Abstract
Hypersensitivity pneumonitis is an environmental lung disease characterized by a diffuse mononuclear cell infiltrate in the lung that can progress to pulmonary fibrosis with chronic exposure to an inhaled Ag. Using a well-established murine model of hypersensitivity pneumonitis, we repeatedly exposed C57BL/6 mice to Saccharopolyspora rectivirgula to investigate whether T cells are required for lung fibrosis. In the absence of αβ T cells, TCRβ−/− mice exposed to S. rectivirgula for 4 wk had markedly decreased mononuclear infiltrates and collagen deposition in the lung compared with wild-type C57BL/6 mice. In contrast to CD8+ T cells, adoptive transfer of CD4+ T cells reconstituted the S. rectivirgula-induced inflammatory and fibrotic response, suggesting that the CD4+ T cell represents the critical αβ T cell subset. Cytokine analysis of lung homogenates at various time points after S. rectivirgula exposure failed to identify a predominant Th1 or Th2 phenotype. Conversely, IL-17 was found in the lung at increasing concentrations with continued exposure to S. rectivirgula. Intracellular cytokine staining revealed that 14% of CD4+ T cells from the lung of mice treated with S. rectivirgula expressed IL-17A. In the absence of IL-17 receptor signaling, Il17ra−/− mice had significantly decreased lung inflammation and fibrosis compared with wild-type C57BL/6 mice. These data are the first to demonstrate an important role for Th17-polarized CD4+ T lymphocytes in the immune response directed against S. rectivirgula in this murine model of hypersensitivity pneumonitis and pulmonary fibrosis.
Hypersensitivity pneumonitis (HP)3 is an environmental lung disease that results from repeated inhalation of aerosolized Ags (1). The etiologic agents are composed of a wide variety of organic particles (e.g., mammalian and avian proteins, fungi, and thermophilic bacteria) and certain small molecular mass volatile and nonvolatile chemical compounds. A classic example of HP is Farmer’s lung, which is caused by the thermophilic actinomycete Saccharopolyspora rectivirgula. This disorder occurs in genetically susceptible individuals who are repeatedly exposed to moldy hay. HP occurs in several clinical forms (e.g., acute, subacute, and chronic), depending on the nature of the Ag, the quantity and duration of exposure, and host/environment interactions (1). The acute form of disease is typically nonprogressive, with spontaneous resolution after cessation of Ag exposure. The subacute and chronic forms of disease result from continued low-level exposure to inhaled Ags. In the chronic subset of patients, pulmonary fibrosis occurs in up to 41% of cases, resulting in irreversible pulmonary dysfunction and right heart failure (2–5). Lung fibrosis has been shown to be an independent predictor of mortality in these patients, with a 5-year mortality of 27% and a median survival of 13 years (6). Histopathologically, this disorder is characterized by a diffuse mononuclear cell infiltration of the lung with poorly formed granulomas located near the bronchovascular bundle (7).
In a well-described murine model of HP, mice treated with S. rectivirgula for 3 days each week by nasal inhalation develop mononuclear infiltrates in a peribronchovascular distribution that approximates the human disease (8–11). The mononuclear infiltrates are predominantly composed of alveolar macrophages and T cells. These T cells have been shown to be important in the immunopathogenesis of this disease, as athymic nude mice that lack T cells develop significantly less severe HP (10). The role of T cells in the development of pulmonary fibrosis, however, is unknown. Evidence from the bleomycin model of lung fibrosis and other models of solid organ fibrosis suggests that type 2 cytokines promote fibrosis through stimulation of fibroblast secretion of extracellular matrix proteins, types I and III collagen, and fibronectin, while the production of type 1 cytokines such as IFN-γ attenuates these fibrotic diseases (12–15). Interestingly, Th1-biased C57BL/6 mice are more susceptible to HP than are Th2-biased DBA/2 mice after repeated exposure to S. rectivirgula (16). Additionally, mice deficient in IFN-γ do not develop significant mononuclear infiltrates consistent with HP after repeated exposure to S. rectivirgula (17), while administration of type 2 cytokines such as IL-4 and IL-10 modulates the severity of HP (18, 19). Although these data suggest that HP induced by S. rectivirgula is a Th1-mediated disease, the cellular source of IFN-γ is unclear, with a recent study suggesting that neutrophil secretion of IFN-γ in the absence of T cell IFN-γ production was sufficient for granuloma formation in response to S. rectivirgula (20). Although mice chronically exposed to S. rectivirgula have type 1 cytokines in their lungs, these mice have also been shown to develop pulmonary fibrosis (21). This paradox remains unresolved. Some reports suggest that a relative decrease in type 1 cytokines (22) may allow the development of pulmonary fibrosis, while other investigators have hypothesized that a transition from a predominantly Th1 to Th2 cytokine milieu occurs in the lung with repeated exposure to an inhaled Ag, promoting the development of lung fibrosis (23).
In this report, we show that αβ-expressing T cells are essential for the development of the peribronchovascular mononuclear infiltrates and resultant pulmonary fibrosis that occur in response to S. rectivirgula. In contrast to CD8+ T cells, lung CD4+ T cells represent the critical αβ T cell subset, expressing an activated effector phenotype and a polarized Th17 cytokine response. After exposure of Il17ra−/− mice to S. rectivirgula, a significantly decreased T cell alveolitis was seen, with an associated decrease in collagen deposition. Taken together, these data are the first to show the importance of a Th17-polarized immune response in S. rectivirgula-induced lung inflammation and the resultant lung fibrosis.
Materials and Methods
Treatment of mice
Six- to 8-wk-old female C57BL/6, TCRβ−/−, and TCRβ−/−δ−/− mice (The Jackson Laboratory) and B6.129-Il17ratm1Koll (Il17ra−/−) mice (Amgen) were treated with 30 μl (150 μg) of S. rectivirgula (American Type Tissue Collection catalogue no. 29034 and a generous gift from Dr. Gary Hunninghake, University of Iowa) or sterile PBS on 3 consecutive days each week for up to 12 consecutive weeks by nasal inhalation. S. rectivirgula was prepared by growing the microorganism in tryptic soy broth at 55°C with constant agitation. The S. rectivirgula culture was centrifuged, resuspended in sterile PBS, and quantified by Lowry method (Sigma-Aldrich) before administration to mice by nasal inhalation. A Limulus amebocyte assay (Sigma-Aldrich) was performed to confirm that the S. rectivirgula preparation and sterile PBS contained <20 μg endotoxin/ml. Mice were lightly anesthetized with isoflurane to allow inhalation of either S. rectivirgula or sterile PBS. These studies were approved by the Animal Care and Use Committee at the University of Colorado Denver.
Preparation of mononuclear cells from lung homogenates
Mice at each time point were sacrificed 24 h after the last treatment with S. rectivirgula as previously described (24). Briefly, the chest cavity was opened using sterile surgical dissection, and the inferior vena cava and abdominal aorta were clamped. The left atrium was opened by incision, and the right ventricle was infused with at least 2 ml of sterile PBS to remove any residual blood from the pulmonary vasculature. The heart and lungs were removed en bloc, and the heart, thymus, and lymph nodes were dissected away from the lungs. The right lung was removed, snap-frozen in liquid nitrogen, and stored at −80°C for collagen quantification. The left lung was cut into small pieces and placed in RPMI 1640 containing 5% FBS, collagenase (Sigma-Aldrich), and DNase (Boehringer Mannheim). After 30 min of collagenase digestion in a 37°C water bath, lungs were further disrupted by aspiration through an 18-gauge needle. The collagenase-digested lungs were layered on top of Ficoll (Accurate Chemical & Scientific) and centrifuged at 1200 rpm for 30 min at room temperature. The interface between the medium and Ficoll was removed and washed twice with RPMI 1640 containing 5% FBS. Total cell and differential cell counts were performed on C57BL/6 and Il17ra−/− mice as previously described (24).
