Abstract
The thoracolumbar fascia attaches to the lumbar spinous processes and encloses the paraspinal muscles to form a muscle compartment. Because muscle spindles can respond to transverse forces applied at a muscle’s surface, we were interested in the mechanical effects this fascia may have on proprioceptive signaling from lumbar paraspinal muscles during vertebral movement. The discharge of paraspinal muscle spindles at rest and in response to muscle history were investigated in the presence and absence of the thoracolumbar fascia in anesthetized cats. Muscle-history was induced by positioning the L6 vertebra in conditioning directions that lengthened and shortened the paraspinal muscles. The vertebra was then returned to an intermediate position for testing the spindles. Neither resting discharge (P= 0.49) nor the effects of muscle history (P>0.30) was significantly different with the fascia intact vs. removed. Our data showed that the thoracolumbar fascia did not influence proprioceptive signaling from lumbar paraspinal muscles spindles during small passive vertebral movements in cats. In addition, comparison of the transverse threshold pressures needed to stimulate our sample of muscle spindles in the cat with the thoracolumbar fascia compartmental pressures measured in humans during previous studies suggests that the thoracolumbar fascia likely does not affect proprioceptive signaling from lumbar paraspinal muscle spindles in humans.
Keywords: lumbar spine, muscle history, muscle spindle, paraspinal muscle, thoracolumbar fascia
Introduction
Fascia is connective tissue investing and interconnecting almost all other bodily tissues, including muscle, solid and hollow viscera, nerves and blood vessels. It helps define muscle compartments, distributes stresses in soft tissue, and reduces occlusal forces on blood vessels and compressive forces on nerves (Benjamin, 2009). It is virtually continuous throughout the organism. In the lumbar region, the posterior layer of the lumbar fascia tightly encloses the back muscles and helps to define a dorsal muscle compartment. We were interested in the mechanical effects that this fascia may have on proprioceptive signaling in the lumbar spine.
In the human lumbar region, the lumbar fascia consists of three layers (Bogduk & Macintosh, 1984). The most superficial or posterior layer is the thickest of the three layers and has been described as having extensive connections arising from the spinous processes and/or intraspinous ligaments and attaching directly to muscles of the upper back and pelvis, and indirectly to abdominal muscles via fusion with the two deeper lumbar fascial layers (Bogduk & Macintosh, 1984; Vleeming et al. 1995; Barker & Briggs, 1999). The superficial layer is the only layer that extends into the thoracic region and is therefore known as the lumbodorsal or thoracolumbar fascia. It has two laminae, a superficial and a deep lamina (Bogduk & Macintosh, 1984; Vleeming et al. 1995; Barker & Briggs, 1999). Positive intracompartmental pressures between the fascia’s inner surface and the erector spinae muscles have been measured, indicating the presence of a physiological space (Carr et al. 1985). These pressures can increase during exercise and changes in posture (Carr et al. 1985; Songcharoen et al, 1994). If the mechanical threshold is reached, mechanically sensitive sensory receptors respond to these loads.
The muscle spindle apparatus is a type of low-threshold mechanoreceptor in muscle. In the lumbar vertebral column, muscle spindle input contributes to positional control of the low back (Brumagne et al. 1999). The lumbar spindle is more sensitive to changes in vertebral position and paraspinal muscle length than its appendicular counterparts are to changes in the limbs (Cao et al. 2009). Its dysfunction has been associated with low back pain (Brumagne et al. 2000) although causal mechanisms are not yet clear. In general, these proprioceptors are well known for providing information about changes in position and movement about a joint (Prochazka, 1996). Their afferent’s resting discharge increases in response to muscle stretch and decreases in response to muscle shortening (Matthews, 1972). Because muscle spindle afferents also respond to transverse pressure when it is sufficient to deform their receptive endings (Bridgman & Eldred, 1964), vertebral movement which increases fascial tension and intracompartmental pressure (ICP) might affect these lumbar proprioceptors by placing transverse loads on the enclosed multifidus, longissimus, and iliocostalis muscles.
To investigate the effects that the thoracolumbar fascia has on lumbar paraspinal muscle spindles we used a previously established model and protocol (Pickar, 1999; Ge et al. 2005) to challenge the lumbar spine’s spindle apparatus in the anesthetized cat. Analogous to the human, the cat’s thoracolumbar fascia has two laminae (Bogduk, 1980). The deep lamina has been considered an aponeurosis because it provides attachment for the longissimus muscle, unlike in the human (Bogduk, 1980). The experimental protocol takes advantage of the spindle’s thixotropic property whereby its responsiveness to vertebral position and movement is differentially affected by the previous flexion vs. extension history of the vertebra to which the paraspinal muscles attach (Ge et al. 2005; Ge & Pickar, 2008). Tests of responsiveness were performed in the same experimental animal in the presence and absence of an intact thoracolumbar fascia. We tested the hypothesis that the history-dependent responsiveness of lumbar paraspinal muscle spindles at rest and during passive movement decreases in the absence of the thoracolumbar fascia.
