Abstract
Objective
Defective smooth muscle expression of LDL receptor-related protein-1 (Lrp1) increases atherosclerosis in hypercholesterolemic mice. This study explored the importance of smooth muscle Lrp1 expression under normolipidemic conditions.
Methods and Results
Smooth muscle cells isolated from control (smLrp1+/+) and smooth muscle-specific Lrp1 knockout (smLrp1−/−) mice were characterized based on morphology, smooth muscle marker protein expression levels, and growth rates in vitro. Vascular functions were assessed by aortic constrictive response to agonist stimulation in situ and neointimal hyperplasia to carotid arterial injury in vivo. The smLrp1−/− smooth muscle cells displayed reduced α-actin and calponin expression and an accelerated growth rate due to sustained phosphorylation of platelet derived growth factor receptor (PRGFR) and protein kinase B/Akt. Vasoconstrictive response to agonist stimulation was impaired in aortic rings isolated from smLrp1−/− mice. Injury-induced neointimal hyperplasia was significantly increased in smLrp1−/−mice. The increase in neointima was associated with corresponding elevated activation of PDGFR signaling pathway.
Conclusions
Smooth muscle expression of Lrp1 is important in maintaining normal vascular functions under normolipidemic conditions. The absence of Lrp1 expression results in greater smooth muscle cell proliferation, deficient contractile protein expression, impairment of vascular contractility, and promotion of denudation-induced neointimal hyperplasia.
Keywords: Lipoprotein receptors, Smooth muscle cell phenotype, Vasocontractility, Growth factor receptor signaling
Low density lipoprotein receptor-related protein-1 (LRP1) is initially identified as an endocytic receptor capable of binding to and mediating the plasma clearance of many ligands including apoE-containing lipoproteins, α2-macroglobulin, and several other protease complexes such as metalloproteinases 13, 2, and 9, and urokinase- and tissue-type plasminogen activators after their complex with plasminogen activator inhibitor type 1.1, 2 The YxxL motif in the carboxyl-terminal intracellular domain of LRP1 serves as endocytosis signal for cellular uptake of bound ligands.3 The cytoplasmic domain of LRP1 also contains two NPxY motifs capable of interacting with adaptor and scaffold proteins for signal transduction purposes.4 In addition, LRP1 also modulates signal transduction functions of platelet derived growth factor (PDGF) and transforming growth factor-β (TGFβ).5–10 The binding of PDGF to its cognate receptor results in tyrosine phosphorylation of the distal NPxY motif of LRP1 by Src and Src family kinases.5, 6 The phosphorylation of the NPxY motif generates a docking site for Shc,11 an adaptor protein that is involved in protein tyrosine kinase signaling, thereby promoting PDGF-induced cell proliferation and migration through Shc-mediated Ras signaling and mitogen-activated protein kinase activation. Direct communications between the extracellular ligand binding domain and the intracellular signaling domain of LRP1 were demonstrated in vitro with results showing apoE binding to LRP1 inhibits its tyrosine phosphorylation by PDGF.5 This apoE-mediated suppression of PDGF-induced LRP1 tyrosine phosphorylation may directly be responsible for its inhibition of PDGF-induced mitogen-activated protein kinase activity and migration of smooth muscle cells.12–14
Recent studies have reported that LRP1 is identical to the type V TGFβ receptor TGFβR(V) and mediates TGFβ inhibition of cell proliferation via Smad protein signaling.15, 16 In myoblasts, LRP1 modulation of TGFβ signaling requires activation of phosphatidylinositol 3-kinase (PI-3K).10 The importance of LRP1 modulating PDGF and TGFβ signaling cascades in vivo was illustrated recently by comparing vascular wall integrity and susceptibility to diet-induced atherosclerosis in LDL receptor defective (Ldlr−/−) mice with or without smooth muscle-specific inactivation of the mouse Lrp1 gene (smLrp1−/−).8, 9 These studies showed that aortas from smLrp1−/−Ldlr−/− mice were distended and dilated in comparison to smLrp1+/+Ldlr−/− mice. Cholesterol feeding of the smLrp1−/−Ldlr−/− mice resulted in massive thickening of the vessel wall in comparison to that observed in cholesterol-fed smLrp1+/+Ldlr−/− mice. The increase in wall thickness in the absence of smLrp1 was attributed to a combination of increased cellular proliferation, foam cell transformation, and cholesterol deposition in the interstitial clefts.8 Vessel wall fibrosis, disruption of the elastic laminae, and aneurysm formation were also observed in the smLrp1−/−Ldlr−/− mice.8 The vascular pathology observed in smLrp1−/−Ldlr−/− mice was significantly improved by blockade of PDGF and/or TGFβ receptor signaling cascades with tyrosine kinase inhibitor Gleevec or PPARγ activation, respectively.9
The expression of LRP1 in vascular smooth muscle cells has been shown to be upregulated under hypercholesterolemic conditions via suppression of sterol regulatory element binding protein-2.17 This increase in LRP1 expression may represent smooth muscle cell response to hypercholesterolemic insults to limit vascular damage, thus the lack of Lrp1 expression in the vessel wall of hypercholesterolemic Ldlr-defective mice may exacerbate atherosclerosis by abolishment of this protective effect. Whether smooth muscle-specific inactivation of Lrp1 affects vessel wall integrity and smooth muscle cell functions in the absence of cholesterol feeding remains unknown. This study was undertaken to address this issue by comparing vascular contractility and response to injury between normolipidemic smLrp1+/+ and smLrp1−/− mice.