Flow cytometry and immunofluorescence analysis
T lymphocytes isolated from the lungs of C57BL/6 mice treated with either S. rectivirgula or sterile PBS were surface stained with mAbs directed against CD3, CD4, CD8, CD44, CD45RB, CD27, CD62L, and CD69 (BD Biosciences). For intracellular cytokine staining, total lung cells were cultured at 1 × 106 cells/ml in complete media containing 10 μg/ml brefeldin A (Sigma-Aldrich), 50 ng/ml PMA (Sigma-Aldrich), and 1 μg/ml ionomycin (Sigma-Aldrich) or S. rectivirgula (10 μg/ml) at 37°C for 4 h. After activation, the cells were washed and stained with mAbs directed against CD3, CD4, and CD8 followed by fixation in 1% paraformaldehyde overnight. Fixed cells were permeabilized for 10 min at room temperature in 0.5% saponin and stained with mAbs directed against IL-17A (eBio-science), IL-17F (eBioscience), IL-22 (R&D Systems), and isotype controls for 30 min at 4°C. Electronic compensation was performed with Ab capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. The lymphocyte population was identified using forward and 90° light scatter patterns, and fluorescence intensity was analyzed using a FACSAria cytometer (BD Immunocytometry Systems) (25, 26). The data files were analyzed using FloJo software (Tree Star).
Adoptive transfer experiments
CD4+ and CD8+ T cells were isolated from spleens of untreated C57BL/6 mice and purified by MACS (Miltenyi Biotec) using negative selection for either CD4+ or CD8+ T lymphocytes. Flow cytometry confirmed >95% purity for each T cell population before adoptive transfer of 5 × 105 cells/100 μl of sterile PBS by tail vein injection into TCRβ−/−δ−/− mice. Briefly, total splenocytes were obtained by pushing spleens through a 70-μm pore size mesh (Falcon) with subsequent resuspension in RBC lysis buffer (eBioscience). Cells were resuspended in buffer and biotinylated Ab cocktail, mixed, and incubated at 4°C for 10 min. Anti-biotin microbeads were added per the manufacturer’s instructions, mixed, and incubated for 15 min at 4°C. The cells were washed and applied to the column per the manufacturer’s instructions. The effluent that contained the unlabeled CD4+ or CD8+ T cells was collected and passed over a second column to further enrich each T cell population. Cell counts for CD4+ and CD8+ T lymphocytes were determined by a Coulter counter (Beckman Coulter).
Histology
C57BL/6, TCRβ−/−, TCRβ−/−δ−/−, and Il17ra−/− mice were sacrificed 24 h after their last exposure to S. rectivirgula. The lungs were removed and infused with 10% formalin, embedded in paraffin, and stained with H&E or Masson trichrome following the manufacturer’s instructions.
Cytokine analysis
Total lung homogenates were prepared by homogenizing whole-lung samples in 500 μl of sterile PBS from C57BL/6 and TCRβ−/−δ−/− mice treated with either S. rectivirgula for 1, 2, 3, and 4 consecutive weeks or sterile PBS for 4 wk. The 100 μl of supernatant from each lung homogenate was analyzed for 20 cytokines and chemokines using the protein multiplex immunoassay kit (BioSource International) as per the manufacturer’s protocol. Briefly, multiplex beads were loaded onto a Millipore MultiScreen BV 96-well filter plate followed by each sample diluted 1/2 with assay diluent. Serial dilutions of cytokine standards were prepared in parallel and added to the plate. After a 2-h incubation on a plate shaker (600 revolutions/min) in the dark at room temperature, the samples were washed and biotinylated anti-mouse multicytokine reporter was added to each well. After incubation on a plate shaker at room temperature for 1 h, the plate was washed and PE-conjugated streptavidin added directly to each well. The plate was incubated for 30 min, washed, and transferred to the Bio-Plex Luminex 100 XYP instrument for analysis. Cytokine concentrations were calculated using Bio-Plex Manager 3.0 software with a five-parameter curve-fitting algorithm applied for standard curve calculations. Activated TGF-β1 in supernatants from whole-lung homogenates was quantified by ELISA according to the manufacturer’s instructions (R&D Systems). IL-17A, IL-23 (eBioscience), IL-17F, and IL-22 (R&D Systems) from whole-lung homogenates were quantified by ELISA according to the manufacturers’ instructions.
Collagen quantification
The collagen content of the right lung from the various mouse strains treated with either S. rectivirgula or sterile PBS was determined using Sirius red staining (24). Sirius red stain allows quantification of types I–V mammalian collagen. Each lung sample was thawed at 4°C in sterile PBS supplemented with protease inhibitors (Sigma-Aldrich), homogenized in 5 ml of 0.5 M acetic acid containing 1 mg of pepsin/10 mg of tissue, and incubated for 24 h at 4°C with constant stirring. After centrifugation at high speed for 10 min, 100 μl of each supernatant was mixed with 1 ml of Sirius red dye reagent, allowed to incubate at room temperature for 30 min, and then centrifuged at high speed for 10 min. After aspiration of the supernatant, the pellet containing the complex of soluble collagen and Sirius red dye reagent was resuspended in 0.5 M NaOH, and the OD was measured using a spectrophotometer. The collagen content in micrograms was calculated from a standard curve generated using known concentrations of collagen per the manufacturer’s instructions.
Statistical analysis
A Mann-Whitney U analysis and one-way ANOVA with a Bonferroni’s multiple comparison test were used to determine whether there were significant differences between the treatment groups at each time point (Prism 4; GraphPad Software). A p value of <0.05 was considered statistically significant.
Results
TCRβ−/− mice repeatedly exposed to S. rectivirgula do not develop severe pulmonary fibrosis
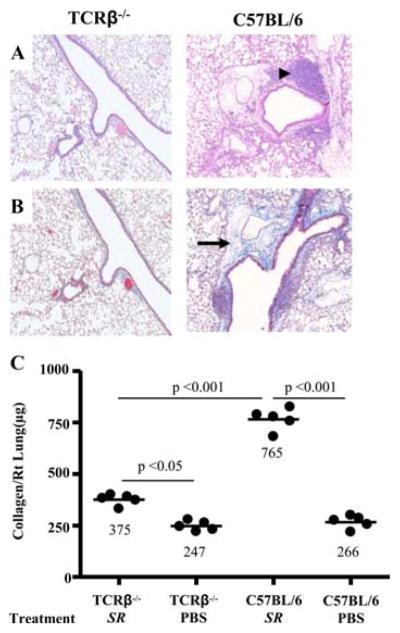
To define the role of αβ T cells in the development of pulmonary fibrosis, TCRβ−/− mice (i.e., mice deficient in CD4+ and CD8+ T cells) were repeatedly exposed to S. rectivirgula for 4 wk. As shown in Fig. 1, A and B, TCRβ−/− mice do not develop mononuclear cell infiltrates or obvious collagen deposition in a peribronchovascular distribution by H&E and Masson trichrome staining, respectively, in the absence of CD4+ and CD8+ T cells. Using the more sensitive Sirius red colorimetric assay, S. rectivirgula-treated TCRβ−/− mice developed a 1.5-fold increase in collagen content in the lung compared with PBS-treated control animals (p < 0.01) but significantly less collagen deposition compared with wild-type (wt) C57BL/6 mice exposed to S. rectivirgula in an identical fashion (p < 0.001) (Fig. 1C). Lungs of TCRβ−/− mice, however, contain a resident γδ T cell population (Vγ1+ and Vγ4+) (27). Interestingly, after 4 wk of exposure to S. rectivirgula, TCRβ−/− mice develop an expanded number of γδ T cells in the lung that includes Vγ6+ γδ T cells compared with PBS-treated TCRβ−/− mice (1.21 ± 0.31 × 105 vs 0.51 ± 0.23 × 105). However, no difference in collagen content was seen in the lung of TCRβ−/− mice compared with mice deficient in both αβ and γδ T cells (TCRβ−/−δ−/− mice) treated with S. rectivirgula for 4 wk (Fig. 2C and data not shown), suggesting that γδ T cells most likely do not have a significant role in attenuating S. rectivirgula-induced pulmonary fibrosis. Conversely, these findings strongly suggest that αβ T cells are essential for the development of lung fibrosis in response to S. rectivirgula. Additionally, the slight increase in collagen deposition in response to S. rectivirgula in the absence of αβ T cells raises the possibility of a T cell-independent mechanism of lung fibrosis in this model.
