Abstract
Background
Strengthening the macrophage glutathione redox buffer reduces macrophage content and decreases the severity of atherosclerotic lesions in LDL receptor-deficient (LDLR−/−) mice, but the underlying mechanisms were not clear. This study examined the effect of metabolic stress on the thiol redox state, chemotactic activity in vivo and the recruitment of macrophages into atherosclerotic lesions and kidneys of LDL-R−/− mice in response to mild, moderate and severe metabolic stress.
Methods and Results
Reduced glutathione (GSH) and glutathione disulfide (GSSG) levels in peritoneal macrophages isolated from mildly, moderately and severe metabolically-stressed LDL-R−/− mice were measured by HPLC, and the glutathione reduction potential (Eh) was calculated. Macrophage Eh correlated with the macrophage content in both atherosclerotic (r2=0.346, P=0.004) and renal lesions (r2=0.480, P=0.001) in these mice as well as the extent of both atherosclerosis (r2=0.414, P=0.001) and kidney injury (r2=0.480, P=0.001). Compared to LDL-R−/− mice exposed to mild metabolic stress, macrophage recruitment into MCP-1-loaded Matrigel plugs injected into LDL-R−/− mice increased 2.6–fold in moderately metabolically-stressed mice and 9.8–fold in severely metabolically-stressed mice. The macrophage Eh was a strong predictor of macrophage chemotaxis (r2=0.554, P<0.001).
Conclusion
Thiol oxidative stress enhances macrophage recruitment into vascular and renal lesions by increasing the responsiveness of macrophages to chemoattractants. This novel mechanism contributes at least in part to accelerated atherosclerosis and kidney injury associated with dyslipidemia and diabetes in mice.
INTRODUCTION
Metabolic disorders such as hypercholesterolemia and diabetes are strongly associated with both macro- and microvascular diseases, a common feature of which is the recruitment of blood monocyte-derived macrophages to sites of vascular injury. While most studies exploring the mechanisms underlying atherosclerosis and other vascular pathologies have focused on the impact of a dysregulated metabolism on the vasculature itself, a number of more recent studies suggest that metabolic disorders may also directly impact monocytes and alter their functionalities in ways that promote and accelerate the disease process. Phenotypical abnormalities in blood monocytes of diabetic patients have been reported, including altered metabolism 1–3, phagocytosis 4;5 and cytokine release 6–8. Furthermore, peritoneal macrophages isolated from either atherosclerosis-prone mice or diabetic mice show altered cytokine and chemokine responses compared with macrophages from healthy control mice 9;10. However, it is not yet well-understood to what extent monocyte dysfunction induced by metabolic diseases contributes to macrophage recruitment and vascular diseases such as atherosclerosis.
The recruitment of blood monocyte-derived macrophages into the vessel wall is considered one of the earliest events in the onset of atherosclerosis 11;12. The mechanisms that trigger macrophage recruitment are not fully understood but prolonged retention and subsequent modification of LDL may play a critical role 13–15. Modified LDL, particularly oxidatively-modified LDL stimulates vascular cells and macrophages to secrete a vast array of inflammatory molecules, including chemokines, which in turn promote and sustain a continuous influx of macrophages into the vasculature 12;16. Studies in transgenic and knockout mice revealed that at least two major chemokine/chemokine receptor systems are involved in the recruitment of macrophages into atherosclerotic lesions, MCP-1/CCL2 and the receptor CCR2, and RANTES/CCL5 and the receptor CCR5. Deficiencies in either the macrophage chemoattractant MCP-1 or its receptor CCR2 reduced the severity of atherosclerosis in different mouse models of atherosclerosis, and the reduction in lesion size was accompanied by a reduction in macrophage accumulation 17–19. Inhibiting the effect of RANTES in high cholesterol diet-fed LDL-R−/− mice with Met-RANTES, a RANTES antagonist, also reduces macrophage infiltration and inhibited lesion formation 20. The reduction in these lesions' macrophage content was associated with a more stable plaque phenotype. Deficiency in the RANTES receptors CCR5 in ApoE−/− mice showed similar effects 21, but other studies suggest that CCR5 contributes primarily to the later stages of lesion development 22;23.
While the individual contributions of these chemoattractants and their receptors to macrophage recruitment and atherogenesis appear to change throughout the maturation of atherosclerotic lesions, these studies suggest that macrophage recruitment can be accelerated either by increasing the chemotactic signals originating from the vasculature or by sensitizing monocytes to these signals, for example by increasing chemokine receptor expression levels and/or activity. Here we examined the hypothesis that metabolic stress promotes macrophage recruitment and atherogenesis by increasing the responsiveness of circulating blood monocytes to chemoattractants, specifically to MCP-1. We compared normolipidemic, hypercholesterolemic and dyslipidemic diabetic LDL-R−/− mice and showed that with each increase in the level of metabolic stress macrophage recruitment into the vasculature and kidneys increased, and the development of vascular and renal lesions accelerated accordingly. Increased monocyte responsiveness to chemoattractants appeared to account at least in part for the observed increase in macrophage recruitment as MCP-1-loaded Matrigel plugs implanted into these mice also showed enhanced macrophage accumulation in response to each increase in the level of metabolic stress, even though the concentration of chemoattractant was identical in each implant. We provide evidence that increased cellular thiol oxidation induced by metabolic stress sensitizes macrophages to MCP-1-induced chemotaxis and that thiol oxidative stress contributes to the enhanced recruitment of macrophages to sites of tissue injury in metabolically-challenged mice.
