Abstract
The interplay between canonical and non-canonical Wnt pathways in development and tumorigenesis is tightly regulated. In this review we will describe the yin and the yang of canonical and non-canonical Wnt signaling pathways during melanocyte development, and melanoma genesis. Canonical Wnt signaling, represented by Wnts such as Wnt1 and Wnt3A, signals via β-catenin to promote melanocyte differentiation and tumor development. Non-canonical Wnt signaling, specifically Wnt5A, regulates canonical pathways, and signals to induce melanoma metastasis. This review will focus on the role of Wnt5A during melanoma progression, and its relationship to canonical Wnt signaling.
Wnts, Their Receptors and Co-receptors: A Motley Crew
The complexity of Wnt signaling in melanoma is due to the vast cohort of Wnts, their receptors, and co-receptors, and the ability of these to regulate each other. The Wnt family consists of over 19 members, all of which are hydrophobic cysteine rich secreted molecules that share a high level of homology. Wnts are typically between 350 and 400 amino acids and have a 20 – 85% amino acid identity. The ligand subtype determines which Wnt signaling pathway will be activated, for example, Wnt1, 3a, and 7 will activate the canonical pathway, whereas Wnt5a, 5b, and 11 will activate the non-canonical pathway (reviewed in Weeraratna, 2005). Depending on the cohorts of receptors available however, some non-canonical Wnts may activate canonical pathways (Mikels and Nusse, 2006). Wnts are involved in a variety of processes in development, which have implications for tumorigenesis. In order to focus on the specific Wnt pathways in melanoma, we have summarized the different Wnts, their genomic localization, and functions in development and cancer in a comprehensive table (Table 1).
Table 1.
| Ligand | Function | Reference |
|---|---|---|
| WNT1 |
|
Rijsewijk et al., 1987; Arheden et al., 1988 |
| WNT2A (WNT13) |
|
Wainwright et al., 1988; Blasband et al., 1992; Klein et al., 2009; Park et al., 2009; Kashani-Sabet et al., 2009; You et al., 2004 |
| WNT2B |
|
Katoh et al., 1996; Rhee et al., 2002; Benhal et al., 2006; Khan et al., 2007 |
| WNT3A |
|
Roelink et al., 1993; Chen et al., 2008; Chein et al., 2009 |
| WNT4 |
|
Ungar, Kelly, and Moon, 1995; Carroll, Wallingford, and Vize, 1999; Jeays-Ward et al., 2003; Miyakoshi et al., 2008; Boyer et al., 2009 |
| WNT5A |
|
Bittner et al., 2000; Weeraratna et al., 2004; Weeraratna et al., 2002; Dissanayake et al., 2007; Dissanayake et al., 2008; O’Connell et al., 2009; Schwartz et al., 2009; Ford et al., 2009; Badiglian Filho et al., 2009 |
| WNT5B |
|
Saitoh and Katoh, 2001; Morioka et al., 2009; Mangioni et al., 2005; Saitoh and Katoh, 2002 |
| WNT6 |
|
Wolda and Moon, 1992; Rankin et al., 1999; Schmidt et al., 2007; Lavery et al., 2008 |
| WNT7A |
|
Riddle et al., 1995; Le Grand et al., 2009; Heasley and Winn, 2008 |
| WNT7B |
|
Rajagopal et al., 2008; Pham et al., 2003 |
| WNT8A |
|
Swiatek et al., 2004 |
| WNT8B |
|
Martinez et al., 2009 |
| WNT9A (14) |
|
Matsumoto et al., 2008 |
| WNT9B (15) |
|
Juriloff et al., 2006; Jezewski et al., 2008 |
| WNT10A |
|
Nawaz et al., 2009; Ordway et al., 2006 |
| WNT10B (WNT12) |
|
Pham et al., 2003 |
| WNT11 |
|
Katoh, 2005; Ulrich et al., 2005; Wai et al., 2002 |
| WNT16 |
|
Mazieres et al., 2005; Casagrande et al., 2006 |
Receptors of the Wnt ligands include the Frizzled (FZD) family of receptors. FZD1 interacts with LRP1 and down-regulates canonical Wnt signaling (Zilberberg et al., 2004). FZD2 is important for the activity of Ror2 (Li et al., 2008), a Wnt co-receptor that we will discuss in detail later in this review. FZD3/9 has been shown to mediate Wnt-3a-dependent neurite outgrowth (Endo et al., 2008) and FZD4 plays a critical role in responses to Wnt signaling in the tumor microenvironment (Planutis et al., 2007). FZD5 has been shown to be involved in melanocytic tumors and prognosis in cutaneous melanoma (Bachmann et al., 2005) and we have shown that inhibiting FZD5 impedes melanoma cell motility (Weeraratna et al., 2002). FZD6 can negatively regulate canonical Wnt signaling (Golan et al., 2004). FZD7 contributes to self-renewal signaling in human embryonic stem cells, and appears to be an important cell surface antigen for ES cells (Melchior et al., 2008). The surface of dimeric CRD domain of the FZD8 receptor has been used to demonstrate the Wnt protein binding site (Voronkov et al., 2008) and FZD10 transactivation causes the activation of the non-canonical Dvl-Rac1-JNK pathway and plays critical roles in the development of synovial sarcomas (Fukukawa et al., 2009). In addition, Wnts also signal through co-receptors such as Cripto, low-density lipoprotein receptor related protein 5/6 (LRP5/6) and the orphan tyrosine kinase receptors ROR1 and ROR2. Cripto, a Nodal co-receptor, has been shown to be a primary target of β-catenin both in embryogenesis as well as in colon carcinoma cell lines and tissues (Morkel et al., 2003). It is regulated by the canonical Wnt signaling pathway through an intronic-exonic enhancer element, and contains three tandem TCF/LEF binding sites (Hamada et al., 2007). Multiple studies have shown the importance of LRP5/6, also known as Arrow, a co-receptor for Wnt signaling (reviewed in Li and Bu, 2005). The availability of these co-receptors, along with the ability of Wnt5A to signal via different FZDs to activate both disparate and similar pathways dependent upon cellular context increases the complexity of Wnt signaling in human disease.
There are also several inhibitors of Wnt signaling that play important roles in development, differentiation and tumor development, such as the Wnt inhibitory factor, WIF-1, dickkopf (DKK1), Kremen (Krm) and the secreted frizzled related protein (sFRP). These can interact with Wnts and receptors to negatively regulate signaling. For example, LRP5/6, when in a ternary complex with Krm and DKK1, can inhibit Wnt/β-catenin signaling (Nakamura and Matsumoto, 2008; Nakamura et al, 2008). Finally, crosstalk between Wnt signaling pathways and other pathways such as Notch, Sonic Hedgehog and the bone morphogenetic proteins (BMPs) compound this complexity. Because of the availability of several excellent reviews on these other pathways (Hsu et al., 2005, Okuyama et al., 2008, Pons and Quintanilla, 2006), we will not address their roles in Wnt signaling in this review, but instead will trace the signaling patterns of Wnt signaling in melanoma development and progression.
There are 3 main Wnt signaling pathways, the canonical pathway, involving β-catenin, the planar cell polarity pathway and the Wnt/ Ca2+pathway (reviewed in Weeraratna, 2005). The planar cell polarity pathway involves the activation of jun-kinase by Wnt5A, but has not yet been demonstrated to play a role in melanoma, thus we will not discuss it in this review. β-catenin activation by Wnts such as Wnt1 and Wnt3A is the best described of the three pathways as it relates to cancer, but the contribution of other Wnt pathways such as the Wnt5A/PKC/Ca2+ pathway to cancer progression is becoming increasingly clearer. As there are many excellent reviews that already exist on canonical Wnt signaling in cancer, and specifically melanoma (Chien et al., 2009, Larue et al., 2009, Larue and Delmas, 2006), we will focus on non-canonical Wnt signaling, specifically the Wnt5A/Ca2+ pathway, and the relationship between these two pathways in melanoma development and progression.
Wnt Signaling In Development And Differentiation
Melanocytes and the Melanocyte Stem Cell
Melanocytes arise from neural crest cells that migrate from the lateral edge of the neural plate to become a variety of cells including glial cells, smooth muscle cells and neurons (Dorsky et al., 1998). Wnt signaling plays a critical role in the development of the neural crest, specifically Wnt1 and Wnt3A (Dunn et al., 2000, Dorsky et al., 1998). Canonical Wnt signaling including Wnts such as Wnt6 (Schmidt et al., 2008) and Wnt8 is required both for the induction of the neural crest, and for its expansion, and controls the expression of a variety of key proteins including Slug and Snail (Sakai et al., 2005, Labonne and Bronner-Fraser, 1998). Wnt1 and Wnt3A also promote the development of neural crest cells into pigment cells. When cells are depleted of these two proteins they become neuronal rather than pigmented cells (Dorsky et al., 1998). Wnt1 signals to melanoblasts in a paracrine manner to increase melanocyte numbers, while Wnt3A and β-catenin can specify neural crest cells to become melanocytes (Dunn et al., 2005). In mice, melanoblasts are concentrated in the hair follicles, where they differentiate into melanocytes, whereas in humans, melanocytes interact with keratinocytes in the epidermis. This is controlled by DKK1 (Yamaguchi et al., 2008) and Wnt signaling via Wnts 1, 3A and 4 (Saitoh et al., 1998).
