Abstract
Testis structure and function in dogs are relatively poorly investigated. The aim of the present study was to carry out a comparative investigation of the stages of the seminiferous epithelium cycle and its duration in different breeds of dog. Fifty-six sexually mature dogs (mongrel, n = 12; pinscher, n = 12; beagle, n = 5; American pit bull, n = 9; poodle, n = 12; and Labrador retriever, n = 6) were analysed. Intratesticular injections of tritiated thymidine were given to determine the duration of spermatogenesis. Orchiectomy was performed at different time periods following injection (1 h, 2 and 4 weeks). Testis fragments were embedded in plastic and routinely prepared for histological and autoradiographic evaluations. Eight stages were characterized based on the acrosome system. Significant (P < 0.05) differences were found for the frequencies of the different stages characterized (except Stages V, VI and VIII), particularly for the mongrel. Stage IV (when spermiation occurs) was the most frequent in all six breeds (∼25%), whereas Stages II and VIII were the least frequent (< 8%). Each spermatogenic cycle and the total duration of spermatogenesis lasted 13.73 ± 0.03 and 61.9 ± 0.14 days, respectively, for the mongrel, poodle, pinscher, beagle, and Labrador retriever. These values were ∼10% lower (P < 0.03) for the American pit bull (12.55 ± 0.26 and 56.5 ± 1.17 days, respectively). To our knowledge, this is the first comprehensive study to perform a careful investigation of stage frequencies and seminiferous epithelium cycle duration in this very important domestic species.
Keywords: Canis familiaris, dog breeds, testis, spermatogenesis, seminiferous epithelium cycle
Introduction
The ancestor of domestic dogs (the wolf, Canis lupus) was domesticated by prehistoric man more than 15 000 years ago. Inbreeding and geographic isolation gave rise to the currently known breeds, such as herders and hunters as well as guard, guide, traction and companion dogs (Björnerfeldt et al. 2006; Cruz et al. 2008).
Spermatogenesis comprises the process of transformation of spermatogonial cells into spermatozoa over an extended period of time within the seminiferous tubule boundaries of the testis (Hess & França, 2007). This process is made up of different cell associations called stages, which are established before puberty and classified based on changes in the shape of the spermatid nucleus, the occurrence of meiotic divisions and the arrangement of spermatids within the germinal epithelium (França & Russell, 1998; França et al. 1998). These stages can also be identified based on the development of the acrosome system and the morphology of developing spermatids (Leblond & Clermont, 1952; Russell et al. 1990; Hess & França, 2007) as well as tubular morphology, in which the shape and location of the spermatid nucleus are the main aspects considered (Amann, 1962; Courot et al. 1970; França & Russell, 1998; Almeida et al. 2006; Leal & França, 2006). Stages of the seminiferous epithelium cycle are considered relatively constant for the same species, although some variation is found in different breeds or strains (Swierstra, 1968; Okwun et al. 1996; França & Russell, 1998). Minor modifications in the expected composition of cell associations and variations in stages frequency also occur (França & Russell, 1998).
The total duration of spermatogenesis is under the control of the germ cell genotype (França et al. 1998) and lasts from 30 to 75 days in mammals (França & Russell, 1998; Hess & França, 2007). Although the length of the spermatogenic cycle is considered a biological constant for species (Clermont, 1972), strain or breed differences are found in the literature regarding cycle length between members of the same species (Salim & Entwistle, 1982; Russell et al. 1990). Testis structure and organization are very similar in mammals. However, each species may exhibit particular morphofunctional characteristics related to phylogenetic aspects, reproductive strategy and/or behaviour (Kerr et al. 2006; Setchell & Breed, 2006).
The domestic dog is a highly important species for mankind, serving, for example, as company, guide, guard, traction and various other functions. Dogs are also very important to biomedical studies. Despite the vast number of dog breeds, spermatogenesis in this species remains poorly investigated (Foote et al. 1972; Amann, 1986; Russell et al. 1990; Paula & Cardoso, 1995). Because variations might occur for different breeds or strains, regarding for instance the duration of spermatogenesis and sperm production (Okwun et al. 1996; França & Russell 1998), we are currently developing a broad-scoped basic comparative study on important testicular parameters for this species. In the present study we chose six different breeds of dog, based on their size and predominance in the southeast region of Brazil, and our aim was to investigate the stages of the seminiferous epithelium cycle and its duration in these breeds.
Materials and methods
Animals
Fifty-six sexually mature dogs (estimated age 1.5–7 years) of six different breeds classified according to body weight (Grandjean & Vasaire, 2001) as small (up to 10 kg; pinscher and poodle), medium (10–25 kg; mongrel and beagle) and large (25–45 kg; pit bull and Labrador), were analysed (Table 1). The dogs came from different locations in the state of Minas Gerais, Brazil. The animals were maintained at the kennel of the Institute of Biological Sciences of the Federal University of Minas Gerais. Prior to orchiectomy, all dogs received an intramuscular injection of xylazine hydrochloride (Vetbrands, Sespo Indústria e Comércio Ltda, Jacareí, São Paulo, Brazil) associated with ketamine (König, König do Brasil Ltda, Santana de Parnaíba, São Paulo, Brazil) at 1.1 and 15 mg per kg of body weight, respectively. As sperm production in dogs appears unaffected by season (Amann, 1986) the animals were not orchiectomized at a particular period of the year. All surgical procedures were performed by veterinarians and followed approved guidelines for the ethical treatment of animals (Ethics Committee in Animal Experimentation from the Federal University of Minas Gerais – CETEA/UFMG).
