Abstract
Cell death is mediated by distinct pathways including apoptosis and oncosis in response to various death signals. To characterize molecules involved in cell death, a panel of mAbs was raised by immunizing mice with apoptotic cells. One of these antibodies, designated anti-Porimin (for pro-oncosis receptor inducing membrane injury), was found to directly induce a unique type of cell death in Jurkat cells. Anti-Porimin defines a 110-kDa cell surface receptor on Jurkat cells. Functionally, anti-Porimin alone rapidly mediates pore formation on the plasma membrane and induces cell death without participation of complement. Both the cellular expression and functional characteristics of the Porimin antigen indicate that it is distinct from the CD95 (Fas/Apo-1) and other cell receptors known to induce apoptosis. Anti-Porimin-mediated cell death was preceded by cell aggregation, formation of plasma membrane pores, and the appearance of membrane blebs. More important, these cells show neither DNA fragmentation nor apoptotic bodies, but display lethal damage of the cell membrane. Cell death by anti-Porimin is distinct from complement-dependent cytolysis or complement-independent apoptosis but is similar to that described for oncosis, a form of cell death accompanied by the membrane damage followed by karyolysis. The induction of cell death by anti-Porimin may represent a unique cell surface receptor-mediated pathway of cell death in the human lymphoid system.
Programmed cell death is an essential process in normal development and the regulation of tissue homeostasis whereas a failure of the cell death machinery contributes to the possible pathogenesis of cancer or other diseases (1). One major focus of cell death research has been the characterization of the role of cell surface receptors and their ligands in mediating signals leading to the elimination of potentially harmful cells from the body. Two well characterized death receptors, the CD95 (Fas/Apo-1) and tumor necrosis factor receptor 1 (TNFR-1), are known to induce apoptosis in lymphoid cells upon binding to their ligands (2–4). These receptors, as well as several recently identified cell death receptors (5–10), have been shown to engage the apoptotic pathway through their interaction with a family of death domain-containing homologous proteins. After engagement of these receptors by specific antibody or their ligands, the death domain orchestrates the assembly of a signaling complex to recruit a series of proapoptotic proteases that cleave structural proteins and interfere with critical repair processes leading to cell death (11).
Cell death by apoptosis has been investigated extensively and shown to be a major mediator of programmed cell death. Nevertheless, other cell death mechanisms have been described during development (12, 13) and under experimental conditions that are distinct from necrosis, the pathological end result of a number of noxious stimuli (14–16). Relying on the morphologic appearance of dying cells, Majno and Joris (17) have found that oncosis and apoptosis define two distinct pathways of cell death. The term oncosis (from ónkos, meaning swelling) was initially coined to characterize ischemic cell death in osteocytes and recently reintroduced to define the cell death lacking apoptotic characteristics. In contrast to apoptosis with cellular shrinkage and nuclear disruption, oncosis is defined in the literature as a form of cell death characterized by cellular swelling, membrane blebbing, and an increase in the membrane permeability. Morphologically, cell death by oncosis leads to necrosis with karyolysis whereas apoptotic cell death leads to necrosis with karyorrhexis, a degenerative change or fragmentation of the cell nucleus (17).
In an effort to investigate the molecular mechanisms of programmed cell death in human T cells, we have characterized molecules involved in cell death by developing mAbs to dying cells. Anti-Porimin, one of these mAbs, efficiently induces a unique type of cell death in Jurkat cells. Cell death mediated by anti-Porimin is distinct from complement-dependent cytolysis or complement-independent apoptosis, but is very similar to the cell death pathway defined for oncosis. The molecular and biochemical characterization of this cell surface receptor and its possible role in the cell death pathways are described.
MATERIALS AND METHODS
Anti-Porimin mAb.
Monoclonal anti-Porimin was generated and characterized as described previously (18). Briefly, BALB/c mice were immunized with Jurkat cells that had been induced to undergo cell death by 1-β-d-arabinofuranosylcytosine. Splenocytes from the immunized mouse were fused with P3-X63-Ag8.653 cells. Anti-Porimin was screened and selected by its reactivity with cell surface antigen and its ability to induce cell death. The mAb was determined to be mouse IgM subclass by using commercial Ig-isotyping reagents (Amersham). Ascites for anti-Porimin was produced in mice, and the mAb was purified from the ascites fluid by using a IgM purification kit from Pierce.
