Abstract
Nonalcoholic fatty liver disease (NAFLD) is one of the commonest causes of chronic liver disease in the United States, and represents several overlapping clinicopathological states, ranging from simple steatosis to nonalcoholic steatohepatitis (NASH). Although dysregulated lipid accumulation occurs across the spectrum of NAFLD, features of liver cell injury such as hepatocyte ballooning, cytoskeletal changes (Mallory-Denk bodies) and hepatocyte apoptosis occur predominantly in NASH, and distinguish NASH from simple steatosis. Indeed, NASH is a more serious form of liver damage, as cirrhosis and / or hepatocellular carcinoma are potential outcomes of NASH, but rarely occurs in individuals with simple steatosis. Hepatic injury and apoptosis that occur in adults is often dysregulated and is accompanied by the accumulation of immune cells, which produce cytokines and growth factors that drives chronic inflammation and may result in fibrosis. The purpose of this review is to summarize the process of apoptosis and roles of putative cytokines in progressive NAFLD.
Keywords: apoptosis, cytokines, inflammation, fibrosis, nonalcoholic steatohepatitis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is now the leading cause of chronic liver disease in the United States (1) . It is closely associated with the metabolic syndrome, which is a constellation of insulin resistance, central obesity, hypertension and dyslipidemia (2). Histologically, NAFLD may range from simple steatosis to steatohepatitis and cirrhosis (3, 4). Individuals with simple steatosis rarely develop significant disease, whereas, nearly 20% of those with nonalcoholic steatohepatitis (NASH) progress to end-stage liver disease (5, 6). Evidence that cirrhosis and hepatocellular carcinoma are more likely to develop in individuals with NASH rather than simple steatosis, suggests that NASH is a more serious form of liver injury (5, 7, 8).
The ‘two-hit’ hypothesis is a widely accepted paradigm to explain the progression of NAFLD, from simple steatosis (fatty liver) to NASH (8). The first hit involves dysregulated hepatic lipid accumulation (steatosis). Second hit(s) include oxidative, metabolic and cytokine stresses that overwhelm hepatocyte survival mechanisms, leading to hepatocyte cell death (apoptosis). Indeed, NASH differs from simple steatosis, mainly, in the degree of hepatocyte injury and apoptosis (9, 10). We have previously proposed that hepatocyte apoptosis is the critical ‘third-hit’ that drives the progression from NASH to cirrhosis (11). Hepatocyte apoptosis triggers regenerative mechanisms to replace dead hepatocytes (12). However, aberrant responses may occur in some individuals, resulting in the activation of hepatic stellate cells (HSC) to myofibroblasts and the hepatic recruitment of pro-inflammatory, pro-fibrogenic immune cells.
In this review, we will discuss the role of apoptosis and impact of putative cytokines in the progression of NAFLD.
Apoptosis
Programmed cell death or apoptosis, is a vital component of normal cellular turnover, and development. It is an ATP-dependent process, characterized by cell shrinkage, chromatin condensation (pyknosis), membrane blebbing and budding (13, 14). When appropriately regulated, the process of apoptosis and/or clearance of apoptotic bodies is limited to specific cells, and is not associated with an inflammatory reaction (15-17). In contrast, apoptosis occurring in adult tissues in response to noxious insults is typically dysregulated, prolonged (18), and inflammatory in nature. Adding to the insult, it may ultimately promote fibrosis (19-21).
Apoptosis is mediated by either the extrinsic (death receptor) pathway or intrinsic (mitochondrial) organelle-based pathway (22). Both pathways converge on a similar execution pathway, which is initiated by the cleavage of caspase-3 (14, 23). Activation of caspases occurs through the cleavage of aspartate residues and requires caspase activity. This proteolytic cascade amplifies the apoptotic signaling pathway and leads to rapid cell death.
In the liver, apoptosis is typically triggered by ligation of surface death receptors (24), including Fas (CD95), tumor-necrosis factor (TNF) receptor 1, and tumor necrosis factor-related apoptosis-inducing ligand receptors 1 and 2 (TRAIL-R1 and -R2) (24, 25). Expression of Fas/CD95 is enhanced in patients with viral hepatitis, alcoholic hepatitis, chronic biliary disease and acute liver failure (26). The binding of ligand to its cognate receptor results in the recruitment of cytoplasmic adaptor molecules, Fas-associated protein with death domain (FADD) and TNFRSF1A-associated via death domain (TRADD), and the subsequent activation of caspase-8 (27-29). Caspase-8, in turn, activates caspase-3, committing the cell to the final, common pathway of apoptosis (14). This pathway was demonstrated when mice that were administered anti-Fas antibodies went on to develop massive hepatocyte apoptosis and die from fulminant hepatic failure (30).
Apoptosis and Inflammation
The link between apoptosis and inflammation was demonstrated in skin and peritoneal experiments as mice injected subcutaneously with anti-Fas antibody developed a robust local inflammatory infiltrate (31), and inoculation of Fas-L expressing tumor cells into the murine peritoneal cavity resulted in an interleukin (IL) - 1β-mediated neutrophilic infiltration (32).