Table 1.
Age and biometric data of different dog breeds
| Breed | Animal | Body weight (kg) | Mean testis weight (g) | Age (year) |
|---|---|---|---|---|
| Mongrel | 1 | 7.5 | 7.2 | 3 |
| 2 | 11 | 10.3 | 2 | |
| 3 | 10 | 9.8 | 4 | |
| 4 | 9 | 6.7 | 1.5 | |
| 5 | 14 | 10.5 | 2 | |
| 6 | 11 | 12 | 1.5 | |
| 7 | 10 | 11.4 | 2 | |
| 8 | 8 | 6.9 | 1.5 | |
| 9 | 10 | 8 | 1.5 | |
| 10 | 14 | 11 | 3 | |
| 11 | 6.5 | 6.5 | 2 | |
| 12 | 12 | 9.7 | 1.5 | |
| (mean ± SEM) | 10.3 ± 0.7 | 8.8 ± 0.6 | 2.1 ± 0.2 | |
| Beagle | 1 | 14 | 12.2 | 7 |
| 2 | 13 | 10 | 2 | |
| 3 | 10 | 9.5 | 6 | |
| 4 | 10 | 8.6 | 4 | |
| 5 | 12 | 9.9 | 3 | |
| (mean ± SEM) | 11.8 ± 0.8 | 10 ± 0.6 | 4.4 ± 0.9 | |
| Poodle | 1 | 9 | 7.5 | 3 |
| 2 | 11 | 8.8 | 3 | |
| 3 | 10 | 8.1 | 2.5 | |
| 4 | 8 | 7.3 | 2 | |
| 5 | 9 | 7.3 | 2.5 | |
| 6 | 11 | 8.52 | 7 | |
| 7 | 7 | 7.7 | 2 | |
| 8 | 8 | 6.3 | 2 | |
| 9 | 5 | 5.3 | 3 | |
| 10 | 8 | 6 | 3 | |
| 11 | 9 | 7.6 | 2 | |
| 12 | 5 | 6.1 | 2 | |
| (mean ± SEM) | 8.3 ± 0.6 | 7.3 ± 0.3 | 2.8 ± 0.4 | |
| Pinscher | 1 | 0.8 | 2.4 | 2 |
| 2 | 1.9 | 2.5 | 3 | |
| 3 | 0.9 | 1.5 | 1.5 | |
| 4 | 1.1 | 1.7 | 6 | |
| 5 | 2.5 | 3.6 | 4 | |
| 6 | 4 | 7.6 | 2 | |
| 7 | 3 | 4.2 | 7 | |
| 8 | 3.5 | 5.5 | 4 | |
| 9 | 4.5 | 6.3 | 2 | |
| 10 | 3 | 4.9 | 1.5 | |
| 11 | 4.5 | 6.4 | 2 | |
| 12 | 4 | 5.8 | 3 | |
| (mean ± SEM) | 2.8 ± 0.4 | 4.4 ± 0.6 | 3.2 ± 0.5 | |
| Labrador | 1 | 30 | 15.7 | 2 |
| 2 | 34 | 24.4 | 2 | |
| 3 | 38 | 21.3 | 4 | |
| 4 | 32 | 18.5 | 3 | |
| 5 | 27.5 | 17.4 | 1.5 | |
| 6 | 28.4 | 16.6 | 1.5 | |
| (mean ± SEM) | 31.7 ± 1.6 | 19 ± 1.3 | 2.3 ± 0.4 | |
| Pit bull | 1 | 28 | 23 | 2 |
| 2 | 25 | 21.3 | 2 | |
| 3 | 25 | 18.5 | 3 | |
| 4 | 24 | 18.2 | 5 | |
| 5 | 25 | 21.4 | 1.5 | |
| 6 | 25 | 20 | 2 | |
| 7 | 25 | 20.8 | 3 | |
| 8 | 30 | 21.9 | 3 | |
| 9 | 27 | 22.6 | 1.5 | |
| (mean ± SEM) | 26 ± 0.6 | 20.9 ± 0.6 | 2.6 ± 0.4 |
Thymidine injections
To estimate the duration of spermatogenesis, intratesticular injections (75 µCi per testis) of tritiated thymidine [thymidine (methyl-3H), specific activity 82.0 Ci mmol−1, Amersham Life Science, UK] were administered near the cauda epididymis prior to orchiectomy. Three time intervals were considered after the thymidine injections (1 h, 14 and 27 days). However, due to very aggressive behaviour, one pit bull was orchiectomized one day earlier (26 instead of 27 days). Following orchiectomy, testes were separated from the epididymis, weighed (Table 1) and cut longitudinally by hand (with a razor blade). The fragments were then fixed by immersion with 4% buffered glutaraldehyde for 12–24 h. Tissue samples measuring 2–3 mm in thickness were routinely processed and embedded in plastic (glycol methacrylate) for histological and autoradiographic analysis, and the procedures used were similar for both methodologies.
