Abstract
We present the first descriptive comparison of the skull, mandible and jaw muscles of the recently recovered Laotian rock rat Laonastes aenigmamus. The gross anatomy of five specimens captured in Laos and internal architecture of the jaw musculature were studied using dissections. The following muscles are described: temporal, masseter, pterygoids, digastric, mylohyoid, geniohyoid and transverse mandibular. The description of the masticatory apparatus of L. aenigmamus offers a rare opportunity to assess the order of establishment of the morphological characters during the evolution of Ctenohystrica. Striking convergences have occurred during the evolution of Diatomyidae and L. aenigmamus presents a unique combination of myological features that corresponds to a mixture of sciurognathous and hystricognathous characters. If L. aenigmamus is a sciurognathous rodent, we have to assume that it independently acquired a pars reflexa of the superficial masseter. We show for the first time that the development of this pars reflexa has occurred several times during the evolution of Ctenohystrica and can no longer be considered a synapomorphic feature of ‘Hystricognathi’. These results bring new insights into the evolution of hystricognathy and have profound implications for the interpretation of the fossil record of early hystricognath rodents.
Keywords: Diatomyidae, hystricognathy, Laos, masticatory muscles, mastication, myology
Introduction
Rodents have undergone an astonishing adaptive radiation through the Cenozoic that led them to represent the most speciose order of mammals (Wilson & Reeder, 2005). New species and genera have recently been described, such as Laonastes aenigmamus (Jenkins et al. 2005) in south east Asia, which represents the sole extant representative of a morphologically distinctive family, the Diatomyidae, considered to have been extinct 11 Ma ago (Dawson et al. 2006). Among extant mammals, rodents show one of the most extreme specializations of the masticatory apparatus, with a single pair of upper and lower incisors highly specialized for gnawing and a small number of cheek teeth for chewing in association with the development of anteroposterior movements (Becht, 1953). The movements of the mandible associated with feeding are performed by the masticatory muscles and are a function of the dental morphology (Butler, 1980; Lazzari et al. 2008), the anatomy of the masticatory apparatus and the shape and position of the temporomandibular joint (Hiiemae, 1967; Crompton & Hiiemae, 1969). In mammals, two main groups of muscles have been recognized (Hiiemae & Houston, 1971). The first group includes muscles innervated by the third division of the fifth cranial (trigeminal) nerve (V3), usually considered ‘the muscles of mastication’ and they include the masseter, temporal, pterygoids, digastric and the mylohyoid (muscles involved in the initiation and the stabilization of jaw movements). The second group includes the ‘accessory muscles of mastication’ and consists of the hyoid musculature, the ‘oral’ muscles (e.g. buccinator) and the muscles of the palate and pharynx. The extreme ecological diversity of rodents has led to many specializations of the jaw musculature.
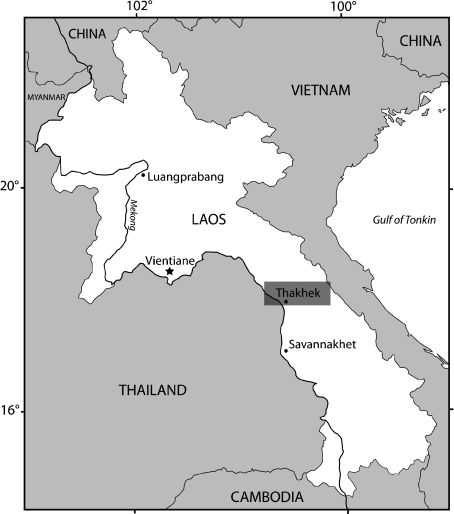
Variation in the distinct ‘parts’ of the masseter and associated modifications in the skull architecture (Brandt, 1855; Simpson, 1945; Wood, 1965) were tentatively used as diagnostic characters to classify the order Rodentia. Four types of layout of the masticatory muscles were recognized by previous authors: protrogomorphy, sciuromorphy, hystricomorphy, and myomorphy (Brandt, 1855; Wood, 1965). Although organization of the masticatory apparatus was recognized early on and used as a diagnostic physical attribute of rodents, many studies showed that these four combinations of masseter muscles cannot be used for the classification of rodents at the subordinal level (e.g. Brandt, 1855; Wood, 1965; Hautier et al. 2008). Considering the direction of the mandibular angular process relative to the plane of the incisors, the order Rodentia was commonly divided into two suborders: Sciurognathi and Hystricognathi (Tullberg, 1899; Chaline & Mein, 1979; Carleton, 1984). The sciurognathous jaws are characterized by an angular process originating in the same plane that includes the alveolus of the incisors. In contrast, in hystricognathous jaws the origin of the angular process is placed distinctly lateral to the plane of the alveolus of the incisors. The living Laotian rock rat Laonastes aenigmamus, recently discovered (Jenkins et al. 2005) in the Lao People’s Democratic Republic (Lao PDR, Thakhek district; Fig. 1), was first considered to be the sole member of a new hystricognathous family, Laonastidae. However, a reexamination of the specimens (Dawson et al. 2006) has shown that this species may represent a surviving member of the extinct family Diatomyidae, i.e. a sciurognathous family. More recently, molecular analyses (Huchon et al. 2007) unambiguously confirmed the paleontological view in demonstrating that L. aenigmamus is the sister group of Ctenodactylidae (within the monophyletic Ctenohystrica) and should be considered a member of Diatomyidae.
Fig. 1.
Location map of the Thakhek market (Thakhek district, Khammouan Province, Lao People’s Democratic Republic).
