SUMMARY
This overview focuses on the (α,α-difluoromethylene)phosphonate mimic of phosphoserine (pCF2Ser) and its application to the study of kinase-mediated signal transduction – pathways of great interest to drug development. The most versatile modes of access to these chemical biological tools are discussed, organized by method of PCF2-C bond. The pCF2-Ser mimic may be site-specifically incorporated into peptides (SPPS) and proteins (expressed protein ligation). This isopolar, dianionic pSer mimic results in a “constitutive phosphorylation” phenotype, and is seen to support native protein-protein interactions that depend upon serine phosphorylation. Signal transduction pathways studied with this chemical biological approach include the regulation of p53 tumor suppressor protein activity, and of melatonin production. Given these successes, the future is bright for the use of such “teflon phospho-amino acid mimics” to map kinase-based signaling pathways.
INTRODUCTION
It has long been known that biological macromolecules undergo kinase-mediated phosphorylation. The reverse step, dephosphorylation, is also usually phosphatase-controlled. Perhaps because the phosphorylation event itself greatly changes the charge distribution and polarity of the substrate, it is often associated with signal transduction/amplification. This is particularly the case for sequence-specific phosphorylations on the amino acid side chains of serine, threonine and tyrosine. The bcr-abl kinase was the first of several to be successfully targeted in a cancer therapeutic drug development, resulting in FDA approval of the drug Gleevec (Imatinib) in 2001. As of this writing, no fewer than eight kinase inhibitors are on the market for cancer chemotherapy (Boros and Boros, 2008; Pytel et al., 2009) and some 150 kinase inhibitors are in clinical trials (Savage and Gingrich, 2009). As such, the elucidation of kinase/phosphatase-controlled signal transduction pathways has emerged as one of the most important front end tasks in the medicinal chemistry arena.
Bioorganic chemists have long sought to develop functionalities that effectively mimic biologically relevant phosphate esters, yet remain inert to phosphatase cleavage. This research domain resembles the peptidomimetics field, in that one seeks to build new organic functional groups that retain key properties of the native structure, yet resist enzymatic bio-degradation. Interestingly, organically bound fluorine has played an important role in both endeavors, surely bolstered by polarity afforded by fluorine in compensating for lost oxygen atoms. For example, fluoroalkenes, in which the electronegative fluorine atom replaces the carbonyl oxygen, can serve as viable peptide isosteres (Welch, 2008). The strength of C-F bonds (O'Hagan, 2008), and their ability to impose unique conformational constraints (Gorres et al., 2008; Nieschalk et al., 1996) are important elements that support such designs. Moreover, the addition of multiple C-F bonds can increase stability to chemical oxidation (DiMagno et al., 1996) and metabolism, and provide additional driving force to enhance binding with macromolecular targets, in aqueous solution (Biffinger et al., 2004).
Dating back to key early reports from the groups of Blackburn (Blackburn et al., 1981), McKenna (McKenna and Shen, 1981) and Burton (Burton and Flynn, 1982) on pyrophosphate mimics in the early 80's, there has emerged an interest in synthesizing α-fluorinated phosphonates as potentially isopolar analogues (Blackburn et al., 1985b) of the corresponding phosphate esters. This has spurred a great effort in methodology development in this area (Benayoud et al., 1996; Blackburn et al., 1985a; Blackburn et al., 1994; Blades et al., 1997; Caplan et al., 2000; Cockerill et al., 2000; Diab et al., 2008; Gautier et al., 2004; Herpin et al., 1996; Hikishima et al., 2006; Lopin et al., 2003; Murano et al., 2005; Nair and Burton, 1997; Ozouf et al., 2004; Pajkert et al., 2008; Pfund et al., 2005; Pignard et al., 2006; Roeschenthaler et al., 2006; Xu et al., 2005; Yokomatsu et al., 2003). The phosphate mimics program in this laboratory was initiated around the goal of establishing methods to access fluorinated phosphonate analogues of sugar phosphates (Berkowitz et al., 2001; Berkowitz et al., 2000a; Berkowitz et al., 1993; Shen et al., 1994), and continues in this direction, with a particular interest, of late, in bivalent sugar phosphonates as ligands for the mannose 6-phosphate-insulin-like growth factor II receptor (M6P/IGF2R) (Berkowitz et al., 2004; Fei et al., 2008).
While phosphorylations at threonine (Di Croce and Shiekhattar, 2008; Kulasekara and Miller, 2007), and certainly tyrosine (Franco and Tamagnone, 2008; Girault, 2006; Grangeasse et al., 2007; Nag and Chaudhary, 2009; Roskoski, 2008; Zhang et al., 2009), are of great importance, this overview focuses on serine phosphorylation (For nice complementary discussions from the groups of Otaka and Ojea, primarily focusing on the chemistry of other phosphono-AA's see: (Fernandez et al., 2006; Otaka et al., 2000; Otaka et al., 2004)). More specifically, we will examine the use of the (α,α-difluoromethylene)phosphonate functionality to mimic the phosphate monoester form of serine residues, in proteins that are subject to regulation via serine phosphorylation. The archetypical example of such a system is represented by the complex interplay of the enzymes glycogen phosphorylase (GP - catabolism), and glycogen synthase (GS - anabolism) cascades associated with glycogen metabolism. These enzymes are each tightly controlled via kinase/phosphatase enzymes that respond to hormonal signals from epinephrine or insulin. For example, epinephrine binding to its cognate receptor results in the activation of adenylate cyclase (Sunahara et al., 1996), and the formation of cAMP, the “universal second messenger.” This leads to the activation of cAMP-dependent protein kinase A, which, in turn, activates phosphorylase kinase, which itself activates glycogen phosphorylase, both activations occuring via enzymatic phosphorylations at serine residues (Toole and Cohen, 2007).
It now appears that the principal phosphorylations are at Ser-14 in GP and at Ser-7 and Ser-640 in GS (Toole and Cohen, 2007). However, the complexity of kinase-phosphatase regulation of this key metabolic branch point is remarkable. For example, in GS alone, nine serine residues can be phosphorylated by regulatory kinases, leading to a theoretical complexity of 512 combinations of distinct Ser/pSer patterns, each potentially with a different kinetic profile. Fortunately, due to hierarchal phosphorylation, the number of configurations of GS observed is considerably lower. The group of Jensen has painstakingly set about to identify and kinetically characterize the most important “phospho-forms” of this enzyme (Jensen and Lai, 2009).
Synthesis of the pCF2-Ser Phosphoserine Mimic
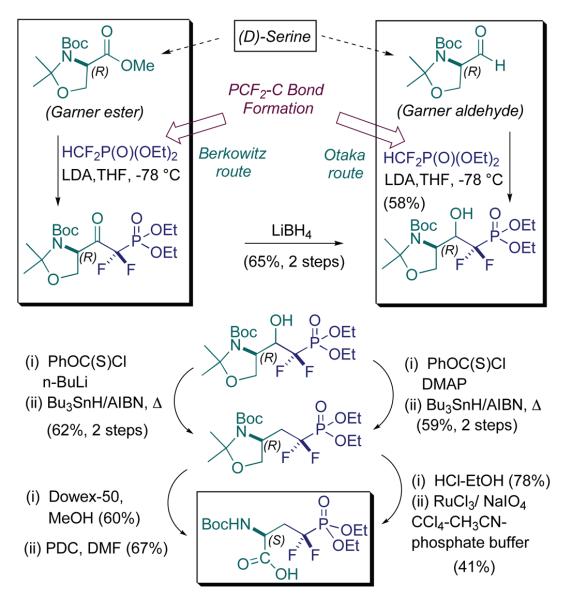
It occurred to us and others that for the study of such signal transduction pathways, it would be useful to have available a stable mimic of the phosphorylated form of the enzyme. Indeed, this led us to develop the first synthesis of the CF2-phosphonate mimic of pSer, in a form suitable for automated peptide synthesis, 15 years ago. Actually, both our first generation, and our second generation synthesis, starting from L-serine, and (R)-isopropylideneglycerol, respectively, were disclosed in that first communication (Schemes 1 and 2) (Berkowitz et al., 1994b). In the following year, the groups of Otaka and Burke collaboratively reported a complementary approach to the same target (Otaka et al., 1995a). In what follows, these syntheses are organized according to PCF2-C bond disconnection, and they are presented along with several more recent approaches, to provide the medicinal chemist with an overview of the routes available to synthesize this “teflon pSer mimic.” This is followed by a look at elegant studies, by the groups of Appella and Cole, respectively, on using this pSer mimic to examine the role of serine phosphorylation in signal transduction.