For the autoradiographic analysis, unstained testis sections (4 µm) were dipped in the autoradiographic emulsion (Kodak NTB-2, Eastman Kodak Company, Rochester, NY) at 43–45 °C. After drying for approximately 1 h at 25 °C, the testis sections were placed in sealed black boxes and stored in a refrigerator at 4 °C for approximately 4 weeks. The sections were then developed in Kodak D-19 (Eastman Kodak Company) solution at 15 °C (Bundy, 1995) and stained with toluidine blue. Analysis of these sections was performed with the aid of light microscopy to detect the most advanced germ cell type labelled at the different time periods following the thymidine injections. Cells were considered labelled when four or more grains were present over the nucleus on a low to moderate background.
Stages of the seminiferous epithelium cycle and cycle length
Seminiferous epithelium cycle stages were characterized based on the development of the acrosome system and morphology of the developing spermatid nucleus. The relative stage frequencies were determined by evaluating at least 250 seminiferous tubule cross-sections per animal (magnification: 400×). The seminiferous tubules examined were chosen at random. Both testes were analysed for each animal. The histological sections used were those with the best quality.
The duration of the spermatogenic cycle was estimated based on the stage frequencies and the most advanced germ cell type labelled at different time periods following the thymidine injections. The total duration of spermatogenesis took into account that approximately 4.5 cycles are necessary for this process to be completed from Type A spermatogonia to spermiation (Amann & Schanbacher, 1983).
Statistical analyses
All data are presented as mean ± SEM and were analysed using anova(Tukey test). Analysis was performed using the statistica 3.11 software program for Windows (StatSoft, Inc., Tulsa, OK). The significance level was set at P < 0.05.
Results
Stages of the seminiferous epithelium cycle and relative stage frequencies
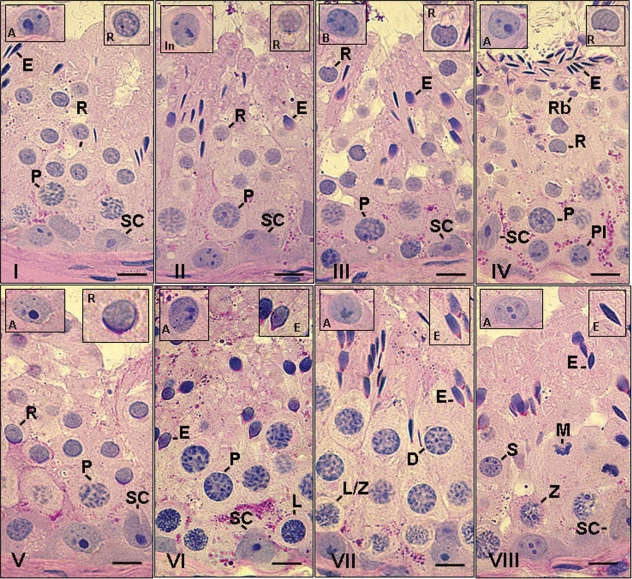
Eight cycle stages were characterized according to the acrosome system in dogs. These stages are shown in Fig. 1and described below.
Fig. 1.
Different stages of the seminiferous epithelium cycle characterized according to the acrosome system in dogs. VIII stages were characterized and the following cell types are indicated: A, Type A spermatogonia; In, intermediate spermatogonia; B, Type B spermatogonia; Pl, pre-leptotene; L, leptotene; Z, zygotene; P, pachytene; D, diplotene; M, meiotic division; R, round spermatids; E, elongated spermatids; Rb, residual bodies; SC, Sertoli cell. In each figure, the inserts in the upper right and left corners show, respectively, a representative spermatid step and spermatogonial type characteristic/predominant of the particular stage illustrated. Bar = 10 µm.
Stage I
Two generations of spermatids were present in this stage: early round spermatids and elongated spermatids. As the proacrosomal granules cannot be seen by a light microscope, the newly formed round spermatids in this stage were characterized by a lack of distinguishing features. However, a juxtanuclear Golgi apparatus was evident. Elongated spermatid bundles were more packed and some were located deep within the epithelium. Young pachytene spermatocytes were located between round spermatids and the basal lamina. Type A spermatogonia were observed in contact with the basal lamina.
Stage II
In dogs, early round spermatids typically have a protuberant acrosomal vesicle in which only occasional proacrosomal granules are seen. At the end of this stage, the small proacrosomal vesicles containing a single acrosome granule were in contact with the nucleus. The elongated spermatids had moved toward the seminiferous tubule lumen. Pachytene spermatocytes were more distant from the basal lamina. A small number of intermediate spermatogonia were present and Type A spermatogonia were occasionally observed in this stage.
