Abstract
Gibbons utilize a number of locomotor modes in the wild, including bipedalism, leaping and, most of all, brachiation. Each locomotor mode puts specific constraints on the morphology of the animal; in some cases these may be complementary, whereas in others they may conflict. Despite several studies of the locomotor biomechanics of gibbons, very little is known about the musculoskeletal architecture of the limbs. In this study, we present quantitative anatomical data of the hind limb for four species of gibbon (Hylobates lar, H. moloch, H. pileatus and Symphalangus syndactylus). Muscle mass and fascicle lengths were obtained from all of the major hind limb muscles and the physiological cross-sectional area was calculated and scaled to remove the effect of body size. The results clearly indicate that, for all of the species studied, the major hip, knee and ankle extensors are short-fascicled and pennate. The major hip and knee flexors, however, are long-fascicled, parallel muscles with relatively small physiological cross-sectional areas. We hypothesize that the short-fascicled muscles could be coupled with a power-amplifying mechanism and are predominantly useful in leaping. The long-fascicled knee and hip flexors are adapted for a wide range of joint postures and can play a role in flexing the legs during brachiation.
Keywords: biomechanics, Hylobates, leaping, muscle architecture, primate, siamang, Symphalangus
Introduction
Gibbons possess a large locomotor repertoire that includes quadrupedal walking and leaping (Fleagle, 1974; Gittins, 1983; Vereecke et al. 2006a), and three sub-modes of torso-orthograde suspensory locomotion: vertical climbing, orthograde clambering and brachiation (categories follow Hunt et al. 1996 and Thorpe & Crompton, 2005, 2006). Of these, brachiation is the most common in the wild, with between half and three-quarters of all locomotion conducted in this way (Fleagle, 1974; Gittins, 1983). Because of the diversity of locomotor modes used by gibbons, the hind limb is likely to be under varying mechanical demands, to which its anatomy is likely to be adapted.
Quantitative anatomical data on primate, and particularly human, hind limbs are abundant (for humans: Alexander & Vernon, 1975; Friederich & Brand, 1990; Fukunaga et al. 1992; for other apes: Thorpe et al. 1999, 2004; Vereecke et al. 2005; Payne et al. 2006a; for other primates: Sigmon & Farslow, 1986). However, quantitative data on the hind limb anatomy of whole gibbon cadavers are limited to a single specimen from which Payne et al. (2006a) made comparisons with the great apes in an evolutionary context. Vereecke et al. (2005) made comparisons between the lower leg and foot of humans, bonobos and gibbons, based on detailed dissections of bonobo and gibbon feet. Although both studies gave a good insight into the comparative anatomy of the ape hind limb, the number of gibbon species and specimens included was very limited (Vereecke et al. 2005, n = 3 from two species; Payne et al. 2006a, n = 1, Hylobates lar). A more extensive quantitative anatomical dataset of gibbon hind limb anatomy is needed in order to obtain a better insight into gibbon morphology and locomotion.
A number of anatomical studies on various mammals have highlighted how gross anatomy can provide insight into muscular force production (Close, 1972; Alexander & Vernon, 1975; Maughan et al. 1983; Brand et al. 1986) and locomotor specialization. Payne et al. (2005) dissected fresh cadaveric hind limbs from seven horses and used macroscopic anatomical measurements [fascicle length (FL), muscle mass, etc.] and published values of maximum isometric stress and contraction velocity to estimate force production in the muscles and tendon stress during locomotion. In agreement with Alexander (1977), Alexander & Vernon (1975) and Alexander et al. (1981), they noted a proximal–distal decrease in muscle volume and FL with a simultaneous increase in tendon volume. Payne et al. (2006a) dissected apes from a number of species (bonobo, gibbon, gorilla and orang-utan), using similar techniques to Payne et al. (2005), and combined this with data on humans and chimpanzees from Thorpe et al. (1999). Muscle architecture data were shown to scale approximately allometrically (as predicted by Alexander, 1977). It was reported that the gibbon was the only ape with a substantial Achilles tendon, and hypothesized that it may be beneficial in returning elastic energy in bipedalism (a hypothesis that was substantiated by Vereecke et al. 2006b). The authors also noted that there was less ‘tapering of the limb distally’ in the African apes, relating this to a need to grasp with the feet.
Muscle architecture data on smaller cursorial quadrupeds (hares and greyhounds; Williams et al. 2007, 2008) also pointed to a prominent distal decrease in muscle volume and FL, linked to an increase in tendon volume. Muscle architecture and muscle moment arm data from a range of cadaveric Macropodoidea (kangaroos and wallabies; Bennett & Taylor, 1995; McGowan et al. 2008) suggest that muscle force scales allometrically with size but that tendon stress is larger in larger animals, reducing the safety factor (see below) and imposing a limit on body size for animals heavily dependent on elastic energy storage for efficient locomotion. The authors use these data to show that large (∼250 kg) extinct kangaroos were ‘likely very limited in locomotor capacity’. More recent studies have used sophisticated imaging techniques to gain insight into in-situ musculoskeletal properties (Miller et al. 2008).
The role of the hind limb in hind limb-dominated locomotion, such as bipedalism and leaping, is quite obvious. It is also likely, however, that it plays a role in powering brachiation through ‘leg-lift’, by which brachiating animals can convert metabolic energy to mechanical energy by lifting the legs during a swing (Preuschoft & Demes, 1984; Bertram & Chang, 2001; Usherwood & Bertram, 2003). This mechanism can be compared with a human using a playground swing, where lifting the legs at the bottom of the arc increases the height of the subsequent swing.
It has long been recognized that the shape (morphometry) of the limbs has a profound effect on the limb's centre of mass and so is an important factor in powering brachiation. Morphometry is also important for hind limb-dominated locomotion as swinging the limb forward incurs a metabolic cost, due to the inertia of the limb itself. This cost can be reduced if the limb is made to swing closer to its natural pendular frequency (NPF) (for a historical review and analysis of measurements of inertial properties see Steudel, 1990, 1996; Preuschoft & Witte, 1991; Preuschoft et al. 1992; Isler et al. 2006; Schoonaert et al, 2007). Shorter pendula swing more rapidly (as NPF = 1 ÷ [2π√l/g], where π and g are constants and l is pendulum length), so animals that swing their limbs faster than the NPF may gain some benefit by decreasing the effective length of their limbs. This reasoning has been cited as the explanation for a proximo-distal decrease in limb muscle mass observed for many animals (see above), particularly those with long or rapidly moving limbs such as cursorial mammals (dogs: Steudel, 1990; horses: Payne et al. 2005; greyhounds: Williams et al. 2008).
By utilizing many of the techniques seen above this study will quantify gibbon hind limb muscle architecture and investigate how the limb is adapted to cope with an extensive locomotor repertoire. Several previous studies (Thorpe et al. 1999; Payne et al. 2005, 2006a; Williams et al. 2007, 2008) did not take pennation angles into account when taking anatomical measurements because these could not be measured accurately and probably had little influence on the physiological cross-sectional area (PCSA). In our study, however, we will take pennation angles into account.
Materials and methods
Subject data
The material used in this study comprised 11 gibbon cadavers of known age and sex (Table 1). Specifically, we employed three white-handed gibbons (H. lar: L1–L3), two pileated gibbons (H. pileatus: P1 and P2), two moloch gibbons (H. moloch: M1 and M2) and four siamangs (Symphalangus syndactylus: S1–S4). All specimens were frozen until required for this study and were eviscerated prior to dissection. Specimens were obtained from The Royal Zoological Society of Antwerp (L1, L3 and S2) and The National Museums of Scotland, Edinburgh (L2, P1, P2, M1, M2, S1, S3 and S4). Most cadavers were eviscerated during post-mortem examination and body mass (prior to evisceration) was not available for all specimens. Therefore, hind limb muscle mass (HLMM) was used to normalize the animals for size. Unfortunately, the iliopsoas muscle was unavailable for dissection, because of its use in a prior study, and was not included in any of the analyses. Data from Payne et al. (2006a) were used to indicate where the iliacus muscle (part of the iliopsoas and an important hip flexor) would be positioned on a graph of PCSA against FL (see below and Table 1).
Table 1.
Details of the 11 gibbon cadavers used in this study
| Specimen | H. lar 1 | H. lar 2 | H. lar 3 | H. pileatus 1 | H. pileatus 2 | H. moloch 1 | H. moloch 2 | S. syndactylus 1 | S. syndactylus 2 | S. syndactylus 3 | S. syndactylus 4 | H. lar 4* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | M | F | M | F | M | M | F | F | F | M | F |
| Age at death (years) | 6 | 26 | 22 | 41 | 25 | 19 | – | 36 | – | 9.4 | > 10 | 16 |
| Mass at death (kg) | 6.3 | 10.6 | 6.5 | 5.2 | – | 5.8 | – | 11.6 | 12.5 | 8.5 | 10.1 | 4.6 |
| HLMM (kg) | 0.49 | 0.65 | 0.40 | 0.39 | 0.27 | 0.32 | 0.43 | 0.78 | 0.76 | 0.38 | 0.81 | 0.22 |
| Femur length (cm) | 20.1 | 22.0 | 20.5 | 21.0 | 20.5 | 19.6 | 20.5 | 21.5 | 19.0 | 21.5 | 20.3 | 18.3 |
| Tibia length (cm) | 19.2 | 18.8 | 16.9 | 18.5 | 19.0 | 17.1 | 19.8 | 21.4 | 21.2 | 20.9 | 19.1 | 15.8 |
| Foot length (cm) | – | 8.8 | 8.8 | 8.5 | 8.4 | 8.0 | 8.7 | 9.8 | 9.3 | 11.1 | 9.9 | 10.2 |
| Abbreviation | L1 | L2 | L3 | P1 | P2 | M1 | M2 | S1 | S2 | S3 | S4 | – |
HLMM, hind limb muscle mass; –, fields where information was unavailable.
H. lar 4 is taken from Payne et al. (2006a). All specimens were obtained post-autopsy and declared free of communicable diseases, although cause of death was not provided to us. Specimens were examined to verify that they were free from any musculoskeletal anomaly.
Scaling and functional muscle groups
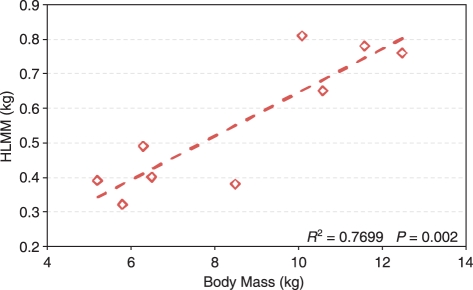
The data were normalized assuming geometric similarity (Alexander et al. 1981; Thorpe et al. 1999; Payne et al. 2006a; Williams et al. 2008). Because of post-mortem evisceration, body mass was unavailable for some subjects and so HLMM was used as a normalizing factor. Masses were scaled directly to HLMM, lengths to HLMM1/3 and areas to HLMM2/3. HLMM correlated significantly with body mass for the subjects where body mass was known (linear regression, P = 0.002, Fig. 1).
Fig. 1.
