Abstract
Lobeline is being tested in clinical trials as a pharmacotherapy for methamphetamine abuse and attention deficit hyperactivity disorder. Preclinical research demonstrates that lobeline produces locomotor hypoactivity apart from its therapeutic effects; however, the hypothesis that there are sex differences in hypoactivity or in the development of tolerance to its locomotor depressant effects has not been investigated. Periadolescent rats were injected with saline to determine baseline locomotor activity. Animals received saline or lobeline (1.0–10 mg/kg) daily for 7 consecutive days (post natal days 29–35), and were challenged with saline 24 h later to assess baseline activity. Lobeline produced hypoactivity in total horizontal activity and center distance travelled. Tolerance developed to the lobeline-induced hypoactivity and sex differences in lobeline tolerance were observed on both measures. Females acquired tolerance to lobeline 5.6 mg/kg at a slower rate than males. Saline challenge revealed a linear dose-dependent trend of hyperactivity on both measures, which indicates that rats exhibited altered locomotor behavior 24 h after the final lobeline treatment. These findings demonstrate sex differences in the hypoactive response to lobeline prior to puberty and suggest that females may experience more locomotor depressant effects than males. Chronic lobeline may induce hyperactivity following cessation of treatment.
Keywords: lobeline, sex differences, locomotor activity, periadolescents, rats
1. Introduction
Novel pharmacotherapies are being developed to help decrease drug taking behavior in individuals exhibiting substance use disorder (SUD; Elkashef et al. 2008; Volkow, 2005). Studies utilizing animal models of drug reward have identified multiple compounds that decrease the motivation to self-administer drugs of abuse and/or alter the response to drug-associated stimuli (Ashby et al. 2003; Caine et al. 1997; Campbell et al. 1999; Campbell et al. 2002; Carroll et al. 2001; Di Ciano et al. 2003; Dwoskin and Crooks, 2002; Farook et al. 2009; Gilbert et al. 2005; Glick et al. 2000; Glick et al. 2008; Goeders et al. 1998; Harrod et al 2001; Harrod et al. 2003; Hart et al. 2008; Kenny et al. 2003; Le Foll and Goldberg, 2005a; Le Foll et al. 2005b; Le Foll et al. 2008; Neugebauer et al. 2007; Pilla et al. 1999; Roberts et al., 1996; Reichel et al. 2009; Sarnyai et al. 2001; Sorensen et al. 2008; Stoops, 2006; Zheng et al. 2006). These candidate pharmacotherapies alter the motivation for abused drugs and drug-associated stimuli by interacting with neuropharmacological processes that influence the organization of goal directed behaviors (Berridge et al. 2009; Kalivas et al. 2005; Koob and La Moal, 2008; Robbins et al. 2008).
Interestingly, preclinical studies also demonstrate that many potential pharmacotherapies can produce unfavorable behavioral effects, like alterations in locomotor activity. Thus, it is common for candidate pharmacotherapeutic drugs to induce hyperactivity or hypoactivity, presumably because these compounds engage and/or inhibit motor systems that functionally overlap with neural circuits that mediate motivated behavior (Bass et al. 2002; Eaves et al. 1985; Geter-Douglass et al. 1997; Gyertyan and Saghy, 2004; Harrod et al. 2001; Harrod et al. 2003; Jarbe et al. 2008; Le Foll et al. 2008; Matsumoto et al. 2008; Miller et al. 2003; Simon et al. 1995; van Vilet et al. 2006; Vickers et al. 2003). Such alterations in locomotor behavior are of interest to preclinical and clinical research because changes in locomotor activity may affect acceptability and compliance of a particular pharmacotherapy, and thus decrease the probability of successful treatment outcomes in individuals who exhibit SUD.
Lobeline, an alkaloidal constituent of Lobelia inflata, is currently in clinical trials for the treatment of psychostimulant abuse (Dwoskin and Crooks, 2002; Harrod et al. 2001; Harrod et al. 2003; Miller et al. 2001; Miller et al. 2003; Neugebauer et al. 2007; Polston et al. 2006; NIDA, 2008) and Attention Deficit Hyperactivity Disorder (ADHD; NIMH, 2008). Hypotheses regarding the mechanisms of lobeline’s therapeutic effects have been proposed for SUD. Data on the effects of lobeline in animal models of ADHD, however, have not been reported. Lobeline decreases the reinforcing effects of methamphetamine and attenuates motivation for intracranial self-stimulation in rats (Harrod et al. 2001; Wellman et al. 2008). Lobeline is hypothesized to decrease the motivation for methamphetamine by altering the storage and release of dopamine through vesicular monoamine transporters (VMAT2) within pathways that organize drug taking behavior (Teng et al.1998; Harrod et al. 2001; Dwoskin and Crooks, 2002; Zheng et al. 2006). Lobeline also exhibits a high affinity for α4β2 nicotinic acetylcholine receptors (Abood et al., 1989; Damaj et al., 1997; Miller et al., 2000), and has been shown to function as a nicotinic acetylcholine receptor antagonist (Benwell and Balfour, 1998; Miller et al., 2000; see Dwoskin and Crooks, 2002). Lobeline is not self-administered and does not support conditioned place preference in rats (Fudala and Iwamoto, 1986; Harrod et al. 2003). Furthermore, repeated lobeline injection does not produce behavioral sensitization or nicotinic receptor upregulation (Bhat et al. 1991; Stolerman et al. 1995; Damaj et al. 1997; Miller et al. 2003). Lobeline’s action as a nicotinic receptor antagonist may contribute to the decreased motivation for rewarding stimuli as administration of lobeline or the nicotinic receptor antagonist mecamylamine decreased alcohol consumption and preference in mice (Farook et al. 2009). Interestingly, lobeline has also been reported to act as a mu opioid receptor antagonist (Milller et al. 2007).
Similar to other candidate pharmacotherapies for SUD, lobeline produces locomotor depressant effects and tolerance develops to the hypoactive locomotor response following repeated treatment in adult male rodents (Stolerman et al. 1995; Damaj et al. 1997; Dwoskin and Crooks, 2002; Harrod et al. 2001; Harrod et al. 2003; Miller et al. 2003). Although substantial preclinical research has examined the pharmacology of lobeline, the mechanisms mediating lobeline-induced hypoactivity are currently unknown. Notably, the lobeline-induced hypoactivity does not appear to be related to its therapeutic effects as a treatment for psychostimulant abuse because lobeline attenuates the reinforcing effects of methamphetamine, and the locomotor stimulant effects of nicotine or cocaine, before and after rats exhibit tolerance to the locomotor depressant effects of this drug (Harrod et al. 2001; Miller et al. 2003; Polston et al. 2006, respectively). Moreover, lobeline decreased alcohol preference and intracranial self stimulation at doses that did not produce hypoactivity in male rodents (Farook et al. 2009; Wellman et al. 2008). Together, these findings show that lobeline decreases the motivational properties of multiple addictive drugs, and produces transient locomotor depressant effects.
To date no studies have explicitly investigated the relative behavioral effects of lobeline in females and males, and thus, nothing is known regarding potential lobeline-induced sex differences in behavior. Sex differences in the locomotor response to drug treatment are well documented, however. Adult female rats are generally reported to exhibit greater drug-induced locomotor activity than males (Becker, 1990, 1999; Chin et al. 2001; Glick et al. 1983; Harrod et al. 2004; Harrod et al. 2005a; Kanyt et al. 1999; van Haaren and Myer, 1991; Walker et al. 2001), and these effects have generally been attributed to the activational effects of gonadal hormones (Becker, 1999; Becker and Hu, 2008; Chin et al. 2002; Harrod et al. 2005a; Sell et al. 2000). Females also exhibit increased plasma and brain levels of drug (i.e., amphetamine, ibogaine, nicotine) following identical dose and route of injection procedures (Becker et al. 1982; Harrod et al. 2007; Pearl et al. 1997; Rosecrans, 1972; also see Bowman et al. 1999), and a greater therapeutic response to potential drug abuse pharmacotherapies, compared to males (Campbell et al. 2002; Carroll et al. 2001; Pearl et al. 1997). The current study therefore tested the general hypothesis that lobeline treatment produces sex differences in locomotor activity.