FIGURE 1.
TCRβ−/− mice do not develop mononuclear infiltrates or severe pulmonary fibrosis. Representative H&E (A) and Masson trichrome (B) staining of lungs from TCRβ−/− and C57BL/6 mice repeatedly treated with S. rectivirgula for 4 consecutive weeks is shown. Data shown represent at least four mice from at least two separate experiments for H&E and Masson trichrome staining (×40). Arrowhead denotes mononuclear infiltrates in the peribronchovascular space. Arrow denotes collagen deposition. C, Quantification of collagen content using Sirius red by colorimetric assay in the lungs of individual TCRβ−/− and C57BL/6 mice treated with S. rectivirgula (SR) or sterile PBS for 4 consecutive weeks. Data compiled from at least two separate experiments.
FIGURE 2.
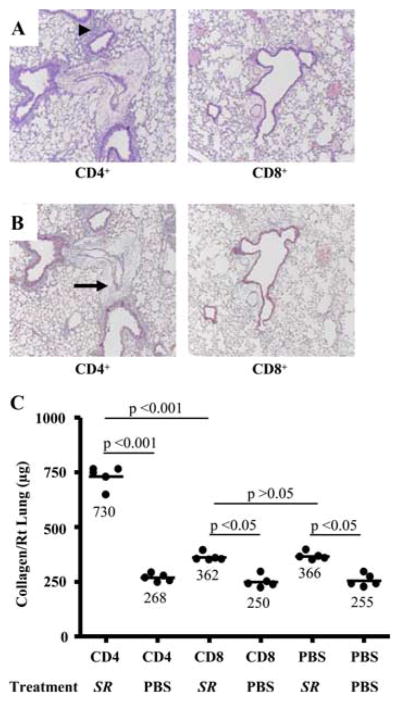
TCRβ−/−δ−/− mice reconstituted with CD4+ T cells develop mononuclear infiltrates and pulmonary fibrosis in a peribronchovascular distribution after treatment with S. rectivirgula. A, Representative H&E staining of lungs from TCRβ−/−δ−/− mice reconstituted with either CD4+ or CD8+ T cells followed by intranasal treatment with S. rectivirgula for 4 consecutive weeks is shown. Arrowhead denotes mononuclear infiltrates in the peribronchovascular space. B, Representative Masson trichrome staining of lungs from TCRβ−/−δ−/− mice reconstituted with either CD4+ or CD8+ T cells followed by intranasal treatment with S. rectivirgula for 4 consecutive weeks. Arrow denotes collagen deposition in a peribronchovascular distribution. Data represent at least four mice from at least two separate experiments for H&E and Masson trichrome staining (×40). C, Quantification of collagen content using Sirius red by colorimetric assay in the lungs of individual TCRβ−/−δ−/− reconstituted with CD4+, CD8+, or sterile PBS by tail vein injection followed by repeated intranasal treatment with S. rectivirgula (SR) or sterile PBS for 4 consecutive weeks. Data compiled from at least two separate experiments.
Adoptive transfer of CD4+ T cells into TCRβ−/− δ−/− mice reconstitutes the S. rectivirgula-induced inflammatory and fibrotic response
For adoptive transfer experiments, we chose TCRβ−/−δ−/− mice (i.e., mice deficient in both αβ- and γδ-expressing T cells) as the recipient animal to eliminate any potential effects due to γδ T cells. To determine whether CD4+ and/or CD8+ T cells are required for the immune response due to S. rectivirgula, TCRβ−/−δ−/− mice were reconstituted with either 5 × 105 CD4+ or CD8+ T cells by tail vein injection and subsequently treated with S. rectivirgula by nasal inhalation. As shown in Fig. 2, mice receiving adoptively transferred CD4+ T cells developed peribronchovascular mononuclear infiltrates upon exposure to inhaled S. rectivirgula compared with control TCRβ−/−δ−/− mice reconstituted with CD4+ T lymphocytes and repeatedly exposed to inhaled sterile PBS. Although CD4+ T cells were found in the lungs and spleens of all mice reconstituted with CD4+ T lymphocytes, there were much higher numbers of CD4+ T cells in the lungs of CD4+-reconstituted TCRβ−/−δ−/− mice exposed repeatedly to S. rectivirgula compared with similar mice treated with inhaled PBS. For example, 1.8 ± 0.3 × 106 CD4+ T cells were recovered from the lungs of CD4+ T cell-reconstituted TCRβ−/−δ−/− mice after S. rectivirgula treatment compared with 0.11 ± 0.02 × 106 CD4+ T cells from the lungs of CD4+ T cell-reconstituted TCRβ−/−δ−/− mice after PBS exposure. Conversely, TCRβ−/−δ−/− mice reconstituted with CD8+ T cells did not show a significant accumulation of cells in the lung after subsequent exposure to either S. rectivirgula or PBS by nasal inhalation (Fig. 2A and data not shown). These data indicate that CD4+ T cells preferentially accumulate in the lung and expand in response to chronic inhalation of S. rectivirgula, suggesting their importance in the S. rectivirgula-induced immune response.
As opposed to mice reconstituted with CD8+ T cells, the adoptive transfer of CD4+ T cells into TCRβ−/−δ−/− mice also resulted in lung fibrosis as detected by Masson trichrome (Fig. 2B). Additionally, quantification of collagen deposition by Sirius red colorimetric assay showed a 2.7-fold increase in collagen content in the lungs of mice reconstituted with CD4+ T cells when repeatedly treated with S. rectivirgula compared with CD4+ T cell-reconstituted control mice treated with PBS by nasal inhalation (p < 0.001) (Fig. 2C). TCRβ−/−δ−/− mice reconstituted with CD8+ T cells also had a slight increase in collagen content in the lung when chronically exposed to S. rectivirgula compared with CD8+ T cell-reconstituted control mice. However, similar levels of collagen deposition were seen in TCRβ−/−δ−/− mice given sterile PBS by tail vein injection upon repeated treatment with S. rectivirgula (Fig. 2C). Therefore, CD8+ T cells most likely do not have a significant role in the development of pulmonary fibrosis. Taken together, these data suggest that CD4+ T cells represent the critical αβ-expressing T cell subset that promotes both lung inflammation and collagen deposition in this murine model of HP.
