Summary
Mirror neurons, as originally described in the macaque, have two defining properties [1] and [2]: They respond specifically to a particular action (e.g., bringing an object to the mouth), and they produce their action-specific responses independent of whether the monkey executes the action or passively observes a conspecific performing the same action. In humans, action observation and action execution engage a network of frontal, parietal, and temporal areas. However, it is unclear whether these responses reflect the activity of a single population that represents both observed and executed actions in a common neural code or the activity of distinct but overlapping populations of exclusively perceptual and motor neurons [3]. Here, we used fMRI adaptation to show that the right inferior parietal lobe (IPL) responds independently to specific actions regardless of whether they are observed or executed. Specifically, responses in the right IPL were attenuated when participants observed a recently executed action relative to one that had not previously been performed. This adaptation across action and perception demonstrates that the right IPL responds selectively to the motoric and perceptual representations of actions and is the first evidence for a neural response in humans that shows both defining properties of mirror neurons.
Results and Discussion
The human capacity to interpret the actions and gestures of others is vital to social interactions. Current theories suggest that this ability is subserved by a “mirror-neuron” system, a network that possesses the unique property of responding to specific actions, regardless of whether they are observed or executed [4]. The mirror-neuron system may therefore provide a neural mechanism through which perceived actions are directly matched with their corresponding representations within an observer's own motor repertoire. Mirror neurons have been directly identified in the macaque ventral premotor cortex (area F5) and inferior parietal lobe (areas PF/PFG) [1], [2] and [5]. In humans, action observation and execution activate homologous areas in the inferior frontal gyrus (IFG) and inferior parietal lobule (IPL), as well as the superior temporal sulcus (STS) [6]. In contrast to the findings from nonhuman primates, however, there currently is no human evidence that has directly established the existence of a single neural population that encodes specific actions during both the observation and execution of action [3].
Here, we used functional magnetic resonance imaging (fMRI) to determine the selectivity of neural populations for particular actions and—crucially—invariance in their responses across the observation and execution of a motor act. fMRI adaptation (or repetition suppression) refers to the observation that repeated presentations of a sensory stimulus consistently reduce blood-oxygen-level dependent (BOLD) responses relative to presentations of a novel stimulus [7], [8] and [9]. Adaptation across two separate stimuli has been taken as evidence for a common neural representation that is invariant to the differences between those stimuli, whereas recovery from adaptation implies selectivity of the neural population to a specific stimulus attribute [8]. The adaptation effect has been demonstrated in many perceptual domains, including the perception of colors [10], shapes [11], and objects [12], and occurs in both lower- and higher-level visual areas [7] and [13]. Recently, fMRI adaptation has also been found during passive action observation [14], [15], [16] and [17] and action execution [16] and [18]. Critically, however, a recent attempt to show adaptation for executed actions that were subsequently observed, or for observed actions that were subsequently executed, failed to reveal any population that responds in such a manner [16]. This missing evidence is crucial, given that it leaves the key properties of macaque mirror neurons—neural specificity and response invariance across action and perception—yet to be demonstrated in humans. If human mirror areas contain neurons that respond to specific actions independently from whether they are observed or executed, their responses should be suppressed during the observation of a previously executed action, relative to one that has not yet been performed. In experiment 1, participants observed and executed a series of actions in two different configurations (Figure 1). In the critical sequence (“Execute-Observe”), participants first performed a set of two to five pantomimed hand actions, and subsequently observed an equivalent number of actions that were either the same as (“Repeated”), or different from (“Novel”), those in the preceding set. In a separate series of scans, we also presented participants with the reverse sequence of events (“Observe-Execute”), such that participants first observed a set of actions and subsequently executed either a Repeated or Novel series of actions. Although potentially interesting, the Observe-Execute sequence was a less critical test of the mirror-system hypothesis, in that the magnitude of repetition suppression during the execution trials could potentially be offset by motor priming effects associated with performing a previously seen action.
Figure 1. Experimental Design of the fMRI Adaptation Paradigm in Experiment 1.
