produced by varied doses of ethanol.
Methods
Subjects
58 male adolescent (P30 on arrival) and 59 adult (P90 on arrival) Wistar rats (Charles River, Wilmington, MA) were used in the present study. The animals were pair-housed within a temperature-controlled (21.5 °C) vivarium with the animals being maintained on a 12-hour light/dark cycle (lights on at 6 a.m.). Upon arrival, animals were weighed and handled daily until the experimental procedures were initiated. Ad libitum food and water were provided for the duration of the experiment. The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals and was reviewed and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
Procedure
For all test days, the animals were weighed and transported to the test area 1.5 hours into their light cycle (i.e. 7:30 a.m.) and were allowed to habituate to the environment for 30 minutes prior to the onset of experimental procedures. Once habituation had occurred, half of the adolescents and adults were injected with a 20% w/v ethanol solution corresponding to 0.75, 1.5, or 3.0 g/kg (IP, N=60), while the other half were administered the same doses of ethanol via gavage (N=57); with the dose of ethanol being counterbalanced according to a Latin square design across days for all animals. Following ethanol administration, at 15, 30, 60 and 120 minutes, the animals were bled from the tip of the tail for later determination of blood alcohol levels (BALs). There was a two to three day interval in between each of the three test days.
Blood Alcohol Levels
Blood was collected from the tip of the tail (0.25 ml) 15, 30, 60 and 120 minutes following ethanol administration of 0.75, 1.5 and 3.0 g/kg. There was no volume replacement during the experiment. Immediately after tail blood collection, samples were centrifuged and plasma alcohol levels were determined using the Analox micro-stat GM7 (Analox Instr. Ltd.; Lunenberg, MA).
Data Analysis
An initial four-way mixed model analysis of variance (ANOVA) was conducted on the BALs of ethanol-naïve adolescents and adults following administration of ethanol (0.75, 1.5 or 3.0 g/kg) via two different routes of administration (IP and gavage) at 15, 30, 60 and 120 minutes post-injection with age, route and dose as the between-subject factors and time as the within-subject factor. Subsequently, to analyze age-related differences within each dose of ethanol, the BALs of adolescents and adults following administration of the ethanol (IP or gavage) were individually analyzed for each of the three ethanol doses using a two-way mixed-model ANOVA with time as the within-subject factor and age as the between-subjects factor. If significant main effects or interactions were found, the appropriate planned comparisons were conducted to identify differences between age groups within each dose.
The large sample size and use of a within-subjects design allowed for the evaluation of order effects due to the administration of different doses of ethanol on three different occasions. Mixed-model two-way ANOVAs were computed on the IP- or gavage-induced BALs produced by 0.75, 1.5 or 3.0 g/kg of ethanol over the 2-hour period for adolescent and adult rats receiving either 0.75, 1.5 or 3.0 g/kg ethanol on the first day of ethanol administration. The within-subjects factor was time and the between-subjects factor was initial ethanol dose (i.e., 0.75, 1.5 or 3.0 g/kg).
For all ANOVAs, if assumptions of sphericity were not met, the Greenhouse-Geisser correction was used to adjust the degrees of freedom in order to establish more conservative F-test critical values for the analysis.
Results
The results of the four-way ANOVA showed main effects for age ( F(1, 105) = 11.751, p ≤ 0.001), route ( F(1, 105) = 40.778, p < 0.001), dose ( F(2, 105) = 392.030, p < 0.001) and time ( F(3, 315) = 49.559, p < 0.001). Significant interactions involving the route of administration were identified: Route x Dose ( F(2, 105) = 15.441, p < 0.001), Route x Time ( F(3, 315) = 7.997, p < 0.001) and Dose x Time x Route ( F(6, 315) = 2.124, p ≤ 0.05), which reflect that the BALs produced by IP and gavage routes varied differentially depending on the dose and the time of measurement. Additionally, there were Time x Age ( F(3, 315) = 7.366, p < 0.001), Dose x Time ( F(6, 315) = 23.666, p < 0.001) and Dose x Time x Age ( F(6, 315) = 2.771, p < 0.05) interactions, which generally reflect that adolescent and adult animals showed differential responses over time to the different doses of ethanol.
