Abstract
Hypersensitivity pneumonitis (HP) is an inflammatory lung disease characterized by a diffuse mononuclear cell infiltrate in the lung that can progress to pulmonary fibrosis with chronic exposure to an inhaled Ag. We previously reported that C57BL/6 mice repeatedly exposed to the ubiquitous microorganism Bacillus subtilis develop mononuclear infiltrates in the lung that contain Vγ6/Vδ1+ γδ T cells. In the absence of this T cell subset, mice treated with B. subtilis had significantly increased collagen deposition in the lung, suggesting a regulatory role for Vγ6/Vδ1+ γδ T cells. To further investigate the role of Vγ6/Vδ1+ γδ T cells in B. subtilis-induced lung fibrosis, we exposed transgenic Vγ6/Vδ1 mice to this microorganism and found decreased collagen content in the lung compared with wild-type C57BL/6 mice. Cytokine analysis of lung homogenates from wild-type C57BL/6 mice demonstrated increased IL-17A concentrations with repeated exposure to B. subtilis. In the absence of IL-17 receptor signaling, IL-17ra−/− mice had delayed clearance of B. subtilis with increased lung inflammation and fibrosis. Although IL-17A was predominantly expressed by Vγ6/Vδ1+ T cells, a compensatory increase in IL-17A expression by CD4+ T cells was seen in the absence of γδ T cells that resulted in similar levels of IL-17A in the lungs of TCRδ−/− and wild-type C57BL/6 mice. In combination, our data suggest an important role for IL-17A-expressing T lymphocytes, both γδ and αβ T cells, in eliminating this microorganism that prevents excessive inflammation and eventual lung fibrosis in this murine model of B. subtilis-induced hypersensitivity pneumonitis.
Hypersensitivity pneumonitis (HP)3 is an inflammatory lung disease that results from repeated inhalation of aerosolized Ags (1). The etiologic agents are composed of a wide variety of organic particles (e.g., mammalian and avian proteins, fungi, and bacteria) and certain small m.w. volatile and nonvolatile chemical compounds. HP occurs in several clinical forms (e.g., acute, subacute, and chronic), depending on the nature of the Ag, the quantity and duration of exposure, and host/environment interactions (1). The acute form of disease is typically nonprogressive, with spontaneous resolution after cessation of Ag exposure. In the chronic subset of patients, pulmonary fibrosis occurs in up to 41% of cases, resulting in irreversible pulmonary dysfunction and right heart failure (2–5). Lung fibrosis has been shown to be an independent predictor of mortality in these patients, with a 5-year mortality of 27% and a median survival of 13 years (6). Histopathologically, this disorder is characterized by a diffuse mononuclear cell infiltration of the lung with poorly formed granulomas located near the bronchovascular bundle (7). Although these mononuclear infiltrates contain large numbers of CD4+ and CD8+ T cells that are important in the pathogenesis of HP, γδ T cells have also been described in the lungs of patients with HP and other granulomatous diseases such as sarcoidosis, but their function remains unknown in these lung disorders (8–10).
γδ T cells are a unique subset of lymphocytes whose function is poorly understood. However, these cells have been implicated in the regulation of the immune response generated against microbial pathogens and allergens (11). The fact that γδ T cells are found in the subepithelium of alveolar and nonalveolar regions of the lung (12) suggests that they play an important role in the immune response directed against inhaled particles such as microbial pathogens (13). For example, mice intratracheally infected with Cryptococcus neoformans develop a transient increase in γδ T cells in the lung that peaks 3–6 days after infection. In the absence of γδ T cells, C. neoformans is cleared more rapidly from the lung, suggesting that γδ T cells may down-regulate the Th1-mediated immune response initiated by C. neoformans at the expense of microbial persistence (14). Similar to pulmonary infection with C. neoformans, mice infected with Pneumocystis carinii or Bordetella pertussis have a transient increase in γδ T cells in the lung and enhanced clearance of these microbial pathogens in the absence of γδ T lymphocytes (15, 16). These data suggest that γδ T cells in these models of lung infection down-regulate the immune response generated against the inhaled pathogen, which may prevent tissue damage. Conversely, in the absence of γδ T cells, mice infected with Nocardia asteroides have a delay in microbial clearance and increased mortality (17, 18). The reason for these contradictory observations is not clear, but all of these studies suggest that γδ T cells are important in pulmonary infections caused by a variety of inhaled microorganisms.
We developed a murine model of HP whereby mice repeatedly exposed to the ubiquitous microorganism Bacillus subtilis develop mononuclear infiltrates in a peribronchovascular distribution and pulmonary fibrosis similar to the human disease (19). Interestingly, these mononuclear infiltrates contain large numbers of γδ T cells that express the Vγ6/Vδ1+ TCR. In the absence of Vγ6/Vδ1+ T cells, mice develop increased numbers of CD4+ and CD8+ T cells and accelerated pulmonary fibrosis, suggesting that these Vγ6/Vδ1+ T lymphocytes protect against collagen deposition caused by chronic exposure to B. subtilis. In this report, we show that transgenic Vγ6/Vδ1 mice repeatedly exposed to B. subtilis develop large expansions of Vγ6/Vδ1+ γδ T cells in lung that associate with reduced numbers of CD4+ T cells and macrophages and less lung fibrosis. Although HP is considered a Th1-mediated disease, we found increased levels of IL-17A that was predominantly expressed by Vγ6/Vδ1+ T cells. In the absence of IL-17 receptor signaling, IL-17ra−/− mice developed increased lung inflammation and collagen deposition compared with wild-type (wt) C57BL/6 mice, with a marked delay in clearance of B. subtilis. Interestingly, in mice deficient in γδ T cells, TCR δ−/− mice had similar levels of IL-17A compared to those of wt C57BL/6 mice, with a compensatory increased expression of IL-17A by CD4+ T cells. Taken together, these data further our understanding of the role of this γδ T cell subset and its secreted cytokines in the immune response directed against B. subtilis.
Materials and Methods
Treatment of mice
Eight-week-old transgenic Vγ6/Vδ1 (R. L. O’Brien) (20, 21), C57BL/6, TCRδ−/− (The Jackson Laboratory), and IL-17ra−/− mice (Amgen) were treated with 30 μl (5 million CFU) of Bacillus subtilis (American Type Culture Collection no. 21332) or sterile PBS on 3 consecutive days each week for up to 4 consecutive weeks by nasal inhalation. All mice have been backcrossed onto a C57BL/6 background for at least 10 generations. B. subtilis was prepared as previously described (19). Briefly, a single colony of B. subtilis was grown in tryptic soy broth at 37°C with constant agitation into log phase, centrifuged, and resuspended in sterile PBS before administration to mice by nasal inhalation. A Limulus amebocyte assay (Sigma-Aldrich) was performed to confirm that the B. subtilis preparation and sterile PBS contained <20 μg of endotoxin per milliliter. Mice were lightly anesthetized with isofluorane to allow inhalation of either B. subtilis or sterile PBS. These studies were approved by the Animal Care and Use Committee at the University of Colorado Denver (Aurora, CO).
Preparation of mononuclear cells from lung homogenates
Mice at each time point were sacrificed 24 h after the last treatment with B. subtilis as previously described (19). Briefly, the chest cavity was opened using sterile surgical dissection and the inferior vena cava and abdominal aorta were clamped. The left atrium was opened by incision, and the right ventricle was infused with at least 2 ml of sterile PBS to remove any residual blood from the pulmonary vasculature. The heart and lungs were removed en bloc, and the heart, thymus, and lymph nodes were dissected away from the lungs. The right lung was removed, snap-frozen in liquid nitrogen, and stored at −80°C for collagen quantification. The left lung was cut into small pieces and placed in RPMI 1640 containing 5% FBS, collagenase (Sigma-Aldrich), and DNase (Boehringer Mannheim). After 30 min of collagenase digestion at 37°C, lungs were further disrupted by aspiration through an 18-gauge needle. The collagenase-digested lungs were layered on top of Ficoll (Accurate Chemical & Scientific) and centrifuged at 1200 rpm for 30 min at room temperature. The interface between the medium and Ficoll was removed and washed twice with RPMI 1640 containing 5% FBS.