Stage III
In this stage, the acrosome was spread slightly over the nucleus of round spermatids and the acrosomal vesicle remained round. The acrosomal vesicle in dogs is indented on the surface of the nucleus and forms an angle of approximately 70°. The elongated spermatid bundles had dissociated and were located very close to the tubular lumen; small residual bodies were also present. A greater number of Type A spermatogonia and intermediate spermatogonia were present, along with pachytene spermatocytes with larger nuclei.
Stage IV
An extensive acrosomal vesicle was seen in round spermatids in this stage. It extended over the nucleus and began to flatten and bend slightly, forming a greater than 90° angle (ranging from ∼90 to ∼110°). Elongated spermatids were undergoing spermiation toward the tubular lumen and large residual bodies were observed just below these cells. Type B spermatogonia were present in contact with the basal lamina. Type A spermatogonia and pre-leptotene spermatocytes (originating from Type B spermatogonia) were occasionally observed in this stage.
Stage V
In this stage, the nuclei of spermatids were still round and the acrosome vesicle formed an angle of approximately 120° (∼110 to ∼150°) over the nucleus. Two generations of primary spermatocytes were present – pre-leptotene/leptotenes located in contact with the basal lamina and pachytenes positioned between round spermatids and pre-leptotene spermatocytes. As all elongated spermatids had under-gone spermiation, this stage only had one generation of spermatids. Type A spermatogonia were also present.
Stage VI
The spermatid nuclei began to elongate and group together. The ratio between the shortest axis (transversal line passing across the nucleus at the equatorial zone) and the longest or longitudinal axis was > 1/3 for this species. At the end of this stage, pachytene spermatocytes transitioned to the diplotene phase of the meiotic prophase. A slightly larger number of Type A spermatogonia was observed.
Stage VII
Elongation of the spermatids was complete during this stage and these cells first formed bundles, with their heads oriented toward the base of the tubule. The ratio between the longest and the shortest axis of the nucleus in dogs was ≤ 1/3. Condensation of the spermatid nucleus (as reflected by staining intensity) was more evident, particularly at the end of this stage. Diplotene spermatocytes were present and young primary spermatocytes were in leptotene. Compared to the former stage, the number of Type A spermatogonia was slightly greater.
Stage VIII
The main feature of this stage was the presence of meiotic figures of the first and second divisions; therefore, secondary spermatocytes and early round spermatids were present. In comparison with the previous stage, nuclei of elongated spermatids had a similar shape. Judging by the staining affinity, condensation of these cells was still occurring and the spermatid bundles were located within Sertoli cell crypts. Zygotene spermatocytes were present and were undergoing the transition into pachytenes at the end of this stage. Compared to the intermediate stages, the number of Type A spermatogonia was substantially increased.
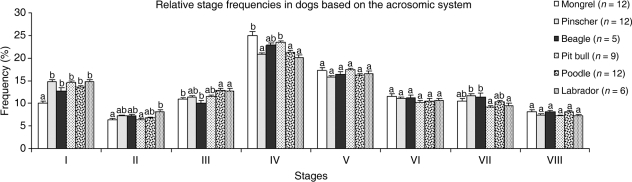
Figure 2displays the stage frequencies for the six breeds of dogs investigated. Significant (P < 0.05) differences were observed between stages (except Stages V, VI and VIII), particularly in the mongrel. However, Stage IV, in which spermiation occurs, was the most frequent (∼25%) in all breeds, whereas Stages II and VIII were the least frequent (< 8%). Table 2 displays the combined frequency of stages in dogs based on the occurrence of meiosis. These frequencies range from 36% to 40% in the pre-meiotic phase (corresponding to Stages V, VI and VII); 52% to 57% in the post-meiotic phase (corresponding to Stages I, II, III and IV); and 7% to 9% during the meiotic divisions (Stage VIII). As the mongrel and American pit bull are situated in the upper and lower range regarding the pre-meiotic and post-meiotic stage frequencies, the values are different (P < 0.05) for these two breeds. The same occurs when the post-meiotic frequencies in the American pit bull and the beagle are compared (P < 0.05) (Table 2).
Fig. 2.
Frequency of the eight stages of the seminiferous epithelium cycle characterized according to the development of the acrosome in the spermatids of the six different breeds investigated. Different letters for the same stage indicate statistical significance (P < 0.05).
Table 2.
Combined frequencies of the seminiferous epithelium, according to the occurrence of meiosis (mean ± SEM)
| Breed | Pre-meiotic | Meiotic | Post-meiotic |
|---|---|---|---|
| Mongrel | 40 ± 0.9a* | 8 ± 0.4a | 52 ± 0.9a |
| Pinscher | 39 ± 1.2ab | 7 ± 0.6a | 54 ± 1.0ab |
| Beagle | 39 ± 1.0ab | 9 ± 0.7a | 52 ± 1.6ac |
| Pit bull | 36 ± 0.4b | 7 ± 0.2a | 57 ± 0.5b |
| Poodle | 37 ± 0.5ab | 8 ± 0.2a | 55 ± 0.6ab |
| Labrador | 37 ± 0.4ab | 7 ± 0.3a | 56 ± 0.6bc |
Different letters for the same column indicate statistical difference (P < 0.05).