METHODS
An expanded Methods section is available online as Supplemental Data.
Mice
The smLrp1−/− mice produced by crossing sm22 promoter-driven cre transgenic mice with Lrp1flox/flox mice8 were backcrossed with wild type C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) for 10 generations to obtain congenic littermates. The animals have free access to water and normal mouse chow (Harlan Teklad, Madison, WI). Male smLrp1+/+ and smLrp1−/−littermates between 6 and 8 weeks of age, and weighed between 25 to 30 grams, were used for all experiments. All procedures were performed in accordance with institutional guidelines.
In Vitro Smooth Muscle Cell Characterization
Aortic smooth muscle cells were isolated from smLrp1+/+ and smLrp1−/− mice using an enzyme dispersion method and were characterized by positive immunostaining with smooth muscle α-actin antibodies (clone 1A4, Sigma) as previously described.14 All experiments were performed with cells between passages 1 and 5. Cell proliferation and migration assays were performed as described previously.14 Proteins were extracted from cells for Western blot analysis with antibodies against vinculin/metavinculin, calponin, caldesmon, smooth muscle α-actin, tubulin and Lrp1. Immunocytofluorescence staining of smooth muscle cells was performed with cells seeded on glass coverslips.
Agonist-induced Vasocontraction
Contractile functions of vascular smooth muscle were evaluated in situ with intact thoracic aortas obtained from age-matched male smLrp1+/+ and smLrp1−/− mice as described.18
Endothelial Denudation of Carotid Arteries and Neointima Evaluation in Mice
The procedures used to induce endothelial denudation in mouse carotid arteries, tissue preparation and evaluation of neointimal formation were performed as described previously.19 Cell proliferation and PDGFR signaling were assessed by incubating deparaffinized tissue sections overnight at 4°C with rabbit anti-Ki67 (Vector Laboratories), goat anti-phospho-PDGFR (Santa Cruz Biotechnology), or rabbit anti-phospho-Akt (Cell Signaling) at a 1:1000 dilution in Antibody Diluent (Zymed).
Statistical Analysis
Values were expressed as mean ± SEM. Multiple comparisons were first tested by Student’s t test or ANOVA. When ANOVA demonstrated significant differences, individual mean differences were analyzed post hoc by Tukey’s multiple comparison method using SigmaStat software. Growth curves and concentration-dependent contractility curves were first fitted with non-linear regression analysis and then compared by ANOVA for repeated measures followed by Bonferroni post hoc analysis to detect differences using Prism software (GraphPad). Values of P<0.05 were considered significant in each case.
RESULTS
Characterization of Wild Type and smLrp1−/− Smooth Muscle Cells
Freshly isolated aortic smooth muscle cells from smLrp1−/− mice were more epithelioid shaped in comparison to the typical spindled-shaped smooth muscle cells from wild type mice. Immunofluorescent localization of smooth muscle-specific α-actin showed the typical filamental bundles spanning the length of quiescent wild type smooth muscle cells (Fig. 1a). In contrast, α-actin filaments were less abundant and were often disrupted in quiescent smooth muscle cells from smLrp1−/− mice (Fig. 1b). The epithelioid cell morphology and low abundance of smooth muscle α-actin filaments in smLrp1−/− cells resembled smooth muscle cells of the synthetic phenotype instead of the typical contractile smooth muscle cell characteristics of freshly isolated aortic smooth muscle cells.20, 21 Western blot analysis of proteins extracted from smLrp1+/+ and smLrp1−/− cells is supportive of this hypothesis, with results showing a slightly reduced level of smooth muscle α-actin and dramatic decrease in the level of calponin in smooth muscle cells without Lrp1 (Fig. 1e). In contrast, vinculin/metavinculin, caldesmon, β-actin, and tubulin levels were not different between smLrp1+/+ and smLrp1−/− smooth muscle cells (Fig. 1e).