Other Antibodies and Reagents.
Monoclonal anti-CD95 (IgM) (19), anti-CD6 (IgM), and mouse IgM (MsIgM) were used as controls in this study and were obtained from Coulter. Affinity-purified goat anti-mouse Ig-fluorescein isothiocyanate (FITC) conjugate for flow cytometry and goat anti-mouse Ig-peroxidase conjugate for immunoblotting assays were purchased from Jackson ImmunoResearch. Propidium iodide and all other chemicals were obtained from Sigma unless otherwise indicated.
Cell Culture and Induction of Cell Death by Anti-Porimin.
Jurkat cells and all other human cell lines used in this study were obtained from the American Type Culture Collection (Manassas, VA) and were maintained in RPMI 1640 medium (BioWhittaker) supplemented with 10% fetal bovine serum, and 2 mM l-glutamine, penicillin (50 units/ml), and streptomycin (50 μg/ml). For induction of cell death, cells were suspended in the culture medium at a density of 5 × 105 cells per ml and were incubated with anti-Porimin in an atmosphere of 5% CO2 at 37°C. For some experiments, the induction of cell death by anti-Porimin was performed in serum-free culture medium PFHM-II (GIBCO/BRL) or in PBS alone.
Cell Staining and Flow Cytometric Analysis.
To test the reactivity of anti-Porimin with Jurkat cells and other human cell types, cells were labeled for flow cytometry by indirect immunofluorescent staining and analyzed by using an Epics Elite flow cytometer (Coulter).
Quantification of Cell Death in Suspension Culture.
The dead cells in suspension culture after exposure to cytotoxic mAb were quantified by flow cytometry and by cell proliferation inhibition assays. For flow cytometry, cells (1 × 106 cells per sample) were washed once with PBS after induction of cell death by mAb and suspended in 1 ml of cold PBS containing propidium iodide at a concentration of 5 μg/ml. Cell death was assessed by determining the intracellular propidium iodide (red) fluorescence with a flow cytometer as described previously (14, 20).
For cell proliferation inhibition assay, Jurkat cells were cultured in 96-well microtiter plates and incubated in the absence or presence of anti-Porimin antibody. After pulsing with 1 μCi (1 μCi = 37 kBq) of [3H]thymidine (NEN) per well for 4 h, cells were harvested and [3H]thymidine incorporation was measured by a Wallac liquid scintillation counter. The reported results represent the means of triplicate determinations and were calculated as follows: cell proliferation inhibition (%) = [1 − (experimental value/medium background value)] × 100.
Immunoblot and Immunoprecipitation.
For immunoblotting assay, the preparation of cell lysate, SDS/PAGE, and protein transfer were done as described previously (18). After incubation with 3% BSA in PBS to block nonspecific binding sites, the nitrocellulose membrane was incubated with anti-Porimin followed by goat anti-mouse Ig-peroxidase conjugate. Immunoblots were visualized by enhanced chemiluminescence assay (Amersham).
For immunoprecipitation, cell surface labeling of Jurkat cells by biotinylation using biotin-7-NHS reagent (Boehringer Mannheim) was performed by a modification of a previously described method (21). Briefly, Jurkat cells (1 × 107 cells per ml) were incubated for 10 min with biotin-7-NHS at a concentration of 80 μg/ml. After two washes with PBS and incubation with 10% fetal bovine serum in PBS for 30 min at 4°C, the cells were washed with PBS and solubilized in 1% Triton X-100 lysis buffer (50 mM Tris⋅HCl, pH 7.6/140 mM NaCl/1 mM EDTA/1 mM phenylmethylsulfonyl fluoride/2 μg/ml aprotinin). Cell lysate was collected after centrifugation at 14,000 rpm for 15 min at 4°C. After preclearance by absorption with Sepharose 4B beads, cell lysates were incubated for 2 h with anti-Porimin that had been coated onto CNBr-activated Sepharose 4B beads (Boehringer Mannheim). Antigen–antibody complexes were pelleted by centrifugation, washed, and then resuspended in sample buffer. Immunoprecipitates were separated by SDS/PAGE and transferred onto a nitrocellulose membrane by electrophoresis. The membrane was blotted with streptavidin-peroxidase conjugate and visualized by chemiluminescence.
