Abstract
Disorders of semantic cognition in different neuropsychological conditions result from diverse areas of brain damage and may have different underlying causes. This study used a comparative case-series design to examine the hypothesis that relatively circumscribed bilateral atrophy of the anterior temporal lobe in semantic dementia (SD) produces a gradual degradation of core semantic representations, whilst a deficit of cognitive control produces multi-modal semantic impairment in a subset of patients with stroke aphasia following damage involving the left prefrontal cortex or regions in and around the temporoparietal area; this condition, which transcends traditional aphasia classifications, is referred to as ‘semantic aphasia’ (SA). There have been very few direct comparisons of these patient groups to date and these previous studies have focussed on verbal comprehension. This study used a battery of object-use tasks to extend this line of enquiry into the non-verbal domain for the first time. A group of seven SA patients were identified who failed both word and picture versions of a semantic association task. These patients were compared with eight SD cases. Both groups showed significant deficits in object use but these impairments were qualitatively different. Item familiarity correlated with performance on object-use tasks for the SD group, consistent with the view that core semantic representations are degrading in this condition. In contrast, the SA participants were insensitive to the familiarity of the objects. Further, while the SD patients performed consistently across tasks that tapped different aspects of knowledge and object use for the same items, the performance of the SA participants reflected the control requirements of the tasks. Single object use was relatively preserved in SA but performance on complex mechanical puzzles was substantially impaired. Similarly, the SA patients were able to complete straightforward item matching tasks, such as word-picture matching, but performed more poorly on associative picture-matching tasks, even when the tests involved the same items. The two groups of patients also showed a different pattern of errors in object use. SA patients made substantial numbers of erroneous intrusions in their demonstrations, such as inappropriate object movements. In contrast, response omissions were more common in SD. This study provides converging evidence for qualitatively different impairments of semantic cognition in SD and SA, and uniquely demonstrates this pattern in a non-verbal expressive domain—object use.
Keywords: semantic dementia, stroke aphasia, semantic memory, semantic cognition, non-verbal, object use
Introduction
Semantic cognition refers to the processes and representations that underlie our understanding and use of the meanings of words, pictures, objects, sounds, faces and events (Rogers et al., 2004; Jefferies and Lambon Ralph, 2006; Lambon Ralph et al., 2007). It plays a critical role in many everyday activities, not only in the verbal domain (for the transmission of meaning between the speaker and listener) but also in a range of non-verbal situations, such as knowing how objects are used (Bozeat et al., 2000; 2002). Impairments of semantic cognition are, consequently, highly debilitating and can arise in a range of disorders including semantic dementia (SD) and some patients with aphasia following a stroke; hereafter referred to as semantic aphasia (SA). The qualitative nature of the impairment, however, is dependent on which component of semantic cognition is affected in a particular patient group.
SD and SA are two disorders of semantic cognition that show striking behavioural and neuroanatomical contrasts. Patients with SD exhibit arguably the most selective disorder of semantic memory (SD, the temporal variant of frontotemporal dementia: Snowden et al., 1989; Hodges et al., 1992), which is associated with relatively circumscribed atrophy affecting the inferior and lateral aspects of the anterior temporal lobes (ATLs) bilaterally (Mummery et al., 2000; Nestor et al., 2006). A central semantic impairment characterizes this disorder such that patients perform poorly on tasks tapping the full range of verbal and non-verbal modalities in both receptive and expressive tasks (Lambon Ralph et al., 1998, 1999; Bozeat et al., 2000; Coccia et al., 2004; Luzzi et al., 2007). Moreover, they exhibit very high correlations and item consistency across different semantic tasks irrespective of the modality probed or other variations in task demands (Bozeat et al., 2000; Jefferies and Lambon Ralph, 2006). In contrast, abilities in all other domains, such as phonology, visual processing and decision-making remain largely preserved. These findings suggest that SD is characterized by progressive degradation of amodal semantic knowledge within the ATL; a view that has been supported by Rogers’ (2004) implemented computational model (see also the theory of ‘convergence zones’; Damasio and Damasio, 1989).