Germ cell labelling
Mongrel, beagle, pinscher, poodle and Labrador retriever
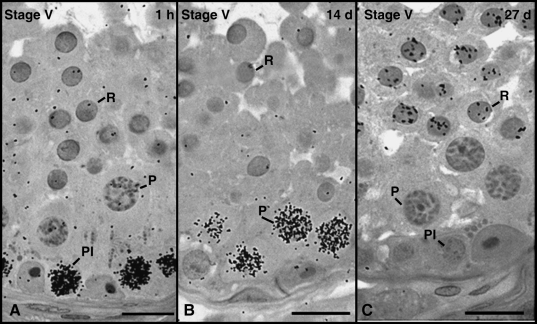
Approximately 1 h after injection, the most advanced labelled germ cells were identified as pre-leptotene spermatocytes or cells in the transition from pre-leptotene to leptotene. These cells were present at the end of Stage V and located in the basal compartment (Fig. 3A). The most advanced germ cell type labelled 14 days after thymidine injection was primary spermatocytes in Stage V (Fig. 3B). At 27 days, round spermatids were the most advanced germ cell labelled and located at the end of Stage V (Fig. 3C).
Fig. 3.
The most advanced labelled germ cell types found after intratesticular thymidine injections in mongrel, beagle, pinscher, poodle and Labrador. At 1–2 h, 14 days and 27 days after injection, these cells were respectively pre-leptotene spermatocytes (Pl) in Stage V (A), pachytene spermatocytes (P) in Stage V (B) and round spermatids (R) in Stage V (C). Bar = 15 µm.
American pit bull
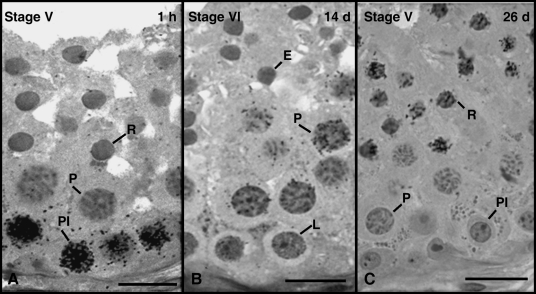
As observed for the other dog breeds investigated, approximately 1 h after injection, pre-leptotene/leptotene spermatocytes were the most advanced labelled germ cells present at the end of Stage V (Fig. 4A). Unlike the other breeds, however, primary spermatocytes in Stage VI were the most advanced germ cell type labelled 14 days after injection in the American pit bull (Fig. 4B). At approximately 26 days (1 day earlier than the other breeds), these cells were also round spermatids present in Stage V (Fig. 4C).
Fig. 4.
In American pit bulls, the most advanced germ cell types labelled 1–2 h, 14 days and 26 days were pre-leptotene spermatocytes (Pl) in Stage V (A), pachytene spermatocytes (P) in Stage VI (B) and round spermatids in Stage V (C), respectively. Bar = 15 µm.
Seminiferous epithelium cycle length
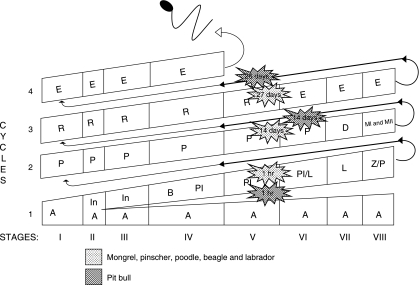
Based on the most advanced labelled germ cell type observed in each time period following thymidine injection and the stage frequencies (Table 3 and Fig. 5), the mean duration of the seminiferous epithelium cycle for the mongrel, beagle, pinscher, poodle and Labrador were estimated as 13.73 ± 0.03 days; this value was 12.55 ± 0.26 days (P < 0.009 or less) for the American pit bull. The duration of the various stages of the cycle was determined taking into account the cycle length and the percentage of occurrence of each stage. The shortest stage was Stage II (∼1 day) and the longest was Stage IV (∼3 days). As approximately 4.5 cycles are necessary for the spermatogenic process to be completed, the total length of spermatogenesis was estimated as 61.9 ± 0.14 days for the mongrel, poodle, pinscher, beagle and Labrador retriever, and 56.5 ± 1.17 days for the American pit bull (P < 0.009 or less).
Table 3.