CD4+ T cells that accumulate in the lung in response to S. rectivirgula express an activated effector phenotype
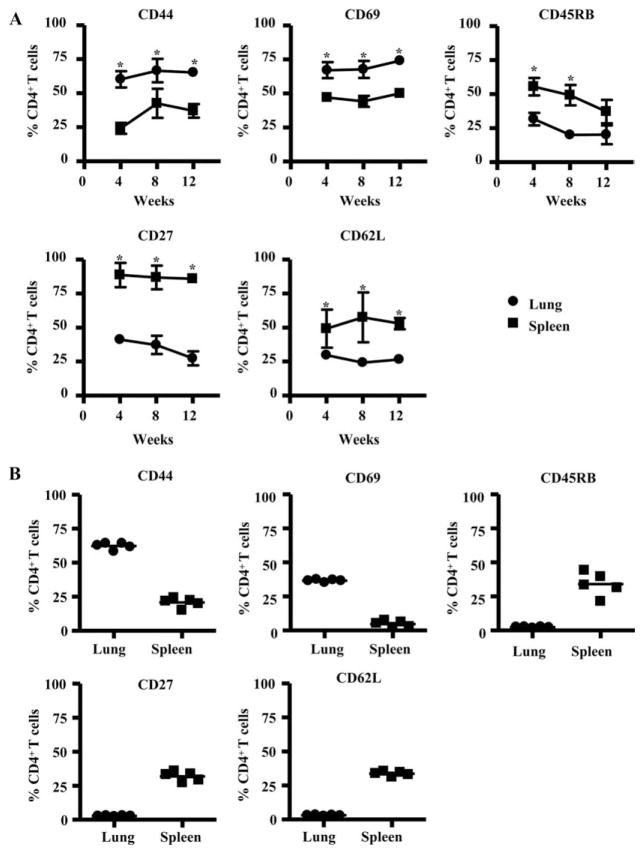
Next, we analyzed the phenotypic markers expressed by CD4+ T cells in the lung and spleen of mice after repeated S. rectivirgula exposure. As compared with splenic CD4+ T cells, CD4+ T lymphocytes in lung were characterized by a significantly increased expression of CD44, a memory cell marker, and CD69, a marker of recent activation (Fig. 3A). Additionally, lung CD4+ T cells had down-regulated the expression of CD45RB, CD27, and CD62L, consistent with an effector T cell phenotype. Adoptively transferred CD4+ T cells that accumulated in the lung of TCRβ−/−δ−/− mice in response to S. rectivirgula exposure also showed an increased expression of CD44 and CD69 and decreased CD45RB, CD27, and CD62L expression compared with CD4+ T cells recovered from the spleen (Fig. 3B). CD4+ T cells that accumulated in the spleen of TCRβ−/−δ−/− mice treated with PBS expressed a similar phenotype compared with CD4+ T cells in the spleen of TCRβ−/−δ−/− mice that were repeatedly exposed to S. rectivirgula after CD4+ T cell reconstitution (data not shown). These findings show that the recruitment of activated effector CD4+ T cells to the lung in response to S. rectivirgula was not due to homeostatic expansion, thus confirming the critical role of this αβ T cell subset in the immune and fibrotic response directed against S. rectivirgula.
FIGURE 3.
CD4+ T cells that accumulate in the lung in response to S. rectivirgula express a highly activated phenotype. A, Expression of CD44, CD69, CD45RB, CD27, and CD62L on CD4+ T cells isolated from the lungs and spleens of C57BL/6 mice treated with S. rectivirgula for 4, 8, and 12 consecutive weeks is shown. Data represent the means ± SD of five mice at each time point treated with S. rectivirgula from at least two separate experiments. *, Denotes a statistically significant (p < 0.05) difference in the percentage of CD4+ T cells that express each surface marker from the lung compared with spleen of C57BL/6 mice treated with S. rectivirgula at each time point. B, Surface expression of CD44, CD69, CD45RB, CD27, and CD62L on CD4+ T cells isolated from the lungs and spleens of TCRβ−/−δ−/− mice reconstituted with CD4+ T cells followed by intranasal treatment with S. rectivirgula for 4 consecutive weeks is shown. Data compiled from at least two separate experiments.
S. rectivirgula exposure induces a Th17-polarized immune response in the lung
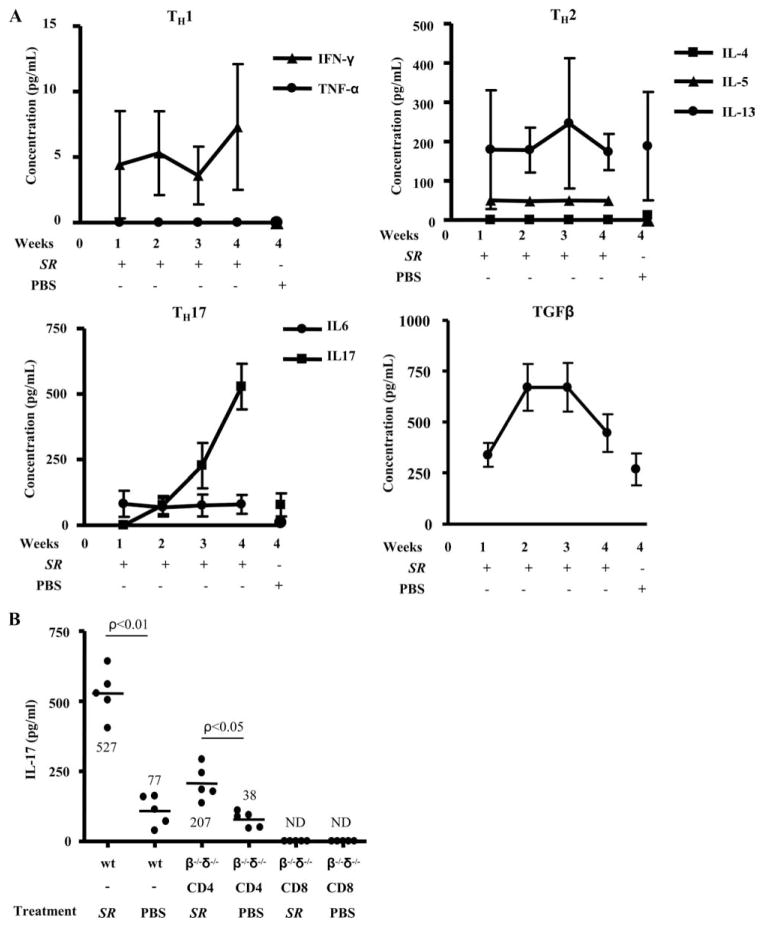
Although HP induced by S. rectivirgula is thought to be a Th1-mediated disease (16, 17), we and others (21) have shown that mice repeatedly exposed to this microorganism develop pulmonary fibrosis. To determine whether specific cytokines correlated with the onset of lung fibrosis, we analyzed the cytokine profile from homogenized lung of mice treated with S. rectivirgula for 1, 2, 3, and 4 wk. As seen in Fig. 4A, IFN-γ levels were increased in the lungs of mice treated with S. rectivirgula at each time point compared with control animals but near the limit of detection of the cytokine bead assay (1 pg/ml) while TNF-α was below the detection limit of the assay. Interestingly, the Th2 cytokine IL-5 was elevated in the lungs of mice treated with S. rectivirgula compared with PBS-treated control mice. IL-13 was found in the lungs of mice treated with S. rectivirgula but levels were not significantly different than controls, whereas IL-4 was below the limit of detection of the assay in both the S. rectivirgula-treated and control animals (Fig. 4A).
FIGURE 4.