MATERIALS AND METHODS
Animals
Female LDL-R−/− mice (B6.129S7-LdlrtmIHer/J, stock no. 002207) on a C57BL/6J background and C57BL/6J mice were obtained from The Jackson Laboratories (Bar Harbor, ME). After one week on a maintenance diet (MD, AIN-93G, BioServ), mice were randomized into three groups and subjected to either mild (MD), moderate (HFD) or severe metabolic stress (STZ+HFD). Mice in the STZ+HFD group were rendered diabetic with intraperitoneal injection of streptozotocin (STZ; 60 mg × kg−1 × day−1) dissolved in citrate buffer (50 mM; pH=4.5) for five consecutive days and, after a two-day rest, again for two consecutive days. Mice in the MD and HFD groups received a comparable volume of citrate buffer. To induce hypercholesterolemia, mice in the HFD and STZ+HFD group were fed a diet supplemented with fat (21% wt/wt) and cholesterol (0.15% wt/wt; AIN-76A, BioServ) beginning at 3 weeks after the first injection, for a total of 12 weeks. The remaining animals (MD group) received MD for 12 weeks. All studies were performed with the approval of the UTHSCSA Institutional Animal Care and Use Committee.
Analysis of Atherosclerosis
After mice were euthanatized, the right atrium was removed and hearts and aortas were perfused with PBS through the left ventricule. Hearts were embedded in OCT and frozen on dry ice. Aortas were fixed over night with 4% paraformaldehyde in PBS, dissected from the proximal ascending aorta to the bifurcation of the iliac artery, and had their adventitial fat removed. For en face analysis, aortas were stained with Oil Red O (ORO), opened longitudinally, pinned flat onto black paper placed over dental wax and digitally photographed at a fixed magnification 24. Total aortic area and lesion areas were calculated using ImagePro Plus 6.0 (Media Cybernetics). As a second measure of atherosclerosis, lesions of the aortic root were analyzed. Serial sections were cut through a 700 μm segment of the aortic root. For each mouse, 8 sections (7 μm) separated by 70 μm were examined. Each section was stained with ORO, counterstained with hematoxylin (Vector Labs, Burlingame, CA) and digitized. Lesion area was measured using ImagePro Plus 6.0 (Media Cybernetics) and expressed as millimeters squared 24. Macrophages were detected with anti-CD68 antibodies (Serotech) 24. In situ labeling of S-glutathionylated proteins in serial sections from the aortic root was performed using the method described by Reynaert et al. 25.
Analysis of Kidney Injury
Kidneys were frozen in OCT, and five cross cryosections separated by an interval of 50 mm were stained with ORO or Masson-Trichrome 26;27, or were processed for immunohistochemical analysis of macrophage content with anti-CD68 antibodies (Serotech) 24.
Blood and Urine Analysis
Mice were fasted overnight prior to glucose and lipid measurements. Glucose was measured biweekly using a Contour®meter (Bayer). For measurements of white blood cell (WBC) and monocyte counts, blood was obtained by retro-orbital bleed after 11 weeks of diet feeding, and differential blood cell counts were performed by the Department of Laboratory Animal Resources at UTHSCSA on a VetScan HM II Analyzer (Abaxis). After peritoneal macrophages were harvested, blood was drawn by cardiac puncture. Plasma cholesterol and triglyceride levels were determined using enzymatic assay kits (Wako Chemicals). Lipoprotein cholesterol distributions were analyzed in individual plasma samples (50 μl) for 8 mice in each group. Plasma samples were separated by size exclusion chromatography on tandem superose 6–10/300GL columns 28. Serum amyloid A (Biosource), leptin and insulin levels (Chrystal Chem) were measured by ELISA. Urine creatinine and albumin concentrations were measured by ELISA (Exocell).
Macrophage Glutathione/Glutathione Disulfide Analysis
Resident peritoneal cells were harvested by lavage, plated, and after 3 h non-adherent cells were removed with multiple washing 24. Adherent cells were cultured overnight prior to cell harvest. Glutathione and glutathione disulfide levels were measured by HPLC and values were normalized to cell DNA levels as described elsewhere 29. The glutathione reduction potential Eh was calculated using the Nernst equation for GSH/GSSH and E0=−264 mV for pH=7.4 30. The mean macrophage volume was estimated at 1.4 × 10−6 μl 31.
In Vivo Matrigel Macrophage Recruitment Assay
Three days prior to the end of the study, each mouse received two Matrigel plugs, one in each flank. Growth factor-reduced Matrigel (BD Biosciences) supplemented with either vehicle or recombinant MCP-1 (300 nM) was injected subcutaneously into the right and left flank of each mouse. The plugs were removed at the time of sacrifice, dissolved, and cells were counted in an automated, video-based hemocytometer (Nexcelom, Lawrence, MA). Cell staining with antibodies directed against macrosialin/CD68 confirmed that >93% of the cells recruited into the Matrigel plugs were macrophages.
Statistics
Data were analyzed using ANOVA (SPSS 16.0). Data were tested for use of parametric or nonparametric post hoc analysis, and multiple comparisons were performed by using the Least Significant Difference method. All data are presented as mean ± SE. Results were considered statistically significant at the P < 0.05 level.