DNA Fragmentation Assay.
Jurkat cells were incubated at 37°C with mAb to induce cell death in suspension culture. At various periods of induction, cells were harvested for DNA extraction in which DNA fragmentation was detected by agarose gel electrophoresis as described previously (22).
Electron Microscopy.
To examine the surface structural changes of cells by scanning electron microscopy, Jurkat cells were treated with anti-Porimin or control antibodies for 1 h at 37°C and fixed in 1.5% glutaraldehyde in 0.1 M cacodylate buffer. The cells were dehydrated in an ascending series of alcohol followed by drying in a Ladd critical point dryer. The samples were coated with palladium coating system and examined by a JEOL JSM-35CF scanning electron microscope.
For transmission electron microscopy, cells were fixed by 1.25% glutaraldehyde in cacodylate buffer containing 1% CaCl2 and postfixed in 1% OsO4. After dehydration and embedding, the samples were sectioned, stained, and examined with a transmission electron microscope.
RESULTS
Anti-Porimin Rapidly Mediates Membrane Blebbing, Pore-Formation, and Increased Permeability of the Plasma Membrane Followed by Cell Death.
In an effort to characterize cell surface molecules involved in the cell death of human lymphoid cells, a panel of mAbs has been raised in the present study by immunizing mice with apoptotic Jurkat cells. One of these antibodies, designated anti-Porimin, was found to react with Jurkat cells and was shown to be cytotoxic for these cells. When Jurkat cells were incubated with anti-Porimin, the cells aggregated in suspension culture within minutes and underwent morphologic changes with damages to the plasma membrane, which was then followed by cell death. Anti-Porimin mediated cell death was complement-independent and was observed after incubation at either 37°C or room temperature.
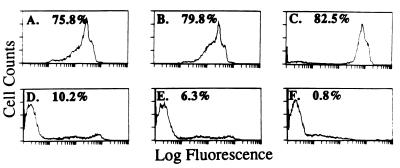
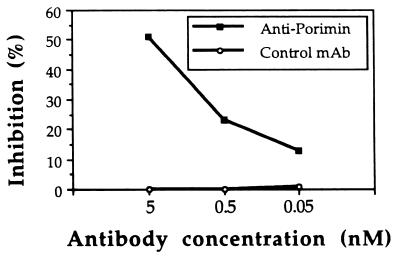
To assess cell death mediated by anti-Porimin, we used propidium iodide as a probe to identify dead cells by flow cytometry. Propidium iodide is a fluorescent compound that, under isotonic conditions, can only enter nonviable cells that lose plasma membrane integrity (14, 20). Thus, by flow cytometric analysis, nonviable cells can be identified as those bearing bright red fluorescence whereas viable cells lack the fluorescent staining. As shown in Fig. 1, anti-Porimin rapidly increased the permeability of Jurkat cells and more than 75% of the cells were stained strongly by propidium iodide as early as 20 min after treatment with anti-Porimin (Fig. 1 A–C). Under identical experimental conditions, fewer than 11% cells took up propidium iodide when incubated for 3 h with anti-CD95, an antibody known to induce apoptotic cell death in Jurkat cells (Fig. 1D). As expected, less than 7% of cells incubated in the presence of an isotype-matched control antibody or cultured in medium alone were stained by propidium iodide (Fig. 1 E and F). The ability of anti-Porimin to induce an increase in membrane permeability of Jurkat cells occurred in regular culture medium, protein-free culture medium, or PBS. Anti-Porimin-mediated cell death was preceded by cell aggregation, morphologic changes, and increased permeability of the plasma membrane. After incubation of cells with anti-Porimin for 18 h, more than 50% of cell proliferation activity was inhibited by anti-Porimin in nanomolar concentrations, as measured by [3H]thymidine incorporation assay (Fig. 2).
Figure 1.
Anti-Porimin causes an increase in the plasma membrane permeability followed by cell death in Jurkat cells. Jurkat cells were treated with anti-Porimin or control antibodies at 1 μg/ml in culture medium and were harvested at various incubation periods for analysis of cell death. After incubation of the cell with propidium iodide in PBS, the intracellular red fluorescence in nonviable cells was analyzed by flow cytometry. (A–C) Cells treated with anti-Porimin for 20 min, 1 h, and 3 h, respectively. (D–F) Cells treated for 3 h with anti-CD95, normal control anti-CD6, or in medium alone. The percentage of nonviable cells that lacked membrane integrity is marked on the profile.