Materials and methods
Preparation
Experiments were performed on 12 deeply anesthetized adult cats (weight: 3.5–4.9 kg). All cats were treated in accordance with the Guiding Principles in the Care and Use of Animals approved by the American Physiological Society. All procedures have been described previously (Pickar, 1999; Ge et al. 2005). Briefly, deep anesthesia was maintained with pentobarbital sodium (35 mg kg−1, i.v.) and additional dosages (∼5 mg kg−1, i.v.) were given when necessary. Cats were mechanically ventilated (model 681; Harvard Apparatus Company, Inc., Millis, MA, USA). A midline incision was made through thoracolumbar fascia at L5 in order to perform an L5 laminectomy and to gain access to the L6 spinal cord for neural recordings from L6 dorsal root. The caudal half of L4 and all of L5 were removed. Except for the incision at L5, the thoracolumbar fascia remained. Arterial pH, Pco2, and Po2 were measured every 90 min using the i-STAT System (i-STAT Corporation, East Windsor, NJ, USA) and were maintained within normal range (pH 7.32–7.43; Pco2, 32–37 mmHg; Po2, > 85 mmHg).
Recording nerve activity from the L6 dorsal rootlets
The dura mater was incised and the L6 dorsal root identified for electrophysiological recordings. The L6 spinal nerve provides innervation to fascicles of the multifidus and longissimus muscles attaching to the L6 vertebra (Bogduk, 1976). L6 dorsal rootlets were cut close to their entrance into the spinal cord and successively placed on a small platform. Thin filaments were teased using sharpened forceps under a dissecting microscope until impulse activity from a single unit with a receptive field in the paraspinal muscles could be identified. Action potentials were sorted using a PC-based data acquisition system (Spike 2, Cambridge Electronic Design, Cambridge, UK). Activity from a putative muscle spindle in the lumbar spine was first identified by the ability to evoke a high frequency in response to gently compressing the lumbar paraspinal tissues. Only those afferents were used whose discharge was highest in response to probing the back muscles compared with the gluteal, hip or leg regions and who responded to manual movement of the L6 vertebra in the cranial–caudal and left–right directions.
Effects of muscle history
While recording afferent activity from lumbar paraspinal muscle spindles, movement about the L6–7 facet joints was introduced by applying controlled displacements at the L6 spinous process in the horizontal plane along both the longitudinal (cranial–caudal) and the transverse (left–right) axes of the spine. Displacements were controlled using an electronic feedback control system (Lever System Model 310; Aurora Scientific, Aurora, ON, Canada). The L6 spinous process was attached to the motor’s drive shaft through a pair of tissue forceps (152.4 mm long) suspended from a pivot at its upper end and clamped via its working end onto the process’s lateral surfaces. To actuate L6 along the longitudinal axis, the lever arm was positioned parallel to the forceps and connected to the forceps via a stiff metal rod 83.0 mm from its pivot. Thus, movement at the L6 spinous process was obligatorily coupled to lever arm movement and determined from lever arm displacement. For the transverse direction, a custom-made bell crank lever transferred the motor’s lever arm movement along the longitudinal axis to a perpendicular movement in the horizontal plane.
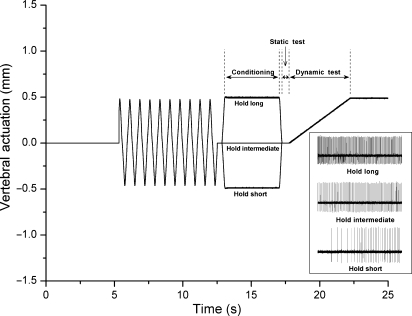
Because the previous lengthening history of a muscle can influence subsequent muscle spindle discharge (Proske et al. 1993), each protocol began by applying identical histories to the L6 vertebra and its attached muscles by moving the L6 vertebra back and forth 10 times (10 mm s−1), rapidly stretching and shortening the attached muscles. Following this deconditioning, a period of controlled muscle history (conditioning) was applied by actuating the vertebra and holding it in a static position that shortened (hold-short), lengthened (hold-long) or maintained the attached muscles at an intermediate (hold-intermediate) position for 4.0 s (see Fig. 1). At the intermediate position, the paravertebral tissues were considered in a neutral position because they exerted no force against the motor’s drive shaft. For technical reasons, protocols were conducted along the longitudinal axis first but the order of the three history protocols was randomized for each axis. To determine the effect of muscle history on muscle spindle discharge, the vertebra was then returned to or remained at the intermediate position for 0.5 s (static test) and then slowly (0.2 mm s−1) moved in a direction that loaded the muscle spindle (dynamic test). Muscle spindle discharge during the static and dynamic tests was compared between hold-intermediate and hold-long or hold-short.