Figure 1.
Characterization of aortic smooth muscle cells from smLrp1+/+ and smLrp1−/− mice. Indirect immunofluorescence of α-actin filaments in smLrp1+/+ (a,c) and smLrp1−/− (b,d) smooth muscle cells after incubation in medium with 0.2% serum for 48 hr (a,b) or after 1 hr stimulation with 15 ng/ml PDGF-bb (c,d). Actin filaments were detected with antibodies against smooth muscle α-actin (cyan color). Magnification = 600×. Panel e shows immunoblot of proteins extracted from smooth muscle cells with specific antibodies against the proteins as indicated.
Different Growth Characteristics Between Wild Type and smLrp1−/− Smooth Muscle Cells
The differences in morphology and protein expression between smooth muscle cells isolated from smLrp1+/+ and smLrp1−/− mice were reflected by their different responses to serum and PDGF-bb stimulation. Whereas PDGF-bb stimulation of wild type smooth muscle cells resulted in α-actin filament disassembly and localization of the α-actin to plasma membranes, with minimal fillapodial extensions observable during the initial 60 min, smLrp1−/− smooth muscle cells challenged with PDGF-bb induced rapid disassembly of α-actin and distinct fillapodial extensions were notable within this same time period (Figs. 1c and 1d). The smLrp1−/− smooth muscle cells also displayed elevated proliferative rates when cultured in vitro. Whereas wild type smooth muscle cells displayed a normal dose-dependent increase in [3H]thymidine incorporation into DNA with maximal 4-fold increase observed at PDGF-bb concentrations of 15 ng/ml (Fig. 2a), smLrp1−/− smooth muscle cells responded robustly to PDGF-bb stimulation with a 5-fold increase in [3H]thymidine incorporation into DNA observed even at 0.15 ng/ml PDGF-bb (Fig. 2a). Greater than 6-fold increase in smLrp1−/− cell proliferation was observed with 1.5 and 15 ng/ml PDGF-bb (Fig. 2a). The increased growth potential of Lrp1-defective smooth muscle cells was confirmed by directly monitoring serum-induced cell growth throughout a 2-week period. Smooth muscle cells isolated from smLrp1−/− mice displayed a shorter doubling time compared to wild type smooth muscle cells (Fig. 2b). The smLrp1−/− cells displayed vigorous growth with significant differences in cell number from wild type cells by day 7 and reaching confluency at day 11 (Fig. 2b). In contrast, wild type smooth muscle cells did not double in number until after 11 days in culture with 10% serum, nevertheless confluency was reached within 2 weeks (Fig. 2b). Similar results showing enhanced growth rates and [3H]thymidine incorporation into cellular DNA were also observed with smooth muscle cells isolated from smLrp1−/− mice with mixed genetic background (online supplement Figure I). The smLrp1−/−smooth muscle cells also displayed enhanced migration in response to PDGF-bb with a 40% increase in the number of migrated cells at 1.5 ng/ml PDGF-bb (Fig. 2c).
Figure 2.
Proliferation and migration of smLrp1+/+ and smLrp1−/− smooth muscle cells. Panel a: Quiescent smooth muscle cells isolated from smLrp1+/+ and smLrp1−/− mice seeded at 2,500 cells per well in 96-well dishes were stimulated with 15 ng/ml PDGF-bb for 18 hr. Incorporation of [3H]thymidine into cellular DNA was determined after cell lysis. Panel b: smooth muscle cells were seeded at 10,000 cells per well in 24-well dishes and cultured in DMEM containing 10% fetal bovine serum. Viable cell number was determined by direct counting. Panel c: quiescent smooth muscle cells were applied to the upper chamber of a modified Boyden chamber in 24-well dishes in which the lower chamber contained 0.6 ml PDGF-bb at the concentrations indicated. The number of cells migrated toward PDGF-bb after 4 hr was determined by cell counting. Data represent mean ± S.D. from triplicate assays performed at least 3 times. * and δ denote significant difference from the smLrp1+/+ group at P<0.05. Panel d: Western blot analysis of proteins extracted from smooth muscle cells isolated from smLrp1+/+ and smLrp1−/−mice after incubation with 15 ng/ml PDGF-bb for the times indicated with antibodies against total- and pY1021-PDGFR-β, total and pS473-Akt, and smooth muscle α-actin. Representative data from 4 different experiments were shown.