RESULTS
Metabolic Stress in LDL-R −/− Mice Promotes the Oxidation of the Glutathione Redox State in Macrophages and Accelerates Atherosclerotic Lesion Formation
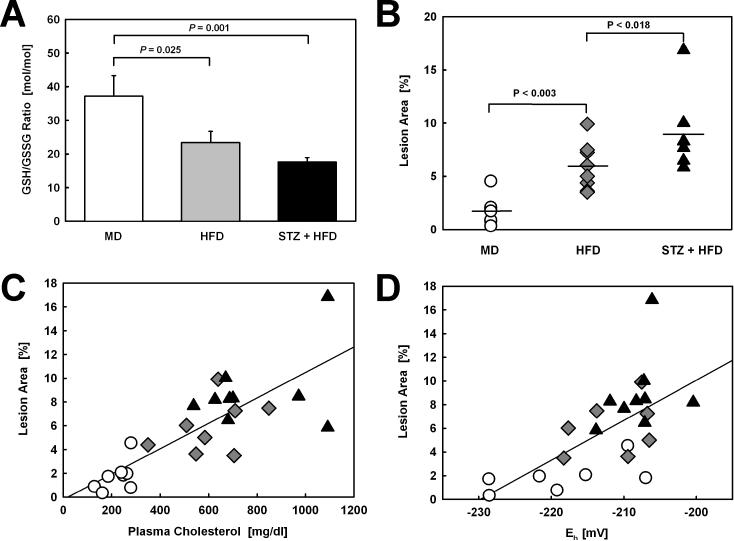
To examine the effect of metabolic stress on the thiol redox state of monocyte-derived macrophages, we determined the GSH/GSSG ratio in peritoneal macrophages isolated from LDL-R−/− mice that were exposed for 12 weeks to either mild (MD), moderate (HFD), or severe metabolic stress (STZ-induced hyperglycemia plus HFD). With increasing metabolic stress, these mice showed a progressive decrease in their macrophage GSH/GSSG ratios, indicating a progressive increase in intracellular (thiol) oxidative stress (Fig. 1A). As expected, increasing metabolic stress increased plasma cholesterol levels (Table 1) and accelerated atherosclerotic lesion formation in both the aortic arch and descending aorta of LDL-R−/− mice (Fig. 1B). Lesion size showed a strong correlation with plasma cholesterol levels in these animals (r2=0.635, P<0.001, Fig, 1C). HFD feeding increased cholesterol in both the IDL/LDL and the VLDL fractions, whereas STZ treatment prior to HFD feeding resulted in only a minor, statistically not significant (P=0.19) further increase in plasma total cholesterol (Table 1), primarily as VLDL cholesterol (Supplemental Fig. I). Both plasma triglycerides and glucose levels were also increased by the STZ treatment, but the differences did not reach statistical significance.
Figure 1. Correlation of Aortic Lesion Surface Area with Plasma Cholesterol and Macrophage Glutathione Reduction Potential.
(A) GSH/GSSG ratios in peritoneal macrophages isolated from LDL-R−/− mice exposed to either mild (MD, n=8), moderate (HFD, n=8) or severe metabolic stress (STZ+HFD, n=10) and (B) lesion surface area in aortas from these metabolically-stressed LDL-R−/− mice. (C) Correlation between plasma cholesterol and aortic lesion surface area (r2=0.635, P<0.001) and (D) between the glutathione reduction potential (Eh) of peritoneal macrophages and aortic lesion surface area (r2=0.414, P=0.001) in mildly (MD, Ο, n=7), modestly (HFD, ◆, n=7) and severely metabolically-stressed LDL-R−/− mice (STZ+HFD, ▴, n=9).
Table 1.
Blood and Plasma Parameters For LDL-R−/− Mice Exposed to Either Mild (MD), Moderate (HFD) or Severe Metabolic Stress (STZ+HFD).
| Parameter | MD (n=8) | HFD (n=8) | STZ + HFD (n=10) |
|---|---|---|---|
| Weight (g) | 19.8 ± 0.9 | 25.6 ± 1.2* | 21.6 ± 0.8§§ |
| Plasma total cholesterol (mg/dl) | 223 ± 20 | 612 ± 53* | 784 ± 70* |
| Plasma triglycerides (mg/dl) | 41.9 ± 6.4 | 58.2 ± 7.2 | 177.3 ± 53.4 |
| Glucose (mg/dl) | 62.8 ± 0.6 | 82.0 ± 4.0** | 149.4 ± 25.4** |
| Albumin/Creatinine (μg/mg) | 2.2 ± 0.6 | 1.7 ± 0.2 | 23.9 ± 13.1** |
| SAA (ng/ml) | 0.78 ± 0.42 | 0.89 ± 0.50 | 2.53 ± 0.89* |
| Leptin (ng/ml) | 15.2 ± 2.2 | 12.6 ± 1.5 | 9.0 ± 2.2§ |
| Insulin (ng/ml) | 0.14 ± 0.03 | 0.14 ± 0.03 | 0.17 ± 0.05 |
| WBC (109/l) | 5.7 ± 0.7 | 7.0 ± 1.4 | 10.9 ± 1.2**,§ |
| Monocytes (109/l) | 0.31 ± 0.06 | 0.26 ± 0.06 | 0.37 ± 0.06 |
Results are expressed as mean ± SE for 8 – 10 mice.
: P < 0.05 versus MD
: P < 0.01 versus MD
: P < 0.05 versus HFD
: P < 0.01 versus HFD.
Lipid, hormone and SAA measurements were performed in plasma samples from overnight-fasted mice. Glucose was measured in whole blood with a glucometer after overnight fasting. White blood cell (WBC) and monocyte counts were obtained with a VetScan HM II Analyzer and determined in blood obtained by retro-orbital bleed after 11 weeks of diet feeding.
Surprisingly, insulin levels were similar in non-diabetic and diabetic mice (Table 1). However, it is unlikely that β-cells recovered from the STZ treatment; as fasting glucose levels peaked four weeks after the first STZ injection, i.e. one week after initiating HFD feeding, and plateaued thereafter. A more likely explanation for the similar insulin levels is that the depletion of β-cells was incomplete. Injecting higher doses of STZ resulted in slightly higher fasting glucose levels in this mouse model, but the survival rate of the HFD-fed animals dropped precipitously at these higher STZ doses. Also, mice were fasted for 15 h to obtain true fasting glucose levels, and this extended fasting period likely contributed to the low insulin levels we observed in the non-diabetic mice.