Fig. 1.
Schematic of the experimental protocol and representative response (inset) of one spindle to three conditioning protocols in the cranial–caudal direction. Loading protocol shows the change in vertebral position relative to the reference position. Note that at the beginning of the static test, the vertebra was positioned identically for each of three protocols.
Each of the six protocols was separated by at least 5 min. Within a cat and across protocols, the intermediate position and the magnitude of vertebral translation during deconditioning, conditioning, and the dynamic test were identical. The magnitude of the conditioning translation was established for each cat by the displacement in the long direction for the sagittal plane that loaded the L6 vertebra 55–70% of the cat’s body weight (BW). The same magnitude of vertebral actuation was used in left–right conditioning for same cat.
After the six history protocols were completed, the thoracolumbar fascia directly above the surface of longissimus and multifidus muscles on the side of nerve recording was resected. The thoracolumbar fascia including both superficial and deep laminae [erector spinae aponeurosis after Bogduk (1980)] was removed between the L6 and S1 dorsal processes and from the midline laterally, sufficient to fully expose the multifidus and longissimus muscles. The sacrocaudalis dorsalis lateralis (lumbococcygeus) muscle lying between the lumbar multifidus and longissimus muscles was removed to improve mechanical isolation of the latter two muscles. All other tissues including the epimysium of underlying multifidus and longissimus muscles were kept intact. To confirm that the source and mechanical threshold of neural activity was from a receptive ending in the lumbar longissimus or multifidus muscles, von Frey hairs (Stoelting Co., Wood Dale, IL, USA) were used, which determined that the most sensitive area for mechanically activating the afferent was in the low back. Transverse pressures applied by the von Frey load were calculated based upon the surface area of the von Frey filament’s tip. Two methods were used to confirm that neural activity was from a muscle spindle: decreased discharge to a muscle twitch and increased discharge to succinylcholine injection (100–300 μg kg−1, intra-arterial).
Following this identification procedure with the thoracolumbar fascia removed, the history protocols were repeated with hold-short, hold-long and hold-intermediate applied in the same order as when the fascia was intact.
Data analysis
Spindle activity was quantified as mean instantaneous frequency (MIF) for the static test and mean frequency (MF) for the dynamic test similar to our previous studies (Ge et al. 2005; Ge & Pickar, 2008). MIF was calculated by averaging the reciprocal of each time interval between consecutive action potentials. MF was calculated by dividing the number of action potentials by the recording duration. Muscle spindle responsiveness during the static and dynamic tests was characterized as a change (Δ) by subtracting MIF or MF of the hold-intermediate protocol from that of the hold-short (ΔMIFshort or ΔMFshort) or hold-long (ΔMIFlong or ΔMFlong) protocols. A positive value indicated an increase in muscle spindle responsiveness; conversely, a negative value indicated a reduction in muscle spindle responsiveness. Values close to zero indicated conditioning had little or no effect.
A paired t-test was used to compare paraspinal muscle spindle discharge before the thoracolumbar fascia was removed with that after removal. Significance level was set at the P < 0.05 level. Assumptions of normality and homogeneity of variance were examined using residual plots. Data are reported as means (lower, upper 95% confidence limit) unless otherwise indicated. Statistical analyses were conducted using sas (version 8; SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
All 12 paraspinal muscle spindle afferents were activated by succinylcholine and silenced by bipolar muscle stimulation. The most sensitive portion of each afferent’s receptive field was located in either the lumbar multifidus (n= 4) or longissimus (n= 8) muscles. Most receptive fields were in deeper parts of these muscles close to the L6–7 facet joint; two were close to L7–S1 facet joint in the longissimus muscle. With the thoracolumbar fascia removed and using von Frey filaments, the transverse force necessary to stimulate these spindles when applied perpendicular to the muscle’s surface ranged between 0.007 and 1.234 N [0.312 (0.478) N; mean (SD)]. By considering the tip diameter of each filament, the applied pressures necessary to stimulate the spindles ranged between 210 and 2378 kPa [881 (846) kPa; mean (SD)] (Table 1). For the cranial–caudal direction, translation of the L6 vertebra in the cranial direction loaded eight muscle spindles and translation in the caudal direction loaded the remaining four spindles. For the left–right direction, leftward translation of the L6 vertebra loaded 11 spindles, whereas rightward translation loaded one spindle despite all spindles being located on the right side. Vertebral displacements ranged from 1.0 to 1.5 mm [1.2 (0.2) mm] which loaded the L6 vertebra 55.3–70.0% BW for displacements in the cranial–caudal direction and 6.5–34.3% BW for the left–right direction. For each cat, identical actuations always produced higher loads in the cranial–caudal compared with the left–right direction [64.9% (4.2%) vs. 18.7% (9.6%) BW].