(A) In the “Execute-Observe” runs, blocks comprised a series of “Execute” trials followed by an equivalent number of “Observe” trials. “Execute” trials required participants to commence a cued action at the onset of a central fixation dot. “Observe” trials required participants to passively observe a presented action. The observed actions were either the same as those previously executed (as illustrated), or different from them.
(B) The “Observe-Execute” runs reversed the order of “Execute” and “Observe” trials.
(A) In the “Execute-Observe” runs, blocks comprised a series of “Execute” trials followed by an equivalent number of “Observe” trials. “Execute” trials required participants to commence a cued action at the onset of a central fixation dot. “Observe” trials required participants to passively observe a presented action. The observed actions were either the same as those previously executed (as illustrated), or different from them.
(B) The “Observe-Execute” runs reversed the order of “Execute” and “Observe” trials.
Stimuli comprised 60 pantomimed hand actions (e.g., shooting a gun, hammering a nail, bouncing a ball), each of which lasted one second and was associated with a two-word cue (e.g., “shoot gun”). Across two preliminary behavioral sessions, participants were trained to recognize and execute the entire set of actions to ceiling performance. During scanning, participants' motor responses were monitored online by an experimenter positioned out of sight by the scanner bore. Participants made errors in performing the learned actions in less than 1% of trials. A repeated-measures analysis of variance on the factors of “Block Type” (Novel, Repeated) and “Sequence” (Execute-Observe, Observe-Execute) revealed no significant difference in error rates between conditions [Block Type, F (1,16) = 0.495, p = 0.492; Sequence F (1,16) = 0.033, p = 0.859; Block Type × Sequence F (1,16) = 0.018, p = 0.896].
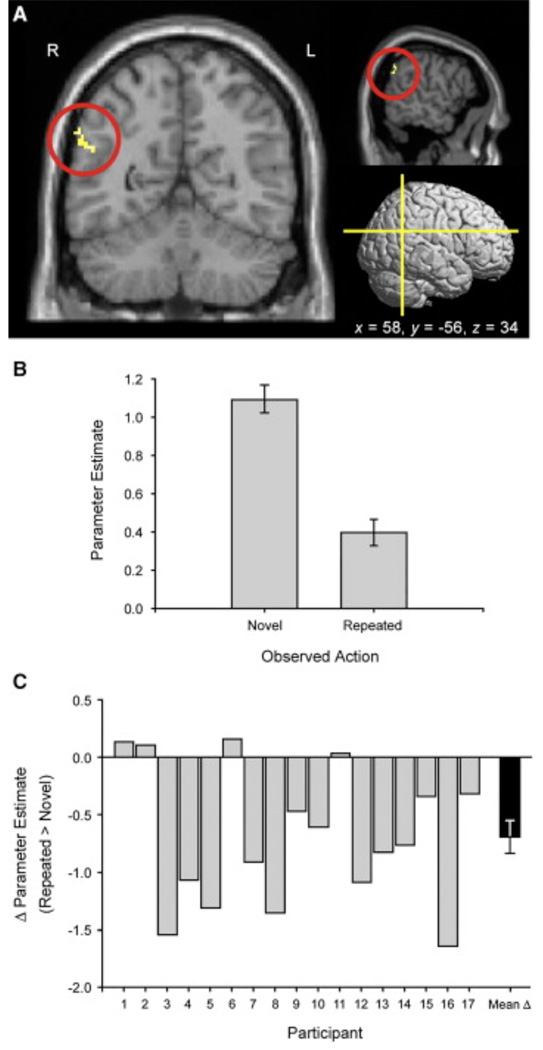
In the Execute-Observe sequence, the critical contrast was between the neural response in action observation areas when participants observed a Novel action and the response in the same region to a Repeated action. To reduce the number of multiple comparisons across voxels, we restricted our analyses a priori to the three principal areas implicated in action observation and the human mirror system (namely, the IFG, IPL, and STS). We measured activity within these anatomically defined regions of interest (ROIs) at a voxel-wise threshold of p < 0.001 and cluster-threshold of p < 0.05 to protect against false positives. Crucially, in this comparison, there was a distinct cluster within the right inferior parietal cortex that showed significantly lower activity when participants observed actions they had just executed (i.e., Repeated actions) than when they observed actions they had not previously executed (Novel actions) (MNI x, y, z: 58, −56, 34, k = 22, p < 0.001; Figure 2). This cluster was located in the posterior part of the supramarginal gyrus (SMG, BA 40), which is thought to be the human homolog of the macaque mirror area PF/PFG [19]. No further significant clusters were found in the IFG or STS, even with more specific ROIs based on the average coordinates of previous studies on the human mirror system [16]. Finally, we repeated our analyses at a whole-brain level. At an uncorrected voxel-wise threshold of p = 0.001, we found other cortical areas, in addition to the right IPL, that exhibited repetition suppression effects Because none of these additional areas has previously been identified in the context of the human mirror system, we conducted a further fMRI study (experiment 2 below) with the aim of testing the specificity of adaptation to the right IPL.