The difficulties in classifying L. aenigmamus (Jenkins et al. 2005; Dawson et al. 2006; Huchon et al. 2007) stem from the fact that it presents a mixture of sciurognathous and hystricognathous characteristics. In favor of hystricognathous affinities are the following characteristics: the hystricomorphous condition of the skull characterized by an enlarged infraorbital foramen; fusion between the incus and malleus; the greatly reduced coronoid process; the multiserial microstructure of incisor enamel; the enlarged fourth premolar and the retention of a deciduous fourth premolar; the posteriorly directed penis with S-bend, and comblike bristles projecting forward over the claws. However, most of the latter characteristics are not confined to Hystricognathi, they are also found in Ctenodactylidae and should be considered synapomorphies of Ctenohystrica (Landry, 1957; Jenkins et al. 2005). The following characteristics are also found in sciurognathous rodents: the jaw of diatomyids seems to be sciurognathous because the angle of the mandible does not originate from the side of the incisor alveolous; the groove for the passage of the pars reflexa of the superficial masseter muscle, from anterior of angular process to medial side of the mandible, is absent; the pterygoid fossa, formed by the divergence of the lateral and the medial pterygoid plate, is not broken through to the orbit, although Jenkins et al. (2005) proposed that the latter fossa is secondarily reduced in L. aenigmamus (i.e. an autapomorphy).
Here, we provide the first complete description of the masticatory apparatus of L. aenigmamus. The discovery of L. aenigmamus offers a rare opportunity to study an original muscular combination among Ctenohystrica and to investigate the evolution of the masticatory apparatus of hystricognathous rodents as a result. These results also have implications for the evolutionary history of early hystricognathous rodents, especially with caviomorph origins. This investigation leads to a discussion of hystricognathy (Tullberg, 1899), which is sometimes confused with sciurognathy, and its phylogenetic implications.
Materials and methods
For this study, we collected five specimens of L. aenigmamus from the Khammouan Province of the Lao People’s Democratic Republic (Lao PDR). All the specimens were captured by villagers from Mauang Village (Thakhek district; Fig. 1) and collected in the Thakhek market (Thakhek district). We also reexamine the morphology of the masticatory muscles of one member of the proposed sister group of L. aenigmamus (Dawson et al. 2006; Huchon et al. 2007), Ctenodactylus vali, because only cursory descriptions appear in the literature (Tullberg, 1899; Landry, 1957). The specimen of C. vali was collected in northern Algeria, Boussaada, 250 km to the south of Alger. The specimens were fixed in formal saline (buffered 4% formaldehyde solution with 0.12 m NaCl) and stored in 70% ethanol. Finally, three females and two males of the Laotian rock rat and one male of gundi were used for dissections and further investigations. The anatomy of the masticatory muscles has been examined by standard dissections. For each masticatory muscle, the positions of the origin and insertion on the skull and the mandible were carefully inspected, as well as the direction of muscle fibers, in order to establish myological maps (Figs 2–4). Digital photographs were taken at each level of dissection using a Nikon Coolpix 995. The division of muscles into separate groups relied on the position and the innervation of individual muscles. The clusters followed here were established by Woods & Howland (1979). The present study focuses on the muscles of mastication proper (masseter, temporal and pterygoid), the anterior supra-hyoid muscles (digastric, geniohyoid and mylohyoid), and the transverse mandibular muscle, an autapomorphic characteristic of eutherian mammals that helps stabilize the mandibular symphysis (Hiiemae & Houston, 1971).
Fig. 2.
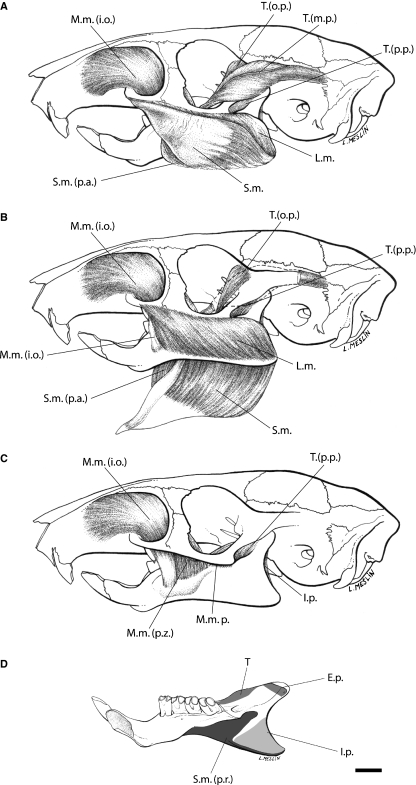
Lateral view of muscles of mastication of Laonastes aenigmamus. (A) Superficial layers, (B) lateral layers, (C) medial layers. (D) Medial view of the mandible of L. aenigmamus, the grey areas indicate attachment to surface of bone. Scale bar = 5 mm. I.p., internal pterygoid; L.m., lateral masseter (pars posterior and pars anterior); M.m., medial masseter; M.m. (i.o.), medial masseter pars infraorbitalis; M.m.p., medial masseter posterior; M.m. (p.z.), medial masseter pars zygomaticomandibularis; S.m., superficial masseter; S.m. (p.a.), superficial masseter pars anterior; T., temporal muscle; T.(m.p.), main part of the temporal muscle; T.(o.p.), orbital part of the temporal muscle; T.(p.p.), posterior part of the temporal muscle. Original artwork by Laurence Meslin, © Laurence Meslin – CNRS.
Fig. 4.
