Abstract
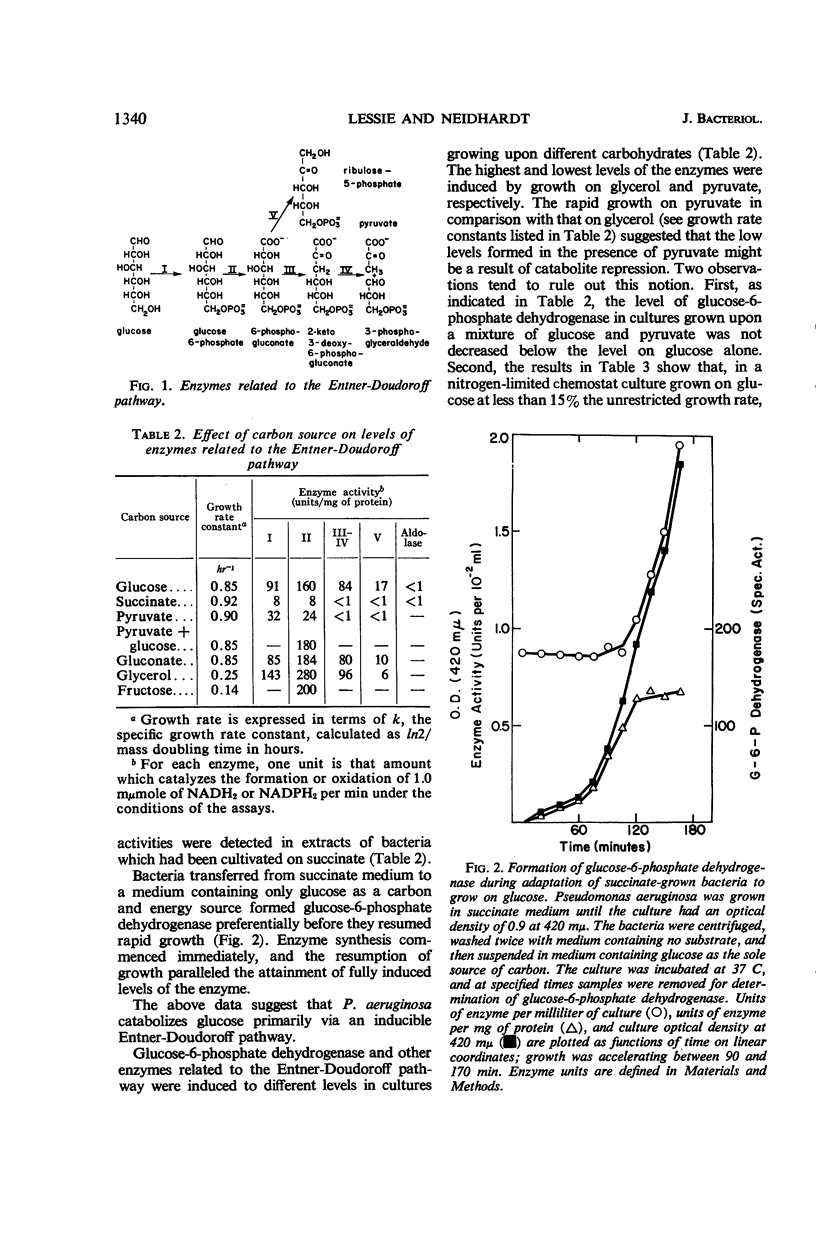
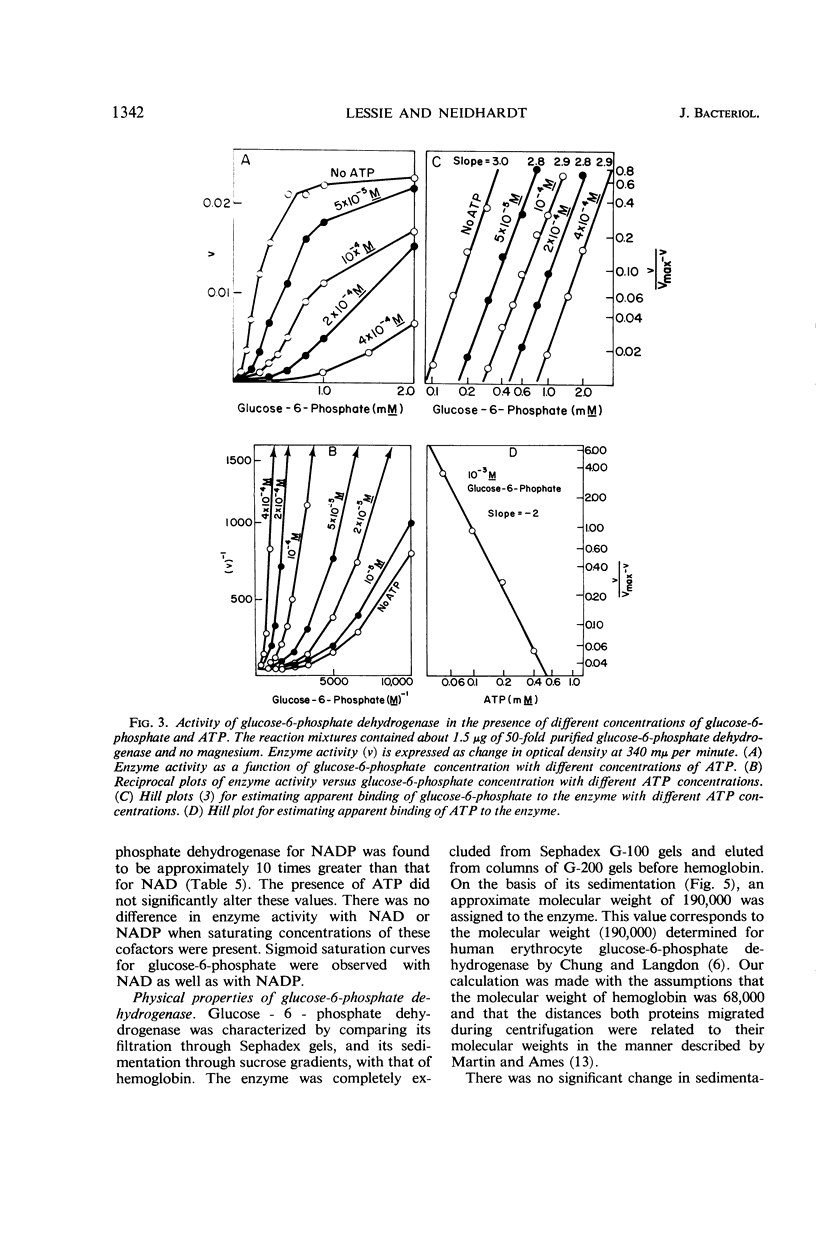
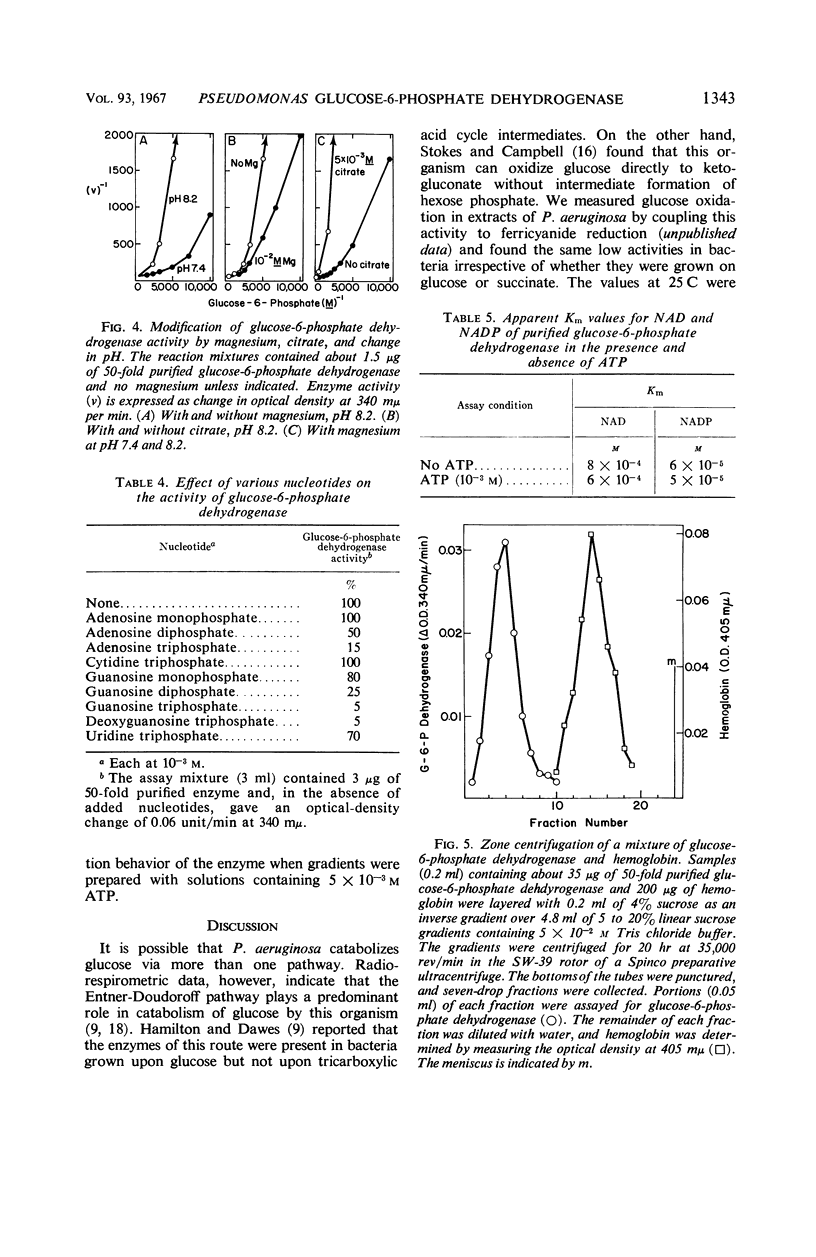
Extracts of Pseudomonas aeruginosa (ATCC 7700) cells grown on glucose, gluconate, or glycerol had enzyme activities related to the Entner-Doudoroff pathway. These activities were present in no more than trace amounts when the bacteria were grown on succinate. Fructose-1,6-diphosphate aldolase could not be detected in extracts of the bacteria grown on any of the above carbon sources. Therefore, it appears that P. aeruginosa degrades glucose via an inducible Entner-Doudoroff pathway. The apparent absence of fructose-1,6-diphosphate aldolase in cells growing on succinate suggests that the bacteria can form hexose and pentose phosphates from succinate by an alternate route. d-Glucose-6-phosphate dehydrogenase, a branch-point enzyme of the Entner-Doudoroff pathway, was purified 50-fold from glucose-grown cells. Its molecular weight, estimated by sucrose density gradient centrifugation, was found to be approximately 190,000. The enzyme was strongly inhibited by adenosine triphosphate, guanosine