Abstract
The cancer-associated anorexia-cachexia syndrome is observed in 80% of patients with advanced-stage cancer, and is one of the major obstacles in chemotherapy. Ghrelin is a orexigenic hormone that has been proposed to prevent anorexia. Aim of the study was to determine whether the addition of the ghrelin agonist growth hormone releasing peptide 2 (GHRP-2) to cytotoxic therapy with 5-fluorouracil (5-FU) prevents the anorexia associated with chemotherapy in cancer cachectic mice. Thirty-three BALB/c female tumour-bearing mice were randomized to receive a solution containing: (a) placebo; (b) GHRP-2; (c) 5-FU; or (d) 5-FU + GHRP-2. Ten BALB/c no tumour-bearing mice received placebo solution. Food intake and survival were checked. Six hours after the drug injection the cumulative food intake was significantly increased in mice treated with the combination of 5-FU + GHRP-2 versus the 5-FU alone (P = 0.0096). On day 3, the cumulative food intake of mice treated with GHRP-2, 5-FU and 5-FU + GHRP-2 significantly increased compared with naive and vehicle groups (P = 0.0007, P = 0.0038 and P = 0.0166, respectively). The median survival time was longer in 5-FU + GHRP-2 treated mice than in those with 5-FU, although it was not significant (18 d versus 15.5 d, P = 0.7). For the first time, we demonstrated that the addition of GHRP-2 to cytotoxic therapy with 5-FU improved appetite in tumour-bearing mice with anorexia/cachexia syndrome in early stage. These data suggest that GHRP-2 may improve the efficacy of therapy and the quality of life of cancer patients thank to the amelioration of their nutritional state.
Keywords: GHS, Ghrelin, Cancer anorexia-cachexia syndrome, Food intake, Chemotherapy, Colon cancer cell line, Murine model
CHEMOTHERAPY-INDUCED ANOREXIA
Chemotherapy is the most effective treatment for most cancer patients because of its systemic distribution. Despite the recent advances in the treatment, many patients do not respond to therapy, and die of their diseases. The cancer-associated anorexia-cachexia syndrome is observed in 80% of patients with advanced-stage cancer, and it is a very powerful prognostic indicator of poor outcome and poor quality of life. This syndrome is also one of the major obstacles in cancer chemotherapy[1].
Ghrelin is the natural ligand of growth hormone secretagogue receptor. Growth hormone releasing peptide 2 (GHRP-2) is a synthetic compound that acts as a potent agonist of GHS receptor[2]. Increasing evidences support that GHRP-2, like ghrelin[3], exerts orexigenic proprieties. Administration of GHRP-2 has shown to increase food intake and body weight in rodents[4] and in healthy men[5]. Since ghrelin has a short half-life, the more stable GHRP-2 was preferred in this experiment.
Aim of the study was to determine whether the addition of GHRP-2 to cytotoxic therapy with 5-fluorouracil (5-FU) prevents anorexia associated with chemotherapy in cancer cachectic mice[6]. Secondary aims were to examine the chronic effects of GHRP-2 on reduced appetite and survival in this animal model.
MURINE MODEL OF CANCER
Six-weeks-old female BALB/c mice (19-24 g) were housed individually in equal plastic cages and received ad libitum standard diet and tap water in a regulated environment. The murine colon cancer cell line (colon 26) was supplied by Dr. Hayashi (Kitazato University, Kanagawa, Japan). Colon 26 cancer cells (5 × 105) were dissolved in a 100 μL volume of PBS containing 0.02% EDTA and then implanted in the abdominal cavity of female BALB/c mice by intraperitoneal administration. Seven days later, when mice manifested the first symptoms of anorexia and cachexia, the tumour-bearing mice were randomized in 4 groups, each of them composed by: (a) 11 mice receiving 5% glucoside solution + PBS (vehicle); (b) 12 mice receiving GHRP-2 + 5% glucoside solution; (c) 12 mice receiving 5FU + PBS; and (d) 15 mice receiving 5-FU + GHRP-2. Ten BALB/c no tumour-bearing mice received 5% glucoside solution + PBS (naïve). The day of randomisation was considered as day 0 of the experiment. GHRP-2 (DalaDβNalAlaTrpDPheLysNH2) was supplied by Prof. Bowers (Tulane University, New Orleans, USA). It was dissolved in PBS and was subcutaneously administered at dose of 10 μg/mouse daily. 5-fluorouracil (Kyowa Hakko Kogyo Co. Ldt, Tokyo, Japan) was dissolved in 5% glucoside solution and then intraperitoneally administered at dose of 100 mg/kg weekly. In the acute experiment, food intake and body weight were measured at 0, 1, 2, 4 and 6 h after the first injection of the drugs and then daily. Food intake was evaluated by subtracting uneaten food from initially pre-measured food and checking for food spillage. All the experiments were approved by the animal care committees at the Kobe University and the Kagoshima University (Japan). Results are expressed as mean ± SE. Analysis of variance (ANOVA) followed by Bonferroni’s t test was used to assess the differences among groups. P-values < 0.05 were considered significant. The survival curves after tumour implantation were analyzed by Kaplan-Meier survival test.