Multimodal semantic impairments are also observed in some patients with aphasia after stroke (Chertkow et al., 1997; Jefferies and Lambon Ralph, 2006). Key to identifying this disorder, all patients with SA exhibit impaired performance across the same range of verbal and non-verbal semantic tasks that are routinely failed by SD patients (e.g. word and picture versions of the Camel and Cactus semantic association task; Bozeat et al., 2000). Although other aspects of aphasia may vary amongst patients, SA cases share many features of their broader neuropsychological profiles with SD—for example, patients with SD and transcortical sensory aphasia (a subtype of SA) both show poor comprehension in the context of fluent speech and good repetition. Similar multimodal semantic impairments in SD and SA, however, follow very different patterns of brain damage. In contrast to the bilateral ATL atrophy in SD, patients with SA commonly have damage affecting the left prefrontal cortex (PFC) and/or areas within the left temporoparietal region (Hart and Gordon, 1990; Chertkow et al., 1997; Berthier, 2001; Saygin et al., 2003; Jefferies and Lambon Ralph, 2006). Although some SA patients have more widespread lesions beyond these key regions, the extent of damage through the temporal lobe is never sufficiently anterior to encroach on important areas of atrophy in SD—crucially the temporal pole is always spared (Noonan et al., 2009).
In a recent comparative case-series study of SD and SA patients, Jefferies and Lambon Ralph (2006) found that the distinct patterns of neural damage in these disorders gave rise to striking differences in the qualitative nature of their respective deficits (Jefferies et al., 2007, 2008). Although the two groups of patients exhibited impairments of equal severity on a range of word- and picture-based tests of conceptual knowledge, the SA patients’ performance was far less consistent than observed in SD and appeared to reflect their compromised semantic control. Unlike the SD group, these patients had relatively good levels of conceptual knowledge but had difficulty shaping activation within the semantic system, impairing their performance on a range of semantic tasks tapping different input/output modalities. Consequently, the SA patients’ performance differed qualitatively from the SD group in the following ways: (i) they were much less consistent across tasks than the SD group—and were strongly influenced by the task demands; (ii) as well as coordinate and superordinate naming errors (e.g. squirrel → ‘rabbit’ or ‘animal’), the SA patients produced associative errors (e.g. squirrel → ‘nuts’) which were almost never observed in SD; (iii) naming and comprehension scores were improved or diminished by the provision of cues or other forms of task constraint (which had little or no effect in SD); (iv) the SA patients exhibited semantic ‘access’ and refractory symptoms in contrast to the ‘storage’ deficits of SD (Warrington and McCarthy 1983; Warrington and Cipolotti 1996; Gotts and Plaut, 2002); and (v) their performance on comprehension tasks was predicted by the control requirements within an individual trial (see Table 1 for a summary of differences between groups). There was an association between patients’ scores on semantic and executive tasks uniquely in the SA group, suggesting that their semantic deregulation was a symptom of a more general executive deficit.
Table 1.
Summary of differences between SD and SA patients
| Symptom | SD | SA |
|---|---|---|
| Lesion | Bilateral anterior temporal | Left prefrontal/ temporoparietal |
| Verbal comprehension | Poor | Poor |
| Non-verbal comprehension | Poor | Poor |
| Consistency/task correlations | ||
| Within task | ✓ | ✓ |
| Between task | ✓ | ✗ |
| Effect of phonemic cueing | ✗ | ✓ |
| Familiarity/frequency effects across tasks | ✓ | ✗ |
| Strong effect for requirement of semantic control | ✗ | ✓ |
| Associative semantic errors in picture naming | ✗ | ✓ |
See Jefferies and Lambon Ralph (2006) for further detail.
When patients with SD are considered alongside those with SA, it is clear that the bilateral ATL as well as the left PFC and temporoparietal region all make a necessary contribution to semantic cognition. Recent findings suggest these contributions are very different, with anterior temporal areas forming a central semantic store of amodal knowledge, and the left PFC and temporoparietal region contributing to semantic control processes. This emerging story is broadly consistent with the functional neuroimaging literature, which reports activation in the same network of frontal, temporal and parietal regions when healthy participants engage in semantically demanding tasks (provided that the findings of both PET and fMRI studies are considered, see Devlin et al., 2000; Visser et al., 2009a,b). All three regions are activated by semantic judgements for both pictures and words (Vandenberghe et al., 1996; Perani et al., 1999; Chee et al., 2000; Postler et al., 2003; Bright et al., 2004), in line with the deficits of SD and SA patients on both picture and word tasks (Jefferies and Lambon Ralph, 2006). Moreover, functional neuroimaging studies show that the key cortical regions affected in patients with SA—areas within the left PFC and temporoparietal region—are both sensitive to the cognitive control demands of semantic tasks (Thompson-Schill et al., 1997; Wagner et al., 2001; Gold and Buckner, 2002; Noppeney et al., 2004), suggesting that these two cortical regions may work in tandem to underpin semantic control (see Discussion section for further details, Garavan et al., 2000; Collette et al., 2005).