This hypothesis was tested using an animal model of periadolescence. Periadolescence is defined as approximately post natal days (PND) 30 – 40, which corresponds to the 10 days prior to puberty (Spear and Brake, 1983). Periadolescent rats were chosen to investigate sex differences in the locomotor depressant effects of lobeline because gonadal hormones present during adulthood further modulate sex differences observed in psychostimulant-induced locomotor behavior (Becker and Hu, 2008). Similar to adults, periadolescent rats exhibit sex differences in the response to abused drugs. Periadolescent females exhibit increased ethanol-induced corticosterone release (Silveri and Spear, 2004) and are more sensitive to the rewarding effects of cocaine than males (Lynch, 2008; Zakharova et al. 2009). Female periadolescent rats show greater cocaine and nicotine-induced behavioral sensitization (Collins and Izenwasser, 2002; Collins and Izenwasser, 2004; Collins et al. 2004) and cocaine-mediated changes in locomotor activity compared to males (Parylak et al. 2008). These findings suggest that organizational effects of gonadal hormones can also mediate sex differences in response to abused drugs (Parylak et al. 2008). In the present experiment, repeated lobeline treatment was administered prior to puberty (e.g., on PND 29–35; Dohler and Wuttke, 1975) to avoid the activational effects of gonadal hormones on lobeline-induced locomotor activity that would otherwise be present in adult animals (Becker and Hu, 2008; Dohler and Wuttke, 1975; Spear, 2000).
Rats were repeatedly administered one of five doses of lobeline for seven consecutive days and the subsequent effect on locomotor activity was measured. Saline injections were administered to all rats on the day prior to and after the lobeline injection phase to determine if baseline activity changed across the experiment. It was hypothesized that females would exhibit greater lobeline-induced hypoactivity (Damaj et al. 1997; Dwoskin and Crooks, 2002; Harrod et al. 2001; Harrod et al. 2003; Miller et al. 2003; Stolerman et al. 1995), and would acquire tolerance to the locomotor attenuating effects more slowly than males. These predictions are based on experiments showing that periadolescent females exhibit greater response to pharmacological treatment compared to males (Collins and Izenwasser, 2004; Collins et al. 2004; Lynch, 2008; Parylak et al. 2008; Silveri and Spear, 2004; Zakharova et al. 2009).
2. Materials and Methods
2.1 Subjects
One hundred and twelve, male (n =56) and female (n =56), Sprague–Dawley rats (Harlan Laboratories Inc., Indianapolis, IN) arrived at the animal care facilities with surrogate dams and litter mates on post-natal day (PND) 20, and were transferred to a colony located in the psychology department at the University of South Carolina. Animals were weaned on PND 21 into fresh plastic cages and were housed four, same sex rats/cage. Rats were pair-housed, same sex, on PND 28 for the remainder of the experiment. Rodent food (Pro-Lab Rat, Mouse Hamster Chow #3000) was provided ad lib. The colony was maintained at 21 ± 2° C, 50% ± 10% relative humidity and a 12L:12D cycle with lights on at 0700 h (EST). The protocol for this research methodology was approved by the Institutional Animal Care and Use Committee at the University of South Carolina.
2.2 Experimental design and procedure
Locomotor activity was chosen as the behavioral measure because previous reports demonstrate that this method is sensitive to lobeline-induced changes in activity (Damaj et al. 1997; Miller et al. 2003; Stolerman et al. 1995). Each treatment group was randomly assigned one male and one female per litter (Holson & Pearce, 1992). Locomotor activity was repeatedly measured in all animals following either saline or lobeline injection. The activity monitors were 16 square (40 × 40 cm) chambers (Kinder Inc., Poway, CA) that detected free movement of animals by infrared photocell interruptions. This equipment used an infrared photocell grid (32 emitter/detector pairs) to measure horizontal and vertical locomotor activity. The chambers were converted into round (~ 40 cm diameter) compartments by adding clear Plexiglas inserts. The photocells were tuned by the manufacturer for the extra perspex width. All activity monitors were located in a single, isolated room. One-to-two males and females from each treatment group were represented in each 60-min activity session. Table 1 shows the total number of rats/group and the basic experimental design.
Table 1.
Experimental design, group designation, n, and injection schedule for the experiment.
| Group | n | Habituation | Saline Baseline |
Saline or Lobeline Injections |
Saline Challenge |
|---|---|---|---|---|---|
| (PND 25 – 27) | (PND 28) | (PND 29 – 35) | (PND 36) | ||
| Males | |||||
| saline | 11 | No injection | saline | saline | saline |
| lobeline 1.0 | 12 | ‘’ | saline | lobeline 1.0 mg/kg | saline |
| lobeline 3.0 | 12 | ‘’ | saline | lobeline 3.0 mg/kg | saline |
| lobeline 5.6 | 11 | ‘’ | saline | lobeline 5.6 mg/kg | saline |
| lobeline 10 | 10 | ‘’ | saline | lobeline 10 mg/kg | saline |
| 56 | |||||
| Females | |||||
| saline | 11 | No injection | saline | saline | saline |
| lobeline 1.0 | 12 | ‘’ | saline | lobeline 1.0 mg/kg | saline |
| lobeline 3.0 | 12 | ‘’ | saline | lobeline 3.0 mg/kg | saline |
| lobeline 5.6 | 11 | ‘’ | saline | lobeline 5.6 mg/kg | saline |
| lobeline 10 | 10 | ‘’ | saline | lobeline 10 mg/kg | saline |
| 56 |
Animals were habituated to locomotor activity chambers for three 60-min sessions, one/day on PND 25–27. No injections were administered during habituation trials. On PND 28 all rats were administered a subcutaneous (sc) saline injection, and five minutes later, were placed into the activity chambers for 60-min to measure baseline activity. Day 28 is referred to as “saline baseline”. On PND 29, rats were injected with saline or lobeline (1.0, 3.0, 5.6, and 10 mg/kg) and were placed into locomotor activity chambers five minutes later. The animals’ locomotor response to saline or lobeline was assessed once daily for 60 minutes across seven consecutive days on PND 29 – 35. All animals received a second saline injection (sc) on PND 36 to determine if the baseline activity changed over the lobeline treatment phase of the experiment. This manipulation is referred to as “saline challenge” for explanatory purposes.
Two dependent measures were used in the present experiment. Total horizontal activity represents all movements detected by the photocells in the horizontal plane. Center distance travelled represents the distance (cm) that animals travel in the centermost portion of the activity chambers. The latter measure was used because although rats exhibit thigmotaxic behavior, they show activity, albeit less, in the center of the chamber. The center distance travelled measure has been a reliable measure of sex differences in locomotor activity (Booze et al., 1999; Harrod et al. 2004; Harrod et al. 2008; Wallace et al., 1996). Center distance travelled was determined with Kinder, Inc. software, which was used to impose a circular region (~24% of total area) in the center of the compartment during the data reduction phase (i.e., following completion of the activity session) of the experiment.
2.3 Drugs
Lobeline sulfate (Sigma-Aldridge, St. Louis, MO) was calculated on the weight of the salt and dissolved in saline two hours prior to injection in a volume of 1 ml/kg. Lobeline solutions were made fresh daily.
2.4 Data Analysis
Time course analyses were conducted on the activity data to examine effects of dose, sex, and the development of tolerance to the hypoactive effects of lobeline over the 7 days of injection. The data were recorded every two minutes of the 60-minute activity session. A mixed model ANOVA (Littell et al. 1997) was used to directly model the mean and slope (the log of the time after placement into the locomotor chamber) of the habituation line as a function of sex, dose, and day. Traditionally, habituation is measured by looking at mean differences between groups in the amount of locomotor activity. These analyses add to the traditional approach an examination of the steepness of the habituation line which could be seen as assessing tolerance to the locomotor depressant effects of lobeline. The advantage of this approach is that the effects of lobeline on both mean levels of activity and tolerance can be examined. Further, this method also allows an assessment of differences in the rate at which activity changes as well as mean levels of activity. The hypothesized lobeline-induced hypoactivity and sex differences in this response are examined through the tests of the main effect of dose and the interaction of dose with sex on average levels of activity and on the slope of the habituation line. The development of tolerance across the week of lobeline administration was investigated by examining linear changes in mean levels of activity and the slope of the habituation line across the week of lobeline administration. The assumption of linearity in the development of tolerance was validated by comparing model fit of an unrestricted model to the model assuming linearity. The advantage of this procedure is that the degrees of freedom are reduced from 6 to 1 for the day effect, providing more power and necessitating fewer contrasts. The error term remained the same, based on seven levels of the within subjects factor (Keppel and Zedeck, 1989). The hypothesis of dose-dependent tolerance to lobeline was tested by examining the interaction of dose with the linear tolerance effect. The hypothesis that there would be sex differences in the development of tolerance was tested with a three way interaction of dose, day, and sex. Thus, all research questions were answered using mixed-model repeated measures ANOVA with sex (2) and dose (5) as between-subjects factors, and day (7) and time (10) as within-subjects factors all predicting the mean and slope of the daily habituation lines. Planned comparisons were conducted for hypothesized interactions that were found to be significant. The first 20 minutes of the activity period were analyzed because visual analysis of individual habituation lines s showed that animals administered lobeline demonstrated consistent trends in activity for the first third of the observation period and that activity in the last two thirds of the period was characterized by much greater variability suggesting that lobeline was no longer producing hypoactivity. This observation was verified with a repeated measures ANOVA comparing absolute deviations from the habituation line (expressed as a percentile) for each minute of observation across the three 20 minute periods of observation. Results showed significantly larger deviations in the second and third periods, F(2,23517) = 205, mean deviations were .48, .82, and 2.46 across the three periods, respectively.