Table 1.
Calculation of pressures applied to a muscle spindle’s receptive field by a von Frey filament
| Muscle spindle afferent | von Frey marking | Forcea (N) | Tip diametera (mm) | Calculated pressure (kPa) |
|---|---|---|---|---|
| 1 | 4.31 | 0.020 | 0.305 | 273.9 |
| 2 | 4.74 | 0.054 | 0.381 | 472.6 |
| 3 | 5.88 | 0.743 | 0.711 | 1873.4 |
| 4 | 4.56 | 0.036 | 0.356 | 357.6 |
| 5 | 4.56 | 0.036 | 0.356 | 357.6 |
| 6 | 3.84 | 0.007 | 0.203 | 209.6 |
| 7 | 5.46 | 0.283 | 0.559 | 1152.2 |
| 8 | 6.1 | 1.234 | 0.813 | 2377.8 |
| 9 | 4.56 | 0.036 | 0.356 | 357.6 |
| 10 | 4.74 | 0.054 | 0.381 | 472.6 |
| 11 | 6.1 | 1.234 | 0.813 | 2377.8 |
| 12 | 4.17 | 0.014 | 0.254 | 286.2 |
Obtained from Stoelting Co., Wood Dale, IL, USA.
1 N mm–2 = 1000 kPa.
Intact thoracolumbar fascia
All afferents had a resting discharge prior to conditioning; mean discharge rate was 30.7 (11.8) imp s−1 (range: 14.0–56.4 imp s−1). The changes in muscle spindle responsiveness to the positional history of the L6 vertebra is shown in Table 2. Hold-long history along either the longitudinal or transverse axis of the spine’s horizontal plane decreased the resting discharge rate of lumbar paraspinal muscle spindles and their responsiveness to passive vertebral movement that stretched the spindle. During the static test, ΔMIFlong decreased by −18.2 (−23.7, −12.6) imp s−1 to the cranial–caudal positional history and −9.4 (−14.9, −3.8) imp s−1 to left–right positional history. During the dynamic test, ΔMFlong decreased by −8.0 (−11.8, −4.1) to cranial–caudal history and −3.3 (−5.5, −1.1) to left–right history. Conversely, hold-short history decreased muscle spindle responsiveness at rest and during vertebral movement (Table 2). However, the absolute magnitude of the responsiveness was substantially smaller compared with hold-long. For the static test, ΔMIFshort increased by 6.2 (4.5, 7.9) imp s−1 to the cranial–caudal history and by 3.8 (2.3, 5.3) imp s−1 to the left–right history. For the dynamic test, ΔMFshort increased by 1.4 (0.1, 2.7) imp s−1 to cranial–caudal history and by 1.9 (0.2, 3.6) imp s−1 to left–right history. For both the static and dynamic test, none of the 95% confidence intervals crossed 0 imp s−1 (Table 2), indicating that the hold-long and hold-short history each significantly altered muscle spindle activity compared to the hold-intermediate history.
Table 2.
History-dependent responsiveness of lumbar paraspinal muscle spindles with thoracolumbar fascia intact and removed
| Cranial–caudal |
Left–right |
||||
|---|---|---|---|---|---|
| Response measure | Conditioning | Intact | Removed | Intact | Removed |
| Static test ΔMIF (imp s−1) | Hold-long | −18.2 (−23.7, −12.6) | −18.8 (−23.7, −14.0) | −9.4 (−14.9, −3.8) | −8.6 (−13.4, −3.7) |
| Hold-short | 6.2 (4.5, 7.9) | 5.7 (3.4, 8.1) | 3.8 (2.3, 5.3) | 4.4 (2.5, 6.3) | |
| Dynamic test ΔMF (imp s−1) | Hold-long | −8.0 (−11.8, −4.1) | −8.6 (−11.0, −6.3) | −3.3 (−5.5, −1.1) | −2.4 (−3.8, −1.1) |
| Hold-short | 1.4 (0.1, 2.7) | 1.0 (−0.4, 2.5) | 1.9 (0.2, 3.6) | 1.7 (0.4, 3.1) | |
Values are reported as means (lower, upper 95% confidence limit).