Figure 2. Locus of fMRI Adaptation within the Right IPL during the “Execute-Observe” Runs of Experiment 1.
(A) Coronal, sagittal, and rendered lateral views of the right IPL cluster, with its peak voxel at MNI 58, −56, 34, p < 0.001.
(B) Parameter estimates for the Observe Novel and Observe Repeated trials across the right IPL cluster.
(C) Magnitude of repetition suppression for the IPL cluster in single participants (gray bars) and across the group (black bar), expressed as the difference in parameter estimates between the observation of a Novel action and that of a Repeated action. Negative differences indicate significant repetition suppression. Error bars indicate ± 1 SEM. The data in (B) and (C) are derived from the statistical test (shown in [A]) by which the right IPL cluster was selected.
(A) Coronal, sagittal, and rendered lateral views of the right IPL cluster, with its peak voxel at MNI 58, −56, 34, p < 0.001.
(B) Parameter estimates for the Observe Novel and Observe Repeated trials across the right IPL cluster.
(C) Magnitude of repetition suppression for the IPL cluster in single participants (gray bars) and across the group (black bar), expressed as the difference in parameter estimates between the observation of a Novel action and that of a Repeated action. Negative differences indicate significant repetition suppression. Error bars indicate ± 1 SEM. The data in (B) and (C) are derived from the statistical test (shown in [A]) by which the right IPL cluster was selected.
Prior to conducting experiment 2, we compared neural activity during the execution of Novel versus Repeated actions for the reverse Observe-Execute sequence of experiment 1. This analysis revealed no significant adaptation effects. The finding of fMRI adaptation within the right SMG for the Execute-Observe sequence, but not for the reverse sequence of Observe-Execute, is perhaps not surprising, given the role of the SMG in motor planning. Previous investigations have found that the SMG is active during motor preparation [20]. Moreover, the SMG is significantly more active when participants execute a prepared movement relative to one that is not prepared [21]. In the Observe-Execute sequence, our analysis revealed increased activity in motor preparation areas during the initial observation period in each block suggesting that participants were preparing (either overtly or covertly) the motor plans for the observed actions should they be cued for later execution. Any neural suppression that might have resulted from performing the Repeated actions would therefore have been offset by augmented activity from having prepared those actions. This situation would not have occurred in the Execute-Observe sequence, in which participants executed their motor responses without having previously observed the repeated actions in each block.
To test the specificity of IPL adaptation in the Execute-Observe sequence, we repeated our analyses with a fresh set of data from a new experimental paradigm. Replicating our initial finding was especially important given the failure of a recent study to detect adaptation effects across the observation and execution of action [16]. In experiment 2, we presented couplets of trials that comprised a single action to be executed followed by a single action to be observed (Figure 3A). To reduce the number of multiple comparisons, we used the right IPL cluster of experiment 1 as a completely independent ROI for experiment 2. We then compared the activity in the Observe Novel relative to the Observe Repeated condition within this cluster. Importantly, as suggested by the findings from experiment 1, neural activity within the right IPL was suppressed when the observed action was identical to a previously executed action [t(8) = 2.97, p < 0.01; Figures 3B and 3C]. To verify the specificity of this effect, we conducted further ROI analyses on the additional regions that had shown repetition suppression in the whole-brain analysis of experiment 1 (at p < 0.001 uncorrected). Critically, we did not observe an adaptation effect in any of these clusters, with the sole exception of the right IPL. This analysis allows us to conclude that the adaptation effect in the right IPL is robust, replicable, and specific to this region.