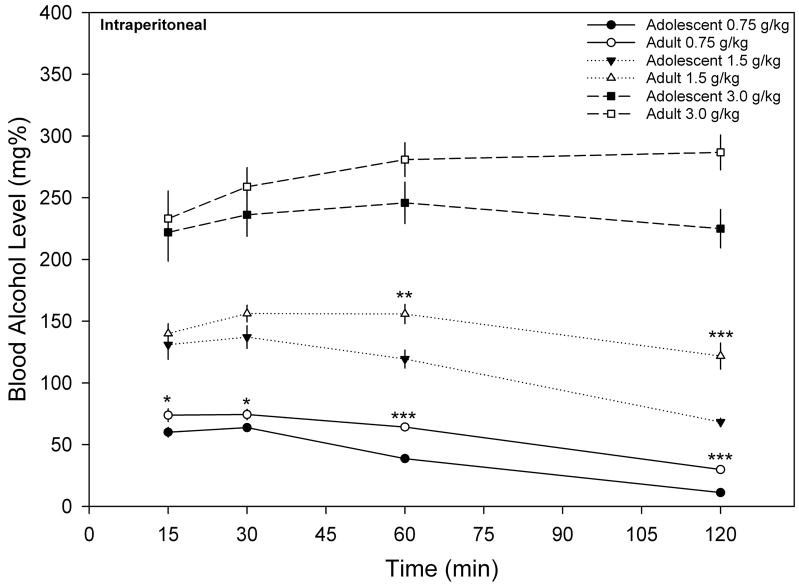
BALs over a two-hour time period following IP injection of ethanol (0.75, 1.5 or 3.0 g/kg) are illustrated in Figure 1. For the 0.75 g/kg ethanol condition, a two-way mixed model ANOVA identified a main effect of time ( F(3, 54) = 187.819, p < 0.001) and age ( F(1, 18) = 23.784, p < 0.001). Post-hoc comparisons identified that the adolescent BALs were significantly lower than the adults ( t(18) = −2.128 to −10.911, ps ≤ 0.05) for the entire two-hour time period following the ethanol administration. When evaluating the 1.5 g/kg condition (see Figure 1), a main effect of time ( F(3, 54) = 33.392, p < .001) and age (F (1, 18) = 9.159, p < 0.05) was found, as well as an Time x Age interaction ( F(3, 54) = 6.086, p < 0.005). Post-hoc comparisons identified that the BALs of adolescents were significantly different from the adults at the 60 ( t(18) = −3.413, p < 0.005) and 120 min time point ( t(18) = −4.847, p < 0.001). Contrary to the differences observed with lower doses of ethanol, the BALs of adolescents and adults following a 3.0 g/kg ethanol injection showed a main effect of time ( F(3, 54) = 6.148, p ≤ 0.001), but no main effect of age or a BAL x Age interaction.
Figure 1.
Mean (± S.E.M.) BALs over a two-hour time period in adolescent (n=10 per dose) and adult (n=10 per dose) rats following the IP administration of 0.75, 1.5 and 3.0 g/kg ethanol (* = p < 0.05, ** = p < 0.01 and *** = p < 0.001 when comparing the BAL of adolescent and adult animals at that particular time point).