Thoracolumbar fascia removed
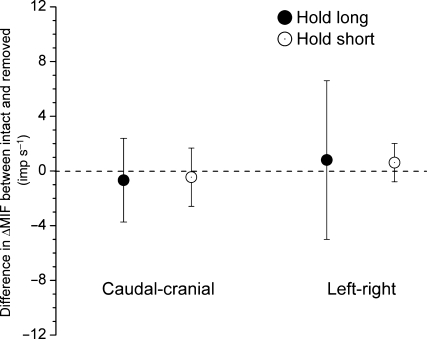
The mean resting muscle spindle discharge of 31.5 (9.8) imp s−1 (range: 14.6–49.2 imp s−1) was similar to that with the intact thoracolumbar fascia (P = 0.49, paired t-test). History-dependent changes still occurred with the fascia removed. For the static test, ΔMIFlong decreased −18.8 (−23.7, −14.0) imp s−1 with the cranial–caudal history and −8.6 (−13.4, −3.7) imp s−1 with the left–right history (Table 2). Compared with the fascia intact, the decreases in ΔMIFlong for the static test were not significantly different for either the cranial–caudal (P = 0.64) or the left–right (P = 0.77) conditioning history (paired t-test, Fig. 2). With the fascia removed, ΔMIFshort increased by 5.7 (3.4, 8.1) imp s−1 to the cranial–caudal direction and by 4.4 (2.5, 6.3) imp s−1 to the left–right direction (Table 2). Compared to intact preparation, no significant difference was found for ΔMIFshort in either the cranial–caudal or left–right conditioning directions (P=0.89 and 0.36, respectively, paired t-test, Fig. 2).
Fig. 2.
Difference in ΔMIF for the static test between conditions with the thoracolumbar fascia intact and removed. Each symbol represents the mean ± 95% confidence interval of 12 observations.
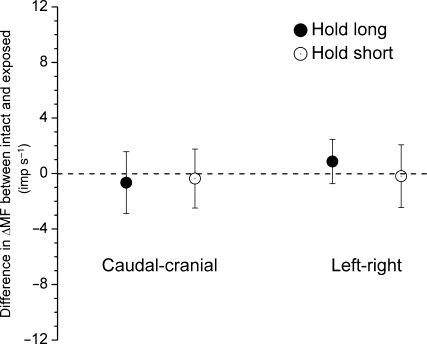
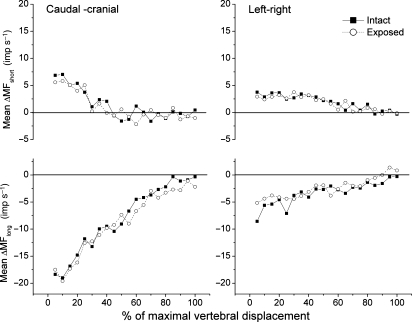
For the dynamic test, ΔMFlong decreased −8.6 (−11.0, −6.3) imp s−1 with cranial–caudal history and −2.4 (−3.8, −1.1) imp s−1 with left–right history (Table 2). Similar to the static test, the decreases in ΔMFlong for the dynamic test with the fascia removed were not significantly different with the fascia intact for the two conditioning directions (P = 0.53 and 0.26, respectively, paired t-test, Fig. 3). ΔMFshort increased by 1.0 (−0.4, 2.5) imp s−1 to cranial–caudal translation and 1.7 (0.4, 3.1) imp s−1 to left–right translation (Table 2). Again, the difference for ΔMFshort between the intact and exposed preparations was not significant for either conditioning direction (P=0.71 and 0.86, respectively, paired t-test, Fig. 3). Figure 4 shows muscle spindle discharge responses to vertebral actuation history over the entire duration of the dynamic test. It provides a comparison of the time course of spindle activity during the dynamic test for the intact and exposed preparations. Time course of spindle responses to the conditioning history was similar in the intact and exposed preparations for both the hold-short and hold-long conditioning directions.
Fig. 3.
Difference in ΔMF for the dynamic test between conditions with the thoracolumbar fascia intact and removed. Each symbol represents the mean ± 95% confidence interval of 12 observations.
Fig. 4.
