Abstract
Colocalization of the classic neurotransmitters serotonin (5-HT) and γ-aminobutyric acid (GABA) (or the enzyme that synthesizes the latter, glutamate decarboxylase) has been reported in a few neurons of the rat raphe magnus-obscurus nuclei. However, there are no data on the presence of neurochemically similar neurons in the brain of non-mammalian vertebrates. Lampreys are the oldest extant vertebrates and may provide important data on the phylogeny of neurochemical systems. The colocalization of 5-HT and GABA in neurons of the sea lamprey brain was studied using antibodies directed against 5-HT and GABA and confocal microscopy. Colocalization of the neurotransmitters was observed in the diencephalon and the isthmus. In the diencephalon, about 87% of the serotonergic cells of the rostral tier of the dorsal thalamus (close to the zona limitans) exhibited GABA immunoreactivity. In addition, occasional cells double-labelled for GABA and 5-HT were observed in the hypothalamic tuberal nucleus and the pretectum. Of the three serotonergic isthmic subgroups already recognized in the sea lamprey isthmus (dorsal, medial and ventral), such double-labelled cells were only observed in the ventral subgroup (about 61% of the serotonergic cells in the ventral subgroup exhibited GABA immunoreactivity). An equivalence between these lamprey isthmic cells and the serotonergic/GABAergic raphe cells of mammals is suggested. Present findings suggest that serotonergic/GABAergic neurons are more extensive in lampreys than in the rat and probably appeared before the separation of agnathans and gnathostomes. Cotransmission by release of 5-HT and GABA by the here-described lamprey brain neurons is proposed.
Keywords: agnathans, cotransmission, GABA, isthmus, serotonin, thalamus, vertebrate evolution
Introduction
The presence of neurons showing immunoreactivity for both serotonin (5-HT) and γ-aminobutyric acid (GABA) [or the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD)] has been reported in a few cells of some raphe nuclei (raphe dorsalis, raphe magnus, raphe obscurus, pontine and supralemniscal nuclei) of the rat brain (Belin et al. 1983; Millhorn et al. 1987, 1988; Stamp & Semba, 1995). Uptake of 3H-GABA by some raphe 5-HT-immunoreactive (5-HT-ir) cells has also been demonstrated (Gamrani et al. 1984). Both GABA and 5-HT can be accumulated by fibres of serotonergic systems such as supra- and subependymal fibre plexuses (Harandi et al. 1986). Such reports represented one of the first indications of the coexistence of ‘canonical neurotransmitters’ in neuronal subsystems and contributed to the general acceptance of the concept of cotransmission (reviewed by Hökfelt et al. 2000; Vergé & Calas, 2000). On the other hand, several studies have indicated that 5-HT exerts a modulatory effect on GABAergic neurotransmission by potentiation of the inhibitory response of neurons to GABA (Xu et al. 1998; Wang et al. 1999; Li et al. 2000; An et al. 2005).
The neuroanatomical organization of the GABAergic and serotonergic systems has been studied in the sea lamprey (serotonin: Antri et al. 2006; Abalo et al. 2007; Barreiro-Iglesias et al. 2008; GABA: Meléndez-Ferro et al. 2001, 2002, 2003; Robertson et al. 2007). However, as far as we are aware there are no studies concerning the possible colocalization of GABA and 5-HT in neurons of the lamprey brain. There is also a notable lack of information on the presence of this type of neurochemical cell (serotonergic/GABAergic neurons) in the brain of non-mammalian vertebrates. The phylogenetic position occupied by lampreys between invertebrate chordates and gnathostome vertebrates makes these animals key subjects for understanding the early evolution of the different neurotransmitter systems.
In the present study, double immunofluorescence methods and confocal microscopy were used to investigate the possible coexistence of 5-HT and GABA in sea lamprey brain neurons. The results indicate for the first time the colocalization of these classical neurotransmitters in lamprey neurons, which were restricted to a few serotonergic populations. Understanding the neuroanatomical organization of serotonergic/GABAergic neurons will provide insights into the co-functions of the neurotransmitters and their possible co-release in lamprey circuits.
Material and methods
Larval sea lampreys (n = 11; 90–140 mm in total body length) were used. The larvae were collected from the river Ulla (Galicia, Spain). Before all experiments, the animals were deeply anaesthetized with 0.05% benzocaine (Sigma, St. Louis, MO) in fresh water. The experiments were approved by the Ethics Committee of the University of Santiago de Compostela and comply with European Community rules on animal care and experimentation.
Immunofluorescence
The larval heads were fixed by immersion in freshly prepared 2% paraformaldehyde, 2.5% glutaraldehyde and 0.5% sodium metabisulphite in 0.05 m Tris buffered saline pH 7.4 (TBS) for 20 h. The samples were rinsed in TBS containing 1% sodium metabisulphite, then cryoprotected with 30% sucrose in TBS, embedded in Tissue Tek (Sakura, Torrance, CA), frozen in liquid nitrogen-cooled isopentane, and cut serially on a cryostat (20 µm thick) in transverse planes. Sections were mounted on subbed glass slides.