Relevant to the liver, inflammation is the critical stage in the progression from steatosis to steatohepatitis (33). The number of inflammatory cells is minimal in simple steatosis, but is significantly up-regulated in individuals with steatohepatitis (34, 35). This increase in inflammatory infiltrate is mirrored by the degree and extent of hepatocyte apoptosis (9, 36). Supporting this, recent studies have shown that hepatocyte apoptosis may directly or indirectly promote inflammation (37-40). Infection with Listeria monocytogenes triggered hepatocyte apoptosis and release of neutrophil chemoattractants (41). Subsequent work demonstrated that MIP2 and IL8 regulate hepatic neutrophil infiltration (42). The use of cathepsin B knock-out mice and pharmacological inhibitors by Canbay et al. demonstrated that apoptosis induced by bile-duct ligation is associated with the production of pro-inflammatory chemokines, CXCL1 and MIP2 (43). Similar observations were noted with experiments using Fas-L agonists (39, 44). The inflammatory infiltrate was composed predominantly of neutrophils; immune recruitment was mediated largely by CXCL1. When investigators inhibited apoptosis using the caspase inhibitor, zDEVD-fmk, they noted a corresponding reduction in CXCL1 and MIP2 production, as well as in the severity of hepatic inflammation.
Ligation of TNF-R1/CD120a triggers nuclear factor κB (NF-κB) activation, up-regulation of pro-inflammatory cytokines and adhesion molecules (25). In the galactosamine/endotoxin shock model, TNF-α mediated, caspase-3 activation, triggered parenchymal cell apoptosis and neutrophil transmigration (38, 45), while supplementation with the caspase-inhibitor abrogated cellular apoptosis, neutrophil transmigration and neutrophil-related injury. These studies lend support to the concept that cellular apoptosis is a signal for inflammatory cell recruitment (38).
Tissue inflammation may similarly ensue during the clearance of apoptotic bodies (15). Apoptotic bodies are typically rapidly phagocytosed by neighboring cells with the process being non-inflammatory, a hallmark of physiological apoptosis (16). In contrast, the engulfment of apoptotic bodies by monocytes or kupffer cells (liver resident macrophages) during liver injury is associated with the up-regulation of the death ligands, CD95, TRAIL and TNF-α (46-48). Complementing this, the depletion of kupffer cells by gadolinium-chloride is associated with impaired apoptotic body engulfment, reduced expression of the chemoattractant, MIP2 and amelioration of liver injury (49).
Experimental evidence for this ‘apoptosis – inflammation’ axis is supported by findings of hepatocyte apoptosis in patients with alcoholic steatohepatitis (50) and NASH (9, 51, 52). In both, hepatocyte apoptosis correlates strongly with clinical and histological disease severity. Additionally, the co-localization of apoptotic hepatocytes with polymorphonuclear cells suggests an apoptosis-dependent immune cell recruitment (53).
Apoptosis and Fibrosis
The nexus between apoptosis and fibrosis was initially explored by Canbay and co-workers (20). Mice treated with bile-duct ligation (BDL) to induce chronic liver injury and fibrosis expressed increased amounts of α-SMA, TGF-β, collagen α 1(I) and TIMP1, compared to sham-operated and Fas-deficient (lpr) mice (54). Phagocytosis of apoptotic bodies by macrophages stimulated the production of TGF-β, a key pro-fibrogenic cytokine (47, 49), whereas treatment with gadolinium chloride to induce macrophage depletion reduced amounts of TGF-β, collagen α 1 (I) and α-SMA after BDL. In a similar fashion, engulfment of apoptotic bodies by primary or immortalized HSC was shown to trigger their own activation and promote fibrosis (48). Both processes could be inhibited pharmacologically with antagonists to PI3-K (LY294002) and p38 MAPK (SB203580). Phagocytosis of apoptotic bodies by HSC was confirmed by recent in vivo data, in 3 different models of liver fibrosis (19). The engulfment of apoptotic bodies triggered NADPH-oxidase activation and superoxide production. In turn, these reactive oxygen species stimulated further apoptosis and enhanced fibrosis (55, 56).
Further evidence for a link between apoptosis and fibrosis is derived from caspase inhibition studies in animals. The administration of the pan-caspase inhibitor, IDN-6556, to rodents subjected to BDL resulted in the attenuation of hepatocyte apoptosis, injury, inflammation and fibrosis (57). Similar observations were recorded in animals treated with an antagonist to Cathepsin B (R-3032) (43).
Apoptosis in NAFLD
Hepatic steatosis occurs as a result of abnormal lipid handling by the liver (58-61) which sensitizes the liver to injury and inflammation (8). Obese ob/ob mice harbor a homozygous mutation in the leptin gene, and are unable to synthesize leptin (62). They develop spontaneous hepatic steatosis, and when injected with anti-Fas antibody, exhibit massive liver injury (63). Similarly, mice fed the carbohydrate diet for 8 weeks develop macrovesicular steatosis and up-regulate expression of the death receptor, Fas (64). Treatment with Jo2 (anti-Fas antibody) enhanced hepatocyte apoptosis, hepatic injury, chemokine production (CXCL1 and MIP2), and infiltration of neutrophils. HepG2 cells cultured in the presence of free fatty acids also developed cellular steatosis, up-regulated Fas expression and were vulnerable to the Fas-L.