Canonical Wnt signaling has been shown to activate the expression of the melanocyte specific micropthalmia transcription factor, MITF-M (Takeda et al., 2000). This occurs via LEF-1 co-operation with MITF in the basic helix-loop-helix-leucine zipper domain. MITF activation in turn activates the expression of melanocytic antigens such as MART1, GP100 etc (Shibahara et al., 2001). This can be inhibited by the expression of DKK1, and interestingly it has been shown that fibroblasts secreting DKK1 can inhibit melanin production in neighboring melanocytes (Yamaguchi et al., 2008). We have also shown that non-canonical Wnt signaling via Wnt5A can inhibit the expression of melanogenic antigens (Dissanayake et al., 2008), supporting data that demonstrate that Wnt5A signaling can antagonize that of Wnt1/3A (Westfall et al., 2003, Topol et al., 2003). This may indicate that where canonical Wnt signaling is important for positioning and differentiation of melanoblasts, non-canonical Wnt signaling, perhaps via suppression of β-catenin, can cause de-differentiation of melanocytes and other cell types to a more stem cell-like state. This is supported by data from the hematopoietic system indicating that Wnt5A maintains hematopoietic stem cells (HSCs) in a quiescent state, thereby increasing both the short and long term repopulation of HSCs. Canonical Wnt signaling is required for the differentiation of these HSCs, and this study indicates that Wnt5A antagonizes the canonical Wnt signaling pathways to maintain pluripotency of the HSCs (Nemeth et al., 2007).
In the skin, stem cells can be found in three main regions- the bulge of the hair follicle, in the interfollicular epidermis and in the sebaceous glands. Evidence indicates that each of these stem cell populations have distinct characteristics (reviewed in Nishikawa and Osawa, 2007). Stem cells found in the hair follicle are essentially undifferentiated melanoblasts. Maintenance of the melanocyte stem cell (MSC) is dependent on a delicate interplay between the factors MITF, Pax3, SOX10, and DCT, and how they regulate stem cell maintenance is quite complex. Pax3 for example, plays a dual role in the regulation of transcription downstream of MITF, acting as a repressor rather than activator of DCT transcription during stem cell maintenance via its ability to bind to LEF1 and inhibit DCT transcription via the repressor Groucho (Lang et al., 2005). However, in the presence of canonical Wnt signaling, Pax3 can signal to activate MITF and cause increases in DCT transcription, and an increase in MSC differentiation. Data from the Herlyn lab has shown that a combination of Wnt3A, SCF and EDN3 are the most critical factors required for the establishment of a melanocyte from a human embryonic stem cell (Fang et al., 2006). Thus it is clear that the tightly regulated exit from and re-entry into the cell cycle relies on a balance of Wnt signaling, as is evidenced by the ability of canonical Wnt signaling to govern MITF transcription, and also by the high levels of Wnt inhibitors that are present in the melanocyte stem cell. Because Wnt5A can signal to inhibit canonical Wnt signaling, it is entirely possible that Wnt5A is involved in maintaining the quiescence of the MSC, just as it is important in maintaining the HSC, however no one has yet studied the presence of Wnt5A in this population of MSCs.
Wnts and Pigmentation: A Colorful Tale
Discussions on Wnt signaling and melanocyte development of necessity involve a consideration of the effects of Wnts on pigmentation. It has been a long held belief that pigmentation is an indicator of poor prognosis for melanoma patients, but recent data suggests that the elements involved in pigment production and differentiation may portend a better prognosis, for a variety of reasons. In an initial microarray study that separated aggressive melanomas from less aggressive ones based on their gene expression profiles, Wnt5A was highly upregulated, and corresponded to a dramatic decrease in MART1 expression (Bittner et al., 2000). Our laboratory has recently shown that Wnt5A signaling, via the activation of PKC and STAT3, can decrease the expression of MITF, and subsequently of MART1, DCT, GP100, etc (Dissanayake et al., 2008). This is coincident with an increase in metastasis, and this data is borne out from data by Hoek et al., who showed that melanoma patients could be divided into a highly metastatic cohort of samples based on a Wnt5A high, MITF low profile, and a less metastatic cohort of samples based on an MITF high, Wnt5A low profile (Hoek et al., 2006). They confirmed that more invasive melanoma samples have less MITF in a follow-up study, and showed that the rates of proliferation also decrease in more invasive melanoma cells (Hoek et al., 2008).
This decrease in MITF, and increase in Wnt5A has multiple consequences. As mentioned above, it may have implications for the role of Wnt5A in the melanocyte stem cell. Since these antigens are involved in pigment production, Wnt5A treatment results in a dramatic decrease in pigmentation of melanoma cells in culture, perhaps allowing these cells to escape early detection by physicians, as is often the case with amelanotic melanomas. Finally, and perhaps most clinically relevant at this time, since MART1, GP100 and the like are presented as antigens at the surface of melanoma cells, allowing for the infiltration of cytotoxic T-cells into the tumor, the down regulation of these antigens heralds an escape from immune surveillance of the tumor. Indeed, we have shown that when cells that are MART1 positive are presented to T-cells that express the receptor for MART1, they activate these cytotoxic T-cells, unless they are first treated with recombinant Wnt5A, which makes them less susceptible to cytolysis. The reverse is also true, when MART1 low, Wnt5A high cells have their Wnt5A silenced by siRNA, MART1 expression increases, and the cells are more susceptible to cytolysis. Staining of a tissue array demonstrates that melanoma metastases express more Wnt5A and less MART1, with the quite remarkable exception of lymph node metastases (Dissanayake et al., 2008). We believe that this indicates that there are two separate modes of melanoma cell migration, one of which may be mediated by Wnt signaling, as described further on in this review.
Just as Wnt5A signaling can decrease the differentiation phenotype of melanoma cells, canonical Wnt signaling can promote it, perhaps with beneficial effects for patient survival. Chien and colleagues have recently shown treating melanoma cells with Wnt3A can decrease their metastatic ability, while simultaneously increasing their pigment, and concomitant with that, their melanogenic antigen expression (Chien et al., 2009). This is in keeping with data from developmental studies, as described above, that show that canonical Wnt signaling is critical for the differentiation of stem cells and melanoblasts into melanocytes (Dorsky et al., 1998). In vivo data from the Chien et al study further indicate that B16 melanoma cells transfected with Wnt3A are less likely to metastasize, and in a melanoma tissue microarray comprised of samples from patients with a 30year follow up nuclear β-catenin correlates to increased survival (Chien et al., 2009). These studies highlight the incredible complexity of the Wnt signaling cascades in melanoma. Below we present a brief overview of the canonical Wnt signaling pathway, and a more in depth review of non-canonical Wnt5A signaling in melanoma.
Wnt Signaling in Melanoma
Canonical Wnt Signaling in Melanoma
The most predominant canonical Wnts in melanoma are Wnt1 and Wnt3A. Wnt1 was first identified as a proto-oncogene activated in mouse model of mammary tumors (Rijsewijk et al., 1987) and mapped to chromosome 12q13 by in situ hybridization (Arheden et al., 1988a). The localization of this gene, the region 12q13-q14, has been reported to be involved in chromosomal rearrangements in a wide range of cancers (Arheden et al., 1988b). Since its discovery, it has been shown to be involved in cell proliferation, motility, patterning, embryonic development, fate determination, and carcinogenesis. Human Wnt3A is located on chromosome 17q21 and was isolated by use of mouse Wnt3 sequences as a probe and comparison between the deduced mouse and human Wnt3 protein sequences showed four changes in 333 amino acids (Roelink et al., 1993). As previously discussed, Wnt3A is critical for melanocyte differentiation, and may promote melanoma differentiation as well. Both Wnt1 and Wnt3A signal to activate β-catenin. Rubinfeld et al first reported evidence of β-catenin mutations resulting in its stabilization in 6 out of 26 melanoma cell lines (Rubinfeld et al., 1997). However, since then, it has been shown that in uncultured melanomas, β-catenin mutations are not very frequent, and in fact β-catenin is often found to be localized in the nucleus of melanoma cells despite the lack of mutations (Rimm et al., 1999). Truncating mutations of the adenomatous polyposis coli (APC) gene, that regulates β-catenin levels, are also rare in melanoma (Worm et al., 2004), and therefore do not account for the elevated levels of β-catenin. Many studies have shown that β-catenin is critical for the malignant transformation of melanocytes, and these are elegantly summarized in excellent reviews from Larue and colleagues as previously mentioned (Larue et al., 2009, Larue and Delmas, 2006). Studies have shown that increasing canonical Wnt signaling in melanocytes leads to their immortalization, increased proliferation, and clonogenic survival (Dunn et al., 2000). Importantly, it has also been shown that β-catenin promotes an escape of melanocytes from senescence via the inactivation of p16, resulting in their immortalization. This co-operates with signaling downstream of N-ras activation to promote melanoma genesis in animal models (Delmas et al., 2007). All of these studies together demonstrate that although β-catenin mutations are uncommon in melanoma, β-catenin activation is a key step in the initial transformation of melanocytes to melanoma.
Although it is abundantly clear that β-catenin activation is required for melanoma genesis, the role of β-catenin and canonical Wnt signaling in melanoma metastasis is quite controversial. A recent study demonstrates that inhibiting GSK-3β, which normally phosphorylates and degrades β-catenin, increases melanogenesis both in B16 cells, and in melanocytes, and that the concomitant increase in β-catenin has inhibitory effects on proliferation (Bellei et al., 2008). Chien et al, have also recently shown that when Wnt3A is activated in melanoma cells, melanoma cell proliferation decreases, and Wnt3A expressing cells are less likely to metastasize (Chien et al., 2009). Studies from Takahashi et al support these data, showing that silencing of β-catenin in B16 melanoma cells actually promotes metastasis (Takahashi et al., 2008). Since Wnt5A can antagonize β-catenin signaling and promote metastasis, but not tumor formation, these data all meld together quite nicely. This brings us to the central point of this review. Both Wnt signaling pathways are critical to melanoma, existing in an opposite and complimentary role as regards melanoma progression. Canonical Wnt signaling is important in the early stages of tumor development, but its continued expression in later stages, due to its promotion of differentiation and deleterious effects on metastasis, may actually bode well for the melanoma patient. Wnt5A signaling, on the other hand, is absent in the early stages of the tumor, and in fact, unpublished data from our laboratory indicates that treating melanocytes with Wnt5A causes them to apoptose, rather than transform. This is consistent with multiple studies from breast cancer (Jonsson et al., 2002), as well as other cancers (Kremenevskaja et al., 2005), where Wnt5A can act as a tumor suppressor. However, once melanocytes have transformed, Wnt5A activation down regulates β-catenin, and promotes metastasis (Figure 1). The role of Wnt5A in melanoma malignancy is described in detail below.