Flow cytometry and immunofluorescence analysis
T lymphocytes isolated from the lungs of mice treated with either B. subtilis or sterile PBS were surface stained with mAbs directed against CD3, CD4, CD8, and Cδ (BD Biosciences). Because Vγ6/Vδ1+ γδ T cells from transgenic Vγ6/Vδ1 mice express CD8α, CD3+ CD8+ T cells were identified by gating on Cδ− and NK1.1− cells and analyzed for expression of CD3+CD8+ T cells. For intracellular cytokine staining, total lung cells were cultured at 1 × 106 cells/ml in complete medium containing 10 μ g/ml brefeldin A (Sigma-Aldrich) at 37°C for 4 h. After activation, the cells were washed and stained with mAbs directed against CD3, CD4, and CD8. γδ T cells were identified by staining with anti-Cδ mAb (BD Biosciences) followed by fixation in 1% paraformaldehyde overnight. γδ T cells that express the Vγ6+ TCR were identified using a mAb, 17D1, that recognizes the Vγ6+ TCR after staining with anti-Cδ mAb (21). Fixed cells were permeabilized for 10 min at room temperature in 0.5% saponin and stained with mAbs directed against IL-17A (eBioscience) and isotype controls for 30 min at 4°C. Electronic compensation was performed with Ab capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. The lymphocyte population was identified using forward and 90° light scatter patterns, and fluorescence intensity was analyzed using a FACSAria cytometer (BD Immunocytometry Systems). The data files were analyzed using FloJo software (Tree Star).
Histology
All mice were sacrificed 24 h after their last exposure to B. subtilis or sterile PBS. The lungs were removed and infused with 10% formalin, embedded in paraffin, and stained with H&E (Dako) or Masson’s trichrome (Sigma-Aldrich) stain following the manufacturer’s instructions.
Cytokine analysis
Total lung homogenates were prepared by homogenizing whole lung samples in 500 μl of sterile PBS from C57BL/6 mice treated with either B. subtilis or sterile PBS for 1, 2, 3 and 4 consecutive weeks. Sterile PBS was infused through the pulmonary vasculature by right heart puncture as described above before preparation of lung homogenates. One hundred microliters of supernatant from each lung homogenate was analyzed for cytokines using the Protein Multiplex Immunoassay kit (BioSource International) per the manufacturer’s protocol. Briefly, Multiplex beads were loaded onto a Millipore MultiScreen BV 96-well filter plate followed by diluting each sample 1/2 with assay diluent. Serial dilutions of cytokine standards were prepared in parallel and added to the plate. After 2 h of incubation on a plate shaker (600 revolutions/minute) in the dark at room temperature, the samples were washed and biotinylated anti-mouse multicytokine reporter was added to each well. After incubation on a plate shaker at room temperature for 1 h, the plate was washed and PE-conjugated streptavidin was added directly to each well. The plate was incubated for 30 min, washed, and transferred to the Bio-Plex Luminex 100 XYP instrument for analysis. Cytokine concentrations were calculated using Bio-Plex Manager 3.0 software with a five parameter curve-fitting algorithm applied for standard curve calculations. Activated TGF-β1 (R&D Systems), IL-22 (R&D Systems), IL-17A (eBioscience), IFN-γ (eBioscience), and IL-23 (eBioscience) in supernatants from whole lung homogenates were quantified by ELISA according to the manufacturer’s instructions.
Collagen quantification
The collagen content of the right lung from mice treated with either B. subtilis or sterile PBS was determined using Sirius red staining (19). Each lung sample was thawed at 4°C in sterile PBS supplemented with protease inhibitors (Sigma-Aldrich), homogenized in 5 ml of 0.5 M acetic acid containing 1 mg of pepsin/10 mg of tissue, and incubated for 24 h at 4°C. After centrifugation at high speed for 10 min, 100 μl of each supernatant was mixed with 900 μl of Sirius red dye reagent, allowed to incubate at room temperature for 30 min, and then centrifuged at high speed for 10 min. After aspiration of the supernatant, the pellet containing the complex of soluble collagen and Sirius red dye reagent was resuspended in 0.5M NaOH, and the OD540 was measured using a spectrophotometer. The collagen content in micrograms was calculated from a standard curve generated using known concentrations of collagen per the manufacturer’s instructions.
Total and differential cell counts
Total and differential cell counts were performed on collagenase-digested lung before purification of mononuclear cells over Ficoll as previously described (19). Briefly, single-cell suspensions of total lung cells were transferred to a glass slide using a cytocentrifuge apparatus and stained with Wright-Giemsa stain (VWR) per manufacturer’s instructions. Epithelial cells were not included in the total cell count while RBCs were removed by RBC lysis. Differential cell counts were performed by counting at least 200 cells under high power field.
Colony counts
Whole lung samples were isolated from mice treated with B. subtilis for 4 consecutive weeks at different time points after the last administration of the microorganism as described above. Colony counts were performed by homogenating whole lung or spleen samples in 500 μl of sterile PBS using a tissue homogenizer. Serial dilutions of the entire lung homogenate were plated on tryptic soy broth agar plates and grown at 37°C for 24 h.
Statistical analysis
A Mann-Whitney U analysis and one-way ANOVA with a Bonferroni’s multiple comparison test were used to determine whether there were significant differences between the treatment groups at each time point (Prism 4; GraphPad Software). p < 0.05 was considered statistically significant.
Results
Altered composition of mononuclear cell infiltrates after B. subtilis inhalation in transgenic Vγ6/Vδ1 mice
To further investigate the potential regulatory role of γδ T cells in lung inflammation and fibrosis, we repeatedly exposed homozygous Vγ6/Vδ1 transgenic (Vγ6+/+) mice to B. subtilis by nasal inhalation for 4 consecutive weeks. Nearly all γδ T cells from Vγ6+/+ mice expressed the canonical Vγ6/Vδ1 TCR (20, 21). Vγ6+/+ mice developed peribronchovascular mononuclear cell infiltrates after B. subtilis exposure, whereas no cellular infiltrates were seen in the lungs of PBS-treated control mice (Fig. 1A). As compared with wt C57BL/6 mice treated in an identical fashion, a 1.4-fold increase in total lung cell numbers was seen in B. subtilis-treated Vγ6+/+ mice (p < 0.01) (Fig. 1B). In addition, a significantly increased number of lung lymphocytes was seen in Vγ6+/+ mice compared with either wt C57BL/6 mice ( p < 0.001) or TCRδ−/− mice (p < 0.001) (Fig. 1B), with a 3-fold increase in Vγ6/Vδ1+ γδ T cells compared with wt C57BL/6 mice (p < 0.001) (Fig. 1C). Interestingly, CD4+ T cells were decreased by 50% in the lungs of Vγ6+/+ mice compared with wt C57BL/6 mice (p < 0.05), but were markedly expanded in TCRδ−/− mice (p < 0.001) (Fig. 1C). CD8+ T cells were not significantly different between Vγ6+/+ and wt C57BL/5 mice but were increased in TCRδ−/− mice in response to chronic treatment with B. subtilis (Fig. 1C). Macrophage numbers were also decreased in Vγ6+/+ mice compared with wt C57BL/6 ( p < 0.05) and TCRδ−/− mice ( p < 0.01) (Fig. 1B). Similar numbers of neutrophils were seen in each group of mice after 4 consecutive weeks of B. subtilis exposure (Fig. 1B). Taken together, these data document a strong response of the transgenic Vγ6/Vδ1−/− γδ T cells to treatment with B. subtilis and the inhibitory effect this T cell subset has on expansion of other cell types such as CD4+ T lymphocytes and macrophages.