Scheme 1.
PCF2-C Bond Formation via Triflate Displacement – Stereocontrol via Chiron Approach from L-Serine
Scheme 2.
PCF2-C Bond Formation via Triflate Displacement – Chiron Approach from (R)-Isopropylidene-glycerol
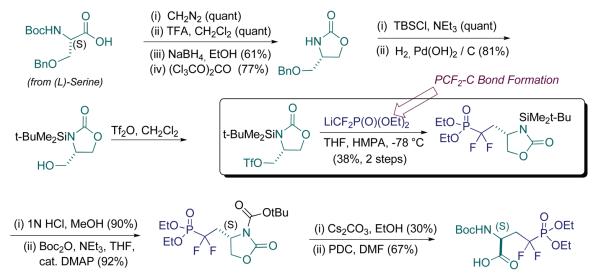
In Scheme 1 is presented our first generation approach to the pCF2-Ser building block for peptide synthesis. A chiron approach was taken, emanating from L-serine. As alluded to earlier, an important observation had been made in our lab that informed our thinking about (α,α-difluoromethylene)phosphonate synthesis. Namely, in early work by Blackburn and coworkers, it was observed that the LiCF2P(O)(OEt)2 anion is unstable to α-elimination to difluorocarbene, at temperatures of approximately −40 °C or higher (Bla ckburn et al., 1987). Therefore, we sought to develop especially efficient ways of capturing this nucleophile, at low T. Indeed, it was found that displacements of primary sugar triflates generally proceed at −78 °C with this rea gent, in a matter of minutes (Berkowitz et al., 1993). This behavior contrasted sharply with the markedly lower reactivity of alkyl halides.
In seeking to apply the triflate displacement approach to the synthesis of the pSer mimic, we were confronted with an issue of functional group cross-compatibility. Namely, would it be possible to carry a masked amino group into the triflate scaffold itself, so that the synthesis could commence with L-serine itself? In fact, the challenge was much greater. Because of the densely functionalized serine framework, the desired pCF2-Ser synthon was to possess an α-triflyloxy, (protected)-β-amino substructure (Scheme 1). It was found that mono-protection of the amine, even with strongly electron-withdrawing N-sulfonyl functionality, did not lead to manageable triflates, perhaps due to neighboring group participation (possible aziridinium ion formation). Thus, bis-protection of the neighboring nitrogen, (i) as an acyclic Bn-N-Ts group, (ii) through incorporation into a 2,5-dimethylpyrrole group, or (iii) through cyclization to the oxazolidinone (as in Scheme 2), and N-benzylation, all led to primary triflates that were stable to chromatography, and could be displaced by LiCF2P(O)(OEt)2. However, in all cases, N-deprotection, in the presence of the resultant fluorinated phosphonate, proved problematic. A solution was found with the latter protection scheme, wherein the N-benzyl protecting group was replaced with a much more labile N-TBS blocking group.
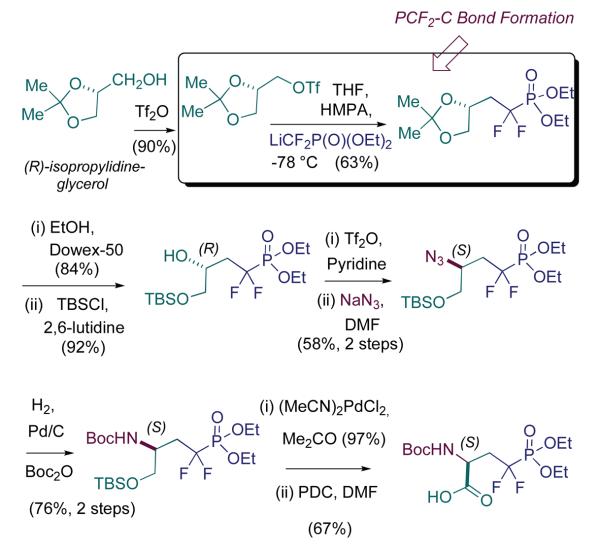
The N-protecting group problem that emerged in this first generation route resulted in a longer synthesis than envisioned, that also suffered from suboptimal N-deprotection conditions. Namely, introduction of the N-BOC functionality serves the dual purpose of activating the oxazolidinone for ring opening, and installing a protecting group appropriate for peptide synthesis. Unfortunately, that ring opening proceeds in modest yield and limits the efficiency of this route. So, the phosphonate team in the lab set about on a parallel route, in which triflate displacement would still be employed for fluorinated phosphonate installation, but upon a glyceryl acetonide scaffold, rather similar to the sugar scaffolds originally employed (Berkowitz et al., 1993). This worked smoothly and allowed for late introduction of nitrogen, via azide displacement upon a secondary triflate (Scheme 2). This remains a rather streamlined and underused route into the pCF2-Ser target. Chemical biologists active in this field are encouraged to examine this isopropylidineglycerol route, if in need of a clean rapid entry into this phosphono-peptide building block.
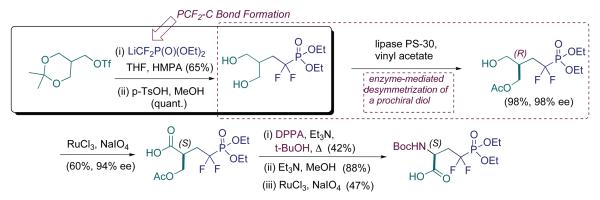
Indeed, highlighting the practicality of this type of approach, the efforts of a phosphonate group in Tokyo, under Shibuya (Yokomatsu et al., 1996), resulted in a conceptually different approach to stereocontrol, while retaining the same sort of triflate displacement chemistry as had been employed in our second generation synthesis (Scheme 3). Cleverly, Yokomatsu et al. utilized the monoacetonide of the C3v-symmetrical tris(hydroxymethyl)methane (THYM) starting material as triflate precursor. Triflate displacement proceeds smoothly. This is followed by acetonide cleavage to produce a 1,3-diol that may be efficiently desymmetrized with several lipases. Note that enzymatic desymmetrization is, of course, an excellent strategy for achieving both high ee and high throughput. The strategy is exceedingly efficient here, and, in our own experience, can be employed with advanced meso intermediates (Berkowitz et al., 2000b; Berkowitz et al., 1996b), relatively deep into total synthetic ventures, particularly with lipase enzymes. Secondly, we have also found that, lipase-mediated hydroxymethyl arm acylation, is a very effective strategy for chiral discrimination for unnatural amino acids. This approach works, even for quaternary amino acid synthons, after reduction of the α-carboxyl to a hydroxymethyl group (Berkowitz et al., 1994a).
Scheme 3.
Shibuya Route: PCF2-C Bond Formation via Triflate Displacement – Enzymatic Desymmetrization
Also in this case, N-introduction occurs late in the synthesis, but via Curtius rearrangement, instead of triflate displacement. The Tokyo route and our second generation triflate displacement route also share the advantage of permitting N-carbamate deprotection, in situ, though in different ways. Interestingly, since the original Shibuya route was published, studies by Guanti and coworkers (Banfi and Guanti, 1998; Banfi et al., 2005), in particular, have produced a number of methods for tris(hydroxymethyl)methane desymmetrization. So, one should be able to enter this triflate displacement route with a single antipode of this type of THYM educt. The resulting phosphonate would possess differentially protected hydroxymethyl arms, streamlining the Tokyo route even further.