NASH, a more advanced lesion than simple steatosis, is characterized by increased hepatocyte injury and apoptosis (9). The same is true in alcoholic steatohepatitis (ASH) (50, 52,65). Livers obtained from individuals with ASH and NASH show enhanced caspase-3 and -7 activation, as well as Fas and TNF-R1 expression. Using immunohistochemical approaches, Ribeiro and co-workers noted that individuals with NASH up-regulated NF-κB expression, a transcription factor that promotes the expression of pro-inflammatory cytokines, death receptors and death ligands such as TNF-α (66, 67). When compared to normal individuals, those with NASH had higher serum levels of TNF-α (68-70). However, studies using the TNFR1 knock-out mice indicated that TNF-α was not always critical for the development of NASH (71-73). Rather, other molecules signaling through the TNF-R superfamily could be involved. For example, livers from patients with excessive alcohol intake show greater induction of TRAIL. When exposed to free fatty acids, hepatocyte-derived cell lines up-regulate TRAIL-receptors (74).
Mice fed the methionine-choline deficient (MCD) diet are commonly used in the study of NASH as they exhibit histologic similarities to human disease (75-77). 8-weeks of MCD treatment result in increased hepatocyte apoptosis by TUNEL-staining and active-caspase-3 assays (Witek, RP et al. Manuscript in submission), with the onset of apoptosis commensurate to the development of steatohepatitis (75). In the latter study, the investigators noted a sustained up-regulation of hepatic p53 tumor suppressor gene. p53 activation was directly associated with Bcl-XL suppression, Bid cleavage, caspase-3 activation and p21 induction. Interestingly, p53 is also known to regulate TRAIL-R expression, and its expression is enhanced in patients with NASH (78) and in obese ob/ob mice (79).
Oxidative stress is one of the second hits believed to mediate the progression to NASH (8, 33). When the amount of ROS overwhelms buffering capacity, DNA mutations, peroxidation of membranes and generation of additional free radicals can occur (80). At low levels, ROS may activate NF-κB to induce synthesis of pro-inflammatory cytokines and death receptor expression (81, 82). In a recent study, rats fed the Lieber-DeCarli high-fat diet (71% of energy from fat) for 6 weeks expressed increased rates of hepatocyte apoptosis that mirrored necro-inflammatory changes and oxidative stress (83). The authors noted higher phosphorylated JNK and Bax (pro-apoptotic protein) compared to controls. JNK activation has been shown to regulate cellular apoptosis (83-85), possibly through the regulation of the Bcl-2 family. In addition, JNK1 has been shown to promote the development of murine NASH (77).
The identification of apoptosis as a critical mediator of inflammation and fibrosis in liver disease is important as it allows the design of future drug therapy and development of noninvasive biomarkers (Figure 1). In this respect, we observed a significant reduction in hepatic fibrosis when genetically obese, diabetic db/db mice were treated with a pan-caspase inhibitor (Witek, RP et al. Manuscript in submission), while Feldstein and colleagues measured serum cytokeratin-18 fragments (a caspase-3-cleavage product) in human subjects and demonstrated a strong correlation with histological severity (10).
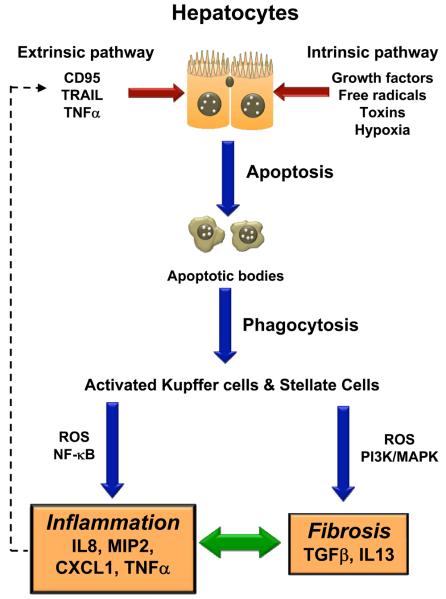
Figure 1. Hepatocyte Apoptosis during liver injury leads to Inflammation and Fibrosis.
During liver injury, ligation of surface death receptors by FasL, TNFα or TRAIL (extrinsic apoptosis pathway), promotes hepatocyte apoptosis, through recruitment of cytoplasmic adaptor molecules and activation of caspase-8. Caspases amplify the apoptotic signaling cascade, and commits the hepatocyte to the final execution (apoptosis) pathway (activated caspase-3). The organelle-based (intrinsic apoptosis pathway), non-receptor mediated apoptosis-pathway involves various stimuli (growth factors, cytokines, free radicals, hypoxia, viral infections and toxins) that disrupt the mitochondrial permeability transition pore, and loss of the mitochondrial transmembrane potential. These changes lead to the release of pro-apoptotic proteins, which initiate the mitochondrial-apoptosis pathway. Clearance of apoptotic bodies occurs through phagocytosis by resident macrophages (Kupffer cells) and hepatic stellate cells. Engulfment of apoptotic bodies is associated with increased ROS production and activation of transcription factors, NF-κB. In turn, this leads to enhanced production of pro-inflammatory cytokines (TNFα) and chemokines (MIP2, IL8). TNFα may further drive the extrinsic apoptosis pathway through a feed-forward, paracrine loop. Phagocytosis of apoptotic bodies also induces the production of the pro-fibrogenic cytokines, TGFβ and IL13, via the PI3K and MAPK pathways.