Figure 3. Experimental Design of the fMRI Adaptation Paradigm and Results for Experiment 2.
(A) The event-related design consisted of sparsely presented pairs of “Execute” and “Observe” trials. The actions presented in the “Observe” trials were either identical to (as illustrated) or different from the previously executed action.
(B) Magnitude of fMRI adaptation across the right IPL cluster defined in experiment 1, in single participants (gray bars) and across the group (black bar), expressed as the difference in parameter estimates between the observation of a Novel action and that of a Repeated action. Negative differences indicate significant adaptation.
(C) Time course of the adaptation effect, shown as a plot of percent signal change against time. Error bars indicate ± 1 SEM. The data in (B) and (C) are independent of the data used to select these voxels and hence provide independent confirmation of the effect shown in Figure 2A.
(A) The event-related design consisted of sparsely presented pairs of “Execute” and “Observe” trials. The actions presented in the “Observe” trials were either identical to (as illustrated) or different from the previously executed action.
(B) Magnitude of fMRI adaptation across the right IPL cluster defined in experiment 1, in single participants (gray bars) and across the group (black bar), expressed as the difference in parameter estimates between the observation of a Novel action and that of a Repeated action. Negative differences indicate significant adaptation.
(C) Time course of the adaptation effect, shown as a plot of percent signal change against time. Error bars indicate ± 1 SEM. The data in (B) and (C) are independent of the data used to select these voxels and hence provide independent confirmation of the effect shown in Figure 2A.
Taken together, the findings from the Execute-Observe tasks in experiments 1 and 2 support the hypothesis of dynamic coupling between perception and action in the right IPL. In particular, the right SMG appears to demonstrate the properties of neural specificity for particular actions and neural abstraction across action and perception. In humans, action-observation studies have shown that the IPL has a gross somatotopic organization, as evident during the observation of mouth, foot, and hand actions [22]. Such findings suggest there is some level of effector specificity within the parietal lobe during action observation. Our findings go beyond these earlier studies by showing that areas within the IPL are also selective for specific actions performed by a single effector, regardless of whether the actions are observed or executed.
Our use of an fMRI adaptation paradigm also allows us to demonstrate the selectivity and invariance of action representations within the IPL at a subvoxel resolution [13] and [23]. Recent investigations have shown that adaptation within single neurons exhibits a greater degree of stimulus selectivity than adaptation at the level of BOLD responses [9]. However, these studies have also concluded that fMRI adaptation across a pair of stimuli implies that these stimuli are likely to excite the same neurons [9] and [23]. In conjunction with cytoarchitectonic studies that suggest homologies between BA 40 and macaque mirror area PF/PFG [19], our data represent strong evidence in favor of the right IPL as a candidate human mirror area. It is worth noting that the IPL adaptation we observed persisted regardless of whether the repeated action was presented within one second (experiment 2) or several seconds (experiment 1) of the initial execution of that action. This outcome implies that action representations in the IPL are temporally robust, at least across the timescales examined in our tasks.
Functionally, the multimodal representations encoded in the IPL could underlie the ability of humans to recognize and imitate the actions and gestures of others. A prominent theory of action recognition is the “Direct-Matching Hypothesis,” which states that an action is understood after it undergoes a process of covert motoric simulation in the observer [24]. The highly specific and modality-independent representations of actions in the right IPL make this area an ideal neural substrate for action recognition. In macaques, a subset of IPL mirror neurons that encode a specific action (e.g., grasping) are sensitive to the purpose of that action (e.g., grasping to place versus grasping to eat) [5]. This suggests a role for IPL mirror neurons in extracting the intentions of others. Furthermore, in humans, many neuroimaging studies have implicated the IPL in action observation [17], [22], [25], [26] and [27] and imitation [28], [29] and [30], and lesions to the inferior parietal cortex have long been known to cause apraxia—an impairment in the ability to recognize or imitate actions in the absence of elementary sensorimotor deficits [31].