All of the aforementioned triflate displacement-based chemistry was carried with diethyl protection across the fluorinated phosphonate moiety, as this is the most well studied synthon. It is worthy of note that we have developed complementary fluorinated phosphonate reagents, carrying both dibenzyl (Berkowitz et al., 1999) and diallyl (Berkowitz and Sloss, 1995) phosphonate protection. Both reagents have performed well in model triflate displacement studies, and may, in the future, allow for adaptation of these routes to the synthesis of pCF2-Ser monomers bearing more convenient side chain protecting groups.
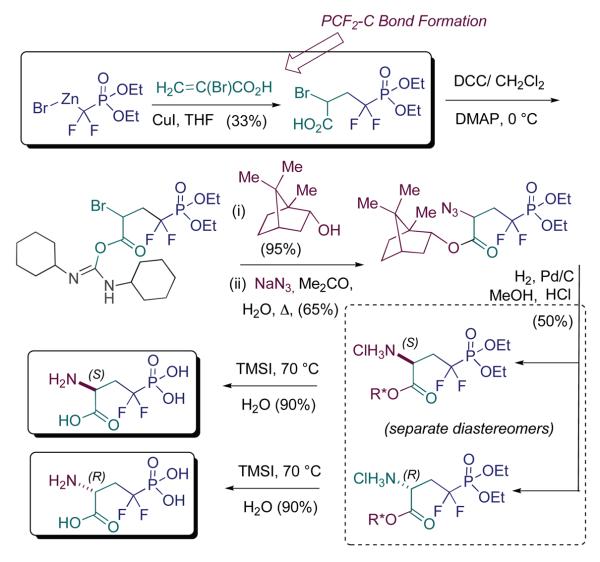
Scheme 4 highlights a route developed jointly by Kawamoto and Campbell (Kawamoto and Campbell, 1997) that features transition metal-mediated alkene addition of (RO)2P(O)CH2ZnBr, similar to a reaction developed by Burton, under both Pd and Cu-catalysis (Yang and Burton, 1992). Related transformations have been developed in the thiono series by Piettre and colleagues, more recently (Lequeux et al., 2001; Pignard et al., 2006). These latter reactions do not require transition metal catalysis, and are routinely run under radical chain conditions. Following (RO)2P(O)CF2-radical addition to α-bromoacrylate, nitrogen is introduced late via bromide displacement. Stereocontrol comes still later, through a classical resolution, via chromatographic separation of the diastereomeric bornyl esters.
Scheme 4.
Kawamoto-Campbell-Burton Route: PCF2-C Bond Formation via Cu-Mediated Resolution of Borneol Esters
Lastly, the original Otaka route (Scheme 5) features a third method of fashioning the PCF2-C linkage, namely via (RO)2P(O)CF2Li addition to carbonyl centers (Otaka et al., 1995a; Otaka et al., 1995b). Specifically, the Kyoto group utilizes the Garner aldehyde (Garner and Park, 1992), and employs a carbonyl addition/Barton ester-type deoxygenation sequence, similar to that described by Martin and coworkers, in non-AA contexts (Martin et al., 1992). Our own carbonyl addition route (Berkowitz et al., 1996a), utilizes the Garner ester as electrophile and, by design, allows the experimentalist to assess three different L-(α,α-difluoromethylene)phosphono AA's, the analogues of pSer, p-allo-Thr and pThr, from a common, β-keto-α,α-difluorophosphonate intermediate, by treatment either with LiBH4 or with MeMgBr, prior to deoxygenation. As can be seen (Scheme 5), if one only wishes to synthesize the pCF2-Ser mimic the two routes are very similar (Corey-Schmidt (PDC-DMF) oxidation vs. RuO4 in final step). Because of the ready accessibility of the Garner ester, this route appears to be the most widely used. The fact that it also provides access to the individual L-phosphonothreonine diastereomers makes this a versatile approach.
Scheme 5.
Berkowitz-Otaka Route: PCF2-C Bond Formation via Carbonyl Add'n – Chiron Approach from D-Serine
Use of the pCF2-Ser Mimic as a Tool for the Study of Signal Transduction
The p53 protein is a tightly regulated tumor suppressor protein (Sherr, 2004), mutated in 50% of human cancers (Bykov et al., 2002; Toledo and Wahl, 2006). The p53 protein, when activated, dissociates from its mdm2 (hdm2 is the human equivalent) binding partner, undergoes conformational change, and acts as a transcription factor, typically leading to either cell cycle arrest or apoptosis (Liebermann et al., 2007). Both appear to be protection mechanisms, especially with the finding that tumor senescence may be associated with inflammatory cytokine up-regulation and tumor clearance (Xue et al., 2007). Activation of p53 occurs in response to cellular stress signals, including DNA damage, osmotic shock (Kishi et al., 2001), oxidative stress (Han et al., 2008) and hyperglycemia. Conversion of the latent form of p53 to its active transcription factor form appears to involve initial phosphorylation (primarily at N-terminal serines) and subsequent acetylation (C-terminal lysines- K320 and K382).
Several experiments underscore the importance of understanding p53 function and regulation, especially for medicinal chemistry programs in the oncology (Bell and Ryan, 2007; Haupt and Haupt, 2006; Vassilev, 2005), and diabetes areas (Fiordaliso et al., 2001). Jacks and coworkers developed a clever genetic construct for temporal control of p53 expression (Ventura et al., 2007). Initially, p53 was held genetically latent, and could be restored via a Cre recombinase mechanism. Restoration of p53 function was shown to lead to tumor regression, with the effect dependent on tumor type. This work pointed to the importance of altered p53 function in tumorigenesis and tumor maintenance. This genetic system, then, serves as a model for pharmacological reactivation of p53. Indeed, along those lines, a couple of interesting early small molecule modulation experiments have come to the fore. For example, small molecules capable of both restoring transcriptional activation function to p53 mutants (Bykov et al., 2002), and capable of inhibiting p53-mdm2 interactions (Vassilev et al., 2004) have been described, each avenue representing a fundamentally new approach to cancer chemotherapy, if viable.
Given the prevalence of p53 abnormalities in cancer, and the importance of serine phosphorylation in its regulation, there has been a very high level of interest in the study of kinase-regulated signal transduction in this system. Among the candidate sites for p53 modulation, via phosphorylation, are included S-6, S-9, S-15, T-18, S-20, S-33, S-37, S-46, at the N-terminus, as well as S-315 and S-392, at the C-terminus. In terms of regulatory complexity, at the level of serine phosphorylation, this system, then, is reminiscent of the glycogen phosphorylase/glycogen synthase pair discussed earlier. Interestingly, at the C-terminus of p53, O-glycosylation may be in direct competition with O-phosphorylation (Clarke et al., 2008; Haltiwanger et al., 1997; Hart et al., 1995; Wells et al., 2003), with the former being particularly important in cases of hyperglycemia (Fiordaliso et al., 2001).
From a chemical biology point of view, one way of examining the effect(s) of post-translational modifications, is to engineer functional variants that model both the “switched “on” and “switched off” states of the macromolecule. While it is easy to genetically engineer “constitutively dephosphorylated” sites by mutating phosphorylation site Ser residues to Ala residues, and this has been shown to be quite effective in the p53 system (Yamauchi et al., 2004), the reverse mutation is unavailable by standard molecular biological techniques, as Asp or Glu are often unsuitable mimics of pSer (Zheng et al., 2003), and there are no better choices available among the proteinogenic AA's. The incorporation of a phosphatase-inert pSer mimic, position-specifically, into a protein of interest, is now possible with expressed protein ligation (EPL) techniques. Moreover, in such endeavors, it appears to be especially advantageous to use phosphono-AA mimics in which a CF2 (as opposed to a CH2) replaces the bridging ester oxygen (Zhang et al., 2003). Among the possible reasons for this advantage, is the aforementioned “isopolarity” of the CF2 unit with the native bridging O. However, pKa effects may be even more important. The simple CH2-phosphono analogue of pSer is expected to have pKa2 of 7-8, whereas its CF2-phosphono counterpart typically displays pKa2 under 6, depending upon molecular context (Berkowitz and Bose, 2001; Berkowitz et al., 2000a; Blackburn et al., 1984). Thus both the native pSer residue and the pCF2-Ser mimic will present as almost exclusively dianionic side chain modifications under most conditions. This ability to bring in such a chemically tuned, hydrolytically stable, pSer mimic, using EPL techniques, will be discussed below, in the context of recent studies of melatonin regulation.