To evaluate the relationship between thiol oxidative stress in macrophages induced by low, modest or severe metabolic stress and atherosclerotic lesion formation, we calculated the glutathione reduction potential (Eh) based on the cellular GSH and GSSG concentrations we determined in peritoneal macrophages isolated from each animal. The correlation between the macrophage glutathione reduction potential and aortic lesion size was statistically highly significant (r2=0.414, P=0.001; Fig. 1D), whereas plasma triglyceride levels (r2=0.345, P=0.003) and plasma glucose concentrations (r2=0.252, P=0.011) were both poorer predictors of atherosclerotic lesion formation.
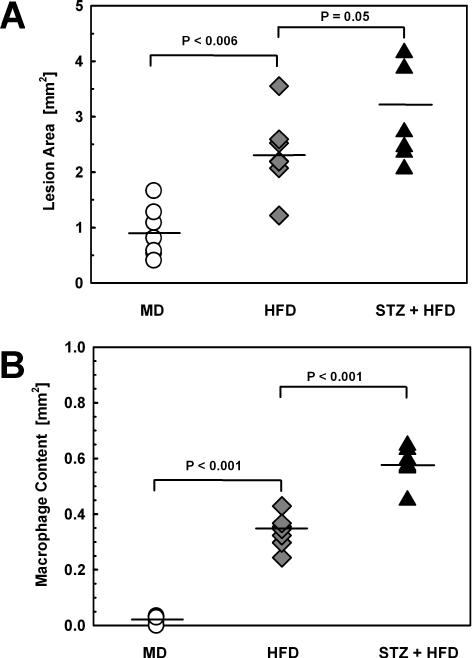
With increasing metabolic stress we also observed an acceleration in atherosclerotic lesion formation in the aortic root (Fig. 2A, Supplemental Fig. II). Increased lesion area was paralleled by an increase in macrophage content in the vessel wall (Fig. 2B, Supplemental Fig. II). Importantly, the macrophage glutathione reduction potential was a strong predictor of macrophage content in the lesions (expressed as mm2: r2=0.346, P=0.004; expressed as % lesion area: r2=0.683, P<0.001), suggesting that thiol oxidative stress in blood monocytes promotes macrophage recruitment into the vascular wall.
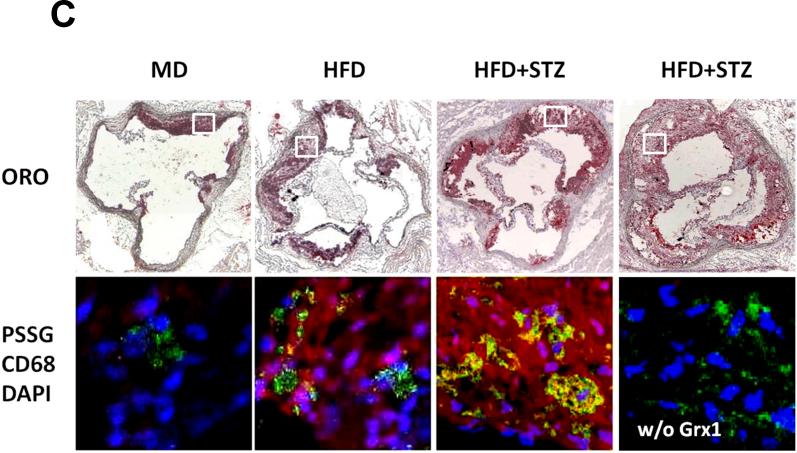
Figure 2. Lesion Area, Macrophage Content ProteinS-Glutathionylation in the Aortic Root.
(A) Lesion area in the aortic roots from LDL-R−/− mice exposed to either mild (MD, Ο, n=8), moderate (HFD, ♦, n=8) or severe metabolic stress (STZ+HFD, ▴, n=10) was measured in eight 7 μm sections separated by 70 μm after ORO staining. Lesion size is expressed mm2. (B) Macrophage content was assessed in adjacent sections by immunohistochemistry with antibodies directed against macrosialin/CD68 and is expressed as macrophage-containing area in mm2 (see Supplement, Figure S1B for representative images). (C) Representative images of three sections taken from three mice per group from the aortic root stained with ORO (upper panels), and enlarged images of selected areas (white box, upper panels) on adjacent sections labeled in situ for S-glutathionylated proteins (red), and stained with a macrophage-specific antiserum directed against CD68 (green) and DAPI (blue) to identify nuclei (lower panels). Colocalization of protein-S-glutathionylation with CD68-positive areas (macrophages), are shown in yellow. Sections in which the glutaredoxin 1-mediated reduction of protein-glutathione mixed disulfides (PSSG) was omitted (w/o Grx1) served as controls for the in situ labeling procedure. Magnification: 600X
Han et al. reported that increasing extracellular cholesterol levels by adding native LDL to THP-1 monocytes increases their chemotactic response to MCP-1 32. We found that plasma cholesterol levels in our mouse models correlated with the macrophage glutathione reduction potentials in these mice (r2=0.527, P=0.01), suggesting that in addition to increasing LDL depositions within the vasculature, elevated plasma cholesterol may also affect lesion size indirectly by promoting thiol oxidative stress in macrophages and by increasing their responsiveness to chemoattractants like MCP-1.
Protein-S-glutathionylation is a reversible posttranslational protein modification involved in redox signaling, and serves as a marker of cellular (thiol) oxidative stress 33. To determine whether thiol oxidative stress is increased in macrophages recruited into atherosclerotic lesions, we incubated aortic sections with glutaredoxin to specifically reduce protein-glutathione mixed disulfides and labeled the released protein thiols with thiol-specific Alexa545-conjugated N-ethyl maleimide. Compared to control mice (MD), we observed increased incorporation of fluorescent label into proteins of aortic lesions from moderately (HFD) or severely metabolically-stressed (STZ+HFD) mice (Fig. 2C), indicating that protein-S-glutathionylation – and thus thiol oxidative stress – increased with increasing levels of metabolic stress. Furthermore, protein-S-glutathionylation also localized to macrophages within these lesion, and intensified with increasing metabolic stress, providing further evidence that macrophages recruited to aortic lesions of metabolically-stressed (STZ+HFD) mice are thiol oxidatively stressed.