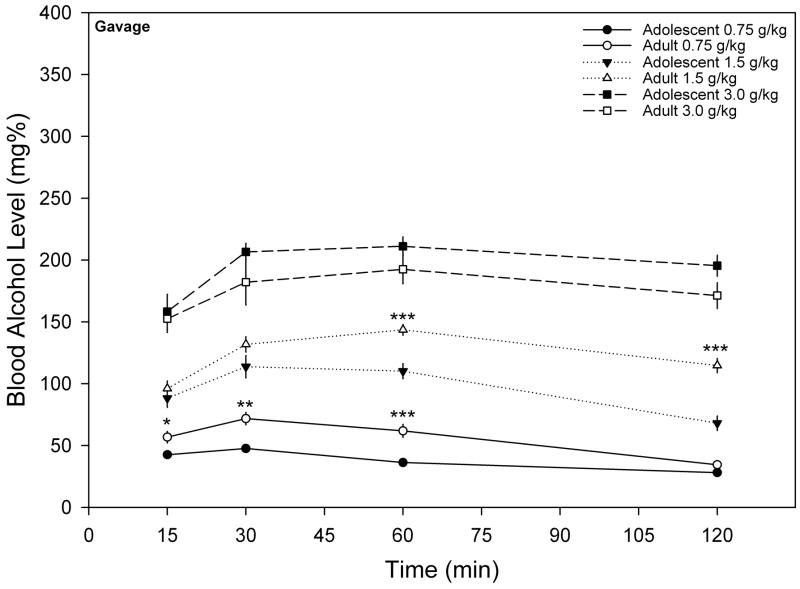
Figure 2 represents the mean BALs over a two-hour time period following administration of ethanol (0.75, 1.5 or 3.0 g/kg) via gavage. The two-way ANOVA conducted on the data following 0.75 g/kg of ethanol indicated main effects for age ( F(1, 17) = 14.150, p < 0.005), time ( F(3, 51) = 76.175, p < 0.001) and an Age x Time interaction ( F(3, 51) = 11.1, p < 0.001). Post-hoc comparisons showed that BALs at the 15, 30 and 60 minute time points for adolescents and adults were significantly different from each other ( t(17) = −2.611 to −4.289, ps < 0.05). The analysis of the 1.5 g/kg dose identified main effects for age ( F(1, 17) = 12.588, p < 0.005), time ( F(3, 51) = 39.992, p < 0.001) and an Age x Time interaction ( F(3, 51) = 7.925, p < 0.001). Post-hoc comparisons showed that the BALs of adolescents and adults at 60 minutes ( t(17) = −4.485, p < 0.001) and 120 minutes (t(17) = −5.577, p < 0.001) were statistically different. Lastly, when the 3.0 g/kg dose was evaluated with the two-way ANOVA, a main effect of time was observed ( F(3, 51) = 14.899, p < 0.001), but no effects of age or an Age x Time interaction were observed.
Figure 2.
Mean (± S.E.M.) BALs for adolescent (n= 9 – 10 per dose) and adult (n = 9 – 10 per dose) rats following administration of 0.75, 1.5 and 3.0 g/kg ethanol via gavage (* = p < 0.05, ** = p < 0.01 and *** = p < 0.001 when comparing the BAL of adolescent and adult animals at that particular time point).