Time course of the dynamic test in 5% increments comparing history-dependent spindle responses with the thoracolumbar fascia intact vs. removed. Top panel represents hold-short conditioning and bottom panel represents hold-long conditioning. X-axis is normalized to the conditioning displacement used for each spindle (see Materials and methods). Velocity of displacement during the dynamic was same for all spindles (0.2 mm s−1).
Discussion
This study showed that removal of the thoracolumbar fascia did not change the activity of paraspinal muscle spindles in the lumbar spine of the cat. These spindles had mechanical pressure thresholds that ranged from 210 to 2378 kPa. Neither their resting discharge nor their responsiveness to a movement protocol that quantified their history-dependence to small, passive vertebral movements was altered. Movements of the L6 vertebra were actuated within the horizontal plane transversely left and right, and longitudinally in the cranial and caudal directions, movements that accompany lateral bending and flexion/extension rotations, respectively (Kanayama et al. 1996; Ochia et al. 2006). The results indicate that the thoracolumbar fascia in the cat did not produce transverse forces sufficient in magnitude to alter the signaling properties of muscle spindles in the paraspinal muscles it encloses.
The literature has suggested various biomechanical roles for the thoracolumbar fascia. Its role as a site of muscle origin for the latissimus dorsi and transversus abdominus is well documented (Bogduk & Macintosh, 1984). A biomechanical role has been proposed wherein the thoracolumbar fascia is thought to help stabilize the lumbar spine by providing the predominant extensor moment during lifting (Gracovetsky et al. 1977). Because the fascia attaches to the spinous processes, an anti-flexion moment could be induced via an increase in its midline longitudinal tension wherein the spinous processes are brought together, creating an extension moment in the low back. One mechanism that might tension the thoracolumbar fascia is contraction of the abdominal muscles to which the fascia attaches (Gracovetsky et al. 1977, 1981). While such a mechanism for anti-flexion seemed reasonable based upon gross anatomy (Macintosh et al. 1987), reconsideration of thoracolumbar anatomy, calculations of extensor moments and measurements of abdominal EMG activity during lifting provide quantitative arguments that makes this mechanism unlikely (Macintosh et al. 1987; McGill & Norman, 1988). For example, the thoracolumbar fascia provides < 4% of the extensor moment caused by contraction of the dorsal paraspinal muscles even during heavy lifting (Macintosh et al. 1987; McGill & Norman, 1988). The hydraulic amplifier is a second mechanism by which fascial tension was proposed to increase (Gracovetsky et al. 1977). The cross-sectional area of contracting paraspinal muscles enclosed by the thoracolumbar fascia would enlarge and thereby load the fascia. Modeling suggests this mechanism is capable of producing a substantial anti-flexion moment (Hukins et al. 1990).
A neurosensory role for the thoracolumbar fascia appears to have first been supported when mechanoreceptive nerve endings were observed in this tissue (Yahia et al. 1992). While the fascia could support longitudinal tension up to 300 N during stretch (Tesh et al. 1987) its actual functional contributions to spinal neurophysiology have not yet been identified. However, changes in this fascia’s innervation have been observed in individuals with low back pain where their thoracolumbar fascia contains few, if any, mechanoreceptive endings (Bednar et al. 1995). Microscopic evidence suggestive of inflammation and ischemia within the fascia was found in association with the deficiency of actual neural end-organs (Bednar et al. 1995).
Even though muscle spindles are clearly sensitive to muscle stretch (Matthews, 1972), they are also sensitive to transverse loading when applied to a muscle. Bridgman & Eldred (1964) were the first to hypothesize and provide evidence that muscle spindles signal a muscle’s contractile state by responding to internal transverse forces as intramuscular pressure increased during muscle contraction. Thus, in addition to a direct mechanosensory role through its innervation, the thoracolumbar fascia could indirectly modulate muscle proprioceptive input to the extent that its loading during vertebral movement transversely loads the paraspinal muscles it encloses. In the passive muscle, application of external transverse forces to a muscle’s surface in order to identify a putative muscle spindle afferent has been a mainstay of neurophysiological approaches. Spindle sensitivity to these transverse forces was clearly evident in the lumbar paraspinal muscles of the cat when we applied von Frey filaments orthogonal to the surface of the multifidus and longissimus muscles. The average mechanical threshold pressure was 0.881 N mm–2 or 881 kPa (range 210–2378 kPa).