Sections were pretreated with 0.2% NaBH4 in distilled water for 45 min at room temperature. The sections were then incubated with a cocktail of a rabbit polyclonal anti-5-HT antibody (dilution 1 : 2500; Incstar, Still Water, MN; code 200800; lot 050017; immunogen: 5-HT-formaldehyde-BSA conjugate) and a mouse monoclonal anti-GABA antibody (dilution 1 : 1200; Sigma; clone GB-69; lot. 075K4795; immunogen: purified GABA conjugated to BSA) and for 3 days at 4 °C. Sections were rinsed in TBS and then incubated with a cocktail of rhodamine isothiocyanate (RICT)-conjugated swine anti-rabbit antibody (diluted 1 : 30; Dako, Glostrup, Denmark) and fluorescein isothiocyanate (FICT)-coupled goat anti-mouse antibody (diluted 1 : 50; Chemicon, Temecula, CA) for 1 h at room temperature. All antibody dilutions were prepared in TBS containing 1% sodium metabisulphite, 15% normal goat serum (NGS), 10% normal swine serum (NSS) and 0.2% Triton X-100. Sections were rinsed in distilled water and then coverslipped with mounting medium for fluorescence (Vectashield; Vector Laboratories, Burlingame, CA).
Controls
The specificity of the anti-5-HT antibody was tested by the supplier, who reported no detectable cross-reactivity with tryptamine, 5-methoxytryptamine, l-tryptophan, 5-hydroxytryptophan, dopamine, norepinephrine or adrenaline. This antibody has been used in recent immunohistochemical studies in the sea lamprey brain and retina (Villar-Cerviño et al. 2006; Abalo et al. 2007; Barreiro-Iglesias et al. 2008) and was previously tested by Western blot in lamprey brain protein extracts in our laboratory (Villar-Cerviño et al. 2006). No protein band was detected in these blots. Moreover, the specificity of this antibody was tested in lamprey in pre-adsorption experiments (Abalo et al. 2007); immunostaining was completely abolished after pre-adsorption of the diluted anti-5-HT antibody with 5-HT-BSA conjugates.
The monoclonal anti-GABA antibody was evaluated for activity and specificity by dot-blot immunoassay by the supplier. No cross-reaction was observed with BSA, l-α-aminobutyric acid, l-glutamic acid, l-aspartic acid, glycine, δ-aminovaleric acid, l-threonine, l-glutamine, taurine, putrescine, l-alanine or carnosine. The antibody showed weak cross-reaction with β-alanine. Furthermore, the sections of the brain and retina of sea lamprey incubated with this antibody revealed the same pattern of immunostaining shown in studies with other anti-GABA antibodies (Meléndez-Ferro et al. 2001, 2002, 2003; Villar-Cerviño et al. 2006; Robertson et al. 2007). In addition, the antibody was previously tested in our laboratory by Western blotting with lamprey brain protein extracts (Villar-Cerviño et al. 2008a). No protein band was stained in these blots. Immunostaining was completely abolished after pre-adsorption of the diluted anti-GABA antibody with a GABA-BSA conjugate (Sigma).
As a further control, some sections were processed as above but without the primary antibody. No immunostaining was observed in any of the corresponding sections.
Photography and cell measurements
Photomicrographs were taken with a laser spectral confocal microscope (Leica TCS-SP2). Data was acquired in the spectral confocal microscope using a narrow window tuned to the specific emission wavelength of each fluorescent marker (FICT, RICT). For a review about advantages of spectral confocal microscopy see Zimmermann et al. (2002). Photomicrographs were adjusted for brightness and contrast with AdobePhotoshop CS (8.0.1) software. Schematic drawings were made with CorelDraw 12 software.
To estimate the size of the 5-HT-ir/GABA-ir cells, the minor axes of 15 perikarya from each cell group were measured with the aid of the Leica confocal software (LCS) in transverse sections. Mean values and standard deviations (SD) are given for each cell group. In the case of the tuberal and pretectal populations, the mean is not given because very few double-labelled cells were observed in the samples. The percentage of 5-HT-ir cells showing GABA immunoreactivity was calculated for those nuclei that showed a high degree of colocalization (the thalamic and ventral isthmic groups) in three samples. For quantitative analysis, only clearly stained neurons were counted. To avoid counting cells twice, series of photographs were aligned with ImageJ (free NIH software), and the positions of labelled cells in pairs of consecutive sections were compared. This unbiased approach made it unnecessary to introduce any correction factor for possible overestimation of cell numbers.
Nomenclature
The nomenclature adopted for the various serotonergic nuclei of the sea lamprey was that used in a previous developmental study carried out in our laboratory, with slight modifications (Abalo et al. 2007).
Results
General organization of the serotonergic and GABAergic systems
Numerous serotonin-ir neuronal bodies are located in discrete groups of cells distributed in the diencephalon, rhombencephalon and the spinal cord of the sea lamprey (Antri et al. 2006; Abalo et al. 2007; Barreiro-Iglesias et al. 2008). GABAergic cells are much more numerous and widely distributed in the sea lamprey brain (Meléndez-Ferro et al. 2001, 2002, 2003; Robertson et al. 2007). Accordingly, the present results on GABA/5-HT colocalization will be described in relation to the 5-HT-ir nuclei. A detailed description of the organization of the serotonergic and GABAergic immunoreactive systems is outside the scope of the present investigation. The topographical organization of the serotonergic populations in large sea lamprey larvae and the location of neurons double-labelled for GABA/5-HT are schematically illustrated in Fig. 1, which is based on the results of Abalo et al. (2007).