Length in days (mean ± SEM) of the seminiferous epithelium cycle in different dog breeds
| Breed | Time after injection | Most advanced germ cell labelled | Stage of the cycle | No. of cycles traversed | Cycle length based on labelling in leptotene | |
|---|---|---|---|---|---|---|
| Mongrel 1 | ||||||
| RT | 14 days | P | V | 1 | 13.5 | |
| LT | 26.9 days | R | V | 2 | 13.9 | |
| Mongrel 2 | ||||||
| RT | 14 days | P | V | 1 | 13.5 | |
| LT | 27 days | R | V | 2 | 13.9 | |
| Mongrel 3 |
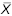 = 13.6 ± 0.08 = 13.6 ± 0.08 |
|||||
| RT | 1–2 h | Pl | V | – | – | |
| LT | 27 days | R | V | 2 | 13.5 | |
| Mongrel 4 | ||||||
| RT | 1–2 h | Pl | V | – | – | |
| LT | 27 days | R | V | 2 | 13.5 | |
| Beagle 1 | ||||||
| RT | 1–2 h | Pl | V | |||
| RT | 14.2 days | P | V | 1 | 14.1 | |
| LT | 27.1 days | R | V | 2 | 13.5 | |
| Beagle 2 |
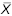 = 13.8 ± 0.17 = 13.8 ± 0.17 |
|||||
| RT | 1–2 h | Pl | V | |||
| RT | 14.2 days | P | V | 1 | 14.1 | |
| LT | 27.1 days | R | V | 2 | 13.5 | |
| Poodle 1 | ||||||
| RT | 1–2 h | Pl | V | – | – | |
| RT | 14.1 days | P | V | 1 | 14 |
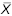 = 13.8 ± 0.20 = 13.8 ± 0.20 |
| LT | 27.1 days | R | V | 2 | 13.6 | |
| Pinscher 1 | ||||||
| RT | 1–2 h | Pl | V | – | – | |
| RT | 14 days | P | V | 1 | 14 |
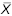 = 13.75 ± 0.25 = 13.75 ± 0.25 |
| LT | 27 days | R | V | 2 | 13.5 | |
| Labrador 1 | ||||||
| RT | 1–2 h | Pl | V | – | – | |
| RT | 14 days | P | V | 1 | 14 | |
| LT | 27 days | R | V | 2 | 13.5 | |
| Labrador 2 |
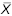 = 13.75 ± 0.20 = 13.75 ± 0.20 |
|||||
| RT | 1–2 h | Pl | V | – | – | |
| RT | 14 days | P | V | 1 | 14 | |
| LT | 27 days | R | V | 2 | 13.5 | |
| Pit Bull 1 | ||||||
| LT | 1–2 h | Pl | V | – | – | |
| LT | 14.1 days | P | VII | 1.2 | 11.8 | |
| Pit Bull 2 | ||||||
| RT | 1–2 h | Pl | V | – | – | |
| RT | 14.1 days | P | VI | 1.1 | 12.7 |
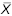 = 12.55 ± 0.26 = 12.55 ± 0.26 |
| LT | 14.1 days | P | VI | 1.1 | 12.7 | |
| Pit Bull 3 | P < 0.03 | |||||
| RT | 1–2 h | Pl | V | – | – | |
| LT | 26.1 days | R | V | 2 | 13 | |
RT, right testis; LT, left testis. Pl, preleptotene; P, pachytene; R, round spermatids.
Fig. 5.
Germ cell composition and the most advanced germ cell type labelled for the different dog breeds investigated in the eight cycle stages and at different time periods (1 h, 14, 26 and 27 days) following tritiated thymidine injections. Roman numerals indicate the stage and Arabic numerals indicate the spermatogenic cycle. The space given for each stage is a proportional representation of its frequency and duration. Letters within each column indicate the germ cell types present in each stage of the cycle. A, Type A spermatogonia; In, intermediate spermatogonia; B, Type B spermatogonia; Pl, pre-leptotene spermatocytes; L, leptotene; Z, zygotene; P, pachytene; D, diplotene; MI/MII, meiotic division; R, round spermatids; E, elongated spermatids.
Discussion
Although the dog is an important domestic animal, there are surprisingly few thorough reports in the literature regarding the testis structure and function of this species (Foote et al. 1972; Amann, 1986; Paula & Cardoso, 1995). This is even more unfortunate considering that there are hundreds of breeds of dog that may exhibit particular characteristics, such as those related to testis function and reproductive capacity (Amann, 1986). Thus, we are currently developing a broad-based study investigating several morphofunctional testis parameters in various dog breeds with remarkable differences in body size.
Specifically for the seminiferous epithelium cycle, the present study revealed that most stages had significant differences in frequency, particularly for the mongrel and American pit bull. This trend was all the more evident when the combined stage frequencies based on the occurrence of meiosis were considered. However, the duration of spermatogenesis differed significantly only for the American pit bull. Each spermatogenic cycle length and the total duration of spermatogenesis were approximately 1 day and 6 days faster in this dog breed, respectively. The results obtained for the duration of spermatogenesis among the other dog breeds investigated were very similar and basically identical to the only data available in the literature on dogs (beagle; Foote et al. 1972).
Our findings are in agreement with investigations showing that the length of the spermatogenic cycle is under the control of the germ cell genotype (França et al. 1998) and that the duration of spermatogenesis does not differ substantially between breeds or strains (Courot et al. 1970; França & Russell, 1998). Thus, at the present moment, we have no explanation regarding the possible mechanisms related to the acceleration of the duration of spermatogenesis in the American pit bull. Studies on mice (Meistrich et al. 1973) and recent investigations on fish (Vilela et al. 2003; Lacerda et al. 2006) have shown that higher temperatures significantly accelerate the duration of spermatogenesis. Based on evidence in the literature, the fish studies hypothesize that this acceleration could be related to alterations in the cell cycle, particularly in the transition of G2-M (Cobb et al. 1999; Liu et al. 2000). However, it remains to be determined whether the germ cell cycle is altered in the American pit bull and/or whether the temperature of the scrotum is higher in this dog breed. In our experience dealing with more than 10 different dog breeds, we found that the scrotum of the American pit bull seems to be more attached to the perineal region than in the other dog breeds and this anatomical peculiarity could result in an elevated scrotum temperature.