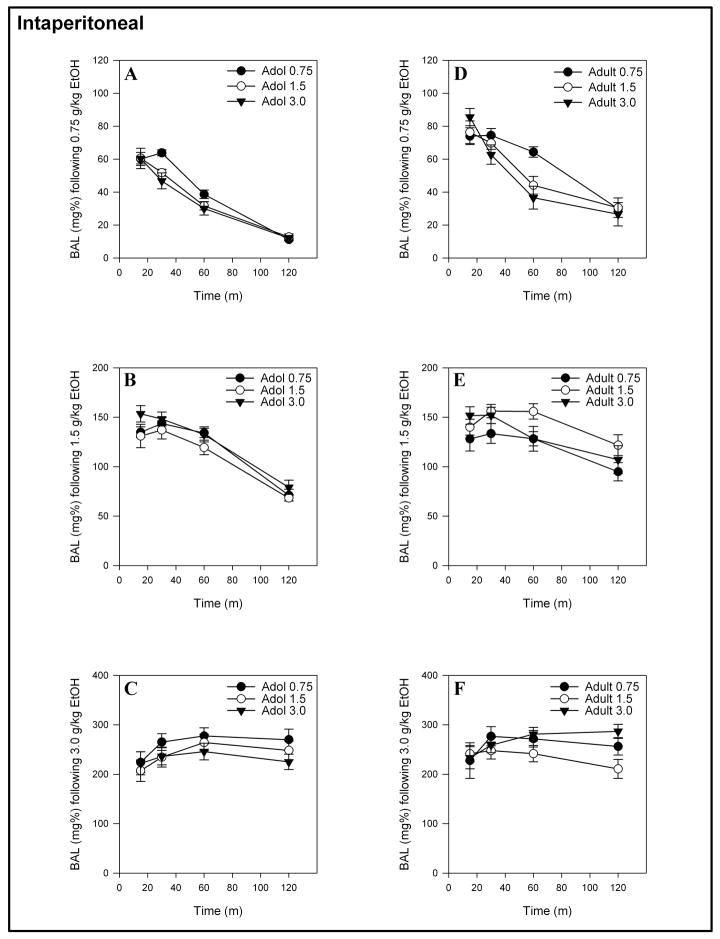
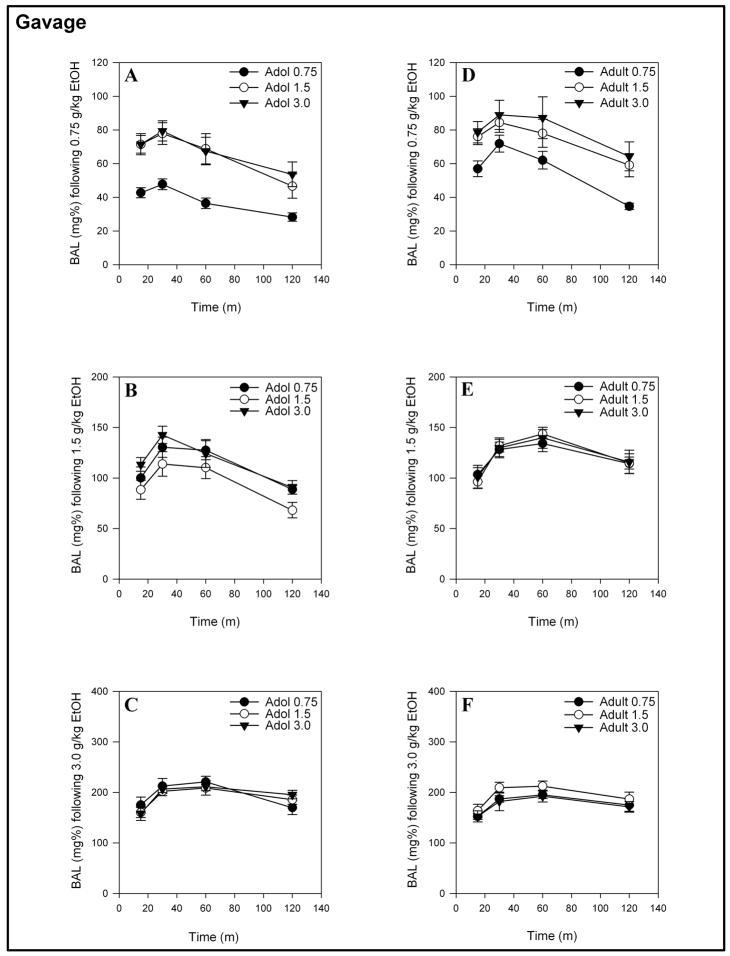
The mean BALs produced by 0.75, 1.5 or 3.0 g/kg ethanol over a 2-hour time period for animals receiving either 0.75, 1.5 or 3.0 g/kg on the first day of ethanol administration are presented in Figures 3 and Figure 4 (for IP or gavage administration, respectively). The two-way ANOVAs that were computed to evaluate order effects for IP-administered ethanol in adolescents and adults showed main effects of time for all doses ( F(3, 81) = 3.732 – 187.54, ps < 0.05 – 0.001) and Time x Initial Dose interactions for BAL’s produced by 0.75 g/kg ethanol ( F(6, 81) = 2.569, p < 0.05 and F(6, 81) = 5.567, p < 0.001 for adolescents and adults, respectively). The Time x Initial Dose interaction reflects that the BALs produced by 0.75 g/kg for those animals receiving 1.5 or 3.0 g/kg ethanol on the first day of exposure were lower than the BALs resulting from 0.75 g/kg ethanol administered to naïve animals. There were no Time x Initial Dose interactions for BALs produced by 1.5 or 3.0 g/kg ethanol, nor were there any main effects of initial dose for adolescents or adults following 0.75, 1.5 or 3.0 g/kg IP administration of ethanol.
Figure 3.
Mean (± S.E.M.) BALs produced by IP administration of 0.75, 1.5 or 3.0 g/kg ethanol for adolescents (A – C) and adults (D – F) receiving 0.75, 1.5 or 3.0 g/kg ethanol on the first day of ethanol exposure.