The saline baseline day and the saline challenge day data were analyzed using standard repeated measures ANOVA techniques, with sex (2) and dose (5) as between-subjects factors, and time (10) and day (2) as within-subjects factors. This analysis was conducted on both the total horizontal activity and center distance travelled data. The significant dose × day interactions, as determined by the overall ANOVA, were further analyzed using multivariate regression analyses. These analyses were conducted to determine if the change in behavior observed following saline challenge represents a linear, quadratic, or cubic trend for both the total horizontal activity and center distance travelled data. An α level of 0.05 was used to determine statistical significance.
3. Results
3.1 Analysis of behavior following repeated lobeline injection
3.1.1 Total horizontal activity
The mixed-model sex × dose × day × time (2 × 5 × 7 × 10) ANOVA revealed no sex difference in overall activity. Most activity occurred at the beginning of the session and then dissipated as a function of time [time: F (1, 7702) = 1562.1, p<0.0001]. This initial activity burst represents exploratory behavior, whereas the subsequent within-session decrease in activity reflects habituation of this response. The slope of the habituation line became steeper across days, suggesting both a within- and between-session habituation of activity [day: F (1, 7702) = 49.0, p<0.0001; day × time: F (1, 7702) = 68.7, p<0.0001].
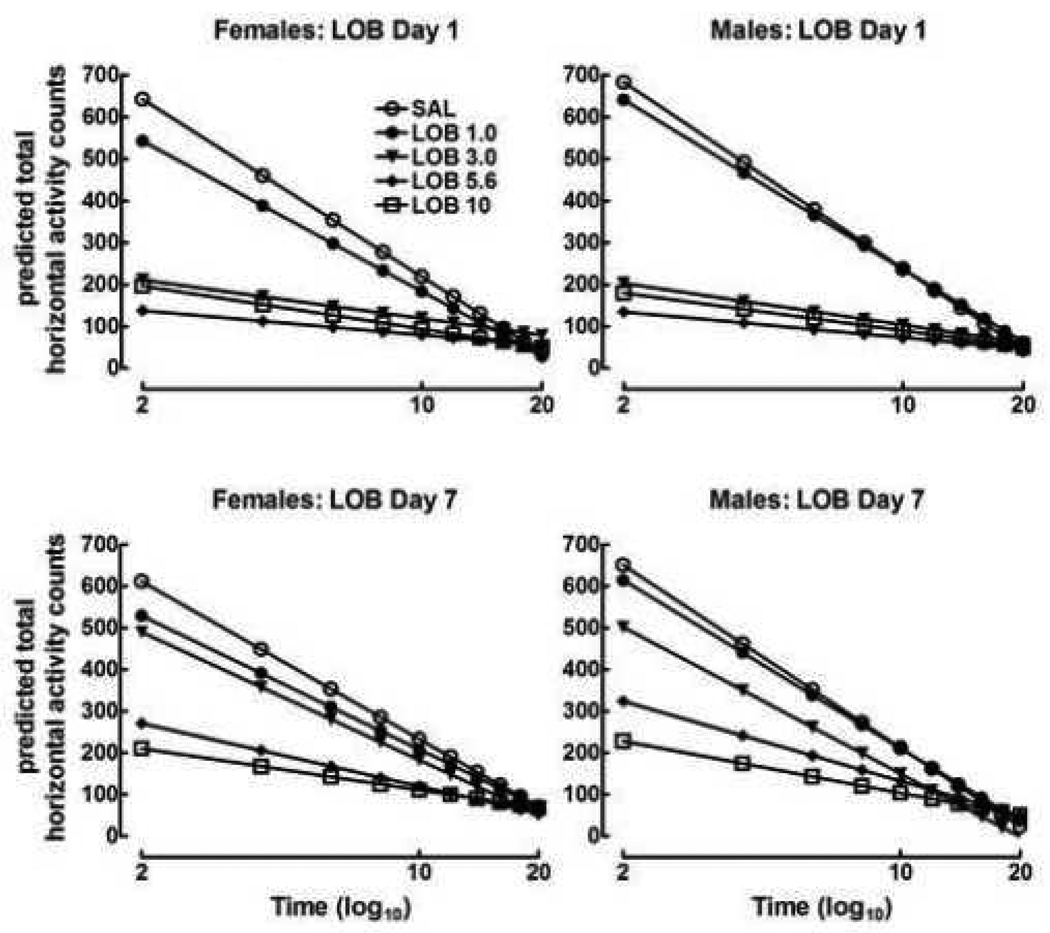
The predicted activity counts for the males and females following acute and repeated pretreatment for the 20 min activity sessions are shown in Figure 1. Since means and slopes of the habituation lines are the outcome, standard error bars for each point are not appropriate, and standard errors for each parameter are provided in text below. Lobeline produced significant hypoactivity relative to saline controls across the seven-day injection phase [dose: F (4, 102) = 68.3, p<0.0001]; however; activity levels increased relative to those following the first injection, suggesting rats exhibited tolerance to the hypoactive effects of repeated lobeline [dose × day: F (4, 7702) = 17.2, p<0.0001]. Tolerance was further demonstrated by significant dose-dependent changes in the slopes of the habituation lines across injection days [dose × day × time: F (4, 7702) = 43.7, p<0.0001]. Thus, not only do the absolute levels of lobeline-induced hypoactivity decrease over days (observed as increased activity), but tolerance was also observed in increasingly precipitous slopes of the habituation lines following repeated lobeline injection (e.g., lines approximate that of saline controls’ lines with repeated injection). Moreover, sex differences in the rate of tolerance varied as a function of dose and repeated treatment [dose × sex × day: F (4, 7702) = 2.9, p<0.05].
Figure 1.
Predicted total horizontal activity counts in male and female periadolescent rats following repeated saline (SAL) or lobeline (LOB; 1.0–10 mg/kg) injection. Rats exhibited tolerance to the repeated effects of LOB and females developed tolerance to repeated LOB 5.6 mg/kg at a slower rate than males (p<0.01). n = 10–12 animals/group.
Planned comparisons revealed that the 1.0 mg/kg group exhibited slightly lower total horizontal activity than the saline group by 34.0 counts (SE = 16.2, p<0.05) and the habituation line is less steep than controls by 37.8 counts/log minute (SE = 17.0, p<0.05); no change in this effect over the course of the trial was observed. There were no sex differences between males and females for this comparison. Animals injected with 3.0 mg/kg exhibited activity levels that were 87.4 counts below the saline group (SE = 16.2, p<0.05) and were less steep by 125.1 counts (SE = 17.0, p<0.05) across the entire trial. However, mean levels of activity increased for the 3.0 mg/kg group by 11 counts per day (SE = 2.8, p<0.001), and the slope of the habituation line decreased (i.e., became steeper) by −26.9 counts per day (SE = 2.5, p<0.05), demonstrating the development of tolerance in this group. There was no effect of sex for the 3.0 mg/kg group either on the average level of activity or on the shape of the habituation line. The 5.6 mg/kg group was on average lower than controls by 144.9 counts (SE = 16.6, p<0.001), and the habituation line is less steep by 183.8 counts (SE = 17.4, p<0.001). The behavior of this group changed dramatically over the course of the trial, exhibiting increased average activity by 5.7 counts per day (SE = 2.8, p<0.05), and a decreased slope of the habituation line by 14.3 counts/log time per day (SE = 2.5, p<0.001). The average level of activity per day for males in the 5.6 mg/kg group increased by 9.7 counts more than it did for females (SE = 3.9, p<0.01), although there was no difference between males and females in the slope of the line. Thus, across days males acquired behavioral tolerance to the 5.6 mg/kg dose at a faster rate than females, but there were not differences within trials. The lower panels of Figure 1 show that the male lobeline 5.6 mg/kg groups exhibited an increased level of activity relative to the female 5.6 mg/kg groups on day 7 of the treatment phase. The 10 mg/kg group exhibited activity which was 142.2 counts below the saline groups (SE = 17.0, p<0.001) over the course of the trial, and the habituation lines are less steep than those of controls by 186.4 counts/log minute (SE = 17.8, p<0.001). However, these differences did not change over the seven injections, and furthermore, did not differ between males and females. Figure 1 shows that neither the height nor slope of the line of the 10 mg/kg group differed between sexes, or between acute and repeated treatment.