Analysis of Th1, Th2, and Th17 cytokines from the lungs of C57BL/6 mice treated with S. rectivirgula. A, Cytokines from supernatants of total lung homogenates were analyzed from C57BL/6 mice treated with S. rectivirgula for 1, 2, 3, and 4 wk compared with control mice treated in an identical fashion with sterile PBS for 4 wk. Data represent the mean ± SD of five mice at each time point treated with S. rectivirgula from at least two separate experiments. B, Supernatants from total lung homogenates of wt C57BL/6 (wt) or TCRβ−/−δ−/− mice reconstituted with either CD4+ or CD8+ T cells were analyzed for IL-17. TCRβ−/−δ−/− mice were treated with either S. rectivirgula (SR) or sterile PBS for 4 consecutive weeks after reconstitution with either CD4+ or CD8+ T cells. Data compiled from at least two separate experiments. ND indicates not detected.
Interestingly, we found increasing amounts of IL-17 in the lungs of mice upon repeated exposure to S. rectivirgula. For example, at 3 and 4 wk, 227 ± 86 pg/ml and 527 ± 87 pg/ml, respectively, were detected in the lung of S. rectivirgula-treated mice (Fig. 4). Additionally, IL-17 was not detected in the spleens of the same animals (data not shown), suggesting a compartmentalized immune response directed against S. rectivirgula in the lung. Importantly, increased IL-17 secretion was also seen in the lung of CD4+ T cell-reconstituted TCRβ−/−δ−/− mice that were subsequently exposed to S. rectivirgula compared with PBS-treated controls (207 ± 68 vs 38 ± 28 pg/ml; p < 0.05) (Fig. 4B). IL-6 and TGF-β1, cytokines important in the differentiation of Th17 cells (28), were also elevated in the lung of S. rectivirgula-exposed mice compared with PBS-treated control mice (Fig. 4A). IL-17 was not present in the lungs of CD8+ T cell-reconstituted TCRβ−/−δ−/− mice that were subsequently treated with S. rectivirgula (Fig. 4B). These data indicate that with repeated exposure to S. rectivirgula, a Th17-polarized immune response predominates, which likely inhibits T cell IFN-γ production.
Th17-polarized CD4+ T cells in the lung differentially express IL-17A and IL-22
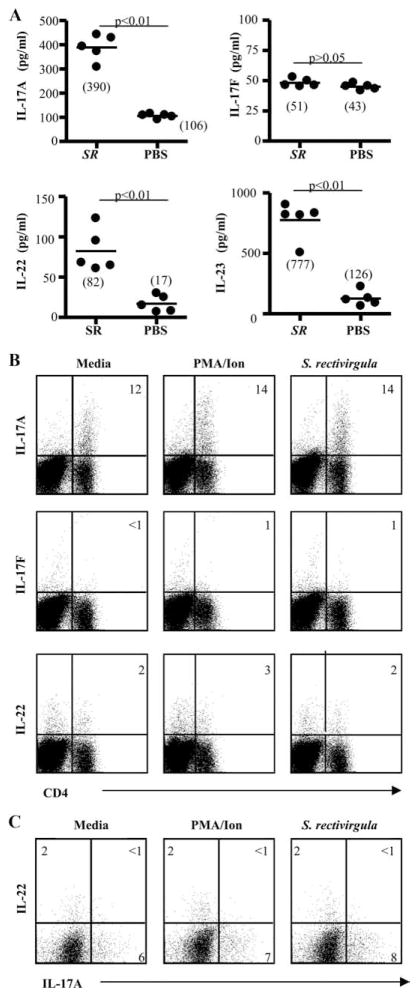
To further characterize Th17 cytokines present in the lung of C57BL/6 mice treated with S. rectivirgula, we performed cytokine analysis for IL-17A, IL-17F, IL-22, and IL-23 on total lung homogenates obtained after 4 wk of treatment. As shown in Fig. 5A, IL-17A and IL-22 were elevated in the lung of S. rectivirgula-treated C57BL/6 mice compared with PBS-treated control mice at 4 wk, with a 3.6-fold increase in IL-17A (p < 0.01) and a 4.8-fold increase in IL-22 (p < 0.01). In mice deficient in γδ T cells (TCRδ−/−), levels of IL-17A in lung homogenates were not significantly different than those in wt C57BL/6 mice treated with S. rectivirgula for 4 wk, suggesting that γδ T cells do not contribute significantly to IL-17A levels in wt C57BL/6 mice (data not shown). There was no difference in the level of IL-17F between S. rectivirgula- and PBS-treated animals. IL-23 was also increased in the lung of S. rectivirgula-treated animals compared with controls (6.2-fold; p < 0.01) (Fig. 5A).
FIGURE 5.
CD4+ T cells that accumulate in the lung in response to S. rectivirgula differentially express IL-17A and IL-22. A, Th17 cytokines from supernatants of total lung homogenates were analyzed from C57BL/6 mice treated with S. rectivirgula or PBS for 4 wk. Data represent the mean ± SD of five mice at each time point treated with S. rectivirgula from at least two separate experiments. B, Representative density plots of CD4+ T cells expressing IL-17A, IL-17F, or IL-22 in response to medium, PMA/ionomycin, or S. rectivirgula (10 μg/ml) are shown. The percentage of CD4+ T cells expressing the respective cytokine is shown in the upper right quadrant of each density plot. C, Representative density plots of CD4+ T cells expressing IL-17A and IL-22 in response to medium, PMA/ionomycin, or S. rectivirgula are shown. The percentage of CD4+ T cells expressing each cytokine is shown in the appropriate quadrant. Data are representative of at least five individual mice treated with S. rectivirgula from at least two separate experiments.
To determine the frequency of IL-17-expressing CD4+ T cells in the lung of S. rectivirgula-treated C57BL/6 mice at 4 wk, we performed intracellular cytokine staining on lung CD4+ T cells for the expression of IL-17A, IL-17F, and IL-22. As seen in Fig. 5B, ~12% of ex vivo CD4+ T cells from the lung of S. rectivirgula-treated C57BL/6 mice expressed IL-17A, regardless of whether the cells were unstimulated or stimulated with either PMA/ionomycin or S. rectivirgula Ag, suggesting that the secretion of IL-17A in response to S. rectivirgula exposure is occurring in an Ag-nonspecific manner. The vast majority of IL-17 expressed was IL-17A, with minimal, if any, IL-17F detected (Fig. 5B). Interestingly, differential expression of IL-17A and IL-22 was seen on lung CD4+ T cells after 4 wk of S. rectivirgula exposure (Fig. 5C). CD8+ T cells did not express IL-17A or IL-22 (data not shown). Consistent with previously published reports (20), we only detected intracellular IFN-γ expression in CD4+ T cells stimulated with PMA/ionomycin at earlier time points but not after 4 wk of treatment with S. rectivirgula (data not shown).