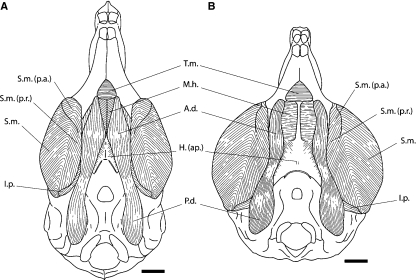
Comparison of the ventral aspect of the masseter and digastric muscles of Laonastes aenigmamus (A) with Ctenodactylus vali (B). Scale bar = 5 mm. A.d., anterior digastric muscle; H.(ap.), aponeuroses of the hyoid cartilage; I.p., internal pterygoid; M.h., mylohyoid muscle; P.d., posterior digastric; S.m., superficial masseter; S.m. (p.a.), superficial masseter pars anterior; S.m. (p.r.), superficial masseter pars reflexa; T.m., transverse mandibular muscle.
The results of the dissections of L. aenigmamus and C. vali dissections were compared with published accounts of other sciurognathous and hystricognathous rodents, including Rattus (Hiiemae & Houston, 1971; Weijs, 1973); Sciurus, Microsciurus, Sciurillus, Tamiasciurus, Tamias, Glaucomys (Ball & Roth, 1995); Paraxerus, Funisciurus, Myosciurus, Heliosciurus, Protoxerus, Funambulus, Callosciurus, Tamiops, Xerus, Atlantoxerus, Ratufa (Thorington & Darrow, 1996); Aplodontia (Hill, 1937; Thorington & Darrow, 1996); Mesocricetus (Gorniak, 1977); Tachyoryctes (Bekele, 1983); Pedetes (Offermans & de Vree, 1989); Reithrodontomys (Rinker & Hooper, 1950); Sigmodon, Oryzomys, Neotoma, Peromyscus (Rinker, 1954); Zapus, Napeozapus, Sicista, Jaculus (Klingener, 1964); Cavia, Chinchilla, Dasyprocta, Erethizon, Thryonomys, Ctenomys, Echimys, Isothrix, Octodon, Petromus, Proechimys (Woods, 1972); Capromys, Geocapromys, Plagiodontia, Myocastor (Woods & Howland, 1979).
Description and comparison of jaw muscles
Jaw muscles of Laonastes aenigmamus
Superficial masseter
The aponeurosis of origin of the superficial masseter forms a round and strong tendinous sheet from the posteroventral surface of the inferior zygomatic root of the maxilla (Figs 2 and 3). This aponeurosis originates from the ventral side of the maxillary zygomatic process as far posterior as the first third of the orbit. A separate anterior part originates from the mesial edge of the lower third of the tendon.
Fig. 3.
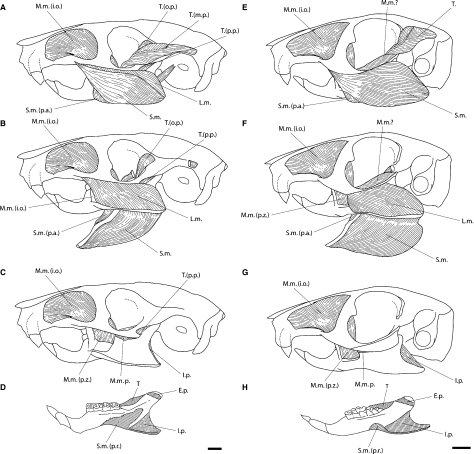
Comparison of the masticatory muscles of Laonastes aenigmamus with Ctenodactylus vali. (A,E) Lateral aspect of the masseter and temporal muscles of L. aenigmamus and C. vali, respectively. (B,F) Lateral aspect of the lateral masseter of L. aenigmamus and C. vali, respectively; superficial masseter and main part of the temporal have been removed. (C,G) Lateral aspect of the medial masseter of L. aenigmamus and C. vali, respectively; the lateral masseter and the orbital part of the temporal have been removed. (D,H) Medial view of the mandible of L. aenigmamus and C. vali, respectively, the hatched areas indicate attachment to surface of bone. Scale bar = 5 mm. E.p., external pterygoid; I.p., internal pterygoid; L.m., lateral masseter (pars posterior and pars anterior); M.m., medial masseter; M.m. (i.o.), medial masseter pars infraorbitalis; M.m.p., medial masseter posterior; M.m. (p.z.), medial masseter pars zygomaticomandibularis; S.m., superficial masseter; S.m. (p.a.), superficial masseter pars anterior; S.m. (p.r.), superficial masseter pars reflexa; T., temporal muscle; T.(m.p.), main part of the temporal muscle; T.(o.p.), orbital part of the temporal muscle; T.(p.p.), posterior part of the temporal muscle.
This muscle is attached on the ventral margin of the angular process of the mandible. The anterior part passes medially and inserts on the ventral side of the alveolar sheath. The rest of the fibers are attached on the mandible via two insertions. The first one is on the ventral and medial ridge of the inflected angle to the tip of the angular process. The second, the Pars reflexa, is continuous with the tendon and inserted medially along the posterior half of the mandible on the pterygoid shelf and ends below the condyle on the condyloid ridge. The posterior half of the Pars reflexa reaches medially the insertion area of the M. pterygoideus internus.
Lateral masseter
This muscle consists of two separate parts, and in some specimens the two parts appear to be rostrally continuous with each other (Figs 2 and 3). Pars anterior arises from the ventral surface of the posterior end of the maxillary part of the zygomatic root and from the ventral surface of the jugal as far posterior as behind the jugal crest. Pars posterior originates from the ventrolateral surface of the maxillary zygomatic process and from the lateral surface of the jugal posteriorly to the tip of the jugal crest.
Pars anterior inserts on the dorsal surface of the masseteric crest from its beginning posterior to the tip of the angle, and some fibers insert partially on the lateral surface of the mandible. Pars posterior is attached on the posterodorsal surface of the crest.