Cytokines in Steatohepatitis
In the recent decades, investigators have defined the critical roles of pro-inflammatory cytokines in the pathogenesis of ASH(50, 86). It was noted that patients with severe ASH exhibited high serum levels of TNF-α (87-89), which correlated with clinical severity. Similar cytokine changes were observed in animal models of alcoholic injury (90, 91). Given that NASH and ASH share common histopathologic features, it is conceivable that similar immunopathogenic mechanisms may be involved in the development of NASH (86).
Tumor-necrosis factor (TNF) -α
TNF-α impairs insulin action in vitro and in vivo (92-95) and individuals with insulin resistance show higher serum levels of TNF-α. Administration of TNF-α to individuals also results in impaired insulin sensitivity (96). The mechanisms responsible for TNF-α effects appear to be related to the sustained activation of inflammatory kinases, such as Jun-N-terminal kinase (JNK) and inhibitor of K-kinase β (IKKβ) (97). JNK activation inhibits the phosphorylation of insulin receptor substrate (IRS)-1 (98, 99) while IKKβ activity leads to the activation of NF-κB and the induction of additional pro-inflammatory cytokines (100). Conversely, neutralization of TNF-α improved hepatic insulin resistance in ob/ob mice through reductions in JNK and IKKβ activities (101, 102). Similarly, probiotic therapy reduced injury and inflammation in ob/ob mice, likely via the down-regulation of JNK and IKKβ. TNF-α also modulates the expression of sterol regulatory element binding proteins (SREBP), transcription factors involved in regulating enzymes of lipid synthesis (103). Levels of SREBP-1c are elevated in ob/ob mice (104). Exogenous TNF-α promotes the expression of SREBP-1c (105) while neutralizing antibodies to TNF-α decreases expression of SREBP-1c.
TNF-α expression is up-regulated in obesity (106) and serum TNF-α levels are increased in patients with NASH (68). Gene expression in adipose tissue and liver are similarly enhanced in NASH, and correlated with the stage of disease (107). More recently, TNF-α polymorphisms have also been noted in individuals with NAFLD compared to the control population (108, 109). Indeed, treatment with metformin and pentoxifylline, drugs which antagonize TNF-α, improve NASH (110, 111). Similar changes in serum and tissue TNF-α levels are observed in animal models of obesity (112) and NASH (113). Moreover, mice genetically deficient in TNF-R1 are resistant to NASH by the MCD and high-carbohydrate diets (71, 73). Specifically, TNF-R deficient mice exhibit reduced kupffer cell activation and fibrogenesis, suggesting a role of TNF-α in modulating HSC activation (102, 114). More recent work by Yamaguchi et al, however, highlighted the possibility that TNF-α alone may be insufficient in the development of fibrosis, as treatment of obese and diabetic db/db mice with diacylglycerol acyltransferase 1 (DGAT1) antisense oligonucleotides resulted in worse fibrosis despite reductions in the amount of steatosis and TNF-α levels (115).
The effects of TNF-α may lie, in part, with its biological relationship with adiponectin, an adipose-tissue derived protein. ob/ob mice have low levels of adiponectin compared with TNF-α (116) and the injection of adiponectin to ob/ob mice reverses NASH and TNF-α levels. Similar changes were observed in KK-Ay mice, another model of NAFLD (117, 118). Individuals with NASH have lower levels of plasma adiponectin compared with controls (119, 120). Importantly, circulating adiponectin levels may inversely correlate with hepatic inflammation (107, 121), while, weight reduction has been shown to increase the ratio of adiponectin to TNF-α and improve NASH (122, 123).
Leptin and Th1 / Th2 cytokines in NASH
Leptin is a highly conserved cytokine-like hormone secreted not only by the adipose tissue, but also activated T cells (124). Leptin binds to the leptin receptor (Ob-R) that stimulates the Janus-kinase signal transduction and activator of transcription (JAK-STAT) signaling pathways (125).
Leptin receptors are found on immune cells and leptin has been shown to modulate T cell responses and viability (126, 127). Obese ob/ob mice are genetically deficient in leptin (128) and spontaneously develop features of the metabolic syndrome and hepatic steatosis. They also develop thymic atrophy and exhibit changes in neurohumoral factors (129) that lead to the selective reduction in hepatic NKT cells (130). Restoration of norepinephrine levels in ob/ob mice reduced NKT cell apoptosis and increased NKT cell numbers (131). NKT cells are critical modulators of the innate and adaptive immune response, and produce both pro-inflammatory (Th1) cytokine (IFN-γ) and anti-inflammatory, pro-fibrogenic (Th2) cytokines (IL4, IL13) (132). Livers from ob/ob mice show significant reductions in IL4 compared with IFN-γ (Th1 polarization) (130). This may explain their relative resistance to fibrosis despite persistent chronic liver injury. The pro-Th1 milieu would also account for their sensitivity to endotoxin-mediated (lipopolysaccharide) hepatotoxicity (113), one of the putative second hits in the progression of NAFLD. When ob/ob mice are corrected for leptin deficiency, they reduce weight, develop less hepatic inflammation but develop fibrosis (133-135), exhibiting features seen in individuals with progressive NASH. In addition to NKT cell numbers, restoration of leptin levels could promote fibrogenesis through increases in TGF-β secretion by macrophages (135, 136). Similarly, ob/ob mice supplemented with norepinephrine develop less injury and lower amounts of pro-inflammatory cytokines, but express increased TGF-β expression, HSC activation and fibrosis (137). Collectively, the current data suggests that the balance of Th1 and Th2 cytokines in the microenvironment may determine disease outcome.