In the p53 area, the most common modus operandi for studying site-specific phosphorylation itself, i.e, for running “phosphorylation-positive” experiments, is a reactive one, rather than a pro-active one. That is to say, rather than effectively generate “constitutive” pSer sites in p53 and examine functional responses to these, one looks at the pSer profile of p53 in response to a stimulus. The analytical tools needed for such studies are a set of antibodies (Ab's) for site-specific phosphorylation variants of p53. For p53, activation usually involves serine phosphorylation, with sites available at both the N- and C-terminal regions.
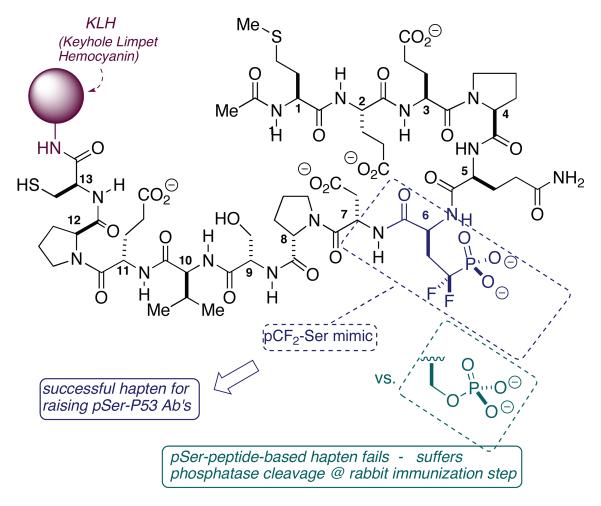
A precedent-setting study in antibody-based mapping technology was carried out by Appella and coworkers at the NIH (Higashimoto et al., 2001; Higashimoto et al., 2000) (Scheme 6). The group sought to specifically examine phosphorylation at Ser-6 and Ser-9 in p53, in response to DNA damage. It was found that one could successfully raise Ab's against pSer-9-containing peptide-KLH-protein conjugate, and that these Ab's, in turn, effectively recognized p53 protein that was specifically phosphorylated at Ser-9. However, the same procedure for producing pSer-6-complementary Ab's failed. Ab's were obtained, but these recognized only p53 samples presenting an unphosphorylated Ser-6-site. It appears that the peptidyl-KLH conjugate suffered phosphatase-mediated Ser-6-O-phosphate cleavage in the rabbit immunization step.
Scheme 6.
Use of the pCF2-Ser Mimic to Study of Position-Specific Phosphorylation of p53 in Response to DNA Damage - Appella
Appella's team astutely recognized that the use of an “isosteric and isopolar,” (Blackburn et al., 1985b; Blackburn et al., 1981) yet hydrolytically stable, pSer mimic might overcome this, otherwise apparently insurmountable, barrier. As is depicted in the scheme, the pCF2-Ser mimic, indeed, served this purpose exceptionally well, producing high titres of Ab that recognized only pSer-6-post-translationally modified (PTM) p53 samples. This experiment demonstrates, at once, the utility of this phosphoserine mimic for overcoming phosphatase cleavage, and its ability to quite accurately mimic the size, polarity and charge distribution of the native PTM. After all, the raised Ab was “remodeled” against the pSer surrogate, and deployed against actual, pSer-modified p53, and showed complete fidelity in recognizing the true functionality.
Using this chemical biological tool, the Appella group was able to establish that Ser-6 is phosphorylated in response to DNA damage induced by both UV light and ionizing radiation (Higashimoto et al., 2000). In subsequent studies, an unexpected interdependence was seen between Ser-6 and Ser-9 phosphorylation (Saito et al., 2003). That is, in the absence of Ser-9 (S9A mutant), no phosphorylation is seen at Ser-6, and vice versa. This work established that the N-terminal transactivation domain of p53 appears to be divisible into four clusters of interdependent serine residues, from the point of view of phosphorylation. This site interdependency model is seen as a mechanism for both control and signal amplification.
As alluded to in the previous discussion, it would be even more powerful if one could generate phosphorylation “knock-in” mutants to complement the phosphorylation “knock-out” mutants typically constructed by Ser to Ala mutations. This would allow one to go beyond analyzing phosphorylation patterns that are generated by native kinases. Rather, one would be generating artificially a “constitutive phosphorylation signal” and examining the downstream consequences of that signal transduction event.
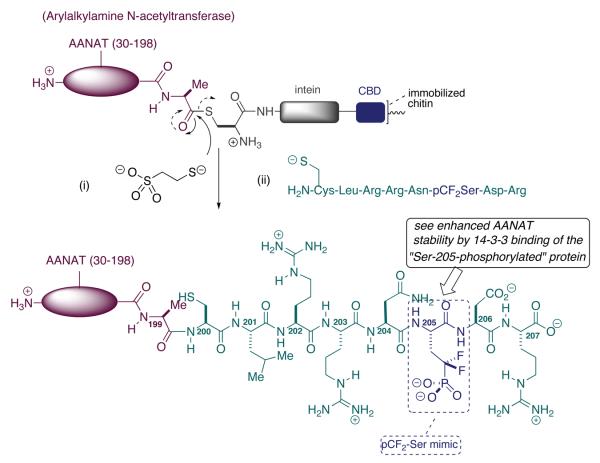
In Scheme 7 is illustrated a seminal study by the group of Philip Cole at Johns Hopkins, that uses the power of EPL to surgically insert the pCF2-Ser mimic of pSer into a protein, at a site of interest (Zheng et al., 2005). The EPL technique (Muir et al., 1998; Schwarzer and Cole, 2005) involves coupling intein technology to cysteine ligation methodology inherent in the parent native chemical ligation procedure (Dawson et al., 1994). EPL may be regarded as the state of the art for chemical biological manipulation of side chain functionality, and is especially useful for studying PTM's and their relation to signal transduction (Flavell and Muir, 2009; Pickin et al., 2008; Rauh and Waldmann, 2007).
Scheme 7.
Use of the pCF2-Ser Mimic to Study the Effects of Position-Specific Phosphorylation of AANAT on the Melatonin Cycle - Cole
In the case at hand, Cole and co-workers were interested in examining closely the regulation of arylalkylamine N-acetyltransferase (AANAT), and enzyme that catalyzes the penultimate step in the biosynthesis of the time-keeping hormone, melatonin (Klein, 2007). Melatonin, N-acetyl-5′-methoxytryptamine, is biosynthesized by the decarboxylation of 5′-hydroxytryptophan to serotonin, followed by N-acylation and O-methylation. It is the N-acylation step that is mediated by AANAT, with acetyl-CoA serving as acyl donor. It had been surmised that phosphorylation at Thr-31, and potentially Ser-205, modulated in vivo acyltransferase activity. Two mechanisms for this effect were in play: (i) improving the catalytic efficiency for the acyl transfer reaction and (ii) increasing the biological half-life of AANAT enzyme.
The mechanism for AANAT stabilization is an interesting one, namely the protein is thought to be shielded from proteosomal proteolysis by association with a specific14-3-3 adaptor protein; namely the zeta-variant. Over 100 signaling proteins have been reported to bind to 14-3-3 proteins, and these interactions are typically associated with pSer or pThr modifications on the signaling protein (Klein et al., 2003; Obsil et al., 2001; Tzivion and Avruch, 2002). In the case at hand, in the dark, AANAT activity and protein levels increase. The putative activating phosphorylation(s) are thought to be mediated by protein kinase A, in response to elevated cAMP levels, in the pineal gland, as a result of a1 or b-adrenergic receptor activation. Thus, through EPL with the title pSer mimic, the Cole group sought to engineer in “constitutive phosphorylation” at position-205, and test for positive interaction between AANAT and its 14-3-3-zeta partner.