Medial masseter
The medial masseter consists of two parts: Pars anterior extends through the enlarged infraorbital foramen on the lateral side of the rostrum (Figs 2 and 3). Its muscle fibers also originate on the medial surface of the maxillary and jugal parts of the zygomatic root. Pars posterior is reduced and originates from the ventromedial surface of the zygomatic process of the squamosal to the posterior end of the jugal.
Most of the fibers of the pars anterior run caudoventrally and insert into a strong tendon attached to the anterior end of the masseteric crest, just ventral to the first molar (i.e. pars infraorbitalis). The rest of the fibers, which originate from the medial surface of the zygomatic arch (i.e. pars zygomaticomandibularis), insert on the masseteric fossa and on the masseteric crest ventral to the first and second molars. The fibers of the pars posterior pass straight rostroventrally to insert on the lateral surface of the mandible, below the abbreviated coronoid process, in a small depression extending from the end of the dentary sheath to the condyle. This muscle is slim and not covered by the fibers of the medial masseter pars anterior. The masseteric nerve runs superficial to the pars posterior.
Temporalis
The muscle is tiny and splits into three parts (Figs 2 and 3). The main part (i.e. pars posterior) corresponds to a prominent bipinnate muscle that originates from the lateral side of a long and narrow temporal fossa and from the lambdoidal crest, the superior and inferior temporal ridge. The orbital part (i.e. pars anterior) comes from the orbital surface of the frontal and squamosal. A posterior part is closely associated with the main one and originates from the anteroventral surface of the zygomatic process of the squamosal bone.
The main part inserts on the dorsal ridge of the ascending ramus of the mandible at the level of a short coronoid process and the posterior end of the third molar. The orbital part is attached on the dorsal ridge and the medial surface of the coronoid process, posteriorly to the insertion of the main part. The posterior part extends on the lateral surface of the coronoid process.
Internal pterygoid
This muscle is divided into two parts and originates from inside and along the margin of the pterygoid fossa (Fig. 3). The larger layer arises from the lateral pterygoid plate, the smaller along the pterygoid process.
Both parts insert on the medial fossa and the dorsal surface of the angular process. The internal pterygoid is visible from the lateral side of the mandible.
External pterygoid
This muscle takes its origin from the external surface of the lateral pterygoid plate but also a part of the surface of the alisphenoid and the maxillary (Fig. 3).
The fibers pass posterodorsally to insert on the medial surface of the neck of the condyloid process.
Digastric
The posterior belly originates from the paraoccipital process (Fig. 4). The origin of the anterior belly is continuous with the posterior digastric. The anterior belly fibers communicate with the posterior ones by a slight constriction and are not separated by a tendon. There is no pronounced attachment to the hyoid bone. The anterior bellies lie side by side in the midline; they are anteriorly separated, allowing the exposure of the transverse mandibular.
The insertion of the posterior belly is into the fibers of the anterior belly. The anterior bellies of the two sides are not in contact with each other but are very close. The insertion is on the linguoventral ridge of the mandible, posterolateral to the symphysis.
Transverse mandibular
This is a small triangular muscle that connects the ventral margin of the two horizontal rami of the mandible posterior to the mandibular symphysis (Fig. 4). Mesially, it is slightly covered by the anterior tip of the anterior bellies of the digastrics. This muscle passes ventrally and inserts on the most anterior fibers of the mylohyoid.
Mylohyoid
The fibers originate from the lingual side of the mandible below the tooth-row and are slightly covered by the insertion of the transverse mandibular near the symphysis (Fig. 4). The muscle is completely covered by both anterior bellies of the digastric. The insertion is on the median fibrous raphe from the most posterior fibers of the transverse mandibular posteriorly to the hyoid.
Geniohyoid
The muscle originates from the medial surface of the mandible above the mandibular symphysis, medial to the origin of the genioglossus. Its fibers are closely associated with those of the genioglossus and are attached to the distolateral surface of the body of the hyoid.
Comparison with C. vali
Laonastes is a member of the rodent clade Ctenohystrica, which includes both sciuragnathous ctenodactylids and hystricognathous rodents. We decided to compare the jaw muscles of L. aenigmamus with the masticatory muscle architecture in C. vali. Landry (1957) and Tullberg (1899) have briefly described the morphology of the masticatory muscles of Ctenodactylus. In C. vali, we observed that the pars anterior of the superficial masseter is present but less individualized than in L. aenigmamus (Fig. 3) and in hystricognathous rodents. This part was not observed by Tullberg (1899) and not mentioned by Landry (1957). A small reflected part passes onto the medial side of the mandible in the area behind and below the sheath of the incisor (Tullberg, 1899; Fig. 3). In Ctenodactylus, the fibers of the lateral masseter (pars posterior) are anchored in a jugal fossa on the ventromedial margin of the posterior end of the jugal. In L. aenigmamus, there is no jugal fossa. In Ctenodactylus vali, the two parts of the internal pterygoid are not separated, but the muscle is large. The digastric muscles are characterized by a clear separation of the two anterior bellies that allows the exposure of the mylohyoid (Fig. 4).
