Abstract
AIM: To evaluate the efficacy of continuous regional arterial infusion therapy (CRAI) with gabexate mesilate and antibiotics for severe acute pancreatitis (SAP).
METHODS: We conducted a prospective study on patients who developed SAP with or without CRAI. Out of 18 patients fulfilled clinical diagnostic criteria for SAP in Japan, 9 patients underwent CRAI, while 9 patients underwent conventional systemic protease inhibitor and antibiotics therapy (non-CRAI). CRAI was initiated within 72 h of the onset of pancreatitis. Gabexate mesilate (2400 mg/d) was continuously administered for 3 to 5 d. The clinical outcome including serum inflammation-related parameters were examined.
RESULTS: The duration of abdominal pain in the CRAI group was 1.9 ± 0.26 d, whereas that in the non-CRAI group was 4.3 ± 0.50. The duration of SIRS in the CRAI group was 2.2 ± 0.22 d, whereas that in the non-CRAI group was 3.2 ± 0.28. Abdominal pain and SIRS disappeared significantly in a short period of time after the initiation of CRAI using gabexate mesilate. The average length of hospitalization significantly differed between the CRAI and non-CRAI groups, 53.3 ± 7.9 d and 87.4 ± 13.9 d, respectively. During the first two weeks, levels of serum CRP and the IL6/IL10 ratio in the CRAI group tended to have a rapid decrease compared to those in the non-CRAI group.
CONCLUSION: The present results suggest that CRAI using gabexate mesilate was effective against SAP.
Keywords: Severe acute pancreatitis, Arterial infusion, Gabexate mesilate, Antibiotics
INTRODUCTION
Severe acute pancreatitis (SAP) remains a lethal disease. It is defined as an inflammatory process of the pancreas with possible peripancreatic tissue, and multi-organ involvement inducing multi-organ dysfunction syndrome (MODS) with an increased mortality rate[1,2]. Continuous regional arterial infusion (CRAI) with protease inhibitor nafamostat mesilate and antibiotics has been proven to be effective as an initial therapy in Japan[2]. However, evidence supporting the benefit of CRAI in treating acute pancreatitis is insufficient, and its advisability according to the JPN guidelines for the management of acute pancreatitis is classed as “Recommendation C”[3]. In the statement, it was described that CRAI with protease inhibitors and antibiotics may possibly reduce the mortality rate and incidence of infectious complications in necrotizing pancreatitis. Actually, until now, most cases have been treated with the protease inhibitor nafamostat mesilate. Here, we performed CRAI using gabexate mesilate to treat SAP, and investigated the clinical benefits including serum inflammation-related parameters such as cytokines and chemokines.
MATERIALS AND METHODS
Patients
The severity of acute pancreatitis was assessed within 48 h of admission according to the diagnostic criteria for the diagnosis of acute pancreatitis by the Research Committee for Intractable Diseases of the Pancreas in Japan by Ministry of Health, Labour and Welfare Japan (Tables 1 and 2)[4-6]. A total of 18 patients fulfilling clinical diagnostic criteria for SAP at six participating institutions were selected for the present study. Nine patients underwent CRAI (CRAI group), while 9 patients underwent conventional systemic protease inhibitor and antibiotics therapy (non-CRAI group). Each institution made the decision to perform CRAI or non-CRAI therapy, so the present study was not a randomized controlled trial. Clinical features of both groups were shown in Table 3. All 9 patients in the CRAI group were men, average age 48.0 ± 13.4 years (mean ± SD). The cause of SAP was alcohol (n = 5), gallstone (n = 1), hyperlipidemia (n = 1), post-endoscopic retrograde cholangiopancreatography (ERCP, n = 1), or unknown (n = 1). On the other hand, 4 of the 9 patients in the non-CRAI group were male and 5 were female (average age of group, 59.9 ± 15.1 years; mean ± SD). Regarding age at onset, no significant difference was observed between CRAI group and non-CRAI group (P = 0.0979). The causes of SAP patients in the non-CRAI group were gallstones (n = 4), alcohol (n = 3), post-ERCP (n = 1), or unknown (n = 1). All patients in both groups were diagnosed as stage 2 SAP. CRAI was initiated within 72 h of the onset of pancreatitis. A 5-Fr shepherd’s catheter was placed in either the celiac artery (including the splenic and gastro-duodenal arteries) or in the supra-mesenteric artery, and gabexate mesilate (2400 mg/d) was continuously administered for 3-5 d. Antibiotics were administered every 12 h (panipenem in 5 patients, meropenem in 2 patients, imipenem in 1 patient, and piperacillin in 1 patient). Catheters were placed in the superior mesenteric, celiac, splenic, and gastroduodenal arteries of 3, 3, 2, and 1 patient, respectively. Complications in one patient comprised thrombosis of the superior mesenteric artery, and warfarin was administered. Carbapenem antibiotics were administered to all patients in the non-CRAI group.