In the Hopkins experiment, the requisite octapeptide for EPL was synthesized by standard solid phase peptide synthesis (SPPS). The sequence represents a modified C-terminus, in that the Ser residue normally at position 205 was replaced by the pCF2-Ser mimic, and a Cys residue was installed in place of the native Ala-200, to enable the cysteine-mediated acyl transfer chemistry that underlies the ligation chemistry in EPL. On the other end, the AANAT protein itself was expressed as a complementary C-terminal (8-) truncated-intein-CBD (chitin binding domain) fusion construct. The CBD domain allowed for facile purification via affinity chromatography. The tag could then be clipped by transthio-esterification with β-mercaptoethanesulfonate, setting the stage for ligation. Incubation of the synthetic, N-Cys-terminated, (α,α,-difluoromethylene)phosphono-peptide proceeded cleanly, presumably via the usual two stage, intermolecular trans-thio-esterification/intramolecular S-N acyl transfer mechanism, providing the targeted semi-synthetic protein (Scheme 7).
Using this construct, the Cole group was able to demonstrate clearly that Ser-205 phosphorylation, though at the C-terminus and not a part of a recognized 14-3-3-zeta consensus sequence, enhances cellular stability of the AANAT protein. Subsequent studies showed that this effect persists in live cells, expanding the domain in which such “teflon phosphates” may be deployed to ask fundamental questions in chemical biology (Szewczuk et al., 2008). Interestingly, from this latter study, it was confirmed that, surprisingly, Ser-205 phosphorylation on AANAT confers a greater binding affinity for 14-3-3-zeta (0.86 μM Kd) than does Thr-31 phosphorylation (1.8 μM Kd). On the other hand, the latter PTM alone improves the catalytic efficiency (kcat/Km) of the acyl transferase by approximately 7-fold, largely by decreasing the Km for serotonin. However, that catalytic improvement is essentially lost when both modifications are present. These observations, when taken together are consistent with a two point (N-terminal pThr and C-terminal pSer) binding model for the interactions between AANAT and its 14-3-3-zeta partner. So, in the end, both mechanisms postulated for increased AANAT activity, improved catalytic efficiency (seen with sole pThr-31 modification), and increased stability to proteolysis through binding to 14-3-3-zeta (seen with sole pSer-205 phosphorylation and with pThr-31/pSer-205 dual phosphorylation) appear to be operative.
CONCLUSIONS
The (α,α-difluoro)methylene phosphonate analogue of L-phosphoserine is available through chemistry employing a M-CF2P(O)(OR)2 equivalent for PCF2-C bond formation via: (i) triflate displacement; (ii) carbonyl addition/reduction or (iii) Cu-mediated alkene addition. Within these approaches, absolute stereochemistry is controlled by (i) starting from an appropriate chiral synthon - L-serine, D-serine or (R)-isopropylideneglycerol; (ii) utilizing a chiral catalyst (lipase P) to desymmetrize an achiral intermediate or (iii) attaching a covalent chiral auxiliary for enantiomer resolution. The pCF2-Ser mimic may be incorporated into peptides utilizing SPPS, either for purposes of raising antibodies to specific pSer modifications, or for installation a “constitutive pCF2-Ser modification” into the native protein itself, using EPL methods. The former application was used to firmly establish a connection between p53 Ser-6 phosphorylation and DNA damage, and the latter clarified the role of Ser-205 phosphorylation of AANAT enzyme stabilization.
This technology adds an important element into the toolbox available to the chemical biologist interested in studying signal transduction. Fundamental mechanistic questions relating to kinase-mediated signal transduction in tumor suppression, and in diurnal regulation of the time-keeping hormone, melatonin, have been addressed. As the aforementioned studies demonstrate, fluorinated phosphonate analogues of phospho-amino acids provide for metabolically stable alternatives to the native phospho-proteins, while retaining the ability to promote protein-protein interactions that depend on that phosphorylation. This makes these unnatural amino acid analogues invaluable tools for the dissection of such signal transduction pathways. With the maturation of semi-synthetic hapten construction, expressed protein ligation and unnatural amino acid mutagenesis (Liu and Schultz, 1999; Xie and Schultz, 2005, 2006), and the importance of kinase-mapping in the present day, the time is ripe for expanded use of this isopolar phosphoserine mimic in chemical biology.
ACKNOWLEDGEMENTS
The authors are indebted to the following funding agencies for support of this research: the American Heart Association, American Chemical Society (PRF), Mizutani Foundation for Glycoscience, NIH, NSF, Alfred P. Sloan Foundation and American Cancer Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Banfi L, Guanti G. Asymmetrized tris(hydroxymethyl)methane and related synthons. Enantioselective preparation and synthetic applications. Eur J Org Chem. 1998:745–757. [Google Scholar]
- Banfi L, Guanti G, Paravidino M, Riva R. Asymmetrized tris(hydroxymethyl)methane as a precursor of N- and O-containing 6-membered heterocycles through ring-closing metathesis. Org Biomol Chem. 2005;3:1729–1737. doi: 10.1039/b502952j. [DOI] [PubMed] [Google Scholar]
- Bell HS, Ryan KM. Targeting the p53 family for cancer therapy: Big brother joins the fight. Cell Cycle. 2007;6:1995–2000. doi: 10.4161/cc.6.16.4614. [DOI] [PubMed] [Google Scholar]
- Benayoud F, deMendonca DJ, Digits CA, Moniz GA, Sanders TC, Hammond GB. Efficient syntheses of (alpha-fluoropropargyl)phosphonate esters. J Org Chem. 1996;61:5159–5164. [Google Scholar]
- Berkowitz DB, Bhuniya D, Peris G. Facile installation of the phosphonate and (alpha,alpha-difluoromethyl)phosphonate functionalities equipped with benzyl protection. Tetrahedron Lett. 1999;40:1869–1872. [Google Scholar]
- Berkowitz DB, Bose M. (alpha-Monofluoroalkyl)phosphonates: a class of isoacidic and “tunable” mimics of biological phosphates. J Fluorine Chem. 2001;112:13–33. [Google Scholar]
- Berkowitz DB, Bose M, Asher NG. A convergent triflate displacement approach to (alpha-monofluoroalkyl)phosphonates. Org Lett. 2001;3:2009–2012. doi: 10.1021/ol015983z. [DOI] [PubMed] [Google Scholar]
- Berkowitz DB, Bose M, Pfannenstiel TJ, Doukov T. alpha-Fluorinated phosphonates as substrate mimics for glucose 6-phosphate dehydrogenase: the CHF stereochemistry matters. J Org Chem. 2000a;65:4498–4508. doi: 10.1021/jo000220v. [DOI] [PubMed] [Google Scholar]
- Berkowitz DB, Choi S, Maeng J-H. Enzyme-assisted asymmetric total synthesis of (−)-podophyllotoxin and (−)-picropodophyllin. J Org Chem. 2000b;65:847–860. doi: 10.1021/jo991582+. [DOI] [PubMed] [Google Scholar]
- Berkowitz DB, Eggen M, Shen Q, Shoemaker RK. Ready access to fluorinated phosphonate mimics of secondary phosphates. Synthesis of the (alpha,alpha-Difluoroalkyl)phosphonate analogs of L-phosphoserine, L-phosphoallothreonine, and L-phosphothreonine. J Org Chem. 1996a;61:4666–4675. doi: 10.1021/jo9604752. [DOI] [PubMed] [Google Scholar]