The most important differences are observed in the arrangement of the temporal muscle. In Ctenodactylus, the temporal muscle is greatly reduced. Tullberg (1899) did not separate parts of this muscle, which were recognized in other studies (Rinker & Hooper, 1950; Rinker, 1954; Hiiemae & Houston, 1971; Woods, 1972; Weijs, 1973; Bekele, 1983; Offermans & de Vree, 1989; Ball & Roth, 1995; Thorington & Darrow, 1996). However, in his drawings, Tullberg (1899) illustrated a part of a muscle that runs from the anterodorsal surface of the zygomatic process of the squamosal bone, very close to the temporal. He referred to this part as the most posterior fibers of the pars anterior of the medial masseter. In his detailed study of the comparative myology of four dipodoid rodents, Klingener (1964) reached the same conclusion for the configuration of the temporal. Woods (1972) reported that ‘the parts of this muscle are difficult to homologize in various rodents’. Tullberg’s part referred to as the most posterior fibers of the medial masseter, pars anterior, appears to be closely associated with the aponeuroses of the temporal (Fig. 3). We consider that this muscular part certainly represents a lateral extension of the main or orbital part of the temporal. As a matter of fact, the fibers of this part insert on the dorsal edge of the mandible situated near the abbreviated coronoid process.
Discussion
Hystricognathous or sciurognathous jaw?
Laonastes aenigmamus presents a masseteric configuration very close to that found in hystricomorphs of the New and Old World (i.e. Hystricognathi [Woods, 1972]). As usual, the superficial masseter takes originates from a round and flat tendon. We showed that the pars anterior of the superficial masseter is clearly individualized in L. aenigmamus. This part is present in Capromys, Chinchilla, Ctenomys, Echimys, Geocapromys, Isothrix, Mesomys, Myocastor, Octodon, Petromus and Proechimys, and is missing in Cavia, Dasyprocta, Erethizon and Thryonomys (Woods, 1972; Woods & Hermanson, 1985). As shown in previous works (e.g. Hiiemae & Houston, 1971; Weijs, 1973; Bekele, 1983; Offermans & de Vree, 1989; Ball & Roth, 1995; Thorington & Darrow, 1996), this part is also missing in sciurognathous rodents. For Woods (1972), ‘this part of the muscle certainly represents a specialized and relatively unusual condition […] found in many, but not all, rodents with a hystricognath jaw’. Tullberg (1899) considered the medial groove and the insertion of pars reflexa of the superficial masseter high up, close to the condyle, to be the characteristics of the tribe ‘Hystricognathi’. The medial groove is clearly absent on the mandible of L. aenigmamus (Dawson et al. 2006), but a significant part of the superficial masseter passes onto the ventral side of the mandible, inserts in the area behind the alveolar sheath and high up below the condyle at the end of the condyloid ridge (Fig. 3). As in most hystricognathous rodents, the anterior fibers of the superficial masseter in L. aenigmamus are partially associated with the anterior part of the lateral masseter, pars posterior. In Capromys, Ctenomys, Echimys, Geocapromys, Isothrix, Mesomys, Myocastor, Octodon, Petromus, Proechimys and Ctenodactylus (see Comparison with Ctenodactylus vali) the pars anterior of the lateral masseter arises from a deep jugal fossa (Woods, 1972; Woods & Hermanson, 1985). The configuration in L. aenigmamus is more similar to that observed in Cavia, Chinchilla, Dasyprocta, Erethizon and Thryonomys, due to the lack of a jugal fossa (Woods, 1972; Woods & Howland, 1979). Laonastes aenigmamus presents a well developed superficial masseter that covers most of the lateral masseter, and as such, it is more similar to the muscular arrangement found in most sciurognathous rodents (e.g. Hiiemae & Houston, 1971; Weijs, 1973).
The arrangement of the temporal muscles (Figs 2 and 3), with a long, narrow main part (i.e. pars posterior) and a small orbital part (i.e. pars anterior), is closest morphologically to that of Proechimys (Woods, 1972). The characteristics of the orbital part (Figs 2 and 3) could be related to the morphology of the cheek teeth of L. aenigmamus. Indeed, Rinker & Hooper (1950) and Klingener (1964) considered the pars anterior to be involved in crushing food, whereas the pars posterior is involved in grinding. They proposed that the reduction of the anterior fibers of the temporal should be associated with the transformation of the molar morphology from tuberculate to flat. The pterygoid muscle in L. aenigmamus is basically the same as in most hystricognathous rodents (Woods, 1972). The two parts of the internal pterygoid muscle are well separated. The mandible of L. aenigmamus lacks a post-condyloid process, which is associated with the development of the external pterygoid muscle. Woods (1972) hypothesized that the presence of a condyloid process is related to flat-crowned teeth and propalinal jaw movement. This hypothesis is clearly violated by considering the case of L. aenigmamus.
Parsons (1896) separated sciuromorphine and hystricomorphine types of digastric muscles. The sciuromorphine type is characterized by the contact of the anterior bellies in the midline. The anterior belly fibers communicate with the posterior ones by a tendon with a strong attachment to the hyoid bone. In the hystricomorphine type, the anterior bellies are clearly separated. They are separated from the posterior bellies by a small constriction without connection with the hyoid bone. Aplodontia (Hill, 1937), Rattus (Weijs, 1973), Mesocricetus (Gorniak, 1977), Tachyoryctes (Bekele, 1983) and Pedetes (Offermans & de Vree, 1989) present a sciuromorphine type. The hystricomorphine type occurs in all hystricognathous rodents (Schumacher, 1961; Woods, 1972; Woods & Howland, 1979) and Ctenodactylus (see Comparison with Ctenodactylus vali and Fig. 4). Laonastes combines both types, the anterior bellies of the digastric are not separated in the midline but there is no pronounced attachment to the hyoid bone (Fig. 4).