CD4+ T cells and lung fibrosis are decreased in the absence of IL-17 receptor signaling
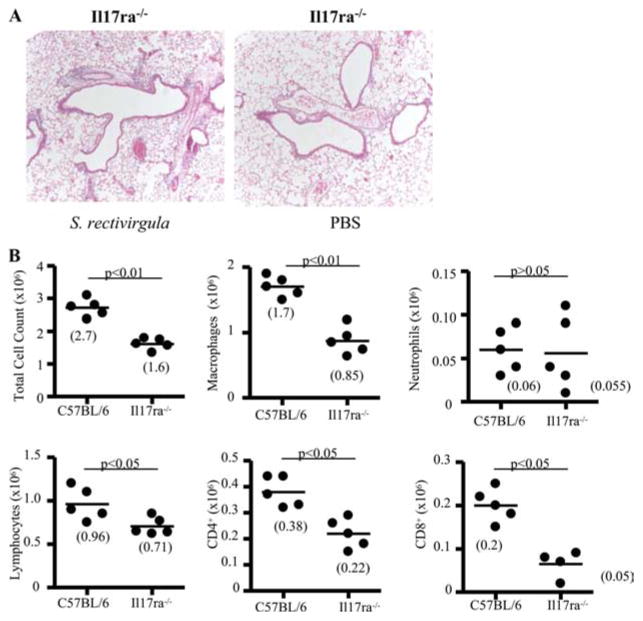
To further define the role of IL-17A, we repeatedly exposed IL-17A receptor-deficient mice (Il17ra−/−) to S. rectivirgula for 4 wk. As shown in Fig. 6A, Il17ra−/− mice had decreased mononuclear infiltrates on H&E staining compared with wt C57BL/6 mice treated in an identical fashion (shown in Fig. 1A). Il17ra−/− mice had a decreased number of total lung cells (1.6 ± 0.18 × 106 vs 2.7 ± 0.28 × 106; p < 0.01) and macrophages (0.85 ± 0.21 × 106 vs 1.7 ± 0.16 × 106; p < 0.01) in the lung compared with wt C57BL/6 mice (Fig. 6B). In contrast, neutrophil counts were not significantly different in the lung of Il17ra−/− compared with wt C57BL/6 mice (Fig. 6B). Lymphocytes (0.96 ± 0.19 × 106 vs 0.71 ± 0.10 × 106; p < 0.05) and CD4+ T cells (0.38 ± 0.06 × 106 vs 0.22 ± 0.07 × 106; p < 0.05) were also significantly decreased in the lung of Il17ra−/− compared with S. rectivirgula-treated wt C57BL/6 mice. Numbers of CD8+ T cells also declined in the lung of Il17ra−/− compared with S. rectivirgula-treated wt C57BL/6 mice without a significant difference in B cell numbers (Fig. 6B and data not shown).
FIGURE 6.
Il17ra−/− mice develop less inflammation in response to S. rectivirgula exposure. A, Representative H&E staining of lungs from Il17ra−/− mice repeatedly treated with S. rectivirgula or PBS for 4 consecutive weeks is shown. Data shown represent at least four mice from at least two separate experiments (×40). B, Il17ra−/− mice treated with S. rectivirgula for 4 consecutive weeks have decreased numbers of total lung cells, macrophages, lymphocytes, and CD4+ and CD8+ T cells compared with wt C57BL/6 mice. Data shown represent at least five mice from at least two separate experiments.
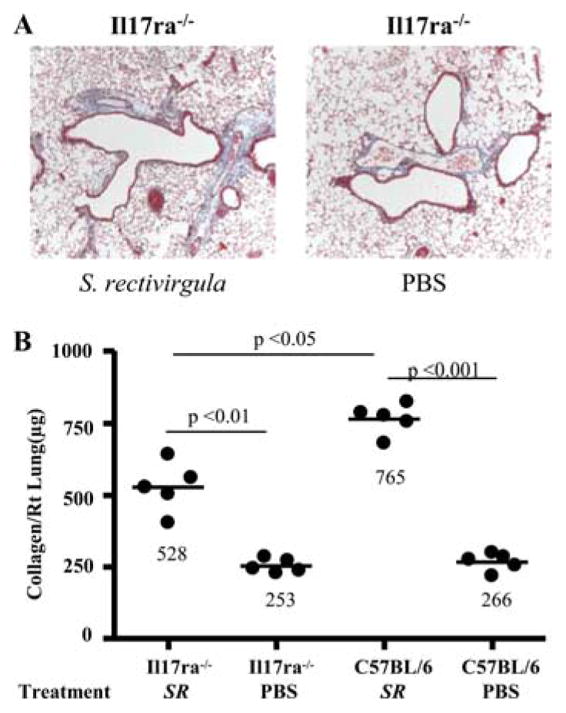
To determine whether IL-17 receptor signaling is necessary for the development of lung fibrosis in response to S. rectivirgula, we analyzed the lungs from Il17ra−/− mice for collagen deposition after 4 wk of treatment. As shown in Fig. 7, there was a 1.4-fold decline in collagen content in the lung of Il17ra−/− mice compared with S. rectivirgula-treated wt C57BL/6 mice (p < 0.05). These data suggest, for the first time, that IL-17 receptor signaling is important for both lung inflammation and fibrosis in this murine model of HP.
FIGURE 7.
Il17ra−/− mice develop less pulmonary fibrosis in response to S. rectivirgula exposure. A, Representative Masson trichrome staining of lungs from Il17ra−/− mice repeatedly treated with S. rectivirgula or PBS for 4 consecutive weeks is shown. Data shown represent at least four mice from at least two separate experiments (×40). B, Quantification of collagen content using Sirius red by colorimetric assay in the lungs of individual Il17ra−/− and wt C57BL/6 mice treated with S. rectivirgula (SR) or PBS for 4 consecutive weeks. Data compiled from at least two separate experiments.
Discussion
Hypersensitivity pneumonitis is an inflammatory lung disease caused by repeated inhalation of a variety of environmental Ags that can progress to lung fibrosis with chronic exposure to the inhaled Ag. Although T cells are important in the pathogenesis of HP, their role in the development of pulmonary fibrosis is poorly understood. Using a murine model of Farmer’s lung, we demonstrate that repeated inhalation of the thermoactinomycete S. rectivirgula results in mononuclear infiltrates and collagen deposition in a peribronchovascular distribution in the lung consistent with earlier reports (16–21). In the absence of CD4+ and CD8+ T lymphocytes, mononuclear infiltrates and collagen deposition are markedly reduced, indicating that αβ T cells are essential for lung fibrosis. Adoptive transfer experiments show that CD4+ T cells represent the critical αβ T cell subset necessary for the development of severe pulmonary fibrosis. With chronic exposure to S. rectivirgula, CD4+ T cells were not Th1 polarized but were rather Th17 polarized with differential expression of IL-17A and IL-22. In the absence of IL-17 receptor signaling, inflammation and lung fibrosis were both diminished. Thus, these studies establish an important role for CD4+ Th17 cells in the immune response directed against S. rectivirgula and for the subsequent development of lung fibrosis.
Numerous studies suggest that IFN-γ is important for granulomatous inflammation in this murine model of HP (17, 20) as well as being protective against collagen deposition in other models of lung fibrosis (22). Additionally, type 1 cytokines have been shown to attenuate collagen deposition while type 2 cytokines promote collagen deposition in numerous models of solid organ fibrosis (15). Despite the fact that HP is considered a Th1-mediated disease (29), it is well established that pulmonary fibrosis develops in both humans and murine models of this disease (6, 21). This paradox, however, remains unresolved. Consistent with these observations, we found very low levels of IFN-γ in the lung and did not find expression of IFN-γ by T cells after the first 2 wk of exposure to S. rectivirgula (P.L.S. and A.P.F., unpublished observation). Additionally, recent studies have shown that the cell responsible for IFN-γ secretion in response to S. rectivirgula is the neutrophil and not the T cell (20). Therefore, IFN-γ expression by neutrophils and not T lymphocytes may be all that is necessary for granuloma formation in response to S. rectivirgula exposure. Although numerous reports suggest that type 2 cytokines modulate the severity of HP in this model (18), we did not find increased levels of IL-4 and IL-13 in the lung of S. rectivirgula-treated mice compared with controls, with the exception of IL-5. IL-5 has been shown to exacerbate bleomycin-induced lung fibrosis, but IL-5−/− mice showed no impairment in fibrosis, suggesting that IL-5 may act as an amplifier rather than as a direct mediator of pulmonary fibrosis (30).