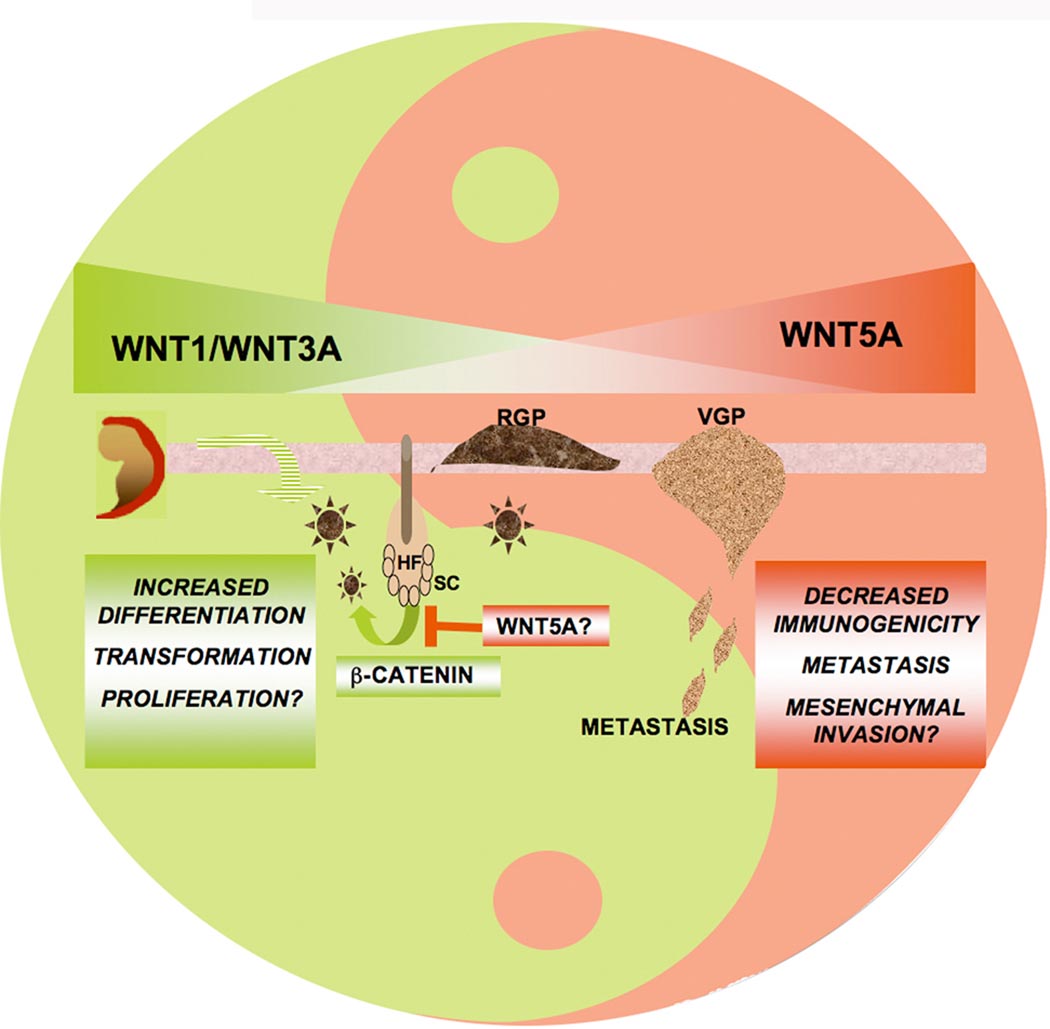
Figure 1. The Yin and Yang of Wnt Signaling in Melanoma Devlopment and Progression.
Both Wnt signaling pathways are critical to melanoma, existing in an opposite and complimentary role. Canonical Wnt signaling is important in the early stages of tumor development during a conversion of melanocytes to radial growth phase melanoma (RGP), but its continued expression in later stages such as vertical growth phase melanoma (VGP), and metastases, due to its promotion of differentiation, may inhibit metastasis. β-catenin is also important for the regeneration of melanocytes, and promotes the entry of quiescent stem cells (SC) from the hair follicle (HF) into the cell cycle. Wnt5A signaling, on the other hand, is absent in the early stages of the tumor, However, once melanocytes have transformed, Wnt5A activation downregulates β-catenin, and promotes metastasis, and may contribute to the maintenance of the stem cell.
Non-canonical Wnt signaling in Melanoma: Spotlight on Wnt5A
Receptor binding and internalization
Microarray studies initially demonstrated the upregulation of Wnt5A in metastatic cells (Bittner et al., 2000). Further studies confirmed that indeed Wnt5A was over expressed in metastatic melanoma tissues, and ectopic expression of Wnt5A could mediate motility in melanoma cells, via activation of PKC (Weeraratna et al., 2002, Dissanayake et al., 2007). Da Forno et al demonstrated that Wnt5A over expression also correlated to decreased survival in melanoma patients. Interestingly this study also demonstrated nevi expressed quite high levels of Wnt5A. Since nevus cells are highly motile, and since Wnt5A is associated with decreases in proliferation (Hoek et al., 2006), the observation of high Wnt5A in nevi is not inconsistent with the behavior of these cells. Wnt5A is known to act very differently in different context- for example, in many cancers it is a tumor suppressor. It may be that in nevi, the interplay of other molecules such as p16, B-Raf and canonical Wnt signaling results in a different cellular context in nevi than that found in melanoma cells. Wnt5A may act to suppress canonical Wnt signaling, also playing a tumor suppressor role to begin with, but once beta-catenin is able to suppress p16 and transform cells, then Wnt5A activation of PKC can promote metastasis. This is supported by the results from Da Forno et al, which show a decrease in Wnt5A expression from nevi to radial growth phase (RGP) melanoma, followed by an increase in vertical growth phase (VGP) and more metastatic lesions (Da Forno et al., 2008). However, an inconsistency highlighted by the presence of Wnt5A in nevi involves the fact that, where high Wnt5A in melanoma is associated with decreases in pigmentation, nevi are highly pigmented, despite the presence of Wnt5A. The same scenario is true for B-Raf, where mutant B-Raf inhibits MIT-F, and B-Raf knockdown increases melanogenesis in melanoma cells (Rotolo et al., 2005). Yet, the large majority of nevi, which are pigmented, have mutant B-Raf (Pollock et al., 2003). A simple explanation for these discrepancies may be the differences in cellular context between nevi and melanoma. Also, since nevi are so highly pigmented, it is possible that small variations in color in these lesions reflect mutant B-Raf or high Wnt5A status, and we do know that the variation in color in nevi is one of the factors used to diagnose malignant transformation. There has been no study done to determine whether nevi with high Wnt5A or mutant B-Raf have less pigment than their low Wnt5A and wild type B-Raf counterparts, which would be a first step in solving this conundrum. It is interesting to note however, that another study demonstrates that, like Wnt5A, B-Raf expression is high in nevi, decreased in RGP melanoma and increased in VGP and more metastatic tumors (Dong et al., 2003). This study suggests that in fact, B-Raf is not involved in melanocytic transformation, but rather in melanoma progression, which is exactly what appears to be the case for Wnt5A.
Over the last decade or so the picture of how Wnt5A signals to mediate melanoma metastasis has become increasingly clearer. First, Wnt5A binds to a very specific cohort of receptors in human melanoma cells- FZD2, FZD5 and ROR2 (Weeraratna et al., 2002, Billiard et al., 2005). FZD2 and 5 are both expressed in melanoma, and it has been shown that blocking the FZD5 receptor using antibodies (Sen et al., 2001) can inhibit invasion of melanoma cells (Weeraratna et al., 2002). However, recent data demonstrate that ROR2, an orphan tyrosine kinase receptor, may be an even more specific receptor for Wnt5A. ROR2 was discovered to be a Wnt co-receptor following the identification of a Frizzled-slike cysteine rich domain in the extracellular region (Saldanha et al., 1998). Wnt5A has been shown to initiate the homodimerization of ROR2 (Liu et al., 2007) and in osteoblasts, ROR2 mediates Wnt5A-induced cell migration through c-Jun N-terminal kinase (JNK) via the actin-binding protein filamin A (Nomachi et al., 2008, Nishita et al., 2006). Wnt5A can interact physically and functionally with ROR2 to mediate downstream signaling, and ROR2 signaling is specific for Wnt5A (Oishi et al., 2003). We have recently shown that ROR2 is highly expressed in metastatic melanoma, and the knockdown of ROR2 inhibits the ability of Wnt5A to signal and mediate metastasis (O’Connell et al, In Submission). This, in combination with all of the above data that identify ROR2 as a very specific receptor for Wnt5A, may have considerable implications for melanoma therapy.