Evolution of the Diatomyidae
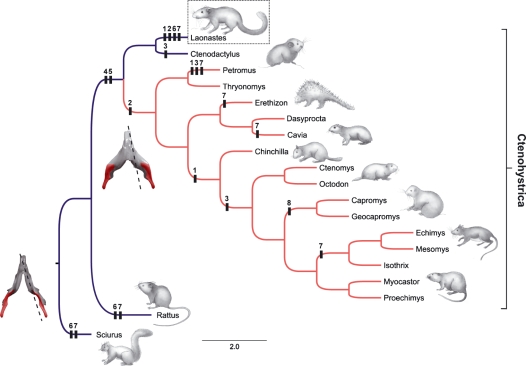
Myological characteristics (i.e. morphology, size, division, innervation and function) have commonly been used to establish phylogenetic relationships of rodents (e.g. Parsons, 1896; Tullberg, 1899; Woods & Hermanson, 1985). However, the use of these characteristics remains controversial. Woods & Hermanson (1985) concluded that ‘all muscles are not equally useful in constructing rodents phylogenies’ and that ‘the masticatory region has been the center of morphological changes in rodent evolution, and there are many examples of convergences and parallelisms in jaw musculature’. As a matter of fact, the most dynamic myological characters were associated with the temporal, digastric and pterygoid muscles (Woods & Howland, 1979; Woods & Hermanson, 1985). We decided not to use the myological characteristics to evaluate phylogenetic relationships but rather to map them onto a phylogenetic tree derived from molecular analyses (Opazo, 2005; Huchon et al. 2007; Fig. 5). Nevertheless, our study shows that myological similarities between diatomyids and Hystricognathi can be added to the skeletal and dental characteristics (Fig. 5): the general organization of the masseter layers; the presence of the pars anterior of the superficial masseter (Character 1; Fig. 5); the pars reflexa of the superficial masseter strongly individualized (Character 2; Fig. 5); the superficial head of the lateral masseter, pars posterior, partially associated with the superficial masseter (Character 4; Fig. 5); the loss of the superficial fibers of the orbital part of the temporal that cover partly the main part (Character 5; Fig. 5); the relative importance of the orbital and main parts of the temporal. However, L. aenigmamus retains some sciurognathous myological characteristics: the superficial masseter is well developed and covers the lateral masseter laterally; the anterior bellies of the digastric are not separated (Character 6; Fig. 5); the pars posterior of the anterior part of the lateral masseter is poorly developed.
Fig. 5.
Mapping of the myological characters on a phylogenetic tree derived from molecular analyses (Opazo, 2005; Huchon et al. 2007). Blue branches, Sciurognathous jaws; Red branches, hystricognathous. The presence of a characteristic in a clade is mapped here. Numbers correspond to myological characters: Character 1: Superficial masseter muscle; presence of the pars anterior well individualized. Character 2: Superficial masseter muscle; presence of the pars reflexa. Character 3: Lateral masseter (pars posterior) muscle; origin in a jugal fossa. Character 4: Lateral masseter (pars posterior) muscle; fibers closely associated with the superficial masseter muscle. Character 5: Temporal muscle; loss of the superficial fibers of the orbital part that cover the main muscle mass. Character 6: Digastric muscle; contact of the anterior bellies (i.e. sciuromorphine type). Character 7: Internal pterygoid muscle; two parts separated. Character 8: Geniohyoid muscle; free floating. Original artwork by Laurence Meslin, © Laurence Meslin – CNRS.
Functional origin of hystricognathy
Laonastes aenigmamus presents a peculiar combination of muscular characteristics that explains the debates concerning its taxonomic position (Jenkins et al. 2005; Dawson et al. 2006). It is the sole extant member of Ctenohystrica, which presents a sciuromorphine arrangement of the digastric muscles. This type certainly represents the primitive condition within rodents. The mandible of L. aenigmamus is characterized by a weak lateral displacement of the angular process and absence of a groove. If the diatomyids are sciurognaths, we have to assume that they independently acquired a pars reflexa of the superficial masseter. For Woods (1972), the passage of pars reflexa might be at the origin of the formation of the groove and was used to define the hystricognathous condition of the jaw (Woods, 1982). This hypothesis is clearly violated in considering the case of L. aenigmamus. If the individualization of a groove is always accompanied by a development of the pars reflexa of the superficial masseter, the contrary is not true. However, by considering the example of L. aenigmamus, this development appears to be linked to a lateral displacement of the angular process. The pars reflexa may have an important role in the stabilization of the mandible during chewing. Most of the caviomorph rodents are characterized by an oblique mastication associated with flattening of the molar occlusal surface (Vassalo & Verzi, 2001). Greaves (1980) noted that a high mandibular condyle is required for the establishment of an oblique chewing movement. However, a low mandibular condyle, i.e. condition that characterizes rodents, implies a decrease of the lateral component of force of the masseter and pterygoid muscles that could explain a lateral displacement of the mandible. Vassalo & Verzi (2001) suggested that such a lateral displacement of the angular process could have occurred during the evolution of hystricognathous rodents and is at the origin of the groove. Thus, the key characteristic defining hystricognathy appears to be related to the mechanics of the masticatory apparatus, especially oblique masticatory movements. The description of the masticatory apparatus of L. aenigmamus offers a rare opportunity to test such a hypothesis as well as to assess the order of establishment of the morphological characteristics of the masticatory apparatus during the evolution of Ctenohystrica. The occlusal surface of the cheek teeth of L. aenigmamus reveals that diatomyids have developed a strong tendency toward propalinal mastication in association with flattening of the molar occlusal surface. We think that such a specialization might explain the lack of the groove on their mandible and its intermediate combination of morphological characteristics.