Although the number of WBC appeared to increase with increasing metabolic stress, we did not observe any statistically significant differences in blood monocyte counts between mildly (MD), moderately (HFD) or severely metabolically-stressed (STZ+HFD) mice (Table 1). This suggests that the increase in macrophage recruitment in these mice was not the result of increased blood monocyte counts.
Hyperglycemia in Dyslipidemic LDL-R−/− Mice Accelerates Glomerular Lipid Deposition, Macrophage Accumulation and Kidney Injury
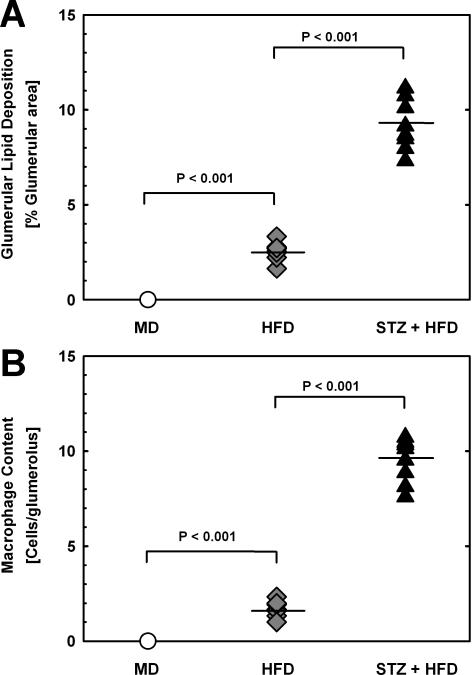
Next, we examined the kidneys of these mice to determine whether the apparent relationship between the macrophage glutathione reduction potential, macrophage recruitment and the severity of vascular lesions also extended to other sites of tissue injury. The kidneys of LDL-R−/− mice exposed to low, modest or severe metabolic stress showed progressive glomerular lipid deposition (Fig. 3A, Supplemental Fig. III), which was accompanied by increased macrophage recruitment into the glomeruli (Fig. 3B, Supplemental Fig. III). Like in the vasculature, we found a highly significant correlation between the macrophage glutathione reduction potential and macrophage accumulation in the kidney (r2=0.481, P=0.001, Supplemental Fig. IV). Increased renal lipid deposition and macrophage accumulation was accompanied by increased fibrosis (Supplemental Fig. III). Interestingly, we also observed a significant correlation between macrophage glutathione reduction potential and renal lipid accumulation in LDL-R−/− mice exposed to mild, modest or severe metabolic stress (r2=0.526, P<0.001, Supplemental Fig. V). Of note, mice from the STZ+HFD group showed a significant increase in the urinary albumin/creatinine ratio (Table 1), indicating that the extent of kidney injury in these severely metabolically-stressed, diabetic mice had resulted in the loss of renal function.
Figure 3. Glomerular Lipid Accumulation and Macrophage Content.
(A) Lipid accumulation in glomeruli was assessed in ORO-stained cross cryosections of kidneys isolated from LDL-R−/− mice exposed to either mild (MD, Ο, n=8), moderate (HFD, ♦, n=8) or severe metabolic stress (STZ+HFD, ▴, n=10). Results are expressed as percent ORO-positive glomerular area. (B) Macrophage recruitment was assessed in adjacent sections by immunohistochemistry, with antibodies directed against macrosialin/CD68 (see Supplement, Figure S2 for representative images). Results are expressed as numbers of macrophages per glomerulus.
Metabolic Stress Enhances Macrophage Chemotactic Activity In Vivo
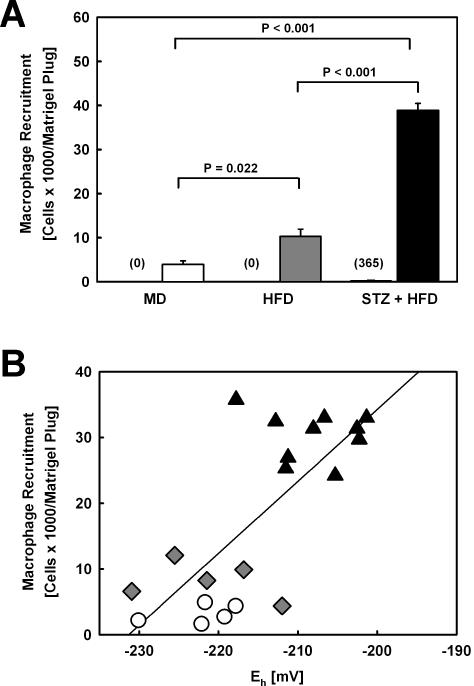
To examine whether metabolic stress changes the responsiveness of macrophages to chemoattractants, we injected mildly (MD), moderately (HFD) or severely metabolically-stressed (STZ+HFD) mice with Matrigel plugs loaded with either vehicle or MCP-1. After 3 days, the plugs were removed and macrophage recruitment into these plugs was quantified. Increasing the level of metabolic stress in LDL-R−/− mice by feeding a HFD, increased macrophage recruitment into MCP-1-loaded Matrigel plugs 2.6-fold (Fig. 4A). No macrophages were detected in vehicle-loaded plugs removed from the opposite flank of either MD-or HFD-fed mice. When we further increased metabolic stress by inducing hyperglycemia in LDL-R−/− mice prior to HFD feeding, we observed an additional 3.8-fold increase in macrophage recruitment. Even though the MCP-1 concentration in the Matrigel injected into all three groups of mice was identical, 9.8-fold more macrophages were recruited into MCP-1-loaded Matrigel plugs isolated from severely metabolically-stressed, diabetic LDL-R−/− mice than into plugs removed from MD-fed LDL-R−/− mice (Fig. 4A). In contrast to mildly (MD) and moderately metabolically-stressed LDL-R−/− mice (HFD), we also detected small numbers of macrophages in vehicle-loaded plugs from severely metabolically-stressed LDL-R−/− mice (Fig. 4A, STZ+HFD, numbers in parentheses). This indicates that the sensitivity of macrophages in these animals was increased to such an extent that the cells even responded to residual chemotactic factors present in the growth factor-poor Matrigel plugs. When we measured GSH and GSSG levels and calculated the glutathione reduction potential of the peritoneal macrophages, we again found a highly significant correlation (r2=0.554, P<0.001) between the macrophage glutathione reduction potential and the number of macrophages recruited into the MCP-1 loaded Matrigel plugs.