The absence of an adaptation effect within the STS is consistent with single-cell data from nonhuman primates, which have thus far failed to identify mirror neurons in this region. Interestingly, however, the focus of adaptation in the right IPL is more ventral than parietal areas typically reported in studies on the human mirror system (such as the anterior intraparietal sulcus, aIPS) [14], [16] and [17]. For example, in a recent study by Dinstein and colleagues, activity in the aIPS was attenuated for actions that were repeatedly observed or repeatedly executed (as might be expected of an area containing mirror neurons) [16]. No such “within-modality” adaptation was found in the IPL cluster described in the present study. Furthermore, Dinstein and colleagues failed to demonstrate adaptation across the observation and execution of action in any cortical area [16], but this may have been because the authors used just three different action types (“rock,” “paper,” and “scissors”), whereas we used 60 different actions overall. The use of a larger stimulus set in our study should have maximized the BOLD response on the initial execution of each new action, thus leading to greater potential for adaptation upon passive observation of the same action.
In conclusion, the mirror-neuron system has received much interest in recent years because of its putative involvement in a range of important cognitive processes, from action understanding [5], observational learning [32] and imitation [33], to socialization [34], theory of mind [35], and empathy [36]. Moreover, dysfunction of the mirror system has been linked with such clinical disorders as apraxia [31], autism [37], and schizophrenia [38]. The link between mirror neurons and this diverse range of cognitive processes and clinical disorders is based on a fundamental hypothesis that there exists a mechanism in the human brain that is capable of encoding observed and executed actions in a common neural format. Until now, however, evidence for such a mechanism has been lacking. Here, we show that the right IPL encodes specific actions, regardless of whether they are executed or passively observed, thus demonstrating for the first time in humans the key properties of mirror neurons as they were first described in the macaque [1] and [2]. A greater understanding of the connectivity and response properties of the right IPL in humans should lead to better models of the mirror system and its teleology.
Supplementary Material
Acknowledgments
The authors wish to thank the scanning staff at St. Vincent's Hospital, Melbourne, for use of the MRI facilities, and Christina Triantafyllou at the Martinos Center for Biomedical Imaging at MIT for assistance with optimizing scan acquisition parameters. This research was supported by the National Health and Medical Research Council (NHMRC) of Australia; the Howard Florey Institute; a University of Melbourne Grant to J.M., R.C., and M.W.; and grant EY13455 to N.K. T.C., R.C., M.W., and J.M. were supported by the NHMRC.
References
- 1.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 2.Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Exp. Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 3.Dinstein I, Thomas C, Behrmann M, Heeger DJ. A mirror up to nature. Curr. Biol. 2008;18:R13–R18. doi: 10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 5.Fogassi L, Ferrari P, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 6.Decety J, Grezes J. Neural mechanisms subserving the perception of human actions. Trends Cogn. Sci. 1999;3:172–178. doi: 10.1016/s1364-6613(99)01312-1. [DOI] [PubMed] [Google Scholar]
- 7.Krekelberg B, Boynton GM, van Wezel RJA. Adaptation: From single cells to BOLD signals. Trends Neurosci. 2006;29:250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Sawamura H, Orban G, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: A single-cell study of the fMRI adaptation paradigm. Neuron. 2006;49:307–318. doi: 10.1016/j.neuron.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Engel SA, Furmanski CA. Selective adaptation to color contrast in human primary visual cortex. J. Neurosci. 2001;21:3949–3954. doi: 10.1523/JNEUROSCI.21-11-03949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kourtzi Z, Kanwisher N. Cortical regions involved in perceiving object shape. J. Neurosci. 2000;20:3310–3318. doi: 10.1523/JNEUROSCI.20-09-03310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- 13.Grill-Spector K, Malach R. fMR-adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychol. (Amst.) 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 14.Shmuelof L, Zohary E. Dissociation between ventral and dorsal fMRI activation during object and action recognition. Neuron. 2005;47:457–470. doi: 10.1016/j.neuron.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 15.Kable JW, Chatterjee A. Specificity of action representations in the lateral occipitotemporal cortex. J. Cogn. Neurosci. 2006;18:1498–1517. doi: 10.1162/jocn.2006.18.9.1498. [DOI] [PubMed] [Google Scholar]