triphosphate, and deoxyguanosine triphosphate, which decreased the apparent binding of glucose-6-phosphate to the enzyme. It is suggested that adenine nucleotide-linked control of glucose-6-phosphate dehydrogenase may regulate the overall catabolism of hexose phosphates and prevent their wasteful degradation under certain conditions requiring gluconeogenesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES: EFFECTORS FOR YEAST PHOSPHOFRUCTOKINASE DO NOT ALTER THE APPARENT KINETIC ORDER OF THE REACTION. Biochem Biophys Res Commun. 1965 Jan 4;18:1–5. doi: 10.1016/0006-291x(65)90872-7. [DOI] [PubMed] [Google Scholar]
- ATKINSON D. E., WALTON G. M. KINETICS OF REGULATORY ENZYMES. ESCHERICHIA COLI PHOSPHOFRUCTOKINASE. J Biol Chem. 1965 Feb;240:757–763. [PubMed] [Google Scholar]
- Atkinson D. E. Biological feedback control at the molecular level. Science. 1965 Nov 12;150(3698):851–857. doi: 10.1126/science.150.3698.851. [DOI] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol. 1966 Aug;92(2):464–469. doi: 10.1128/jb.92.2.464-469.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG A. E., LANGDON R. G. Human erythrocyte glucose 6-phosphate dehydrogenase. I. Isolation and properties of the enzyme. J Biol Chem. 1963 Jul;238:2309–2316. [PubMed] [Google Scholar]
- ENTNER N., DOUDOROFF M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952 May;196(2):853–862. [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- HAYAISHI O., GEFTER M., WEISSBACH H. Adenosine diphosphate-dependent threonine dehydrase activity in extracts of Clostridium tetanomorphum. J Biol Chem. 1963 Jun;238:2040–2044. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- STOKES F. N., CAMPBELL J. J. R. The oxidation of glucose and gluconic acid by dried cells of Pseudomonas aeruginosa. Arch Biochem. 1951 Jan;30(1):121–125. [PubMed] [Google Scholar]
- Sable H. Z. Biosynthesis of ribose and deoxyribose. Adv Enzymol Relat Areas Mol Biol. 1966;28:391–460. doi: 10.1002/9780470122730.ch7. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- WANG C. H., STERN I. J., GILMOUR C. M. The catabolism of glucose and gluconate in Pseudomonas species. Arch Biochem Biophys. 1959 Apr;81(2):489–492. doi: 10.1016/0003-9861(59)90229-2. [DOI] [PubMed] [Google Scholar]