- Berkowitz DB, Eggen M, Shen Q, Sloss DG. Synthesis of (alpha,alpha-difluoroalkyl)phosphonates by displacement of primary triflates. J Org Chem. 1993;58:6174–6176. [Google Scholar]
- Berkowitz DB, Maeng J-H, Dantzig AH, Shepard RL, Norman BH. Chemoenzymic and ring E-modular approach to the (−)-podophyllotoxin skeleton. Synthesis of 3′,4′,5′-tridemethoxy-(−)-podophyllotoxin. J Am Chem Soc. 1996b;118:9426–9427. [Google Scholar]
- Berkowitz DB, Maiti G, Charette BD, Dreis CD, MacDonald RG. Mono- and bivalent ligands bearing mannose 6-phosphate (M6P) Surrogates: Targeting the M6P/Insulin-Like Growth Factor II Receptor. Org Lett. 2004;6:4921–4924. doi: 10.1021/ol0479444. [DOI] [PubMed] [Google Scholar]
- Berkowitz DB, Pumphrey JA, Shen Q. Enantiomerically enriched alpha-vinyl amino acids via lipase-mediated reverse transesterification. Tetrahedron Lett. 1994a;35:8743–8746. [Google Scholar]
- Berkowitz DB, Shen Q, Maeng J-H. Synthesis of the (alpha,alpha-difluoroalkyl)phosphonate analog of phosphoserine. Tetrahedron Lett. 1994b;35:6445–6448. [Google Scholar]
- Berkowitz DB, Sloss DG. Diallyl (Lithiodifluoromethyl)phosphonate: A new reagent for the introduction of the (difluoromethylene)phosphonate functionality. J Org Chem. 1995;60:7047–7050. [Google Scholar]
- Biffinger JC, Kim HW, DiMagno SG. The polar hydrophobicity of fluorinated compounds. ChemBioChem. 2004;5:622–627. doi: 10.1002/cbic.200300910. [DOI] [PubMed] [Google Scholar]
- Blackburn GM, Brown D, Martin SJ. A novel synthesis of fluorinated phosphonoacetic acids. J Chem Res, Synopses. 1985a:92–93. [Google Scholar]
- Blackburn GM, Brown D, Martin SJ, Parratt MJ. Studies on selected transformations of some fluoromethanephosphonate esters. J Chem Soc, Perkin. 1987;1:181–186. [Google Scholar]
- Blackburn GM, Eckstein F, Kent DE, Perree TD. Isopolar vs. isosteric phosphonate analogs of nucleotides. Nucleosides Nucleotides. 1985b;4:165–167. [Google Scholar]
- Blackburn GM, England DA, Kolkmann F. Monofluoro- and difluoromethylenebisphosphonic acids: isopolar analogs of pyrophosphoric acid. J Chem Soc, Chem Commun. 1981:930–932. [Google Scholar]
- Blackburn GM, Jakeman DL, Ivory AJ, Willliamson MP. Synthesis of phosphonate analogs of 1,3-bisphosphoglyceric acid and their binding to yeast phosphoglycerate kinase. Bioorg Med Chem Lett. 1994;4:2573–2578. [Google Scholar]
- Blackburn GM, Kent DE, Kolkmann F. The synthesis and metal binding characteristics of novel, isopolar phosphonate analogs of nucleotides. J Chem Soc, Perkin. 1984;1:1119–1125. [Google Scholar]
- Blades K, Lequeux TP, Percy JM. A reproducible and high-yielding cerium-mediated route to alpha,alpha-difluoro-beta-ketophosphonates. Tetrahedron. 1997;53:10623–10632. [Google Scholar]
- Boros LG, Boros TF. Use of metabolic pathway flux information in anticancer drug design. Ernst Schering Found Symp Proc. 2008:189–203. doi: 10.1007/2789_2008_094. [DOI] [PubMed] [Google Scholar]
- Burton DJ, Flynn RM. Difluoromethanebis(phosphonic acid) Application: US 80-143995, 4330486. University of Iowa Research Foundation; USA: 1982. p. 5. [Google Scholar]
- Bykov VJN, Issaeva N, Shilov A, Hultcrantz M, Pugacheva L, Chumakov P, Bergman J, Wiman KG, Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- Caplan NA, Pogson CI, Hayes DJ, Blackburn GM. The synthesis of novel bisphosphonates as inhibitors of phosphoglycerate kinase (3-PGK) Perkin. 2000;1:421–437. doi: 10.1016/s0960-894x(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Clarke AJ, Hurtado-Guerrero R, Pathak S, Schuttelkopf AW, Borodkin V, Shepherd SM, Ibrahim AFM, van Aalten DMF. Structural insights into mechanism and specificity of O-GlcNAc transferase. EMBO J. 2008;27:2780–2788. doi: 10.1038/emboj.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill GS, Easterfield HJ, Percy JM, Pintat S. Facile syntheses of building blocks for the construction of phosphotyrosine mimetics. Perkin. 2000;1:2591–2599. [Google Scholar]
- Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- Di Croce L, Shiekhattar R. Thrilling transcription through threonine phosphorylation. Nat Cell Biol. 2008;10:5–6. doi: 10.1038/ncb0108-5. [DOI] [PubMed] [Google Scholar]
- Diab SA, Sene A, Pfund E, Lequeux T. Efficient synthesis of fluorophosphonylated alkyles by ring-opening reaction of cyclic sulfates. Org Lett. 2008;10:3895–3898. doi: 10.1021/ol801443s. [DOI] [PubMed] [Google Scholar]
- DiMagno SG, Dussault PH, Schultz JA. Fluorous biphasic singlet oxygenation with a perfluoroalkylated photosensitizer. J Am Chem Soc. 1996;118:5312–5313. [Google Scholar]
- Fei X, Connelly CM, MacDonald RG, Berkowitz DB. A set of phosphatase-inert “molecular rulers” to probe for bivalent mannose 6-phosphate ligand-receptor interactions. Bioorg Med Chem Lett. 2008;18:3085–3089. doi: 10.1016/j.bmcl.2007.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MC, Diaz A, Guillin JJ, Blanco O, Ruiz M, Ojea V. Diastereoselective synthesis of 2-amino-4-phosphonobutanoic acids by electrophilic substitution and tin-Peterson olefination of bis-lactim ethers derived from cyclo-[L-AP4-D-Val] J Org Chem. 2006;71:6958–6974. doi: 10.1021/jo061072x. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- Flavell RR, Muir TW. Expressed protein ligation (EPL) in the study of signal transduction, ion conduction, and chromatin biology. Acc Chem Res. 2009;42:107–116. doi: 10.1021/ar800129c. [DOI] [PubMed] [Google Scholar]
- Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep. 2008;9:865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner P, Park JM. 1,1-Dimethylethyl (S)- or (R)-4-formyl-2,2-dimethyl-3-oxazolidinecarboxylate: a useful serinal derivative. Org Synth. 1992;70:18–28. [Google Scholar]
- Gautier A, Lopin C, Garipova G, Kalinina I, Salcedo C, Balieu S, Piettre SR. Analogues of nucleotides and oligonucleotides featuring difluorophosphonate, difluorophosphonothioate and difluorophosphinate functional groups. J Fluorine Chem. 2004;125:1745–1756. [Google Scholar]
- Girault J-A. Signalling by tyrosine phosphorylation in the nervous system. Mol Biol Neuron. (2nd Ed) 2006:271–289. [Google Scholar]
- Gorres KL, Edupuganti R, Krow GR, Raines RT. Conformational preferences of substrates for human prolyl 4-hydroxylase. Biochemistry. 2008;47:9447–9455. doi: 10.1021/bi8009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grangeasse C, Cozzone AJ, Deutscher J, Mijakovic I. Tyrosine phosphorylation: an emerging regulatory device of bacterial physiology. Trends Biochem Sci. 2007;32:86–94. doi: 10.1016/j.tibs.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Busby S, Grove K, Li S, Mason D, Medina L, Moloney D, Philipsberg G, Scartozzi R. O-glycosylation of nuclear and cytoplasmic proteins: regulation analogous to phosphorylation? Biochem Biophys Res Commun. 1997;231:237–242. doi: 10.1006/bbrc.1997.6110. [DOI] [PubMed] [Google Scholar]