The study by Jefferies and Lambon Ralph (2006) provided a theoretical framework that integrates the findings from SD and SA patients, and allows the neuropsychological literature to align with functional neuroimaging studies. In particular, Jefferies and Lambon Ralph highlighted the critical role played by regulatory control processes in semantic cognition. These findings were reinforced in a subsequent study which, using a battery of mostly word-based tasks, uncovered important characteristics of a semantic control disorder (Noonan et al., 2009). SA patients in this study performed poorly on semantic judgement tasks when selection was made more difficult with highly distracting, semantically related foils but also when accessing less pre-potent aspects of semantic knowledge. Accuracy on a synonym judgment task, for example, was much lower when a probe item (e.g. ‘fire’) had to be matched with a subordinate associate (e.g. ‘rifle’) compared to a more dominant correlate (e.g. ‘hot’). The ability to control or shape activation within the semantic system is not only essential for language based tasks but also for activities in the non-verbal domain. Consider, for example, how different facets of knowledge relating to a piano become significant when using it as an instrument compared to moving the object around; in the first instance it is necessary to understand the relationship between individual keys and corresponding sounds in order to make the fine motor movements that produce musical notes, whereas information about the object's overall size, shape and weight is essential for the latter activity (Saffran, 2000). Although damage to a core control component would be expected to produce semantic impairment across a full range of modalities, SA has largely been explored using only verbal tasks (e.g. Jefferies and Lambon Ralph, 2006; Noonan et al., 2009), which is potentially problematic given that these patients were drawn from the wider aphasic population. In the current study, therefore, we extended the case-series comparison approach to an indisputably non-verbal domain—object use (previously employed to examine the SD group by Bozeat et al., 2002). By directly comparing SA and SD patients on the same object-use battery, we can establish whether SA patients have genuine non-verbal difficulties and also if there are qualitative differences between the groups consistent with the hypothesis that patients with SD suffer from a gradual degradation of core semantic representations, whilst the semantic impairment in SA reflects poor cognitive control. If our hypothesis is correct then the object use results should parallel the characteristics observed in the verbal domain specifically:
SA patients should be less sensitive to the frequency/familiarity of objects than SD patients;
The level of object use knowledge in receptive tasks in SA should depend to a large extent on the semantic control demands of each task such that patients exhibit more impaired performance when making judgements about objects on the basis of specific attributes defined by the task, rather than global semantic similarities. Patients with SD, in contrast, would be expected to perform more consistently across different tasks;
The effect of control demands on SA patients’ object use knowledge should also be observable in the expressive domain. Consequently, their ability to use objects should depend to a greater extent on the control requirements of the task, rather than the involvement of conceptual knowledge per se. Although everyday object use demonstration requires semantic knowledge, for example, its relatively straightforward nature means that SA patients should perform more accurately on this task than a non-semantic but executively demanding mechanical puzzles test. The opposite pattern would be expected from the SD group given their intact performance on standard executive tasks in the face of impoverished semantic knowledge (Jefferies and Lambon Ralph, 2006); and
SA patients should make errors that reflect a lack of top-down control over object use, such as inappropriate object movements, which have not previously been noted as a central characteristic of object use demonstrations given by SD patients (Bozeat et al., 2002).
Method
Participants
Seven SA patients with semantic impairment in the context of stroke aphasia were recruited from stroke clubs and speech and language therapy services in Manchester, UK (previously reported in Jefferies and Lambon Ralph, 2006). Patients with verbal comprehension deficits were initially screened and enrolled in the study if they scored at least two standard deviations below the control mean for both picture and word tests of semantic association [Camel and Cactus Task (CCT), Bozeat et al., 2000; Table 5]. Control data for this task were collected from 20 participants who were matched in education to the SA group and were of a similar age (mean years in education: control: 10.8; SA: 10.7; mean age in years: control: 71.8; SA: 62). The CCT assesses associative semantic knowledge by asking participants to match a probe item with a semantically related target (e.g. camel → cactus) while rejecting three other possible responses (e.g. tree, sunflower, rose). The CCT was a suitable screening tool because it provided a measure of comprehension in the verbal domain but also more non-verbally using picture stimuli. Further, because the task employs a relatively difficult four alternative-forced-choice (AFC) paradigm it can detect milder semantic impairments that might be missed by a similar 2AFC association task [Pyramids and Palm Trees (PPT) task; Howard and Patterson, 1992]; all but one case in this study, however, failed both association tasks (Table 5).
