Abstract
OBJECTIVES
Obesity is often associated with hypertriglyceridemia and elevated free fatty acids (FFAs) which are independent risk factors for cardiovascular disease and diabetes. While impairment of cholesterol homeostasis is known to induce toxicity in macrophages, the consequence of altered fatty acid homeostasis is not clear.
METHODS AND RESULTS
Long chain acyl CoA synthetases (ACSLs) play a critical role in fatty acid homeostasis by channeling fatty acids to diverse metabolic pools. We treated mouse peritoneal macrophages (MPMs) with VLDL or FFAs in the presence of triacsin C, an inhibitor of the three ACSL isoforms present in macrophages. Treatment of macrophages with VLDL and triacsin C resulted in reduced TG accumulation but increased intracellular FFA levels which induced lipotoxicity characterized by induction of apoptosis. Treatment of MPMs with the saturated fatty acid stearic acid in the presence of triacsin C increased intracellular stearic acid and induced apoptosis. Stromal vascular cells collected from high fat diet-fed mice displayed foam cell morphology and exhibited increased mRNA levels of macrophage markers and ACSL1. Importantly, all of these changes were associated with increased FFA level in AT.
CONCLUSIONS
Inhibition of ACSLs during fatty acid loading results in apoptosis via accumulation of FFAs. Our data have implications in understanding the consequences of dysregulated fatty acid metabolism in macrophages.
Keywords: VLDL, foam cells, free fatty acids, triacsin C, long chain acyl CoA synthetases, stearic acid, apoptosis
Obesity and the associated metabolic dysregulations such as dyslipidemia and elevated plasma free fatty acids (FFAs) contribute to increased incidence of cardiovascular disease and type 2 diabetes.1, 2 Macrophages are cells of the innate immune system, traditionally thought to participate predominantly in immune disorders. However, in the past 2 decades, a role for macrophages in lipid homeostasis and in metabolic diseases has been established. It is well known that free cholesterol induces an inflammatory response and apoptosis in macrophages, and that apoptotic macrophages contribute to atherosclerotic lesion formation;3 however, the consequences of FFA accumulation in macrophages are not clear.
Long chain acyl CoA synthetases (ACSLs) play a crucial role in regulating fatty acid metabolism by converting FFAs into fatty acyl CoA derivatives via a process called fatty acid activation. This modification is required for any FFA to undergo further metabolism. Activated fatty acids can enter several metabolic pathways such as β-oxidation; desaturation; or esterfication into triglycerides, phospholipids or cholesterol esters. Because of the crucial role of ACSLs in activating fatty acids, and in partitioning them to diverse metabolic pools, we hypothesized that inhibition of ACSLs would impair fatty acid homeostasis in macrophages.
Five different isoforms of ACSL - 1, 3, 4, 5 and 6 - have been identified in humans and rodents.4 Mouse peritoneal macrophages (MPMs) predominantly express ACSL1, although ACSL 3 and 4 are also expressed to some extent.5 Triacsins are potent inhibitors of ACSLs and the inhibitory potential of triacsin C varies among the different ACSL isoforms. Triacsin C has been shown to inhibit ACSL 1, 3 and 4 but does not inhibit ACSL 5 or 6.6–8 Thus, triacsin C can inhibit all of the isoforms of ACSL present in macrophages.
Taking advantage of this inhibitor, we demonstrate that blocking the activity of ACSLs during fatty acid loading leads to induction of apoptosis which is due, at least in part, to accumulation of intracellular FFAs. We also show that SVCs derived from obese adipose tissue (AT) display foam cell morphology and exhibit increased mRNA levels of macrophage markers and ACSL1. All of these changes were associated with increased local FFA levels in AT. These findings highlight the importance of ACSLs in regulating fatty acid homeostasis in macrophages and have implications for potential mechanisms by which AT macrophages respond to increased fatty acid flux in obese AT.
METHODS
Fatty acid treatment
We previously reported that fatty acids at 90 μM concentration induce a pro-inflammatory response and/or apoptosis in endothelial cells.9, 10 Therefore, in most of the experiments, MPMs were treated with individual FFAs at 90 μM concentration or an equimolar mixture of the long chain fatty acids palmitic acid, stearic acid, oleic acid, and linoleic acid at a total final concentration of 90 μM. The fatty acids were first dissolved in ethanol and then added to DMEM with 5% FBS and MPMs were treated with fatty acids for 24 h in the presence or absence of triacsin C (5 μM). This resulted in a fatty acid to albumin ratio of 3:1 which is within a physiological range.11 This method of fatty acid treatment was employed for most of the experiments unless otherwise indicated. In separate experiments, MPMs were also treated with FFAs complexed to fatty acid free BSA using serum free DMEM as described earlier.12 Briefly, fatty acids were first dissolved in ethanol and pre-equilibrated with BSA at 37°C for 1.5 h at a molar ratio of 5:1 (fatty acid:albumin). Fatty acid-albumin complex solution was freshly prepared prior to each experiment.