- Han E-S, Muller FL, Perez VI, Qi W, Liang H, Xi L, Fu C, Doyle E, Hickey M, Cornell J, et al. The in vivo gene expression signature of oxidative stress. Physiol Genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Greis KD, Dong LYD, Blomberg MA, Chou T-Y, Jiang M-S, Roquemore EP, Snow DM, Kreppel LK, et al. O-linked Nacetylglucosamine: The “Yin-Yang” of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv Exp Med Biol. 1995;376:115–123. [PubMed] [Google Scholar]
- Haupt S, Haupt Y. Importance of p53 for cancer onset and therapy. Anti-Cancer Drugs. 2006;17:725–732. doi: 10.1097/01.cad.0000217422.52208.fa. [DOI] [PubMed] [Google Scholar]
- Herpin TF, Houlton JS, Motherwell WB, Roberts BP, Weibel J-M. Preparation of some new anomeric carbohydrate difluoromethylenephosphonates via phosphonyl radical addition to gem-difluoroenol ethers. Chem Commun. 1996:613–614. [Google Scholar]
- Higashimoto Y, Saito S.i., Tong X-H, Hong A, Sakaguchi K, Anderson CW, Appella E. Synthesis of a difluoromethyl-phosphoserine (F2Pab)-containing peptides for generating specific antibodies to phosphorylation sites. Peptides. 2001;2000:869–870. [Google Scholar]
- Higashimoto Y, Saito S.i., Tong X-H, Hong A, Sakaguchi K, Appella E, Anderson CW. Human p53 is phosphorylated on serines 6 and 9 in response to DNA damage-inducing agents. J Biol Chem. 2000;275:23199–23203. doi: 10.1074/jbc.M002674200. [DOI] [PubMed] [Google Scholar]
- Hikishima S, Isobe M, Koyanagi S, Soeda S, Shimeno H, Shibuya S, Yokomatsu T. Synthesis and biological evaluation of 9-(5′,5′-difluoro-5′-phosphonopentyl)guanine derivatives for PNP-inhibitors. Bioorg Med Chem. 2006;14:1660–1670. doi: 10.1016/j.bmc.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Jensen J, Lai Y-C. Regulation of muscle glycogen synthase phosphorylation and kinetic properties by insulin, exercise, adrenaline and role in insulin resistance. Arch Physiol Biochem. 2009;115:13–29. doi: 10.1080/13813450902778171. [DOI] [PubMed] [Google Scholar]
- Kawamoto AM, Campbell MM. A new method for the synthesis of a phosphonic acid analog of phosphoserine via a novel 1,1-difluorophosphonate intermediate. J Fluorine Chem. 1997;81:181–186. [Google Scholar]
- Kishi H, Nakagawa K, Matsumoto M, Suga M, Ando M, Taya Y, Yamaizumi M. Osmotic shock induces G1 arrest through p53 phosphorylation at Ser33 by activated p38MAPK without phosphorylation at Ser15 and Ser20. J Biol Chem. 2001;276:39115–39122. doi: 10.1074/jbc.M105134200. [DOI] [PubMed] [Google Scholar]
- Klein DC. Arylalkylamine N-acetyltransferase: “the timezyme”. J Biol Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- Klein DC, Ganguly S, Coon SL, Shi Q, Gaildrat P, Morin F, Weller JL, Obsil T, Hickman A, Dyda F. 14-3-3 proteins in pineal photoneuroendocrine transduction: How many roles? J Neuroendocrinol. 2003;15:370–377. doi: 10.1046/j.1365-2826.2003.01000.x. [DOI] [PubMed] [Google Scholar]
- Kulasekara HD, Miller SI. Threonine phosphorylation times bacterial secretion. Nat Cell Biol. 2007;9:734–736. doi: 10.1038/ncb0707-734. [DOI] [PubMed] [Google Scholar]
- Lequeux T, Lebouc F, Lopin C, Yang H, Gouhier G, Piettre SR. Sulfanyl- and selanyldifluoromethylphosphonates as a source of phosphonodifluoromethyl radicals and their addition onto alkenes. Org Lett. 2001;3:185–188. doi: 10.1021/ol006746j. [DOI] [PubMed] [Google Scholar]
- Liebermann DA, Hoffman B, Vesely D. p53 Induced growth arrest versus apoptosis and its modulation by survival cytokines. Cell Cycle. 2007;6:166–170. doi: 10.4161/cc.6.2.3789. [DOI] [PubMed] [Google Scholar]
- Liu DR, Schultz PG. Progress toward the evolution of an organism with an expanded genetic code. Proc Natl Acad Sci U S A. 1999;96:4780–4785. doi: 10.1073/pnas.96.9.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopin C, Gouhier G, Piettre SR. First synthesis of S,S-dialkyl difluorophosphonodithioates and difluorophosphonotrithioates. Tetrahedron Lett. 2003;44:8837–8840. [Google Scholar]
- Martin SF, Dean DW, Wagman AS. A general method for the synthesis of 1,1-difluoroalkylphosphonates. Tetrahedron Lett. 1992;33:1839–1842. [Google Scholar]
- McKenna CE, Shen P-D. Fluorination of methanediphosphonate esters by perchloryl fluoride. Synthesis of fluoromethanediphosphonic acid and difluoromethanediphosphonic acid. J Org Chem. 1981;46:4573–4576. [Google Scholar]
- Muir TW, Sondhi D, Cole PA. Expressed protein ligation: A general method for protein engineering. Proc Natl Acad Sci U S A. 1998;95:6705–6710. doi: 10.1073/pnas.95.12.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano T, Kobayakawa H, Yuasa Y, Yokomatsu T, Shibuya S. Improved synthesis of 1,3-propanediol derivatives having a diethoxyphosphoryldifluoroethyl functional group at the 2-position: application to chemoenzymatic synthesis of novel acyclic nucleotide analogues of adenosine bisphosphates. Synthesis. 2005:187–192. [Google Scholar]
- Nag K, Chaudhary A. Mediators of tyrosine phosphorylation in innate immunity: from host defense to inflammation onto oncogenesis. Curr Signal Transduction Ther. 2009;4:76–81. doi: 10.2174/15743620978816750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair HK, Burton DJ. Facile synthesis of fluorinated phosphonates via photochemical and thermal reactions. J Am Chem Soc. 1997;119:9137–9143. [Google Scholar]
- Nieschalk J, Batsanov AS, O'Hagan D, Howard JAK. Synthesis of monofluoro- and difluoromethylenephosphonate analogs of snglycerol-3-phosphate as substrates for glycerol-3-phosphate dehydrogenase and the x-ray structure of the fluoromethylenephosphonate moiety. Tetrahedron. 1996;52:165–176. [Google Scholar]
- O'Hagan D. Understanding organofluorine chemistry. An introduction to the C-F bond. Chem Soc Rev. 2008;37:308–319. doi: 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]
- Obsil T, Ghirlando R, Klein DC, Ganguly S, Dyda F. Crystal structure of the 14-3-3Œ∂:serotonin N-acetyltransferase complex: a role for scaffolding in enzyme regulation. Cell. 2001;105:257–267. doi: 10.1016/s0092-8674(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Otaka A, Mitsuyama E, Kinoshita T, Tamamura H, Fujii N. Stereoselective synthesis of CF2-substituted phosphothreonine mimetics and their incorporation into peptides using newly developed deprotection procedures. J Org Chem. 2000;65:4888–4899. doi: 10.1021/jo000169v. [DOI] [PubMed] [Google Scholar]
- Otaka A, Mitsuyama E, Watanabe J, Watanabe H, Fujii N. Synthesis of fluorine-containing bioisosteres corresponding to phosphoamino acids and dipeptide units. Biopolymers. 2004;76:140–149. doi: 10.1002/bip.10570. [DOI] [PubMed] [Google Scholar]
- Otaka A, Miyoshi K, Burke TR, Jr., Roller PP, Kubota H, Tamamura H, Fujii N. Synthesis and application of N-Boc-L-2-amino-4-(diethylphosphono)-4,4-difluorobutanoic acid for solid-phase synthesis of nonhydrolyzable phosphoserine peptide analogs. Tetrahedron Lett. 1995a;36:927–930. [Google Scholar]
- Otaka A, Miyoshi K, Kaneko M, Burke TR, Jr., Roller PP, Tamamura H, Fujii N. Synthesis and biological activity of phosphatases resistant phosphoamino acid mimetics containing peptides. Pept Chem. 1995b;32:9–12. [Google Scholar]