Table 5.
Assessments of general semantic function
| Task | Max | Control mean (SD) | SD mean | SA mean | SA |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N.Y. | S.C. | P.G. | B.B. | M.E. | L.S. | K.A. | |||||
| Word PPT | 52 | 51.1 (1.1) | 40 | 41 | 42 | 51 | 43 | 35 | 39 | 39 | 44 |
| Picture PPT | 52 | 51.2 (1.4) | 22 | 40 | 47 | 50 | 42 | 41 | 29 | 31 | 44 |
| Naming | 64 | 62.3 (1.6) | 22 | 21 | 55 | 28 | 46 | 10 | 5 | 5 | 0 |
| WPM | 64 | 63.7 (0.5) | 40 | 49 | 60 | 59 | 58 | 54 | 50 | 37 | 26 |
| Word CCT | 64 | 60.7 (2.06) | 40a | 36 | 39 | 56 | 40 | 30 | 34 | 16 | 36 |
| Picture CCT | 64 | 58.9 (3.1) | 49a | 34 | 36 | 46 | 44 | 38 | 13 | 16 | 46 |
| Category fluency (6) | – | 95.7 (16.5) | 23 | 19 | 25 | 17 | 4 | 13 | 25 | 11 | 0 |
Each patient enrolled in the study had chronic impairment from a stroke at least a year previously (Table 2, Fig. 1). Four were transcortical sensory aphasia (TSA) patients. The remainder had less fluent speech and/or poorer repetition. All participants spoke English as their first language. Hearing was not explicitly tested but any patients with age-related hearing loss used their hearing aids during testing. Educational level was not a criterion for selection so the control participants were matched on this variable. In order to assess the same patients in this study that have previously been examined with tasks in the verbal domain (Jefferies and Lambon Ralph, 2006; Noonan et al., 2009), hemiplegia was not a criterion for exclusion. Given the uniquely practical nature of the tasks employed in this study, however, we looked for associations between object use and patients’ degree of hemiplegic impairment. Four indicators were used as measures of limb equivalence, which were: limb coordination, strength, proprioception and skin sensation (light and sharp touch, see McLeod and Lance, 1989). Each patient was awarded a score out of five where a low score indicated more severe hemiplegia (Table 2). As limb equivalence scores showed no correlation with accuracy for either semantic or non-semantic object use tasks assessed in this study (i.e. object use demonstration: r = 0.06, NS; mechanical puzzles task: r = 0.09, NS; see below for details), hemiplegia will not be considered further in the following analyses.
Table 2.
Background details for SA patients
| Case | Age | Sex | Education (leaving age) | Aetiology of stroke | Years since stroke | Aphasia type | BDAE compre hension percentile | BDAE fluency percentile | BDAE repetition percentile | Non-word repetition (%) | Word repetition (%) | Limb equivalence score (max = 5)a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N.Y. | 63 | M | 15 | 4.5 | Conduction | 47 | 37 | 40 | 40 | 81 | 0 | |
| S.C. | 76 | M | 16 | Haemorrhage | 5.5 | Anomic/TSA | 37 | 90 | 60 | 87 | 98 | 5 |
| P.G. | 59 | M | 18 | Subarachnoid haemorrhage | 5 | TSA | 20 | 40 | 80 | 73 | 91 | 2 |
| B.B. | 55 | F | 16 | Subarachnoid haemorrhage | 2.5 | Mixed transcortical | 10 | 17 | 55 | 83 | 96 | 4 |
| M.E. | 36 | F | 16 | Subarachnoid haemorrhage | 6.5 | TSA | 33 | 100 | 100 | 93 | 100 | 4 |
| L.S. | 71 | M | 15 | 3 | TSA | 13 | 90 | 90 | 90 | 96 | 3 | |
| K.A. | 74 | M | 14 | Thomboembolic/ partial haemorrhage | 1 | Global | 0 | 23 | 0 | 0 | 0 | 3 |
Patients arranged in order of their word–picture matching scores from the Cambridge 64 item battery. BDAE = Boston Diagnostic Aphasia Examination (Goodglass, 1983). Comprehension scores derived from word discrimination, commands and complex ideational material. Fluency percentile is derived from phrase length, melodic line and grammatical form ratings. Repetition percentile is average of word and sentence repetition. Transcortical sensory aphasia (TSA) was defined as good or intermediate fluency/repetition and poorer comprehension. Aphasia classifications were confirmed by an experienced speech and language therapist. Word/non-word repetition: Tests 8 and 9 from Psycholinguistic Assessments of Language Processing in Aphasia (PALPA, Kay et al., 1992).