Internalization
In the above-mentioned study on ROR2 and Wnt5A in melanoma, we further demonstrate that, in order to signal, Wnt5A must first bind to ROR2, after which the complex of ROR2 and Wnt5A are internalized via clathrin-coated endosomes (O’Connell et al, In Submission). This corroborates data from chondrocytes that show that ROR2 is internalized via rab5 positive endosomes (Akbarzadeh et al., 2008). Additionally, ROR2 is very similar in structure to neurotrophic tyrosine kinases (Wilson et al., 1993), and these too have been shown to signal from with clathrin-coated endosomes (Howe et al., 2001). Other Wnt receptors have been shown to be internalized via the endosome as well. It has been shown by Pfeiffer et al that canonical Wnt proteins such as Wingless are first secreted to the cell surface via the golgi, then endocytosed and compartmentalized into endosomes, from which they can be recycled to the surface (Pfeiffer et al., 2002). It may be that this process requires Wntless (WLS), a receptor that appears to be critical for endosome-golgi trafficking of Wnt proteins, and that is present in the golgi and the endosomes (Banziger et al., 2006, Bartscherer et al., 2006, Goodman et al., 2006). ROR2 is also present in both of these organelles in Wnt5A-high cells, raising the question whether it too may play a similar role in Wnt5A trafficking. The localization of ROR2 is dependent on the amount of Wnt5A present- in the presence of high amounts of Wnt5A, there is more internalized ROR2. Inhibition of clathrin inhibits internalization of ROR2, and subsequently motility (O’Connell et al, In Submission). Interestingly, results from two different groups indicate a similar discrepancy between the localization of WLS, with one group finding it largely in the subcellular organelles/ golgi (Banziger et al., 2006) and the other at the plasma membrane (Bartscherer et al., 2006). Both groups used different cell types, and our current results suggest that based on the Wnt context of the cells, subcellular localization of Wnt receptors may vary greatly, perhaps accounting for the observed discrepancy in the above-mentioned case. From our data it appears that this process of Wnt/receptor internalization is PKC dependent, resulting in a positive feedback loop between Wnt5A signaling and activation. Witze et al have also demonstrated that in order to mediate migration of melanoma cells, rab4 internalization of Wnt5A is required, and that this too is PKC dependent (Witze et al., 2008). Since ROR2 has serine threonine phosphorylation sites (Kani et al., 2004), it is entirely possible that it can be phosphorylated by PKC, thereby increasing its rate of auto-phosphorylation and activation.
G-Protein Coupled Signaling, And Second Messenger Activation
It has been shown that Wnt5 A is pertussis toxin sensitive, implicating the proteins Gαi and Gαo in its activation (Dejmek et al., 2003). The frizzled receptors contain a seven-transmembrane region that is consistent with G-protein coupled receptors (Schulte and Bryja, 2007). It has also been shown that RTKs can activate G-proteins (Dejmek et al., 2003), so it is also possible that Wnt5A activation of G-proteins may be downstream of ROR2. To further complicate matters, ligand stimulation can increase RTK activation via changes in metalloproteinase (Shah et al., 2006). Since we have previously shown that Wnt5A can increase MMP-2 activation (O’Connell et al., 2008), Wnt5A activation of G-proteins may also occur upstream of ROR2, such that both modes of activation may propagate the other resulting in a positive autocrine feedback loop. Regardless, the ultimate effect is the activation of PLCγ, phospholipid turnover in the membrane, and production of inositol triphosphate and di-acyl glycerol. These ultimately act to increase the release of calcium from its intracellular stores, and activate PKC (Kuhl et al., 2000b). Calcium activation is responsible for the activation of CAMKII and subsequent phosphorylation events (Kuhl et al., 2000a). Inhibition of PKC and of Ca2+ can modulate the cellular effects of Wnt5A. In other cellular systems, increases in calcium have been shown to activate the calcium sensing receptor, which in turn can activate secretion of Wnt5A, and increase expression of ROR2 (Macleod et al., 2007). In melanoma, we have previously demonstrated that Wnt5A increases levels of CAMKII phosphorylation (Dissanayake et al., 2007), and increased CAMKII has been shown to protect against Trail-induced apoptosis in melanoma (Xiao et al., 2005). More recently we have shown that Wnt5A-mediated calcium activation also increases the activity of the calpain protease family. This in turn can cleave the cytoskeletal protein filamin, which has been shown to be required for ROR2-Wnt5A mediated filopodia formation in migratory osteoblast cells. Our data indicate that filamin cleavage is associated with increased motility in melanoma, and this cleavage requires active calcium and calpain activity (O'Connell et al., 2009). All of these data indicate a critical role for calcium signaling in melanoma metastasis.
The downstream signaling target that we have studied most extensively in our laboratory is PKC. Our data indicates that the isoforms of PKC most affected by Wnt5A signaling in melanoma cells are the calcium sensitive PKC isoforms (Weeraratna et al., 2002), PKC α and β, and to some extent, µ. Wnt signaling does not appear to affect the total pool of PKC phosphorylation but instead causes a shift from non-phosphorylated to phosphorylated PKC (Dissanayake et al., 2007). PKC activity has been shown to increase the migration of melanoma cells, and its inhibition can decrease melanoma metastasis (Nakamura et al., 2003, Mapelli et al., 1994, Legg et al., 2002, Dennis et al., 1998). We show that many effects of Wnt5A, such as activation of an EMT, down regulation of metastasis suppressors such as Kiss-1, and upregulation of metastasis activators such as CD44, are dependent upon PKC (Dissanayake et al., 2007). Further, inhibition of PKC results in a decrease in Wnt5A-mediated melanoma motility (O'Connell et al., 2008, Dissanayake et al., 2007). PKC mediates many of the effects of Wnt5A, and is also responsible for its continued over expression, as PKC activation is capable of stabilizing Wnt5A mRNA, which in turn increases levels of Wnt5A, which likely binds to its receptors to activate more PKC (Jonsson et al., 1998). Secretion of Wnt5A thus promotes autocrine signaling, and we have recently demonstrated that this is augmented by the presence of heparan sulfate proteoglycans as described below.
Positive Feedback- Sequestering Wnt5A At The Surface Of The Cell
Cell surface HSPGs have also been demonstrated to play a role in Wnt signaling. Heparan sulfate proteoglycans (HSPGs) are ubiquitously expressed on almost every type of cell with their major function being to bind extracellular ligands using their heparan sulfate glycosaminoglycan (GAG) side chains. The sulfation status of the GAG chains determines to which specific portion of the GAG chains ligands, such as Wnt, will attach. GAG chains are targets for many effector molecules including growth factors and their receptors, cell-cell adhesion molecules, proteolytic enzymes, protease inhibitors, and extracellular matrix proteins (reviewed in Delehedde et al., 2001). The binding to this vast array of molecules subsequently facilitates protein interactions and downstream events, i.e. enhanced or reduced formation of ligand-receptor complexes subsequently influencing intracellular signaling. In vivo functional studies of HSPGs in Drosophila have demonstrated the critical roles of HSPGs in several major signaling pathways, including Wnt, fibroblast growth factor (FGF), Hedgehog (Hh) and TGF-β (Lin and Perrimon, 2000, Baeg et al., 2001). HSPGs have also been shown to be essential in coordinating the extracellular distribution of Wg, the Drosophila homolog of Wnt (Baeg et al., 2001). Further, in embryogenesis, the heparan-specific N-acetyl glucosamine sulfatases, QSulf1, is induced by Sonic hedgehog in myogenic somite progenitors and is required for the activation of MyoD, a Wnt-induced regulator of muscle specification (Dhoot et al., 2001). QSulf1 has been shown to remodel the 6-O sulfation states of cell surface HSPGs to promote Wnt signaling (Ai et al., 2006).
There are two types of cell surface HSPGs known as glypicans and syndecans (Lopes et al., 2006, Filmus et al., 2008). Glypicans are attached to the cell surface via a GPI anchor, while syndecans consist of extracellular, transmembrane, and cytoplasmic domains. GAG side chains are joined to the core protein via a tetrasaccharide linker attached to serine residues. Recently, our laboratory has demonstrated that HSPG expression, especially syndecan 1 and syndecan 4, correlates to metastatic potential (O’Connell et al., In Submission). Removal of HSPG GAG chains caused a decrease in endogenous Wnt5A levels and an increase in Wnt5A protein in the media of heparinase treated cells. Further, Wnt5A downstream targets were decreased. In addition, both motility and invasive capability were reduced in the heparinase treated samples, which could be restored by treatment with excess Wnt5A, but not Wnt3A. A similar effect was seen when syndecan 1 and 4 core protein levels were reduced. Motility, invasiveness, and Wnt5A signaling were all decreased upon syndecan 1 and 4 knockdown. Cell surface biotinylation assays and immunofluorescent microscopy (respectively) indicate that Wnt5A interacts with syndecans at the surface of the cells, but that syndecans can also be internalized into endosomes along with Wnt5A, perhaps as part of a complex of Wnt5A/ROR2/Syndecan (O’Connell et al., In Submission). The ability of syndecans to sequester Wnt5A at the surface of the cell and promote its signaling greatly contributes to the observed downstream effects of Wnt5A on melanoma cell motility. All of these data together tell the story of Wnt5A binding to ROR2, subsequent internalization and activation of signaling, which increases mRNA stabilization of Wnt5A, its increased secretion, its capture by HSPGs, and re-presentation to the receptor, resulting in a positive feedback loop, with multiple downstream effects that promote a metastatic phenotype (Figure 2).