- Ozouf P, Binot G, Pommelet J-C, Lequeux TP. Syntheses of omega-hydroxy-alpha,alpha-difluoromethylphosphonates by oxacycle ring-opening reactions. Org Lett. 2004;6:3747–3750. doi: 10.1021/ol048549g. [DOI] [PubMed] [Google Scholar]
- Pajkert R, Kolomeitsev AA, Milewska M, Roeschenthaler G-V, Koroniak H. TiCl4 and Grignard reagent-promoted ring-opening reactions of various epoxides: synthesis of gamma-hydroxy-alpha,alpha-difluoromethylenephosphonates. Tetrahedron Lett. 2008;49:6046–6049. [Google Scholar]
- Pfund E, Lequeux T, Masson S, Vazeux M, Cordi A, Pierre A, Serre V, Herve G. Efficient synthesis of fluorothiosparfosic acid analogues with potential antitumoral activity. Bioorg Med Chem. 2005;13:4921–4928. doi: 10.1016/j.bmc.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Pickin KA, Chaudhury S, Dancy BCR, Gray JJ, Cole PA. Analysis of protein kinase autophosphorylation using expressed protein ligation and computational modeling. J Am Chem Soc. 2008;130:5667–5669. doi: 10.1021/ja711244h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignard S, Lopin C, Gouhier G, Piettre SR. Phosphonodifluoromethyl and phosphonothiodifluoromethyl radicals. Generation and addition onto alkenes and alkynes. J Org Chem. 2006;71:31–37. doi: 10.1021/jo051511c. [DOI] [PubMed] [Google Scholar]
- Pytel D, Sliwinski T, Poplawski T, Ferriola D, Majsterek I. Tyrosine kinase blockers: new hope for successful cancer therapy. Anti-Cancer Agents Med Chem. 2009;9:66–76. doi: 10.2174/187152009787047752. [DOI] [PubMed] [Google Scholar]
- Rauh D, Waldmann H. Linking chemistry and biology for the study of protein function. Angew Chem. (Int Ed) 2007;46:826–829. doi: 10.1002/anie.200602979. [DOI] [PubMed] [Google Scholar]
- Roeschenthaler G-V, Kukhar VP, Belik MY, Mazurenko KI, Sorochinsky AE. Diastereoselective addition of diethyl difluoromethylphosphonate to enantiopure sulfinimines: synthesis of alpha,alpha-difluoro-beta-aminophosphonates, phosphonic acids, and phosphonamidic acids. Tetrahedron. 2006;62:9902–9910. [Google Scholar]
- Roskoski R. VEGF receptor protein-tyrosine kinases: Structure and regulation. Biochem Biophys Res Commun. 2008;375:287–291. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- Saito S.i., Yamaguchi H, Higashimoto Y, Chao C, Xu Y, Fornace AJ, Jr., Appella E, Anderson CW. Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J Biol Chem. 2003;278:37536–37544. doi: 10.1074/jbc.M305135200. [DOI] [PubMed] [Google Scholar]
- Savage MJ, Gingrich DE. Advances in the development of kinase inhibitor therapeutics for Alzheimer's disease. Drug Dev Res. 2009;70:125–144. [Google Scholar]
- Schwarzer D, Cole PA. Protein semisynthesis and expressed protein ligation: chasing a protein's tail. Curr Opin Chem Biol. 2005;9:561–569. doi: 10.1016/j.cbpa.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Shen Q, Sloss DG, Berkowitz DB. Displacement of sugar triflates with C-nucleophiles: D-glucopyranose and D-ribofuranose chain extension and functionalization. Synth Commun. 1994;24:1519–1530. [Google Scholar]
- Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- Szewczuk LM, Tarrant MK, Sample V, Drury WJ, Zhang J, Cole PA. Analysis of serotonin N-acetyltransferase regulation in vitro and in live cells using protein semisynthesis. Biochemistry. 2008;47:10407–10419. doi: 10.1021/bi801189d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- Toole BJ, Cohen PTW. The skeletal muscle-specific glycogen-targeted protein phosphatase 1 plays a major role in the regulation of glycogen metabolism by adrenaline in vivo. Cell Signalling. 2007;19:1044–1055. doi: 10.1016/j.cellsig.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Tzivion G, Avruch J. 14-3-3 Proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem. 2002;277:3061–3064. doi: 10.1074/jbc.R100059200. [DOI] [PubMed] [Google Scholar]
- Vassilev LT. p53 Activation by small molecules: Application in oncology. J Med Chem. 2005;48:4491–4499. doi: 10.1021/jm058174k. [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumor regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- Welch JT. Fluoroolefin dipeptide isosteres: structure, syntheses, and applications. Fluorine and Health. 2008:699–735. [Google Scholar]
- Wells L, Whalen SA, Hart GW. O-GlcNAc: a regulatory post-translational modification. Biochem Biophys Res Commun. 2003;302:435–441. doi: 10.1016/s0006-291x(03)00175-x. [DOI] [PubMed] [Google Scholar]
- Xie J, Schultz PG. Adding amino acids to the genetic repertoire. Curr Opin Chem Biol. 2005;9:548–554. doi: 10.1016/j.cbpa.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Xie J, Schultz PG. A chemical toolkit for proteins - an expanded genetic code. Nature Rev Mol Cell Biol. 2006;7:775–782. doi: 10.1038/nrm2005. [DOI] [PubMed] [Google Scholar]
- Xu Y, Aoki J, Shimizu K, Umezu-Goto M, Hama K, Takanezawa Y, Yu S, Mills GB, Arai H, Qian L, et al. Structure-activity relationships of fluorinated lysophosphatidic acid analogues. J Med Chem. 2005;48:3319–3327. doi: 10.1021/jm049186t. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Suzuki K, Kodama S, Watanabe M. Stabilization of alanine-substituted p53 protein at Ser15, Thr18, and Ser20 in response to ionizing radiation. Biochem Biophys Res Commun. 2004;323:906–911. doi: 10.1016/j.bbrc.2004.08.175. [DOI] [PubMed] [Google Scholar]
- Yang ZY, Burton DJ. A novel and practical preparation of alpha,alpha-difluoro functionalized phosphonates from iododifluoromethylphosphonate. J Org Chem. 1992;57:4676–4683. [Google Scholar]
- Yokomatsu T, Kato J, Sakuma C, Shibuya S. Stereoselective synthesis of highly-functionalized cyclohexene derivatives having a diethoxyphosphoryldifluoromethyl functionality from cyclohex-2-enyl-1-phosphates. Synlett. 2003:1407–1410. [Google Scholar]
- Yokomatsu T, Sato M, Shibuya S. Lipase-catalyzed enantioselective acylation of prochiral 2-(omega-phosphono)alkyl-1,3-propanediols: application to the enantioselective synthesis of omega-phosphono-alpha-amino acids. Tetrahedron: Asymmetry. 1996;7:2743–2754. [Google Scholar]
- Zhang G, Xu C-F, Neubert TA. Analysis of protein-tyrosine phosphorylation by mass spectrometry. Compr Anal Chem. 2009;52:297–313. [Google Scholar]
- Zhang Z, Shen K, Lu W, Cole PA. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J Biol Chem. 2003;278:4668–4674. doi: 10.1074/jbc.M210028200. [DOI] [PubMed] [Google Scholar]
- Zheng W, Schwarzer D, LeBeau A, Weller JL, Klein DC, Cole PA. Cellular stability of serotonin N-acetyltransferase conferred by phosphonodifluoromethylene alanine (Pfa) substitution for Ser-205. J Biol Chem. 2005;280:10462–10467. doi: 10.1074/jbc.M412283200. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhang Z, Ganguly S, Weller JL, Klein DC, Cole PA. Cellular stabilization of the melatonin rhythm enzyme induced by nonhydrolyzable phosphonate incorporation. Nat Struct Biol. 2003;10:1054–1057. doi: 10.1038/nsb1005. [DOI] [PubMed] [Google Scholar]