Hind limb muscle mass (HLMM) against body mass for the individuals where body mass was known. Dashed line shows linear regression.
For part of the analysis, muscles were categorized into functional groups, which are given in Table 2, together with the abbreviations used for the hind limb muscles used in the analyses. A weighted harmonic mean was used to calculate group averages of FL; this technique takes the mass of each muscle into account when calculating a mean (for a more detailed description see: Alexander et al. 1981; Thorpe et al. 1999; Payne et al. 2006a).
Table 2.
Functional muscle groups, their constituent muscles and abbreviations (Abr.)
| Thigh |
Shank |
||||
|---|---|---|---|---|---|
| Group | Constituent muscles | Abr. | Group | Constituent muscles | Abr. |
| Hip extensors | Gluteus superficialis | GSu | Plantarflexors | Gastrocnemius lateralis | GaL |
| Gluteus medius | GMe | Gastrocnemius medialis | GaM | ||
| Gluteus minimus | GMi | Soleus | Sol | ||
| Adductors | Adductor magnus | AdM | Tibialis posterior | TiP | |
| Adductor longus | AdL | Dorsiflexors | Tibialis anterior | TiA | |
| Adductor brevis | AdB | Digital flexors (and plantar flexors) | Flexor tibialis | FlT | |
| Pectineus | Pec | Flexor fibularis | FlF | ||
| Quadratus femoris | QuF | Digital extensors (and dorsiflexors) | Extensor hallucis longus | EHL | |
| Knee extensors | Rectus femoris | ReF | Extensor digitorum longus | EDL | |
| Vastus lateralis | VLa | Everters (and plantarflexors) | Peroneus longus | PeL | |
| Vastus intermedius | VIn | Peroneus brevis | PeB | ||
| Vastus medius | VMe | ||||
| Hip rotators | Obturator internus | ObI | |||
| Obturator externus | ObE | ||||
| Piriformis | Pir | ||||
| Knee flexors and hip extensors (hamstrings) | Semitendinosus | SeT | |||
| Semimembranosus | SeM | ||||
| Biceps femoris (both heads) | BFL/S | ||||
| Bi-articular knee and hip flexors | Gracilis | Gra | |||
| Sartorius | Sar | ||||
| Uni-articular knee flexor | Popliteus | Pop | |||
The plantaris muscle was inconsistently present and was not included in the analysis (see text); the iliopsoas was not available for dissection and was also excluded from the analysis.
Anatomical measurements
Hind limb muscles were removed systematically and measurements of isolated muscles were taken. Measurements of mass were taken to the nearest 0.1 g, using an electronic scale (Radwag, Poland, accurate to 0.01 g), whereas measurements of length were taken to the nearest 0.1 mm using a set of digital Vernier callipers (Mitutoyo, UK, accurate to 0.01 mm). Measurements included: muscle tendon unit (MTU) mass and MTU length, muscle belly length and mass. The muscle was then cut along its tendon in order to determine the orientation of the muscle fascicles and the length of internal tendon. FL was measured at three points along the muscle belly and the mean was calculated. Photographs of pennate muscles were taken using a digital camera (Nikon D40) so that the pennation angle (θ) could be measured using custom-written software (LabVIEW 8.2, National Instruments). The pennation angle was measured at 10 points along the muscle belly (to account for internal variation) and the mean was calculated. For muscles with an external tendon, the tendon was removed and a uniform section of known length was weighed; this weight was divided by section length and the density of mammalian tendon (1.12 g cm−3; Ker et al. 1988) in order to estimate the cross-sectional area. The tendon length (TL) was measured from its most proximal fibres (in the muscle) to its most distal fibres (insertion on the bone). Muscle function was estimated from the position of the muscle on the skeleton and its line of action.
Muscle physiological cross-sectional area and fascicle length
The PCSA of a muscle is affected by its pennation angle (Alexander, 1968; Burkholder et al. 1994; Thorpe et al. 1999; Payne et al. 2006a). It can be directly related to the maximum isometric force (FMAX) generating capacity by multiplying it by the maximum isometric stress of vertebrate skeletal muscle (0.3 MPa; Wells, 1965; Fukunaga et al. 2001; Medler, 2002). Although this method is commonly used in functional anatomy it should be noted that the value of maximum isometric stress has been shown to vary between muscles of mammalian species (0.1–0.3 Mpa, Josephson, 1993; Hiroyuki et al. 1996) and so caution should be exercised when making hypotheses based on estimations of FMAX.
The PCSA was estimated using:
where m is the muscle belly mass, ρ is muscle density (1.06 g cm−3; Mendez & Keys, 1960) and l is the muscle FL. Previous studies (Thorpe et al. 1999; Payne et al. 2006a) have observed that pennation angles are close to 20° in most ape limb muscles, suggesting that θ has little effect on the PCSA [as Cos(20°) ≈ 1]. However, pennation angle was included in our calculation of PCSA because the pennation angle of many gibbon hind limb muscles exceeded 20° (maximum θ = 39°, implying a 22% reduction in PCSA), which was considered substantial enough to be taken into account.
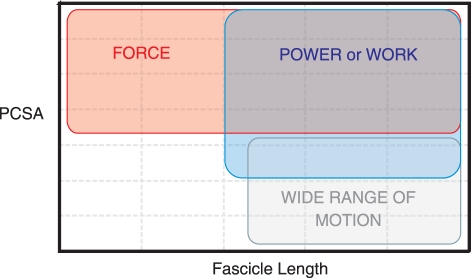
Muscle function can be estimated by the position of the muscle or muscle group on a graph of PCSA against FL (Fig. 2; see also Williams et al. 2008). Muscles at the top of the graph, with high PCSA, can produce high levels of force (as PCSA is directly related to FMAX). The maximum contraction distance is proportional to FL, so muscles on the right-hand side of the graph, where FL is high, can contract over a wide range of motion. Muscles with both high PCSA and long fascicles are capable of producing high levels of work (force × distance); these sit in the middle or at the top on the right-hand side of the graph. Contraction velocity, and therefore power (as power = work ÷ time), can also be said to increase with FL (Zajac, 1989) but only where all other variables are fixed, e.g. of two muscles with identical physiological properties but different lengths, the longer muscle should contract more rapidly (and hence produce more power) as they have a greater number of sarcomeres in series (Zajac, 1989, 1992). In reality, however, the muscle fibre type has a much greater influence on contraction velocity and a myriad of other factors have a profound effect on muscle power output (temperature: Marsh & Bennett, 1986; activation pattern: Biewener, 1998; muscle fibre type: Altringham & Johnson, 1990; Widrick et al. 1996; architectural gear ratio and pennation angle: Alexander, 1996; Azizi et al. 2007).
Fig. 2.
Muscle function estimated by position on a graph of physiological cross-sectional area (PCSA) against fascicle length.
Muscles on the bottom right-hand side of the graph, with low PCSA and high FL, produce a modest force over a wide range of motion. Finally, the function of muscles on the bottom of the left-hand side of the graph (low PCSA, short fascicles) is debatable. Their function may be related to the stabilization of joints (Williams et al. 2008), they may be used for precision movements or it may simply be that this is the ‘default’ position of unspecialized muscle and muscles specialized for force, power or work deviate from this.
Tendon function
The function of a tendon can be estimated from its gross morphology, where long thin tendons are indicative of elastic energy storage (‘compliant’ MTU) and short thick tendons imply large amounts of muscular contraction and therefore work (i.e. ‘stiff’ MTUs; Ker et al. 1988; Williams et al. 2007; McGowan et al. 2008). The safety factor is an index that links the force-producing capabilities of the muscle to the force-resisting capabilities of the tendon. MTUs with a large PCSA : tendon cross-sectional area (TCSA) ratio can place the tendon under large amounts of stress, eliciting large tendon strain and therefore enabling elastic energy storage. MTUs with a relatively smaller PCSA : TCSA ratio have a lower propensity to stretch the tendon and, hence, are less likely to be associated with elastic energy storage. The safety factor gives an indication of how close the tendon comes to rupture when the muscle undergoes FMAX. A safety factor of 1 implies that if the muscle contracts with FMAX this will be just enough to cause the tendon to fail, whereas a safety factor of 2 suggests that FMAX is half of the force required to rupture the tendon, etc. We estimated the safety factor as follows:
 |
where the maximum tendon stress is 100 MPa (again, this value has been shown to vary between species; Pollock & Shadwick, 1994) and the maximum isometric stress of skeletal muscle is 0.3 MPa (Wells, 1965; Medler, 2002).
A second estimate of tendon function can be made by devising a ratio of TL : FL. Muscles with long fascicles and short tendons possess a large amount of control over the tendon as the fascicles can negate any tendon strain by muscular contraction. In this context, muscles with low TL : FL ratios can be termed ‘stiff’. MTUs with short fascicles and long tendons (high TL : FL) are less able to contract to negate tendon strain because of the relatively shorter fascicles. These MTUs can be termed ‘compliant’ and are more likely to be associated with elastic energy storage. The TL : FL ratio was calculated using:
By including the pennation angle (θ) in our results we calculate an ‘effective fascicle length’ (EFL) by which we divide TL to give a ratio of TL : EFL. In muscles with parallel fibres θ was 0.
Results
Descriptive anatomy
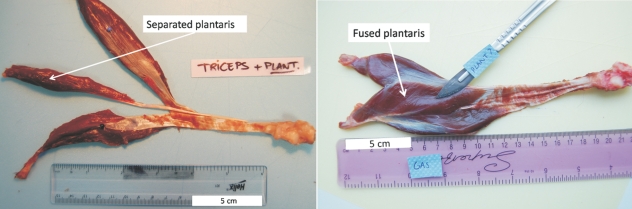
There were few qualitative differences in organization of the hind limb musculature between the different species and between individuals of the same species. One obvious variation was the presence of a plantaris muscle, which was either absent (4 of 11 specimens), completely or partially fused with the lateral head of the gastrocnemius (5/11), or completely separate (2/11, Fig. 3). When present, the thin tendon ran at the medial side of the Achilles tendon and inserted separately on the posterior side of the tuber calcanei.
Fig. 3.
Photographs showing the presence of separated (specimen H. lar 2) and fused (specimen S. syn 1) plantaris muscles.
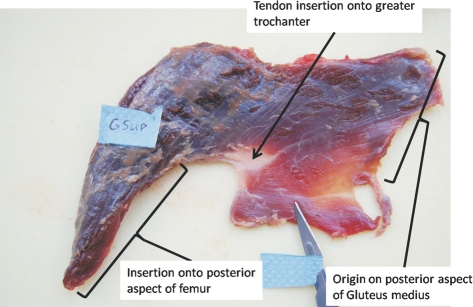
The gluteus superficialis (called gluteus maximus in humans) was irregularly shaped and had fascicles that were orientated in different directions relative to the insertion tendon (Fig. 4). The muscle had a thin, sheet-like origin, originating on the posterior side of the gluteus medius, across the width of the ilium. It became progressively thicker around the hip joint. A small portion of the belly inserted with an internal tendon onto the greater trochanter and another small part passed down the lateral side of the hip until the proximal end of the femoral diaphysis where in some specimens it was associated with a thickened fascia, probably homologous to the tensor fascia lata in humans. Most of the muscle belly passed posterior to the hip and inserted directly onto the posterior aspect of the femur, without a tendon.