As hepatic NKT cells are a predominant source of Th2 cytokines, IL4 and IL13, depletion of NKT numbers would imply a dearth of pro-fibrogenic factors. NKT cells accumulate in chronic viral hepatitis (138-140), primary biliary cirrhosis (141, 142) and Wilson's disease (143). Indeed, hepatic and circulating NKT cells from individuals with chronic viral hepatitis show enhanced IL4 and IL13 production (138). IL13 has been shown to activate hepatic stellate cells via IL13-Ra2 (144) and activate macrophages via the alternative pathway (145). In the TNBS model of chronic colitis, IL13 signaling has been found to initiate a cascade of pro-fibrogenic events that involve TGF-β activation and myofibroblast production of collagen (146); conversely, antagonism of IL13 signaling ameliorated murine schistosomiasis hepatic fibrosis (147). Recent work in our laboratory have shown that wild-type mice with intact leptin signaling possess greater number of NKT cells and exhibit greater fibrosis when treated with the MCD diet for 8 weeks, and αGalCer-activated NKT cells promote hepatic stellate cell activation in vitro (unpublished). Explanted livers from patients with NASH cirrhosis also contain up to 4-fold more NKT cells than normal human livers (unpublished). Further studies will be needed to determine if NKT-associated cytokines such as IL4 and IL13 regulate NASH progression. The identification of such cytokines could potentially provide novel targets for NASH therapy (Table 1).
Table 1.
Summary of cytokines in NAFLD
| Th1 Pro- inflammatory |
Th2 Pro- Fibrogenic |
Major source(s) of cytokines and other known function(s) |
|
|---|---|---|---|
| IL4 | − | + | S: Th2 lymphocytes, NKT F: Promotes Th2 differentiation |
| IL10 | − | − / + | S: Monocytes, Th2 lymphocytes, (Foxp3) Treg, NKT F: Down-regulates Th1 cytokine expression Suppresses antigen-presentation |
| IL13 | − | + | S: Th2 lymphocytes, NKT F: Induces secretion of TGF-β Alternative macrophage activation Allergic inflammation, IgE secretion |
| IFN γ | + | − | S: Th1 lymphocytes, NK and NKT F: Promotes Th1 and suppresses Th2 differentiation Classical macrophage activation Anti-viral and Anti-tumor activity |
| TNF α | + | − | S: macrophage, lymphoid and other tissues F: inflammatory, apoptotic activity, cell survival, proliferation and differentiation via NF-κB, MAPK and caspase activity |
| Leptin | + | + | S: adipose tissue, liver, brain, muscle, T cells F: regulate energy balance T cell survival and response (Th1 vs Th2 cytokines) |
| TGF β | − | + | S: (Foxp3) Treg, immune cells, stellate and epithelial cells F: apoptosis, cell cycle regulation, angiogenesis Suppress lymphocyte activation (immunosuppression) |
+: pro; -: anti; S: source; F: function
Summary
NASH develops in a subgroup of individuals with NAFLD, and differs from simple steatosis with regard to the degree of hepatocyte injury and apoptosis. Hepatocyte apoptosis results in the release of factors that promote the recruitment of inflammatory cells and trigger the deposition of type 1 collagen by hepatic myofibroblasts. Studies have shown that the degree of hepatocyte apoptosis may be assessed by serum measurements of cytokeratin-18 fragments (a caspase-3-cleavage product) in human subjects, and the use of caspase inhibitors may ameliorate the amount of fibrosis in vivo. NASH is also characterized by high levels of pro-inflammatory cytokines such as TNF-α, which promotes hepatic insulin resistance and drives the progression from simple steatosis to NASH. TNF-α may activate downstream kinases that induce further cytokine production in a feed-forward loop, while attenuating the expression and activity of adiponectin. In aggregate, the balance of Th1 (IFN γ) and Th2 (IL4, IL13) cytokines in the microenvironment may play a critical role in shaping disease outcomes.