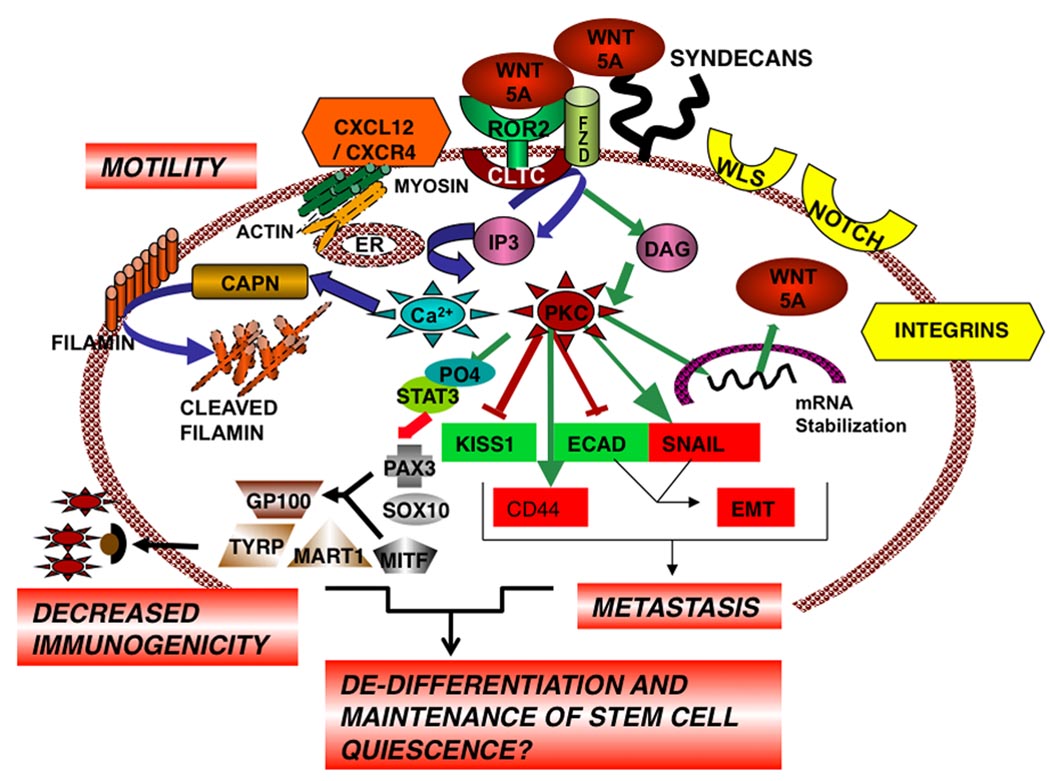
Figure 2. Wnt5A Signaling in Melanoma Metastasis.
Wnt5A binds to ROR2, perhaps via Frizzled, and is internalized via clathrin (CLTC), activating Ca2+ and PKC signaling which increases mRNA stabilization of Wnt5A and its increased secretion. Secreted Wnt5A is sequestered to the surface of the cell by HSPGs, and re-presented to its receptor, resulting in a positive feedback loop, with multiple downstream effects that promote a metastatic phenotype, including an EMT, upregulation of CD44, loss of Kiss-1, downregulation of melanogenic antigens, and cytoskeletal changes. See text for details.
Downstream Signaling: Course To Motility
Signaling via calcium and PKC can activate a host of downstream signal transduction pathways that account for the pleiotropic effects of Wnt5A. One consequence of both calcium and PKC signaling is a change in cell shape due to alterations of the cytoskeleton. We have previously shown that ectopically expressing Wnt5A in melanoma cells can cause changes in actin (Weeraratna et al., 2002). Microarray analysis of Wnt5A overexpressing cells also indicated changes in other cytoskeletal proteins such as vimentin (Dissanayake et al., 2007). Concomitant with these changes are change in the shape of the cell, switching from a rounder and more cobblestone-like appearance to a long and spindly shape. In melanoma cells, the transcriptional repressor Snail has been shown to mediate many of the changes leading to the acquisition of a metastatic phenotype, including upregulation of MMP2, and activation of Notch signaling (Nyormoi and Bar-Eli, 2003, Kuphal et al., 2005). Previous studies also indicated that ectopic over expression of Snail could upregulate MMP-2 and Wnt5A in squamous cell carcinomas (Yokoyama et al., 2003, Taki et al., 2003). In addition, in keeping with our observations that Wnt5A often acts in a positive feedback loop with its downstream elements, we showed Wnt5A could also upregulate Snail and vimentin and down-regulate e-cadherin in a β-catenin independent manner, leading to a dramatic change in cell shape (Dissanayake et al., 2007). These data further highlight the importance of the loss of E-cadherin for malignant transformation and progression of melanoma (Herlyn et al., 2000), as it is one of the few changes that are consistent between these somewhat disparate Wnt signaling pathways.
Wnt5A and the Cytoskeleton: Anchors Aweigh
In order for cells to undergo an EMT, as well as to become motile, further changes in the cytoskeleton have to occur. Witze et al showed that in melanoma cells exposed to a chemokine gradient, specifically CXCL12, Wnt5A signaling could control the direction migration of melanoma cells to CXCL12 via the redistribution of the melanoma cell adhesion molecule (MCAM) into a polarized structure that involved both actin and myosin (Witze et al., 2008). We have also shown that this is true for other systems, where Wnt5A is critical for the signaling from the CXCL12-CXCR4 axis in human T-cells, causing their directional migration (Ghosh et al., 2009). Since CXCR4 over expression is common in metastatic melanoma (Robledo et al., 2001), the Wnt5A regulation of this signaling axis in both T-cells and melanoma cells may have significant implications for immune response to melanoma. Wnt5A also affects other components of the cytoskeleton, and we have recently shown that highly metastatic melanoma cells express large amounts of the protein Filamin (O’Connell et al., 2009). Filamin has been shown to mediate the motility of osteoblasts via a Wnt5A/ ROR2 dependent mechanism (Nishita et al., 2006, Nomachi et al., 2008). We examined our melanoma cells for Filamin expression in the context of Wnt5A and metastasis, and found that while there was an increase in Filamin in metastatic cells, it was distributed in a diffuse manner across the cell, rather than in ordered filaments around the cell. Since Wnt5A activates calcium, and Filamin contains cleavage sites for a calcium activated protease known as calpain, we investigated whether Wnt5A induced calcium increased activated calpain cleavage of Filamin. We found that this was the case, and that the cleavage of Filamin was essential for migration. Interestingly, silencing of Filamin inhibited migration, indicating that cleaved Filamin is not merely a degradation product of increased calpain activity but may also play a role in signaling to promote metastasis (O’Connell et al., 2009).
Effects of Wnt5A on Cell Adhesion: To Dock or Not To Dock?
In the aforementioned study, Wnt5A-mediated cleavage of Filamin caused it to move away from the periphery of the cells. This indicated to us that there may be some effects on cell adhesion. We believe that many of the differences between the roles of Wnt5A in breast cancer and melanoma may rely on the adhesion patterns in these different cell types. Work from the Andersson lab has shown that in breast cancer, Wnt5A enhances the adhesion of breast cancer cells via src, activating collagen, which induces the discoidin domain receptor (DDR1) (Jonsson and Andersson, 2001). This increased adhesion to collagen prevented the migration of the Wnt5A transfectants through collagen matrices. Increased Wnt5A, and subsequently, adhesion of breast cancer predicts a favorable outcome (Jonsson et al., 2002). Conversely, in melanoma increased adhesion predicts a less favorable outcome. For example, β1 integrins promote CXCR4 mediated binding of tumor cells to endothelial cells (Cardones et al., 2003). Upregulation of other cell adhesion molecules such as MCAM etc., are also affected/ respond to CXCR4 signaling (Witze et al., 2008) as previously mentioned, and Wnt5A over expression increases melanoma cell adhesion (Weeraratna et al., 2002), while simultaneously promoting metastasis, supporting the notion that, unlike breast cancer cells, melanomas are pushed towards invasion by increases in adhesion.
The increased ability of CXCR4 to mediate melanoma metastasis via increases in adhesion of tumor cells to endothelial cells (Cardones et al., 2003) and the ability of Wnt5A to mediate CXCR4 signaling in T-cells (Ghosh et al., 2009) leads to a discussion of what at first seemed to be an anomaly in our data. We have previously demonstrated that Wnt5A suppresses MART1 expression, and that the ratio of Wnt5A to MART1 was dramatically increased in visceral metastases (Dissanayake et al., 2008). However, in lymph node metastases there was very little expression of Wnt5A and high expression of MART1. This may simply be due to the microenvironment of the lymph node, however, it is also very interesting to start to consider the routes of metastasis, and what mediates them. It has been postulated that two types of melanoma invasion exist- the amoeboid form of invasion, which is protease independent and does not involve molecules such as CD44, and the mesenchymal form of invasion which involves an EMT, MMP-2 activation and secretion, and upregulation of CD44 (Parri et al., 2009). Since all of these are upregulated by Wnt5A, it is interesting to speculate that Wnt5A specifically mediates the mesenchymal form of invasion. Certainly all visceral metastases do not require dissemination through the lymph nodes. Pulmonary metastases in mice implanted with subcutaneous tumors have been shown to occur, despite the absence of lymph node metastasis (Rebhun et al., 2008). The interactions between Wnt5A, CXCR4, and the EMT are certainly suggestive of a mesenchymal, hematogenous rather than amoeboid, lymphatic form of metastasis.
Wnt5A As A Therapeutic Target For Melanoma
Almost ten years after the association of Wnt5A with highly aggressive melanoma, we have come to an increased appreciation of its importance in melanoma metastasis. The fact that it down regulates melanogenic antigens that could otherwise be used as targets for immunotherapy implies that if Wnt5A was first down regulated, the efficacy of these immunotherapies could be increased. Small peptide agonists of Wnt5A such as FOXY-5 have been used to successfully target breast cancer metastasis in vivo (Safholm et al., 2008, Ford et al., 2009), and one imagines that modifications of such peptides such that they act as antagonists would be also possible, and effective in melanoma therapy. However, targeting secreted ligands poses its own set of challenges, and because the FZD receptors are ubiquitously expressed, and can signal to activate both the canonical and non-canonical Wnt signaling pathways, they are not very attractive targets. ROR2, however, due to its specificity for Wnt5A (Oishi et al., 2003) is a very interesting target, more so because it bears great homology to neurotrophic tyrosine kinase receptors (NTRKs) (Wilson et al., 1993). NTRKs have increased expression during the progression of several kinds of cancers, and inhibitors to NTRKs have been successfully used to target prostate cancer metastasis in experimental models (Weeraratna et al., 2001, Weeraratna et al., 2000). Another tyrosine kinase of potential interest in melanoma is the Bruton’s tyrosine kinase (BTK), which has been shown to inhibit β-catenin signaling in colorectal cancer (James et al., 2009), implying that its activation may mimic a more Wnt5A-high like phenotype, and thus it may potentially be over expressed in melanoma. It is clear that current therapies for melanoma are not as effective as would be desired, and this is likely due to the heterogeneity of the tumor, where some cells are targeted but others are missed, and these can proceed to even further malignancy. With immunotherapy for example, targeting MART1 positive cells, overlooks the highly aggressive Wnt5A positive cells (that have downregulated their MART1). Therefore, targeting these types of tyrosine kinase receptors may have beneficial effects for melanoma therapy, especially as adjuvant agents for currently existing immuno- and chemotherapy.