Other methods are described in detail in the supplemental data (available online at http://atvb.ahajournals.org).
RESULTS
Inhibition of ACSLs during VLDL loading reduces triglyceride accumulation and increases intracellular FFA concentrations in MPMs
MPMs were incubated with VLDL in the presence or absence of triacsin C and lipid accumulation was analyzed by Oil Red O staining. As we previously reported,13 incubation of MPMs with VLDL by itself resulted in a dramatic increase in neutral lipid accumulation. However, staining was greatly reduced in cells treated with both VLDL and triacsin C (5 μM), suggesting that inhibition of ACSL activity nearly abolished the accumulation of neutral lipids (Fig. 1A). Analysis of the lipid profiles in these cells by gas chromatography (GC) revealed that treatment with VLDL plus triacsin C reduced triglyceride (TG) accumulation by 73% (P<0.01) compared to treatment with VLDL alone. In contrast, intracellular FFAs were increased 2-fold (P<0.001) compared to the VLDL-treated group (Fig. 1B). The levels of cholesterol esters, unesterified cholesterol, and phospholipids were not altered by treatment with VLDL and triacsin C.
Figure 1. Inhibition of ACSL during VLDL loading induces FFA accumulation.
MPMs were pretreated for 30 min with 5 μM triacsin C, followed by co-treatment with 100 μg/ml of VLDL for an additional 6 h. Controls were treated with DMSO (vehicle for TC) in the absence of TC or VLDL. (A) Cells were stained with Oil Red O to detect neutral lipids. Magnification, 10×. (B) Cells were analyzed for lipid content by GC. Data are presented as mean ± SEM of three individual samples. VL=VLDL, TC=triacsin C, TG=triglyceride, FFA=free fatty acids, CE=cholesterol esters, UC=unesterified cholesterol, PL=phospholipids.
#P<0.001 vs. control and TC, P<0.01 vs. VL+TC
*P<0.05 vs. TC and P<0.01 vs. control
^P<0.001 vs. all
Inhibition of ACSLs during VLDL loading induces apoptosis in MPMs
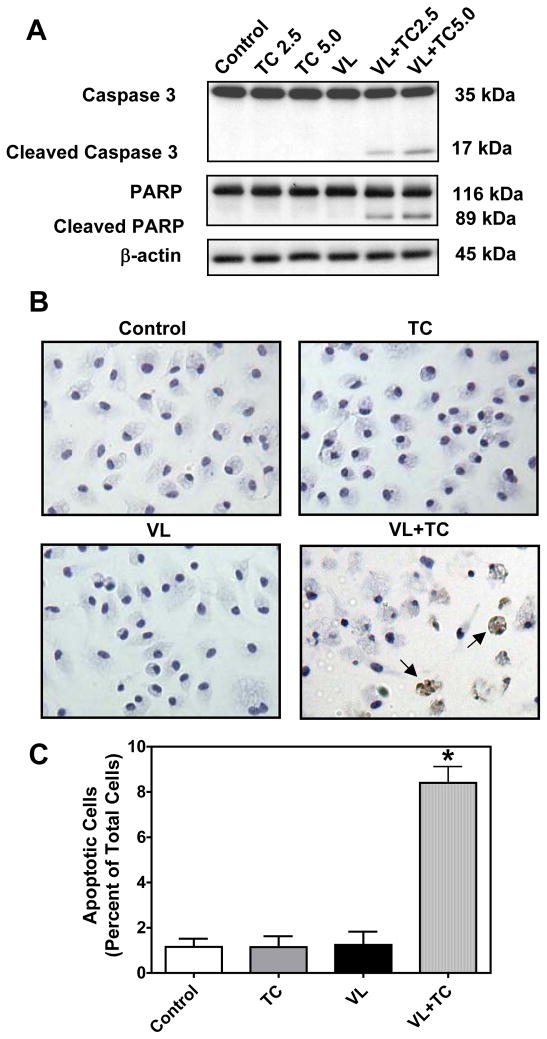
Treatment of MPMs with VLDL in the presence of 2.5 and 5 μM triacsin C for 6 h increased the cleavage of the apoptotic marker pro-caspase 3 (35 kDa), to its 17 kDa active form in a dose-dependent manner (Fig. 2A). Along with the activation of caspase 3, cleavage of Poly (ADP-ribose) polymerase (PARP, 116 kDa), another apoptotic marker, to its 89 kDa fragment was observed in MPMs treated with VLDL in the presence of triacsin C. Apoptotic death was also confirmed by immunohistochemical staining of cleaved caspase 3 in MPMs treated with VLDL in the presence of triacsin C (Fig. 2B). Quantification of cleaved caspase 3 stained cells revealed that 8.4 ± 0.7% of cells were apoptotic in the VLDL plus triacsin C treated cells as compared to less than 2% of cells in the control groups (Fig. 2C, P<0.001).
Figure 2. Inhibition of ACSL during VLDL loading induces apoptosis.