Fig. 4.
The gluteus superficialis muscle, with indication of thigh insertion and tensor fascia lata.
The adductor muscles were fused to varying degrees in all of the specimens, making identification and separation difficult. The adductor longus was quite distinct and the easiest to identify, whereas the adductor brevis was very difficult to identify as a separate muscle and was therefore treated as part of the adductor magnus in the analyses.
A detailed qualitative description of the hind limb anatomy of gibbons has been published previously (Bischoff, 1870; Kanagsuntheram, 1952; Sigmon & Farslow, 1986; Vereecke et al. 2005) and we refer to those publications for a full anatomical description of the gibbon hind limb musculature. Mean anatomical data are presented for each species in Table 3 and Appendix 1.
Table 3.
Mean ( ) anatomical measurements with standard deviations (σ) for each species
) anatomical measurements with standard deviations (σ) for each species
|
H. lar |
H. pileatus |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fascicle length (cm) |
θ (°) |
PCSA (cm2) |
Fascicle length (cm) |
θ (°) |
PCSA (cm2) |
|||||||
| Muscle |  |
σ |  |
σ | 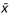 |
σ | 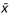 |
σ | 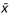 |
σ | 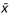 |
σ |
| THIGH | ||||||||||||
| Gluteus superficialis | 9.7 | 0.3 | – | – | 6.0 | 1.3 | 8.1 | 0.5 | – | – | 4.4 | 0.4 |
| Gluteus medius | 3.8 | 0.7 | 24.6 | – | 8.3 | 1.2 | 3.8 | 0.2 | 27.6 | – | 6.1 | 1.1 |
| Gluteus minimus | 2.8 | 0.3 | – | – | 2.5 | 1.3 | 3.2 | – | – | – | 2.1 | – |
| Pectineus | 4.8 | 0.2 | p | p | 0.6 | 0.2 | 5.1 | 0.4 | p | p | 0.5 | 0.0 |
| Obturator internus | 2.2 | 0.8 | 30.9 | – | 3.3 | 1.1 | 1.3 | 0.1 | – | – | 4.5 | 0.3 |
| Obturator externus | 4.0 | 3.4 | – | – | 2.0 | 1.1 | 2.0 | – | – | – | 2.2 | – |
| Piriformis | 4.2 | 0.4 | 28.5 | – | 1.4 | 0.1 | 2.7 | 0.1 | – | – | 1.8 | 0.1 |
| Adductor magnus | 13.9 | 1.7 | 29.1 | 5.8 | 4.6 | 1.3 | 12.5 | 0.9 | – | – | 3.2 | 1.3 |
| Adductor longus | 8.0 | 0.6 | 22.8 | – | 0.7 | 0.2 | 9.8 | 2.9 | – | – | 0.9 | 0.4 |
| Adductor brevis | 9.0 | – | p | p | 1.1 | – | 5.6 | – | p | p | 0.6 | – |
| Quadratus femoris | 3.4 | 0.6 | p | p | 1.4 | 0.4 | 3.3 | – | p | p | 0.7 | – |
| Rectus femoris | 3.9 | 0.3 | 21.0 | 0.3 | 3.9 | 0.3 | 3.2 | 0.2 | 17.2 | 0.1 | 2.8 | 0.2 |
| Vastus medialis | 4.6 | – | 25.5 | 0.3 | 3.8 | – | 3.4 | 0.1 | 21.8 | – | 6.5 | 2.6 |
| Vastus intermedius | 3.6 | 0.8 | 25.9 | 4.9 | 10.0 | 1.5 | 3.0 | – | 17.5 | – | 10.9 | – |
| Vastus lateralis | 4.3 | 0.5 | 30.7 | 5.3 | 11.9 | 1.4 | 3.2 | 0.1 | 22.3 | 3.8 | 10.3 | 2.4 |
| Gracilis | 19.2 | 0.9 | p | p | 0.5 | 1.4 | 16.9 | 4.2 | p | p | 0.4 | 0.0 |
| Sartorius | 21.4 | 1.7 | p | p | 0.7 | 0.8 | 21.5 | 1.1 | p | p | 0.4 | 0.1 |
| Semimembranosus | 11.3 | 1.8 | 29.4 | – | 0.7 | 0.1 | 9.0 | 0.3 | 18.2 | – | 0.9 | 0.1 |
| Semitendinosus | 18.0 | 2.3 | p | p | 0.8 | 0.2 | 16.5 | 4.3 | p | p | 0.5 | 0.1 |
| Biceps femoris (long head) | 7.6 | 2.9 | p | p | 1.1 | 0.1 | 7.5 | 0.3 | p | p | 1.0 | 0.1 |
| Biceps femoris (short head) | 3.8 | 1.2 | – | – | 1.7 | 0.3 | 4.1 | – | 15.3 | – | 1.0 | – |
| SHANK | ||||||||||||
| Tibialis anterior | 4.4 | 1.2 | 26.1 | 10.8 | 1.6 | 0.2 | 3.3 | 0.0 | 12.9 | – | 1.5 | 0.1 |
| Extensor digitorum longus | 4.9 | 2.2 | 15.7 | 2.3 | 0.8 | 0.2 | 4.3 | 0.3 | 16.1 | – | 0.7 | 0.1 |
| Extensor hallucis longus | 5.5 | 1.1 | 14.6 | 2.4 | 0.4 | 0.0 | 5.8 | 0.3 | – | – | 0.3 | 0.0 |
| Peroneus longus | 1.9 | 0.1 | 23.5 | 4.0 | 2.6 | 0.2 | 1.6 | 0.7 | 18.4 | – | 1.5 | 1.0 |
| Peroneus brevis | 1.5 | 0.2 | 24.9 | – | 0.9 | 1.2 | 1.4 | 0.2 | – | – | 0.9 | 0.2 |
| Soleus | 2.6 | 0.1 | 27.7 | 0.3 | 3.4 | 0.3 | 2.6 | 0.2 | 23.0 | 5.4 | 1.7 | 0.2 |
| Gastrocnemius medialis | 3.0 | 0.4 | 27.4 | – | 3.5 | 1.0 | 3.0 | 0.4 | – | – | 2.0 | 0.3 |
| Gastrocnemius lateralis | 3.4 | 0.1 | – | – | 4.3 | 0.3 | 3.2 | 0.0 | 26.6 | – | 3.2 | 0.4 |
| Tibialis posterior | 1.7 | 0.4 | 20.2 | – | 3.1 | 0.7 | 1.3 | 0.7 | 27.9 | – | 3.7 | 0.9 |
| Flexor tibialis | 2.9 | 0.4 | 21.8 | – | 1.8 | 0.6 | 2.3 | 0.9 | 17.3 | 1.1 | 1.6 | 0.8 |
| Flexor fibularis | 3.8 | 0.2 | 30.1 | 2.0 | 4.5 | 1.1 | 3.8 | 0.2 | 23.3 | 2.7 | 3.1 | 0.2 |
| Popliteus | 1.6 | 0.2 | 29.9 | – | 1.7 | 0.2 | 1.4 | 0.1 | 24.3 | 3.2 | 2.0 | 0.3 |
|
H. moloch |
S. syndactylus |
|||||||||||
| Fascicle length (cm) |
θ (°) |
PCSA (cm2) |
Fascicle length (cm) |
θ (°) |
PCSA (cm2) |
|||||||
| Muscle | 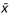 |
σ | 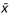 |
σ | 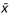 |
σ | 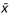 |
σ | 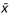 |
σ | 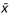 |
σ |
| THIGH | ||||||||||||
| Gluteus superficialis | 7.4 | 1.6 | – | – | 5.2 | 0.3 | 9.0 | 3.2 | – | – | 9.3 | 4.1 |
| Gluteus medius | 3.6 | 1.4 | 29.1 | 5.0 | 5.8 | 0.7 | 4.2 | 1.1 | 28.4 | 4.3 | 10.1 | 1.3 |
| Gluteus minimus | 3.3 | 1.4 | – | – | 1.7 | 1.1 | 4.0 | 1.5 | 20.5 | 3.5 | 2.0 | 0.9 |
| Pectineus | 4.2 | 0.4 | p | p | 0.5 | 0.3 | 6.2 | 0.4 | p | p | 1.0 | 0.1 |
| Obturator internus | 1.8 | 0.0 | – | – | 2.6 | 1.4 | 3.1 | 0.4 | 35.7 | 5.3 | 3.6 | 1.3 |
| Obturator externus | 1.8 | 0.4 | – | – | 2.2 | 0.3 | 3.1 | 0.4 | 30.9 | 0.4 | 3.6 | 0.7 |
| Piriformis | 4.2 | 0.5 | – | – | 0.8 | 0.3 | 3.9 | 1.3 | – | – | 1.9 | 0.5 |
| Adductor magnus | 10.9 | 1.5 | 24.7 | – | 4.1 | 0.3 | 12.2 | 0.8 | 32.3 | 3.7 | 6.7 | 2.0 |
| Adductor longus | 7.5 | 0.3 | – | – | 0.6 | 0.0 | 8.9 | 1.3 | – | – | 1.2 | 0.2 |
| Adductor brevis | 6.2 | – | p | p | 0.3 | – | 7.4 | 1.2 | p | p | 0.9 | 0.8 |
| Quadratus femoris | 3.6 | 0.0 | p | p | 0.9 | 0.3 | 4.8 | 1.1 | p | p | 1.6 | 0.6 |
| Rectus femoris | 3.0 | 0.9 | 24.7 | 7.6 | 2.8 | 0.2 | 5.0 | 0.4 | 18.9 | 7.4 | 4.0 | 1.3 |
| Vastus medialis | 4.3 | 2.4 | – | – | 3.4 | 1.2 | 4.6 | 1.5 | 18.5 | 3.6 | 4.8 | 1.5 |
| Vastus intermedius | 3.4 | – | – | – | 4.6 | – | 3.8 | 0.4 | 22.4 | – | 12.3 | 3.3 |
| Vastus lateralis | 4.1 | 1.2 | 21.2 | 1.1 | 5.5 | 3.3 | 5.0 | 0.5 | 27.3 | 8.1 | 9.7 | 1.6 |
| Gracilis | 16.1 | 2.2 | p | p | 0.5 | 0.0 | 19.6 | 2.0 | p | p | 1.0 | 0.5 |
| Sartorius | 18.9 | 0.6 | p | p | 0.6 | 0.1 | 20.8 | 1.3 | p | p | 1.0 | 0.4 |
| Semimembranosus | 8.2 | 1.9 | 26.1 | – | 0.9 | 0.0 | 9.9 | 1.0 | 23.9 | 5.0 | 1.3 | 0.5 |
| Semitendonosus | 14.0 | 0.3 | p | p | 0.8 | 0.1 | 17.4 | 2.9 | p | p | 1.5 | 0.6 |
| Biceps femoris (long head) | 8.5 | 1.9 | p | p | 1.0 | 0.0 | 9.0 | 2.0 | p | p | 1.9 | 0.9 |
| Biceps femoris (short head) | 3.6 | 0.5 | – | – | 1.2 | 0.0 | 6.8 | 3.3 | – | – | 1.5 | 1.0 |
| SHANK | ||||||||||||
| Tibialis anterior | 3.0 | 0.2 | 24.6 | – | 1.8 | 0.4 | 4.5 | 1.4 | 20.9 | 2.9 | 3.1 | 0.8 |
| Extensor digitorum longus | 3.7 | 0.1 | 17.7 | – | 0.8 | 0.2 | 5.8 | 1.2 | 15.8 | 4.6 | 1.0 | 0.4 |
| Extensor hallucis longus | 5.2 | 0.1 | – | – | 0.3 | 0.0 | 5.4 | 1.3 | 17.3 | 5.5 | 0.6 | 0.3 |
| Peroneus longus | 1.7 | 0.5 | 19.6 | – | 2.2 | 0.1 | 3.2 | 0.7 | 21.2 | 2.5 | 2.8 | 1.0 |
| Peroneus brevis | 1.2 | 0.2 | – | – | 1.4 | 0.2 | 2.0 | 0.5 | 20.7 | 1.6 | 1.5 | 0.7 |
| Soleus | 2.3 | 0.3 | 24.8 | – | 3.0 | 0.5 | 3.3 | 0.4 | 23.2 | 0.9 | 4.9 | 1.5 |
| Gastrocnemius medialis | 3.2 | 0.1 | 25.3 | 1.1 | 1.8 | 0.6 | 3.9 | 0.6 | 24.9 | – | 4.9 | 1.8 |
| Gastrocnemius lateralis | 3.6 | 0.0 | 22.5 | – | 2.7 | 0.8 | 4.1 | 1.0 | – | – | 5.9 | 0.8 |
| Tibialis posterior | 1.6 | 0.3 | – | – | 2.8 | 0.0 | 2.7 | 1.3 | 28.4 | 12.6 | 3.4 | 1.0 |
| Flexor tibialis | 2.5 | 1.2 | 20.5 | – | 1.1 | 0.2 | 4.7 | 1.9 | 21.3 | 2.2 | 2.5 | 1.3 |
| Flexor fibularis | 4.2 | 0.8 | – | – | 2.3 | 0.9 | 5.9 | 0.5 | 24.6 | 1.8 | 3.4 | 0.8 |
| Popliteus | 1.2 | 0.2 | 32.4 | – | 1.8 | 0.4 | 2.6 | 1.9 | 19.8 | 4.6 | 3.1 | 1.2 |
PCSA, physiological cross-sectional area; –, data were unavailable; p, parallel fascicled muscle.