Figure 2.
Inhibition of cell proliferation by anti-Porimin. Jurkat cells were cultured in triplicate samples in a 96-well microtiter plate containing 5 × 104 cells per well in a total of 200 μl of medium and incubated for 18 h with either anti-Porimin or normal control mAb at the indicated concentrations. Background controls were determined by incubation of cells in medium alone. The inhibition of cell proliferation was measured by [3H]thymidine incorporation as described in Materials and Methods.
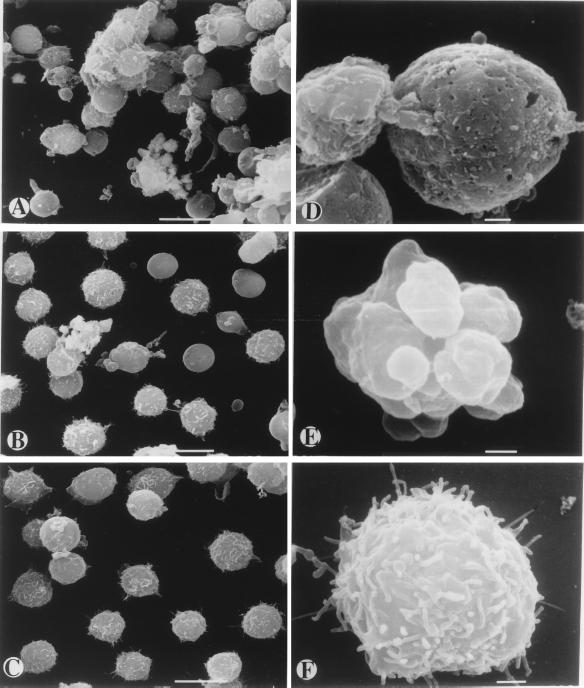
To further investigate the lethal effects of anti-Porimin antibody on the cell membrane, Jurkat cells were treated with the antibody for 1 h and examined for cell surface structural changes by scanning electron microscopy (Fig. 3). In the presence of anti-Porimin, Jurkat cells rapidly aggregated in clusters in suspension culture and morphologically displayed a loss of surface microvilli. The induction of cell aggregation by anti-Porimin occurred within minutes and lasted until the cells had died. More important, these cells were found to have cellular swelling followed by shrinkage, membrane blebs, and surface wrinkling or dents before cell death. Many pores were formed on the plasma membrane of those cells that were marked for the cell death (Fig. 3 A and D). In contrast, apoptotic cell death mediated by anti-CD95 was morphologically distinct and characterized by a decrease in cell size and evidence of membrane budding. Although the apoptotic cells also displayed a loss in surface microvilli, the integrity of the plasma membrane remained preserved under comparable conditions because no obvious membrane pores were observed on these cells (Fig. 3 B and E). As expected, cells treated with normal control antibody preserved uniform morphology and normal cell surface structures (Fig. 3 C and F).
Figure 3.
Scanning electron micrographs of cell death mediated by anti-Porimin in Jurkat cells. Jurkat cells were incubated for 1 h in the presence of anti-Porimin or control mAbs and prepared for scanning electron microscopy. Cells treated with anti-Porimin (A and D), apoptotic antibody anti-CD95 (B and E), or the isotype-matched control antibody anti-CD6 (C and F) were examined by scanning electron microscopy at ×2,000 (A–C) (bars = 10 μm) or ×11,000 original magnifications (D–F) (bars = 1 μm).
Anti-Porimin Identifies a 110-kDa Cell Surface Receptor on Jurkat Cells.