- 16.Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. J. Neurophysiol. 2007;98:1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. J. Neurosci. 2006;26:1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 19.Von Economo C. The cytoarchitectonics of the human cerebral cortex. London: Oxford University Press; 1929. [Google Scholar]
- 20.Krams M, Rushworth MFS, Deiber M-P, Frackowiack RSJ, Passingham RE. The preparation, execution and suppression of copied movements in the human brain. Exp. Brain Res. 1998;120:386–398. doi: 10.1007/s002210050412. [DOI] [PubMed] [Google Scholar]
- 21.Deiber M-P, Ibanez V, Sadato N, Hallet M. Cerebral structures participating in motor preparation in humans: A positron emission tomography study. J. Neurophysiol. 1996;75:233–247. doi: 10.1152/jn.1996.75.1.233. [DOI] [PubMed] [Google Scholar]
- 22.Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz R, Zilles K, Rizzolatti G, Freund H. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- 23.Grill-Spector K. Selectivity of adaptation in single units: Implications for fMRI experiments. Neuron. 2006;49:170–171. doi: 10.1016/j.neuron.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 25.Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro C, Rizzolatti G. Neural circuits involved in the recognition of actions performed by nonconspecifics: An fMRI study. J. Cogn. Neurosci. 2004;16:114–126. doi: 10.1162/089892904322755601. [DOI] [PubMed] [Google Scholar]
- 26.Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Hum. Brain Mapp. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong TT-J, Williams MA, Cunnington R, Mattingley JB. Selective attention modulates inferior frontal gyrus activity during action observation. Neuroimage. 2008;40:298–307. doi: 10.1016/j.neuroimage.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Peigneux P, Van der Linden M, Garraux G, Laureys S, Degueldre C, Aerts J, Del Fiore G, Moonen G, Luxen A, Salmon E. Imaging a cognitive model of apraxia: The neural substrate of gesture-specific cognitive processes. Hum. Brain Mapp. 2004;21:119–142. doi: 10.1002/hbm.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka S, Inui T, Iwaki S, Konishi J, Nakai T. Neural substrates involved in imitating finger configurations: An fMRI study. Neuroreport. 2001;12:1171–1174. doi: 10.1097/00001756-200105080-00024. [DOI] [PubMed] [Google Scholar]
- 30.Chaminade T, Meltzoff AN, Decety J. An fMRI study of imitation: Action representation and body schema. Neuropsychologia. 2005;43:115–127. doi: 10.1016/j.neuropsychologia.2004.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldenberg G, Karnath H-O. The neural basis of imitation is body part specific. J. Neurosci. 2006;26:6282–6287. doi: 10.1523/JNEUROSCI.0638-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrosini L, Graziano A, Mandolesi L, Neri P, Molinari M, Leggio MG. Watch how to do it! New advances in learning by observation. Brain Res. Brain Res. Rev. 2003;42:252–264. doi: 10.1016/s0165-0173(03)00176-0. [DOI] [PubMed] [Google Scholar]
- 33.Iacoboni M. Understanding others: Imitation, language, and empathy. In: Hurley S, Chater N, editors. Perspectives on Imitation: From Neuroscience to Social Science. Volume 1. Cambridge, MA: MIT Press; 2005. pp. 77–99. [Google Scholar]
- 34.Oberman LM, Pineda JA, Ramachandran VS. The human mirror neuron system: A link between action observation and social skills. Social Cognitive and Affective Neuroscience. 2007;2:62–66. doi: 10.1093/scan/nsl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallese V. The ‘Shared Manifold’ Hypothesis: From mirror neurons to empathy. J. Conscious. Stud. 2001;8:33–50. [Google Scholar]
- 37.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 38.Arbib MA, Mundhenk TN. Schizophrenia and the mirror system: An essay. Neuropsychologia. 2005;43:268–280. doi: 10.1016/j.neuropsychologia.2004.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.