Figure 4.
Mean (± S.E.M.) BALs produced by gavage-administered 0.75, 1.5 or 3.0 g/kg ethanol for adolescents (A – C) and adults (D – F) receiving 0.75, 1.5 or 3.0 g/kg ethanol on the first day of ethanol exposure.
As observed with an IP route of administration, the two-way ANOVA computed on the gavage data showed main effects of time for both adolescents and adults ( F(3, 75–78) = 12.284 – 99.003, ps < 0.001) and a Time x Initial Dose interaction for adolescents when measuring the response to 0.75 g/kg ethanol ( F(6,75) = 2.824, p < 0.05). In contrast to the BAL data from IP-administered ethanol, there were main effects of initial dose for the gavage-induced BALs of adolescent and adult animals following 0.75 g/kg ( F(2, 25) = 8.797, p ≤ 0.001 and F(2, 26) = 3.403, p < 0.05, respectively). The main effect of initial dose that was observed in gavage-treated animals identified that those animals receiving either 1.5 or 3.0 g/kg initially had a much higher BAL across the entire time course than the naive animals receiving 0.75 g/kg ethanol. There were no other Time x Initial Dose interactions or main effects of initial dose for adolescent or adult animals receiving 1.5 or 3.0 g/kg ethanol.
Discussion
The purpose of this experiment was to evaluate whether male adolescent and adult Wistar rats display pharmacokinetic differences in response to acute ethanol administered via gavage or an IP injection. Overall, there were significant age- and route of administration-related differences and as expected, also main effects of dose and time on BALs. While the general BAL distributions from each dose of ethanol are comparable regardless of route of administration, there are some noticeable differences. One of the most striking dissociations was the reduced BALs across all time points for the high dose of ethanol (3.0 g/kg) following administration by gavage, which is reflected statistically by the Dose x Route interaction. In addition, the peak BALs appear to be shifted to the right for both age groups following administration of the lowest dose, and for the adult animals following administration of the moderate dose of ethanol by gavage (which is also supported by the Route x Time interaction). It is interesting to note that although the peak times are shifted, maximum recorded BALs for the lower doses (i.e., 0.75 and 1.5 g/kg) are comparable with each other regardless of route of administration. These data are relatively consistent with the peak BALs previously established in adult male Wistar rats following IP administration of 1.5 (Schulteis and Liu 2006) and 3.0 g/kg ethanol (Morse et al. 2000;Schulteis and Liu 2006).
When evaluating BALs to assess age- and route of administration-related differences in the response to 0.75 g/kg ethanol, adolescent animals showed a significantly reduced BAL in comparison to adults across the measured time points, an effect that was not altered by the route of administration. Following administration of 1.5 g/kg by IP injection or gavage, adolescent and adult animals showed comparable BALs for at least 30 minutes that then diverged to the point of being significantly different from each other at the 1 and 2 hour time points. In contrast to the two lower doses, no statistically significant differences were seen between adolescent and adult animals following administration of 3.0 g/kg ethanol over the two hour time period.
Thus, following low dose ethanol administration, adolescent BALs were distinctly different from adults. For moderate doses of ethanol, initial similarities between adolescent and adult BALs showed divergence one-hour after injection, with adolescent BALs being considerably lower than adults by the two hour time-point. Lastly, for high doses of ethanol, BALs for adults and adolescents were statistically comparable across the entire time frame. The response to the high dose of ethanol could be reflecting a ceiling effect produced by excessive ethanol levels that are temporarily overwhelming the mechanisms involved in ethanol elimination. It follows that if one was to measure BALs for longer than two hours (after administration of 3.0 g/kg), it might be expected that adolescent and adult elimination rates would diverge to the point of being statistically different as they did following injection of 1.5 g/kg ethanol. However, one should be cautioned against making such assumptions because although adult Sprague-Dawley rats show higher BALs for the first two hours following 2.5 g/kg ethanol when compared to adolescents, the adults then display faster ethanol elimination rates after the two-hour time point (Kelly et al. 1987). An additional factor to consider is that the blood collection regimen resulted in a higher proportion of blood volume being collected from adolescents in comparison to adults. Although the dissociable BALs seen between adolescents and adults would be difficult to explain in relation to the differences in blood volume collection, this is an important consideration for future study designs that utilize multiple blood samples over a short period of time.