GHRELIN AGONIST GHRP-2 REVERSES CHEMOTHERAPY-INDUCED ANOREXIA
The median survival time was longer in the group treated with 5-FU + GHRP-2 than in the group treated with 5-FU, although it was not significant (18 d versus 15.5 d, P = 0.7) as shown in Figure 1. At day 0, 6 h after the drug injection, the cumulative food intake was significantly increased in mice treated with the combination of 5-FU + GHRP-2 versus the 5-FU alone (P = 0.0096, Figure 2A). At day 0, 4 h after the drug injection, the cumulative body weight of the group treated with 5-FU + GHRP-2 showed a reduced loss of body weight compared with the group treated with 5-FU (P = 0.0074, Figure 2B). At day 2, the groups treated with 5-FU and 5-FU + GHRP-2 showed a higher loss of cumulative body weight compared with the group treated with GHRP-2 alone (P = 0.0003 and P < 0.0001, respectively, Figure 3). However, from day 3 to 5, the cumulative food intake of mice treated with 5-FU and 5-FU + GHRP-2 significantly increased compared with naive and vehicle groups (P = 0.0038 and P = 0.0166, respectively, Figure 3). The cumulative food intake was significantly increased in mice treated with GHRP-2 respect to naïve and vehicle groups throughout the observation period (P = 0.0007 and P = 0.0004, respectively, Figure 3). We could not follow the body weight changes since the tumour-bearing mice developed ascites after the inoculation of cancer cells into the abdominal cavity.
Figure 1.
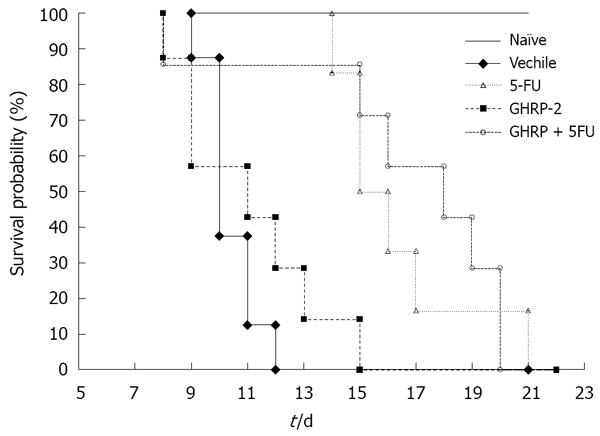
Kaplan-Meier curve of overall survival in colon 26-bearing mice (Naïve: 10 mice; Vehicle: 11 mice; GHRP-2: 12 mice; 5-FU: 12 mice; 5-FU + GHRP-2: 15 mice).
Figure 2.
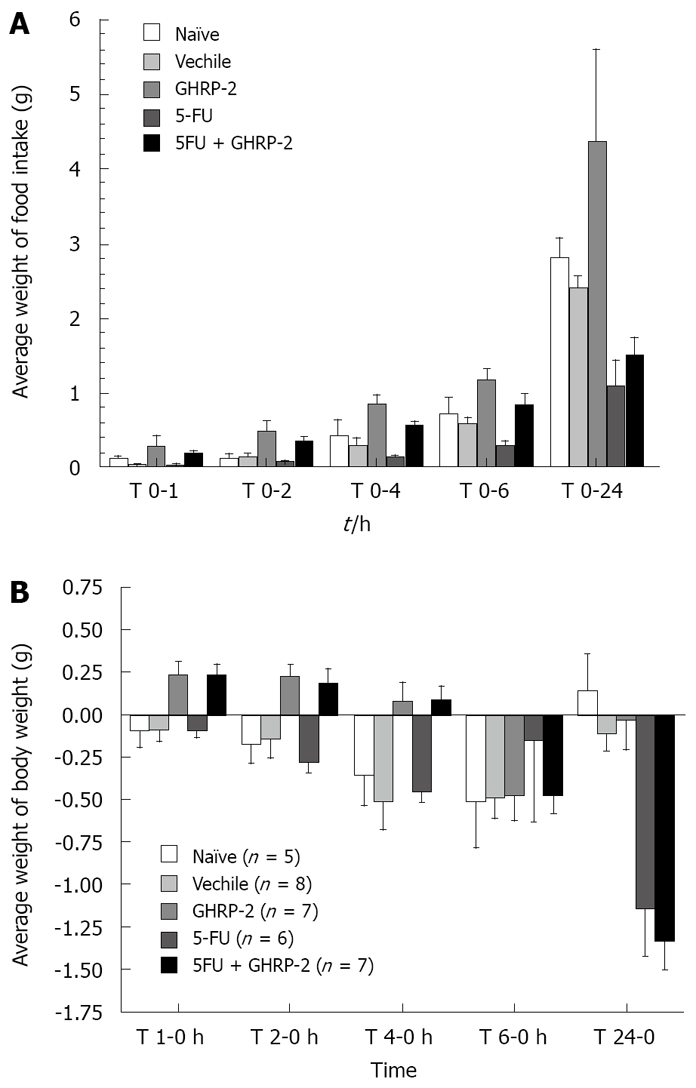
GHRP-2 reverses reduced food intake and body weight by 5-FU administration in colon 26-bearing mice-acute experiments. A: Food intake; B: Body weight.