These findings demonstrate that lobeline produced significant hypoactivity in male and female periadolescent rats and further indicates that females exhibited a slower rate of tolerance than males to the hypoactive effects of lobeline if injected with 5.6 mg/kg, but not the 1.0 or 3.0 mg/kg doses of lobeline. No evidence of tolerance or sex differences was evident in the hypoactive effects of 10 mg/kg lobeline.
3.1.2 Center distance travelled following repeated lobeline injection
A mixed-model sex × dose × day × time (2 × 5 × 7 × 10) ANOVA, identical to the ANOVA analyzing total horizontal activity, was conducted on the lobeline pretreatment phase of the experiment. There was no sex difference in overall center distance travelled. The most distance travelled in the center of the chambers occurred during the beginning of the session and then dissipated as a function of time [time: F (1, 7702) = 567.9, p<0.0001]. Like the horizontal measure, this initial activity burst represents exploratory behavior, whereas the subsequent within-session decrease in activity reflects habituation of this response. For distance in the center, average cm travelled did not change across days [day: F (1, 7702) = 3.25, p=0.07], but the slope of the habituation line became steeper across days, suggesting both a within- and between-session habituation of activity [day × time: F (1, 7702) = 8.24, p<0.01].
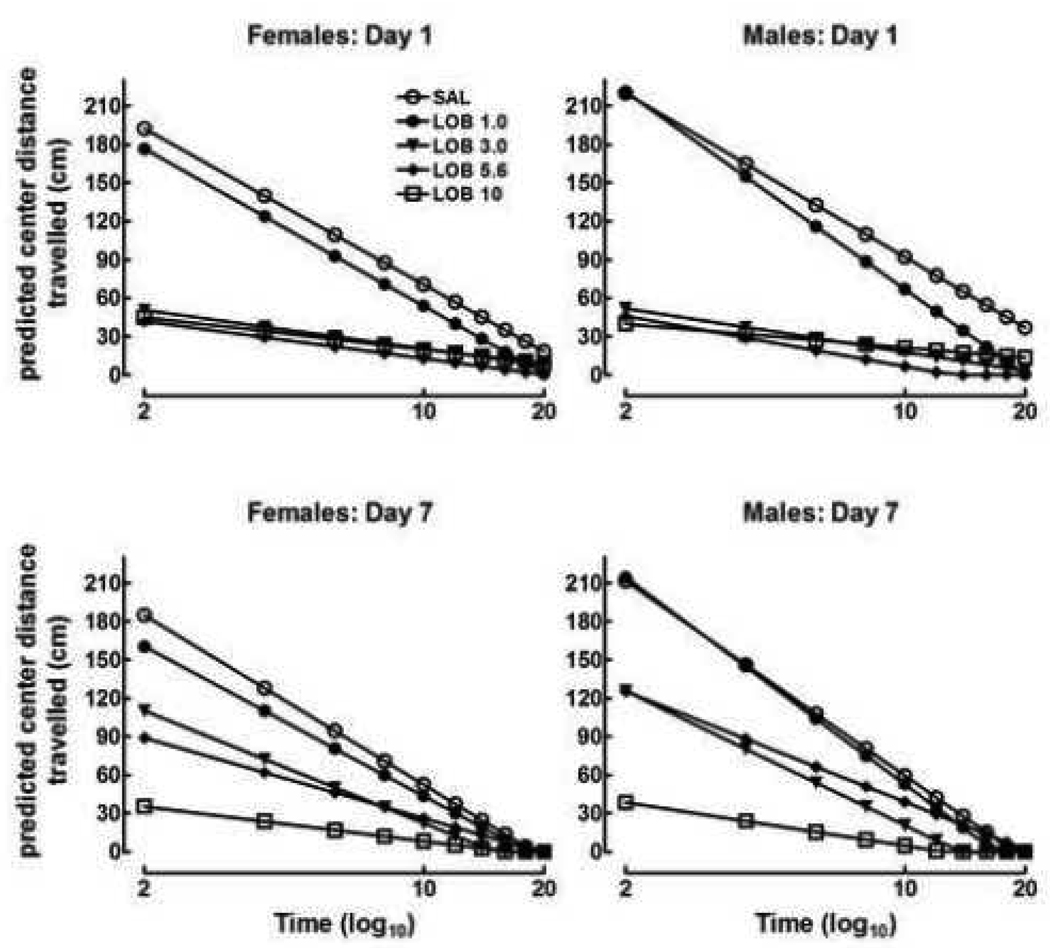
The predicted center distance travelled for the males and females for the 20 min activity session are shown in Figure 2. Lobeline significantly decreased the distance travelled in the center of the apparatus relative to saline controls across the seven-day injection phase [dose: F (4, 102) = 26.8, p<0.0001]; however; the distance travelled increased relative to those following acute injection, indicating that rats exhibited tolerance to the hypoactive effects of repeated lobeline [dose × day: F (4, 7702) = 3.1, p<0.05]. Tolerance was further demonstrated by significant dose-dependent changes in the slopes of the habituation lines across injection days [dose × day × time: F (4, 7702) = 6.85, p<0.0001]. Thus, not only does the absolute level of lobeline-induced hypoactivity decrease over days (observed as increased center distance travelled), but tolerance was also observed in increasingly precipitous slopes of the habituation lines following repeated lobeline injection. Moreover, sex differences in the rate of tolerance varied as a function of dose and repeated treatment [dose × sex × day: F (4, 7702) = 2.6, p<0.05].
Figure 2.
Predicted center distance travelled in male and female periadolescent rats following repeated saline (SAL) or lobeline (LOB; 1.0–10 mg/kg) injection. Rats exhibited tolerance to the repeated effects of LOB and females developed tolerance to repeated LOB 5.6 mg/kg at a slower rate than males (p<0.01). n = 10–12 animals/group.
Planned comparisons revealed that the 1.0 mg/kg group did not have significantly lower time in the center than the saline group by 11.7 cm (SE = 7.5, p=0.13), but the habituation line is less steep than controls by 20.2 cm/log minute (SE = 7.8, p<0.05). No change in this effect was observed over the course of the trial, demonstrating no tolerance to 1.0 mg/kg. There were no differences in center distance travelled for the 1.0 mg/kg dose of lobeline. Animals injected with 3.0 mg/kg lobeline exhibited activity levels that were 27.9 cm below the saline group (SE = 27.9, p<0.05) and were less steep by 40.8 cm (SE = 7.78, p<0.05) across the entire trial. Mean levels of distance travelled did not change across days, however the slope of the habituation line decreased (i.e., became steeper) by −4.84 cm per day (SE = 1.46, p<0.05), demonstrating the development of tolerance in this group. There was no effect of sex for the 3.0 mg/kg group either on the average level of activity or on the shape of the habituation line. The 5.6 mg/kg group was on average lower than controls by 36.4 cm (SE = 7.7, p<0.05), and the habituation line is less steep by 52.8 cm (SE = 8.0, p<0.05). The behavior of this group did not change significantly over the course of the trial. The average level of distance travelled per day for males in the 5.6 mg/kg group increased by 5.5 cm more than it did for females (SE = 1.78, p<0.05) over the course of the pretreatment phase, although there was no difference between males and females in the slope of the line. Thus, males acquired behavioral tolerance to the 5.6 mg/kg dose more quickly relative to females. Figure 2 shows that the male lobeline 5.6 mg/kg group showed an increased level of activity relative to the female lobeline 5.6 mg/kg groups on day 7 of the treatment phase (see lower panels). The 10 mg/kg group exhibited activity which was 70.7 cm below the saline groups (SE = 5.62, p<0.05) over the course of the trial, and the habituation lines are less steep than those of controls by 54.7 cm/log minute (SE = 8.1, p<0.05). However, these differences did not change over the seven injections, and furthermore, did not differ between males and females. Figure 2 shows that neither the height nor slope of the line describing the behavior of the 10 mg/kg group differed between females or males, or across repeated treatment.