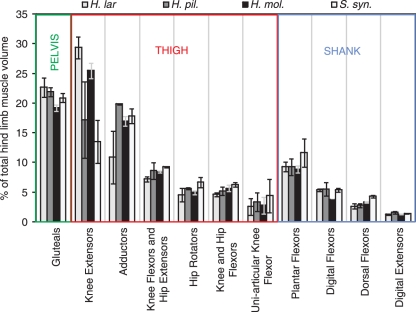
Hind limb muscle volume
The gluteals (hip extensors), adductors and quadriceps (knee extensors) muscles made up the majority of the hind limb muscle volume (HLMV), together accounting for 58 ± 4% of the total volume (Fig. 5). The lar and moloch gibbons both had larger quadriceps than gluteals (lar: 29% vs. 23% of HLMV; moloch: 26% vs. 19% of HLMV for quadriceps vs. gluteals, respectively), a trend not seen in the pileated gibbon or siamang (pileated: 19% vs. 22% of HLMV; siamang: 14% vs. 21% of HLMV for quadriceps vs. gluteals, respectively). There were few other interspecific differences in muscle volume make-up. The adductor group was the third largest functional muscle group in all of the species, despite including the adductor magnus, which was the largest single hind limb muscle (regardless of whether it was fused with the other adductors) in all species (14.6 ± 0.2% of HLMV). The largest muscle group on the distal limb segment was the plantarflexor group (9.8 ± 1.4% of HLMV), consisting of the triceps surae. The other muscle groups on the shank made up 5% or less of HLMV.
Fig. 5.
The contribution of each functional group to total hind limb muscle volume and the position of each group on the skeleton. Error bars denote the standard error of the mean.
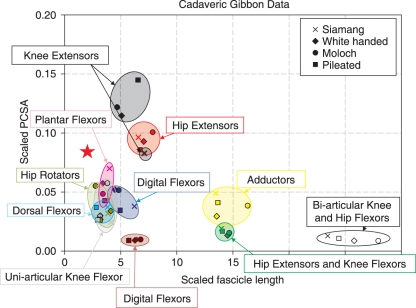
Muscle physiological cross-sectional area and fascicle length
The gibbon hip (gluteals) and knee (quadriceps) extensors showed a relatively higher PCSA and relatively shorter fascicles than the other functional muscle groups of the hind limb (Fig. 6). The knee extensors of the siamang had a relatively lower PCSA than the other gibbon species, suggesting that the quadriceps of the siamangs have a lower propensity for force production. The muscles with the longest fascicles were the bi-articular knee and hip flexors (i.e. sartorius and gracilis), which had a small PCSA. There were no muscles with both high PCSA and long fascicles. The majority of muscle groups (plantarflexors, dorsiflexors, knee flexors, digital flexors, hip rotators and digital extensors) had short fascicles and relatively small PCSAs, putting them on the lower left-hand side of Fig. 6.
Fig. 6.
Plot of physiological cross-sectional area (PCSA) (scaled to hind limb muscle mass – HLMM2/3) against fascicle length (scaled to HLMM1/3) for gibbon hind limb muscles. Different colours represent different muscle groups: black, knee extensors; red, hip extensors; blue, digital flexors; yellow, adductors; green, hip extensors and knee flexors; mauve, digital extensors; grey, uni-articular knee flexor; cyan, dorsal flexors; pink, plantar flexors; olive, hip rotators; open symbols, bi-articular knee and hip flexors. Different symbols represent different species: cross, siamang; diamond, lar gibbon (white-handed gibbon); circle, moloch gibbon; square, pileated gibbon. The red star represents the position of the iliacus muscle from Payne et al. (2006).
The iliacus muscle taken from Payne et al. (2006a) had relatively short fascicles and an intermediate PCSA, positioning it at the middle/left-hand side of the graph (red star, Fig. 6).
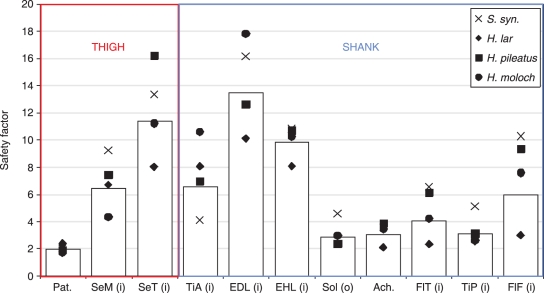
Tendon anatomy
The tendons of the hind limb muscles of gibbons display a range of safety factors, implying varying tendon function throughout the hind limb (Fig. 7). The knee flexors and hip extensors (semimembranosus and semitendinosus), dorsal flexors (tibialis anterior), digital extensors (extensor digitorum longus and extensor hallucis longus) and digital flexors (flexor tibialis and flexor fibularis) all had safety factors above 4 (semimembranosus, 6.4; semitendinosus, 11.4; tibialis anterior, 6.6; extensor digitorum longus, 13.5; extensor hallucis longus, 9.8; flexor tibialis, 4.1; flexor fibularis, 6.0). Tendons with lower safety factors include the patellar tendon of the quadriceps (Pat., 2.2) and the Achilles tendon of the triceps (Achilles, 3.1), as well as the tendon of origin of the soleus (2.9) and the tendon of insertion of tibialis posterior (3.1). The safety factor was highly variable between species, and no pattern was observed suggesting that one species had consistently higher or lower safety factors than any other.
Fig. 7.
Estimated safety factors for tendons in the hind limb. i, insertion tendon; o, tendon of origin; Pat., Patellar; Ach., Achilles. Bars represent the mean of all species. Symbols represent the mean from each species. See Table 2 for muscle name abbreviations.
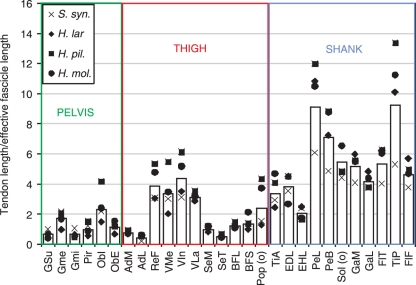
Generally, MTUs on the pelvis and thigh had TL : EFL ratios of around or less than 1 (Fig. 8). There was one notable exception to this: the quadriceps had relatively longer tendons and more pennate and shorter fascicles than other muscles on the thigh (e.g. adductor magnus), giving a higher TL : EFL ratio (interspecific means of 3.87, 3.84, 4.36 and 3.12 for rectus femoris, vastus medius, vastus intermedius and vastus lateralis, respectively vs. 0.88 interspecific mean for all other thigh muscles). Muscles on the distal limb segment (shank and foot) had higher TL : EFL ratios than MTUs on the hip and thigh (interspecific mean for all muscles: pelvis, 0.88; thigh, 1.99; shank, 5.15). The highest TL : EFL ratios were seen in tibialis posterior and peroneus longus (interspecific means of 9.23 and 9.13, respectively).
Fig. 8.
Tendon length divided by effective fascicle length (see text for calculation) for all muscles with an appreciable tendon in the hind limb. Bars represent inter-species mean. Symbols represent individual species means. See Table 2 for muscle name abbreviations.
Discussion
Gluteus superficialis and adductor magnus
The morphology of the gluteus superficialis was similar to that described by Sigmon & Farslow (1986) and Stern (1972), where the authors correlated specific regions of the muscle with human equivalents (e.g. the presence of a pars tensorica is hypothesized as an equivalent of tensor fascia lata). The gibbon's gluteus superficialis had a similar morphology to that of the African great apes (Sigmon & Farslow, 1986; Payne et al. 2006a). Because the muscle is made up of several functional parts with varying fibre orientations, its function is likely to be diverse. The position of the muscle in gibbons (i.e. mainly posterior to the hip joint) suggests that its main role is hip extension, although it may also play some role in abduction and/or stabilizing the hip joint because it also covers the lateral aspect of the hip joint.
The high degree of fusion in the adductor muscles observed in most of our gibbon specimens has not been described by other authors (Sigmon & Farslow, 1986; Payne et al. 2006a). All of the adductor muscles appear to perform the same role (thigh adduction) and probably work together, allowing varying degrees of fusion.
The unpredictability of the presence of a plantaris muscle in gibbons has also been documented by other authors (Sigmon & Farslow, 1986; Vereecke et al. 2005). This muscle is known to be vestigial in many ape species (Sigmon & Farslow, 1986). The plantaris is a dedicated plantarflexor and inverter of the foot, yet its small size and variable presence suggest that it is of minor importance for foot motion.
Muscle volume: adaptations for vertical climbing, orthograde clambering and leaping?