Acknowledgments
Funding: RO1 DK077794 and RO1 DK053792 to Anna Mae Diehl
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 3.Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126:137–145. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 5.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 7.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–378. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 9.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 10.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 11.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 16.Kurosaka K, Takahashi M, Watanabe N, Kobayashi Y. Silent cleanup of very early apoptotic cells by macrophages. J Immunol. 2003;171:4672–4679. doi: 10.4049/jimmunol.171.9.4672. [DOI] [PubMed] [Google Scholar]
- 17.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 19.Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–443. doi: 10.1002/hep.21093. [DOI] [PubMed] [Google Scholar]
- 20.Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- 21.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 22.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 23.Thornberry NA. Caspases: key mediators of apoptosis. Chem Biol. 1998;5:R97–103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- 24.Faubion WA, Gores GJ. Death receptors in liver biology and pathobiology. Hepatology. 1999;29:1–4. doi: 10.1002/hep.510290101. [DOI] [PubMed] [Google Scholar]
- 25.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 26.Galle PR, Krammer PH. CD95-induced apoptosis in human liver disease. Semin Liver Dis. 1998;18:141–151. doi: 10.1055/s-2007-1007150. [DOI] [PubMed] [Google Scholar]
- 27.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 28.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 29.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. Embo J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 31.Biancone L, Martino AD, Orlandi V, Conaldi PG, Toniolo A, Camussi G. Development of inflammatory angiogenesis by local stimulation of Fas in vivo. J Exp Med. 1997;186:147–152. doi: 10.1084/jem.186.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 33.Syn WK, Teaberry V, Choi SS, Diehl AM. Similarities and differences in the pathogenesis of alcoholic and nonalcoholic steatohepatitis. Semin Liver Dis. 2009;29:200–210. doi: 10.1055/s-0029-1214375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lalor PF, Faint J, Aarbodem Y, Hubscher SG, Adams DH. The role of cytokines and chemokines in the development of steatohepatitis. Semin Liver Dis. 2007;27:173–193. doi: 10.1055/s-2007-979470. [DOI] [PubMed] [Google Scholar]
- 35.Hubscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450–465. doi: 10.1111/j.1365-2559.2006.02416.x. [DOI] [PubMed] [Google Scholar]
- 36.Jaeschke H. Inflammation in response to hepatocellular apoptosis. Hepatology. 2002;35:964–966. doi: 10.1053/jhep.2002.0350964. [DOI] [PubMed] [Google Scholar]
- 37.Maher JJ, Scott MK, Saito JM, Burton MC. Adenovirus-mediated expression of cytokine-induced neutrophil chemoattractant in rat liver induces a neutrophilic hepatitis. Hepatology. 1997;25:624–630. doi: 10.1002/hep.510250322. [DOI] [PubMed] [Google Scholar]
- 38.Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Parenchymal cell apoptosis as a signal for sinusoidal sequestration and transendothelial migration of neutrophils in murine models of endotoxin and Fas-antibody-induced liver injury. Hepatology. 1998;28:761–767. doi: 10.1002/hep.510280324. [DOI] [PubMed] [Google Scholar]
- 39.Faouzi S, Burckhardt BE, Hanson JC, Campe CB, Schrum LW, Rippe RA, Maher JJ. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem. 2001;276:49077–49082. doi: 10.1074/jbc.M109791200. [DOI] [PubMed] [Google Scholar]
- 40.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 41.Rogers HW, Callery MP, Deck B, Unanue ER. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 42.Ebe Y, Hasegawa G, Takatsuka H, Umezu H, Mitsuyama M, Arakawa M, Mukaida N, et al. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol Int. 1999;49:519–532. doi: 10.1046/j.1440-1827.1999.00910.x. [DOI] [PubMed] [Google Scholar]
- 43.Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, Taniai M, et al. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112:152–159. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–1717. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 45.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- 46.Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC, Liles WC. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol. 1997;159:1594–1598. [PubMed] [Google Scholar]
- 47.Geske FJ, Monks J, Lehman L, Fadok VA. The role of the macrophage in apoptosis: hunter, gatherer, and regulator. Int J Hematol. 2002;76:16–26. doi: 10.1007/BF02982714. [DOI] [PubMed] [Google Scholar]
- 48.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 49.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 50.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 51.Ziol M, Tepper M, Lohez M, Arcangeli G, Ganne N, Christidis C, Trinchet JC, et al. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol. 2001;34:254–260. doi: 10.1016/s0168-8278(00)00047-7. [DOI] [PubMed] [Google Scholar]
- 52.Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–253. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 53.Jaeschke H. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol. 2002;27:23–27. doi: 10.1016/s0741-8329(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 54.Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Trevijano ER, Iraburu MJ, Fontana L, Dominguez-Rosales JA, Auster A, Covarrubias-Pinedo A, Rojkind M. Transforming growth factor beta1 induces the expression of alpha1(I) procollagen mRNA by a hydrogen peroxide-C/EBPbeta-dependent mechanism in rat hepatic stellate cells. Hepatology. 1999;29:960–970. doi: 10.1002/hep.510290346. [DOI] [PubMed] [Google Scholar]
- 56.Nieto N, Friedman SL, Greenwel P, Cederbaum AI. CYP2E1-mediated oxidative stress induces collagen type I expression in rat hepatic stellate cells. Hepatology. 1999;30:987–996. doi: 10.1002/hep.510300433. [DOI] [PubMed] [Google Scholar]