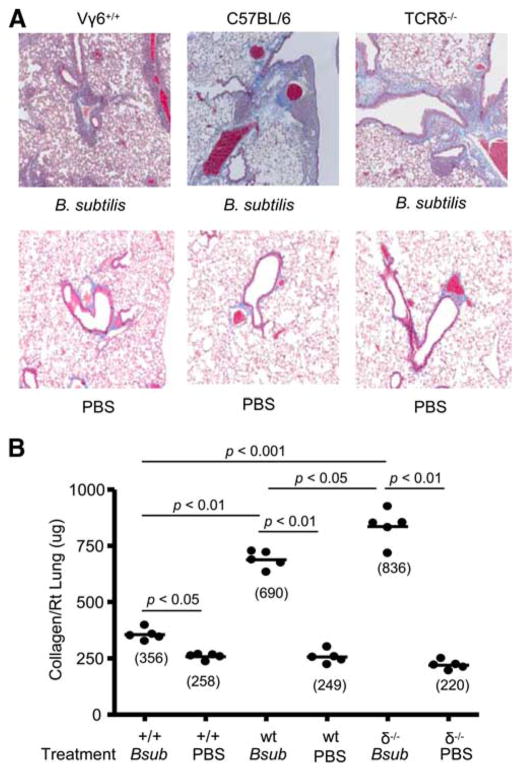
FIGURE 1.
Transgenic Vγ6+/+ mice repeatedly exposed to B. subtilis develop mononuclear infiltrates. A, Representative H&E of lungs from transgenic Vγ6+/+, wt C57BL/6, and TCRδ−/− mice repeatedly exposed to inhaled B. subtilis or PBS for 4 consecutive weeks is shown. Vγ6+/+ mice develop mononuclear infiltrates in both the peribronchovascular and the interstitial spaces. Data shown represent at least five mice from at least two separate experiments (original magnification × 100). B, Total and differential cell counts were performed on total lung homogenates from Vγ6+/+ (+/+), wt C57BL/6 (wt), and TCRδ−/− (δ−/−) mice repeatedly treated with inhaled B. subtilis for 4 consecutive weeks. C, Vγ6/Vδ1+, CD4+, and CD8+ T cell counts were performed on total lung homogenates from Vγ6+/+ (+/+), wt C57BL/6 (wt), and TCRδ−/− (δ−/−) mice repeatedly treated with inhaled B. subtilis for 4 consecutive weeks. Data were compiled from at least two separate experiments.
Vγ6/Vδ1 transgenic mice have attenuated lung fibrosis after B. subtilis exposure
We have previously shown that in the absence of Vγ6/Vδ1+ T cells, lung fibrosis is accelerated in mice chronically exposed to B. subtilis (19). To determine whether γδ T cells prevent collagen deposition in the lung, we repeatedly treated Vγ6+/+ mice with B. subtilis for 4 consecutive weeks. As shown in Fig. 2A, Vγ6+/+ mice repeatedly exposed to B. subtilis showed significantly decreased collagen deposition in the peribronchovascular space by Masson’s trichrome staining compared with both wt C57BL/6 and TCRδ−/− mice treated in an identical fashion. Using the more sensitive Sirius red colorimetric assay for collagen deposition, B. subtilis-treated Vγ6+/+ mice developed a 1.9-fold decrease in collagen content in the lung compared with wt C57BL/6 mice (p < 0.01) and a 2.3-fold decrease compared with mice deficient in γδ T cells (TCRδ−/− mice) (p < 0.001) (Fig. 2B). Interestingly, Vγ6+/+ mice had increased collagen deposition in the lung compared with PBS-treated control animals (mean ± SD; 356 ± 26 pg/ml vs 258 ± 12 pg/ml; p < 0.05) (Fig. 2B), suggesting that Vγ6/Vδ1+ γδ T cells can significantly attenuate but not completely abrogate B. subtilis-induced lung fibrosis.
FIGURE 2.
Transgenic Vγ6+/+ mice repeatedly exposed to B. subtilis develop less lung fibrosis. A, Representative trichrome staining of lungs from transgenic Vγ6+/+, wt C57BL/6, and TCRδ−/− mice repeatedly treated with inhaled B. subtilis or PBS for 4 consecutive weeks is shown. Data shown represent at least five mice from at least two separate experiments (original magnification × 100). B, Quantification of collagen content using Sirius red colorimetric assay in the lungs of individual transgenic Vγ6+/+ (+/+), wt C57BL/6 (wt), and TCRδ−/− (δ−/−) mice treated with B. subtilis (Bsub) or PBS for 4 consecutive weeks. Data were compiled from at least two separate experiments.
B. subtilis exposure induces a Th17-polarized immune response in the lung
Although HP is considered a Th1-mediated disease (22), lung fibrosis is seen in both humans and murine models of this disease (6), suggesting that other cytokines may be important for the development of B. subtilis-induced disease. We analyzed the cytokine profile from homogenized lungs of mice treated with B. subtilis and PBS for 1, 2, 3, and 4 wk. Because there was no statistically significant difference in cytokine levels in PBS-treated control animals after 1, 2, 3, or 4 wk of treatment, only the 4-wk time point is shown. As seen in Fig. 3A, very low levels of both IFN-γ and TNF-α were detected in the lungs of mice treated with B. subtilis at each time point compared with controls. IL-5, a Th2 cytokine, was elevated in the lungs of mice after 2 wk of treatment with B. subtilis, but IL-5 levels were not statistically significant at any other time points compared with PBS-treated control mice. IL-13 was also found in the lungs of mice treated with B. subtilis, but the levels were not significantly different from controls, whereas IL-4 was below the detection limit of the assay in both the B. subtilis-treated and control animals (Fig. 3A).
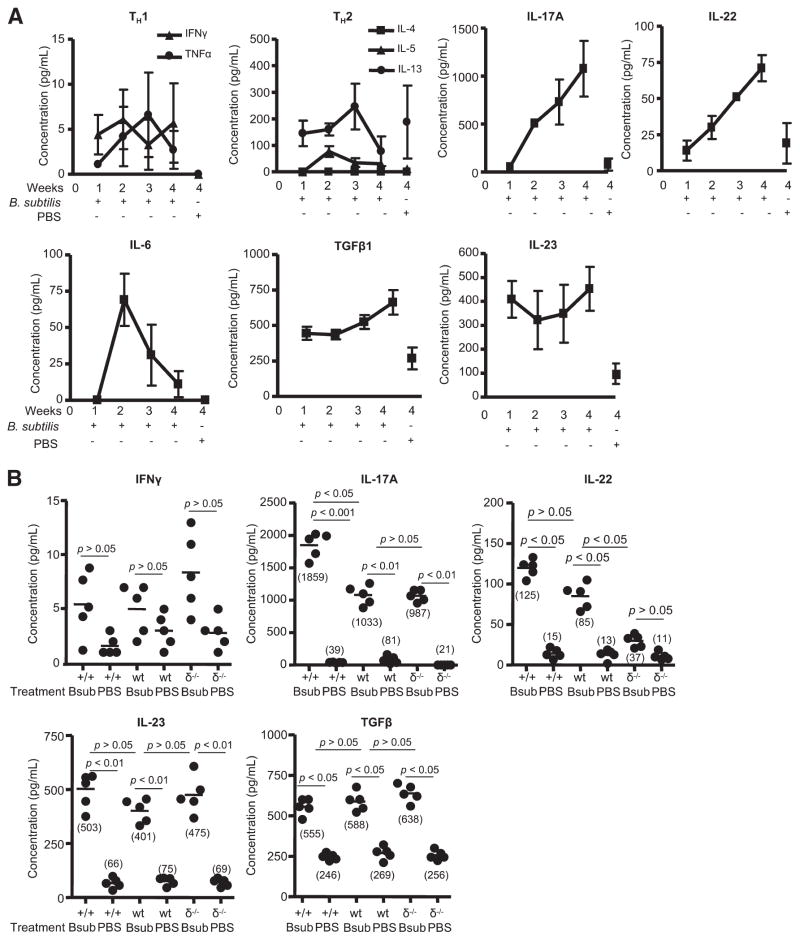
FIGURE 3.