These findings show that lobeline produced significant decreases in center distance travelled in male and female periadolescent rats. Furthermore, these findings indicate that females exhibited a slower rate of tolerance than males to the hypoactive effects of lobeline if injected with lobeline 5.6 mg/kg, but not lobeline 1.0 or 3.0 mg/kg. No evidence of tolerance or a sex difference was evident in the 10 mg/kg group.
3.2 Analysis of the saline baseline and saline challenge data
3.2.1 Total Horizontal Activity
Repeated measures sex × dose × day × time (2 × 5 × 2 × 10) ANOVA was conducted on the absolute data from the saline baseline and saline challenge days to determine if rats’ total horizontal activity changed as a function of sex, repeated lobeline treatment, and time. There were no significant main effects or interactions with the factors of sex or time. Activity was similar across groups during the initial saline baseline measurement [F (4, 107) < 1.0, p > 0.05]. Saline challenge, however, resulted in altered locomotor activity in some, but not all of the groups previously injected with lobeline. Rats repeatedly treated with lobeline exhibited increased activity following saline challenge relative to saline baseline [day × dose: F (4, 102) = 5.4, p = 0.001]. Thus, although there were no differences between saline controls across the saline baseline and challenge days [F (1, 21) <1.0, p > 0.05], rats treated with repeated lobeline exhibited a dose-dependent increase in total horizontal activity following saline injection.
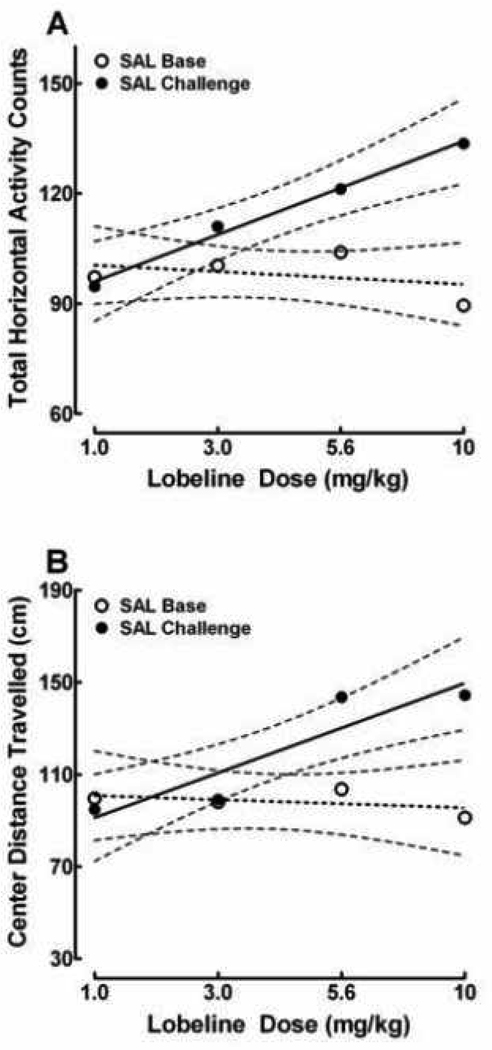
The baseline and challenge data were converted to percent of the saline control and were analyzed using multivariate regression with contrast analyses to determine if the change in behavior observed following saline challenge represents a linear, quadratic, or cubic trend. Day (saline baseline and saline challenge) was the within-subjects factor and dose (1.0, 3.0, 5.6, 10 mg/kg) was the between-subjects factor in the analysis. The slopes for the regression lines were significantly different on the baseline and challenge days [day × dose: F (3, 86) = 5.6, p = 0.002] indicating that total horizontal activity observed on saline challenge increased relative to saline baseline. The hyperactive response followed a significant linear trend [day × dose: F (1, 86) = 15.8, p = 0.0001] which indicates that the hyperactivity observed following saline challenge increased as a function of lobeline dose. That is, animals repeatedly treated with lobeline exhibited a dose-dependent hyperactive response following saline challenge. The day × dose interaction is illustrated in Figure 3A.
Figure 3.
Total horizontal counts (A) and center distance travelled (B) during saline (SAL) baseline (open circles) and SAL challenge (filled circles) in animals repeatedly treated with lobeline (LOB) 1.0, 3.0, 5.6, or 10 mg/kg during the seven-day treatment phase of the experiment. The SAL Baseline occurred one day prior to the first day of LOB or saline control injection. SAL Challenge was administered one day after the seventh LOB or SAL injection. Multiple regression analyses, which were performed on the percent of SAL control animal’s data, demonstrate that the dose-dependent response was characterized as a linear trend for both the total horizontal activity [day × dose: F (1, 86) = 15.8, p = 0.0001], and center distance travelled data [day × dose: F (1, 86) = 14.8, p = 0.0001]. The data represent the first 20 minutes of the activity session. The dotted lines represent 95% confidence intervals. n = 20–24 animals/dose.
3.2.2 Center Distance Travelled
Repeated measures sex × group × day × time (2 × 5 × 2 × 10) ANOVA, identical to that performed on the total horizontal activity, was conducted on the saline baseline and saline challenge data. There were no main effects or interactions including the factor of sex. Center distance travelled was similar across groups during the initial saline baseline measurement [F (4, 107) < 1.0, p > 0.05]. However, the significant main effect of day shows that rats exhibited a significant change in baseline of center distance travelled between the saline baseline and saline challenge days [day: F (1, 102) = 13.9, p = 0.001]. This change was observed as decreased center distance travelled. Thus, the saline controls exhibited means (± SEM) of 928.8 (± 128.9) and 634.0 (±72.2) cm travelled on the saline baseline and saline challenge days, respectively [F (1, 21) = 6.6, p<0.05]. Following saline challenge, the saline, 1.0, and 3.0 mg/kg groups exhibited similar activity of 636.0 ± (72.3), 601.1 (± 72.9), 628.8 (± 58.6), respectively, whereas the animals treated with 5.6 and 10 mg/kg lobeline exhibited increased activity relative to saline controls during the saline challenge day [day × group: F (4, 102) = 2.6, p = 0.001]. Those means were 910.3 (± 71.0) and 915.4 (±93.1) cm travelled, respectively. These findings show that the cm travelled in the center decreased from saline baseline to saline challenge in control and lobeline 1.0 and 3.0 mg/kg groups. The decrease was not observed in the lobeline 5.6 and 10 mg/kg groups. Rather, the latter groups exhibited significant hyperactivity on saline challenge, relative to controls.
The baseline and challenge data were converted to percent of the saline control and were analyzed using multivariate regression with contrast analyses to determine if the change in behavior observed following saline challenge represents a linear, quadratic, or cubic trend. The analysis revealed a significant main effect of day which shows that the center distance travelled increased on the challenge day relative to saline baseline [day: F (1, 86) = 12.7, p = 0.0006]. Furthermore, the slopes for the regression lines were significantly different on the baseline and challenge days [day × dose: F (3, 86) = 5.6, p = 0.002] indicating that total horizontal activity observed on saline challenge increased relative to saline baseline as a function of lobeline dose. The dose-dependent response was characterized as a linear trend [day × dose: F (1, 86) = 14.8, p = 0.0001]. That is, animals repeatedly treated with lobeline exhibited a hyperactive response that was particularly evident in the 5.6 and 10 mg/kg groups following saline challenge. The day × dose interaction is illustrated in Figure 3B.
4. Discussion
The current study investigated sex differences in lobeline-induced hypoactivity and furthermore, determined if males and females exhibited different rates of tolerance to lobeline’s locomotor depressant effects. The results from the lobeline treatment phase replicate previous findings showing that lobeline produced hypoactivity in a dose-dependent manner in adult rodents (Damaj et al.1997; Harrod et al. 2001; 2003; 2004; Miller et al. 2003; Stolerman et al. 1995). Furthermore, the present findings are also in accord with previous research demonstrating tolerance to the locomotor depressant effects after repeated administration (Damaj et al.1997; Harrod et al. 2001; Miller et al.2003; Stolerman et al. 1995). In the present experiment, rats given repeated injection of lobeline 3.0 mg/kg showed nearly complete tolerance, and those receiving lobeline 5.6 mg/kg exhibited only partial tolerance after seven treatments. Interestingly, however, rats did not exhibit tolerance to the hypoactive effects repeatedly produced by lobeline 10 mg/kg. Damaj et al. (1997) demonstrated that mice administered lobeline (15 mg/kg) 2×/day exhibited tolerance to its hypoactive effects after 10 days of treatment. Taken together, these findings suggest that greater than seven exposures are needed to investigate tolerance to the hypoactive effects of lobeline at concentrations in the range of 10 mg/kg and above in rats, according to the dependent measures used in the present experiment.