Regarding the characterization of the stages of the cycle according to the acrosome system, the results of the present study were generally similar to the only report on dogs (beagle; Russell et al. 1990). A study carried out by Foote et al. (1972) used the tubular morphology system for the characterization of cycle stages, which renders a precise comparison with the data obtained in the present work very difficult.
There is evidence in the literature showing that the stage frequencies grouped in pre-meiotic and post-meiotic phases of spermatogenesis may be phylogenetically determined among members of the same mammalian family (França & Russell, 1998; Almeida et al. 2006; Leal & França, 2006; Costa et al. 2008; Leal & França, 2008). In fact, besides being very similar to other studies on dogs (Foote et al. 1972; Russell et al. 1990), the data obtained in the present investigation are very similar to those for the wolf (Bitencourt et al. 2007), which is a close relative of the dog.
The age range for the different dog breeds investigated in the present study was considered appropriate. This assumption is based on a study in the literature investigating several testis parameters, including the number of germ cells and Sertoli cells in cross-sections of seminiferous tubules in mongrel dogs evaluated from early sexual maturity to senescence. The authors found that these parameters decreased significantly after 7 years of age and even more clearly after 10 years (Paula & Cardoso, 1995).
Knowledge on the length of the spermatogenic cycle is fundamental to estimates on spermatogenic efficiency (daily sperm production per gram of testis) and comparative studies between different mammalian species (Hess & França, 2007). According to a study using xenogenic (rats to mice) spermatogonial transplantation (França et al. 1998), the determination of cycle length is under the control of the germ cell genotype. A similar conclusion was reached using xenografts from porcine and ovine testis (Zeng et al. 2006). In most mammals, each spermatogenic cycle lasts 9–12 days, whereas the total duration of spermatogenesis lasts 40–54 days (Hess & França, 2007). Therefore, the duration of spermatogenesis found for dogs is in the upper range in comparison with previously investigated mammalian species.
The present study is the most comprehensive investigation to date on the seminiferous epithelium cycle and its duration in dogs. This study is part of an important, extensive comparative investigation currently underway at our laboratory involving several breeds of dog. Significant differences were found in the frequencies of some stages, specifically for the mongrel and American pit bull. Whereas the duration of spermatogenesis was very similar for five dog breeds investigated, this parameter was significantly shorter in the American pit bull. Data from the present study contribute toward a better understanding of testis function in dogs and are fundamental to the determination of spermatogenic efficiency in this important domestic species.
Acknowledgments
Technical help from Adriano Moreira and Mara Lívia Santos is highly appreciated. Financial support from the Minas Gerais State Foundation (FAPEMIG) and the Brazilian National Research Council (CNPq) is gratefully acknowledged.
References
- Almeida FF, Leal MC, França LR. Testis morphometry, duration of spermatogenesis, and spermatogenic efficiency in the wild boar (Sus scrofa scrofa. Biol Reprod. 2006;75:792–799. doi: 10.1095/biolreprod.106.053835. [DOI] [PubMed] [Google Scholar]
- Amann RP. Reproductive capacity of dairy bulls. III. The effect of ejaculation frequency, unilateral vasectomy, and age on spermatogenesis. Am J Anat. 1962;110:49–67. doi: 10.1002/aja.1001100106. [DOI] [PubMed] [Google Scholar]
- Amann RP. Reproductive physiology and endocrinology of the dog. In: Morrow DA, editor. Current Therapy in Theriogenology. London: Saunders; 1986. pp. 531–538. [Google Scholar]
- Amann RP, Schanbacher BD. Physiology of male reproduction. J Anim Sci. 1983;57:380–403. [PubMed] [Google Scholar]
- Bitencourt VL, Paula TAR, Matta SLP, Fonseca CC, Benjamin LA, Costa DS. The seminiferous epithelium cycle and daily spermatic production in the adult maned wolf (Chrysocyon brachyurus, Illiger, 1811) Micron. 2007;38:584–589. doi: 10.1016/j.micron.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Björnerfeldt S, Webster MT, Vilà C. Relaxation of selective constraint on dog mitochondrial DNA following domestication. Genome Res. 2006;16:990–994. doi: 10.1101/gr.5117706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy DC. Photographic emulsions and processing. In: Stumpf WE, Solomon HF, editors. Autoradiography and Correlative Imaging. San Diego: Academic Press; 1995. pp. 49–57. [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals, seminiferous epithelium cycle and spermatogonial review. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Cobb J, Cargile B, Handel MA. Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev Biol. 1999;205:49–64. doi: 10.1006/dbio.1998.9101. [DOI] [PubMed] [Google Scholar]