Figure 4. Macrophage Chemotactic Activity in Metabolically Stress Mice.
(A) Matrigel plugs filled with either vehicle (left bar, number of cells given in parentheses) or MCP-1 (300 nM, right bar) were injected into the left and right flanks, respectively, of LDL-R−/− mice exposed to either mild (MD, □ n=5), moderate (HFD, ■, n=5) or severe metabolic stress (STZ+HFD, ■, n=10). After three days the plugs were removed, dissolved and cells were counted. Immunostaining with antibodies directed against macrosialin/CD68 confirmed that > 93% of the cells recruited into the Matrigel plugs were macrophages. (B) Correlation between glutathione reduction potential (Eh) of peritoneal macrophages isolated from mildly (MD, Ο, n=5), modestly (HFD, ♦, n=5) and severely metabolically-stressed LDL-R−/− mice (STZ+HFD, ▴, n=10) and macrophage numbers recruited into Matrigel plugs implanted for 3 days into the same animals (r2=0.554, P<0.001).
DISCUSSION
In this study we examined whether metabolic stress induced by hypercholesterolemia alone or hyperlipidemia plus hyperglycemia accelerates atherosclerosis and renal injury by promoting thiol oxidative stress in macrophages and increasing the recruitment of macrophages to sites of tissue injury. We found that with each additional level of metabolic stress, macrophage accumulation increased in both atherosclerotic lesions and in kidneys of LDL-R−/− mice. Analysis of the macrophage glutathione reduction potential (Eh) revealed that metabolic stress promotes intracellular thiol oxidation and that oxidation of the macrophage glutathione reduction state correlates with both accelerated macrophage recruitment and increased lesion severity. Macrophage recruitment into MCP-1-loaded Matrigel plugs implanted in these mice was also accelerated with each additional level of metabolic stress, suggesting that increased cellular thiol oxidation induced by metabolic stress amplifies macrophage responses to chemotactic signals. These studies identified a novel thiol-dependent mechanism that contributes to macrophage recruitment and the development of atherosclerotic lesions.
Macrophages are continually recruited into atherosclerotic lesions, and macrophage accumulation appears to increase in proportion to lesion size 34. Although the rate of macrophage recruitment depends on 1) the nature of chemoattractants released by the injured tissue and 2) the size of chemotactic gradient, our data suggest that there is a third key factor determining the extent of macrophage accumulation and thus lesion size: the intensity of the monocytes' response to a chemotactic signal. The increase in chemotactic activity we observed in response to increased metabolic stress could not be fully accounted for by a single cardiovascular risk factor, e.g. plasma cholesterol, triglycerides, or glucose levels, suggesting that the overall metabolic state rather than any individual metabolite determines the monocyte responsiveness to chemoattractants. Monocytes appear to act as sensors of metabolic stress and their chemotactic activity may reflect cardiovascular risk, a concept, if confirmed, that would have important diagnostic and therapeutic implications.
Changes in the cellular redox environment not only initiate (or inhibit) individual signaling pathways but dictate a cell's fate with regard to function, differentiation, proliferation and survival 35. The cellular glutathione reduction potential serves as a key indicator of a cell's redox environment 35. Previously, we showed that increased expression of macrophage glutathione reductase activity reduces the severity of atherosclerosis in LDL-R−/− mice, providing evidence that the glutathione redox state in macrophages plays a critical role in the development and progression of atherosclerotic lesions 24. The strong correlations we observed in this study between the macrophage glutathione reduction potential (Eh) and 1) macrophage chemotactic activity in vivo, 2) macrophage accumulation in vascular and renal lesions, and 3) the severity of atherosclerotic lesions and renal injury, confirm that the level of thiol oxidation in macrophages appears to be a critical determinant for both the extent of macrophage recruitment and the rate of lesion development.
Our in vivo chemotaxis assay demonstrated that macrophage chemotactic activity increased with increasing levels of metabolic stress, even though the concentration of chemoattractant, i.e. MCP-1, was identical in all Matrigel plugs. Two mechanisms could have contributed to this increase in macrophage recruitment: increased numbers of blood monocytes and/or increased responsiveness of monocytes to chemoattractants. We observed no statistically significant increases in blood monocyte counts in metabolically-stressed mice, indicating that chronic metabolic stress rendered monocytes-derived macrophages hyperresponsive to MCP-1. Increased cell surface expression of the MCP-1 receptor CCR2 could account for the detected increase in macrophage responsiveness to MCP-1. In support of this hypothesis, Han et al. reported that CCR2 transcript levels are elevated 2-fold in human blood monocytes isolated from hypercholesterolemic subjects as compared with monocytes from normolipidemic subjects, although cell surface expression of CCR2 protein was not determined in this study 32. Increased surface expression of CCR2, however, was detected on monocytes from diabetic patients 36. The lack of a commercially available antibody directed against murine CCR2 prevented us from directly testing whether CCR2 surface expression in macrophages was increased in our metabolically-stressed mice, but the increase in macrophage chemotactic activity we observed in moderately and severely metabolically-stressed mice would be consistent with increased CCR2 surface expression.