MPMs were pretreated for 30 min with 2.5 μM and 5 μM triacsin C followed by co-treatment with 100 μg/ml of VLDL for an additional 6 h. (A) Western blot analysis was performed for markers of apoptosis. Representative samples from two experiments performed in triplicate wells are shown. (B) Immunohistochemical analysis for cleaved caspase 3. Arrows indicate cells positive for cleaved caspase 3. (C) Cleaved caspase 3 positive cells were counted in 3 fields from 3 different wells for each treatment group. Data are expressed as percentage of total cells. VL=VLDL, TC=triacsin C.
*P<0.001 vs. all
Inhibition of ACSLs during fatty acid loading leads to accumulation of intracellular FFAs
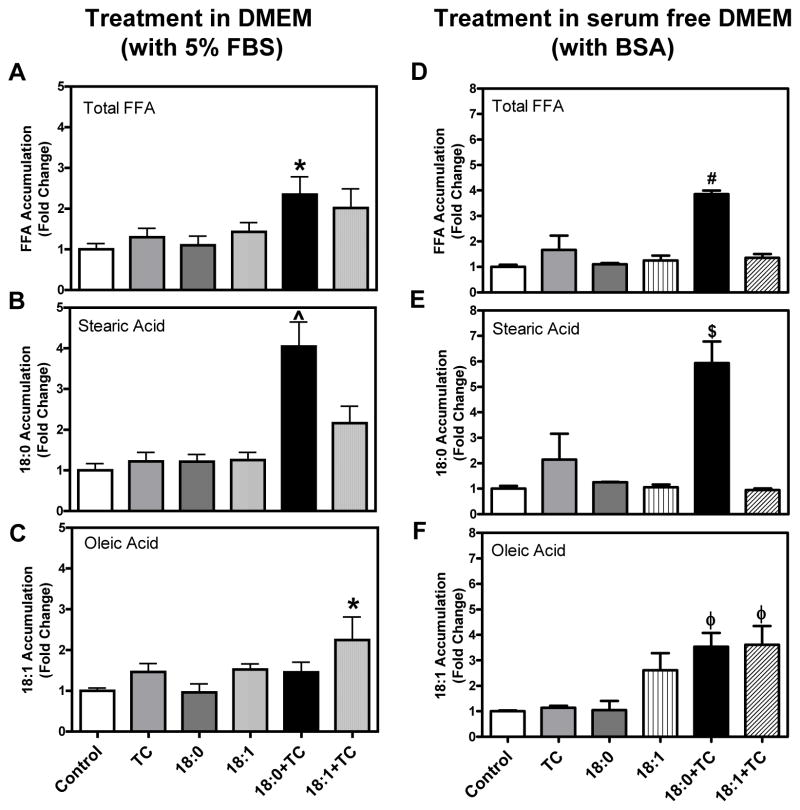
MPMs treated with the saturated fatty acid, stearic acid, in the presence or absence of triacsin C for 6 h C did not result in an increase the total intracellular FFA content. However, stearic acid treatment by itself did lead to a significant increase specifically in intracellular stearic acid levels (P<0.001) as compared to control treatment groups and exhibited an even further increase in the presence of triacsin C (Supplemental Figure I). Signs of apoptosis were not detected at this time point (data not shown). Interestingly, prolonged exposure to stearic acid and triacsin C for 24 h led to a significant increase in total intracellular FFA accumulation (Fig. 3A, P<0.05). It is interesting to note that while the stearic acid level was decreased to baseline levels in stearic acid alone treated cells at the 24 h time point, a sustained increase (4.1-fold) in stearic acid accumulation was exhibited by cells treated with stearic acid plus triacsin C (Fig. 3B, P<0.001). Unlike stearic acid, oleic acid treatment in the presence of triacsin C only showed a trend towards an increase the total intracellular FFA content (Fig. 3A). However, oleic acid plus triacsin C led to a significant 2.2-fold increase specifically in oleic acid accumulation (Fig. 3C, P<0.05).
Figure 3. MPMs loaded with stearic acid oroleic acid during ACSL inhibition accumulatestearic acid and oleic acid, respectively.
(A–C) MPMs were pretreated for 30 min with 5 μM triacsin C followed by co-treatment with stearic acid (18:0) or oleic acid (18:1) at a concentration of 90 μM in DMEM with 5% FBS for an additional 24 h. Controls were treated with DMSO and ethanol (vehicles for TC and fatty acids, respectively) in the absence of TC and fatty acids. (D–F) MPMs were treated with fatty acids complexed to albumin in serum free DMEM. Cells were analyzed for (A&D) total FFAs, (B&E) stearic acid, and (C&F) oleic acid content by GC. Stearic and oleic acid levels are expressed as the fold change of the mass amount present in the total FFA fraction compared to control. Data are presented as mean ± SEM of 3–6 individual samples.