a Four hemiplegia indicators were used to generate a score of limb equivalence (limb coordination, strength, proprioception and skin sensation, see McLeod et al., 1989). A low score indicates more severe hemiplegia.
Figure 1.
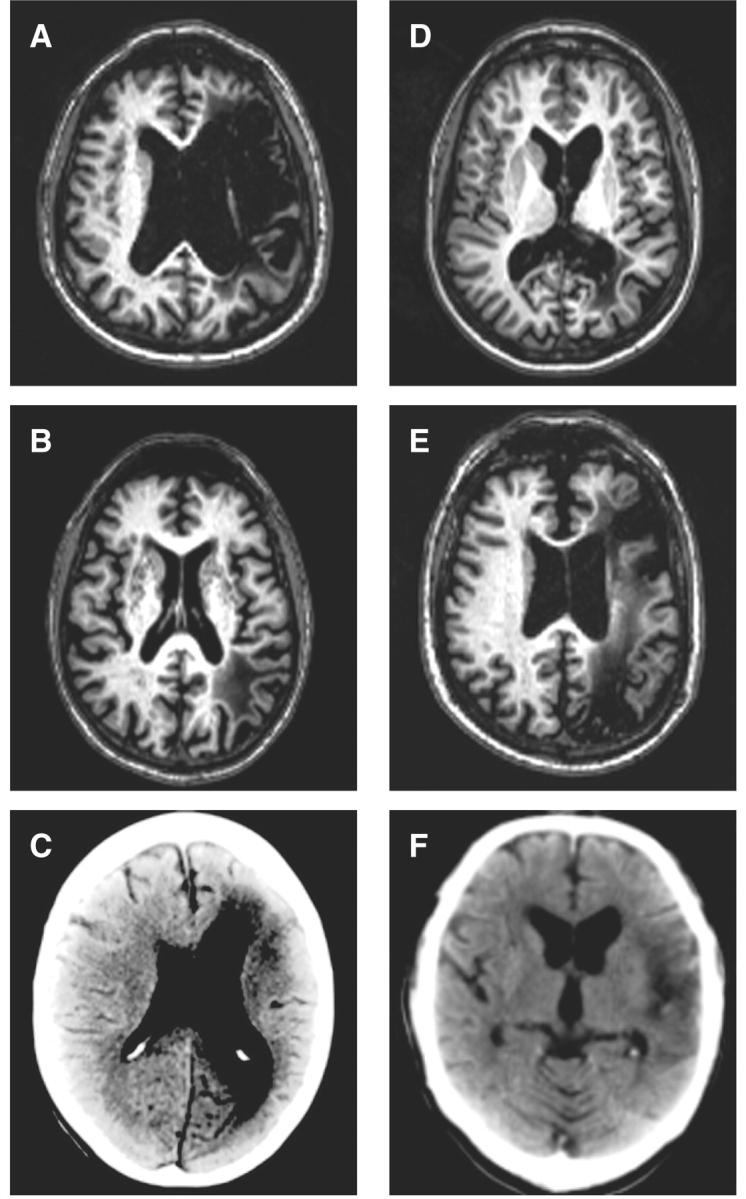
Neuroimaging for the SA group. (A) N.Y. (MRI); (B) S.C. (MRI); (C) B.B. (CT); (D) M.E. (MRI); (E) L.S. (MRI); (F) K.A. (CT). One SA patient (P.G.) was unable to have an MRI scan for medical reasons.
Lesion analyses
CT/MRI scans were available for 6/7 SA patients. A previous CT scan was not available for P.G. but the associated neuroradiology report described a left frontal lesion and no explicit reference was made to posterior damage. Contraindications prevented further MR scanning in this case. For the remaining six cases, scans were manually traced onto Damasio's standardized templates (Damasio and Damasio, 1989). It is important to highlight that SAH could have caused widespread disruption in some patients, which might have contributed to their neuropsychological impairments. However, the following analyses will only provide details of patients’ focal lesions.