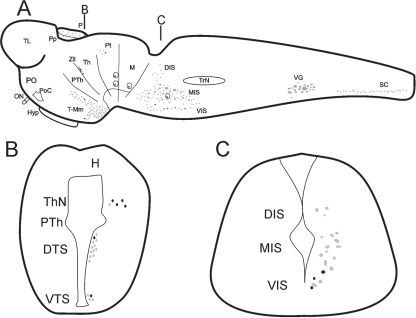
Fig. 1.
Schematic drawings of sagittal (A) and transverse (B and C) sections of the brain of a larval sea lamprey showing the distribution of double-labelled serotonin/GABA immunoreactive cells (black) in comparison with the distribution of serotonergic cells (grey). (A) Sagittal section of the larval brain/spinal cord (SC) showing the organization of the serotonergic nuclei and the location of the 5-HT-ir/GABA-ir cells within these nuclei. The black lines indicate approximately the positions of the transverse sections shown in (B) and (C). (B) Transverse section of the diencephalon showing the location of the 5-HT-ir/GABA-ir cells of the thalamic nucleus (ThN) and the dorsal and ventral tuberal subpopulation (DTS and VTS, respectively). (C) Transverse section of the rostral rhombencephalon showing the location of the 5-HT-ir/GABA-ir cells in the lamprey isthmus. Note the presence of double-labelled cells only in the ventral isthmic subpopulation (VIS) and the absence of this type of cell in the medial (MIS) and dorsal (DIS) subpopulations. H, habenula; Hyp, hypophysis; M, mesencephalon; ON, optic nerve; P, pineal organ; PO, preoptic region; PoC, postoptic commissure; Pp, parapineal organ, Pt, pretectum, PTh, prethalamus, Th, thalamus, TL, telencephalon, T-Mm, tuberal-mammillary nuclei, TrN, trigeminal nucleus; VG, vagal nucleus; Zli, zona limitans intrathalamica.
Serotonin-ir cells were observed in the diencephalon, rhombencephalon and the spinal cord. Present observations in paraformaldehyde/glutaraldehyde-fixed samples did not reveal any differences in the 5-HT-ir system, as found in previous reports in paraformaldehyde-fixed samples (Abalo et al. 2007). In the diencephalon, 5-HT-ir cells were observed in four nuclei (Fig. 1A,B): the pretectal area [group I of Antri et al. (2006)], the thalamus [zona limitans intrathalamica nucleus of Abalo et al. (2007)] and the tuberal and mammillary regions of the hypothalamus. Two cell nuclei were observed in the rhombencephalon (Fig. 1A,C): one in the caudal rhombencephalon [group III of Antri et al. (2006); vagal group of Barreiro-Iglesias et al. (2008)] and another in the rostral rhombencephalon, the isthmic nucleus [group II of Antri et al. (2006)]. Serotonin-ir cells were also observed in the ventral margin of the spinal cord (Fig. 1A). The telencephalon and mesencephalon of the larvae studied did not exhibit 5-HT-ir cells. The general pattern of GABA-ir cell groups observed in the brain of large larvae of the sea lamprey coincided with that previously shown in smaller larvae, by other authors (Meléndez-Ferro et al. 2002, 2003), and will not be described.
Colocalization of 5-HT and GABA in lamprey neurons
Double immunofluorescence experiments in tissue fixed in a paraformaldehyde/glutaraldehyde mixture revealed the presence of GABA-ir neurons in addition to 5-HT-ir neurons in most regions of the sea lamprey brain containing serotonergic cells. However, colocalization of GABA and 5-HT immunoreactivities in cells was only observed in a few serotonergic groups (Figs 2–4). Double-labelled cells were mainly observed in the thalamic (Fig. 2) and isthmic serotonergic nuclei (Fig. 3). Occasional double-labelled cells were observed in another two diencephalic nuclei, the tuberal and pretectal nuclei (Fig. 4). No double-labelled cells were observed in the serotonergic nucleus of the mammillary recess, in the vagal 5-HT-ir reticular nucleus or in the ventromedial region of the spinal cord (i.e. those of the spinal cord; Fig. 4C).
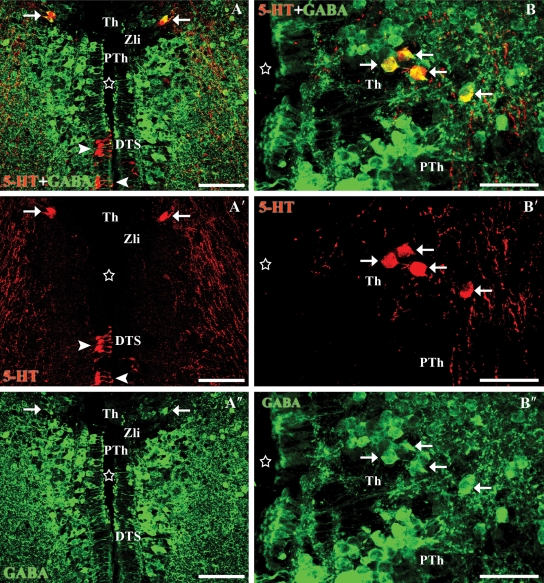
Fig. 2.
Transverse sections through the thalamic and tuberal nuclei of the diencephalon. (A-A″) Photomicrographs of a transverse section of the diencephalon showing double-labelled cells (5-HT in red, GABA in green) in the thalamus (white arrows). Note the presence of a gap without labelled cells between the thalamus and the prethalamus that may correspond to the zona limitans intrathalamica (Zli). Note the absence of double-labelled cells in the dorsal tuberal subgroup of the hypothalamus (arrowheads). (B-B″) Photomicrographs of a transverse section of the dorsal diencephalon showing double-labelled cells in the thalamus (white arrows). Stars indicate the ventricle. All photomicrographs are Z-stack projections from confocal images. Scale bars: 40 µm.