Conclusions
We showed that striking convergences have occurred in the evolution of Diatomyidae. Despite numerous myological similarities with hystricognathous rodents, L. aenigmamus is the sole member of Ctenohystrica that has retained a sciuromorphine type of digastric muscles. The pars reflexa of the superficial masseter, considered by Tullberg (1899) to be characteristic of hystricognathous jaws, is present in L. aenigmamus despite its sciurognathous condition. We showed that this character could no longer be considered a synapomorphic feature of ‘Hystricognathi’. The development of the pars reflexa has occurred several times during the evolution of Ctenohystrica, and this feature is usually accompanied by the development of a groove and a lateralization of the angular process. This result has profound implications for the interpretation of the fossil record of early hystricognathous rodents. For instance, Marivaux et al. (2002) proposed an early dispersal of hystricognathous rodents to South America from Asia. From now on, paleontological studies and paleogeographical interpretations will have to take into consideration that other members of Ctenohystrica, like diatomyids, could have developed a pars reflexa of the superficial masseter convergently with hystricognathous rodents and might have achieved an apparent hystricognathous condition of the jaw. The definition of hystricognathy is complex and requires access to complete material. In fact, we considered that the mandible of L. aenigmamus presents an original combination of morphological characteristics among rodents that cannot be considered sciurognathous, any more than hystricognathous. Darwin (1859) spent two chapters of On the Origin of Species apologizing for the lack of ‘transitional’ forms, those with intermediate morphological features between two major groups of organisms. Our study shows that L. aenigmamus could be conceived of as a ‘missing link’ between sciurognathous and hystricognathous rodents. It becomes necessary to revisit the entire morphological diversity of the mandible of extant and extinct hystricognathous rodents and this might lead to a redefinition of hystricognathy.
Acknowledgments
We are very grateful to villagers from Mauang Village (Thakhek district) who captured the specimens of L. aenigmamus. We would like to thank Komsorn Lauprasert and the Department of Biology of the Faculty of Science of the Mahasarakham University (Tambon Khamriang Kantarawichai district, Thailand). We are also indebted to Tida Saenyamoon, Romain Liard, Jean Leloeuff (Musée des Dinosaures, Esperaza), Christel Souillat, Suravech Suteethorn (Institut des Sciences de l’Evolution de Montpellier) and Julien Claude (Institut des Sciences de l’Evolution de Montpellier) for their contributions to and advice for the field work.
We thank Zoubir Harrat (Institut Pasteur d’Algérie, service d’Ecoépidémiologie parasitaire, annexe de Sidi Fredj Dely Ibrahim) and his collaborators for providing the specimen of Ctenodactylus vali.
We owe gratitude to François Catzeflis (Institut des Sciences de l’Evolution de Montpellier) for his interesting discussions on dissections and for access to facilities. We thank Marika Tilak and the Laboratory of Molecular Phylogeny (Institut des Sciences de l’Evolution de Montpellier) for the molecular identification of the specimen of C. vali. We also thank Laurence Meslin (Institut des Sciences de l’Evolution de Montpellier) for drawings.
We would like to thank all the Laboratory of Paleontology (Institut des Sciences de l’Evolution de Montpellier). We thank especially Monique Vianey-Liaud, Jacques Michaux, Fabrice Lihoreau, Laurent Marivaux, Rodolphe Tabuce, Helder Gomes Rodrigues, and Pierre-Henri Fabre for their suggestions on earlier versions of the manuscript. We are also indebted to two anonymous reviewers, Erik Seiffert (Department of Anatomical Sciences, Stony Brook University), as well as the associated editors for their contribution to improve the manuscript. This is a publication of the Institut des Sciences de l’Evolution de Montpellier (Unité Mixte de Recherche 5554 du Centre National de la Recherche Scientifique).
References
- Ball SS, Roth VL. Jaw muscles of New World squirrels. J Morphol. 1995;224:265–291. doi: 10.1002/jmor.1052240303. [DOI] [PubMed] [Google Scholar]
- Becht G. Comparative biologic-anatomical researches on mastication in some mammals, I and II. Proc Koninklijke Nederlandse Akademie van Wetenschappen. 1953;56:508–527. [Google Scholar]
- Bekele A. The comparative functional morphology of some head muscles of the rodents Tachyoryctes splendens and Rattus rattus. Mammalia. 1983;47:395–419. [Google Scholar]
- Brandt JK. Beiträge zur nahern Kenntnissder Säugethiere Russlands. Mémoires de l’Académie Impériale des Sciences de St. Petersbourg. 1855;69:1–375. [Google Scholar]
- Butler PM. Functional aspects of the evolution of rodent molars. Paleovertebrata, Mémoire du Jubilée R. Lavocat. 1980:249–262. [Google Scholar]
- Carleton MD. Introduction to rodents. In: Anderson S, Knox J, editors. Orders and Families of Recent Mammals of the World. New York: J. Wiley and Sons; 1984. pp. 255–265. [Google Scholar]
- Chaline J, Mein P. Les Rongeurs et l’Évolution. Paris: Doin; 1979. [Google Scholar]