Analysis of Th1, Th2, and Th17 cytokines from the lungs of wt C57BL/6 mice treated with B. subtilis. A, Cytokines from supernatants of total lung homogenates were analyzed from wt C57BL/6 mice treated with B. subtilis for 1, 2, 3, and 4 wk compared with control mice treated in an identical fashion with sterile PBS for 4 wk. Data represent the mean ± SD of five mice at each time point treated with B. subtilis or sterile PBS from at least two separate experiments. B, Cytokines from supernatants of total lung homogenates were analyzed by ELISA from Vγ6+/+ (+/+), wt C57BL/6 (wt), and TCRδ−/− (δ−/−) mice treated with either B. subtilis (Bsub) or PBS for 4 wk. Data represent the mean ± SD of five mice treated with B. subtilis or sterile PBS from at least two separate experiments.
As opposed to type 1 and 2 cytokines, we found increasing amounts of IL-17A in the lungs of mice with repeated exposure to B. subtilis, with a 14-fold increase in IL-17A in the lung of B. subtilis-treated mice compared with control animals (p < 0.001) after 4 wk of treatment (Fig. 3A). IL-22 was also elevated in the lungs of B. subtilis-treated C57BL/6 mice with a 3.7-fold increase in IL-22 (p < 0.01) compared with PBS-treated control mice after 4 wk of treatment (Fig. 3A). In addition, IL-17A and IL-22 were not detected in the spleens of the same animals (data not shown), suggesting a compartmentalized Th17 immune response directed against B. subtilis in the lung. IL-6, a cytokine important for the commitment of Th17 cells, increased rapidly after the second week of exposure but steadily declined with continued exposure to B. subtilis, suggesting that commitment to the Th17 lineage occurs in the early stages of exposure to this microorganism (Fig. 3A). TGF-β1, also important in the differentiation of Th17 cells (24), was 2.6-fold higher in the lung of B. subtilis-exposed mice compared with PBS-treated control mice after 4 wk of treatment (Fig. 3A). In addition, IL-23, essential for expansion and maintenance of committed Th17 cells, was elevated in the lungs of C57BL/6 mice with repeated exposure to B. subtilis for 4 wk compared with control animals (4.6-fold) (Fig. 3A).
Because increasing numbers of Vγ6/Vδ1+ T cells correlated with decreased lung fibrosis, we next compared Th1, Th2, and Th17 cytokines from lung homogenates of Vγ6+/+, wt C57BL/6, and TCRδ−/− mice after 4 wk of treatment with either B. subtilis or PBS. Low levels of IFN-γ and TNF-α were detected in the lungs of Vγ6+/+, wt C57BL/6, and TCRδ−/− mice but were not significantly different from controls after 4 wk of treatment (Fig. 3B and data not shown). For Th2 cytokines, IL-4 was below the detection limit of the assay while IL-5 and IL-13 levels were similar to those of PBS-treated control animals (data not shown). Conversely, levels of the Th17-associated cytokines IL-6, IL-23, and TGF-β1 were all increased in the lungs of Vγ6+/+ and TCRδ−/− mice treated with B. subtilis compared with control animals, but they were similar to those in wt C57BL/6 mice treated in an identical fashion (Fig. 3B and data not shown). Interestingly, IL-17A levels were further increased in the lungs of B. subtilis-treated Vγ6+/+mice compared with both wt C57BL/6 and TCRδ−/− mice with similar concentrations of IL-17A in TCRδ−/− and wt C57BL/6 mice (Fig. 3B). In addition, IL-22 levels were increased in Vγ6+/+ mice compared with wt C57BL/6 mice, whereas in the absence of γδ T cells, TCRδ−/− mice had significantly decreased concentrations of IL-22 in the lung compared with both Vγ6+/+ and wt C57BL/6 mice (Fig. 3B). These data indicate that a Th17-polarized immune response predominates in the lungs of animals repeatedly exposed to B. subtilis.
Expression of IL-17A by Vγ6/Vγ1+ γδ T cells and conditionally by CD4+ T cells
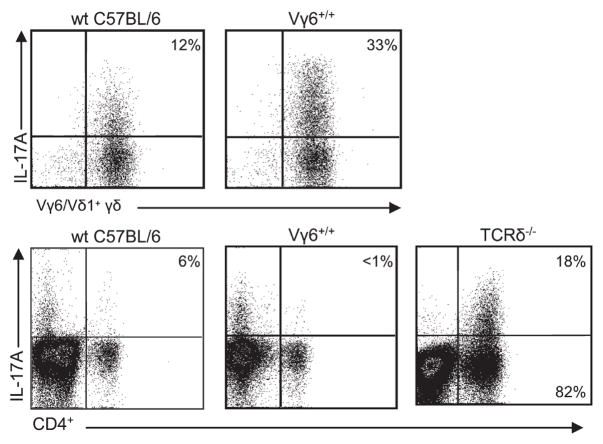
Because IL-17A was the predominant cytokine identified in response to B. subtilis and has been shown to be expressed by numerous cell types including γδ, CD4+, and CD8+ T cells (24), we analyzed these T lymphocyte populations in the lungs of mice for IL-17A. As shown in Fig. 4, a higher percentage of Vγ6/Vδ1+ T cells express IL-17A compared with CD4+ T cells in both Vγ6+/+ and wt C57BL/6 mice while CD8+ T cells did not express IL-17A (data not shown). Interestingly, in the absence of γδ T cells, CD4+ T cells isolated from the lungs of B. subtilis-treated TCRδ−/− mice had increased expression of IL-17A (Fig. 4), which may account for the similar levels of IL-17A in the lungs of wt C57BL/6 and TCRδ−/− mice (Fig. 3B). Therefore, in the absence of Vγ6/Vδ1+ T cells there is not only a 5-fold increase in the number of CD4+ T lymphocytes but also a 13-fold increase in the number of CD4+ T cells expressing IL-17A, suggesting a crucial role for IL-17A in the immune response directed against B. subtilis.
FIGURE 4.
IL-17A expression by Vγ6/Vδ1+ and CD4+ T cells. Representative density plots of intracellular IL-17A expression in Vγ6/Vδ1+ or CD4+ T cells from Vγ6+/+, wt C57BL/6, or TCRδ−/− treated with B. subtilis for 4 wk are shown. Vγ6/Vδ1+ and CD4+ T cells were isolated from the lungs of individual mice and placed in medium with brefeldin A for 4 h. The percentage of Vγ6/Vδ1+ or CD4+ T cells expressing IL-17A is shown in the upper right quadrant of each density plot. Data are representative of at least five individual mice treated with B. subtilis from at least two separate experiments.