The absence of significant type 1 and 2 cytokine expression in the lungs of mice treated with S. rectivirgula raised the possibility that Th17 cytokines were involved in the immune response directed against S. rectivirgula. With the emergence of Th17 cytokines, diseases that where once considered Th1 mediated have been, in fact, shown to be Th17 mediated (31). For example, in a murine model of multiple sclerosis, mice deficient in IL-17 had delayed onset of experimental autoimmune encephalitis with markedly diminished severity (28). Expression of IL-17 has also been detected in the sera and target tissues of patients with various autoimmune diseases, including rheumatoid arthritis and systemic lupus erythematosus as well as asthma (25, 26, 32). In this regard, the predominant cytokine identified in the lungs of mice treated with S. rectivirgula was IL-17. IL-17-secreting T cells belong to a unique subset of CD4+ helper T lymphocytes, Th17 cells, which are characterized by the production of IL-17A and IL-17F as well as IL-21, IL-22, TNF-α, IL-6, and GM-CSF but not IL-4 or IFN-γ. In this model, we found that CD4+ T cells expressed IL-17A and IL-22 but not IL-4, IFN-γ, TNF-α, or IL-17F. Interestingly, 12% of CD4+ T cells expressed IL-17A ex vivo without further antigenic stimulation, which most likely occurs as a result of recent exposure to the S. rectivirgula microorganism. In contrast, in a murine model of pathogen-mediated arthritis, only 0.9% of CD4+ T cells isolated from the inflamed joint expressed IL-17 ex vivo and only up to 3.9% with Ag stimulation, although IL-17 is required for disease development (33). Additionally, we did not find expression of IL-17F by CD4+ T cells, and IL-17F levels in the lung were not different by ELISA between S. rectivirgula- and PBS-treated mice at 4 wk, suggesting that in this model redundant functions of IL-17A and IL-17F may not be required. Taken together, our findings suggest that CD4+ T cells differentiate toward a Th17 phenotype and thus inhibit the development of a Th1 response early in the course of disease.
IL-17 is a proinflammatory cytokine that is thought to increase inflammation by recruiting cells, particularly neutrophils, to the site of infection to assist in pathogen clearance (34, 35). Consistent with these reports, mice deficient in IL-17 receptor signaling (Il17ra−/−) had less inflammation, by H&E staining, with decreased numbers of macrophages and lymphocytes, including CD4+ and CD8+ T cells. Interestingly, the number of neutrophils was not significantly different in the absence of IL-17 receptor signaling, suggesting that neutrophil recruitment to the lung is not affected by IL-17 receptor signaling in this model. Neutrophil numbers, however, are not markedly elevated in the lung of mice repeatedly exposed to S. rectivirgula even in the presence of IL-17 receptor signaling. Therefore, IL-17A must serve other distinct functions in this model of HP. Il17ra−/− mice repeatedly treated with S. rectivirgula not only had diminished inflammation but also less lung fibrosis compared with wt C57BL/6 mice. These data suggest that IL-17 receptor signaling plays a role in the development of pulmonary fibrosis. Histologic analysis of lungs from transgenic mice with forced IL-17 expression showed focal mononuclear cell infiltrates containing CD4+ T lymphocytes as well as collagen deposition in the subepithelium of bronchioles adjacent to these lymphocytic aggregates (36). These data suggest that CD4+ T cells promote collagen deposition in the lung through expression of IL-17. The role of IL-17 in lung fibrosis, however, may occur indirectly through activation or recruitment of other inflammatory cells to the lung. Myofibroblasts are a primary collagen-producing cell when activated and are present in the lung of S. rectivirgula-treated C57BL/6 mice (P.S.L. and A.P.F, unpublished observation). Myofibroblasts can be generated from a variety of cell types, including resident mesenchymal cells, epithelial and endothelial cells, and circulating fibrocytes derived from bone marrow stem cells (15). IL-17 has been shown to stimulate secretion of IL-6, IL-8, and MCP-1 in human colonic subepithelial myofibroblasts (37). Therefore, it is plausible that IL-17A activates subepithelial myofibroblasts in the lung, resulting in increased collagen deposition in the lung. Alternatively, TGF-β and IL-6 are necessary for the differentiation of naive CD4+ T lymphocytes into Th17 cells in mice via the transcription factor RORγt (38–42). TGF-β has also been shown to be a critical cytokine for the development of pulmonary fibrosis (15, 43) and is elevated in the lung in response to S. rectivirgula. Therefore, TGF-β may promote differentiation of CD4+ T cells into Th17 cells with the adverse consequence of promoting collagen deposition in the lung in response to chronic exposure to S. rectivirgula.
A small percentage of lung CD4+ Th17 cells also expressed IL-22. Interestingly, we found that the CD4+ T cells that express IL-17A differentially express IL-22, indicating the existence of two distinct subpopulations of CD4+ Th17 cells with potentially differing functions. IL-22 has been shown to be an effector cytokine of the Th17 lineage that acts synergistically with IL-17A or IL-17F to induce expression of antimicrobial peptides on keratinocytes (44). More recently, IL-22 has been shown to be important for mucosal host defense against Klebsiella pneumonia in the lung (45). In this model, IL-22 increased lung epithelial cell proliferation and increased transepithelial resistance to injury whereas IL-17A was important for induction of G-CSF, neutrophil recruitment, and clearance of the microorganism (45). IL-22 has also been shown to increase expression of inflammatory cytokines but not proliferation or collagen synthesis in subepithelial myofibroblasts in inflammatory bowel disease (46). These data suggest that CD4+ T cells that express IL-17A and IL-22 are most likely important for mucosal immunity against extracellular microorganisms but may promote pulmonary fibrosis through activation or recruitment of other cell types to the lung in response to an inhaled microorganism.
In summary, we show that activated CD4+ T cells that express IL-17A and IL-22 are essential for the development of lung inflammation and fibrosis induced by repeated inhalation of S. rectivirgula. Although the mechanism by which these CD4+ Th17 cells promote pulmonary fibrosis is not completely understood, these data suggest that CD4+ T cells that express IL-17A and IL-22 are most likely important for mucosal immunity against S. rectivirgula, with the adverse effect of pulmonary fibrosis due to recruitment of other cell types or the necessity of TGF-β for the development of the Th17 lineage. Studies are ongoing to determine the mechanism by which CD4+ T cells promote the development of pulmonary fibrosis and the exact roles of IL-17A and IL-22 in this S. rectivirgula-induced model of HP and pulmonary fibrosis.
Footnotes
Abbreviations used in this paper: HP, hypersensitivity pneumonitis; wt, wild type.
Disclosures
The authors have no financial conflicts of interest.
This work was supported by National Institutes of Health Grants HL62410 and ES011810 (to A.P.F.) and HL89766 (to P.L.S.).