Increased activity in the center of an open field apparatus is a putative measure of decreased anxiety. In the injection phase of the present experiment, rats showed a dose-dependent decrease in center distance travelled, followed by a progressive increase on this measure after repeated lobeline treatment (1.0–5.6 mg/kg). One explanation for this trend is that lobeline produces anxiolytic effects that alter the basal thigmotaxic behavior of the rat. Thus, according to this explanation, rats progressively spend more time exploring the center of the locomotor chamber because lobeline decreases anxiety. Lobeline has been shown to produce anxiolytic effects in rats according to research that utilized an elevated plus maze (Brioni et al. 1993). To our knowledge, no studies have investigated the possible anxiolytic effect of lobeline in an open field arena, and it isn’t clear if the increased distance travelled in the center of the locomotor activity chambers used in the present experiment is related to the potential anxiolytic effects of lobeline. A more conservative suggestion is that the increased center distance travelled reflects tolerance to the hypoactive effects of lobeline. Thus, as animals acquired tolerance to the locomotor depressant effects (i.e., as indicated by the mean total horizontal activity measure) rats exhibited a corresponding increase in exploration of the chamber.
Sex differences in the development of tolerance to lobeline’s hypoactive effects were observed. According to both dependent measures, females exhibited increased sensitivity to the hypoactive effects of lobeline as indicated by a slower rate of tolerance to repeated injection of 5.6 mg/kg when compared to males. These sex differences were not likely mediated by the activational effects of gonadal hormones because lobeline was repeatedly administered prior to puberty (Dohler and Wuttke, 1975). These findings add to a growing body of research suggesting that the activational effects of gonadal hormones are not the only factor mediating sex differences in the behavioral response to pharmacological treatment, and that differences in brain organization between males and females contribute to the sex differences in response to drug administration (Collins and Izenwasser, 2004; Collins et al. 2004; Hu et al. 2004; Kuhn et al. 2001; Lynch, 2008; Parylak et al. 2008; Silveri and Spear, 2004; Zakharova et al. 2009).
Sex differences in dopamine neuropharmacology may account for the slower acquisition of tolerance to the lobeline-induced hypoactivity by females, relative to males. Lobeline alters dopamine storage and release by inhibiting the VMAT2 in striatal preparations (Grady et al. 1992; Teng et al. 1998; Zheng et al. 2007). Sex differences in striatal dopamine concentrations have been reported in mice treated with the VMAT2 inhibitor, reserpine. Thus, adult males showed greater reserpine-induced striatal dopamine concentrations compared to females (Ji et al. 2007). Walker et al. (2000) demonstrated that electrically stimulated increases in striatal dopamine release and reuptake was greater in adult female rats compared to males, and that this effect occurred independently of variations in the estrous cycle. Furthermore, females exhibit greater striatal dopamine transporter density than males (Rivest et al. 1995), and males express increased striatal D1 and D2 receptors relative to females throughout the transition of periadolescence to adolescence (e.g., PND 25 –40; Andersen and Teicher, 2000). These findings suggest that lobeline could induce inhibition of dopamine release or dopamine storage in a sex-dependent manner via activity with the VMAT2, thus yielding sex differences in locomotor activity. Further studies using periadolescent rats will be important to test the hypothesis that sex differences in lobeline-induced hypoactivity are mediated by lobeline-induced modulation of dopamine release and storage by VMAT2.
All rats in the present experiment were administered saline injections 24 h before and after the lobeline treatment phase to determine if the baseline levels of activity changed over the course of the experiment. There were no changes in baseline activity according to the total horizontal activity measure; however, rats treated with lobeline during the treatment phase exhibited a hyperactive behavioral response during saline challenge. This hyperactive response was observed as a linear trend (day × dose), suggesting that the hyperactivity, which was observed only on the saline challenge day, was directly related to the treatment dose of lobeline administered during the injection phase of the experiment. Thus, repeated lobeline pretreatment produced hyperactivity of total horizontal activity and center distance travelled following saline challenge, and this effect was observed independently of sex.
One explanation for the hyperactivity exhibited during saline challenge is that repeated lobeline injection may have suppressed monoaminergic activity during the pretreatment phase of the experiment (Clarke and Reuben, 1996). As previously mentioned, lobeline alters dopamine storage and release by inhibiting VMAT2 (Dwoskin and Crooks, 2002; Grady et al. 1992; Teng et al., 1998; Zheng et al., 2007). Because the saline challenge occurred 24 hours after the final lobeline injection, a rebound in monoaminergic neurotransmission may have induced a hyperactive response. A second explanation is related to Pavlovian conditioning processes. Previous research has extensively investigated the relationship between contextual conditioning and drug effects (Anagnostaras and Robinson, 1996; Bevins and Palmatier, 2003; Hinson and Poulos, 1981; Siegel, 1975). These experiments demonstrate that repeated drug injection administered amongst the same contextual cues produces a conditional response (CR) that mimics the unconditional response (UR) produced by the drug (Anagnostaras and Robinson, 1996; Hinson and Poulos, 1981; Pavlov, 1927), or results in a CR that is in opposition to the UR (Siegel, 1975; Siegel et al. 2000). The compensatory conditioned response theory of drug tolerance predicts that the development of tolerance to a particular drug effect following repeated injection will result in a CR that is opposite of the UR (Siegel, 1975). The present findings demonstrate a robust hyperactive response following the absence of lobeline, and suggest that further experiments, which assess the associative versus non-associative nature of contextual conditioning in locomotor activity studies, are needed (Baker and Tiffiany, 1985; Besheer et al. 2004; Bevins and Palmatier, 2004; Poulos and Cappell, 1991). This finding may have clinical implications for individuals using lobeline as a pharmacotherapy for SUD and ADHD because successful treatment of these disorders may require chronic lobeline treatment.
The present experiment demonstrates that periadolescent females developed tolerance to the locomotor depressant effects of lobeline more slowly than males. These findings strongly suggest that cessation of chronic lobeline treatment may produce further changes in locomotor activity, specifically, hyperactivity. Lobeline-induced fluctuations in overt locomotor behavior, which may occur during lobeline treatment or following cessation of treatment, could produce negative experiences by altering the individual’s daily activities. Interruption of ongoing behavior by a pharmacological treatment might therefore decrease compliance with the pharmacotherapy. Lobeline is currently approved for clinical trials for SUD and ADHD in adults (NIDA, 2008; NIMH, 2008). These findings of the present experiment suggest that female individuals who use lobeline as a pharmacotherapy may experience more lobeline-induced locomotor depressant effects, compared to males. The current study investigated repeated lobeline using a periadolescent model to determine if lobeline produced sex differences in locomotor activity prior to sexual maturity because gonadal hormones have been shown to modulate drug effects in females relative to males (Lynch et al. 2002; Becker an d Hu, 2008). Preclinical research, which elucidates the processes that mediate lobeline-induced sex differences in behavior, will provide important information for future hypotheses regarding the application of lobeline to females and males as a pharmacotherapy for SUD and ADHD.