In accordance with previous research using Sprague-Dawley rats to evaluate the ethanol elimination rate of adolescents and adults (Hollstedt et al. 1980;Kelly et al. 1987;Silveri and Spear 2000), the present data collected from Wistar rats showed age-dependent differences in ethanol pharmacokinetics. Furthermore, comparable time- and dose-dependent differences, as well as Dose x Time x Age interactions were found in the present study when compared to data collected using other strains of rats (Silveri and Spear 2000). One considerable difference between the present data set and that generated previously using Sprague Dawley rats was the inclusion of data in the analysis from rats that were pre-adolescent (i.e., P16) that potentially could be driving much of the age-related effects that were observed (Silveri and Spear 2000). In addition, there were differences in the ages of the “adults” used in each study. Although an adolescent age approximating P30 was included in all studies, the previous studies considered younger ages (P56 – P60) as adults (Hollstedt et al. 1980;Kelly et al. 1987;Silveri and Spear 2000), whereas the present study used a time point of P90 for adults. This age difference is not inconsequential, because depending on the gender of the rat, adolescence could be considered to extend as far as P55 to P60 (Smith 2003;Spear 2007). By selecting an adult age of P90 in the present study, adolescents can reliably be compared to adults.
Questions regarding age-related differences in ethanol pharmacokinetics as being a putative determinant of differences in behavior have been raised (Silveri and Spear 2000;Spear 2007). The results of the present study would suggest that under lower ethanol dose conditions, pharmacokinetic differences could be contributing to differential effects on brain and behavior. This is particularly important in regards to the assessment of adolescent neuroadaptations and/or neurotoxicity in response to ethanol (Spear 2007), as well as the development of self-administration models which are trying to model human alcohol abuse (Bell et al. 2006;Doremus et al. 2005;Martinetti et al. 2006;Walker et al. 2008). Based on the minimal differences between adolescent and adult BALs that were observed following administration of 1.5 and 3.0 g/kg ethanol during the first hour, one might be inclined to infer that increases in self-administration of ethanol would result in comparable increases in BALs for adolescents and adults. In contrast, the results observed following the 0.75 g/kg dose of ethanol suggest otherwise. Indeed, when controlling for weight, identical ethanol doses resulted in significantly different BALs across the entire two hour time course for adolescents and adults, regardless of route. The fact that this lower dose produces BALs in the range of those defining binge drinking in humans (NIAAA Advisory Council 2004) and that have been posited as a putative goal for animal models of binge drinking (Ji et al. 2008;Walker et al. 2008), highlights the need to measure not only behavior, but BALs when comparing the effects of ethanol on adolescents and adults.
To evaluate whether prior dosing with ethanol altered the subsequent response to varied doses of ethanol, order effects analyses were conducted on the BALs produced by different doses of ethanol depending on whether a particular dose of ethanol was administered on the first day or administered after exposure to one of the alternate ethanol doses. When evaluating the IP route of administration, it was identified that both adolescent and adult animals receiving 1.5 or 3.0 g/kg on the first day of injections showed an altered BAL profile over the 2-hour period following administration of 0.75 g/kg when compared to naïve animals receiving 0.75 g/kg ethanol. This appears to be consistent with research focusing on pharmacokinetic ethanol tolerance (Silvers et al. 2003); although at higher doses this phenomenon was not observed. Furthermore, it has been posited that much of the behavioral tolerance to higher doses of ethanol that has been previously observed is not exclusively attributable to pharmacokinetic tolerance (Spear and Varlinskaya 2005). The peak BALs and BAL time course following gavage-administered ethanol were dissociable from the observed tolerance produced by IP-administered ethanol. Both adolescent and adult animals that received either 1.5 or 3.0 g/kg ethanol on the first day of administration showed significantly escalated BALs produced by subsequent 0.75 g/kg ethanol administration in comparison to those animals receiving 0.75 g/kg ethanol on the first day of administration. One interpretation of this phenomenon would be that pre-exposure to high doses of ethanol decreases gastric metabolic efficiency to low doses of ethanol, however further research will have to be conducted to systematically evaluate this effect. Given that order effects were observed, particularly in the case of large dose-related differences seen following gavage-administered ethanol, the importance of measuring BALs when animals are subjected to multiple administrations of ethanol was identified. In addition, the existence of order effects also suggests that pharmacokinetic studies ought to utilize a between-subjects design to protect against excessive variance.