References
- 1.Selman M. Hypersensitivity pneumonitis. In: Schwarz MI, King TE Jr, editors. Interstitial Lung Disease. 3. B. C. Decker; Hamilton, Ontario: 2003. pp. 452–484. [Google Scholar]
- 2.Bourke SJ, Banham SW, Carter R, Lynch P, Boyd G. Longitudinal course of extrinsic allergic alveolitis in pigeon breeders. Thorax. 1989;44:415–418. doi: 10.1136/thx.44.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalancette M, Carrier G, Laviolette M, Ferland S, Rodrique J, Begin R, Cantin A, Cormier Y. Farmer’s lung: long-term outcome and lack of predictive value of bronchoalveolar lavage fibrosing factors. Am Rev Respir Dis. 1993;148:216–221. doi: 10.1164/ajrccm/148.1.216. [DOI] [PubMed] [Google Scholar]
- 4.Monkare S, Haahtela T. Farmer’s lung: a 5-year follow-up of eighty-six patients. Clin Allergy. 1987;17:143–151. doi: 10.1111/j.1365-2222.1987.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshizawa Y, Ohtani Y, Hayakawa H, Sato A, Suga M, Ando M. Chronic hypersensitivity pneumonitis in Japan: a nationwide epidemiologic survey. J Allergy Clin Immunol. 1999;103:315–320. doi: 10.1016/s0091-6749(99)70507-5. [DOI] [PubMed] [Google Scholar]
- 6.Vourlekis JS, Schwarz MI, Cherniack RM, Curran-Everett D, Cool CD, Tuder RM, King TE, Jr, Brown KK. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med. 2004;116:662–668. doi: 10.1016/j.amjmed.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Reyes CN, Wenzel FJ, Lawton BR, Emanuel DA. The pulmonary pathology of farmer’s lung disease. Chest. 1982;81:142–146. doi: 10.1378/chest.81.2.142. [DOI] [PubMed] [Google Scholar]
- 8.Denis M, Cormier Y, Laviolette M. Murine hypersensitivity pneumonitis: a study of cellular infiltrates and cytokine production and its modulation by cyclosporin A. Am J Respir Cell Mol Biol. 1992;6:68–74. doi: 10.1165/ajrcmb/6.1.68. [DOI] [PubMed] [Google Scholar]
- 9.Denis M, Cormier Y, Laviolette M, Ghadirian E. T cells in hypersensitivity pneumonitis: effects of in vivo depletion of T cells in a mouse model. Am J Respir Cell Mol Biol. 1992;6:183–189. doi: 10.1165/ajrcmb/6.2.183. [DOI] [PubMed] [Google Scholar]
- 10.Takizawa H, Ohta K, Horiuchi T, Suzuki N, Ueda T, Yamaguchi M, Yamashita N, Ishii A, Suko M, Okudaira H, et al. Hypersensitivity pneumonitis in athymic nude mice: additional evidence of T cell dependency. Am Rev Respir Dis. 1992;146:479–484. doi: 10.1164/ajrccm/146.2.479. [DOI] [PubMed] [Google Scholar]
- 11.Takizawa H, Suko M, Kobayashi N, Shoji S, Ohta K, Nogami M, Okudaira H, Miyamoto T, Shiga J. Experimental hypersensitivity pneumonitis in the mouse: histologic and immunologic features and their modulation with cyclosporin A. J Allergy Clin Immunol. 1988;81:391–400. doi: 10.1016/0091-6749(88)90907-4. [DOI] [PubMed] [Google Scholar]
- 12.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on human lung fibroblasts: implication in asthma. J Clin Invest. 1998;101:2129–2139. doi: 10.1172/JCI741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fertin C, Nicolas JF, Gillery P, Kalis B, Banchereau J, Maquart FX. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol. 1991;37:823–829. [PubMed] [Google Scholar]
- 14.Tiggelman AM, Boers W, Linthorst C, Sala M, Chamuleau RA. Collagen synthesis by human liver (myo)fibroblasts in culture: evidence for a regulatory role of IL-1β, IL-4, TGFβ and IFNγ. J Hepatol. 1995;23:307–317. [PubMed] [Google Scholar]
- 15.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudmundsson G, Monick MM, Hunninghake GW. IL-12 modulates expression of hypersensitivity pneumonitis. J Immunol. 1998;161:991–999. [PubMed] [Google Scholar]
- 17.Gudmundsson G, Hunninghake GW. Interferon-γ is necessary for the expression of hypersensitivity pneumonitis. J Clin Invest. 1997;99:2386–2390. doi: 10.1172/JCI119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler NS, Monick MM, Yarovinsky TO, Powers LS, Hunninghake GW. Altered IL-4 mRNA stability correlates with Th1 and Th2 bias and susceptibility to hypersensitivity pneumonitis in two inbred strains of mice. J Immunol. 2002;169:3700–3709. doi: 10.4049/jimmunol.169.7.3700. [DOI] [PubMed] [Google Scholar]
- 19.Gudmundsson G, Bosch A, Davidson BL, Berg DJ, Hunninghake GW. Interleukin-10 modulates the severity of hypersensitivity pneumonitis in mice. Am J Respir Cell Mol Biol. 1998;19:812–818. doi: 10.1165/ajrcmb.19.5.3153. [DOI] [PubMed] [Google Scholar]
- 20.Nance S, Cross R, Yi AK, Fitzpatrick EA. IFN-γ production by innate immune cells is sufficient for development of hypersensitivity pneumonitis. Eur J Immunol. 2005;35:1928–1938. doi: 10.1002/eji.200425762. [DOI] [PubMed] [Google Scholar]
- 21.Denis M, Cormier Y, Fournier M, Tardif J, Laviolette M. Tumor necrosis factor plays an essential role in determining hypersensitivity pneumonitis in a mouse model. Am J Respir Cell Mol Biol. 1991;5:477–483. doi: 10.1165/ajrcmb/5.5.477. [DOI] [PubMed] [Google Scholar]
- 22.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukacs NW, Hogaboam C, Chensue SW, Blease K, Kunkel SL. Type 1/type 2 cytokine paradigm and the progression of pulmonary fibrosis. Chest. 2001;120:5S–8S. doi: 10.1378/chest.120.1_suppl.s5. [DOI] [PubMed] [Google Scholar]
- 24.Simonian PL, Roark CL, Diaz del Valle F, Palmer BE, Douglas IS, Ikuta K, Born WK, O’Brien RL, Fontenot AP. Regulatory role of γδ T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. 2006;177:4436–4443. doi: 10.4049/jimmunol.177.7.4436. [DOI] [PubMed] [Google Scholar]
- 25.Linden A, Hoshino H, Laan M. Airway neutrophils and interleukin-17. Eur Respir J. 2000;15:973–977. doi: 10.1034/j.1399-3003.2000.15e28.x. [DOI] [PubMed] [Google Scholar]
- 26.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–593. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 27.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O’Brien RL, Gelfand EW, Born WK. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 28.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki H, Ando M, Brazer W, Center DM, Cruikshank WW. Polarized type 1 cytokine profile in bronchoalveolar lavage T cells of patients with hypersensitivity pneumonitis. J Immunol. 1999;163:3516–3523. [PubMed] [Google Scholar]
- 30.Huaux F, Liu T, McGarry B, Ullenbruch M, Xing Z, Phan SH. Eosinophils and T lymphocytes possess distinct roles in bleomycin-induced lung injury and fibrosis. J Immunol. 2003;171:5470–5481. doi: 10.4049/jimmunol.171.10.5470. [DOI] [PubMed] [Google Scholar]
- 31.Bettelli E, Oukka M, Kuchroo VK. Th-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 32.Shahrara S, Huang Q, Mandelin AM, 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 35.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andoh A, Ogawa A, Bamba S, Fujiyama Y. Interaction between interleukin-17-producing CD4+ T cells and colonic subepithelial myofibroblasts: what are they doing in mucosal inflammation? J Gastroenterol. 2007;42(Suppl 17):29–33. doi: 10.1007/s00535-006-1926-7. [DOI] [PubMed] [Google Scholar]
- 38.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the Th17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 41.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 43.Denis M, Ghadirian E. Transforming growth factor-β is generated in the course of hypersensitivity pneumonitis: contribution to collagen synthesis. Am J Respir Cell Mol Biol. 1992;7:156–160. doi: 10.1165/ajrcmb/7.2.156. [DOI] [PubMed] [Google Scholar]
- 44.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andoh A, Bamba S, Fujiyama Y, Brittan M, Wright NA. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol. 2005;40:1089–1099. doi: 10.1007/s00535-005-1727-4. [DOI] [PubMed] [Google Scholar]