*P<0.05 vs. control and 18:0
^P<0.05 vs. 18:1+TC and P<0.001 vs. all others
#P<0.01 vs. TC and P<0.001 vs. all others
$P<0.001 vs. control, 18:1, 18:1+TC, and P<0.01 vs. TC and 18:0
φP<0.05 vs. control and TC
Similar to stearic acid treatment using DMEM with 5% FBS, stearic acid complexed to BSA and treated using serum free DMEM also significantly increased both the total intracellular FFA fraction as well as the specific stearic acid accumulation (Fig. 3D & E, P<0.001 vs. control). Interestingly, oleic acid accumulation was significantly increased not only in oleic acid plus triacsin C treated cells but also in stearic acid plus triacsin C treated cells (Fig. 3F, P<0.05). Taken together, stearic acid plus triacsin C led to a significant increase in total intracellular FFA content and specific stearic acid accumulation regardless of the treatment method.
Inhibition of ACSLs during saturated but not unsaturated fatty acid loading induces apoptosis in MPMs
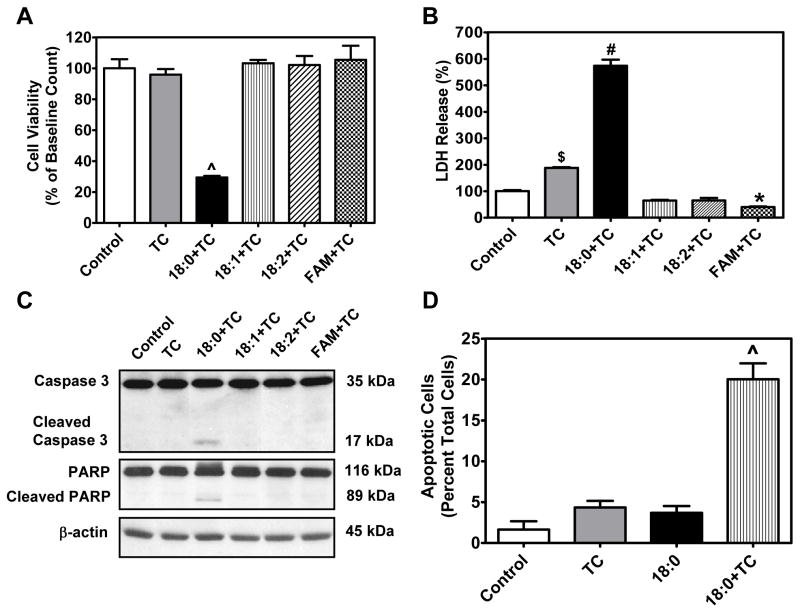
To determine whether accumulation of intracellular FFAs, as seen in stearic acid plus triacsin C treated cells, also induces lipotoxicity, we analyzed markers of apoptosis. In addition to stearic acid, MPMs were treated with other free fatty acids such as oleic acid (18:1) or linoleic acid (18:2) individually, or with a mixture of fatty acids in the presence of triacsin C for 24 h (all at 90 μM). Cell viability, as determined by MTT assay, was dramatically reduced in cells treated with stearic acid plus triacsin C (P<0.001) but was not reduced by treatment with any other fatty acid tested (Fig. 4A). In line with the MTT assay, a profound increase in lactate dehydrogenase (LDH) release, a marker of cell death, was exhibited by cells treated with stearic acid and triacsin C for 24 h (P<0.001). A mild increase in LDH release was also seen in cells treated with triacsin C alone as compared to untreated control cells. In addition, MPMs treated with a mixture of fatty acids in the presence of triacsin C showed a small but significant (P<0.05) decrease in LDH activity compared to controls (Fig. 4B).
Figure 4. Differential effects of long chain fatty acids on cell viability and apoptosis during ACSL inhibition.
MPMs were pretreated for 30 min with 5 μM triacsin C followed by co-treatment with 90 μM stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2) or a mixture (22.5 μM each of palmitic acid, stearic acid, oleic acid and linoleic acid) for an additional 24 h. Cell viability was determined by (A) MTT assay and (B) LDH assay. Data are expressed as mean ± SEM of 4–6 individual samples. (C) Western blot analysis was carried out for cleaved caspase 3 and cleaved PARP. Representative bands from two experiments performed in triplicate are shown. (D) Immunohistochemical analysis for cleaved caspase-3. Cleaved caspase 3 positive cells were counted in 3 fields from 3 different wells for each treatment group. Data are expressed as percentage of total cells. TC = triacsin C, FAM=fatty acid mixture.
^P<0.001 vs. all #P<0.01 vs. TC and P<0.001 vs. all others
$P<0.01 vs. 18:0+TC and P<0.001 vs. all others
*P<0.05 vs. control
Cells treated with stearic acid and triacsin C demonstrated cleavage of caspase 3 and PARP; however, neither individual unsaturated fatty acids nor mixtures of fatty acids induced apoptosis in the presence of triacsin C (Fig. 4C). Quantification of cleaved caspase 3 stained cells revealed that 20.0 ± 1.9% of cells exhibited features of apoptosis upon treatment with both stearic acid and triacsin C (P<0.001, Fig. 4D).
We have also determined the dose-dependent effect of stearic acid in inducing apoptosis in the presence of triacsin C and noted that stearic acid induced apoptosis at a concentration ranging from 30 to 90 μM in the presence of triacsin C (Supplemental Figure II).