IL17ra−/− mice have increased inflammation
To further define the role of IL-17A in the immune response to repeated exposure to B. subtilis, we treated IL-17ra−/− mice with B. subtilis for 4 wk. In the absence of IL-17A receptor signaling, B. subtilis-treated IL-17ra−/− mice had increased lung inflammation by H&E (Fig. 5A) with a 1.5-fold increase in total cell numbers (p < 0.05), a 1.7-fold increase in lymphocytes (p < 0.05), and 1.9-fold expansion of macrophages (p < 0.05) compared with the lungs of wt C57BL/6 mice treated in an identical fashion (Fig. 5B). A decreased number of neutrophils in the lungs of IL-17ra−/− compared with wt C57BL/6 mice (p < 0.05) was also seen (Fig. 5B). Consistent with an expanded number of total lymphocytes, there was a significant increase in Vγ6/Vδ1+ T cells in the lungs of IL-17ra−/− compared with wt C57BL/6 mice (p < 0.01) after 4 wk of treatment with B. subtilis (Fig. 5C). In addition, CD4+ (p < 0.01) and CD8+ (p < 0.05) T cells were also expanded in the lungs of IL-17ra−/− compared with the lungs of wt C57BL/6 mice (Fig. 5C).
FIGURE 5.
IL17ra−/−mice develop increased mononuclear infiltrates in response to B. subtilis. A, Representative H&E staining of lungs from IL17ra−/− mice repeatedly treated with inhaled B. subtilis for 4 consecutive weeks shows increased mononuclear infiltrates compared with PBS-treated controls. Data shown for H&E represent at least four mice from at least two separate experiments (original magnification × 100). Arrow denotes a mononuclear infiltrate in a peribronchovascular distribution. B, Total cell and lymphocyte, macrophage, and neutrophil counts from the lungs of B. subtilis-treated IL17ra−/− or wt C57BL/6 mice repeatedly treated with inhaled B. subtilis for 4 consecutive weeks are shown. C, Vγ6/Vδ1+, CD4+, and CD8+ T cell counts from the lung of B. subtilis-treated IL17ra−/− or wt C57BL/6 mice repeatedly treated with inhaled B. subtilis for 4 consecutive weeks are depicted. Data are representative of at least five individual mice treated with B. subtilis from at least two separate experiments.
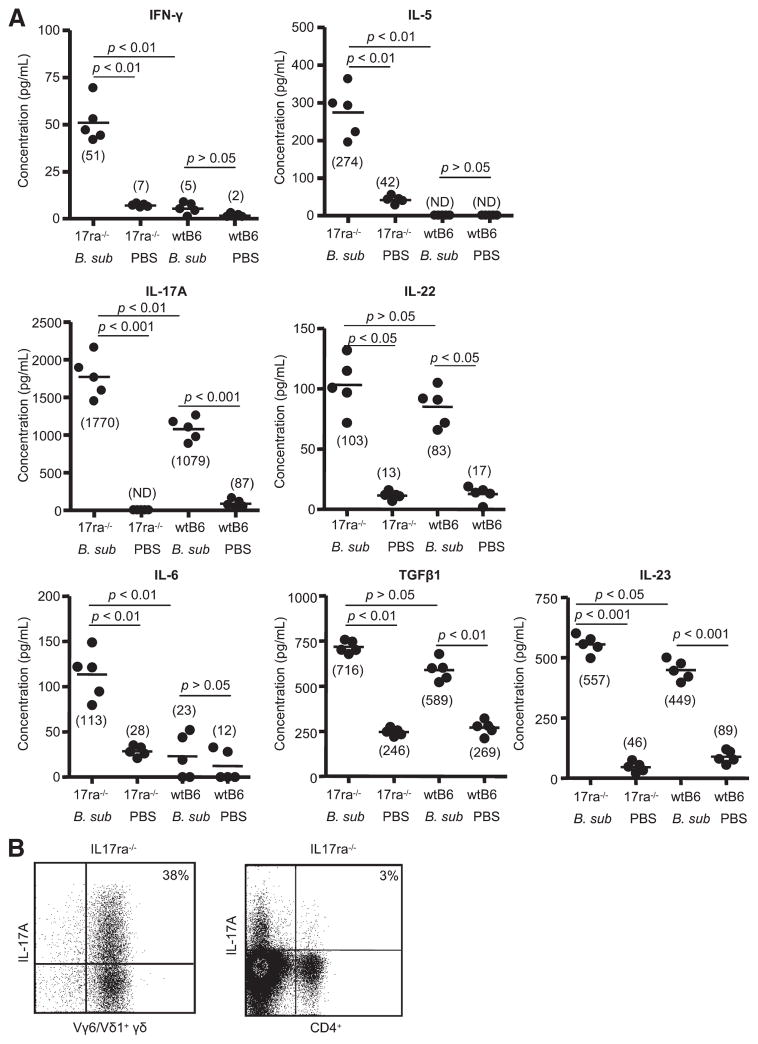
In the absence of IL-17A receptor signaling, B. subtilis-treated IL-17ra−/− mice also had increased levels of inflammatory cytokines. As shown in Fig. 6A, IFN-γ and IL-5 were increased in the lungs of B. subtilis-treated mice compared with PBS-treated controls as well as wt C57BL/6 mice treated with either B. subtilis or PBS. IL-6, IL-17A, and IL-23 were also elevated in the lungs of B. subtilis-treated IL-17ra−/− mice compared with wt C57BL/6 mice treated with B. subtilis (Fig. 6A). Although there was a trend toward higher levels of IL-22 and TGF-β1 levels in the lungs of B. subtilis-treated IL-17ra−/− mice compared with wt C57BL/6 mice, the differences were not statistically significant (Fig. 6A). Vγ6/Vδ1+ γδ T cells remained the cell type predominantly responsible for IL-17A expression in IL-17ra−/− mice treated with B. subtilis for 4 wk (Fig. 6B).
FIGURE 6.
Increased inflammatory cytokines in the lung of IL17ra−/− mice treated with B. subtilis. A, Cytokines from supernatants of total lung homogenates were analyzed from IL17ra −/− or wt C57BL/6 mice treated with B. subtilis (B sub) or PBS for 4 wk. Data represent the mean ± SD of five mice treated with B. subtilis or sterile PBS from at least two separate experiments. B, Representative density plots of intracellular IL-17A expression in Vγ6/Vδ1+ or CD4+ T cells from IL17ra −/−mice treated with B. subtilis for 4 wk. Vγ6/Vδ1+ and CD4+ T cells were isolated from the lungs of individual mice and placed in medium with brefeldin A for 4 h. The percentage of Vγ6/Vδ1+ or CD4+ T cells expressing IL-17A is shown in the upper right quadrant of each density plot. Data are representative of at least five individual mice treated with B. subtilis from at least two separate experiments.