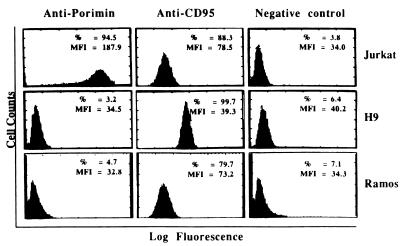
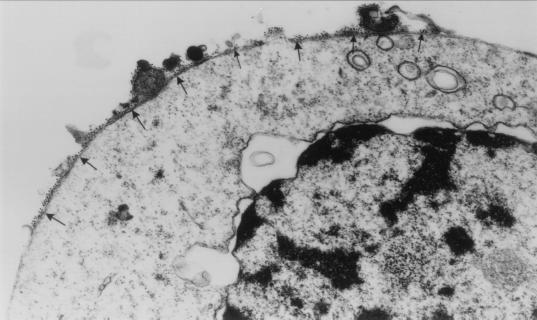
The reactivity of anti-Porimin with Jurkat cells was defined initially by cell proliferation inhibition and by flow cytometry. Fig. 4 represents flow cytometric profiles of the Porimin expression on Jurkat cells and other human cells tested, in comparison with the expression of CD95 antigen. Almost 95% of Jurkat cells were found to express the Porimin antigen on the cell surface, and the mean fluorescence intensity (MFI) was always very high. All six subclones of Jurkat cells obtained from the American Type Culture Collection were shown to express this cell surface receptor, and all could be induced to undergo cell death by anti-Porimin. In contrast, human peripheral blood lymphocytes and other hematopoietic cell lines, including H9 (T cell line), CEM (T cell line), Ramos (B cell line), and the erythroleukemia line K562 cells, showed little reactivity. CD95, on the other hand, could be readily identified on activated peripheral blood lymphocytes and a number of malignant lymphoid cell lines including Jurkat, H9, and Ramos (Fig. 4). As determined by transmission electron microscopy, the antigen defined by anti-Porimin was localized onto the surface of the plasma membrane in Jurkat cells (Fig. 5).
Figure 4.
The cellular expression of Porimin antigen on Jurkat cells. Cells were stained with anti-Porimin or control mAbs followed by goat anti-mouse Ig-FITC conjugate, and analyzed by flow cytometry. The percentage of positive cells (%) and the mean fluorescence intensity (MFI) of cells are indicated in each profile.
Figure 5.
Localization of the Porimin antigen by immunoelectron microscopy. Jurkat cells were labeled with anti-Porimin followed by goat anti-mouse Ig conjugated to 10-nm gold particles and examined by transmission electron microscopy (×35,000 original magnification). The gold particles are localized onto the surface of plasma membrane (arrows) of the cell.
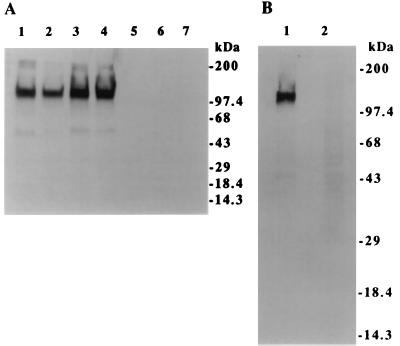
Molecular characteristics of the Porimin antigen were determined by immunoblots and by immunoprecipitation with lysates of Jurkat cells (Fig. 6). By immunoblot analysis, anti-Porimin detected a specific protein band with a molecular mass of approximately 110 kDa in the cell lysates prepared from all subclones of Jurkat cells tested, including E6–1, JA1, J. Cam1.6, and J45.01 (Fig. 6A, lanes 1–4). The 110-kDa protein band could not be detected in those cells that did not express the cell surface antigen (Fig. 6A, lanes 5–7).
Figure 6.
Anti-Porimin defines a 110-kDa antigen in the lysate of Jurkat cells by immunoblot (A) or immunoprecipitation assay (B). For immunoblotting assay, cell lysates from Jurkat subclones E6–1, JA1, J. Cam1.6, and J45.01 (lanes 1–4) or from H9 (lane 5), CEM (lane 6), or K562 cells (lane 7) were blotted with anti-Porimin and visualized by chemiluminescence assay. The relative migration of protein standards is indicated on the right. For immunoprecipitation assay, Jurkat cells were biotinylated and then lysed in a lysis buffer. Immunoprecipitates from the cell lysates by anti-Porimin (lane 1) or control antibody MsIgM (lane 2) were analyzed by Western blotting assay by using streptavidin-peroxidase conjugate.
These findings were confirmed further by immunoprecipitation studies in which Jurkat cells were labeled with biotin before the preparation of cell lysates (Fig. 6B). Immunoprecipitates from the cell lysates by mAbs were separated by SDS/PAGE and transferred onto a nitrocellulose membrane, which was blotted with Streptavidin-peroxidase conjugate and visualized by chemiluminescence assay. Anti-Porimin precipitated a single protein band with a molecular mass of approximately 110 kDa from Jurkat cell lysate (Fig. 6B, lane 1). As expected, no protein band was observed in the precipitates by the negative control antibody (Fig. 6B, lane 2).