Conclusions
The canonical and non-canonical Wnt pathways signal along an intricate course from melanocyte development to tumor progression. Canonical Wnt signaling sets the course for melanocyte, development, differentiation and tumor initiation, where non-canonical Wnt signaling is activated later in the tumor, tacking away from a β-catenin positive phenotype, and setting the sails for a course of tumor progression and metastasis, and perhaps de-differentiation. Just as a realistic sailor adjusts their sails to the changing wind, so do melanoma cells adjust to changes in Wnt, in order to pursue the most favorable course for a speedy progression. One can imagine that in an invincible host, Wnts could tack back and forth forever, from tumor initiation, to metastasis, and re-colonization of distant sites, but in the fallible human host, metastasis is fatal. It has taken us several years to just begin to understand the complex roles of the disparate Wnt signaling pathways in melanoma. It is hoped that we will one day come to a clearer knowledge of the complexity of Wnt signaling in melanoma, such that we can target these pathways to effectively treat this disease.
Acknowledgements
MPO and ATW are supported by the Intramural Research Program of the National Institute on Aging. We sincerely apologize to all the authors whose wonderful work was not cited here due to space constraints, and the more editorial rather than encyclopedic nature of this review. We also apologize for the liberal use of sailing-related puns.
References
- Ai X, Do AT, Kusche-Gullberg M, Lindahl U, Lu K, Emerson CP., Jr Substrate specificity and domain functions of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and QSulf2. J Biol Chem. 2006;281:4969–4976. doi: 10.1074/jbc.M511902200. [DOI] [PubMed] [Google Scholar]
- Akbarzadeh S, Wheldon LM, Sweet SM, Talma S, Mardakheh FK, Heath JK. The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PLoS ONE. 2008;3:e1873. doi: 10.1371/journal.pone.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arheden K, Mandahl N, Strombeck B, Isaksson M, Mitelman F. Chromosome localization of the human oncogene INT1 to 12q13 by in situ hybridization. Cytogenet Cell Genet. 1988a;47:86–87. doi: 10.1159/000132513. [DOI] [PubMed] [Google Scholar]
- Arheden K, Tommerup N, Mandahl N, et al. Amplification of the human putative oncogene INT1 in primary retinoblastoma tumors. Cytogenet Cell Genet. 1988b;48:174–177. doi: 10.1159/000132619. [DOI] [PubMed] [Google Scholar]
- Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, β-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11:8606–8614. doi: 10.1158/1078-0432.CCR-05-0011. [DOI] [PubMed] [Google Scholar]
- Badiglian Filho L, Oshima CT, De Oliveira Lima F, De Oliveira Costa H, De Sousa Damiao R, Gomes TS, Goncalves WJ. Canonical and noncanonical Wnt pathway: a comparison among normal ovary, benign ovarian tumor and ovarian cancer. Oncol Rep. 2009;21:313–320. [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Bellei B, Flori E, Izzo E, Maresca V, Picardo M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell Signal. 2008;20:1750–1761. doi: 10.1016/j.cellsig.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Benhaj K, Akcali KC, Ozturk M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 2006;15:701–707. [PubMed] [Google Scholar]
- Billiard J, Way DS, Seestaller-Wehr LM, Moran RA, Mangine A, Bodine PV. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol Endocrinol. 2005;19:90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Blasband A, Schryver B, Papkoff J. The biochemical properties and transforming potential of human Wnt-2 are similar to Wnt-1. Oncogene. 1992;7:153–161. [PubMed] [Google Scholar]
- Boyer A, Paquet M, Lague MN, Hermo L, Boerboom D. Dysregulation of WNT/CTNNB1 and PI3K/AKT signaling in testicular stromal cells causes granulosa cell tumor of the testis. Carcinogenesis. 2009;30:869–878. doi: 10.1093/carcin/bgp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Wallingford JB, Vize PD. Dynamic patterns of gene expression in the developing pronephros of Xenopus laevis. Dev Genet. 1999;24:199–207. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<199::AID-DVG3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin. Cancer Res. 2003;63:6751–6757. [PubMed] [Google Scholar]
- Casagrande G, Te Kronnie G, Basso G. The effects of siRNA-mediated inhibition of E2A-PBX1 on EB-1 and Wnt16b expression in the 697 pre-B leukemia cell line. Haematologica. 2006;91:765–771. [PubMed] [Google Scholar]
- Chen K, Fallen S, Abaan HO, et al. Wnt10b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr Blood Cancer. 2008;51:349–355. doi: 10.1002/pbc.21595. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Moore EC, Lonsdorf AS, et al. Activated Wnt/β-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejmek J, Dib K, Jonsson M, Andersson T. Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int J Cancer. 2003;103:344–351. doi: 10.1002/ijc.10752. [DOI] [PubMed] [Google Scholar]
- Delehedde M, Lyon M, Sergeant N, Rahmoune H, Fernig DG. Proteoglycans: pericellular and cell surface multireceptors that integrate externa stimuli in the mammary gland. J Mammary Gland Biol Neoplasia. 2001;6:253–273. doi: 10.1023/a:1011367423085. [DOI] [PubMed] [Google Scholar]
- Delmas V, Beermann F, Martinozzi S, et al. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 2007;21:2923–2935. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JU, Dean NM, Bennett CF, Griffith JW, Lang CM, Welch DR. Human melanoma metastasis is inhibited following ex vivo treatment with an antisense oligonucleotide to protein kinase C-alpha. Cancer Lett. 1998;128:65–70. doi: 10.1016/s0304-3835(98)00052-4. [DOI] [PubMed] [Google Scholar]
- Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- Dissanayake SK, Olkhanud PB, O’Connell MP, et al. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68:10205–10214. doi: 10.1158/0008-5472.CAN-08-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake SK, Wade M, Johnson CE, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282:17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Phelps RG, Qiao R, et al. BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res. 2003;63:3883–3885. [PubMed] [Google Scholar]
- Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- Dunn KJ, Brady M, Ochsenbauer-Jambor C, Snyder S, Incao A, Pavan WJ. WNT1 and WNT3a promote expansion of melanocytes through distinct modes of action. Pigment Cell Res. 2005;18:167–180. doi: 10.1111/j.1600-0749.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- Dunn KJ, Williams BO, Li Y, Pavan WJ. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc Natl Acad Sci U S A. 2000;97:10050–10055. doi: 10.1073/pnas.97.18.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Beauchamp E, Woods D, Taylor WG, Toretsky JA, Uren A, Rubin JS. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism. Mol Cell Biol. 2008;28:2368–2379. doi: 10.1128/MCB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Leishear K, Nguyen TK, et al. Defining the conditions for the generation of melanocytes from human embryonic stem cells. Stem Cells. 2006;24:1668–1677. doi: 10.1634/stemcells.2005-0414. [DOI] [PubMed] [Google Scholar]
- Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9:224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CE, Ekstrom EJ, Andersson T. Wnt-5a signaling restores tamoxifen sensitivity in estrogen receptor-negative breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:3919–3924. doi: 10.1073/pnas.0809516106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ford CE, Ekstrom EJ, Howlin J, Andersson T. The WNT-5a derived peptide, Foxy-5, possesses dual properties that impair progression of ERalpha negative breast cancer. Cell Cycle. 2009;8:1838–1842. doi: 10.4161/cc.8863. [DOI] [PubMed] [Google Scholar]
- Fukukawa C, Nagayama S, Tsunoda T, Toguchida J, Nakamura Y, Katagiri T. Activation of the non-canonical Dvl-Rac1-JNK pathway by Frizzled homologue 10 in human synovial sarcoma. Oncogene. 2009;28:1110–1120. doi: 10.1038/onc.2008.467. [DOI] [PubMed] [Google Scholar]
- Ghosh MC, Collins GD, Vandanmagsar B, et al. Activation of Wnt5A signaling is required for CXCL12-mediated T-cell migration. Blood. 2009 doi: 10.1182/blood-2008-08-175869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan T, Yaniv A, Bafico A, Liu G, Gazit A. The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt. β-catenin signaling cascade. J Biol Chem. 2004;279:14879–14888. doi: 10.1074/jbc.M306421200. [DOI] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Hamada S, Watanabe K, Hirota M, et al. β-Catenin/TCF/LEF regulate expression of the short form human Cripto-1. Biochem Biophys Res Commun. 2007;355:240–244. doi: 10.1016/j.bbrc.2007.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley LE, Winn RA. Analysis of Wnt7a-stimulated JNK activity and cJun phosphorylation in non-small cell lung cancer cells. Methods Mol Biol. 2008;468:187–196. doi: 10.1007/978-1-59745-249-6_14. [DOI] [PubMed] [Google Scholar]
- Herlyn M, Berking C, Li G, Satyamoorthy K. Lessons from melanocyte development for understanding the biological events in naevus and melanoma formation. Melanoma Res. 2000;10:303–312. doi: 10.1097/00008390-200008000-00001. [DOI] [PubMed] [Google Scholar]
- Hoek KS, Eichhoff OM, Schlegel NC, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- Hoek KS, Schlegel NC, Brafford P, et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 2006;19:290–302. doi: 10.1111/j.1600-0749.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Rovinsky S, Penmatcha S, Herlyn M, Muirhead D. Bone morphogenetic proteins in melanoma: angel or devil? Cancer Metastasis Rev. 2005;24:251–263. doi: 10.1007/s10555-005-1575-y. [DOI] [PubMed] [Google Scholar]