Alternatively, thiol oxidative stress may increase CCR2 sensitivity toward MCP-1 and/or signaling in response to MCP-1 binding. An example of how thiol oxidative stress and protein-S-glutathionylation can affect chemokine signaling was illustrated by Kanda et al. for PDGF receptor β (PDGFRβ) 37. PDGF-B, a PDGFRβ agonist and mitogen, is also a potent monocyte chemoattractant involved in tissue repair and wound healing. The authors show that S-glutathionylation of active site cysteine residues of LMW-PTP inhibits the ability of the phosphatase to counterregulate PDGFRβ activation, thus promoting hyperactivation of the receptor and its downstream signaling pathways in response to agonist binding. MCP-1 signaling through CCR2 and the subsequent internalization of the receptor is regulated by G-protein-related kinases (GRK) GRK2 and GRK3 38. Both GRK2 activity and protein levels are attenuated by oxidative stress, suggesting a potential role for thiol oxidation in the regulation of both CCR2 surface expression and MCP-1-dependent signaling 39. Loss of GRK2 activity and the subsequent dysregulation of the protein kinase/phosphatase steady state controlling CCR2 activity would be expected to promote CCR2 surface retention and to hyperactivate the receptor in response to MCP-1. Amplification of MCP-1-induced signaling by (thiol) oxidative stress would provide an additional mechanism for the enhanced chemotactic activity of macrophages observed in metabolically-stressed mice.
In summary, these studies provide evidence that increased macrophage responsiveness to chemoattractants induced by thiol oxidative stress is a novel mechanism contributing to increased macrophage recruitment and accelerated atherosclerosis and renal injury associated with metabolic disorders. Our data underscore the critical role of the macrophage glutathione redox state in regulating macrophage chemotaxis and identify the glutathione-dependent antioxidant system in monocytes as a potential therapeutic target for the prevention and treatment of atherosclerosis.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Wuqiong Ma for her technical assistance and Kirsten Gallagher for her comments and critical reading of the manuscript.
FUNDING SOURCES This work was supported by grants to R.A. from the NIH (HL-70963) and the American Heart Association (0855011F).
ABBREVIATIONS
- Eh
glutathione reduction potential
- Grx
glutaredoxin
- GSH
reduced glutathione
- GSSG
glutathione disulfide (oxidized glutathione)
- HFD
high fat diet
- HPLC
high performance liquid chromatography
- MCP-1
monocyte chemotactic protein-1
- MD
maintenance diet
- ORO
Oil Red O
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- SAA
serum amyloid A
- STZ
streptozotocin
- WBC
white blood cells
Footnotes
DISCLOSURES There are no conflicts to disclose.
REFERENCES
- 1.Noritake M, Katsura Y, Shinomiya N, Kanatani M, Uwabe Y, Nagata N, Tsuru S. Intracellular hydrogen peroxide production by peripheral phagocytes from diabetic patients. Dissociation between polymorphonuclear leucocytes and monocytes. Clin Exp Immunol. 1992;88:269–274. doi: 10.1111/j.1365-2249.1992.tb03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josefsen K, Nielsen H, Lorentzen S, Damsbo P, Buschard K. Circulating monocytes are activated in newly diagnosed type 1 diabetes mellitus patients. Clin Exp Immunol. 1994;98:489–493. doi: 10.1111/j.1365-2249.1994.tb05517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill HR, Hogan NA, Rallison ML, Santos JI, Charette RP, Kitahara M. Functional and metabolic abnormalities of diabetic monocytes. Adv Exp Med Biol. 1982;141:621–628. doi: 10.1007/978-1-4684-8088-7_61. [DOI] [PubMed] [Google Scholar]
- 4.Katz S, Klein B, Elian I, Fishman P, Djaldetti M. Phagocytotic activity of monocytes from diabetic patients. Diabetes Care. 1983;6:479–482. doi: 10.2337/diacare.6.5.479. [DOI] [PubMed] [Google Scholar]
- 5.Geisler C, Almdal T, Bennedsen J, Rhodes JM, Kolendorf K. Monocyte functions in diabetes mellitus. Acta Pathol Microbiol Immunol Scand [C] 1982;90:33–37. doi: 10.1111/j.1699-0463.1982.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 6.Luger A, Schernthaner G, Urbanski A, Luger TA. Cytokine production in patients with newly diagnosed insulin-dependent (type I) diabetes mellitus. Eur J Clin Invest. 1988;18:233–236. doi: 10.1111/j.1365-2362.1988.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 7.Ohno Y, Aoki N, Nishimura A. In vitro production of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77:1072–1077. doi: 10.1210/jcem.77.4.8408455. [DOI] [PubMed] [Google Scholar]
- 8.Salvi GE, Collins JG, Yalda B, Arnold RR, Lang NP, Offenbacher S. Monocytic TNF alpha secretion patterns in IDDM patients with periodontal diseases. J Clin Periodontol. 1997;24:8–16. doi: 10.1111/j.1600-051x.1997.tb01178.x. [DOI] [PubMed] [Google Scholar]
- 9.Netea MG, Demacker PN, Kullberg BJ, Boerman OC, Verschueren I, Stalenhoef AF, van der Meer JW. Increased interleukin-1alpha and interleukin-1beta production by macrophages of low-density lipoprotein receptor knock-out mice stimulated with lipopolysaccharide is CD11c/CD18-receptor mediated. Immunology. 1998;95:466–472. doi: 10.1046/j.1365-2567.1998.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zykova SN, Jenssen TG, Berdal M, Olsen R, Myklebust R, Seljelid R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes. 2000;49:1451–1458. doi: 10.2337/diabetes.49.9.1451. [DOI] [PubMed] [Google Scholar]