Cell Death Mediated by Anti-Porimin Antibody Exhibits Neither DNA Fragmentation nor Apoptotic Bodies, but Displays Lethal Damages to the Cell Membrane.
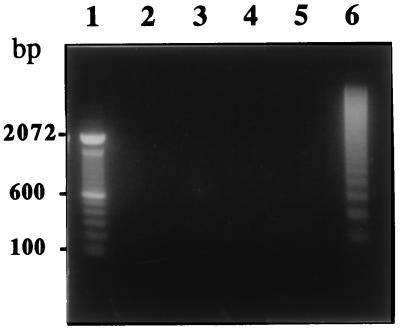
The internucleosomal cleavage of chromatin into DNA fragments is a common feature of apoptosis and serves as a characteristic marker for apoptotic cell death. To test whether cell death by anti-Porimin induces the degradation of internucleosomal DNA, Jurkat cells were incubated with anti-Porimin at 37°C for various times and analyzed for DNA fragmentation by agarose gel electrophoresis (Fig. 7). DNA extracted from anti-Porimin-treated Jurkat cells showed no evidence of DNA fragmentation even when these cells were treated with the antibody for 18 h, at which time there was pronounced cell death (lanes 2–4). Under comparable conditions, cells treated with anti-CD95 for only 2 h clearly exhibited a ladder pattern of DNA fragments by agarose gel electrophoresis (lane 6). These findings are in keeping with both transmission and scanning electron microscopy (Fig. 3 and data not shown), where the main feature of cell death induced by anti-Porimin was destruction of the plasma membrane and the dilatation of mitochondria. Nuclear damage including karyolysis represented a late event in the anti-Porimin-mediated cell death of Jurkat cells. In contrast, apoptotic Jurkat cells by anti-CD95 displayed cytoplasmic condensation, nuclear disruption, and the formation of apoptotic bodies.
Figure 7.
No DNA fragmentation is detected in the cell death mediated by anti-Porimin. After the treatment of Jurkat cells with anti-Porimin or control mAbs for various periods of time as indicated, DNA was extracted from these cells and analyzed on a 1.4% agarose gel, with DNA equivalent to 2 × 106 cells per lane. The DNA samples were prepared from cells pretreated with anti-Porimin for 2, 9, and 18 h (lanes 2–4, respectively), or with nonapoptotic anti-CD6 for 18 h (lane 5) or apoptotic anti-CD95 for 2 h (lane 6). The relative mobility of a 100-bp DNA ladder is shown as molecular weight standards (lane 1).
DISCUSSION
We describe a mAb, designated anti-Porimin, that defines a 110-kDa cell surface receptor (Porimin) on human T leukemia Jurkat cells and that rapidly induces these cells to undergo a unique form of cell death. The induction of cell death by anti-Porimin was preceded by cell aggregation, morphologic changes, pore formation in the plasma membrane, and the appearance of membrane blebs. Anti-Porimin-mediated cell death showed no evidence of DNA fragmentation or formation of apoptotic bodies but did, however, rapidly cause plasma membrane injury followed by cell death. The nature of the anti-Porimin cytotoxicity was complement-independent and distinct from apoptosis. The induction of cell death by anti-Porimin may represent a unique cell surface receptor-mediated pathway for cell death in human lymphoid cells.