Elevated PDGF Signaling Pathway in smLrp1−/− Smooth Muscle Cells
The mechanism responsible for the increased growth rates of smLrp1−/− smooth muscle cells compared to wild type cells was explored by determining their initial cell signaling response to PDGF-bb stimulation. The incubation of wild type smooth muscle cells with PDGF-bb resulted in rapid tyrosine phosphorylation of PDGFR-β with peak induction occurring at 2.5 min followed by a return to basal levels after 30 min (Fig. 2d). In contrast, smLrp1−/− smooth muscle cells displayed significantly higher level of PDGFR-β expression and phosphorylation throughout the same period. High levels of PDGFR-β expression and phosphorylation was sustained throughout 10 min of PDGF-bb incubation and their levels were significantly above basal level even after 30 min of incubation (Fig. 2d). The sustained elevation of PDGFR-β phosphorylation in smLrp1−/− smooth muscle cells also resulted in their elevated and sustained Akt phosphorylation in response to PDGF-bb in comparison to wild type smooth muscle cells (Fig. 2d).
Medial Thickening and PDGFR-β activation in aortas of smLrp1−/− mice
Consistent with results of isolated smooth muscle cells incubated in vitro, histochemical staining of aortas from smLrp1−/− mice documented a doubling of medial thickness characterized by increased number of cell nuclei and disruption of elastic layers in comparison to the aortas of smLrp1+/+ mice (Fig. 3a,b). Analysis of aortic extracts revealed increased PDGFR-β expression and activation as well as decreased calponin expression in the vessel wall of smLrp1−/− mice (Fig. 3c). Residual Lrp1 expression observed in the aortic extract of smLrp1−/− mice was most likely due to Lrp1 expression in non-smooth muscle cells in the vessel wall.
Figure 3.
Analysis of morphology and protein expression in aortas from smLrp1+/+ and smLrp1−/−mice. Panel a: Representative hematoxylin and eosin (H&E) or Verhoeff-Van Geison (VVG) staining of aortas from smLrp1+/+ and smLrp1−/− mice. Panel b: Morphometric data of medial thickness from 5 mice in each group. Panel c: Western blot analysis of proteins expressed in aortas of smLrp1+/+ and smLrp1−/− mice.
Defective Aortic Contraction in smLrp1−/− Mice
The reduced expression level of the contractile protein calponin in aortas of smLrp1−/−mice suggested that normal contractile function of the vessel wall may be compromised due to Lrp1 deficiency. Therefore, aortic rings were prepared from smLrp1+/+ and smLrp1−/− mouse aortas to evaluate their vasoconstrictive response to stimulation. Whereas aortic rings from smLrp1+/+ mice displayed concentration-dependent increase of contractile force in response to KCl and phenylephrine stimulation, with maximal force achieved with 50 mM and 1 μM, respectively, aortic rings from smLrp1−/− mice failed to reach 50% level of force generation at any concentrations of KCl and phenylephrine tested (Fig. 4).
Figure 4.
Vasoreactivity of aortic rings from smLrp1+/+ and smLrp1−/− mice. Aortic rings prepared from 15-week old male smLrp1+/+ (closed symbols) and smLrp1−/− (open symbols) mice were mounted on tungsten wires and then attached to a differential capacitor force transducer. The aortic rings were then incubated with KCl or phenylephrine at the concentrations as indicated and force generation was recorded. The data represent mean ± SE of results from 8 different aortas from each group. δ and δδ denote significant difference from the smLrp1+/+ curves at P<0.01 and P=0.05, respectively.
Smooth Muscle Lrp1 Deficiency Promotes Neointimal Hyperplasia after Endothelial Denudation
The impact of the increased proliferative rates of smLrp1−/− smooth muscle cells on vascular response to injury was explored by comparing neointimal formation in the vessel wall of smLrp1+/+ and smLrp1−/− mice after endothelial denudation. In these experiments, the carotid arteries of chow-fed smLrp1+/+ and smLrp1−/− mice were denuded of their endothelium mechanically with an epon resin-modified catheter probe and whole neck sections were processed after 14 days for histological analysis.22 Results showed minimal neointimal formation in smLrp1+/+ mice after endothelial denudation (online supplement Fig. II), which is consistent with previous reports of resistance to injury-induced neointimal hyperplasia in the C57BL/6 mouse strain.23 In contrast, the injured carotid arteries of smLrp1−/− mice showed significant thickening of the vessel wall.