The gluteals, quadriceps and adductors made up the majority of the HLMV of gibbons. Having large (voluminous) muscles in these areas might give insight into the specialization of the gibbon hind limb. Gluteals and quadriceps are hip and knee extensors, respectively, which might be important in several activities such as bipedalism, vertical climbing and leaping. The volume of muscle dedicated to knee and hip extension in the gibbon is likely to be useful for some or all of these activities. Moreover, having a large proportion of muscle mass situated proximally (hip and thigh) will minimize the inertia of the swinging limb during locomotion, thus reducing metabolic work (Steudel, 1996 and see below for further discussion).
Adductor muscles are traditionally associated with keeping the limbs underneath the body (Alexander, 1996). During vertical climbing and orthograde clambering the limbs are often outside the projection of the body's centre of gravity. Therefore, arboreal animals should possess enlarged adductor muscles to cope with the increased muscular demand of such activities (Preuschoft, 2002; Isler, 2005). In gibbons, in which these modes form up to 35% of locomotion (Fleagle, 1976), the adductors made up a similar proportion of the HLMV as reported for other non-human apes (interspecific mean; gibbons: 16.4 ± 5.5% HLMV; bonobos: 18.5%; chimpanzee: 18.8%; gorilla: 22.5%; orang-utan: 16.7%; data on great apes from Thorpe et al. 1999 and Payne et al., 2006a), suggesting that limb adduction has a similar importance across the non-human apes. Humans have significantly smaller adductor muscles (≈ 7% of HLMV, based on estimates from Thorpe et al. 1999 and assuming that each hind limb in humans makes up 19% of the total body mass; Zihlman, 1992), which is probably associated with osteological adaptations to bipedal walking, e.g. the bicondylar or valgus angle of the knees (i.e. medio-distal inclination of the femur, Jones et al. 1992).
Functional implications of the gibbon's hind limb anatomy
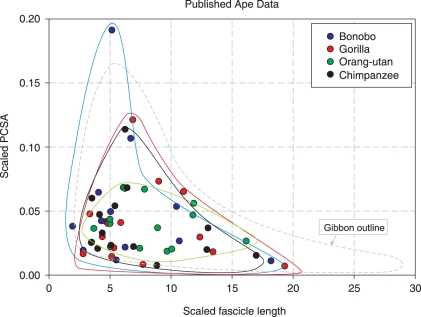
The short-fascicled, large-PCSA anatomy of the hip and knee extensors implies that the muscles are suitable for high muscular force production but not for exerting much muscular work. It is possible that gibbons use high levels of force (as distinct from power) for a number of activities, including the dissipation of energy during landing. In this case, the muscles have to work eccentrically to decelerate the gibbon during landing, reducing the magnitude of the forces associated with landing, although our estimations suggest that the patellar tendon would be close to rupture during maximal eccentric loading (see ‘Tendon anatomy: elastic energy storage or ideal mass distribution?’ below and Westing et al. 1991; Demes et al. 1999). However, the short-fascicled large PCSA hip and knee extensors may still be able to produce high levels of power at the joint by means of a power-amplifying mechanism, as observed in several primate genera (Galago: Aerts, 1998; bonobo: Scholz et al. 2006). Power amplifiers usually take one of two forms; some galagos are very proficient leapers and use a tendinous mechanism, where the patellar tendon stores elastic strain energy during pre-stretch, which is released rapidly prior to push-off, amplifying power generation (Alexander, 1995; Aerts, 1998). Bonobos utilize short-fascicled hip and knee extensors (Payne et al. 2006a) coupled with small muscle moment (lever) arms at the hip and knee joints (Payne et al. 2006b) to turn relatively small fascicular contractions into relatively large joint movements (see also Alexander, 1995; Fukunaga et al. 2001). Recent research has shown that bonobos are expert leapers and it is suggested that they use this ‘amplifying’ mechanism for propulsion generation in leaping (Scholz et al. 2006). Our results indicate that gibbons, which are also very able leapers (Fleagle, 1976; Gittins, 1983), have hip and knee extensors that fall into positions on a PCSA against FL graph that are similar to bonobos (Fig. 9). This leads us to hypothesize that both gibbons and bonobos may use a similar mechanism, coupling their short-fibred, large PCSA hip and knee extensors to short muscle moment arms, in order to enhance leaping performance. It is interesting to note that none of the other apes, of which none are remarkable jumpers, have hind limb muscles with similar relative PCSAs as the bonobo or gibbon. Also, although all of the published data (Fig. 9) are scaled in the same way as our gibbon data, these take no account of pennation angle, meaning that the gap between the gibbon's and bonobo's relative PCSA is probably exaggerated, which further strengthens our hypothesis.
Fig. 9.
Relative physiological cross-sectional area (PCSA) against relative fascicle length for all non-human ape species (see text for scaling parameters). Data for chimpanzee are from Thorpe et al. (1999), data from bonobo, gorilla and orang-utan are from Payne et al. (2006a).The coloured shapes visualize the position of the muscles of each species. Blue, bonobos; grey dotted, gibbon; red, gorilla; black, common chimpanzee; green, orang-utan.
Within gibbons, the knee extensors of the siamang have a relatively smaller PCSA than those of other gibbons (Fig. 6). If knee extensors are indeed used to power leaping in gibbons this would suggest that siamangs are less adept or less frequent leapers than the other gibbon species. In support of this, field reports (Fleagle, 1976) indicate that siamangs indeed spend proportionally less time leaping than other species of gibbon (6% vs. 15% for lar gibbons; see ‘Interspecific differences’ below for further discussion).
The bi-articular hip and knee flexors (gracilis and sartorius) in gibbons have relatively longer fascicles than those of any of the non-human ape species (Fig. 9), which may reflect a higher propensity for positioning the hind limb in a wider range of postures. The rapid locomotion of the gibbons through an unstable three-dimensional environment may mean that being able to move the limbs over a wide range of motion has advantages in reaching a branch and avoiding a fall. They are also vertical climbers and orthograde clamberers; it is likely that limb placement is highly variable during this form of locomotion and long-fascicled muscles should provide some aid to this. Indeed, the long-fascicled, low-PCSA muscles of the hind limb of orang-utans (where orthograde clambering is a major activity; Thorpe & Crompton 2005, 2006) have been attributed to varied limb placement during orthograde clambering by Hunt et al. (1996) and Payne et al. (2006a), although when scaled to mass1/3 the FLs do not seem extraordinary in comparison to other ape species (Fig. 9). The hind limb position is also thought to be important in powering brachiation (Bertram & Chang, 2001) where ‘leg-lift’ raises the centre of mass during the swing phase, resulting in an increase in mechanical energy or a reduction in collisional energy loss on the next swing (Usherwood & Bertram, 2003). Long-fascicled muscles allow a greater range of hind limb motion, enabling a greater upward displacement of the body's centre of mass during the swing and a greater mechanical energy benefit for brachiating gibbons. Hip flexors probably play an important role in this leg-lift. However, data on a major hip flexor, the iliopsoas, were not available, which means that we have underestimated the mass of muscle used to flex the hip and the force involved in these movements. Published data indicate that the iliacus (part of the iliopsoas) is short fascicled with a large PCSA in comparison with the other hip flexors (i.e. rectus femoris, sartorius and gracilis), suggesting that the muscle is unlikely to increase the range of motion of the hip significantly but that it will increase the amount of force available for leg-lift (Fig. 6).
Tendon anatomy: elastic energy storage or ideal mass distribution?
Overall, the tendons in the distal hind limb segment were relatively longer (with respect to FL; high TL : EFL) than those in the proximal limb segment (Fig. 8 and Appendix 1). Longer tendons allow muscle force to be transmitted to the distal limb without the burden of extra muscle mass placed distally, which is detrimental to efficient locomotion (through increased limb inertia; Steudel, 1996). Long tendons also allow short-fascicled muscles to produce force more efficiently by combining isometric muscle contraction with tendon strain, thus keeping the muscle fascicles at optimum length for efficiency (Alexander, 1996). The thickness of tendons with respect to PCSA is shown by the safety factor, where relatively thick tendons have a high safety factor and thinner tendons have a lower safety factor.
One method of power amplification for muscles is the sudden release of elastic energy previously stored in a tendon (Alexander, 1995; Aerts, 1998) and the safety factor can be used to estimate whether this is likely to be the case. The safety factors of the tendons in the gibbon hind limb varied greatly, suggesting different functions (Fig. 7). The lowest safety factor, and hence highest potential tendon stress, was found for the patellar tendon, suggesting that it may be used for elastic energy storage. As the patella tendon is associated with the knee extensors (quadriceps), a low safety factor in this tendon supports the hypothesis that leaping may be powered by a tendinous mechanism. This hypothesis is further supported by the relatively long tendons and relatively short fascicles of the vasti and rectus femoris (Fig. 8), suggesting a relatively ‘compliant’ MTU. Interestingly, our estimations of the safety factor are based on maximum isometric stress, which may be exceeded during eccentric loading (e.g. during cyclical locomotion or landing), further reducing the safety factor (Ker et al. 1988; Westing et al. 1991) and potentially making the patellar tendon vulnerable to rupture under high eccentric loads.
The perceived ‘compliance’ (based on a high TL : EFL) of the MTUs in the distal hind limb could simply be a by-product of minimizing inertia in the distal limb. By using the TL : EFL in combination with the safety factor we can gain some insight into whether or not the distal MTUs may be used to store elastic strain energy or are merely a by-product of limb inertia optimization. The Achilles tendon in gibbons has a very low safety factor and a high TL : EFL ratio, which is due to the remarkably long length of the Achilles tendon in gibbons compared with that of other non-human apes (Payne et al. 2006a; Vereecke & Aerts, 2008). A compliant triceps MTU may play a number of roles during gibbon locomotion including energy storage during bipedalism or leaping (Vereecke et al. 2006b; Vereecke & Aerts, 2008). Alternatively, it may be used to transfer force to the distal limb from the powerful vasti, as in the Galago (Aerts, 1998). It is difficult to know from gross anatomy alone what the function of the Achilles tendon is, especially as it is likely to have a variety of roles given the gibbon's extensive locomotor repertoire. However, the large PCSA of the triceps surae suggests a significant role in hind-limb-dominated locomotion. Further data on tendon properties (Young's modulus, ultimate tensile strength, etc.) and muscle fibre type are needed to yield further insight into the triceps’ role in power production, force transfer and weight support.
Interspecific differences
The few studies investigating the locomotor behaviour of wild gibbons (Whitmoor, 1975; Fleagle, 1976; Gittins, 1983) indicate that there are few interspecific differences in locomotor repertoire, which could explain why only a few interspecific differences in myology were observed in our study. One notable difference was the smaller mean PCSA of the knee extensors of the siamang compared with other gibbon genera. The volume of the knee extensor muscles of siamangs was less than the volume of hip extensor muscles, a pattern also observed in the pileated gibbon but not in the lar gibbon or moloch gibbon. Like siamangs, pileated gibbons spend relatively little time leaping (ca. 5% of their locomotor time; Whitmoor, 1975), yet pileated gibbons had the highest PCSA in their knee extensor muscles of any of our subjects. This suggests that the quadriceps PCSA might not be as good an indicator of leaping frequency as muscle volume, although our sample size is too small to draw any definite conclusions about this. Of the gibbons in our sample, those that spend a greater proportion of time leaping (lar gibbons and moloch gibbons; Fleagle, 1976) had a large muscle volume dedicated to knee extension, and those that spend proportionally less time leaping (siamangs and pileated gibbons; Whitmoor, 1975; Fleagle, 1976) had less muscle volume associated with knee extension.