Figure 3.
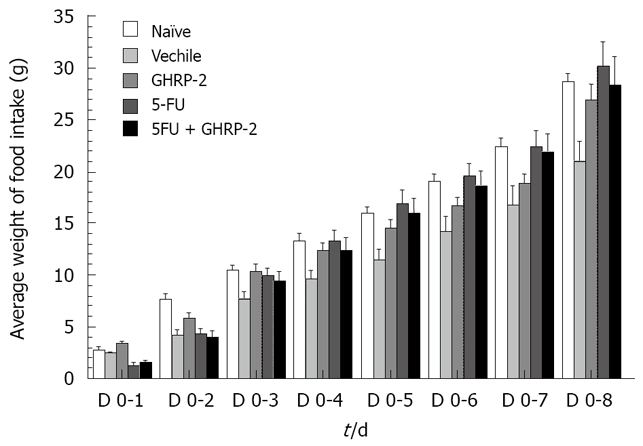
GHRP-2 increases cumulative food intake compared to vehicle controls in colon 26-bearing mice-chronic experiments.
THERAPEUTIC ROLE OF GHRP-2 IN CANCER ANOREXIA-CACHEXIA
Up to 50% of cancer patients report changes in eating behavior at time of diagnosis, leading to weight loss[3]. This study suggests that the addition of GHRP-2 to cytotoxic therapy with 5-FU improved appetite in tumour-bearing mice with anorexia/cachexia syndrome in early stages, although a statistically significant improvement in survival was not achieved compared with mice treated with 5-FU. It is likely that GHRP-2 may overcome any resistance to the appetite-stimulating effects of ghrelin in cachectic animals and cancer patients with appetite loss. In our experiment GHRP-2 has shown to be a short-lasting, acute potent agent, which mimics the orexigenic effects of ghrelin[4,5]. In this study, we demonstrated for the first time that GHRP-2 reversed loss of food intake and body weight in tumour-bearing mice treated with chemotherapy. It is likely that preventing loss of appetite and weight associated with chemotherapy helps mice remain in a relatively balanced condition of electrolytes and hydration, thereby decreasing the side effect and increasing the efficacy of 5-FU. The characteristic of a long acting drug which allows to increased interval of time between two consecutive administrations of the drug, may be a further improve the quality of life in cancer patients.
This study has some limitations. The first is the difficulty to determinate if the survival and the weight loss experienced by tumour-bearing mice was due to tumour burden or to the efficacy or side effect of the 5-FU administration. Secondary, although GHRP-2 did increase the cumulative food intake in tumour-bearing mice, there was a lack of significant difference among groups in survival analysis which should be examined under various chemotherapy conditions and tumour models.
Due to the amelioration of the nutritional state, and of the side effects of chemotherapy, GHRP-2 may offer an interesting treatment for cachexia associated with cancer in order to improve the efficacy of therapy and the quality of life of cancer patients.
Acknowledgments
The authors would like to thank Ueno N, PhD, MD and Professor Mantovani G for the scientific support and for their valuable comments.
Footnotes
Supported by (in part) A Grant-in-Aid for Scientic Research (B:16390208) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to A.I.)
S- Editor Xiao LL E- Editor Ma WH
References
- 1.Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin. 2002;52:72–91. doi: 10.3322/canjclin.52.2.72. [DOI] [PubMed] [Google Scholar]
- 2.Bowers CY, Momany FA, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology. 1984;114:1537–1545. doi: 10.1210/endo-114-5-1537. [DOI] [PubMed] [Google Scholar]
- 3.Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 4.Tschop M, Statnick MA, Suter TM, Heiman ML. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology. 2002;143:558–568. doi: 10.1210/endo.143.2.8633. [DOI] [PubMed] [Google Scholar]
- 5.Laferrere B, Abraham C, Russell CD, Bowers CY. Growth hormone releasing peptide-2 (GHRP-2), like ghrelin, increases food intake in healthy men. J Clin Endocrinol Metab. 2005;90:611–614. doi: 10.1210/jc.2004-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeliger H, Guba M, Koehl GE, Doenecke A, Steinbauer M, Bruns CJ, Wagner C, Frank E, Jauch KW, Geissler EK. Blockage of 2-deoxy-D-ribose-induced angiogenesis with rapamycin counteracts a thymidine phosphorylase-based escape mechanism available for colon cancer under 5-fluorouracil therapy. Clin Cancer Res. 2004;10:1843–1852. doi: 10.1158/1078-0432.ccr-1176-3. [DOI] [PubMed] [Google Scholar]