- 11.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 12.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 13.Williams KJ, Tabas I. Lipoprotein retention--and clues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–1540. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- 14.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg D, Witztum JL. Lipoproteins, lipoprotein oxidation, and atherogenesis. In: Chien KR, editor. Molecular Basis of Cardiovascular Disease. W.B. Sanders Co.; Philadelphia, PA: 1998. [Google Scholar]
- 17.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 18.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 20.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 21.Braunersreuther V, Zernecke A, Arnaud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Luckow B, Mach F, Weber C. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27:373–379. doi: 10.1161/01.ATV.0000253886.44609.ae. [DOI] [PubMed] [Google Scholar]
- 22.Kuziel WA, Dawson TC, Quinones M, Garavito E, Chenaux G, Ahuja SS, Reddick RL, Maeda N. CCR5 deficiency is not protective in the early stages of atherogenesis in apoE knockout mice. Atherosclerosis. 2003;167:25–32. doi: 10.1016/s0021-9150(02)00382-9. [DOI] [PubMed] [Google Scholar]
- 23.Potteaux S, Combadiere C, Esposito B, Lecureuil C, Ait-Oufella H, Merval R, Ardouin P, Tedgui A, Mallat Z. Role of bone marrow-derived CC-chemokine receptor 5 in the development of atherosclerosis of low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:1858–1863. doi: 10.1161/01.ATV.0000231527.22762.71. [DOI] [PubMed] [Google Scholar]
- 24.Qiao M, Kisgati M, Cholewa J, Zhu W, Smart EJ, Sulistio MS, Asmis R. Increased expression of cytosolic and mitochondrial glutathione reductase in macrophages inhibits atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1375–1382. doi: 10.1161/ATVBAHA.107.142109. [DOI] [PubMed] [Google Scholar]
- 25.Reynaert NL, Ckless K, Guala AS, Wouters EF, Van D, V, Janssen-Heininger YM. In situ detection of S-glutathionylated proteins following glutaredoxin-1 catalyzed cysteine derivatization. Biochim Biophys Acta. 2006;1760:380–387. doi: 10.1016/j.bbagen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Q, Egashira K, Inoue S, Usui M, Kitamoto S, Ni W, Ishibashi M, Hiasa KK, Ichiki T, Shibuya M, Takeshita A. Vascular endothelial growth factor is necessary in the development of arteriosclerosis by recruiting/activating monocytes in a rat model of long-term inhibition of nitric oxide synthesis. Circulation. 2002;105:1110–1115. doi: 10.1161/hc0902.104718. [DOI] [PubMed] [Google Scholar]
- 27.Ni W, Egashira K, Kitamoto S, Kataoka C, Koyanagi M, Inoue S, Imaizumi K, Akiyama C, Nishida KI, Takeshita A. New anti-monocyte chemoattractant protein-1 gene therapy attenuates atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2001;103:2096–2101. doi: 10.1161/01.cir.103.16.2096. [DOI] [PubMed] [Google Scholar]
- 28.Daugherty A, Rateri DL. Presence of LDL receptor-related protein/alpha 2-macroglobulin receptors in macrophages of atherosclerotic lesions from cholesterolfed New Zealand and heterozygous Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb. 1994;14:2017–2024. doi: 10.1161/01.atv.14.12.2017. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Qiao M, Mieyal JJ, Asmis LM, Asmis R. Molecular mechanism of glutathione-mediated protection from oxidized LDL-induced cell injury in human macrophages: Role of glutathione reductase and glutaredoxin. Free Radic Biol Med. 2006;41:775–785. doi: 10.1016/j.freeradbiomed.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 31.Poulter LW, Turk JL. Rapid quantitation of changes in macrophage volume induced by lymphokine in vitro. Clin Exp Immunol. 1975;19:193–199. [PMC free article] [PubMed] [Google Scholar]
- 32.Han KH, Tangirala RK, Green SR, Quehenberger O. Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler Thromb Vasc Biol. 1998;18:1983–1991. doi: 10.1161/01.atv.18.12.1983. [DOI] [PubMed] [Google Scholar]
- 33.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25:332–346. [PMC free article] [PubMed] [Google Scholar]
- 34.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 36.Mine S, Okada Y, Tanikawa T, Kawahara C, Tabata T, Tanaka Y. Increased expression levels of monocyte CCR2 and monocyte chemoattractant protein-1 in patients with diabetes mellitus. Biochem Biophys Res Commun. 2006;344:780–785. doi: 10.1016/j.bbrc.2006.03.197. [DOI] [PubMed] [Google Scholar]
- 37.Kanda M, Ihara Y, Murata H, Urata Y, Kono T, Yodoi J, Seto S, Yano K, Kondo T. Glutaredoxin modulates platelet-derived growth factor-dependent cell signaling by regulating the redox status of low molecular weight protein-tyrosine phosphatase. J Biol Chem. 2006;281:28518–28528. doi: 10.1074/jbc.M604359200. [DOI] [PubMed] [Google Scholar]
- 38.Aragay AM, Mellado M, Frade JM, Martin AM, Jimenez-Sainz MC, Martinez A, Mayor F., Jr. Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proc Natl Acad Sci U S A. 1998;95:2985–2990. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lombardi MS, Kavelaars A, Penela P, Scholtens EJ, Roccio M, Schmidt RE, Schedlowski M, Mayor F, Jr., Heijnen CJ. Oxidative stress decreases G protein-coupled receptor kinase 2 in lymphocytes via a calpain-dependent mechanism. Mol Pharmacol. 2002;62:379–388. doi: 10.1124/mol.62.2.379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.