Fig. 4.
Transverse sections through the dorsal tuberal subgroup and the pretectal nucleus of the diencephalon and the spinal cord. (A-A″) Photomicrographs of a transverse section of the hypothalamus showing a 5-HT-ir (red)/GABA-ir (green) cerebrospinal fluid-contacting cell of the dorsal tuberal subgroup (white arrow). (B-B″) Photomicrographs of a transverse section of the caudal diencephalon showing a couple of double-labelled cells in the pretectal nucleus (white arrows). Note that the cells of the pretectum were faintly immunoreactive for the anti-5-HT antibody (red). (C) Photomicrograph of a transverse section of the spinal cord illustrating the absence of double-labelled cells (the thick arrow indicates 5-HT-ir cells and the arrowheads indicate GABA-ir cells). Stars indicate the ventricle. All photomicrographs are Z-stack projections from confocal images. Scale bars: (A) 20 µm, (B) 25 µm, (C) 40 µm.
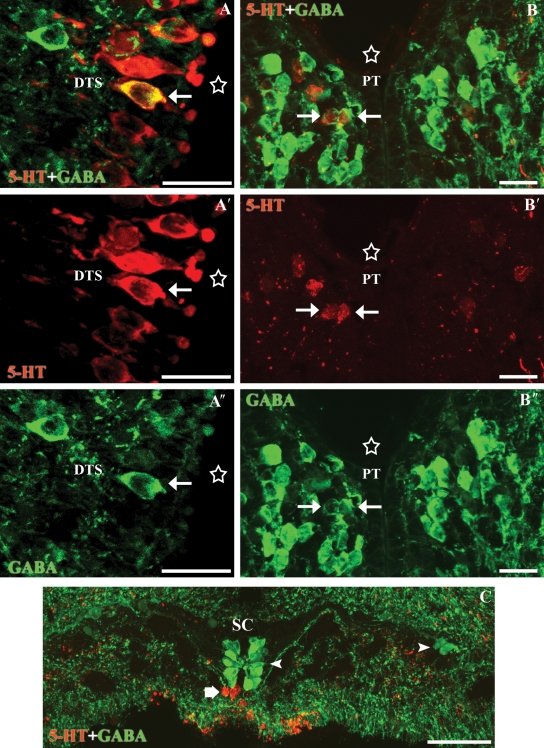
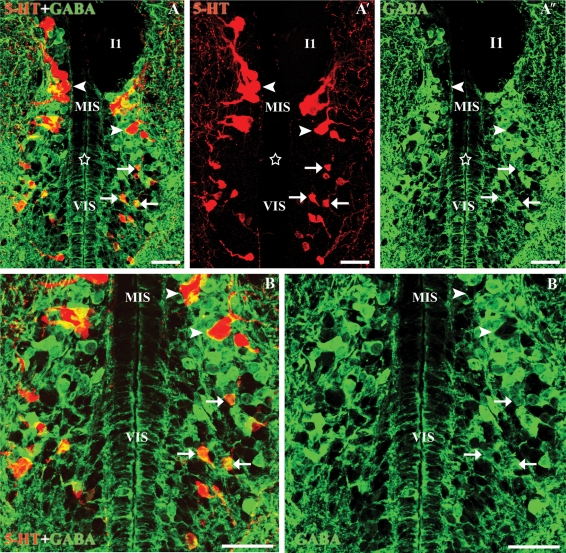
Fig. 3.
Transverse sections through the isthmic region of the rhombencephalon. (A-A″) Photomicrographs of a transverse section of the rostral rhombencephalon showing 5-HT-ir (red) and GABA-ir (green) cells. Note the presence of double-labelled cells in the ventral isthmus (white arrows) and the absence of double-labelled cells in the medial isthmic subgroup (arrowheads). (B-B′) Enlargements of Fig.2A-A″. Stars indicate the ventricle. Note that the anti-5-HT immunofluorescence labelling fills the somas, whereas the GABA labelling is more concentrated at the border of the neuronal somas. I1, Müller isthmic giant cell. All photomicrographs are Z-stack projections from confocal images. Scale bars: 50 µm.
In the alar diencephalon, numerous GABAergic cells were observed in nuclei of the prethalamus, thalamus and pretectum, whereas serotonergic cells were only observed in a thalamic nucleus [the 5-HT-ir nucleus of the zona limitans intrathalamica of Abalo et al. (2007)] and in the pretectum. A number of serotonergic cells in the thalamus were double-labelled with the anti-5-HT and anti-GABA antibodies. Serotonin was mainly colocalized with GABA in a row of cells of the thalamus (prosomere 2) (Fig. 2) close to its rostral limit with the prethalamus (prosomere 3). In all, 87% of the 5-HT-ir cells of this thalamic nucleus were also GABA-ir. The perikarya of these 5-HT-ir/GABA-ir cells were rounded or polygonal, and 10.04 ± 2.57 µm in size. The region devoid of any GABA-labelled cells that is intercalated between the prethalamic and thalamic GABA-ir populations probably corresponds to the zona limitans intrathalamica separating both prosomeres (Fig. 2). The GABA-ir population of the rostral part of the thalamus was far more abundant than that of 5-HT-ir cells, so that the GABA-ir cells outside the zona limitans were 5-HT negative. A nucleus of faint 5-HT-ir round cells was observed in the pretectum of large larvae (i.e. prosomere 1). Occasional cells of this pretectal nucleus (between 7 and 9 µm in size) were double-labelled for 5-HT and GABA (Fig. 4B-B″).