IL17ra−/− mice have increased lung fibrosis with bacterial persistence in the lung
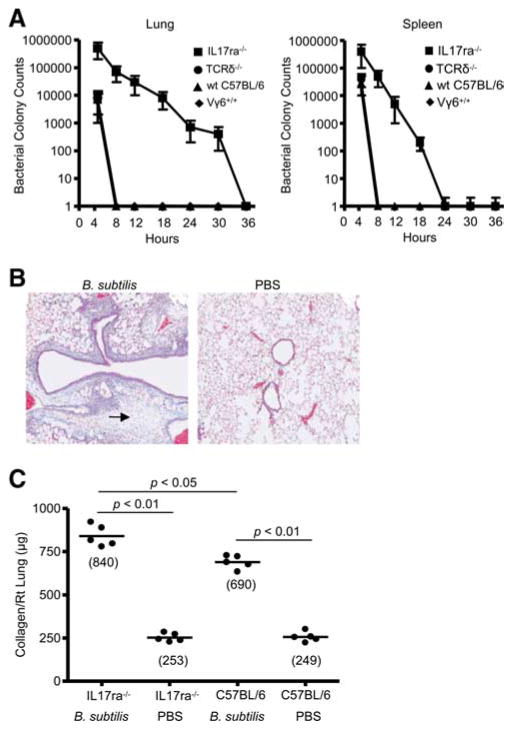
We previously reported that wt C57BL/6 and mice deficient in Vγ6/Vδ1+ T cells (Vγ4−/−6−/− mice) clear B. subtilis from the lung with similar efficiency (18, 19). To determine whether IL-17A receptor signaling is necessary for bacterial clearance, we performed colony counts on whole lung homogenates at different time points after inhalation of the microorganism. As shown in Fig. 7A, Vγ6+/+, wt C57BL/6, and TCR δ−/− mice were able to clear B. subtilis from the lung and spleen 8 h after exposure. In contrast, B. subtilis persisted in the lungs of IL-17ra−/− mice up to 30 h after inhalation of B. subtilis.
FIGURE 7.
IL17ra−/− mice develop increased lung fibrosis in response to persistent B. subtilis infection. A, B. subtilis colony counts from lung homogenates of individual IL17ra−/−, TCRδ−/−, wt C57BL/6, and Vγ6+/+ mice. All mice were treated with B. subtilis for 3 consecutive days each week for 4 consecutive weeks and analyzed for the persistence of B. subtilis in the lung and spleen at different time points after last exposure to B. subtilis. Data represent the mean ± SD colony counts from at least four mice at each time point treated with B. subtilis from at least two separate experiments. B, Representative Masson’s trichrome staining of lungs from IL17ra−/− mice repeatedly treated with inhaled B. subtilis for 4 consecutive weeks shows collagen deposition in a peribronchovascular distribution. Arrow denotes collagen deposition in a peribronchovascular distribution. Data shown for Masson’s trichrome represent at least four mice from at least two separate experiments (original magnification × 100). C, Quantification of collagen content using Sirius red colorimetric assay shows increased collagen deposition in the lungs of individual IL17ra−/− compared with wt C57BL/6 mice treated with B. subtilis or PBS for 4 consecutive weeks. Data compiled from at least two separate experiments.
To determine whether IL-17ra−/− mice developed accelerated lung fibrosis due to increased inflammation, we analyzed collagen content in the lung using both Masson’s trichrome staining and a Sirius red colorimetric assay. As shown in Fig. 7B, IL-17ra−/− mice developed collagen deposition in a peribronchovascular distribution compared with PBS-treated control animals. By Sirius red colorimetric assay, IL-17ra−/− mice developed a statistically significant increase in collagen content in the lung compared with wt C57BL/6 mice (840 ± 62 μg vs 690 ± 38 μg; p < 0.05) treated in an identical fashion with B. subtilis for 4 wk (Fig. 7C). Taken together, these data suggest that in the absence of IL-17A signaling, IL-17ra−/− mice fail to efficiently eliminate B. subtilis from the lung, which results in increased inflammation and accelerated fibrosis.
Discussion
Hypersensitivity pneumonitis is an inflammatory lung disease caused by repeated inhalation of a variety of environmental Ags that can progress to lung fibrosis with chronic exposure to the inhaled Ag. Although T cells are important in the pathogenesis of HP, their role in the development of pulmonary fibrosis remains poorly understood (25). We previously reported that C57BL/6 mice repeatedly exposed to B. subtilis develop lung fibrosis with increased numbers of CD4+ and CD8+ T cells in the lung and a large expansion of Vγ6/Vδ1+ γδ T cells (19). In the absence of γδ T cells, Vγ4−/−6−/− mice had increased numbers of CD4+ and CD8+ T lymphocytes with accelerated lung fibrosis upon repeated exposure to inhaled B. subtilis, suggesting that Vγ6/Vδ1+ T cells protect against lung fibrosis by limiting the expansion of αβ T cells. In the present study, we build on these studies using Vγ6/Vδ1 transgenic mice. With chronic exposure to B. subtilis, Vγ6/Vδ1 transgenic mice have further increased numbers of Vγ6/Vδ1+ T cells in the lung with fewer CD4+ T cells and macrophages and less lung fibrosis compared with wt C57BL/6 mice. Although HP is considered a Th1-mediated disease, we found increasing levels of the Th17 cytokine IL-17A, which was predominantly expressed by Vγ6/Vδ1+ T cells. In the absence of IL-17 signaling, B. subtilis persisted in the lung, resulting in increased inflammation and fibrosis and suggesting an important role for IL-17A in eliminating B. subtilis.
To investigate the mechanisms by which γδ T lymphocytes attenuate B. subtilis-induced lung inflammation and fibrosis, we initially analyzed lung homogenates for type 1 and 2 cytokines. In a number of models of solid organ fibrosis, Th1 and Th2 cytokines differentially regulate organ fibrosis (26). Numerous studies suggest that the type 1 cytokine IFN-γ is important for the granulomatous inflammation seen in HP (17, 20) as well as protective against collagen deposition in the bleomycin-induced model of lung fibrosis (27). Therefore, we hypothesized that γδ T cells prevent fibrotic lung disease through the expression of IFN-γ. We found, however, very low levels of IFN-γ in the lung and did not find significant expression of IFN-γ by γδ, CD4+, or CD8+ T cells at any of several time points examined (P. L. Simonian and A. P. Fontenot, unpublished observation). In contrast to type 1 cytokines, type 2 cytokines have been reported to promote lung fibrosis (26). We found increased levels of the type 2 cytokine IL-5 compared with PBS-treated control mice. IL-5 has been shown to exacerbate bleomycin-induced lung fibrosis, but IL-5−/− mice showed no impairment in fibrosis, suggesting that IL-5 may act as an amplifier rather than a direct mediator of pulmonary fibrosis (28). In addition, we did not find increased levels of other type 2 cytokines such as IL-4 and IL-13 compared with controls. These data suggest that in this model of B. subtilis-induced HP, type 2 cytokines play at most a minor role in promoting lung fibrosis.
The absence of significant type 1 and 2 cytokine expression in the lungs of mice treated with B. subtilis raised the possibility that other T cell cytokines were involved in the immune response directed against B. subtilis. Numerous studies show an important role for IL-17 expressed by CD4+ T cells in mucosal immunity against lung pathogens (29), whereas IL-17 expression by γδ T cells is important in pulmonary tuberculosis (23), collagen-induced arthritis (30), and Listeria monocytogenes-induced liver disease (31). In this regard, the predominant cytokine identified in the lungs of wt C57BL/6 mice treated with B. subtilis was IL-17A, which was expressed predominantly by Vγ6/Vδ1+ T cells. The fact that Th17 cytokines were identified in the lung in the absence of type 1 and 2 cytokines was not surprising. Numerous reports have recently shown that IL-6 and TGF-β are necessary for the development of T cells that express Th17 cytokines such as IL-17A and IL-22 while directly inhibiting the differentiation of Th1 and Th2 cells (24). TCR δ−/− and wt C57BL/6 mice, however, had similar levels of IL-17A in the lung associated with a compensatory increase in the number of IL-17A-expressing CD4+ T cells, suggesting that this T cell cytokine is critical for the immune response directed against B. subtilis. Numerous studies demonstrate that IL-17A is important for neutrophil recruitment to the site of inflammation and assists in pathogen clearance (24, 32). Consistent with these reports, we found a decreased number of neutrophils in the lung of B. subtilis-treated IL-17ra−/− mice compared with wt C57BL/6, Vγ6+/+, and TCRδ−/− mice, indicating that IL-17A signaling is important for neutrophil recruitment to the lung in response to repeated exposure to B. subtilis. Interestingly, Vγ6+/+ mice treated with B. subtilis had further increased levels of IL-17A in the lung but a similar clearance rate of B. subtilis from the lung as compared with wt C57BL/6 and TCRδ−/− mice. The presence of nearly identical numbers of neutrophils in Vγ6+/+ mice treated with B. subtilis compared with wt C57BL/6 and TCRδ−/− mice suggests that a threshold concentration of IL-17A may be required for neutrophil recruitment to lung and pathogen removal. In addition, B. subtilis persisted in the lungs of IL-17ra−/− mice compared with wt C57BL/6, Vγ6+/+ and TCR δ−/− mice, suggesting that IL-17A signaling is necessary for efficient pathogen clearance, most likely through neutrophil recruitment.