Morphometric analysis of injured and uninjured carotid arteries from 8 mice in each group confirmed the lack of neointimal formation 14 days after endothelial denudation of the carotid arteries of smLrp1+/+ mice, whereas a significant neointimal area was observed after endothelial denudation of the smLrp1−/− carotid arteries (Fig. 5a). Morphometric measurements revealed consistently larger medial area and thickness in the contralateral uninjured and the injured carotid arteries of smLrp1−/− mice compared to that observed in smLrp1+/+ mice (Figs. 5b,c). Luminal area measurements were not different between injured arteries of smLrp1+/+ and smLrp1−/− mice.
Figure 5.
Measurements of neointimal area (panel a), medial area (b), and medial thickness (c) of control (open bars) and injured (filled bars) carotid arteries from wild type smLrp1+/+ and smLrp1−/− mice. Tissues were analyzed 14 days after endothelial denudation. The data represent the mean ± SEM from 10 mice in each group. Bars with different letters in each graph indicate significant difference at P<0.05.
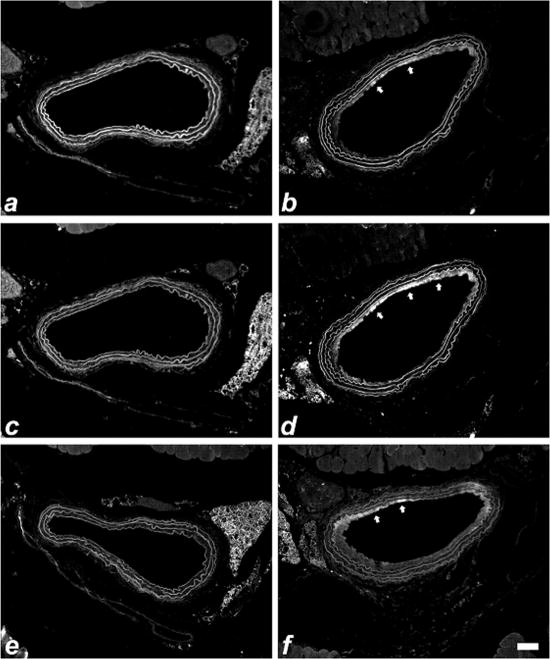
Immunofluorescent characterization of the neointima in injured arteries of smLrp1−/− mice revealed the presence of the cell proliferative marker Ki67 14 days after endothelial denudation (Fig. 6). As reported previously,22 smooth muscle cell proliferation in the neointima has already subsided 14 days after endothelial denudation in wild type C57BL/6 smLrp1+/+ mice (Data not shown). The prolonged neointimal hyperplasia observed in the injured arteries of smLrp1−/− mice coincided with the persistent PDGFR-β activation of cell signaling events with both phospho-PDGFR-β and phospho-Akt epitopes readily detectable in the intima of injured arteries of smLrp1−/− mice (Fig. 6). Interestingly, Ki67, phospho-PDGFR-β, and phospho-Akt epitopes were below the detected limits in the uninjured carotid arteries of smLrp1−/− mice (Fig. 6).
Figure 6.
Immunofluorescent detection of Ki-67 (panels a, b), phospho-PDGFR-β (panels c, d), and phospho-Akt (e,f) in control (a,c,e) and injured carotid arteries (b,d,f) of smLrp1−/− mice 14 days after endothelial denudation. The elastic laminae is visible around the circumference of the vessel. Positive immunoreactivity appears in the neointima with the most intensive staining areas highlighted with arrows. Scale bar represents 50 μm.
DISCUSSION
Arterial smooth muscle cells are heterogeneous and can be separated into at least 2 distinct phenotypes: one displaying epithelioid morphology with a relatively fast growth rate and another displaying spindle morphology that grows slower but has the capacity to contract.21, 24 The spindle-shaped smooth muscle cells also have a more mature smooth muscle cell phenotype with greater abundance of smooth muscle α-actin, calponin, h-caldesmon, and the presence of metavinculin.21 In the present study, we showed that smooth muscle cells devoid of Lrp1 displayed an epithelioid morphology with relatively less α-actin filaments than the typical smooth muscle cells isolated from aortas of wild type mice. Importantly, the smLrp1−/− smooth muscle cells were depleted of calponin, similar to that observed in synthetic but not contractile smooth muscle cells. The morphologic and protein expression differences between smLrp1+/+ and smLrp1−/− smooth muscle cells are reflected by their functional differences in response to stimulation. The aortas isolated from smLrp1−/− mice, with calponin-depleted smooth muscle cells, were defective in constrictive response to KCl and phenylephrine stimulation in situ. In addition, the smLrp1−/− smooth muscle cells displayed accelerated growth rates in response to growth factor and serum stimulation in vitro, thickening of the aorta in vivo, and increased proliferation resulting in neointimal hyperplasia after endothelial denudation.