Acknowledgements
The authors are grateful for the technical assistance offered by Lauren Ballina, Alexandra Basilakos, Ryan Lacy, and Rachel Singleton. We also appreciate Nichole Neugebauer’s and Charles Mactutus’ comments on early versions of the manuscript. This research was made possible by NIDA grant DA021287 (SBH) and by a Research Productivity Scholar award granted by the University of South Carolina (K21).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abood LG, Banerjee S, Kanne DB. Sites, mechanisms, and structural characteristics of the brain's nicotine receptor. J Subst Abuse. 1989;1:259–271. [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110(6):1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000 Jan;24(1):137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Paul M, Gardner EL, Heidbreder CA, Hafan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48(3):154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Baker TB, Tiffany ST. Morphine tolerance as habituation. Psychol Rev. 1985 Jan;92(1):78–108. [PubMed] [Google Scholar]
- Bass CE, Griffin G, Grier M, Mahadevan A, Razdan RK, Martin BR. SR-141716A-induced stimulation of locomotor activity. A structure-activity relationship study. Pharmacol Biochem Behav. 2002 Dec;74(1):31–40. doi: 10.1016/s0091-3057(02)00945-0. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5(2):157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64(4):803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008 Jan;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. Epub 2007 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamineelicited rotational behavior. Eur J Pharmacol. 1982 May 7;80(1):65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The influence of lobeline on nucleus accumbens dopamine and locomotor responses to nicotine in nicotine-pretreated rats. Br J Pharmacol. 1998 Nov;125(6):1115–1119. doi: 10.1038/sj.bjp.0702161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009 Feb;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. Epub 2009 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Palmatier M. Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev. 2004 Sep;3(3):143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 2004 Feb;172(1):108–117. doi: 10.1007/s00213-003-1621-9. Epub 2003 Oct 3. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Turner SL, Selvaag SR, Marks MJ, Collins AC. Regulation of brain nicotinic receptors by chronic agonist infusion. J Neurochem. 1991 Jun;56(6):1932–1939. doi: 10.1111/j.1471-4159.1991.tb03450.x. [DOI] [PubMed] [Google Scholar]
- Booze RM, Wood ML, Welch MA, Berry S, Mactutus CF. Estrous cyclicity and behavioral sensitization in female rats following repeated intravenous cocaine administration. Pharmacol Biochem Behav. 1999 Nov;64(3):605–610. doi: 10.1016/s0091-3057(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. J Pharmacol Exp Ther. 1999 Sep;290(3):1316–1323. [PubMed] [Google Scholar]
- Brioni JD, O'Neill AB, Kim DJ, Decker MW. Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. Eur J Pharmacol. 1993 Jul 6;238(1):1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport. 1997 Jul 7;8(9–10):2373–2377. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1999 Apr;143(2):209–214. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Depend. 2002 Mar 1;66(1):61–69. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Campbell UC, Heideman P. Ketoconazole suppresses food restriction-induced increases in heroin self-administration in rats: sex differences. Exp Clin Psychopharmacol. 2001 Aug;9(3):307–316. doi: 10.1037//1064-1297.9.3.307. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, Perrotti LI, Quiñones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002 Jul 26;945(1):123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Fletcher H, Perrotti LI, Jenab S, Quiñones-Jenab V. Sex differences in cocaine-induced behavioral sensitization. Cell Mol Biol. 2001 Sep;47(6):1089–1095. [PubMed] [Google Scholar]
- Clarke PB, Reuben M. Release of [3H]-noradrenaline from rat hippocampal synaptosomes by nicotine: mediation by different nicotinic receptor subtypes from striatal [3H]-dopamine release. Br J Pharmacol. 1996 Feb;117(4):595–606. doi: 10.1111/j.1476-5381.1996.tb15232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002 Sep 20;138(1):27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004 Mar;46(3):349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetaminestimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res. 2004 Nov 25;153(2):175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Patrick GS, Creasy KR, Martin BR. Pharmacology of lobeline, a nicotinic receptor ligand. Journal of Pharmacology Exp Ther. 1997;282(1):410–419. [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attentuation of cue-controlled cocaine-seeking by selective D3 dopamine receptor antagonist SB-277011A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin, and gonadal steroids in pubertal male and female rats. 1975;97(4):898–907. doi: 10.1210/endo-97-4-898. Endo. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63(2):89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- Eaves M, Thatcher-Britton K, Rivier J, Vale W, Koob GF. Effects of corticotropin releasing factor on locomotor activity in hypophysectomized rats. Peptides. 1985 Sep–Oct;6(5):923–926. doi: 10.1016/0196-9781(85)90323-7. [DOI] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Subst Abus. 2008;29(3):31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Gaddis JG, Littleton JM, Barron S. Lobeline, a nicotinic partial agonist attenuates alcohol consumption and preference in male C57BL/6J mice. Physiol Behav. 2009 Jun 22;97(3–4):503–506. doi: 10.1016/j.physbeh.2009.02.031. Epub 2009 Mar 5. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Further studies on nicotine-induced conditioned place preference in the rat. Pharmacol Biochem Behav. 1986 Nov;25(5):1041–1049. doi: 10.1016/0091-3057(86)90083-3. [DOI] [PubMed] [Google Scholar]
- Geter-Douglass B, Katz JL, Alling K, Acri JB, Witkin JM. Characterization of unconditioned behavioral effects of dopamine D3/D2 receptor agonists. J Pharmacol Exp Ther. 1997 Oct;283(1):7–15. [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Hinds PA, Shapiro RM. Cocaine-induced rotation: sex-dependent differences between left- and right-sided rats. Science. 1983 Aug 19;221(4612):775–777. doi: 10.1126/science.6879177. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport. 2000 Jun 26;11(9):2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration. Eur J Pharmcol. 2008 Dec 3;599(1–3):91–95. doi: 10.1016/j.ejphar.2008.09.038. Epub 2008 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Peltier RL, Guerin GF. Ketoconazole reduces low dose cocaine self-administration in rats. Drug Alcohol Depend. 1998 Dec 1;53(1):67–77. doi: 10.1016/s0376-8716(98)00108-2. [DOI] [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992 Sep;59(3):848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Gyertyán I, Sághy K. Effects of dopamine D3 receptor antagonists on spontaneous and agonist-reduced motor activity in NMRI mice and Wistar rats: comparative study with nafadotride, U 99194A and SB 277011. Behav Pharmacol. 2004 Jul;15(4):253–262. doi: 10.1097/01.fbp.0000137857.26150.ab. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Fitting S, Hasselrot U, Booze RM. Intra-accumbal Tat1-72 alters acute and sensitized responses to cocaine. Pharmacol Biochem Behav. 2008 Oct;90(4):723–729. doi: 10.1016/j.pbb.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Mactutus CF. Sex differences in nicotine levels following repeated intravenous injection in rats are attenuated by gonadectomy. Pharmacol Biochem Behav. 2007;86(1):32–36. doi: 10.1016/j.pbb.2006.12.004. Epub 2006 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Welch M, Browning CE, Mactutus CF. Acute and repeated intravenous cocaineinduced locomotor activity is altered as a function of sex and gonadectomy. Pharmacol Biochem Behav. 2005a;82(1):170–181. doi: 10.1016/j.pbb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Browning CE, Welch M, Booze RM. Home cage observations following acute and repeated IV cocaine in intact and gonadectomized rats. Neurotoxicol Teratol. 2005b Nov–Dec;27(6):891–896. doi: 10.1016/j.ntt.2005.07.004. Epub 2005 Oct 7. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, Booze RM. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacol Biochem Behav. 2004;78(3):581–592. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Green TA, Gehrke BJ, Bardo MT. Lobeline does not serve as a reinforcer in rats. Psychopharmacology (Berl) 2003;165(4):397–404. doi: 10.1007/s00213-002-1289-6. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2001;298(1):172–179. [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008 Mar;33(4):761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Hinson RE, Poulos CX. Sensitization to the behavioral effects of cocaine: modification by Pavlovian conditioning. Pharmacol Biochem Behav. 1981;15(4):559–562. doi: 10.1016/0091-3057(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, LeMay BJ, Olszewska T, Vemuri VK, Wood JT, Makriyannis A. Intrinic effects of AM 4113, a putative neutral CB1 receptor selective antagonist, on open-field behaviors in rats. Pharmacol Biochem Behav. 2008 Nov;91(1):84–90. doi: 10.1016/j.pbb.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, McDermott JL, Dluzen DE. Sex differences in K+-evoked striatal dopamine output from superfused striatal tissue fragments of reserpine-treated CD-1 mice. J Neuroendocrinol. 2007 Sep;19(9):725–731. doi: 10.1111/j.1365-2826.2007.01581.