Table 1.
Criteria for grading the severity of acute pancreatitis in Japan[4]
| Prognostic factors | Clinical signs | Laboratory data |
| Prognostic factor I (2 points for each positive factor) | Shock | BE ≤ -3 mmol/L |
| Respiratory failure | Ht ≤ 30% (after hydration) | |
| Mental disturbance | BUN ≥ 40 mg/dL or creatinine ≥ 2.0 mg/dL | |
| Severe infection | ||
| Hemorrhagic diathesis | ||
| Prognostic factor II (1 points for each positive factor) | PaO2 ≤ 60 mmHg (room air) | |
| FBS ≥ 200 mg/dL | ||
| Total protein ≤ 60 g/L | ||
| LDH ≥ 700 IU/L | ||
| Ca ≤ 7.5 mg/dL | ||
| Prothrombin time ≥ 15 s | ||
| Platelet count ≤ 1 × 105/mm3 | ||
| CT grade IV or V | ||
| Prognostic factor III | SIRS score ≥ 3 (2 points) | |
| Age ≥ 70 yr (1 point) |
BE: Base excess; Ht: Hematocrit; BUN: Blood urea nitrogen; FBS: Fasting blood sugar; LDH: Lactate dehydrogenase; SIRS: Systemic inflammatory response syndrome. CT grade IV or V: Presence of diffuse and uneven density in the pancreatic parenchyma or the presence of inflammatory changes extending beyond the border of the pancreas. Severity score: Sum of the points for the positive prognostic factors is defined as the severity score. Standardized criteria: Severe, presence of more than one prognostic factor I, and/or the presence of more than two prognostic factor II (severity score ≥ 2 points); Moderate, presence of one prognostic factor II (severity score = 1 point); Mild, acute pancreatitis without prognostic factor I or II (severity = 0 point).
Table 2.
Stage classification of acute pancreatitis and mortality rate in 2003 in Japan[4]
| Stage | Severity score | Severity | No. of patients(%) | Died | Mortality rate (%) |
| Stage 0 | 0 point | Mild | 943 (53.3) | 1 | 0.1 |
| Stage 1 | 1 point | Moderate | 280 (15.8) | 2 | 0.7 |
| Stage 2 | 2-8 points | Severe I | 455 (25.7) | 17 | 3.7 |
| Stage 3 | 9-14 points | Severe II | 63 (3.6) | 16 | 25.4 |
| Stage 4 | ≥ 15 points | Most severe | 27 (1.5) | 16 | 59.3 |
| Total | 1786 (100) | 52 | 2.9 |
In 2004, nationwide survey of patients with acute pancreatitis in Japan who visited the hospitals in the year 2003 (from January 1 to December 31) was performed by stratified random sampling method. From the first survey, the total number of patients treated for acute pancreatitis in Japan in the year 2003 was estimated as 35 300 (95% confidence interval, 30 500-40 000). Clinical records of 1768 patients with acute pancreatitis were obtained in the second survey for analysis of etiology and outcome. Number of patients who died of acute pancreatitis or related complications.
Table 3.
The clinical features of patients
| CRAI group (n = 9) | Non-CRAI group (n = 9) | |
| Mean age | 48.0 ± 13.4 | 59.8 ± 15.1 |
| Gender (male/female) | 9/0 | 4/5 |
| Cause of pancreatitis | ||
| Alcoholic | 5 | 3 |
| Biliary | 1 | 4 |
| Hyperlipidemia | 1 | 0 |
| post-ERCP | 1 | 1 |
| Idiopathic | 1 | 1 |
| Severity score | ||
| Mean score (range) | 4.0 (2-7) | 3.6 (2-7) |
Measured parameters
The duration of abdominal pain and of systemic inflammatory response syndrome (SIRS) as well as the length of hospitalization were recorded. As biochemical markers of pancreatitis, the levels of serum pancreatic amylase (P-amylase), the white blood counts (WBC), and C-reactive protein (CRP) were examined on day 0 (onset of pancreatitis), day 1, day 3, day 7, and day 14. ELISAs were performed to determine serum IL-6, IL-8, IL-10, TNF-α, and MCP-1 concentrations on day 0 (onset of pancreatitis), day 1, day 3, day 7, and 14. Samples were determined with commercially available kits according to the manufacturer’s instructions for human IL-6, human IL-8, human IL-10, human TNF-α, and human MCP-1 (Biosource, Camarillo, CA, USA).