- 57.Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- 58.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 59.Charlton M, Sreekumar R, Rasmussen D, Lindor K, Nair KS. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 60.Miele L, Grieco A, Armuzzi A, Candelli M, Forgione A, Gasbarrini A, Gasbarrini G. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am J Gastroenterol. 2003;98:2335–2336. doi: 10.1111/j.1572-0241.2003.07725.x. [DOI] [PubMed] [Google Scholar]
- 61.Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Prog Lipid Res. 2009;48:1–26. doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Friedman JM, Leibel RL, Siegel DS, Walsh J, Bahary N. Molecular mapping of the mouse ob mutation. Genomics. 1991;11:1054–1062. doi: 10.1016/0888-7543(91)90032-a. [DOI] [PubMed] [Google Scholar]
- 63.Siebler J, Schuchmann M, Strand S, Lehr HA, Neurath MF, Galle PR. Enhanced sensitivity to CD95-induced apoptosis in ob/ob mice. Dig Dis Sci. 2007;52:2396–2402. doi: 10.1007/s10620-006-9148-7. [DOI] [PubMed] [Google Scholar]
- 64.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 65.Ribeiro PS, Cortez-Pinto H, Sola S, Castro RE, Ramalho RM, Baptista A, Moura MC, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 67.Green DR. Overview: apoptotic signaling pathways in the immune system. Immunol Rev. 2003;193:5–9. doi: 10.1034/j.1600-065x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 68.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, et al. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006;26:39–45. doi: 10.1111/j.1478-3231.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 70.Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303–306. doi: 10.1136/gut.2003.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dela Pena A, Leclercq I, Field J, George J, Jones B, Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–1674. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Memon RA, Grunfeld C, Feingold KR. TNF-alpha is not the cause of fatty liver disease in obese diabetic mice. Nat Med. 2001;7:2–3. doi: 10.1038/83316. [DOI] [PubMed] [Google Scholar]
- 73.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 74.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farrell GC, Larter CZ, Hou JY, Zhang RH, Yeh MM, Williams J, dela Pena A, et al. Apoptosis in experimental NASH is associated with p53 activation and TRAIL receptor expression. J Gastroenterol Hepatol. 2009;24:443–452. doi: 10.1111/j.1440-1746.2009.05785.x. [DOI] [PubMed] [Google Scholar]
- 76.Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J Gastroenterol Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- 77.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 78.Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:6198–6202. doi: 10.3748/wjg.v12.i38.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yahagi N, Shimano H, Matsuzaka T, Sekiya M, Najima Y, Okazaki S, Okazaki H, et al. p53 involvement in the pathogenesis of fatty liver disease. J Biol Chem. 2004;279:20571–20575. doi: 10.1074/jbc.M400884200. [DOI] [PubMed] [Google Scholar]
- 80.Mansouri A, Fromenty B, Berson A, Robin MA, Grimbert S, Beaugrand M, Erlinger S, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96–102. doi: 10.1016/s0168-8278(97)80286-3. [DOI] [PubMed] [Google Scholar]
- 81.Cao Q, Mak KM, Lieber CS. Cytochrome P4502E1 primes macrophages to increase TNF-alpha production in response to lipopolysaccharide. Am J Physiol Gastrointest Liver Physiol. 2005;289:G95–107. doi: 10.1152/ajpgi.00383.2004. [DOI] [PubMed] [Google Scholar]
- 82.Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54:3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Ausman LM, Russell RM, Greenberg AS, Wang XD. Increased apoptosis in high-fat diet-induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J Nutr. 2008;138:1866–1871. doi: 10.1093/jn/138.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 85.Marderstein EL, Bucher B, Guo Z, Feng X, Reid K, Geller DA. Protection of rat hepatocytes from apoptosis by inhibition of c-Jun N-terminal kinase. Surgery. 2003;134:280–284. doi: 10.1067/msy.2003.237. [DOI] [PubMed] [Google Scholar]
- 86.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 87.Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917–920. doi: 10.7326/0003-4819-112-12-917. [DOI] [PubMed] [Google Scholar]
- 88.McClain CJ, Barve S, Barve S, Deaciuc I, Hill DB. Tumor necrosis factor and alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:248S–252S. doi: 10.1097/00000374-199805001-00006. [DOI] [PubMed] [Google Scholar]
- 89.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 90.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 91.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 92.Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169–175. [PubMed] [Google Scholar]
- 93.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 94.Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- 95.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 96.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- 97.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 99.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 100.Steinberg GR. Inflammation in obesity is the common link between defects in fatty acid metabolism and insulin resistance. Cell Cycle. 2007;6:888–894. doi: 10.4161/cc.6.8.4135. [DOI] [PubMed] [Google Scholar]
- 101.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 102.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 103.Weber LW, Boll M, Stampfl A. Maintaining cholesterol homeostasis: sterol regulatory element-binding proteins. World J Gastroenterol. 2004;10:3081–3087. doi: 10.3748/wjg.v10.i21.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 105.Endo M, Masaki T, Seike M, Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c) Exp Biol Med (Maywood) 2007;232:614–621. [PubMed] [Google Scholar]