These threshold pressures can be considered in relationship to transverse pressures that might develop at the muscle surface with an intact thoracolumbar fascia. The space enclosed by the paraspinal muscles and the fascia’s inner surface can be considered a compartment. In the relaxed standing human, two studies report similar ICPs of ∼ 5 mmHg (0.67 kPa) (Carr et al. 1985; Songcharoen et al. 1994). During 90° low back flexion, presumably when flexion–relaxation has occurred so that paraspinal muscles are silent and the fascia had been maximally loaded by axial stretch, ICP reaches no more than 34 mmHg (4.5 kPa) (Carr et al. 1985; Songcharoen et al. 1994). During erector spinae contraction sufficient to produce lumbar extension; ICP increases to no more than 21 mmHg (2.8 kPa) and then further increases to 60 mmHg (8.0 kPa) when accompanied by a valsalva maneuver (Carr et al. 1985). With either the spine flexed at 30° during an 80% maximal voluntary isometric extension effort, or with the lumbosacral junction flexed and legs straight while lifting an 18.2-kg weight, or with the hips flexed and the back straight while lifting 18.2 kg, ICPs never exceed 52 mmHg (6.9 kPa) (Carr et al. 1985; Songcharoen et al. 1994).
While intracompartmental pressures in the cat have not been determined, ICPs measured in humans during the variety of lumbar positions and loadings summarized above were always at least two orders of magnitude below the average mechanical threshold pressure that stimulated spindle afferents in our cat preparation. Even the lowest threshold pressure we measured in the cat (209.7 kPa, Table 1) was more than an order of magnitude (∼ 26×) greater than the highest ICP measured in the human lumbar spine (8.0 kPa). The invasive procedures we used to determine threshold pressure in the cat spindle have not been applied in humans. Assuming the range of threshold pressures in the passive spindle of the human are similar to those in the cat [e.g. see Kakuda, 2000)], it seems unlikely that the thoracolumbar fascia loading that occurs during physical activity generates ICPs sufficiently high to stimulate passive paraspinal muscle spindle. However, unlike other mechanosensory receptors, the sensitivity of muscle spindles can be increased by the central nervous system through the action of gamma-motoneurons. Increased gamma drive might increase spindle sensitivity to thoracolumbar fascial compartment pressures relative to the passive state of muscle spindles studied here. To our knowledge, little if anything is known about gamma-motoneuronal control of spindles in muscles of the axial skeleton.
In addition to these functional implications, this study advances an important issue in animal welfare. Institutional Animal Care and Use Committees are mandated by the Public Health Service to refine, reduce and replace the use of animals in research to minimize the number used to answer a scientific question (Pitts, 2002). When using animal preparations that require invasive procedures, it is desirable to keep a preparation as physiological as possible so that interpretations may be generalized. In an animal model used to study proprioceptive mechanisms in the spine (Pickar, 1999; Ge et al. 2005; Cao et al. 2009), the thoracolumbar fascia has been kept intact until the end of the experimental protocol; only then is it opened to identify the recorded neuron. This approach yields only one data point per experimental animal. In the current study, because the thoracolumbar fascia played no role in transmitting loads that affected proprioceptive signaling properties from paraspinal muscle spindles, it can be removed without sacrificing the physiology. This knowledge allows more muscle spindles to be studied in each experimental animal, thus reducing the number of animals required to achieve the needed spindle sample size, reducing the cost of performing a study, and reducing the time necessary to complete a study.
Acknowledgments
The authors thank Mr. Randall Sozio for technical work, Dr. Cynthia Long and Mr. Ying Cao for statistical assistance. This work was supported by NIH grant NS46818. The work was conducted in a facility constructed with support from Research Facilities Improvement Grant Number C06 RR15433 from the National Center for Research Resources, National Institute of Health.