In summary, BALs following varied doses of ethanol administration via two different routes of administration were measured in ethanol-naïve adolescent and adult Wistar rats over a two-hour time period. The pharmacokinetic responses of adolescent animals were dissociable from adult responses, a fact that was clearly recognized when measuring BALs following injection of low to moderate doses of ethanol. Furthermore, there were route of administration- and initial dose-dependent dissociations in the metabolism of low doses of ethanol. This information should be useful for the development of animal models of alcohol abuse by identifying that regardless of route of administration (for gavage and IP), under lower ethanol administration conditions, enhanced adolescent ethanol administration (experimenter- or self-administered) could result in comparable BALs, a fact that should be incorporated into the interpretation of future adolescent / adult comparisons. Additionally, under conditions of high dose ethanol administration, absorption via the gastric route can produce markedly lower BALs when compared to IP administration.
Acknowledgments
Support for this research was provided by a National Institute on Alcohol Abuse and Alcoholism grant awarded to CLE (AA006059 & AA014339). The authors would like to thank Derek Wills, Greta Berg, Maury Cole and Tony Kerr for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vidal JM, Spear NE, Molina JC. Adolescent rats discriminate a mild state of ethanol intoxication likely to act as an appetitive unconditioned stimulus. Alcohol. 2003;30:45–60. doi: 10.1016/s0741-8329(03)00093-4. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hollstedt C, Rydberg U, Olsson O, Buijten J. Effects of ethanol on the developing rat. I. Ethanol metabolism and effects on lactate, pyruvate, and glucose concentrations. Med Biol. 1980;58:158–163. [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Bonthius DJ, West JR. Developmental changes in alcohol pharmacokinetics in rats. Alcohol Clin Exp Res. 1987;11:281–286. doi: 10.1111/j.1530-0277.1987.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Land C, Spear NE. Ethanol impairs memory of a simple discrimination in adolescent rats at doses that leave adult memory unaffected. Neurobiol Learn Mem. 2004;81:75–81. doi: 10.1016/j.nlm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Martinetti MP, Lowery EG, Vona SR, Wichnick AM, Adler RA, Finch DG. Limited-access consumption of ascending ethanol concentrations in alcohol-preferring, nonpreferring, and Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:836–843. doi: 10.1111/j.1530-0277.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Dev Alcohol. 2005;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- Morse AC, Schulteis G, Holloway FA, Koob GF. Conditioned place aversion to the “hangover” phase of acute ethanol administration in the rat. Alcohol. 2000;22:19–24. doi: 10.1016/s0741-8329(00)00099-9. [DOI] [PubMed] [Google Scholar]
- NIAAA Advisory Council. NIAAA Council approves definition of binge drinking. NIAAA Newsletter. 2004;3:5. [Google Scholar]
- Philpot R, Kirstein C. Developmental differences in the accumbal dopaminergic response to repeated ethanol exposure. Ann N Y Acad Sci. 2004;1021:422–426. doi: 10.1196/annals.1308.056. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008 doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Liu J. Brain reward deficits accompany withdrawal (hangover) from acute ethanol in rats. Alcohol. 2006;39:21–28. doi: 10.1016/j.alcohol.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. Modeling Adolescent Development and Alcohol Use in Animals. Alcohol Research and Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995a;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995b;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- U.S.Department of Health and Human Services. National Survey on Drug Use and Health, 2004. Washington, DC: 2005. [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcohol Clin Exp Res. 2002a;26:960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002b;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Hori M, Sorimachi Y, Watanabe T, Yano T, Yasuhara M. Increase of rat alcohol drinking behavior depends on the age of drinking onset. Alcohol Clin Exp Res. 2002;26:63S–65S. doi: 10.1097/01.ALC.0000026977.19902.61. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]