Statistical analysis
Results are expressed as mean ± SE. We analyzed the duration of abdominal pain, SIRS as well as the length of hospitalization using the proportional hazard model. Serum pancreatic enzymes, inflammation-related parameters, cytokines and chemokines were analyzed using the non-parametric Mann-Whitney U test. P values < 0.05 were considered significant. Pearson’s correlation analysis was used to calculate correlations between the data.
RESULTS
Duration of abdominal pain, SIRS, and hospitalization
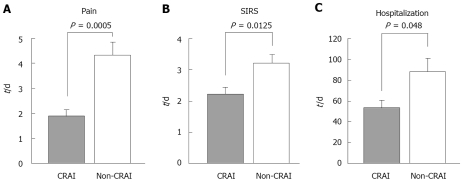
The duration of abdominal pain in the CRAI group was 1.9 ± 0.26 d (range, 1-3), whereas the duration in the non-CRAI group was 4.3 ± 0.50 (range, 3-8). Abdominal pain disappeared significantly in a short period of time after the initiation of CRAI with the protease inhibitor (P = 0.0005, Figure 1A). Similarly, SIRS disappeared significantly and shortly after the initiation of CRAI (P = 0.0125, Figure 1B). The duration of SIRS in the CRAI group was 2.2 ± 0.22 d (range, 1-3), whereas the duration in the non-CRAI group was 3.2 ± 0.28 (range, 2-4). The average length of hospitalization significantly differed between both groups, 53.3 ± 7.9 and 87.4 ± 13.9 d for the CRAI and non-CRAI, respectively. Patients in the CRAI group discharged significantly in a short period of time after the initiation of CRAI with gabexate mesilate (P = 0.048, Figure 1C).
Figure 1.
Changes in clinical parameters. The duration of abdominal pain (A) and of systemic inflammatory response syndrome (SIRS) (B) as well as the length of hospitalization (C) were investigated. Grey columns represent data for continuous regional arterial infusion using gabexate mesilate with antibiotics (CRAI-group) and white columns for non-CRAI group. Values are expressed as mean ± SE.
Changes in serum inflammation-related parameters
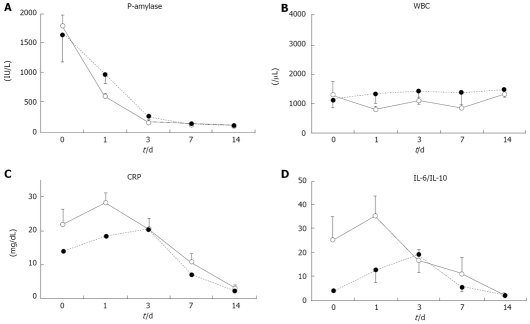
P-amylase and WBC quickly decreased, with no significant differences between the groups (Figure 2A and B). During the first two weeks of therapy, levels of serum CRP in the CRAI group rapidly decreased (Figure 2C). IL-6 and IL-10 in the CRAI group rapidly decreased in the same manner as the IL-6/IL-10 ratio (Figure 2D). On the other hand, both CRP and IL-6/IL-10 in the non-CRAI group tended to decrease slowly with a 2-d delay in peak values compared to those in the CRAI group, with no significant differences between the groups. Levels of serum IL-8, TNF-α, and MCP-1 over time did not significantly differ between the two groups (data not shown).
Figure 2.
Changes in serum inflammation-related parameters. The levels of serum pancreatic amylase (P-amylase) (A), the white blood counts (WBC) (B), C-reactive protein (CRP) (C, and IL-6/IL-10 ratio D) were examined on days 0 (onset of pancreatitis), 1, 3, 7, and 14. Straight lines give data for continuous regional arterial infusion using gabexate mesilate with antibiotics (CRAI-group) and dotted lines for non-CRAI group. Values are expressed as mean ± SE. No significant differences between the groups were observed.