The lack of significant interspecific differences in the locomotor anatomy of our population could also be attributable to the limited sample size and age of the specimens. Although this was the largest sample number in any quantitative myological study on gibbons to date (11 individuals), the sample number for each species was still relatively low for the purpose of addressing interspecific variation (four siamangs, three lar gibbons, two pileated gibbons and two moloch gibbons). Hence the primary aim of this study was not to investigate interspecific differences in myology but to quantify the general anatomy of the gibbon hind limb and link it to the major locomotor modes utilized by these species. The small interspecific differences that were noted indicate that the presented anatomical data are valid for all studied gibbon species and we would expect all hylobatids to present a similar hind limb musculature as quantified in this study.
All of our animals were kept in captivity, and the area in which they lived is small compared with their natural home range (Milton & May, 1976), so it is likely that they were not as physically active as wild animals. As all of our cadavers were captive animals it is probable that they were subject to similar limitations of activity. At least four of our specimens were over 25 years old (three were of unknown age) and it is likely that this has some effect on the absolute values of some muscle masses, although none died of musculoskeletal pathologies. Unfortunately, these are unavoidable limitations when working with endangered species (the gibbons represented in this study are specified as endangered or critically endangered on the IUCN Red List, IUCN, 2008), as specimens are very difficult to obtain. We would like to underline that, due to these limitations, the provided anatomical data are very valuable as they provide a quantitative database of the hind limb musculature of the gibbon. Such databases are valuable tools for a number of studies investigating comparative anatomy, evolutionary biomechanics and human evolution.
Conclusion
This study has investigated how the gibbon hind limb may cope with the varying mechanical demands placed upon it by the gibbon's varied locomotor repertoire. The short-fascicled, high-PCSA hip and knee extensors are likely to play a role in leaping, potentially via a power-amplifying mechanism using the relatively compliant patellar tendon, whereas the long-fascicled knee and hip flexors enable a wide range of limb positions for support and centre of mass position. Further analyses of moment arms and tendon properties, as well as kinematics and kinetics of gibbon locomotion, are needed to provide further evidence of this hypothesis.
Acknowledgments
The authors would like to thank The National Museums of Scotland and The Royal Zoological Society of Antwerp for loaning the cadaveric specimens and The Royal Society for travel funding. This project was supported by a PhD demonstratorship to A.J.C. from the Department of Human Anatomy and Cell Biology, The University of Liverpool.
Appendix 1.
Mean anatomical data on hind limb muscles collected for each species
|
H. lar | |||||||
|---|---|---|---|---|---|---|---|
| Tendon length (cm) |
|||||||
| Mass (g) | MTU length (cm) | Origin | Insertion | Mean fascicle length (cm) | Ext. tendon length (cm) | Belly mass (g) | |
| THIGH | |||||||
| Gluteus superficialis | 61.5 | 19.6 | – | 6.4 | 13.2 | – | 61.5 |
| Gluteus medius | 38.1 | 10.6 | – | 3.2 | 5.9 | – | 38.1 |
| Gluteus minimus | 7.4 | 5.3 | – | 1.6 | 3.5 | – | 7.4 |
| Pectineus | 2.8 | 6.5 | – | 0.7 | 5.3 | – | 2.8 |
| Obturator internus | 8.4 | 6.3 | – | 2.8 | 3.6 | – | 8.4 |
| Obturator externus | 7.4 | 6.5 | – | 2.2 | 4.4 | – | 7.4 |
| Piriformis | 6.7 | 7.8 | – | 2.1 | 6.2 | – | 4.5 |
| Adductor magnus | 78.6 | 21.1 | – | 8.2 | 13.8 | – | 78.5 |
| Adductor longus | 6.1 | 12.6 | 1.8 | 3.5 | 8.7 | – | 6.1 |
| Adductor brevis | 10.6 | 10.2 | – | – | 9.8 | – | 3.5 |
| Quadratus femoris | 4.8 | 4.7 | – | – | 3.8 | – | 4.8 |
| Rectus femoris | 17.1 | 20.7 | 6.3 | 10.9 | 9.1 | 2.8 | 16.9 |
| Vastus medialis | 19.3 | 18.8 | – | 8.2 | 8.4 | 2.6 | 19.2 |
| Vastus intermedius | 42.1 | 23.0 | – | 11.6 | 8.0 | – | 42.1 |
| Vastus lateralis | 63.2 | 19.1 | 7.0 | 10.8 | 8.2 | 3.1 | 62.9 |
| Gracilis | 9.1 | 23.6 | 0.8 | 4.4 | 21.7 | – | 9.1 |
| Sartorius | 15.3 | 25.7 | 2.2 | – | 24.8 | – | 15.3 |
| Semimembranosus | 9.8 | 23.5 | 9.0 | 7.6 | 13.1 | 4.7 | 9.5 |
| Semitendinosus | 17.2 | 27.0 | 6.4 | 6.2 | 19.0 | 3.8 | 17.0 |
| Biceps femoris (long head) | 10.0 | 23.2 | 6.4 | 8.5 | 9.7 | 1.6 | 9.5 |
| Biceps femoris (short head) | 6.5 | 9.3 | – | 3.5 | 5.3 | – | 6.5 |
| SHANK | |||||||
| Tibialis anterior | 8.7 | 15.7 | – | 9.5 | 7.9 | 3.2 | 8.3 |
| Extensor digitorum longus | 4.2 | 24.6 | – | 19.9 | 7.6 | 3.8 | 3.9 |
| Extensor hallucis longus | 2.8 | 20.4 | – | 12.9 | 7.9 | 5.8 | 2.7 |
| Peroneus longus | 5.8 | 22.0 | – | 18.7 | 5.4 | 9.7 | 5.6 |
| Peroneus brevis | 2.2 | 12.3 | – | 9.6 | 4.0 | – | 1.3 |
| Soleus | 10.5 | 17.5 | 14.7 | 7.1 | 5.9 | 2.4 | 10.2 |
| Gastrocnemius medialis | 12.7 | 20.0 | 6.3 | 15.6 | 5.9 | – | 11.8 |
| Gastrocnemius lateralis | 16.8 | 19.1 | 4.3 | 14.7 | 6.4 | – | 16.8 |
| Plantaris | 6.9 | 18.1 | – | 15.3 | 5.0 | – | 2.3 |
| Tibialis posterior | 6.4 | 20.0 | – | 16.3 | 4.7 | 6.0 | 6.1 |
| Flexor tibialis | 6.1 | 23.6 | – | 16.7 | 7.3 | 5.0 | 5.8 |
| Flexor fibularis | 21.2 | 26.7 | – | 18.7 | 7.9 | 6.6 | 20.6 |
| Popliteus | 3.1 | 5.6 | 1.7 | 2.0 | 2.6 | – | 3.1 |
|
H. pileatus | |||||||
| Tendon length (cm) |
|||||||
| Mass (g) | MTU length (cm) | Origin | Insertion | Mean fascicle length (cm) | Ext. tendon length (cm) | Est. belly mass (g) | |
| THIGH | |||||||
| Gluteus superficialis | 38.0 | 16.1 | – | 4.4 | 10.9 | – | 38.0 |
| Gluteus medius | 27.7 | 11.3 | – | 6.8 | 6.3 | – | 27.7 |
| Gluteus minimus | 5.0 | 7.5 | – | 1.4 | 5.4 | – | 5.0 |
| Pectineus | 2.5 | 5.1 | – | – | 4.9 | – | 2.5 |
| Obturator internus | 7.8 | 6.9 | – | 4.4 | 3.2 | – | 7.8 |
| Obturator externus | 5.2 | 5.1 | – | 2.0 | 2.8 | – | 2.6 |
| Piriformis | 5.6 | 8.0 | – | 3.8 | 4.4 | – | 5.6 |
| Adductor magnus | 49.0 | 21.5 | – | 10.3 | 13.0 | 5.8 | 48.9 |
| Adductor longus | 11.3 | 11.7 | – | 4.4 | 8.7 | – | 11.3 |
| Adductor brevis | 3.7 | 7.2 | – | – | 5.7 | – | 1.9 |