- James RG, Biechele TL, Conrad WH. Bruton's tyrosine kinase revealed as a negative regulator of Wnt-β-catenin signaling. Sci Signal. 2009;2:ra25. doi: 10.1126/scisignal.2000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Jezewski PA, Fang PK, Payne-Ferreira TL, Yelick PC. Zebrafish Wnt9b synteny and expression during first and second arch, heart, and pectoral fin bud morphogenesis. Zebrafish. 2008;5:169–177. doi: 10.1089/zeb.2007.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M, Smith K, Harris AL. Regulation of Wnt5a expression in human mammary cells by protein kinase C activity and the cytoskeleton. Br J Cancer. 1998;78:430–438. doi: 10.1038/bjc.1998.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M, Andersson T. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Cell Sci. 2001;114:2043–2053. doi: 10.1242/jcs.114.11.2043. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62:409–416. [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Mcmahon AP, Carroll TJ, Lidral AC. Wnt9b is the mutated gene involved in multifactorial nonsyndromic cleft lip with or without cleft palate in A/WySn mice, as confirmed by a genetic complementation test. Birth Defects Res A Clin Mol Teratol. 2006;76:574–579. doi: 10.1002/bdra.20302. [DOI] [PubMed] [Google Scholar]
- Kani S, Oishi I, Yamamoto H, et al. The receptor tyrosine kinase Ror2 associates with and is activated by casein kinase Iepsilon. J Biol Chem. 2004;279:50102–50109. doi: 10.1074/jbc.M409039200. [DOI] [PubMed] [Google Scholar]
- Katoh M, Hirai M, Sugimura T, Terada M. Cloning, expression and chromosomal localization of Wnt-13, a novel member of the Wnt gene family. Oncogene. 1996;13:873–876. [PubMed] [Google Scholar]
- Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- Khan NI, Bradstock KF, Bendall LJ. Activation of Wnt/β-catenin pathway mediates growth and survival in B-cell progenitor acute lymphoblastic leukaemia. Br J Haematol. 2007;138:338–348. doi: 10.1111/j.1365-2141.2007.06667.x. [DOI] [PubMed] [Google Scholar]
- Klein D, Demory A, Peyre F, et al. Wnt2 acts as an angiogenic growth factor for non-sinusoidal endothelial cells and inhibits expression of stanniocalcin-1. Angiogenesis. 2009 doi: 10.1007/s10456-009-9145-5. [DOI] [PubMed] [Google Scholar]
- Kremenevskaja N, Von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24:2144–2154. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000a;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000b;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Kuphal S, Palm HG, Poser I, Bosserhoff AK. Snail-regulated genes in malignant melanoma. Melanoma Res. 2005;15:305–313. doi: 10.1097/00008390-200508000-00012. [DOI] [PubMed] [Google Scholar]
- Labonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Lang D, Lu MM, Huang L, et al. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- Larue L, Delmas V. The WNT/B-catenin pathway in melanoma. Front Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- Larue L, Luciani F, Kumasaka M, Champeval D, Demirkan N, Bonaventure J, Delmas V. Bypassing melanocyte senescence by β-catenin: A novel way to promote melanoma. Pathol Biol (Paris) 2009 doi: 10.1016/j.patbio.2008.11.003. In Press. [DOI] [PubMed] [Google Scholar]
- Lavery DL, Martin J, Turnbull YD, Hoppler S. Wnt6 signaling regulates heart muscle development during organogenesis. Dev Biol. 2008;323:177–188. doi: 10.1016/j.ydbio.2008.08.032. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg JW, Lewis CA, Parsons M, Ng T, Isacke CM. A novel PKC-regulated mechanism controls CD44 ezrin association and directional cell motility. Nat Cell Biol. 2002;4:399–407. doi: 10.1038/ncb797. [DOI] [PubMed] [Google Scholar]
- Li C, Chen H, Hu L, et al. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with FZD2. BMC Mol Biol. 2008;9:11. doi: 10.1186/1471-2199-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bu G. LRP5/6 in Wnt signaling and tumorigenesis. Future Oncol. 2005;1:673–681. doi: 10.2217/14796694.1.5.673. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Role of heparan sulfate proteoglycans in cell-cell signaling in Drosophila. Matrix Biol. 2000;19:303–307. doi: 10.1016/s0945-053x(00)00073-1. [DOI] [PubMed] [Google Scholar]
- Lin YC, You L, Xu Z, et al. Wnt inhibitory factor-1 gene transfer inhibits melanoma cell growth. Hum Gene Ther. 2007;18:379–386. doi: 10.1089/hum.2006.005. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bhat RA, Seestaller-Wehr LM, et al. The orphan receptor tyrosine kinase Ror2 promotes osteoblast differentiation and enhances ex vivo bone formation. Mol Endocrinol. 2007;21:376–387. doi: 10.1210/me.2006-0342. [DOI] [PubMed] [Google Scholar]
- Lopes CC, Dietrich CP, Nader HB. Specific structural features of syndecans and heparan sulfate chains are needed for cell signaling. Braz J Med Biol Res. 2006;39:157–167. doi: 10.1590/s0100-879x2006000200001. [DOI] [PubMed] [Google Scholar]
- Macleod RJ, Hayes M, Pacheco I. Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G403–G411. doi: 10.1152/ajpgi.00119.2007. [DOI] [PubMed] [Google Scholar]
- Mangioni S, Vigano P, Lattuada D, Abbiati A, Vignali M, Di Blasio AM. Overexpression of the Wnt5b gene in leiomyoma cells: implications for a role of the Wnt signaling pathway in the uterine benign tumor. J Clin Endocrinol Metab. 2005;90:5349–5355. doi: 10.1210/jc.2005-0272. [DOI] [PubMed] [Google Scholar]
- Mapelli E, Banfi P, Sala E, Sensi M, Supino R, Zunino F, Gambetta RA. Effect of protein kinase C inhibitors on invasiveness of human melanoma clones expressing different levels of protein kinase C isoenzymes. Int J Cancer. 1994;57:281–286. doi: 10.1002/ijc.2910570225. [DOI] [PubMed] [Google Scholar]
- Martinez G, Wijesinghe M, Turner KN, et al. Conditional mutations of β-catenin and APC reveal roles for canonical Wnt signaling in lens differentiation. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-3567. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Miki R, Nakayama M, Tatsumi N, Yokouchi Y. Wnt9a secreted from the walls of hepatic sinusoids is essential for morphogenesis, proliferation, and glycogen accumulation of chick hepatic epithelium. Dev Biol. 2008;319:234–247. doi: 10.1016/j.ydbio.2008.04.021. In Press. [DOI] [PubMed] [Google Scholar]
- Mazieres J, You L, He B, et al. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene. 2005;24:5396–5400. doi: 10.1038/sj.onc.1208568. [DOI] [PubMed] [Google Scholar]
- Melchior K, Weiss J, Zaehres H, et al. The WNT receptor FZD7 contributes to self-renewal signaling of human embryonic stem cells. Biol Chem. 2008;389:897–903. doi: 10.1515/BC.2008.108. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi T, Takei M, Kajiya H, Egashira N, Takekoshi S, Teramoto A, Osamura RY. Expression of Wnt4 in human pituitary adenomas regulates activation of the β-catenin-independent pathway. Endocr Pathol. 2008;19:261–273. doi: 10.1007/s12022-008-9048-9. [DOI] [PubMed] [Google Scholar]
- Morioka K, Tanikawa C, Ochi K, et al. Orphan receptor tyrosine kinase ROR2 as a potential therapeutic target for osteosarcoma. Cancer Sci. 2009;100:1227–1233. doi: 10.1111/j.1349-7006.2009.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkel M, Huelsken J, Wakamiya M, et al. Â-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Effect of PKC412, an inhibitor of protein kinase C, on spontaneous metastatic model mice. Anticancer Res. 2003;23:1395–1399. [PubMed] [Google Scholar]
- Nakamura T, Matsumoto K. The functions and possible significance of Kremen as the gatekeeper of Wnt signalling in development and pathology. J Cell Mol Med. 2008;12:391–408. doi: 10.1111/j.1582-4934.2007.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz S, Klar J, Wajid M, et al. WNT10A missense mutation associated with a complete Odonto-Onycho-Dermal Dysplasia syndrome. Eur J Hum Genet. 2009 doi: 10.1038/ejhg.2009.81. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A. 2007;104:15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Osawa M. Generating quiescent stem cells. Pigment Cell Res. 2007;20:263–270. doi: 10.1111/j.1600-0749.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- Nishita M, Yoo SK, Nomachi A, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein Filamin A. J Biol Chem. 2008;283:27973–27981. doi: 10.1074/jbc.M802325200. [DOI] [PubMed] [Google Scholar]
- Nyormoi O, Bar-Eli M. Transcriptional regulation of metastasis-related genes in human melanoma. Clin Exp Metastasis. 2003;20:251–263. doi: 10.1023/a:1022991302172. [DOI] [PubMed] [Google Scholar]
- O'Connell MP, Fiori JL, Baugher KM, et al. Wnt5A activates the calpainmediated cleavage of Filamin A. J Invest Dermatol. 2009;129:1782–1789. doi: 10.1038/jid.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MP, French AD, Leotlela PD, Weeraratna AT. Assaying Wnt5A-mediated invasion in melanoma cells. Methods Mol Biol. 2008;468:243–253. doi: 10.1007/978-1-59745-249-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Okuyama R, Tagami H, Aiba S. Notch signaling: its role in epidermal homeostasis and in the pathogenesis of skin diseases. J Dermatol Sci. 2008;49:187–194. doi: 10.1016/j.jdermsci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Ordway JM, Bedell JA, Citek RW, et al. Comprehensive DNA methylation profiling in a human cancer genome identifies novel epigenetic targets. Carcinogenesis. 2006;27:2409–2423. doi: 10.1093/carcin/bgl161. [DOI] [PubMed] [Google Scholar]