- Crompton AW, Hiiemae K. Functional occlusion in tribosphenic molars. Nature. 1969;222:678–679. doi: 10.1038/222678b0. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Marivaux L, Li C, Beard C, Métais G. Laonastes aenigmamus and the ‘Lazarus effect’ in recent mammals. Science. 2006;311:1456–1458. doi: 10.1126/science.1124187. [DOI] [PubMed] [Google Scholar]
- Gorniak GC. Feeding in golden hamsters, Mesocricetus auratus. J Morphol. 1977;154:427–458. doi: 10.1002/jmor.1051540305. [DOI] [PubMed] [Google Scholar]
- Greaves WS. The mammalian jaw mechanism – the high glenoid cavity. Am Nat. 1980;116:432–440. [Google Scholar]
- Hautier L, Michaux J, Marivaux L, Vianey-Liaud M. The evolution of the zygomasseteric construction in Rodentia, as revealed by a geometric morphometric analysis of the mandible of Graphiurus (Rodentia, Gliridae) Zool J Linn Soc. 2008;154:807–821. [Google Scholar]
- Hiiemae K. Masticatory function in the mammals. J Dent Res. 1967;46:883–893. doi: 10.1177/00220345670460054601. [DOI] [PubMed] [Google Scholar]
- Hiiemae K, Houston WJB. The structure and function of the jaw muscles in the rat (Rattus norvegicus L.) I. Their anatomy and internal architecture. Zool J Linn Soc. 1971;50:75–99. [Google Scholar]
- Hill JE. Morphology of the pocket gopher mammalian genus Thomomys. Univ Calif Pub Zool. 1937;42:81–171. [Google Scholar]
- Huchon D, Chevret P, Jordan U, Kilpatrick CW, Ranwez V, Jenkins PD, et al. Multiple molecular evidences for a living mammalian fossil. Proc Natl Acad Sci U S A. 2007;104:7495–7499. doi: 10.1073/pnas.0701289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins PD, Kilpatrick CW, Robinson MF, Timmins RJ. Morphological and molecular investigations of a new family, genus and species of rodent (Mammalia: Rodentia: Hystricognatha) from Lao PDR. Syst Biodiv. 2005;2:419–454. [Google Scholar]
- Klingener DJ. The comparative myology of four dipodoid rodents (Genus Zapus, Napeozapus, Sicista and Jaculus) Misc Pub Mus Zool Univ Mich. 1964;124:1–100. [Google Scholar]
- Landry SO. The interrelationships of the New and Old World hystricomorph rodents. Univ Calif Pub Zool. 1957;56:1–118. [Google Scholar]
- Lazzari V, Tafforeau P, Aguilar J-P, Michaux J. Topographic maps applied to comparative molar morphology: the case of murine and cricetine dental plans (Rodentia, Muroidea) Paleobiology. 2008;34:46–64. [Google Scholar]
- Marivaux L, Vianey-Liaud M, Welcomme JL, Jaeger JJ. The role of Asia in the origin and diversification of Hystricognathous rodents. Zool Scr. 2002;31:225–239. [Google Scholar]
- Offermans M, de Vree F. Morphology of the masticatory apparatus in the springhare, Pedetes capensis. J Mammal. 1989;70:701–711. [Google Scholar]
- Opazo JC. A molecular timescale for caviomorph rodents (Mammalia, Hystricognathi) Mol Phyl Evol. 2005;37:932–937. doi: 10.1016/j.ympev.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Parsons FG. Myology of rodents. Part 2. Proc Zool Soc London. 1896;426:159–192. [Google Scholar]
- Rinker GC. The comparative myology of the mammalian genera Sigmodon, Oryzomys, Neotoma, and Peromyscus (Cricetinae), with remarks on their intergeneric relationships. Misc Pub Mus Zool Univ Mich. 1954;83:1–124. [Google Scholar]
- Rinker GC, Hooper ET. Notes on the cranial musculature of two subgenera of Reithrodontomys (harvest mice) Misc Pub Mus Zool Univ Mich. 1950;528:1–11. [Google Scholar]
- Schumacher GH. Funktionelle morphologie der kaumuskulatur. Jena: Gustav Fisher Verlag; 1961. [Google Scholar]
- Simpson GG. Principles of classification and a classification of mammals. Bull Am Mus Nat Hist. 1945;85:172–174. [Google Scholar]
- Thorington RW, Darrow K. Jaw muscles of Old World squirrels. J Morphol. 1996;230:145–165. doi: 10.1002/(SICI)1097-4687(199611)230:2<145::AID-JMOR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tullberg T. Ueber das System der Nagetiere: ein phylogenetische Studie. Nova Acta Reg Soc Sc Upsal. 1899;18:1–514. [Google Scholar]
- Vassalo AI, Verzi DH. Patrones craneanos y modalidades de masticacion en roedores caviomorfos (Rodentia, Caviomorpha) Bol Soc Biol Concepción Chile. 2001;72:145–151. [Google Scholar]
- Weijs WA. Morphology of the muscles of mastication in the albino rat, Rattus norvegicus (Berkenhout, 1769) Acta Morphol Neerl Scand. 1973;11:321–340. [PubMed] [Google Scholar]
- Wilson D, Reeder D. Mammal species of the world. Baltimore: Johns Hopkins University Press; 2005. [Google Scholar]
- Wood AE. Grades and clades among rodents. Evolution. 1965;19:115–130. [Google Scholar]
- Woods CA. Comparative mycology of jaw, hyoid, and pectoral appendicular regions of the New and Old World hystricomorph rodents. Bull Am Mus Nat Hist. 1972;147:117–198. [Google Scholar]
- Woods CA. The history and classification of South American hystricognath rodents: reflections on the far away and long ago. In: Mares MA, Genoways HH, editors. Mammalian biology in South America. The Pymatuning Symposia in Ecology. Pittsburgh: University of Pittsburgh; 1982. pp. 377–392. [Google Scholar]
- Woods CA, Hermanson JW. Myology of hystricognath rodents: an analysis of form, function and phylogeny. In: Luckett WP, Hartenberger J-L, editors. Evolutionary Relationships among Rodents, a Multidisciplinary Analysis. New York: Plenum Press; 1985. pp. 515–548. [Google Scholar]
- Woods CA, Howland EB. Adaptative radiation of capromyid rodents: anatomy of the masticatory apparatus. J Mammal. 1979;60:95–115. [Google Scholar]