- Costa GMJ, Chiarini-Garcia H, Morato RG, Alvarenga RLLS, França LR. Duration of spermatogenesis and daily sperm production in the jaguar (Panthera onca. Theriogenology. 2008;70:1136–1146. doi: 10.1016/j.theriogenology.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Courot M, Hochereau DE, Reviers MT, Ortavant R. Spermatogenesis. In: Johnson AD, Gomes WR, Vandemark NL, editors. The Testis. New York: Academic Press; 1970. pp. 339–432. [Google Scholar]
- Cruz F, Webster MT, Vilà C. The legacy of domestication: accumulation of deleterious mutations in the dog genome. Mol Biol Evol. 2008;25:2331–2336. doi: 10.1093/molbev/msn177. [DOI] [PubMed] [Google Scholar]
- Foote RH, Swierstra EE, Hunt WL. Spermatogenesis in the dog. Anat Rec. 1972;175:341–351. doi: 10.1002/ar.1091730309. [DOI] [PubMed] [Google Scholar]
- França LR, Russell LD. The testis of domestic animals. In: Martínez F, Regadera J, editors. Male Reproduction. A Multidisciplinary Overview. Madrid: Churchill Livingstone; 1998. pp. 197–219. 1st edn (eds. [Google Scholar]
- França LR, Ogawa T, Avarbock MR, Brinster RL, Russell LD. Germ cell genotype control cells cycle during spermatogenesis in the rat. Biol Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- Grandjean D, Vasaire JP. Dog breeds. In: Gallitelli B, editor. The Royal Canin Dog Encyclopedia. Paris: Aniwa Publishing; 2001. pp. 45–46. [Google Scholar]
- Hess RA, França LR. Spermatogenesis and cycle of the seminiferous epithelium. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austen, TX: Landes Bioscience; 2007. pp. 1–15. [Google Scholar]
- Kerr JB, Loveland KL, O’Bryan MK, Kretser DM. Cytology of the testis and intrinsic control mechanisms. In: Neill JD, editor. Physiology of Reproduction. Amsterdam: Elsevier; 2006. pp. 827–947. [Google Scholar]
- Lacerda SMSN, Batlouni SR, Da Silva SGB, Homem CSP, França LR. Germ cell transplantation in fish: the Nile-tilapia model. Anim Reprod. 2006;3:146–159. [Google Scholar]
- Leal MC, França LR. The seminiferous ephithelium cycle length in the black tufted-ear marmoset (Callithrix penicillata) is similar to humans. Biol Reprod. 2006;74:616–624. doi: 10.1095/biolreprod.105.048074. [DOI] [PubMed] [Google Scholar]
- Leal MC, França LR. Slow increase of Sertoli cell efficiency and daily sperm production causes delayed establishment of full sexual maturity in the rodent Chinchilla lanigera. Theriogenology. 2008;71:509–518. doi: 10.1016/j.theriogenology.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–584. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Liao C, Wolgemuth DJ. A role for cyclin A1 in the activation of MPF and G2–M transition during meiosis of male germ cells in mice. Dev Biol. 2000;224:388–400. doi: 10.1006/dbio.2000.9776. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Eng VWS, Loir M. Temperature effects on the kinetics of spermatogenesis in the mouse. Cell Tissue Kinet. 1973;6:379–393. doi: 10.1111/j.1365-2184.1973.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Okwun OE, Igboeli G, Ford JJ, Lunstra DD, Johnson L. Number and function of Sertoli cells, number and yield of spermatogonia, and daily sperm production in three breeds of boar. J Reprod Fert. 1996;107:137–149. doi: 10.1530/jrf.0.1070137. [DOI] [PubMed] [Google Scholar]
- Paula TAR, Cardoso FM. Alterações etárias na espermatogênese do cão II. População celular dos túbulos seminíferos e rendimento espermatogênico. Arq Brasil Med Vet Zoot. 1995;4:19–30. [Google Scholar]
- Russell LD, Ettlin RA, Sinha-Hikim AP. Histological and Histopathological Evaluation of the Testis. Vienna, IL: Cache River Press; 1990. [Google Scholar]
- Salim B, Entwistle KW. Duration of the seminiferous epithelial cycle in hybrid Bos indicusx Bos taurusbulls. J Reprod Fert. 1982;66:729–734. doi: 10.1530/jrf.0.0660729. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Breed WG. Anatomy, vasculature and innervation of the male reproductive tract. In: Neill JD, editor. Physiology of Reproduction. Amsterdam: Elsevier; 2006. pp. 771–825. [Google Scholar]
- Swierstra EE. Cytology and duration of the cycle of seminiferous epithelium of the boar, duration of spermatozoa transit through the epididymis. Anat Rec. 1968;161:171–186. doi: 10.1002/ar.1091610204. [DOI] [PubMed] [Google Scholar]
- Vilela DAR, Silva SGB, Peixoto MTD, Godinho HP, França LR. Spermatogenesis in teleost: insights from the Nile tilapia (Oreochromis niloticus) model. Fish Physiol Biochem. 2003;28:187–189. [Google Scholar]
- Zeng W, Avelar GF, Rathi R, França LR, Dobrinski I. The length of the spermatogenic cycle is conserved in porcine and ovine testis xenografts. J Androl. 2006;27:527–533. doi: 10.2164/jandrol.05143. [DOI] [PubMed] [Google Scholar]