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005 Mar 3;45(5):647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kanýt L, Stolerman IP, Chandler CJ, Saigusa T, Pöğün S. Influence of sex and female hormones on nicotine-induced changes in locomotor activity in rats. Pharmacol Biochem Behav. 1999 Jan;62(1):179–187. doi: 10.1016/s0091-3057(98)00140-3. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine selfadministration but not nicotine- and cocaine-induced facilitation of brain reward functions in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Keppel G, Zedeck S. Data analysis for research designs. Cranbury, NJ: Worth Publishers; 1989. [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kuhn CM, Walker QD, Kaplan KA, Li ST. Sex, steroids, and stimulant sensitivity. Ann N Y Acad Sci. 2001 Jun;937:188–201. doi: 10.1111/j.1749-6632.2001.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wertheim CE, Goldberg SR. Effects of baclofen on conditioned rewarding and discrimative stimulus effects of nicotine in rats. Neurosci Lett. 2008 Oct 10;443(3):236–240. doi: 10.1016/j.neulet.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preference through a mechanism that does not involve discrimination-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medicaitions for drug Dependence. J Pharmacol Exp Ther. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for Mixed Models. Cary NC: SAS Institute; 1997. [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008 Apr;197(2):237–246. doi: 10.1007/s00213-007-1028-0. Epub 2007 Dec 8. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. Attenuation of methamphetamine-induced effects through the antagonism of sigma (sigma) receptors: Evidence from in vivo and vitro studies. Eur Neurpsychophamacol. 2008;18(12):871–881. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Dwoskin LP. Lobeline inhibits nicotine-evoked [(3)H]dopamine overflow from rat striatal slices and nicotine-evoked (86)Rb(+) efflux from thalamic synaptosomes. Neuropharmacology. 2000 Oct;39(13):2654–2662. doi: 10.1016/s0028-3908(00)00140-4. [DOI] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, Acri JB, Dwoskin LP. Lobeline inhibits the neurochemical and behavioral effects of amphetamine. J Pharmacol Exp Ther. 2001;296(3):1023–1034. [PubMed] [Google Scholar]
- Miller DK, Harrod SB, Green TA, Wong MY, Bardo MT, Dwoskin LP. Lobeline attenuates locomotor stimulation induced by repeated nicotine administration in rats. Pharmacol Biochem Behav. 2003;74(2):279–286. doi: 10.1016/s0091-3057(02)00996-6. [DOI] [PubMed] [Google Scholar]
- Miller DK, Lever JR, Rodvelt KR, Baskett JA, Will MJ, Kracke GR. Lobeline, a potential pharmacotherapy for drug addiction, binds to mu opioid receptors and diminishes the effects of opioid receptor agonists. Drug Alcohol Depend. 2007 Jul 10;89(2–3):282–291. doi: 10.1016/j.drugalcdep.2007.02.003. Epub 2007 Mar 21. [DOI] [PubMed] [Google Scholar]
- National Institutes on Drug Abuse (NIDA) Ph1 Lobeline Interaction Study – 1 (Clinical Trials Identifier: NCT00439504) Principle Investigator: Jones, R, MD Langley Porter Psychiatric Institute, National Institutes on Drug Abuse; 2008
- National Institute of Mental Health (NIMH) Effectiveness of the Non-Stimulant Medication Lobeline in Improving Symptoms of Attention Deficit Hyperactivity Disorder in Adults. (Clinical Trials Identifier: NCT00664703) Principle Investigator: Martin, C., MD General Clinical Research Center, University of Kentucky; 2008
- Neugebauer NM, Harrod SB, Stairs DJ, Crooks PA, Dwoskin LP, Bardo MT. Lobelane decreases methamphetamine self-administration in rats. Eur J Pharmacol. 2007;571(1):33–38. doi: 10.1016/j.ejphar.2007.06.003. Epub 2007 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29(1):17–22. doi: 10.1016/j.ntt.2006.11.003. Epub 2006 Nov 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008 May;89(3):314–323. doi: 10.1016/j.pbb.2008.01.003. Epub 2008 Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes: An Investigation of Physiological Activity of the Cerebral Cortex. In: Anrep GV, translator and editor. London: Oxford University Press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl SM, Hough LB, Boyd DL, Glick SD. Sex differences in ibogaine antagonism of morphine-induced locomotor activity and in ibogaine brain levels and metabolism. Pharmacol Biochem Behav. 1997 Aug;57(4):809–815. doi: 10.1016/s0091-3057(96)00383-8. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behavior by a partial dopamine D3 receptor agonist. Nature. 1999 Jul 22;400(6742):371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Polston JE, Cunningham CS, Rodvelt KR, Miller DK. Lobeline augments and inhibits cocaine-induced hyperactivity in rats. Life Sci. 2006;79(10):981–990. doi: 10.1016/j.lfs.2006.05.006. Epub 2006 May 17. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Cappell H. Homeostatic theory of drug tolerance: a general model of physiological adaptation. Psychol Rev. 1991 Jul;98(3):390–408. doi: 10.1037/0033-295x.98.3.390. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Grant KM, Bevins RA. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug Alcohol Depend. 2009 Feb 1;100(1–2):54–62. doi: 10.1016/j.drugalcdep.2008.09.006. Epub 2008 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest R, Falardeau P, Di Paolo T. Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res. 1995 Sep 18;692(1–2):269–272. doi: 10.1016/0006-8993(95)00611-s. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008 Oct;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996 Oct;15(4):417–423. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology. 1972;6:863–870. doi: 10.1016/0028-3908(72)90045-7. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001 Jun;53(2):209–243. [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000 Jun;293(3):879–886. [PubMed] [Google Scholar]
- Siegel S. Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psychol. 1975;89(5):498–506. doi: 10.1037/h0077058. [DOI] [PubMed] [Google Scholar]
- Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol. 2000 Aug;8(3):276–293. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Characterizing the ontogeny of ethanol-associated increases in corticosterone. Alcohol. 2004 Feb;32(2):145–155. doi: 10.1016/j.alcohol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Simon P, Hemet C, Ramassamy C, Costentin J. Non-amphetaminic mechanism of stimulant locomotor effect of modafinil in mice. Eur Neuropsychopharmacol. 1995 Dec;5(4):509–514. doi: 10.1016/0924-977x(95)00041-m. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983 Mar;16(2):83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Sørensen G, Sager TN, Petersen JH, Brennum LT, Thøgersen P, Hee Bengtsen C, Thomsen M, Wörtwein G, Fink-Jensen A, Woldbye DP. Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. Psychopharmacology (Berl) 2008 Jul;199(1):37–46. doi: 10.1007/s00213-008-1069-z. Epub 2008 May 15. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Mirza NR. Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology (Berl) 1995;117(4):430–437. doi: 10.1007/BF02246215. [DOI] [PubMed] [Google Scholar]
- Stoops WW. Aripiprazole as a potential pharmacotherapy for stimulant dependence: human laboratory studies with d-amphetamine. Exp Clin Psychopharmacol. 2006 Nov;14(4):413–421. doi: 10.1037/1064-1297.14.4.413. [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Dwoskin LP. Lobeline displaces [3H]dihydrotetrabenazine binding and releases [3H]dopamine from rat striatal synaptic vesicles: comparison with d-amphetamine. J Neurochem. 1998;71(1):258–265. doi: 10.1046/j.1471-4159.1998.71010258.x. [DOI] [PubMed] [Google Scholar]
- Van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991 Aug;39(4):923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Van Vilet SA, Jongsma MJ, Vanwersch RA, Olivier B, Philippens IH. Behavioral effects of modafinil in marmoset monkeys. Psychopharmacology (Berl) 2006 May;185(4):443–440. doi: 10.1007/s00213-006-0340-4. Epub 2006 Mar 21. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain in obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003 Apr;167(1):103–111. doi: 10.1007/s00213-002-1384-8. Epub 2003 Mar 11. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Methamphetamine Abuse - Testimony before the Senate Subcommittee on Labor, Health and Human Services, Education, and Related Agencies - Committee on Appropriations. 2005 ( http://www.nida.nih.gov/Testimony/4-21-05Testimony.html)
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001 Jul;25(1):118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Mactutus CF, Booze RM. Repeated intravenous cocaine administration: locomotor activity and dopamine D2/D3 receptors. Synapse. 1996 Jul;23(3):152–163. doi: 10.1002/(SICI)1098-2396(199607)23:3<152::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Elliott AE, Barbee S, Hollas CN, Clifford PS, Nation JR. Lobeline attenuates progressive ratio breakpoint scores for intracranial self-stimulation in rats. Physiol Behav. 2008 Mar 18;93(4–5):952–957. doi: 10.1016/j.physbeh.2007.12.018. Epub 2007 Dec 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology (Berl) 2006 Sep;188(1):18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009 Mar;92(1):131–134. doi: 10.1016/j.pbb.2008.11.002. Epub 2008 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Zheng G, Deaciuc AG, Zhan CG, Dwoskin LP, Crooks PA. Computational neural network analysis of the affinity of lobeline and tetrabenazine analogs for the vesicular monoamine transporter-2. Bioorg Med Chem. 2007;15(8):2975–2992. doi: 10.1016/j.bmc.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dwoskin LP, Crooks PA. Vesicular monoamine transporter 2: role as a novel target for drug development. AAPSJ. 2006;8(4):E682–E692. doi: 10.1208/aapsj080478. [DOI] [PMC free article] [PubMed] [Google Scholar]