Scarce 5-HT-ir/GABA-ir cells were observed in nuclei of the basal diencephalon. In the hypothalamus, 5-HT-ir bipolar cells were observed in the tuberal region, where dorsal and ventral serotonergic subpopulations were recognizable. The neurons of the dorsal hypothalamic subgroup were more numerous and more intensely stained than those of the ventral subgroup; most of the cells in the dorsal subgroup exhibited intraventricular dendrites with a club-shaped end and processes coursing laterally, and are therefore cerebrospinal fluid-contacting (CSF-c) cells (paraventricular organ). GABA-ir CSF-c cells of similar appearance were also observed in the periventricular hypothalamus, including the regions containing 5-HT-ir neurons. Occasional CSF-c cells in the dorsal hypothalamic subpopulation (between 7 and 8 µm in minor diameter) showed immunoreactivity for both 5-HT and GABA (Fig. 4A-A″). Occasional bipolar cells of the ventral subpopulation (between 8 and 9 µm in size) were also 5-HT-ir/GABA-ir (not shown).
In the isthmus (rostral rhombencephalon), a complex group of serotonergic cells was observed. Three subpopulations of 5-HT-ir cells were recognized in this region: the dorsal, medial and ventral isthmic subpopulations (cf. Abalo et al. 2007). Experiments using immunofluorescence methods revealed numerous GABA-ir neurons intermingled with the medial and ventral subpopulations, whereas such cells were absent from the area occupied by the dorsal serotonergic subpopulation. Double-labelled cells were observed in the ventral subpopulation (Fig. 3). These cells were rounded or fusiform and small (8.56 ± 1.09 µm), and were located in the periventricular cell layer. They represent about the 61% of the total serotonergic cells of the ventral subgroup. By comparison, the larger 5-HT-ir cells of the medial isthmic subgroup were not GABA-ir, although numerous GABA-ir cells were intermingled with these serotonergic cells.
In the caudal rhombencephalon, the 5-HT-ir cells of the vagal nucleus were intermingled with GABA-ir cells but no double-labelled cells were observed in this region (not shown). Double-labelled cells were not observed in the spinal cord; 5-HT-ir cells were observed in a ventromedial position away from the spinal cord central canal, whereas the nearest GABA-ir cells were of CSF-c type and were observed around the central canal (Fig. 4C).
Discussion
Organization of the serotonergic/GABAergic cells in lampreys: comparison with mammals
The present investigation is the first demonstration of the colocalization of 5-HT and GABA immunoreactivities in neurons of the larval lamprey brain. Although the different 5-HT-ir neuronal populations observed at the end of the larval stage are the same as in adults (Abalo et al. 2007), studies in adult lampreys will be necessary to determine whether the pattern of colocalization of these neurotransmitters change during metamorphosis. As far as we are aware, colocalization of 5-HT and GABA has not been reported in the brain of other non-mammalian vertebrates. The absence of studies of this cell type in non-mammalian species may be related to the different fixatives required for immunostaining with the anti-5-HT and anti-GABA antibodies (paraformaldehyde-based and glutaraldehyde-based fixatives, respectively). In the present study, fixation with a paraformaldehyde and glutaraldehyde mixture was developed to carry out the double immunofluorescence experiments. Moreover, the results obtained with the different controls (Western blotting and pre-adsorption with neurotransmitter-BSA conjugates; see Material and methods section) indicate that the antibodies were highly specific for 5-HT and GABA, respectively.
Studies in the rat have reported that neurons double-labelled with 5-HT-ir/GABA-ir or with 5-HT-ir/GAD-ir represent a small or very small percentage of the 5-HT-ir cells in the brainstem raphe nuclei (Belin et al. 1983; Millhorn et al. 1987; Millhorn et al. 1988; Stamp & Semba, 1995). These scarce serotonergic/GABAergic cells were mainly observed in the raphe dorsalis nucleus (Belin et al. 1983), or in the raphe magnus nucleus and raphe obscurus nucleus (Millhorn et al. 1987; Millhorn et al. 1988; Stamp & Semba, 1995); very few cells double-labelled with 5-HT-ir and GAD-ir were reported in the pontine raphe nuclei and supralemniscal nucleus (Stamp & Semba, 1995). In all these rat raphe nuclei a mixture of GABA-ir neurons and 5-HT-ir neurons was observed (Stamp & Semba, 1995). In the lamprey brain, the 5-HT-ir populations were largely intermingled with GABA-ir neurons (with the exception of the dorsal isthmic subpopulation), suggesting a conserved pattern. As regards the possible equivalences between the serotonergic reticular groups of the lamprey rhombencephalon with the raphe-reticular populations of mammals, considerable uncertainty is introduced by the non-migration of 5-HT-ir cells toward the raphe during lamprey development (Abalo et al. 2007), which is unlike that observed in all jawed vertebrates, including cartilaginous fishes (Carrera et al. 2008). In the sea lamprey rhombencephalon, 5-HT-ir/GABA-ir neurons were observed in the ventral isthmic subgroup (present results), which extends between the isthmus and trigeminal levels. We previously suggested that the serotonergic reticular nuclei of lampreys are homologues of the caudal and rostral parts of the serotonergic raphe/reticular complex of jawed vertebrates (Barreiro-Iglesias et al. 2008). This suggests a level equivalence with the neurochemically similar neurons of the rat raphe dorsalis/raphe magnus, although possible homology between the 5-HT-ir/GABA-ir cells of the lamprey ventral isthmus and those of the rat brainstem raphe is uncertain. In the medial isthmic and the caudal (vagal) reticular serotonergic populations, GABA is not colocalized with serotonin. These nuclei contain spinal-projecting serotonergic cells (Barreiro-Iglesias et al. 2008). Occasional serotonergic cells of the ventral isthmic subnucleus were found to project to the rostral spinal cord (Barreiro-Iglesias et al. 2008), but whether any of them colocalize GABA has not been investigated.