High fat diet feeding leads to increased FFAs and macrophage accumulation in AT
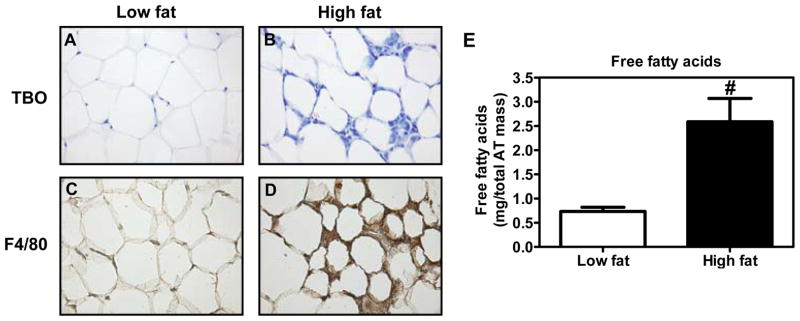
Because ACSLs play an important role in regulating fatty acid homeostasis in macrophages, we next attempted to determine the physiological significance of these enzymes on macrophage fatty acid homeostasis. It is well established that AT in obesity releases excessive FFAs14–16 and that macrophages accumulate in greater numbers in obese AT.17, 18 We fed wild type C57BL/6 mice with a low fat (LF) or a high fat (HF) diet for 16 wks and analyzed the perigonadal AT. As expected, the morphology of AT derived from HF diet fed mice analyzed by TBO staining suggests the presence of more macrophages compared to LF diet fed mice (Fig. 5A & B). Further analysis of AT sections for F4/80, a macrophage surface marker, demonstrated that AT derived from HF diet fed mice exhibited a profound increase in macrophage accumulation (Fig. 5C & D) and a 3.4-fold increase in local FFA concentrations (P<0.01, Fig. 5E).
Figure 5. Analysis of macrophage markers and FFA content in AT.
(A-D) Histological analysis of AT morphology. Wild type C57BL/6 mice were fed low fat (A&C) or high fat (B&D) diet for 16 wk. Perigonadal AT samples collected at sacrifice were fixed and paraffin embedded. Sections (7 μM) were stained with TBO (A&B), or immunostained with an antibody against F4/80 (C&D). 20X magnification. (E) Concentration of FFAs in AT of low fat and high fat diet fed mice as measured by GC analysis. Student’s t-test was carried out for statistical analysis. Values are mean ± SEM of 6 samples in each group.
#P<0.01
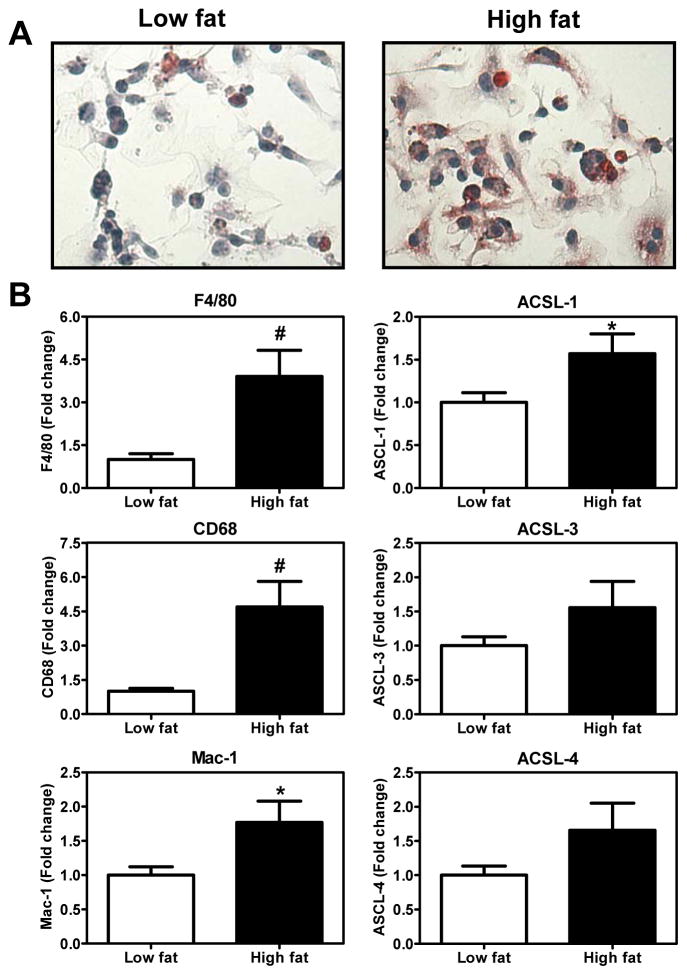
SVCs from HF diet fed mice exhibit foam cell like morphology associated with increased ACSL1
Interestingly, SVCs from HF diet fed mice exhibited features of foam cell morphology after staining with Oil Red O (Fig. 6A). In addition, there was an increase in the mRNA levels of the macrophage markers F4/80, CD68 and Mac-1. More importantly, the SVCs derived from the AT of HF diet fed mice displayed a significant increase in the expression of ACSL1 and a trend towards an increase in ACSL 3 and 4 (Fig. 6B). Thus, the macrophage enriched SVCs collected from HF diet fed mice exhibited signs of lipid deposition which was also associated with an increased expression of ACSL1.