The rapid growth rate of smLrp1−/− smooth muscle cells in comparison to that observed with wild type cells is consistent with the sustained elevated levels of phosphorylated PDGFR-β in the absence of Lrp1. Previous studies have already shown elevated PDGFR-β level and phosphorylation in smooth muscle cells isolated from mice deficient in both Lrp1 and LDL receptor.8, 9 The current study revealed similarly elevated PDGFR-β expression and activation in Lrp1-negative cells without LDL receptor deficiency. Accordingly, Lrp1 regulation of PDGFR-β level and activity is not dependent on LDL receptor expression or hypercholesterolemia. The increased expression level of PDGFR-β in smLrp1−/− smooth muscle cells also confirms the established role of Lrp1 in regulating PDGFR-β clearance of PDGF-bb. Both Lrp1 and PDGFR-β are located in lipid raft-rich membrane domains where cell signaling pathways initiate.5, 25 Ligand binding to PDGFR-β induces receptor clustering, and the receptor-ligand complex is translocated to clathrin coated pits and then internalized to lysosomes for degradation and cell signal termination. Recent studies showed a direct interaction between Lrp1 and PDGFR-β in endosomal compartments.7 Thus, in the absence of Lrp1, PDGFR-β internalization and inactivation may be compromised resulting in increased PDGFR-β level at the cell surface and enhanced cell response to PDGF-bb. Results demonstrating a direct role of Lrp1 in promoting PDGFR-β endocytosis and turnover7, 26 are supportive of this hypothesis. However, it is important to note that Lrp1 regulation of PDGFR-β level and activation is cell type specific since PDGFR-β expression is lower in Lrp1-negative than Lrp1-positive fibroblasts.26, 27 The mechanism responsible for this cell type specific difference in Lrp1-modulation of PDGFR-β level remains to be identified.
The current study also demonstrated that the increased expression and activity of PDGFR-β in Lrp1-deficient vessel wall promotes neointimal hyperplasia after endothelial denudation. Comparison of injured carotid arteries between smLrp1+/+ and smLrp1−/− mice revealed the increase in medial area as well as presence of a neointima in the latter group. Medial thickening of the vessel wall was also observed in the contralateral uninjured carotid arteries as well as in the aortas of smLrp1−/− mice without surgery. Since Ki-67 epitopes were not detected in the medial layer of uninjured vessels, the increased medial thickness is likely due to increased extracellular matrix as a consequence of constitutive TGFβ receptor activation in the absence Lrp1.9 However, we cannot rule out the possibility of elevated medial smooth muscle cell proliferation in smLrp1−/− mice due to the limited sensitivity of the histological immunodetection method used. The elevated levels of proliferating smooth muscle cells were evident upon endothelial injury with positive immunostaining for Ki-67 epitope, phospho-PDGFRβ and phospho-Akt.
The decreased expression of the contractile protein calponin in smLrp1−/− smooth muscle cells resulted in compromised vascular functions as demonstrated by defective vasoconstrictive response of smLrp1−/− mouse aortas to KCl and phenylephrine stimulation. Previous studies have shown the down-regulation of calponin expression and conversion of contractile to synthetic phenotype of smooth muscle cells after treatment with PDGF-bb and interleukin-1β (IL-1β).28 Although PDGF-bb treatment alone was not sufficient and synergistic IL1-β activity to induce calponin down-regulation, the difference between PDGF-bb and PDGF-bb/IL-1β treatment was the transient induction of PDGFR and Akt phosphorylation in the former and their sustained phosphorylation in the latter.28 The sustained activation of Akt was shown to be effective in converting contractile smooth muscle cells to that of a synthetic phenotype.28 Thus, the constitutive activation of PDGFR-β signaling pathway and activation of Akt is likely responsible for the decreased expression of calponin and reduced vasocontractive response of smLrp1−/− mice.