Apoptosis and oncosis represent two distinct pathways leading to cell death. During normal development and in the process of homeostasis of multicellular organisms, large numbers of cells die by programmed cell death through apoptosis pathway. Apoptotic cell death usually displays a decrease in cell size, chromatin condensation, DNA fragmentation, membrane budding, and formation of apoptotic bodies. On the other hand, cell death by mechanisms other than apoptosis often is referred to as necrosis or accidental cell death. Unfortunately, the term necrosis does not define a mode of cell death but rather it refers to morphologic changes secondary to cell death induced by many mechanisms, including apoptosis. Recently, Majno and Joris (17) and others (16, 23) redefined the concept of cell death and proposed the term oncosis to describe a form of cell death distinct from apoptosis. The term oncosis, derived from ónkos, meaning swelling, initially was used to define ischemic cell death with swelling in osteogenic cells. In contrast with apoptosis, oncosis currently is defined as a form of cell death accompanied by cellular swelling, membrane blebbing, and increased membrane permeability, whereas DNA fragmentation is shown in a nonspecific fashion in oncosis. Cell death by oncosis may result in necrosis with karyolysis whereas apoptotic cell death leads to necrosis with karyorrhexis and cell shrinkage (17, 24). Cell death mediated by anti-Porimin in the present study displays most, if not all, characteristic features of oncosis but not those described for apoptosis. Anti-Porimin defined a 110-kDa cell surface receptor on Jurkat cells and rapidly mediated the formation of membrane pores followed by cell death. Porimin-mediated cell death, however, showed no evidence of DNA fragmentation. Furthermore, the cellular expression and biochemical characteristics of the Porimin antigen showed that it is distinct from CD95 and its ligand CD95L (2, 3, 25, 26), as well as other cell surface receptors or ligands known to induce apoptosis including DR3 (also known as Apo-3/WSL-1/TRAMP) (5, 6, 27, 28), DR4 (7), DR5 and its decoy receptors (8–10, 29), and TRAIL (also called Apo2L), the ligand for both DR4 and DR5 (30, 31). Our results also indicate that anti-Porimin triggers cell death in a manner distinct from that which occurs in apoptosis but consistent with that described in oncosis. Whether this antibody-defined cell surface receptor is unique to the Jurkat cell or can be found in other cell types undergoing oncosis requires further study.
Studies on the mechanisms of apoptotic cell death have been greatly stimulated by the recent identification of several cell death receptors and signaling pathways triggered by these receptors. During apoptosis, a cysteine protease cascade is activated by signaling through the death receptor–ligand interaction, and the activated proteases in turn cleave various cellular components leading to morphologic changes characteristic of apoptosis (11). In contrast, the molecular and biochemical mechanisms underlying oncosis are unknown although some believe that oncosis is a result of a failure of the ionic pumps in the plasma membrane and decreased levels of cellular ATP (17, 24). Whether cell death by anti-Porimin requires the activation of proteases and whether the antibody has any effect on the ionic events of the plasma membrane remain to be characterized. Nevertheless, several studies have documented that a number of cells die in a manner consistent with oncosis, and the current state of knowledge of oncosis is evolving rapidly. Cell death by oncosis has been found in human macrophages infected with virulent Shigella flexneri (15), in murine B16 melanoma cells treated in vivo with cyclophosphamide (32), and in ocular diseases (33). In addition, several reports have suggested that the cell death of intersegmental muscles in the moth (13), in human peripheral blood lymphocytes treated with a high dose of staphylococcal toxin (14), or in antibody RE2-mediated cell death of murine cells was not characteristic of apoptosis (34). Cell death mediated by a high dose of Staphylococcal toxin or by antibody RE2 displayed lethal damages to the cell membranes but showed no evidence of DNA fragmentation in target cells (14, 34). As shown in this study, cell death mediated by anti-Porimin displays many common features described in oncosis. Still to be defined is the nature of the structures identified by anti-Porimin and whether or not these disparate mediators of oncosis participate in a conserved biochemical pathway that mediates cell death.
In conclusion, our results indicate that anti-Porimin defines a cell surface receptor mediating cell death by oncosis. Thus, anti-Porimin may be useful as a cell surface marker to define cell subsets or as an oncosis-inducing reagent to generate cell death distinct from apoptosis. Determination of the molecular structure of Porimin antigen may provide a clue as to its functional role in the induction of cell death. Finally, characterization of the molecular and biochemical events in the Porimin-mediated cell death certainly will contribute to a general understanding of cell death by oncosis.
Acknowledgments
We thank Herbert Levine for assistance in flow cytometric analysis and Aruna Seth for helpful discussion. This work was supported by Grant AI 12069 from the National Institutes of Health.