The rat diencephalon does not contain serotonergic cells (Lidov & Molliver, 1982; Wallace & Lauder, 1983, 1992), which precludes comparison with diencephalic serotonergic groups of lampreys. Like in lampreys, the diencephalon of other fishes contains abundant CSF-c serotonergic cells in the paraventricular organ, and transient or permanent pretectal-thalamic serotonergic populations have been reported in some teleosts and elasmobranchs (see Ekström et al. 1985; Kaslin & Panula, 2001; Carrera et al. 2008). However, the absence of data on GABA/serotonin colocalization in other fish species prevents comparison with lampreys. Thus, it is not clear whether or not the presence of 5-HT-ir/GABA-ir cells in the diencephalon is a characteristic exclusive to lampreys.
Previous studies of the serotonergic system of the developing and adult sea lamprey described the presence of a 5-HT-ir nucleus in or close to the zona limitans intrathalamica (Zli; Pombal & Puelles, 1999; Meléndez-Ferro et al. 2002; Abalo et al. 2007), which is considered a stretched basal-analogous territory separating the prosomeres p2 and p3. The serotonergic zona limitans population appears early on in lamprey development (Meléndez-Ferro et al. 2002; Abalo et al. 2007). This cell population was apparently located at the rostral limit of the band of GABA-ir cells of the dorsal thalamus (Meléndez-Ferro et al. 2002), but codistribution or colocalization of these substances was not investigated. Here we show that most of the zona limitans 5-HT-ir cells were also GABA immunoreactive and that double-labelled cells are located at the rostral border of the thalamic GABA-ir cell band. Accordingly, this appears to be a unique characteristic of this lamprey population.
Several double immunofluorescence studies of the sea lamprey have shown that GABA is colocalized with different neurotransmitters in cells of different brain/spinal cord nuclei (5-HT: present results; dopamine: Rodicio et al. 2008; glycine: Villar-Cerviño et al. 2008a; aspartate: Villar-Cerviño et al. 2008b). This indicates that GABAergic populations of lamprey do not represent morphologically and neurochemically homogeneous groups and reveals a complex relationship between the GABAergic system and other neurotransmitter systems in the most primitive line of vertebrates, the agnathans. Furthermore, 5-HT is colocalized with dopamine in ventromedial cells of the lamprey spinal cord (Schotland et al. 1995), again indicating that serotonergic populations in lampreys are neurochemically heterogeneous as regards the presence of other classical neurotransmitters in the cell.
Functional implications of the colocalization of 5-HT and GABA in lamprey neurons
Both 5-HT and GABA are stored in synaptic vesicles of neurons (revised by Siegel et al. 2006). The presence of both neurotransmitters in the here-described lamprey neurons suggests that both neurotransmitters may be co-released from these neurons at the synaptic cleft. GABA is a major inhibitory neurotransmitter of the central nervous system, and acts on GABA(A) receptors to elicit inward Cl−currents (Li et al. 2000). Some studies have shown that 5-HT changes the response of rat neurons to GABA by postsynaptic potentiation of the GABA-induced Cl− current (Xu et al. 1998; Wang et al. 1999; Li et al. 2000). This effect appears to be mediated by the activation of 5-HT2 receptors and subsequent activation of the PKC-dependent phosphorylation of the GABA(A) receptor (An et al. 2005).
If these results also apply to lamprey, the present colocalization data would suggest that the double-labelled 5-HT/GABA-ir neurons found mainly in the lamprey isthmus and thalamus are a specialized type of neuron that may potentiate the GABA inhibitory effects on postsynaptic neurons via co-release of serotonin, possibly contrasting with effects produced by GABA-only or serotonin-only synapses. However, the targets of these 5-HT/GABA-ir lamprey neurons are not known, which precludes further speculation.
Concluding remarks
The present investigation is the first demonstration of the presence of both 5-HT and GABA in neurons of the brain of a non-mammalian vertebrate. Serotonergic/GABAergic cells of the lamprey ventral isthmus may be homologues of serotonergic/GABAergic raphe neurons of the rat. However, the thalamic serotonergic/GABAergic cells appear to be characteristic of lampreys. Neurochemical and anatomical data from the present study suggest that the colocalization of 5-HT and GABA in neurons appeared in evolution before the separation of agnathans and gnathostomes.