Despite the fact that TCR δ−/− mice had similar levels of IL-17A, neutrophil numbers, and pathogen clearance as C57BL/6 mice, these mice, like Vγ4−/−6−/− mice (19), develop accelerated lung fibrosis in response to repeated exposure to B. subtilis. In addition, Vγ6+/+ mice had increased levels of IL-17A with similar rates of bacterial clearance as wt C57BL/6 and TCR δ−/− mice, yet decreased lung fibrosis. Together, these findings suggest that Vγ6/Vδ1+ γδ T cells may possess other mechanisms that attenuate lung inflammation and fibrosis independently of IL-17A expression. Vγ6/Vδ1+ T cells may regulate the immune response directed against B. subtilis by limiting the expansion of other inflammatory cell types including macrophages and CD4+ T cells, thus promoting the resolution of lung inflammation and possibly fibrosis. Numerous studies suggest that γδ T cells are important for terminating the host immune response directed against infectious agents (33, 34). In a murine model of L. monocytogenes infection, cytotoxic γδ T cells induced apoptosis of peritoneal macrophages, resulting in a return to normal macrophage homeostasis and the prevention of chronic inflammation (35). Consistent with these reports, we found decreased numbers of macrophages in the lungs of transgenic Vγ6/Vδ1 mice compared with mice deficient in γδ T cells (TCR δ−/−). Therefore, it is possible that Vγ6/Vδ1+ T cells suppress inflammation and pulmonary fibrosis by limiting the expansion and/or activation of macrophages.
We also observed a further reduction in the number of CD4+ T cells in the lungs of B. subtilis-treated transgenic Vγ6/Vδ1 mice compared with wt C57BL/6 mice treated in an identical fashion. These data are consistent with our previous report that showed increased numbers of CD4+ T cells and accelerated lung fibrosis in the absence of γδ T lymphocytes (19). Although a decline in the number of CD4+ T cells in the lung associates with less lung fibrosis in transgenic Vγ6/Vδ1 mice, Vγ6/Vδ1+ T cells may not directly regulate the expansion of CD4+ T cells in these mice. As opposed to wt C57BL/6 mice, transgenic Vγ6/Vδ1 mice have Vγ6/Vδ1+ T cells in the lung before B. subtilis exposure that may prevent the expansion of B. subtilis-specific CD4+ T cells in the lung upon repeated Ag treatment. In addition, although very few αβ T cells are found in the lung before treatment with B. subtilis, transgenic Vγ6/Vδ1 mice have fewer αβ T cells than wt C57BL/6 mice in the spleen and other immune organs. Therefore, although increasing numbers of CD4+ T cells in the lung correlate with accelerated lung fibrosis, it remains to be established whether Vγ6/Vδ1+ T cells directly regulate the expansion of CD4+ T cells in mice repeatedly exposed to B. subtilis.
In another murine model of HP and lung fibrosis, we recently reported C57BL/6 mice repeatedly exposed to Saccharopolyspora rectivirgula in an identical fashion as B. subtilis also accumulate Th17 cytokines in the lung (36). Interestingly, we and others (37) showed that in the absence of IL-17 receptor signaling (IL-17ra−/−) as well as in IL-17 deficient mice (IL-17−/−), inflammation and collagen deposition induced by S. rectivirgula were markedly diminished. These data suggest that in this model Th17 cytokines promote inflammation and lung fibrosis consistent with other murine models of autoimmune disease such as experimental autoimmune encephalitis (38). In the S. rectivirgula model of HP, however, γδ T cells are only found at very low numbers and do not appear to protect against lung fibrosis. In addition, CD4+ T cells were the predominant cell type expressing Th17 cytokines in response to S. rectivirgula (36). In the absence of IL-17 receptor signaling however, IL-17ra−/− mice developed a heterogeneous expansion of γδ T cells that correlated with decreased numbers of macrophages and CD4+ T cells and with lung fibrosis (P. L. Simonian and A. P. Fontenot, unpublished observation). These data further suggest that the presence of γδ T cells may attenuate lung fibrosis by limiting the expansion of inflammatory cells in the lung in response to microbial invasion, which is independent of IL-17A.
In addition to IL-17A, we also found increased levels of IL-22 in the lungs of wt C57BL/6 mice with repeated exposure to B. subtilis exposure. Interestingly, Vγ6+/+ mice had further increased levels of IL-22 in the lung after repeated exposure to B. subtilis compared with wt C57BL/6 mice, whereas in the absence of γδ T cells (TCRδ−/− mice), IL-22 concentrations are markedly decreased in the lung, which inversely correlated with the amount of B. subtilis-induced lung fibrosis. Although the function of IL-22 in this model is not known, IL-22 has been shown to act synergistically with IL-17A or IL-17F to induce expression of antimicrobial peptides on keratinocytes (39). More recently, IL-22 has been shown to be important for mucosal host defense against Klebsiella pneumoniae in the lung (40). In this model, IL-22 increased lung epithelial cell proliferation and increased transepithelial resistance to injury, whereas IL-17A was important for induction of G-CSF, neutrophil recruitment, and clearance of the microorganism (40). IL-22 has also been shown to increase expression of inflammatory cytokines but not proliferation or collagen synthesis in sub-epithelial myofibroblasts in inflammatory bowel disease (41). Studies are ongoing to determine the role of IL-22 in this murine model of HP and lung fibrosis.
In summary, the expansion of Vγ6/Vδ1+ γδ T cells in the lung in response to B. subtilis exposure significantly reduces lung inflammation and the resultant fibrosis. As opposed to type 1 or 2 cytokines, we found increased levels of IL-17A expressed by Vγ6/Vδ1+ T cells and, in their absence, CD4+ T cells. In the absence of IL-17A signaling, neutrophil numbers were reduced and B. subtilis persisted in the lung of IL-17ra−/− mice, resulting in accelerated lung inflammation and fibrosis. These data suggest an important role for IL-17A-expressing T lymphocytes, both γδ and CD4+ T cells, in eliminating this microorganism, thus preventing excessive lung inflammation and eventual fibrosis in this murine model of HP.
Footnotes
Abbreviations used in this paper: HP, hypersensitivity pneumonitis; wt, wild type.
Disclosures
The authors have no financial conflict of interest.
This work is supported by National Institutes of Health Grants HL62410 and ES011810 (to A.P.F.) and HL89766 (to P.L.S.).