DISCUSSION
Protease inhibitors are widely applied to treat acute pancreatitis in Japan; but since randomized controlled trials (RCTs) are difficult to conduct on patients with acute pancreatitis, only five RCTs have been examined gabexate mesilate[7-11]. The results of a meta-analysis of 4 among 5 trials were negative, and indicated that gabexate mesilate does not lower rates of surgical intervention or mortality. One of the reason was considered as follows; the protease inhibitors used to treat acute necrotizing pancreatitis cannot easily reach the pancreas when administered intravenously, and, because of ischemia or impaired microcirculation, they hardly penetrate into pancreatic tissue[12,13]. However, Chen et al[11] conducted an RCT and reported that continuous intravenous administration of high doses of gabexate mesilate (2400 mg/d) decreased the incidence of complications and mortality. On the other hand, since Takeda et al described arterial infusion with a protease inhibitor together with an antibiotic in Japan, severe acute pancreatitis has been treated by CRAI with nafamostat mesilate, and whereas an RCT has not been conducted, the usefulness of CRAI has been documented[14-17]. This strategy suppresses early inflammation and infection in pancreatic tissue, which controls subsequent systemic inflammation. The level of protease inhibitor in pancreatic tissues after CRAI using nafamostat was 5-fold higher than that delivered by intravenous injection, and trypsin activities in pancreatic tissues are significantly suppressed by CRAI[18]. On the other hand, the level of protease inhibitor in pancreatic tissues after CRAI using gabexate mesilate was 32-fold higher than that delivered by intravenous injection[19]. However, until now, CRAI using gabexate mesilate has not been examined sufficiently. In the present study, therefore, we investigated the usefulness of CRAI using gabexate mesilate for patients with SAP. The reasons for using gabexate mesilate were as follows: (1) Gabexate mesilate is the only intravenous protease inhibitor that has been proven effective in an RCT[11,20]. (2) Gabexate mesilate has a higher anticoagulant capacity than nafamostat mesilate[21]. (3) Gabexate mesilate induces less hyperkalemia even at high doses compared to nafamostat mesilate[22]. (4) In Japan, most studies on CRAI have used nafamostat mesilate, and more needs to be understood about gabexate mesilate.
All patients in this study had stage 2 pancreatitis, and since the severity was relatively mild, the patients were discharged in good health without requiring surgical intervention. The duration of pain, SIRS, and hospitalization was shorter for the CRAI group than the non-CRAI group. Previous studies of CRAI evaluated the mortality rates and surgical intervention in lethal SAP; but the present study suggested that CRAI is also effective against relatively milder forms of non-lethal SAP.
Blood cytokines and chemokines play important roles in the progression of severe acute pancreatitis. Local release of the proinflammatory cytokines, IL-18, TNF-α, and IL-1 upregulates IL-6. Levels of anti-inflammatory cytokines such as IL-10 also increase to maintain homeostasis. Excessive proinflammatory responses advance SIRS, and activated neutrophils and endothelial cells damage multiple organs. Ohmoto et al[23] reported that, during the healing process of acute pancreatitis, the IL-10/IL-6 ratio initially decreased, but increased as the pancreatitis improved. Put another way, IL-6/IL-10 ratio reveals an increase in a more severe stage of acute pancreatitis. We found here that IL-6 and IL-10 levels quickly increased and then decreased with therapy. The changes in the IL-6/IL-10 ratio were the same as those in CRP, but the ratio tended to decrease 2 d earlier in the CRAI group than in the non-CRAI group. These findings suggested that CRAI using gabexate mesilate effectively treats acute pancreatitis regarding biochemical features. On the other hand, changes in other proinflammatory cytokines such as IL-8 and TNF-α were not significant. However, among patients with relatively mild stage 2 SAP in the present study, the release of these cytokines in tissues was insufficient to increase and reflect in their blood concentrations.
Essentially, a large-scale RCT should be necessary to verify the effects of CRAI; but to conduct such a study on patients with highly lethal SAP seems to be unethical in Japan. A future RCT might consider enrolling patients with stage 2 pancreatitis that is relatively mild and less fatal than in the present study. In conclusion, the present results suggest that CRAI using gabexate mesilate was effective against SAP in terms of yielding clinical benefits for patients with SAP.