The decrease in vasoconstrictive response and the elevated injury-induced neointimal hyperplasia observed in smLrp1−/− mice cannot be attributed to the role of Lrp1 in lipoprotein metabolism since both smLrp1+/+ and smLrp1−/− mice used in these experiments were maintained on a low fat/low cholesterol diet and displayed similar plasma cholesterol levels. Previously, increase in vascular occlusion in the absence of smooth muscle Lrp1 was also observed in cholesterol-fed LDL receptor-deficient mice.8 In view of recent studies illustrating different mechanisms for the pathogenesis of injury-induced neointimal hyperplasia and lipid-induced atherosclerosis,23, 29 these results indicated the importance of Lrp1 expression in vascular protection against both injury-induced and diet-induce vascular occlusion. Importantly, the increased in neointimal formation after endothelial denudation of smLrp1−/− mice, a model with minimal involvement of lymphocytes and macropahges,29, 30 indicates Lrp1 protection against vascular occlusion is a direct effect of Lrp1 function in smooth muscle cells in limiting hyperplasia independent of inflammatory response.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This research was supported by NIH grants HL61332 and DK74932 to D.Y.H., a Pre-Doctoral Fellowship (0415267B) from the Ohio valley Affiliates of the American Heart Association (AHA) to Z.W.Q.M., and a Post-Doctoral Fellowship (0825316F) from the South Central Affiliate of the AHA to L.Z. NIH also provided research support for J.H.
Footnotes
DISCLOSURES
None.
References
- 1.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by Cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101:689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strickland DK, Gonias SL, Argraves WS. Diverse roles for the LDL receptor family. Trends Endocrinol Metabol. 2002;13:66–74. doi: 10.1016/s1043-2760(01)00526-4. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor related protein. J Biol Chem. 2000;275:17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 4.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RGW, Herz J. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in caveolae. J Biol Chem. 2002;277:15507–15513. doi: 10.1074/jbc.M200428200. [DOI] [PubMed] [Google Scholar]
- 6.Loukinova E, Ranganathan S, Kuznetsov S, Gorlatova N, Migliorini MM, Loukinov D, Ulery PG, Mikhailenko I, Lawrence DA, Strickland DK. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function between LRP and the PDGF. J Biol Chem. 2002;277:15499–15506. doi: 10.1074/jbc.M200427200. [DOI] [PubMed] [Google Scholar]
- 7.Newton CS, Loukinova E, Mikhailenko I, Ranganathan S, Gao Y, Haudenschild C, Strickland DK. Platelet-derived growth factor receptor-beta (PDGF-beta) activation promotes its association with the low density lipoprotein receptor-related protein (LRP). Evidence for co-receptor function. J Biol Chem. 2005;280:27872–27878. doi: 10.1074/jbc.M505410200. [DOI] [PubMed] [Google Scholar]
- 8.Boucher P, Gotthardt M, Li W-P, Anderson RGW, Herz J. LRP: Role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 9.Boucher P, Li W-P, Matz RL, Takayama Y, Auwerx J, Anderson RGW, Herz J. LRP1 functions as an atheroprotective integrator of TGFbeta and PDGF signals in the vascular wall: implications for marfan syndrome. PLoS ONE. 2007;2:e448. doi: 10.1371/journal.pone.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J Biol Chem. 2007;282:18842–18850. doi: 10.1074/jbc.M700243200. [DOI] [PubMed] [Google Scholar]
- 11.Barnes H, Larsen B, Tyers M, van der Geer P. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (LRP1) associates with the adaptor protein SHC in SRC-transformed cells. J Biol Chem. 2001;276:19119–19125. doi: 10.1074/jbc.M011437200. [DOI] [PubMed] [Google Scholar]
- 12.Ishigami M, Swertfeger DK, Granholm NA, Hui DY. Apolipoprotein E inhibits platelet-derived growth factor-induced vascular smooth muscle cell migration and proliferation by suppressing signal transduction and preventing cell entry to G1 phase. J Biol Chem. 1998;273:20156–20161. doi: 10.1074/jbc.273.32.20156. [DOI] [PubMed] [Google Scholar]
- 13.Swertfeger DK, Bu G, Hui DY. Low density lipoprotein receptor-related protein mediates apolipoprotein E inhibition of smooth muscle cell migration. J Biol Chem. 2002;277:4141–4146. doi: 10.1074/jbc.M109124200. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Hui DY. Apolipoprotein E Binding to Low Density Lipoprotein Receptor-related Protein-1 Inhibits Cell Migration via Activation of cAMP-dependent Protein Kinase A. J Biol Chem. 2003;278:36257–36263. doi: 10.1074/jbc.M303171200. [DOI] [PubMed] [Google Scholar]