References
- 1.Selman M. Hypersensitivity pneumonitis. In: Schwarz MI, King TE Jr, editors. Interstitial Lung Disease. 3. B. C. Decker; Hamilton, Ontario: 2003. pp. 452–484. [Google Scholar]
- 2.Bourke SJ, Banham SW, Carter R, Lynch P, Boyd G. Longitudinal course of extrinsic allergic alveolitis in pigeon breeders. Thorax. 1989;44:415–418. doi: 10.1136/thx.44.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalancette M, Carrier G, Laviolette M, Ferland S, Rodrique J, Begin R, Cantin A, Cormier Y. Farmer’s lung: long-term outcome and lack of predictive value of bronchoalveolar lavage fibrosing factors. Am Rev Respir Dis. 1993;148:216–221. doi: 10.1164/ajrccm/148.1.216. [DOI] [PubMed] [Google Scholar]
- 4.Monkare S, Haahtela T. Farmer’s lung: a 5-year follow-up of eighty-six patients. Clin Allergy. 1987;17:143–151. doi: 10.1111/j.1365-2222.1987.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshizawa Y, Ohtani Y, Hayakawa H, Sato A, Suga M, Ando M. Chronic hypersensitivity pneumonitis in Japan: a nationwide epidemiologic survey. J Allergy Clin Immunol. 1999;103:315–320. doi: 10.1016/s0091-6749(99)70507-5. [DOI] [PubMed] [Google Scholar]
- 6.Vourlekis JS, Schwarz MI, Cherniack RM, Curran-Everett D, Cool CD, Tuder RM, King TE, Jr, Brown KK. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med. 2004;116:662–668. doi: 10.1016/j.amjmed.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Reyes CN, Wenzel FJ, Lawton BR, Emanuel DA. The pulmonary pathology of farmer’s lung disease. Chest. 1982;81:142–146. doi: 10.1378/chest.81.2.142. [DOI] [PubMed] [Google Scholar]
- 8.Raulf M, Liebers V, Steppert C, Baur X. Increased γδ-positive T-cells in blood and bronchoalveolar lavage of patients with sarcoidosis and hypersensitivity pneumonitis. Eur Respir J. 1994;7:140–147. doi: 10.1183/09031936.94.07010140. [DOI] [PubMed] [Google Scholar]
- 9.Forrester JM, Newman LS, Wang Y, King TE, Jr, Kotzin BL. Clonal expansion of lung Vδ1+ T cells in pulmonary sarcoidosis. J Clin Invest. 1993;91:292–300. doi: 10.1172/JCI116184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trentin L, Migone N, Zambello R, di Celle PF, Aina F, Feruglio C, Bulian P, Masciarelli M, Agostini C, Cipriani A, et al. Mechanisms accounting for lymphocytic alveolitis in hypersensitivity pneumonitis. J Immunol. 1990;145:2147–2154. [PubMed] [Google Scholar]
- 11.Born WK, Jin N, Aydintug MK, Wands JM, French JD, Roark CL, O’Brien RL. γδ T lymphocytes-selectable cells within the innate system? J Clin Immunol. 2007;27:133–144. doi: 10.1007/s10875-007-9077-z. [DOI] [PubMed] [Google Scholar]
- 12.Wands JM, Roark CL, Aydintug MK, Jin N, Hahn YS, Cook L, Yin X, Dal Porto J, Lahn M, Hyde DM, et al. Distribution and leukocyte contacts of γδ T cells in the lung. J Leukocyte Biol. 2005;78:1086–1096. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 13.Nanno M, Shiohara T, Yamamoto H, Kawakami K, Ishikawa H. γδ T cells: firefighters or fire boosters in the front lines of inflammatory responses. Immunol Rev. 2007;215:103–113. doi: 10.1111/j.1600-065X.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 14.Uezu K, Kawakami K, Miyagi K, Kinjo Y, Kinjo T, Ishikawa H, Saito A. Accumulation of γδ T cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. J Immunol. 2004;172:7629–7634. doi: 10.4049/jimmunol.172.12.7629. [DOI] [PubMed] [Google Scholar]
- 15.Steele C, Zheng M, Young E, Marrero L, Shellito JE, Kolls JK. Increased host resistance against Pneumocystis carinii pneumonia in γδ T-cell-deficient mice: protective role of gamma interferon and CD8+ T cells. Infect Immun. 2002;70:5208 –5215. doi: 10.1128/IAI.70.9.5208-5215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zachariadis O, Cassidy JP, Brady J, Mahon BP. γδ T cells regulate the early inflammatory response to Bordetella pertussis infection in the murine respiratory tract. Infect Immun. 2006;74:1837–1845. doi: 10.1128/IAI.74.3.1837-1845.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam S, King DP, Beaman BL. Increase of γδ T lymphocytes in murine lungs occurs during recovery from pulmonary infection by Nocardia asteroides. Infect Immun. 2001;69:6165–6171. doi: 10.1128/IAI.69.10.6165-6171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King DP, Hyde DM, Jackson KA, Novosad DM, Ellis TN, Putney L, Stovall MY, Van Winkle LS, Beaman BL, Ferrick DA. Cutting edge: protective response to pulmonary injury requires γδ T lymphocytes. J Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- 19.Simonian PL, Roark CL, Diaz del Valle F, Palmer BE, Douglas IS, Ikuta K, Born WK, O’Brien RL, Fontenot AP. Regulatory role of γδ T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. 2006;177:4436–4443. doi: 10.4049/jimmunol.177.7.4436. [DOI] [PubMed] [Google Scholar]
- 20.Sim GK, Olsson C, Augustin A. Commitment and maintenance of the αβ and γδ T cell lineages. J Immunol. 1995;154:5821–5831. [PubMed] [Google Scholar]
- 21.Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn YS, Born WK, Tigelaar RE, O’Brien RL. Subset-specific, uniform activation among Vγ6/Vδ1+γδ T cells elicited by inflammation. J Leukocyte Biol. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- 22.Yamasaki H, Ando M, Brazer W, Center DM, Cruikshank WW. Polarized type 1 cytokine profile in bronchoalveolar lavage T cells of patients with hypersensitivity pneumonitis. J Immunol. 1999;163:3516–3523. [PubMed] [Google Scholar]
- 23.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 24.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 25.Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukocyte Biol. 2008;83:237–244. doi: 10.1189/jlb.0707504. [DOI] [PubMed] [Google Scholar]
- 26.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huaux F, Liu T, McGarry B, Ullenbruch M, Xing Z, Phan SH. Eosinophils and T lymphocytes possess distinct roles in bleomycin-induced lung injury and fibrosis. J Immunol. 2003;171:5470–5481. doi: 10.4049/jimmunol.171.10.5470. [DOI] [PubMed] [Google Scholar]
- 29.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, et al. IL-17A produced by γδ T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 33.Fu YX, Roark CE, Kelly K, Drevets D, Campbell P, O’Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J Immunol. 1994;153:3101–3115. [PubMed] [Google Scholar]
- 34.Carding SR, Egan PJ. The importance of γδ T cells in the resolution of pathogen-induced inflammatory immune responses. Immunol Rev. 2000;173:98 –108. doi: 10.1034/j.1600-065x.2000.917302.x. [DOI] [PubMed] [Google Scholar]
- 35.Egan PJ, Carding SR. Downmodulation of the inflammatory response to bacterial infection by γδ T cells cytotoxic for activated macrophages. J Exp Med. 2000;191:2145–2158. doi: 10.1084/jem.191.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, Born WK, O’Brien RL, Fontenot AP. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182:657– 665. [PMC free article] [PubMed] [Google Scholar]
- 37.Joshi AD, Fong DJ, Oak SR, Trujillo G, Flaherty KR, Martinez FJ, Hogaboam CM. IL-17 mediated immunopathogenesis in experimental hypersensitivity pneumonitis. Am J Resp Crit Care Med. doi: 10.1164/rccm.200811-1700OC. In press. [DOI] [PubMed] [Google Scholar]
- 38.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566 –577. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 39.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andoh A, Bamba S, Fujiyama Y, Brittan M, Wright NA. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol. 2005;40:1089–1099. doi: 10.1007/s00535-005-1727-4. [DOI] [PubMed] [Google Scholar]