| Quadratus femoris | 2.4 | 3.3 | – | – | 3.3 | – | 2.4 |
| Rectus femoris | 11.7 | 20.8 | 14.7 | 16.4 | 8.3 | 1.8 | 10.2 |
| Vastus medialis | 19.8 | 21.8 | – | 17.2 | 8.3 | 2.4 | 9.4 |
| Vastus intermedius | 33.7 | 23.6 | – | 17.3 | 7.7 | – | 33.7 |
| Vastus lateralis | 37.9 | 23.1 | 12.5 | 10.8 | 8.3 | – | 37.9 |
| Gracilis | 6.8 | 20.7 | – | 4.5 | 17.3 | 4.4 | 6.7 |
| Sartorius | 9.8 | 23.6 | 4.1 | – | 21.7 | – | 9.8 |
| Semimembranosus | 9.1 | 22.7 | 9.5 | 9.9 | 11.7 | 6.6 | 8.8 |
| Semitendinosus | 10.5 | 23.6 | – | 9.9 | 18.6 | 4.5 | 10.2 |
| Biceps femoris (long head) | 8.4 | 22.4 | 8.6 | 10.3 | 11.0 | 2.7 | 8.1 |
| Biceps femoris (short head) | 4.2 | 11.0 | 6.1 | 5.4 | 6.1 | – | 4.2 |
| SHANK | |||||||
| Tibialis anterior | 5.9 | 20.4 | – | 13.4 | 7.6 | 3.9 | 5.4 |
| Extensor digitorum longus | 3.9 | 23.6 | – | 18.1 | 9.0 | 5.0 | 3.4 |
| Extensor hallucis longus | 1.8 | 17.8 | – | 9.2 | 8.0 | 5.1 | 1.7 |
| Peroneus longus | 2.7 | 11.8 | 3.9 | 17.7 | 4.5 | 9.2 | 2.2 |
| Peroneus brevis | 1.5 | 13.1 | – | 11.1 | 6.1 | 2.4 | 1.2 |
| Soleus | 5.3 | 16.9 | – | 11.5 | 5.8 | 4.7 | 5.2 |
| Gastrocnemius medialis | 8.3 | 19.9 | 9.0 | 14.8 | 6.2 | 5.0 | 6.9 |
| Gastrocnemius lateralis | 11.8 | 20.4 | 8.0 | 10.9 | 6.7 | 5.0 | 11.8 |
| Plantaris | – | – | – | – | – | – | – |
| Tibialis posterior | 6.1 | 19.6 | – | 17.9 | 4.9 | 13.1 | 5.3 |
| Flexor tibialis | 4.1 | 21.7 | – | 13.6 | 6.4 | 6.4 | 3.5 |
| Flexor fibularis | 15.4 | 22.9 | – | 17.4 | 8.3 | 6.3 | 13.6 |
| Popliteus | 3.2 | 7.8 | 5.6 | – | 3.1 | 1.4 | 3.2 |
|
H. moloch | |||||||
| Tendon length (cm) |
|||||||
| Mass (g) | MTU length (cm) | Origin | Insertion | Mean fascicle length (cm) | Ext. tendon length (cm) | Est. belly mass (g) | |
| THIGH | |||||||
| Gluteus superficialis | 41.1 | 14.7 | – | 3.1 | 10.0 | – | 41.1 |
| Gluteus medius | 24.4 | 10.0 | – | 5.3 | 5.6 | – | 24.4 |
| Gluteus minimus | 5.5 | 4.9 | – | 1.5 | 3.5 | – | 5.5 |
| Pectineus | 2.3 | 5.1 | – | – | 4.3 | – | 2.3 |
| Obturator internus | 6.2 | 5.8 | – | 3.6 | 3.1 | – | 6.2 |
| Obturator externus | 4.8 | 4.9 | – | 2.5 | 2.7 | – | 4.8 |
| Piriformis | 3.9 | 7.0 | – | 3.3 | 4.7 | – | 3.9 |
| Adductor magnus | 53.5 | 18.6 | – | 7.1 | 11.9 | – | 53.5 |
| Adductor longus | 5.0 | 11.4 | 1.5 | 4.1 | 7.7 | – | 5.0 |
| Adductor brevis | 2.3 | 6.2 | – | – | 6.2 | – | 1.2 |
| Quadratus femoris | 3.6 | 4.5 | – | – | 3.6 | – | 3.6 |
| Rectus femoris | 9.9 | 18.8 | 11.6 | 12.9 | 7.0 | 2.8 | 9.6 |
| Vastus medialis | 15.4 | 21.1 | – | 13.6 | 7.5 | – | 15.2 |
| Vastus intermedius | 21.5 | 20.2 | – | 16.3 | 7.3 | – | 21.5 |
| Vastus lateralis | 23.9 | 17.5 | 8.9 | 12.2 | 7.5 | 3.1 | 23.4 |
| Gracilis | 8.4 | 20.0 | – | 4.4 | 17.0 | – | 8.4 |
| Sartorius | 12.1 | 22.5 | 3.6 | 4.4 | 19.4 | – | 12.1 |
| Semimembranosus | 8.2 | 22.3 | 8.6 | 8.8 | 10.1 | 4.7 | 8.2 |
| Semitendinosus | 13.9 | 22.5 | 2.9 | 6.1 | 15.5 | 3.8 | 13.7 |
| Biceps femoris (long head) | 9.5 | 22.4 | 9.4 | 8.9 | 10.4 | 1.6 | 9.1 |
| Biceps femoris (short head) | 4.7 | 7.0 | – | 7.5 | 4.6 | – | 4.7 |
| SHANK | |||||||
| Tibialis anterior | 6.5 | 18.3 | – | 12.8 | 6.3 | 3.2 | 6.2 |
| Extensor digitorum longus | 3.1 | 21.0 | – | 9.5 | 7.3 | 3.8 | 3.1 |
| Extensor hallucis longus | 1.6 | 16.7 | – | 8.6 | 7.3 | 5.8 | 1.6 |
| Peroneus longus | 4.6 | 20.0 | – | 16.9 | 4.5 | 9.7 | 4.2 |
| Peroneus brevis | 1.7 | 14.2 | – | 10.2 | 3.8 | – | 1.7 |
| Soleus | 8.6 | 16.9 | 13.8 | – | 5.9 | 2.4 | 8.1 |
| Gastrocnemius medialis | 8.1 | 19.6 | 7.6 | 15.9 | 5.8 | 9.3 | 6.7 |
| Gastrocnemius lateralis | 11.1 | 19.7 | 6.7 | 14.2 | 6.4 | 11.1 | 11.1 |
| Plantaris | 2.4 | 7.5 | – | – | 2.9 | – | 1.2 |
| Tibialis posterior | 5.6 | 19.5 | – | 16.1 | 5.3 | 5.6 | 5.2 |
| Flexor tibialis | 3.3 | 18.5 | – | 14.2 | 6.0 | 5.0 | 3.0 |
| Flexor fibularis | 12.2 | 22.0 | – | 16.6 | 7.4 | 6.6 | 11.1 |
| Popliteus | 2.7 | 5.7 | 3.9 | – | 2.3 | – | 2.7 |
|
S. syndactylus | |||||||
| Tendon length (cm) |
|||||||
| Mass (g) | MTU length (cm) | Origin | Insertion | Mean fascicle length (cm) | Ext. tendon length (cm) | Est. belly mass (g) | |
| THIGH | |||||||
| Gluteus superficialis | 78.2 | 18.5 | 6.0 | 7.9 | 11.0 | – | 78.2 |
| Gluteus medius | 52.8 | 13.7 | – | 7.9 | 7.1 | – | 52.8 |
| Gluteus minimus | 8.3 | 6.5 | – | 3.4 | 4.3 | – | 8.3 |
| Pectineus | 6.8 | 7.1 | – | 6.5 | 6.5 | – | 5.1 |
| Obturator internus | 13.7 | 7.7 | – | 4.9 | 4.3 | – | 13.7 |
| Obturator externus | 13.1 | 7.0 | – | 3.8 | 4.0 | – | 13.1 |
| Piriformis | 8.2 | 9.9 | – | 4.7 | 5.5 | – | 8.2 |
| Adductor magnus | 101.9 | 20.6 | 3.1 | 8.5 | 13.8 | 5.1 | 101.6 |
| Adductor longus | 10.1 | 12.1 | 2.4 | 1.4 | 10.0 | – | 10.1 |
| Adductor brevis | 6.2 | 8.3 | – | 2.3 | 7.3 | – | 3.1 |
| Quadratus femoris | 7.5 | 5.3 | – | 1.0 | 4.8 | – | 7.5 |
| Rectus femoris | 22.9 | 21.3 | 13.4 | 14.4 | 9.4 | 1.5 | 22.1 |
| Vastus medialis | 24.0 | 19.1 | – | 12.8 | 8.6 | – | 12.0 |
| Vastus intermedius | 52.6 | 21.4 | – | 10.8 | 8.3 | – | 39.5 |
| Vastus lateralis | 56.4 | 19.2 | 8.0 | 13.6 | 8.5 | – | 28.2 |
| Gracilis | 20.7 | 24.2 | 1.4 | 5.3 | 20.0 | 3.2 | 20.6 |
| Sartorius | 22.4 | 25.2 | 4.9 | 5.7 | 21.7 | 4.6 | 22.3 |
| Semimembranosus | 15.5 | 23.6 | 10.5 | 8.2 | 11.4 | 6.6 | 15.0 |
| Semitendinosus | 29.6 | 26.3 | – | 8.5 | 19.0 | 4.3 | 29.2 |
| Biceps femoris (long head) | 18.2 | 24.7 | 11.0 | 9.7 | 11.4 | 4.8 | 17.9 |
| Biceps femoris (short head) | 8.7 | 12.0 | – | 6.5 | 6.7 | – | 8.7 |
| SHANK | |||||||
| Tibialis anterior | 15.8 | 19.1 | – | 12.0 | 8.0 | 2.7 | 15.1 |
| Extensor digitorum longus | 7.3 | 25.8 | – | 18.7 | 9.3 | 12.3 | 6.3 |
| Extensor hallucis longus | 3.4 | 19.0 | – | 12.1 | 7.4 | 9.0 | 3.1 |
| Peroneus longus | 10.5 | 21.9 | – | 17.8 | 6.8 | 7.5 | 9.6 |
| Peroneus brevis | 3.9 | 11.4 | – | 9.1 | 3.9 | 4.0 | 3.6 |
| Soleus | 19.3 | 17.3 | 13.4 | – | 6.6 | 3.8 | 18.7 |
| Gastrocnemius medialis | 25.8 | 20.1 | 7.8 | 14.3 | 7.0 | 4.7 | 23.3 |
| Gastrocnemius lateralis | 25.7 | 20.5 | 9.5 | 14.5 | 7.2 | 5.5 | 18.7 |
| Plantaris | - | – | – | – | – | 0.0 | |
| Tibialis posterior | 11.2 | 20.0 | – | 12.6 | 6.5 | 4.9 | 10.4 |
| Flexor tibialis | 12.3 | 22.9 | – | 17.5 | 8.2 | 11.2 | 11.5 |
| Flexor fibularis | 25.5 | 26.6 | – | 20.2 | 9.4 | 14.3 | 23.6 |
| Popliteus | 11.1 | 10.9 | 4.0 | 11.4 | 4.3 | – | 11.1 |
–, data were unavailable; MTU, muscle tendon unit.