References
- Barker PJ, Briggs CA. Attachments of the posterior layer of lumbar fascia. Spine. 1999;24:1757–1764. doi: 10.1097/00007632-199909010-00002. [DOI] [PubMed] [Google Scholar]
- Bednar DA, Orr FW, Simon GT. Observations on the pathomorphology of the thoracolumbar fascia in chronic mechanical back pain. A microscopic study. Spine. 1995;20:1161–1164. doi: 10.1097/00007632-199505150-00010. [DOI] [PubMed] [Google Scholar]
- Benjamin M. The fascia of the limbs and back – a review. J Anat. 2009;214:1–18. doi: 10.1111/j.1469-7580.2008.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. The lumbosacral dorsal rami of the cat. J Anat. 1976;122:653–662. [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. The dorsal lumbar muscles of the cat. Anat Anz. 1980;148:55–67. [PubMed] [Google Scholar]
- Bogduk N, Macintosh JE. The applied anatomy of the thoracolumbar fascia. Spine. 1984;9:164–170. doi: 10.1097/00007632-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Bridgman CF, Eldred E. Hypothesis for a pressure-sensitive mechanism in muscle spindles. Science. 1964;143:481–482. doi: 10.1126/science.143.3605.481. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Lysens R, Swinnen S, et al. Effect of paraspinal muscle vibration on position sense of the lumbosacral spine. Spine. 1999;24:1328–1331. doi: 10.1097/00007632-199907010-00010. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Cordo P, Lysens R, et al. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25:989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- Cao DY, Pickar JG, Ge W, et al. Position sensitivity of feline paraspinal muscle spindles to vertebral movement in the lumbar spine. J Neurophysiol. 2009;101:1722–1729. doi: 10.1152/jn.90976.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D, Gilbertson L, Frymoyer J, et al. Lumbar paraspinal compartment syndrome. A case report with physiologic and anatomic studies. Spine. 1985;10:816–820. [PubMed] [Google Scholar]
- Ge W, Pickar JG. Time course for the development of muscle history in lumbar paraspinal muscle spindles arising from changes in vertebral position. Spine J. 2008;8:320–328. doi: 10.1016/j.spinee.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Long CR, Pickar JG. Vertebral position alters paraspinal muscle spindle responsiveness in the feline spine: effect of positioning duration. J Physiol. 2005;569:655–665. doi: 10.1113/jphysiol.2005.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracovetsky S, Farfan HF, Lamy C. A mathematical model of the lumbar spine using an optimized system to control muscles and ligaments. Orthop Clin North Am. 1977;8:135–153. [PubMed] [Google Scholar]
- Gracovetsky S, Farfan HF, Lamy C. The mechanism of the lumbar spine. Spine. 1981;6:249–262. doi: 10.1097/00007632-198105000-00007. [DOI] [PubMed] [Google Scholar]
- Hukins DWL, Aspden RM, Hickey DS. Thoracolumbar fascia can increase the efficiency of the erector spinae muscles. Clin Biomech. 1990;5:30–34. doi: 10.1016/0268-0033(90)90029-6. [DOI] [PubMed] [Google Scholar]
- Kakuda N. Response of human muscle spindle afferents to sinusoidal stretching with a wide range of amplitudes. J Physiol. 2000;527(Pt 2):397–404. doi: 10.1111/j.1469-7793.2000.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama M, Abumi K, Kaneda K, et al. Phase lag of the intersegmental motion in flexion-extension of the lumbar and lumbosacral spine. An in vivo study. Spine. 1996;21:1416–1422. doi: 10.1097/00007632-199606150-00004. [DOI] [PubMed] [Google Scholar]
- Macintosh JE, Bogduk N, Gracovetsky S. The biomechanics of the thoracolumbar fascia. Clin Biomech. 1987;2:78–83. doi: 10.1016/0268-0033(87)90132-X. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. Baltimore: The Williams & Wilkins Co; 1972. [Google Scholar]
- McGill SM, Norman RW. Potential of lumbodorsal fascia forces to generate back extension moments during squat lifts. J Biomed Eng. 1988;10:312–318. doi: 10.1016/0141-5425(88)90060-x. [DOI] [PubMed] [Google Scholar]
- Ochia RS, Inoue N, Renner SM, et al. Three-dimensional in vivo measurement of lumbar spine segmental motion. Spine. 2006;31:2073–2078. doi: 10.1097/01.brs.0000231435.55842.9e. [DOI] [PubMed] [Google Scholar]
- Pickar JG. An in vivo preparation for investigating neural responses to controlled loading of a lumbar vertebra in the anesthetized cat. J Neurosci Methods. 1999;89:87–96. doi: 10.1016/s0165-0270(99)00060-6. [DOI] [PubMed] [Google Scholar]
- Pitts M. Institutional Animal Care and Use Committee Guidebook. 2002. ARENA/OLAW 2nd, 97–101. Available at: http://grants.nih.gov/grants/olaw/GuideBook.pdf (accessed on 10 July 2009) [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology. Bethesda, MD: American Physiological Society; 1996. pp. 89–127. [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol. 1993;41:705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Songcharoen P, Chotigavanich C, Thanapipatsiri S. Lumbar paraspinal compartment pressure in back muscle exercise. J Spinal Disord. 1994;7:49–53. [PubMed] [Google Scholar]
- Tesh KM, Dunn JS, Evans JH. The abdominal muscles and vertebral stability. Spine. 1987;12:501–508. doi: 10.1097/00007632-198706000-00014. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine. 1995;20:753–758. [PubMed] [Google Scholar]
- Yahia L, Rhalmi S, Newman N, et al. Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand. 1992;63:195–197. doi: 10.3109/17453679209154822. [DOI] [PubMed] [Google Scholar]