ABBREVIATIONS
- Porimin
pro-oncosis receptor-inducing membrane injury
- MFI
mean fluorescence intensity
References
- 1.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Yonehara S, Ishii A, Yonehara M. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trauth B C, Klas C, Peters A M J, Matzku S, Möller P, Falk W, Debatin K-M, Krammer P H. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 4.Tartaglia L A, Rothe M, Hu Y-F, Goeddel D V. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- 5.Chinnaiyan A M, O’Rourke K, Yu G-L, Lyons R H, Garg M, Duan D R, Xing L, Gentz R, Ni J, Dixit V M. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 6.Kitson J, Raven T, Jiang Y-P, Goeddel D V, Giles K M, Pun K-T, Grinham C J, Brown R, Farrow S N. Nature (London) 1996;384:372–375. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 7.Pan G, O’Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 8.Pan G, Ni J, Wei Y-F, Yu G-L, Gentz R, Dixit V M. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan J P, Marsters S A, Pitti R M, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray C L, Baker K, Wood W I, et al. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 10.Walczak H, Degli-Esposti M A, Johnson R S, Smolak P J, Waugh J Y, Boiani N, Timour M S, Gerhart M J, Schooley K A, Smith C A, et al. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata S. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 12.Clarke P G H. Anat Embryol. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz L M, Smith S W, Jones M E E, Osborne B A. Proc Natl Acad Sci USA. 1993;90:980–984. doi: 10.1073/pnas.90.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Infect Immunity. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Prada C M, Hoover D L, Tall B D, Venkatesan M M. Infect Immunity. 1997;65:1486–1496. doi: 10.1128/iai.65.4.1486-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trump B F, Berezesky I K, Chang S H, Phelps P C. Toxicol Pathol. 1997;25:82–88. doi: 10.1177/019262339702500116. [DOI] [PubMed] [Google Scholar]
- 17.Majno G, Joris I. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, Ao Z, Seth A, Schlossman S F. J Immunol. 1996;157:3980–3987. [PubMed] [Google Scholar]
- 19.Robertson M J, Manley T J, Pichert G, Cameron C, Cochran K J, Levine H, Ritz J. Leuk Lymphoma. 1995;17:51–61. doi: 10.3109/10428199509051703. [DOI] [PubMed] [Google Scholar]
- 20.Ross D D, Joneckis C C, Ordóñez J V, Sisk A M, Wu R K, Hamburger A W, Nora R E. Cancer Res. 1989;49:3776–3782. [PubMed] [Google Scholar]
- 21.Cole S R, Ashman L K, Ey P L. Mol Immunol. 1987;24:699–705. doi: 10.1016/0161-5890(87)90051-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Robertson M J, Schlossman S F. Cell Immunol. 1995;165:161–167. doi: 10.1006/cimm.1995.1201. [DOI] [PubMed] [Google Scholar]
- 23.Kane A B. Am J Pathol. 1995;146:1–2. [PMC free article] [PubMed] [Google Scholar]
- 24.Trump B F, Berezesky I K. Biochim Biophys Acta. 1996;1313:173–178. doi: 10.1016/0167-4889(96)00086-9. [DOI] [PubMed] [Google Scholar]
- 25.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S-I, Sameshima M, Hase A, Seto Y, Nagata S. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 26.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 27.Marsters S A, Sheridan J P, Donahue C J, Pitti R M, Gray C L, Goddard A D, Bauer K D, Ashkenazi A. Curr Biol. 1996;6:1669–1676. doi: 10.1016/s0960-9822(02)70791-4. [DOI] [PubMed] [Google Scholar]
- 28.Bodmer J-L, Burns K, Schneider P, Hofmann K, Steiner V, Thome M, Bornand T, Hahne M, Schröter M, Becker K, et al. Immunity. 1997;6:79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 29.Degli-Esposti M A, Smolak P J, Walczak H, Waugh J, Huang C-P, DuBose R F, Goodwin R G, Smith C A. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiley S R, Schooley K, Smolak P J, Din W S, Huang C-P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C A, Goodwin R G. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 31.Pitti R M, Marsters S A, Ruppert S, Donahue C J, Moore A, Ashkenazi A. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 32.Kuwashima Y. Anticancer Res. 1996;16:2997–3000. [PubMed] [Google Scholar]
- 33.Heathcote J G. Can J Ophthalmol. 1995;30:298–300. [PubMed] [Google Scholar]
- 34.Matsuoka S, Asano Y, Sano K, Kishimoto H, Yamashita I, Yorifuji H, Utsuyama M, Hirokawa K, Tada T. J Exp Med. 1995;181:2007–2015. doi: 10.1084/jem.181.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]