COMMENTS
Background
Severe acute pancreatitis (SAP) remains a lethal disease. Protease inhibitors are widely applied to treat acute pancreatitis in Japan; but the protease inhibitors used to treat acute necrotizing pancreatitis cannot easily reach the pancreas when administered intravenously, and, because of ischemia or impaired microcirculation, they hardly penetrate into pancreatic tissue. Recently, continuous regional arterial infusion (CRAI) with the protease inhibitor nafamostat mesilate and antibiotics has proven effective as an initial therapy. CRAI has been applied to treat SAP, but the evidence of its value is still scarce.
Research frontiers
The article focuses on the efficacy of CRAI using gabexate mesilate and antibodies for SAP.
Innovations and breakthroughs
The present study shows the efficacy of CRAI using gabexate mesilate for SAP, and the clinical benefits and sequential changes in serum inflammation-related parameters such as cytokines and chemokines. Abdominal pain and SIRS disappeared significantly in a short period of time after the initiation of CRAI with a protease inhibitor compared to non-CRAI. The average length of hospitalization significantly decreased with CRAI and patients discharged significantly in a shorter period of time after the initiation of CRAI with gabexate mesilate compared to non-CRAI.
Applications
CRAI using gabexate mesilate was shown to be effective against SAP in terms of clinical benefits for patients with SAP, and thus may provide a new strategy of treatment for SAP.
Peer review
Effect of continuous regional arterial infusion therapy with gabexate and antibiotics for SAP is very interesting clinical research. Known that SAP may have high mortality, some new modalities of therapy which improve prognosis of patients are welcome.
Acknowledgments
The authors thank Mr. Rife SE and Mr. Matsuo H for their contribution to this article.
Footnotes
Supported by Grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, No. 20590808; The Research Committee of Intractable Diseases of the Pancreas, provided by the Ministry of Health, Labour, and Welfare Japan, No. 50253448
Peer reviewer: Anton Vavrecka, MD, Clinic of Gastroenterology, SZU, NSP SV.CAM, Antolska 11, Bratislava 85107, Slovakia
S- Editor Zhong XY L- Editor Mihm S E- Editor Yin DH
References
- 1.Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 2.Al Mofleh IA. Severe acute pancreatitis: pathogenetic aspects and prognostic factors. World J Gastroenterol. 2008;14:675–684. doi: 10.3748/wjg.14.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda K, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Sekimoto M, Hirota M, Kimura Y, Isaji S, et al. JPN Guidelines for the management of acute pancreatitis: medical management of acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:42–47. doi: 10.1007/s00534-005-1050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otsuki M, Hirota M, Arata S, Koizumi M, Kawa S, Kamisawa T, Takeda K, Mayumi T, Kitagawa M, Ito T, et al. Consensus of primary care in acute pancreatitis in Japan. World J Gastroenterol. 2006;12:3314–3323. doi: 10.3748/wjg.v12.i21.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koizumi M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Sekimoto M, Hirota M, Kimura Y, Takeda K, et al. JPN Guidelines for the management of acute pancreatitis: diagnostic criteria for acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:25–32. doi: 10.1007/s00534-005-1048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa M, Hirota M, Hayakawa T, Matsuno S, Watanabe S, Atomi Y, Otsuki M, Kashima K, Koizumi M, Harada H, et al. Development and use of a new staging system for severe acute pancreatitis based on a nationwide survey in Japan. Pancreas. 2002;25:325–330. doi: 10.1097/00006676-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Valderrama R, Perez-Mateo M, Navarro S, Vazquez N, Sanjose L, Adrian MJ, Estruch J. Multicenter double-blind trial of gabexate mesylate (FOY) in unselected patients with acute pancreatitis. Digestion. 1992;51:65–70. doi: 10.1159/000200877. [DOI] [PubMed] [Google Scholar]