Acknowledgments
This study was financially supported by the Ministerio de Educación y Ciencia (BFU2004-01080) and the Xunta de Galicia (PGIDIT05P XIC20004PN), and a predoctoral contract (María Barbeito contract) from the Xunta de Galicia to A. Barreiro-Iglesias. We thank the Consellería de Medio Ambiente (Dirección Xeral de Montes e Medio Ambiente Natural) of the Xunta de Galicia for kind permission to capture the animals used in this study. We also thank the Servicio de Microscopía Electrónica and Mercedes Rivas Cascallar (University of Santiago de Compostela) for confocal microscope facilities and assistance.
Author contributions
A. Barreiro-Iglesias contributed to the concept/design of the study, acquisition of experimental data, data analysis/interpretation and drafting of the manuscript. M. E. Cornide-Petronio contributed to the acquisition of experimental data. R. Anadón contributed to the data analysis/interpretation and critical revision of the article. M. C. Rodicio contributed to the concept/design of the study, the data analysis/interpretation, critical revision and final approval of the article.
References
- Abalo XM, Villar-Cheda B, Meléndez-Ferro M, Pérez-Costas E, Anadón R, Rodicio MC. Development of the serotonergic system in the central nervous system of the sea lamprey. J Chem Neuroanat. 2007;34:29–46. doi: 10.1016/j.jchemneu.2007.03.010. [DOI] [PubMed] [Google Scholar]
- An J, Chen CH, Guan BC, Tang M, Yu CG, Li ZW. 5-HT2 receptor mediated the potentiation of GABA-activated current in the membrane of the dorsal root ganglion neurons of rat. Yao Xue Xue Bao. 2005;40:1–7. [PubMed] [Google Scholar]
- Antri M, Cyr A, Auclair F, Dubuc R. Ontogeny of 5-HT neurons in the brainstem of the lamprey, Petromyzon marinus. J Comp Neurol. 2006;495:788–800. doi: 10.1002/cne.20910. [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Villar-Cerviño V, Anadón R, Rodicio MC. Development and organization of the descending serotonergic brainstem-spinal projections in the sea lamprey. J Chem Neuroanat. 2008;36:77–84. doi: 10.1016/j.jchemneu.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Belin MF, Nanopoulos D, Didier M, et al. Immunohistochemical evidence for the presence of gamma-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 1983;275:329–339. doi: 10.1016/0006-8993(83)90994-0. [DOI] [PubMed] [Google Scholar]
- Carrera I, Molist P, Anadón R, Rodríguez-Moldes I. Development of the serotoninergic system in the central nervous system of a shark, the lesser spotted dogfish Scyliorhinus canicula. J Comp Neurol. 2008;511:804–831. doi: 10.1002/cne.21857. [DOI] [PubMed] [Google Scholar]
- Ekström P, Nyberg L, van Veen T. Ontogenetic development of serotoninergic neurons in the brain of a teleost, the three-spined stickleback. An immunohistochemical analysis. Dev Brain Res. 1985;17:209–224. doi: 10.1016/0165-3806(85)90145-2. [DOI] [PubMed] [Google Scholar]
- Gamrani H, Harandi M, Belin MF, Dubois MP, Calas A. Direct electron microscopic evidence for the coexistence of GABA uptake and endogenous serotonin in the same rat central neurons by coupled radioautographic and immunocytochemical procedures. Neurosci Lett. 1984;48:25–30. doi: 10.1016/0304-3940(84)90283-0. [DOI] [PubMed] [Google Scholar]
- Harandi M, Didier M, Aguera M, Calas A, Belin MF. GABA and serotonin (5-HT) pattern in the supraependymal fibers of the rat epithalamus: combined radioautographic and immunocytochemical studies. Effect of 5-HT content on [3H]GABA accumulation. Brain Res. 1986;370:241–249. doi: 10.1016/0006-8993(86)90479-8. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Arvidsson U, Cullheim S, et al. Multiple messengers in descending serotonin neurons: localization and functional implications. J Chem Neuroanat. 2000;18:75–86. doi: 10.1016/s0891-0618(99)00037-x. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Panula P. Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio. J Comp Neurol. 2001;440:342–377. doi: 10.1002/cne.1390. [DOI] [PubMed] [Google Scholar]
- Li H, Lang B, Kang JF, Li YQ. Serotonin potentiates the response of neurons of the superficial laminae of the rat spinal dorsal horn to gamma-aminobutyric acid. Brain Res Bull. 2000;52:559–565. doi: 10.1016/s0361-9230(00)00297-5. [DOI] [PubMed] [Google Scholar]
- Lidov HGW, Molliver ME. Immunohistochemical study of the development of serotoninergic neurons in the rat CNS. Brain Res Bull. 1982;9:559–604. doi: 10.1016/0361-9230(82)90164-2. [DOI] [PubMed] [Google Scholar]
- Meléndez-Ferro M, Pérez-Costas E, Rodríguez-Muñoz R, Gómez-López MP, Anadón R, Rodicio MC. GABA immunoreactivity in the olfactory bulbs of the adult sea lamprey Petromyzon marinusL. Brain Res. 2001;893:253–260. doi: 10.1016/s0006-8993(00)03316-3. [DOI] [PubMed] [Google Scholar]