Figure 6. Analysis of SVCs collected from AT.
(A) SVCs were stained with Oil Red O for neutral lipid accumulation. (B) mRNA samples were collected from the SVCs and analyzed for macrophage markers and ACSLs by real time PCR. Data are mean ± SEM of 13–15 samples in each group. Student’s t-test was carried out for statistical analysis.
*P<0.05 and #P<0.01
DISCUSSION
We and others have previously reported that macrophages efficiently take up FFAs derived from lipoprotein lipase mediated hydrolysis of TG-rich VLDL, which, in turn, are esterified and stored as intracellular TGs. 13, 19–22 Because conversion of fatty acids into fatty acyl CoAs is required for their esterification into TGs, we attempted to determine the impact of inhibiting fatty acid activation on fatty acid homeostasis in macrophages. In the current study, fatty acid loading was achieved using both TG-rich VLDL and albumin bound FFAs, and fatty acid activation was inhibited by triacsin C, which inhibits all three ACSL isoforms expressed in macrophages. We demonstrate that VLDL treatment in the presence of triacsin C leads to accumulation of intracellular FFAs and induction of apoptosis. Furthermore, we demonstrate that not only VLDL but also the FFA, stearic acid, in the presence of triacsin C, leads to accumulation of intracellular FFAs and induction of apoptosis. In addition, we demonstrate that SVCs derived from obese AT exhibit foam cell morphology, and show increased mRNA expression of ACSL1, and these changes occur concurrently with increased local FFA levels in AT. Our current data suggest that ACSLs play a critical role in regulating fatty acid homeostasis in macrophages and have implications in understanding the mechanisms by which AT macrophages respond to elevated local FFA levels.
Because TG-rich VLDL is an important physiological source of FFAs for macrophages,13, 19–22 we first treated macrophages with VLDL in the presence or absence of triacsin C. We found that VLDL treatment in the presence of triacsin C results in redistribution of cellular fatty acids by decreasing TGs and increasing FFAs (Fig. 1). Our data also show that partitioning of fatty acids into a “free” pool rather than into the storage form as TGs, leads to lipotoxicity in macrophages (Fig. 2). Traditionally, TG accumulation itself has been considered to be responsible for lipotoxicity. However, several reports suggest that TG accumulation may actually protect cells from the toxic effects of lipids by preventing the accumulation of FFAs and their metabolites such as ceramide and diacylglycerol.23, 24 This notion is further supported by the observation that even prolonged exposure to high levels of TG-rich VLDL in the absence of triacsin C failed to reduce cell viability in MPMs.25 These data have implications for situations in which macrophage ACSL activity is insufficient for the fatty acid load, especially when macrophages are exposed to high levels of fatty acids, such as in obesity or hypertriglyceridemia.
Comparison of the FFA accumulation under different conditions (VLDL vs. FFA exposure and 6 h vs. 24 h) reveals important biological information regarding the toxicity of intracellular FFA accumulation. First, 6 h exposure of MPMs to stearic acid in the presence of triacsin C led to a 5.1-fold increase in stearic acid accumulation in the cells (Supplemental Fig. I), and no increase in total FFA accumulation, yet did not induce apoptosis (data not shown). Second, treatment of MPMs with VLDL plus triacsin C for 6 h led to a 5.2-fold increase in total FFAs and extensive apoptosis (Figs. 1B and 2). Third, treatment with stearic acid plus triacsin C for 24 h led to accumulation of both total FFAs (2.3-fold) and stearic acid (4.0-fold) and also resulted in extensive apoptosis (Figs. 3A & B and 4D & E). In fact, the extent of apoptosis induced by stearic acid plus triacsin C treatment was much higher compared to treatment with VLDL plus triacsin C. One possible reason for this is the difference in exposure time between these two treatment conditions. Fourth, treatment of oleic acid plus triacsin C for 24 h led to a 2-fold increase in oleic acid and minimal increases in total FFA and did not induce apoptosis (Figs. 3A & C and Fig. 4C). Taken together, these data indicate that three elements of fatty acid exposure are important for their lipotoxic effects in MPMs: the source of fatty acids, the amount of total FFAs that accumulate, and the length of exposure time to intracellular FFAs.