- Ordway JM, Budiman MA, Korshunova Y, et al. Identification of novel high-frequency DNA methylation changes in breast cancer. PLoS One. 2007;2:e1314. doi: 10.1371/journal.pone.0001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JK, Song JH, He TC, Nam SW, Lee JY, Park WS. Overexpression of Wnt-2 in colorectal cancers. Neoplasma. 2009;56:119–123. doi: 10.4149/neo_2009_02_119. [DOI] [PubMed] [Google Scholar]
- Parri M, Taddei ML, Bianchini F, Calorini L, Chiarugi P. EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res. 2009;69:2072–2081. doi: 10.1158/0008-5472.CAN-08-1845. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr Biol. 2002;12:957–962. doi: 10.1016/s0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- Planutis K, Planutiene M, Moyer MP, Nguyen AV, Perez CA, Holcombe RF. Regulation of norrin receptor frizzled-4 by Wnt2 in colon-derived cells. BMC Cell Biol. 2007;8:12. doi: 10.1186/1471-2121-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Pons M, Quintanilla M. Molecular biology of malignant melanoma and other cutaneous tumors. Clin Transl Oncol. 2006;8:466–474. doi: 10.1007/s12094-006-0046-4. [DOI] [PubMed] [Google Scholar]
- Rajagopal J, Carroll TJ, Guseh JS, et al. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–1634. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J, Strachan T, Lako M, Lindsay S. Partial cloning and assignment of WNT6 to human chromosome band 2q35 by in situ hybridization. Cytogenet Cell Genet. 1999;84:50–52. doi: 10.1159/000015212. [DOI] [PubMed] [Google Scholar]
- Rebhun RB, Lazar AJ, Fidler IJ, Gershenwald JE. Impact of sentinel lymphadenectomy on survival in a murine model of melanoma. Clin Exp Metastasis. 2008 doi: 10.1007/s10585-008-9141-y. [DOI] [PubMed] [Google Scholar]
- Rhee CS, Sen M, Lu D, et al. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–6605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C. Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell. 1995;83:631–640. doi: 10.1016/0092-8674(95)90103-5. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER. Frequent nuclear/cytoplasmic localization of β-catenin without exon 3 mutations in malignant melanoma. Am J Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo MM, Bartolome RA, Longo N, et al. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–45105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- Roelink H, Wang J, Black DM, Solomon E, Nusse R. Molecular cloning and chromosomal localization to 17q21 of the human WNT3 gene. Genomics. 1993;17:790–792. doi: 10.1006/geno.1993.1412. [DOI] [PubMed] [Google Scholar]
- Rotolo S, Diotti R, Gordon RE, et al. Effects on proliferation and melanogenesis by inhibition of mutant BRAF and expression of wild-type INK4A in melanoma cells. Int J Cancer. 2005;115:164–169. doi: 10.1002/ijc.20865. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Safholm A, Tuomela J, Rosenkvist J, Dejmek J, Harkonen P, Andersson T. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin Cancer Res. 2008;14:6556–6563. doi: 10.1158/1078-0432.CCR-08-0711. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Hansen LA, Vogel JC, Udey MC. Characterization of Wnt gene expression in murine skin: possible involvement of epidermis-derived Wnt-4 in cutaneous epithelial-mesenchymal interactions. Exp Cell Res. 1998;243:150–160. doi: 10.1006/excr.1998.4152. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Katoh M. Molecular cloning and characterization of human WNT5B on chromosome 12p13.3 region. Int J Oncol. 2001;19:347–351. doi: 10.3892/ijo.19.2.347. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Katoh M. Expression and regulation of WNT5A and WNT5B in human cancer: up-regulation of WNT5A by TNFalpha in MKN45 cells and upregulation of WNT5B by β-estradiol in MCF-7 cells. Int J Mol Med. 2002;10:345–349. [PubMed] [Google Scholar]
- Sakai D, Tanaka Y, Endo Y, Osumi N, Okamoto H, Wakamatsu Y. Regulation of Slug transcription in embryonic ectoderm by β-catenin-Lef/Tcf and BMP-Smad signaling. Dev Growth Differ. 2005;47:471–482. doi: 10.1111/j.1440-169X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- Saldanha J, Singh J, Mahadevan D. Identification of a Frizzled-like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein Sci. 1998;7:1632–1635. doi: 10.1002/pro.5560070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Mcgonnell I, Allen S, Patel K. The role of Wnt signalling in the development of somites and neural crest. Adv Anat Embryol Cell Biol. 2008;195:1–64. doi: 10.1007/978-3-540-77727-4. [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Sen M, Chamorro M, Reifert J, Corr M, Carson DA. Blockade of Wnt-5A/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum. 2001;44:772–781. doi: 10.1002/1529-0131(200104)44:4<772::AID-ANR133>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Shah BH, Shah FB, Catt KJ. Role of metalloproteinase-dependent EGF receptor activation in alpha-adrenoceptor-stimulated MAP kinase phosphorylation in GT1-7 neurons. J Neurochem. 2006;96:520–532. doi: 10.1111/j.1471-4159.2005.03585.x. [DOI] [PubMed] [Google Scholar]
- Shibahara S, Takeda K, Yasumoto K, Udono T, Watanabe K, Saito H, Takahashi K. Microphthalmia-associated transcription factor (MITF): multiplicity in structure, function, and regulation. J Investig Dermatol Symp Proc. 2001;6:99–104. doi: 10.1046/j.0022-202x.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- Swiatek W, Tsai IC, Klimowski L, Pepler A, Barnette J, Yost HJ, Virshup DM. Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nishikawa M, Suehara T, Takiguchi N, Takakura Y. Gene silencing of β-catenin in melanoma cells retards their growth but promotes the formation of pulmonary metastasis in mice. Int J Cancer. 2008;123:2315–2320. doi: 10.1002/ijc.23812. [DOI] [PubMed] [Google Scholar]
- Takeda K, Yasumoto K, Takada R, et al. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000;275:14013–14016. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- Taki M, Kamata N, Yokoyama K, Fujimoto R, Tsutsumi S, Nagayama M. Down-regulation of Wnt-4 and up-regulation of Wnt-5a expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Cancer Sci. 2003;94:593–597. doi: 10.1111/j.1349-7006.2003.tb01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, et al. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Voronkov AE, Baskin Ii, Palyulin VA, Zefirov NS. Molecular model of the Wnt protein binding site on the surface of dimeric CRD domain of the hFZD8 receptor. Dokl Biochem Biophys. 2008;419:75–78. doi: 10.1134/s1607672908020087. [DOI] [PubMed] [Google Scholar]
- Wai DH, Schaefer KL, Schramm A, et al. Expression analysis of pediatric solid tumor cell lines using oligonucleotide microarrays. Int J Oncol. 2002;20:441–451. [PubMed] [Google Scholar]
- Wainwright BJ, Scambler PJ, Stanier P, et al. Isolation of a human gene with protein sequence similarity to human and murine int-1 and the Drosophila segment polarity mutant wingless. Embo J. 1988;7:1743–1748. doi: 10.1002/j.1460-2075.1988.tb03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeraratna AT. A Wnt-er wonderland--the complexity of Wnt signaling in melanoma. Cancer Metastasis Rev. 2005;24:237–250. doi: 10.1007/s10555-005-1574-z. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Arnold JT, George DJ, Demarzo A, Isaacs JT. Rational basis for Trk inhibition therapy for prostate cancer. Prostate. 2000;45:140–148. doi: 10.1002/1097-0045(20001001)45:2<140::aid-pros8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Dalrymple SL, Lamb JC, Denmeade SR, Miknyoczki S, Dionne CA, Isaacs JT. Pan-trk inhibition decreases metastasis and enhances host survival in experimental models as a result of its selective induction of apoptosis of prostate cancer cells. Clin Cancer Res. 2001;7:2237–2245. [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, ladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Goberdhan DC, Steller H. Dror, a potential neurotrophic receptor gene, encodes a Drosophila homolog of the vertebrate Ror family of Trk-related receptor tyrosine kinases. Proc Natl Acad Sci U S A. 1993;90:7109–7113. doi: 10.1073/pnas.90.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolda SL, Moon RT. Cloning and developmental expression in Xenopus laevis of seven additional members of the Wnt family. Oncogene. 1992;7:1941–1947. [PubMed] [Google Scholar]
- Worm J, Christensen C, Gronbaek K, Tulchinsky E, Guldberg P. Genetic and epigenetic alterations of the APC gene in malignant melanoma. Oncogene. 2004 doi: 10.1038/sj.onc.1207647. [DOI] [PubMed] [Google Scholar]
- Xiao C, Yang BF, Song JH, Schulman H, Li L, Hao C. Inhibition of CaMKII-mediated c-FLIP expression sensitizes malignant melanoma cells to TRAIL-induced apoptosis. Exp Cell Res. 2005;304:244–255. doi: 10.1016/j.yexcr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Passeron T, Hoashi T, et al. Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/β-catenin signaling in keratinocytes. Faseb J. 2008;22:1009–1020. doi: 10.1096/fj.07-9475com. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Kamata N, Fujimoto R, et al. Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int J Oncol. 2003;22:891–898. [PubMed] [Google Scholar]
- You L, He B, Xu Z, et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64:5385–5389. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- Zilberberg A, Yaniv A, Gazit A. The low density lipoprotein receptor-1, LRP1, interacts with the human frizzled-1 (HFz1) and down-regulates the canonical Wnt signaling pathway. J Biol Chem. 2004;279:17535–17542. doi: 10.1074/jbc.M311292200. [DOI] [PubMed] [Google Scholar]