- 15.Huang SS, Ling T-Y, Tseng W-F, Huang Y-H, Tang F-M, Leal SM, Huang JS. Cellular growth inhibition by IGFBP-3 and TGF-beta1 requires LRP-1. FASEB J. 2003;17:2068–2081. doi: 10.1096/fj.03-0256com. [DOI] [PubMed] [Google Scholar]
- 16.Tseng W-F, Huang SS, Huang JS. LRP-1/TbetaR-V mediates TGF-beta1-induced growth inhibition in CHO cells. FEBS Letters. 2004;562:71–78. doi: 10.1016/S0014-5793(04)00185-1. [DOI] [PubMed] [Google Scholar]
- 17.Llorente-Cortes V, Otero-Vinas M, Sanchez S, Rodriguez C, Badimon L. Low density lipoprotein upregulates low density lipoprotein receptor related protein expression in vascular smooth muscle cells. Possible involvement of sterol regulatory element binding protein 2-dependent mechanism. Circulation. 2002;106:3104–3110. doi: 10.1161/01.cir.0000041434.28573.0b. [DOI] [PubMed] [Google Scholar]
- 18.Lalli J, Harrer JM, Luo W, Kranias EG, Paul RJ. Targeted Ablation of the Phospholamban Gene Is Associated With a Marked Decrease in Sensitivity in Aortic Smooth Muscle. Circ Res. 1997;80:506–513. doi: 10.1161/01.res.80.4.506. [DOI] [PubMed] [Google Scholar]
- 19.Zhu B, Kuhel DG, Witte DP, Hui DY. Apolipoprotein E inhibits neointimal hyperplasia after arterial injury in mice. Am J Pathol. 2000;157:1839–1848. doi: 10.1016/S0002-9440(10)64823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bochaton-Piallat ML, Clowes AW, Clowes MM, Fischer JW, Redard M, Gabbiani F, Gabbiani G. Cultured arterial smooth muscle cells maintain distinct phenotypes when implanted into carotid artery. Arterioscler Thromb Vasc Biol. 2001;21:949–954. doi: 10.1161/01.atv.21.6.949. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Fan Y-S, Chow LH, Van Den Diepstraten C, van der Veer E, Sims SM, Pickering JG. Innate Diversity of Adult Human Arterial Smooth Muscle Cells: Cloning of Distinct Subtypes From the Internal Thoracic Artery. Circ Res. 2001;89:517–525. doi: 10.1161/hh1801.097165. [DOI] [PubMed] [Google Scholar]
- 22.Zhu B, Zhao G, Witte DP, Hui DY, Fagin JA. Targeted overexpression of IGF-1 in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury. Endocrinology. 2001;142:3598–3606. doi: 10.1210/endo.142.8.8331. [DOI] [PubMed] [Google Scholar]
- 23.Kuhel DG, Zhu B, Witte DP, Hui DY. Distinction in genetic determinants for injury-induced neointimal hyperplasia and diet-induced atherosclerosis in inbred mice. Arerioscler Thromb Vasc Biol. 2002;22:955–960. doi: 10.1161/01.atv.0000017994.77066.75. [DOI] [PubMed] [Google Scholar]
- 24.Shanahan CM, Weissberg PL. Smooth muscle cell phenotypes in atherosclerotic lesions. Curr Opin Lipidol. 1999;10:507–513. doi: 10.1097/00041433-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Ying YS, Anderson RGW. Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc Natl Acad Sci USA. 1997;94:13666–13670. doi: 10.1073/pnas.94.25.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takayama Y, May P, Anderson RGW, Herz J. Low density lipoprotein receptor-related protein 1 (LRP1) controls endocytosis and c-CBL-mediated ubiquitination of the platelet-derived growth factor receptor beta (PDGFbeta) J Biol Chem. 2005;280:18504–18510. doi: 10.1074/jbc.M410265200. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Arandjelovic S, Gonias SL. Effects of low density lipoprotein receptor-related protien-1 on the expression of platelet-derived growth factor beta-receptor in vitro. J Cell Biochem. 2004;93:1169–1177. doi: 10.1002/jcb.20288. [DOI] [PubMed] [Google Scholar]
- 28.Chen C-N, Li i-SJ, Yeh Y-t, Lee P-L, Usami S, Chien S, Chiu J-J. Synergistic roles of platelet-derived growth factor-BB and interleukin-1beta in phenotypic modulation of human aortic smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:2665–2670. doi: 10.1073/pnas.0510973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets. 2008;9:251–260. doi: 10.2174/138945008783755601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu B, Reardon CA, Getz GS, Hui DY. Both Apolipoprotein E and Immune Deficiency Exacerbate Neointimal Hyperplasia After Vascular Injury in Mice. Arterioscler Thromb Vasc Biol. 2002;22:450–455. doi: 10.1161/hq0302.105377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.