Because the lipotoxic effect was seen only with stearic acid, it is possible that the apoptotic response we noted with VLDL treatment may also be caused by the stearic acid present in VLDL triglycerides. We previously reported that palmitic acid, stearic acid, oleic acid and linoleic acid were the predominant fatty acids found in VLDL used for treating macrophages 13. The amount of stearic acid in the VLDL triglyceride was 15 μM; whereas the minimum concentration of stearic acid we used to induce apoptosis in the current study was 30 μM. However, even with a lower stearic acid content (15 μM) and at an earlier time point (6 h), VLDL plus TC treatment led to stearic acid accumulation to levels only slightly lower than those in cells treated with stearic acid (90 μM) plus triacsin C treatment for 24 h (2.24 ± 0.28 vs. 3.2 ± 0.47 μg/mg protein, respectively). Thus, stearic acid accumulation accompanied by an increase in total FFA concentration may contribute to the apoptotic response seen in VLDL plus triacsin C treated cells at 6 h. However, the role of palmitic acid, another saturated fatty acid present in VLDL13 cannot be ruled out.
Another interesting observation of our current study is that when MPMs were treated with fatty acids in serum free medium, a significant increase in oleic acid accumulation was noted in stearic acid plus triacsin C treated cells (Fig. 3C). The increased oleic acid levels in stearic acid plus triacsin C treated cells suggests the involvement of stearoyl coA desaturase-1 (SCD-1) in converting stearic acid to oleic acid via a desaturation reaction.26 Because desaturation reactions also require fatty acid activation, our data indicate that even when ACSLs are inhibited, stearic acid activation occurs to some extent. In fact, fatty acid activation can also be carried out by another family of enzymes, the very long chain acyl CoA synthetases (ACSVLs),27 which are not inhibited by triacsin C.28 These enzymes can activate exogenous fatty acids during their transmembrane transport 27. On the other hand, in the absence of ACSL activity, the endogenous oleic acid produced intracellularly from stearic acid via SCD-1 activity may not be activated and metabolized further, thus leading to their intracellular accumulation.
It should also be noted that oleic acid accumulated to a lesser degree in oleic acid plus triacsin C treated cells compared to stearic acid accumulation in stearic acid plus triacsin C treated cells. As mentioned, even in the absence of ACSL activity, a portion of exogenous oleic acid can still be activated via ACSVLs. It is possible that the activated oleic acid may be preferentially partitioned to β-oxidation as opposed to stearic acid which appears to go through a desaturation reaction, thus accounting for the difference in the extent of its accumulation. However, we cannot rule out the possibility that the degree of inhibition by triacsin C may not be the same for stearic acid and oleic acid substrates. Further studies are needed to better understand the inhibitory potential of triacsin C on different fatty acids.
In order to gain insight into the role of ACSLs in regulating fatty acid homeostasis in vivo, we analyzed SVCs collected from lean and obese AT and noted that SVCs from obese AT exhibited features of foam cell transformation. As SVCs contain several other cell types, we analyzed them for markers of macrophages and found that all the macrophage markers were significantly higher in SVCs from obese AT compared to lean AT. SVCs from obese AT also exhibited an increased mRNA expression of ACSL1. Overexpression of ACSL1 has been shown to increase TG synthesis in liver.29 Because the increased ACSL1 expression in SVCs is also associated with increased neutral lipid accumulation and an increase in local FFA levels in AT, our data suggest that AT macrophages respond to increased FFA levels in AT by overexpressing ACSL1, which, in turn, regulates fatty acid homeostasis in AT macrophages.
Taken together, we have demonstrated that inhibition of ACSLs during fatty acid loading leads to intracellular FFA accumulation which results in apoptosis. Our data also demonstrated that increased local FFA levels in obese AT were associated with foam cell transformation of adipose tissue macrophages and these changes were accompanied by an increased expression of ACSL1. These findings provide insight into the biological importance of ACSLs in regulating fatty acid homeostasis in macrophages found in sites that are exposed to very high levels of FFAs and/or TGs, and indicate that perturbations in ACSL activity may lead to lipotoxic effects of FFAs in the context of obesity.
Supplementary Material
Acknowledgments
We would like to acknowledge the technical assistance of Corey Webb. Lipid profiles and staining for cleaved caspase 3 were performed at the lipid core and the immunohistochemistry core, respectively, of the Mouse Metabolic Phenotyping Center at Vanderbilt University (DK59637).
Source of funding
This project was supported by the National Institutes of Health (HL089466).