References
- Aerts P. Vertical jumping in Galago senegalensis: the quest for an obligate mechanical power amplifier. Philos Trans R Soc B Biol Sci. 1998;353:1607–1620. [Google Scholar]
- Alexander RM. Animal Mechanics. London, UK: Sidgwick and Jackson; 1968. [Google Scholar]
- Alexander RM. Allometry of the limbs of antelopes (Bovidae) J Zool. 1977;183:125–146. [Google Scholar]
- Alexander RM. Leg design and jumping technique for humans, other vertebrates and insects. Philos Trans R Soc B Biol Sci. 1995;347:235–248. doi: 10.1098/rstb.1995.0024. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Principles of Animal Locomotion. Princeton: Princeton University Press; 1996. [Google Scholar]
- Alexander RM, Vernon A. The dimensions of the knee and ankle muscles and the forces they exert. J Hum Mov Stud. 1975;1:115–123. [Google Scholar]
- Alexander RM, Jayes AS, Maloiy GM, Wathuta E. Allometry of the leg muscles of mammals. J Zool. 1981;194:227–267. [Google Scholar]
- Altringham JD, Johnson IA. Modelling muscle power output in a swimming fish. J Exp Biol. 1990;148:395–402. [Google Scholar]
- Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. Proc Natl Acad Sci U S A. 2008;105:1745–1750. doi: 10.1073/pnas.0709212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MB, Taylor GC. Scaling of elastic strain energy in kangaroos and the benefits of being big. Nature. 1995;378:56–59. doi: 10.1038/378056a0. [DOI] [PubMed] [Google Scholar]
- Bertram JEA, Chang Y-H. Mechanical energy oscillations of two brachiation gaits: Measurement and simulation. Am J Phys Anthropol. 2001;115:319–326. doi: 10.1002/ajpa.1088. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:73–87. doi: 10.1016/s0305-0491(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Bischoff LW. Beiträge zur Anatomie des Hylobates leuciscusund zu einer vergleichenden Anatomie der Muskeln der Affen und des Menschen. Abh K Bayer Akad Wiss. Math-physik Klasse. 1870;10(3):198–297. [Google Scholar]
- Brand RA, Pederson DR, Friederich JA. The sensitivity of muscle force predictions to changes in physiological cross-sectional area. J Biomech. 1986;19:589–596. doi: 10.1016/0021-9290(86)90164-8. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972;52:129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Demes B, Fleagle JG, Jungers WL. Takeoff and landing forces of leaping strepsirhine primates. J Hum Evol. 1999;37:279–292. doi: 10.1006/jhev.1999.0311. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. The dynamics of the brachiating siamang (Hylobates[Symphalangus]syndactylus. Nature. 1974;248:259–260. doi: 10.1038/248259a0. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. Locomotion and posture of the Malayan siamang and implications for hominoid evolution. Folia Primatol. 1976;26:245–269. doi: 10.1159/000155756. [DOI] [PubMed] [Google Scholar]
- Friederich JA, Brand RA. Muscle fascicle architecture in the human lower limb. J Biomech. 1990;23:91–95. doi: 10.1016/0021-9290(90)90373-b. [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Miyatani M, Tachi M, Kouzaki M, Kawakami Y, Kanehisa H. Muscle volume is a major determinant of joint torque in humans. Acta Physiol Scand. 2001;172:249–255. doi: 10.1046/j.1365-201x.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Roy RR, Shellock FG, et al. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res. 1992;10:926–934. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]
- Gittins SP. Use of the forest canopy by the agile gibbon. Folia Primatol. 1983;40:134–144. doi: 10.1159/000156095. [DOI] [PubMed] [Google Scholar]
- Hiroyuki A, Kozaburo H, Masaaki S. Data Book on Mechanical Properties of Living Cells, Tissues and Organs. Tokyo: Springer; 1996. [Google Scholar]
- Hunt KD, Cant JGH, Gebo D, Rose MD, Walker SE, Youlatos D. Standardized descriptions of primate locomotor and postural modes. Primates. 1996;37:363–387. [Google Scholar]
- Isler K. 3D-Kinematics of vertical climbing in hominoids. Am J Phys Anthropol. 2005;126:66–81. doi: 10.1002/ajpa.10419. [DOI] [PubMed] [Google Scholar]
- Isler K, Payne RC, Günther MM, et al. Inertial properties of hominoid limb segments. J Anat. 2006;209:201–218. doi: 10.1111/j.1469-7580.2006.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. http://www.iucnredlist.org.
- Jones S, Martin R, Pilbeam D. The Cambridge Encyclopedia of Human Evolution. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Josephson RK. Contraction dynamics and power output of skeletal muscle. Ann Rev Physiol. 1993;55:527–546. doi: 10.1146/annurev.ph.55.030193.002523. [DOI] [PubMed] [Google Scholar]
- Kanagsuntheram R. Observations on the anatomy of the hoolock gibbon. Ceylon J Sci. 1952;5:11–64. [Google Scholar]
- Ker RF, Alexander RM, Bennet M. Why are mammalian tendons so thick? J Zool. 1988;216:309–324. [Google Scholar]
- Marsh RL, Bennett AF. Thermal dependence of contractile properties of skeletal muscle from the lizard Sceloporus occidentalis with comments on methods for fitting and comparing force-velocity curves. J Exp Biol. 1986;126:63–77. doi: 10.1242/jeb.126.1.63. [DOI] [PubMed] [Google Scholar]
- Maughan RJ, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983;338:37–47. doi: 10.1113/jphysiol.1983.sp014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CP, Skinner J, Biewener AA. Hind limb scaling of kangaroos and wallabies (superfamily Macropodoidea): implications for hopping performance, safety factor and elastic savings. J Anat. 2008;212:153–163. doi: 10.1111/j.1469-7580.2007.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medler S. Comparative trends in shortening velocity and force production in skeletal muscles. Am J Regul Integr Comp Physiol. 2002;283:R368–378. doi: 10.1152/ajpregu.00689.2001. [DOI] [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Miller CE, Basu C, Fritsch G, Hildebrandt T, Hutchinson JR. Ontogenetic scaling of foot musculoskeletal anatomy in elephants. J R Soc Interface. 2008;5:465–475. doi: 10.1098/rsif.2007.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton K, May ML. Body weight, diet and home range area in primates. Nature. 1976;259:459–462. doi: 10.1038/259459a0. [DOI] [PubMed] [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, Smith NC, Wilson AM. Functional specialisation of pelvic limb anatomy in horses (Equus caballus. J Anat. 2005;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. Morphological analysis of the hindlimb in apes and humans. I. Muscle architecture. J Anat. 2006a;208:709–724. doi: 10.1111/j.1469-7580.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. Morphological analysis of the hindlimb in apes and humans. II. Moment arms. J Anat. 2006b;208:725–742. doi: 10.1111/j.1469-7580.2006.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CM, Shadwick RE. Allometry of muscle, tendon, and elastic energy storage capacity in mammals. Am J Regul Integr Comp Physiol. 1994;266:R1022–1031. doi: 10.1152/ajpregu.1994.266.3.R1022. [DOI] [PubMed] [Google Scholar]
- Preuschoft H. What does ‘arboreal locomotion’ mean exactly and what are the relationships between ‘climbing’, environment and morphology? Z Morphol Anthropol. 2002;83:171–188. [PubMed] [Google Scholar]
- Preuschoft H, Demes B. Biomechanics of brachiation. In: Preuschoft H, Chivers DJ, Brockelman WY, Creel N, editors. The Lesser Apes. Edinburgh: Edinburgh University Press; 1984. pp. 96–118. (eds), pp. [Google Scholar]
- Preuschoft H, Witte H. Origines de la bipédie chez les hominids. Paris: 1991. Biomechanical reasons for the evolution of hominin body shape; pp. 59–77. Editions du CNRS. [Google Scholar]
- Preuschoft H, Witte H, Demes B. Topics in Primatology Volume 3 Evolutionary Biology, Reproductive Endocrinology and Virology. Tokyo: University of Tokyo Press.; 1992. Biomechanical factors: the influence of overall body shape of large apes and humans. [Google Scholar]
- Scholz MN, D’Août K, Bobbert MF, Aerts P. Vertical jumping performance of bonobo (Pan paniscus) suggests superior muscle properties. Proc R Soc B Biol Sci. 2006;273:2177–2184. doi: 10.1098/rspb.2006.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonaert K, D’Août K, Aerts P. Morphometrics and inertial properties in the body segments of chimpanzees (Pan troglodytes. J Anat. 2007;210:518–531. doi: 10.1111/j.1469-7580.2007.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon BA, Farslow DL. The primate hindlimb. Comp Primate Biol. 1986;1:671–718. [Google Scholar]
- Stern JT. Anatomical and functional specialisations of the human gluteus maximus. Am J Phys Anthropol. 1972;36:315–339. doi: 10.1002/ajpa.1330360303. [DOI] [PubMed] [Google Scholar]
- Steudel K. The work and energetic cost of locomotion. I. The effects of limb mass distribution in quadrupeds. J Exp Biol. 1990;154:273–285. doi: 10.1242/jeb.154.1.273. [DOI] [PubMed] [Google Scholar]
- Steudel K. Limb morphology, bipedal gait, and the energetics of hominid locomotion. Am J Phys Anthropol. 1996;99:345–355. doi: 10.1002/(SICI)1096-8644(199602)99:2<345::AID-AJPA9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH. The locomotor ecology of wild orang-utans (Pongo pygmaeus abelii) in the Gunung Leuser ecosystem, Sumatra, Indonesia: a multivariate analysis using log-linear modelling. Am J Phys Anthropol. 2005;127:58–78. doi: 10.1002/ajpa.20151. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH. Orang-utan positional behavior and the nature of arboreal locomotion in Hominoidea. Am J Phys Anthropol. 2006;131:384–401. doi: 10.1002/ajpa.20422. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH, Gunther MM, Ker RF, Alexander RM. Dimensions and moment arms of the hind- and forelimb muscles of common chimpanzees (Pan troglodytes. Am J Phys Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH, Wang WJ. Stresses exerted in the hindlimb muscles of common chimpanzees (Pan troglodytes) during bipedal locomotion. Folia Primatol. 2004;75:253–265. doi: 10.1159/000078937. [DOI] [PubMed] [Google Scholar]
- Usherwood JR, Bertram JEA. Understanding brachiation: insight from a collisional perspective. J Exp Biol. 2003;206:1631–1642. doi: 10.1242/jeb.00306. [DOI] [PubMed] [Google Scholar]
- Vereecke EE, Aerts P. The mechanics of the gibbon foot and its potential for elastic energy storage during bipedalism. J Exp Biol. 2008;211:3661–3670. doi: 10.1242/jeb.018754. [DOI] [PubMed] [Google Scholar]
- Vereecke EE, D’Août K, Payne R, Aerts P. Functional analysis of the foot and ankle myology of gibbons and bonobos. J Anat. 2005;206:453–476. doi: 10.1111/j.1469-7580.2005.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke EE, D’Aout K, Aerts P. Locomotor versatility of the white-handed gibbon (Hylobates lar): a spatiotemporal analysis of the bipedal, tripedal and quadrupedal gaits. J Hum Evol. 2006a;50:552–567. doi: 10.1016/j.jhevol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Vereecke EE, D’Aout K, Aerts P. The dynamics of hylobatid bipedalism: evidence for an energy-saving mechanism? J Exp Biol. 2006b;209:2829–2838. doi: 10.1242/jeb.02316. [DOI] [PubMed] [Google Scholar]
- Wells JB. Comparison of mechanical properties between slow and fast mammalian muscle. J Physiol. 1965;178:252–269. doi: 10.1113/jphysiol.1965.sp007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westing SH, Cresswell AG, Thorstensson A. Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur J Appl Physiol. 1991;62:104–108. doi: 10.1007/BF00626764. [DOI] [PubMed] [Google Scholar]
- Whitmoor TC. Tropical Rain Forests of the Far East. Oxford: Clarendon Press; 1975. [Google Scholar]
- Widrick JJ, Trappe SW, Costill DL, Fitts RH. Force-velocity and force-power properties of single muscle fibers from elite master runners and sedentary men. Am J Physiol Cell Physiol. 1996;271:676–683. doi: 10.1152/ajpcell.1996.271.2.C676. [DOI] [PubMed] [Google Scholar]
- Williams SB, Payne RC, Wilson AM. Functional specialisation of the pelvic limb of the hare (Lepus europeus. J Anat. 2007;210:472–490. doi: 10.1111/j.1469-7580.2007.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Wilson AM, Rhodes L, Andrews J, Payne RC. Functional anatomy and muscle moment arms of the pelvic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris. J Anat. 2008;213:361–372. doi: 10.1111/j.1469-7580.2008.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon: properties, models, scaling and application to biomechanics and motor control. Criti Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]
- Zajac FE. How musculotendon architecture and joint geometry affect the capacity of muscles to move and exert force on objects: A review with application to arm and forearm tendon transfer design. J Hand Surg. 1992;17:799–804. doi: 10.1016/0363-5023(92)90445-u. [DOI] [PubMed] [Google Scholar]
- Zihlman AL. Locomotion as a life history character: the contribution of anatomy. J Hum Evol. 1992;22:315–325. [Google Scholar]