- Meléndez-Ferro M, Pérez-Costas E, Villar-Cheda B, et al. Ontogeny of gamma-aminobutyric acid-immunoreactive neuronal populations in the forebrain and midbrain of the sea lamprey. J Comp Neurol. 2002;446:360–376. doi: 10.1002/cne.10209. [DOI] [PubMed] [Google Scholar]
- Meléndez-Ferro M, Pérez-Costas E, Villar-Cheda B, Rodríguez-Muñoz R, Anadón R, Rodicio MC. Ontogeny of gamma-aminobutyric acid-immunoreactive neurons in the rhombencephalon and spinal cord of the sea lamprey. J Comp Neurol. 2003;464:17–35. doi: 10.1002/cne.10773. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Hökfelt T, Seroogy K, Oertel W, Verhofstad AA, Wu JY. Immunohistochemical evidence for colocalization of gamma-aminobutyric acid and serotonin in neurons of the ventral medulla oblongata projecting to the spinal cord. Brain Res. 1987;410:179–185. doi: 10.1016/s0006-8993(87)80043-4. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Hökfelt T, Seroogy K, Verhofstad AA. Extent of colocalization of serotonin and GABA in neurons of the ventral medulla oblongata in rat. Brain Res. 1988;461:169–174. doi: 10.1016/0006-8993(88)90736-6. [DOI] [PubMed] [Google Scholar]
- Pombal MA, Puelles L. Prosomeric map of the lamprey forebrain based on calretinin immunocytochemistry, Nissl stain, and ancillary markers. J Comp Neurol. 1999;414:391–422. [PubMed] [Google Scholar]
- Robertson B, Auclair F, Ménard A, Grillner S, Dubuc R. GABA distribution in lamprey is phylogenetically conserved. J Comp Neurol. 2007;503:47–63. doi: 10.1002/cne.21348. [DOI] [PubMed] [Google Scholar]
- Rodicio MC, Villar-Cerviño V, Barreiro-Iglesias A, Anadón R. Colocalization of dopamine and GABA in spinal cord neurones in the sea lamprey. Brain Res Bull. 2008;76:45–49. doi: 10.1016/j.brainresbull.2007.10.062. [DOI] [PubMed] [Google Scholar]
- Schotland J, Shupliakov O, Wikstrom M, et al. Control of lamprey locomotor neurons by colocalized monoamine transmitters. Nature. 1995;374:266–268. doi: 10.1038/374266a0. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Albers RW, Brady ST, Price DL. Basic Neurochemistry. Molecular, Cellular, and Medical Aspects. San Diego: Elsevier Academic Press; 2006. [Google Scholar]
- Stamp JA, Semba K. Extent of colocalization of serotonin and GABA in the neurons of the rat raphe nuclei. Brain Res. 1995;677:39–49. doi: 10.1016/0006-8993(95)00119-b. [DOI] [PubMed] [Google Scholar]
- Vergé D, Calas A. Serotoninergic neurons and serotonin receptors: gains from cytochemical approaches. J Chem Neuroanat. 2000;18:41–56. doi: 10.1016/s0891-0618(99)00050-2. [DOI] [PubMed] [Google Scholar]
- Villar-Cerviño V, Abalo XM, Villar-Cheda B, et al. Presence of glutamate, glycine, and gamma-aminobutyric acid in the retina of the larval sea lamprey: comparative immunohistochemical study of classical neurotransmitters in larval and postmetamorphic retinas. J Comp Neurol. 2006;499:810–827. doi: 10.1002/cne.21136. [DOI] [PubMed] [Google Scholar]
- Villar-Cerviño V, Barreiro-Iglesias A, Anadón R, Rodicio MC. Distribution of glycine immunoreactivity in the brain of adult sea lamprey (Petromyzon marinus). Comparison with gamma-aminobutyric acid. J Comp Neurol. 2008a;507:1441–1463. doi: 10.1002/cne.21634. [DOI] [PubMed] [Google Scholar]
- Villar-Cerviño V, Barreiro-Iglesias A, Anadón R, Rodicio MC. Aspartate immunoreactivity in the telencephalon of the adult sea lamprey: comparison with GABA immunoreactivity. Brain Res Bull. 2008b;75:246–250. doi: 10.1016/j.brainresbull.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Wallace JA, Lauder JM. Development of the serotoninergic system in the rat embryo: an immunocytochemical study. Brain Res Bull. 1983;10:459–479. doi: 10.1016/0361-9230(83)90144-2. [DOI] [PubMed] [Google Scholar]
- Wallace JA, Lauder JM. Development of the serotoninergic systems in rat and chick embryos. In: Björklund A, Hökfelt T, Tohyama M, editors. Handbook of Chemical Neuroanatomy. Ontogeny of Transmitters and Peptides in the CNS. Amsterdam: Elsevier; 1992. pp. 619–645. (eds), vol 10. [Google Scholar]
- Wang DS, Xu TL, Li JS. 5-HT potentiates GABA- and glycine-activated chloride currents on the same neurons in rat spinal cord. J Hirnforsch. 1999;39:531–537. [PubMed] [Google Scholar]
- Xu TL, Pang ZP, Li JS, Akaike N. 5-HT potentiation of the GABA(A) response in the rat sacral dorsal commissural neurones. Br J Pharmacol. 1998;124:779–787. doi: 10.1038/sj.bjp.0701896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann T, Rietdorf J, Girod A, Georget V, Pepperkok R. Spectral imaging and linear un-mixing enables improved FRET efficiency with a novel GFP2-YFP FRET pair. FEBS Lett. 2002;531:245–249. doi: 10.1016/s0014-5793(02)03508-1. [DOI] [PubMed] [Google Scholar]