- 8.Yang CY, Chang-Chien CS, Liaw YF. Controlled trial of protease inhibitor gabexelate mesilate (FOY) in the treatment of acute pancreatitis. Pancreas. 1987;2:698–700. doi: 10.1097/00006676-198711000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Buchler M, Malfertheiner P, Uhl W, Scholmerich J, Stockmann F, Adler G, Gaus W, Rolle K, Beger HG. Gabexate mesilate in human acute pancreatitis. German Pancreatitis Study Group. Gastroenterology. 1993;104:1165–1170. doi: 10.1016/0016-5085(93)90288-n. [DOI] [PubMed] [Google Scholar]
- 10.Messori A, Rampazzo R, Scroccaro G, Olivato R, Bassi C, Falconi M, Pederzoli P, Martini N. Effectiveness of gabexate mesilate in acute pancreatitis. A metaanalysis. Dig Dis Sci. 1995;40:734–738. doi: 10.1007/BF02064970. [DOI] [PubMed] [Google Scholar]
- 11.Chen HM, Chen JC, Hwang TL, Jan YY, Chen MF. Prospective and randomized study of gabexate mesilate for the treatment of severe acute pancreatitis with organ dysfunction. Hepatogastroenterology. 2000;47:1147–1150. [PubMed] [Google Scholar]
- 12.Inoue K, Hirota M, Kimura Y, Kuwata K, Ohmuraya M, Ogawa M. Further evidence for endothelin as an important mediator of pancreatic and intestinal ischemia in severe acute pancreatitis. Pancreas. 2003;26:218–223. doi: 10.1097/00006676-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Mikami Y, Fukuyama S, Egawa S, Sunamura M, Ishibashi T, Sato A, Masamune A, Matsuno S. Pancreatic ischemia associated with vasospasm in the early phase of human acute necrotizing pancreatitis. Pancreas. 2005;30:40–49. [PubMed] [Google Scholar]
- 14.Takeda K, Matsuno S, Sunamura M, Kakugawa Y. Continuous regional arterial infusion of protease inhibitor and antibiotics in acute necrotizing pancreatitis. Am J Surg. 1996;171:394–398. doi: 10.1016/S0002-9610(97)89617-1. [DOI] [PubMed] [Google Scholar]
- 15.Anai H, Sakaguchi H, Uchida H, Matsuo N, Tanaka T, Yoshioka T, Ohishi H, Murao Y, Miyamoto S. Continuous arterial infusion therapy for severe acute pancreatitis: correlation between CT arteriography and therapeutic effect. J Vasc Interv Radiol. 1999;10:1335–1342. doi: 10.1016/s1051-0443(99)70240-x. [DOI] [PubMed] [Google Scholar]
- 16.Imaizumi H, Kida M, Nishimaki H, Okuno J, Kataoka Y, Kida Y, Soma K, Saigenji K. Efficacy of continuous regional arterial infusion of a protease inhibitor and antibiotic for severe acute pancreatitis in patients admitted to an intensive care unit. Pancreas. 2004;28:369–373. doi: 10.1097/00006676-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Matsuno S, Ogawa M, Watanabe S, Atomi Y. Continuous regional arterial infusion (CRAI) therapy reduces the mortality rate of acute necrotizing pancreatitis: results of a cooperative survey in Japan. J Hepatobiliary Pancreat Surg. 2001;8:216–220. doi: 10.1007/s005340170019. [DOI] [PubMed] [Google Scholar]
- 18.Kakugawa Y, Takeda K, Sunamura M, Kawaguchi S, Kobari M, Matsuno S. [Effect of continuous arterial infusion of protease inhibitor on experimental acute pancreatitis induced by closed duodenal loop obstruction] Nippon Shokakibyo Gakkai Zasshi. 1990;87:1444–1450. [PubMed] [Google Scholar]
- 19.Satoh H, Harada M, Tashiro S, Shiroya T, Imawaka H, Machii K. The effect of continuous arterial infusion of gabexate mesilate (FOY-007) on experimental acute pancreatitis. J Med Invest. 2004;51:186–193. doi: 10.2152/jmi.51.186. [DOI] [PubMed] [Google Scholar]
- 20.Pederzoli P, Cavallini G, Falconi M, Bassi C. Gabexate mesilate vs aprotinin in human acute pancreatitis (GA.ME.P.A.). A prospective, randomized, double-blind multicenter study. Int J Pancreatol. 1993;14:117–124. doi: 10.1007/BF02786117. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, Shibata A. The comparative study of nafamostat mesilate (FUT-175), gabexate mesilate (FOY) and heparin on anticoagulant and antifibrinolytic action. Jpn J Clin Exp Med. 1988;65:127–134. [Google Scholar]
- 22.Muto S, Imai M, Asano Y. Effect of nafamostat mesilate on Na+ and K+ transport properties in the rabbit cortical collecting duct. Br J Pharmacol. 1993;109:673–678. doi: 10.1111/j.1476-5381.1993.tb13626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohmoto K, Yamamoto S. Serum interleukin-6 and interleukin-10 in patients with acute pancreatitis: clinical implications. Hepatogastroenterology. 2005;52:990–994. [PubMed] [Google Scholar]