Footnotes
Disclosures
None
References
- 1.Smith SC., Jr Multiple risk factors for cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S3–S11. doi: 10.1016/j.amjmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120:S3–8. doi: 10.1016/j.amjmed.2006.11.012. discussion S29–32. [DOI] [PubMed] [Google Scholar]
- 3.Yao PM, Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem. 2000;275:23807–23813. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- 4.Mashek DG, Bornfeldt KE, Coleman RA, Berger J, Bernlohr DA, Black P, DiRusso CC, Farber SA, Guo W, Hashimoto N, Khodiyar V, Kuypers FA, Maltais LJ, Nebert DW, Renieri A, Schaffer JE, Stahl A, Watkins PA, Vasiliou V, Yamamoto TT. Revised nomenclature for the mammalian long-chain acyl-CoA synthetase gene family. J Lipid Res. 2004;45:1958–1961. doi: 10.1194/jlr.E400002-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Askari B, Kanter JE, Sherrid AM, Golej DL, Bender AT, Liu J, Hsueh WA, Beavo JA, Coleman RA, Bornfeldt KE. Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-gamma-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes. 2007;56:1143–1152. doi: 10.2337/db06-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Horn CG, Caviglia JM, Li LO, Wang S, Granger DA, Coleman RA. Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: identification of a novel variant of isoform 6. Biochemistry. 2005;44:1635–1642. doi: 10.1021/bi047721l. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Lewin TM, Coleman RA. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J Biol Chem. 2001;276:24667–24673. doi: 10.1074/jbc.M010793200. [DOI] [PubMed] [Google Scholar]
- 8.Mashek DG, McKenzie MA, Van Horn CG, Coleman RA. Rat long chain acyl-CoA synthetase 5 increases fatty acid uptake and partitioning to cellular triacylglycerol in McArdle-RH7777 cells. J Biol Chem. 2006;281:945–950. doi: 10.1074/jbc.M507646200. [DOI] [PubMed] [Google Scholar]
- 9.Saraswathi V, Wu G, Toborek M, Hennig B. Linoleic acid-induced endothelial activation: role of calcium and peroxynitrite signaling. J Lipid Res. 2004;45:794–804. doi: 10.1194/jlr.M300497-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Meerarani P, Ramadass P, Toborek M, Bauer HC, Bauer H, Hennig B. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor alpha. Am J Clin Nutr. 2000;71:81–87. doi: 10.1093/ajcn/71.1.81. [DOI] [PubMed] [Google Scholar]
- 11.Potter BJ, Sorrentino D, Berk PD. Mechanisms of cellular uptake of free fatty acids. Annu Rev Nutr. 1989;9:253–270. doi: 10.1146/annurev.nu.09.070189.001345. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 13.Saraswathi V, Hasty AH. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res. 2006;47:1406–1415. doi: 10.1194/jlr.M600159-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Lionetti L, Mollica MP, Lombardi A, Cavaliere G, Gifuni G, Barletta A. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr Metab Cardiovasc Dis. 2009;19:146–152. doi: 10.1016/j.numecd.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MD. Adipose tissue metabolism -- an aspect we should not neglect? Horm Metab Res. 2007;39:722–725. doi: 10.1055/s-2007-990274. [DOI] [PubMed] [Google Scholar]
- 16.Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev. 2002;18 (Suppl 2):S5–9. doi: 10.1002/dmrr.254. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates SR, Murphy PL, Feng ZC, Kanazawa T, Getz GS. Very low density lipoproteins promote triglyceride accumulation in macrophages. Arteriosclerosis. 1984;4:103–114. doi: 10.1161/01.atv.4.2.103. [DOI] [PubMed] [Google Scholar]
- 20.Evans AJ, Sawyez CG, Wolfe BM, Connelly PW, Maguire GF, Huff MW. Evidence that cholesteryl ester and triglyceride accumulation in J774 macrophages induced by very low density lipoprotein subfractions occurs by different mechanisms. J Lipid Res. 1993;34:703–717. [PubMed] [Google Scholar]
- 21.Palmer AM, Nova E, Anil E, Jackson K, Bateman P, Wolstencroft E, Williams CM, Yaqoob P. Differential uptake of subfractions of triglyceride-rich lipoproteins by THP-1 macrophages. Atherosclerosis. 2005;180:233–244. doi: 10.1016/j.atherosclerosis.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 22.Sofer O, Fainaru M, Schafer Z, Goldman R. Regulation of lipoprotein lipase secretion in murine macrophages during foam cell formation in vitro. Effect of triglyceride-rich lipoproteins. Arterioscler Thromb. 1992;12:1458–1466. doi: 10.1161/01.atv.12.12.1458. [DOI] [PubMed] [Google Scholar]
- 23.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianturco SH, Bradley WA, Gotto AM, Jr, Morrisett JD, Peavy DL. Hypertriglyceridemic very low density lipoproteins induce triglyceride synthesis and accumulation in mouse peritoneal macrophages. J Clin Invest. 1982;70:168–178. doi: 10.1172/JCI110590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Kurdi-Haidar B, Oram JF. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J Lipid Res. 2004;45:972–980. doi: 10.1194/jlr.M400011-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Watkins PA. Very-long-chain acyl-CoA synthetases. J Biol Chem. 2008;283:1773–1777. doi: 10.1074/jbc.R700037200. [DOI] [PubMed] [Google Scholar]
- 28.Hall AM, Smith AJ, Bernlohr DA. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem. 2003;278:43008–43013. doi: 10.1074/jbc.M306575200. [DOI] [PubMed] [Google Scholar]
- 29.Parkes HA, Preston E, Wilks D, Ballesteros M, Carpenter L, Wood L, Kraegen EW, Furler SM, Cooney GJ. Overexpression of acyl-CoA synthetase-1 increases lipid deposition in hepatic (HepG2) cells and rodent liver in vivo. Am J Physiol Endocrinol Metab. 2006;291:E737–744. doi: 10.1152/ajpendo.00112.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.