- 106.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, Fernandez-Escalante JC, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 108.Valenti L, Fracanzani AL, Dongiovanni P, Santorelli G, Branchi A, Taioli E, Fiorelli G, et al. Tumor necrosis factor alpha promoter polymorphisms and insulin resistance in nonalcoholic fatty liver disease. Gastroenterology. 2002;122:274–280. doi: 10.1053/gast.2002.31065. [DOI] [PubMed] [Google Scholar]
- 109.Tokushige K, Takakura M, Tsuchiya-Matsushita N, Taniai M, Hashimoto E, Shiratori K. Influence of TNF gene polymorphisms in Japanese patients with NASH and simple steatosis. J Hepatol. 2007;46:1104–1110. doi: 10.1016/j.jhep.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 110.Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–2368. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 111.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 112.Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89–104. doi: 10.1055/s-2001-12932. [DOI] [PubMed] [Google Scholar]
- 113.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008;103:1036–1042. doi: 10.1111/j.1572-0241.2007.01709.x. [DOI] [PubMed] [Google Scholar]
- 115.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, et al. Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis. Hepatology. 2008;47:625–635. doi: 10.1002/hep.21988. [DOI] [PubMed] [Google Scholar]
- 116.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 118.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 119.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 120.Musso G, Gambino R, Biroli G, Carello M, Faga E, Pacini G, De Michieli F, et al. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438–2446. doi: 10.1111/j.1572-0241.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 121.Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 122.Gabriely I, Barzilai N. Surgical removal of visceral adipose tissue: effects on insulin action. Curr Diab Rep. 2003;3:201–206. doi: 10.1007/s11892-003-0064-3. [DOI] [PubMed] [Google Scholar]
- 123.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. Jama. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 124.Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, Matarese G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoteit MA, Anania FA. Treatment of fibrosis in nonalcoholic fatty liver disease. Curr Gastroenterol Rep. 2007;9:47–53. doi: 10.1007/s11894-008-0020-0. [DOI] [PubMed] [Google Scholar]
- 126.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 127.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, Fantuzzi G. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci U S A. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 129.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guebre-Xabier M, Yang S, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- 131.Li Z, Oben JA, Yang S, Lin H, Stafford EA, Soloski MJ, Thomas SA, et al. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434–441. doi: 10.1002/hep.20320. [DOI] [PubMed] [Google Scholar]
- 132.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 133.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ, Lang T, et al. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399–1410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- 135.Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–213. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 136.Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, et al. Kupffer Cells Mediate Leptin-Induced Liver Fibrosis. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Commins SP, Marsh DJ, Thomas SA, Watson PM, Padgett MA, Palmiter R, Gettys TW. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology. 1999;140:4772–4778. doi: 10.1210/endo.140.10.7043. [DOI] [PubMed] [Google Scholar]
- 138.de Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, Monno A, Nuti S, et al. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol. 2004;173:1417–1425. doi: 10.4049/jimmunol.173.2.1417. [DOI] [PubMed] [Google Scholar]
- 139.Nuti S, Rosa D, Valiante NM, Saletti G, Caratozzolo M, Dellabona P, Barnaba V, et al. Dynamics of intra-hepatic lymphocytes in chronic hepatitis C: enrichment for Valpha24+ T cells and rapid elimination of effector cells by apoptosis. Eur J Immunol. 1998;28:3448–3455. doi: 10.1002/(SICI)1521-4141(199811)28:11<3448::AID-IMMU3448>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 140.Durante-Mangoni E, Wang R, Shaulov A, He Q, Nasser I, Afdhal N, Koziel MJ, et al. Hepatic CD1d expression in hepatitis C virus infection and recognition by resident proinflammatory CD1d-reactive T cells. J Immunol. 2004;173:2159–2166. doi: 10.4049/jimmunol.173.3.2159. [DOI] [PubMed] [Google Scholar]
- 141.Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 142.Harada K, Isse K, Tsuneyama K, Ohta H, Nakanuma Y. Accumulating CD57 + CD3 + natural killer T cells are related to intrahepatic bile duct lesions in primary biliary cirrhosis. Liver Int. 2003;23:94–100. doi: 10.1034/j.1600-0676.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- 143.Kinebuchi M, Matsuura A, Ohya K, Abo W, Kitazawa J. Contribution of Va24Vb11 natural killer T cells in Wilsonian hepatitis. Clin Exp Immunol. 2005;139:144–151. doi: 10.1111/j.1365-2249.2005.02664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shimamura T, Fujisawa T, Husain SR, Kioi M, Nakajima A, Puri RK. Novel role of IL-13 in fibrosis induced by nonalcoholic steatohepatitis and its amelioration by IL-13R-directed cytotoxin in a rat model. J Immunol. 2008;181:4656–4665. doi: 10.4049/jimmunol.181.7.4656. [DOI] [PubMed] [Google Scholar]
- 145.Deepak P, Kumar S, Acharya A. Interleukin-13-induced type II polarization of inflammatory macrophages is mediated through suppression of nuclear factor-kappaB and preservation of IkappaBalpha in a T cell lymphoma. Clin Exp Immunol. 2007;149:378–386. doi: 10.1111/j.1365-2249.2007.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146.Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135:2003–2013. doi: 10.1053/j.gastro.2008.08.055. 2013 e2001-2007. [DOI] [PubMed] [Google Scholar]
- 147.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34:273–282. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]