Common areas of damage were revealed in the left prefrontal and/or left temporoparietal region; 4/6 patients had damage to both regions. The remaining two cases had damage affecting the temporoparietal but not frontal region. Table 3 gives a breakdown of patients’ lesions relative to regions of interest defined by previous functional neuroimaging and neuropsychological studies of semantic cognition (Hart and Gordon, 1990; Demb et al., 1995; Chertkow et al., 1997; Thompson-Schill et al., 1997; Wagner et al., 2001; Vigneau et al., 2006). Patients showed considerable destruction of perisylvian language areas, with the highest occurrence of damage in BA44 of the frontal lobe and BA37 of the posterior temporal lobe. When white matter disruption was considered, inferior parietal structures (BA 39/40) were also commonly implicated. Some cases exhibited more widespread lesions such as M.E. who, in addition to serious destruction of the posterior occipitotemporal area and white matter immediately underlying the cortex in angular and supramarginal gyri, also displayed damage in middle and inferior temporal as well as fusiform gyri. Nonetheless, where lesions did extend beyond the two key areas of damage in SA, they rarely impinged on more anterior portions of the temporal lobe and always spared the temporal pole—a critical region of atrophy in SD.
Table 3.
SA patients’ lesion characteristics and patterns of cooccurrence
| Case | L frontal lesion | L temporo-parietal lesion | DLPFC |
orbIFC | trIFG | opIFG | STG | MTG | ITG | FG | POT | AG | SMG | TP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA9 | BA46 | BA47 | BA45 | BA44 | BA22 | BA21 | BA20 | BA36 | BA37 | BA39 | BA40 | BA38 | |||
| N.Y. | ✓ | ✓ | 1 | 1 | 2 | 2 | 2 | 1 | – | – | – | – | 2 | 2 | – |
| S.C. | ✗ | ✓ | – | – | – | – | – | – | – | 2 | – | 2 | 2 | w | – |
| B.B.a | ✓ | ✓ | – | – | 2 | 2 | 2 | 2 | – | – | – | – | – | – | – |
| M.E. | ✗ | ✓ | – | – | – | – | – | – | 2 | 2 | 2 | 2 | w | w | – |
| L.S. | ✓ | ✓ | 2 | 1 | 1 | 2 | 2 | – | 2 | 2 | – | 2 | 2 | 1 | – |
| K.A. | ✓ | ✓ | – | – | – | – | 2 | 2 | 1 | – | – | 2 | – | 2 | – |
| Percentage of patients with grey matter damage | 33 | 33 | 50 | 50 | 67 | 50 | 50 | 50 | 17 | 67 | 50 | 50 | 0 | ||
| Percentage of patients with grey or white matter damage | 33 | 33 | 50 | 50 | 67 | 50 | 50 | 50 | 17 | 67 | 67 | 83 | 0 | ||
All data previously reported by (Noonan et al., 2009). Patients arranged in order of their word-picture matching scores from the Cambridge 64 item battery. Quantification of lesion: 2 = complete destruction/serious damage to cortical grey matter; 1 = partial destruction/mild damage to cortical grey matter; w = damage confined to white matter immediately underlying cortex.
a BB showed additional signs of ventricular enlargement in the left hemisphere.
DLPFC = dorsolateral prefrontal cortex; orbIFG = pars orbitalis in inferior frontal gyrus; trIFG = pars triangularis in inferior frontal gyrus; opIFG = pars opercularis in inferior frontal gyrus; TP = temporal pole; STG = superior temporal gyrus; MTG = middle temporal gyrus; ITG = inferior temporal gyrus; FG = fusiform gyrus; POT = posterior occipitotemporal area; SMG = supramarginal gyrus; AG = angular gyrus.
The SA cases were compared with eight SD patients recruited through the Memory and Cognitive Disorders Clinic at Addenbrooke's Hospital, Cambridge, UK who were previously described by Bozeat et al.. (2002). The SD patients showed bilateral ATL atrophy and met all of the diagnostic criteria for SD, including anomia, impairment in single-word comprehension and impoverished semantic knowledge with relative preservation of phonology, syntax, visuospatial abilities and day-to-day memory (Hodges et al., 1992, 1995). The SD group was age and education matched to our SA patients (mean age in years: SA: 62; SD: 64; mean years in education: SA: 10.7; SD: 10.5). Ten healthy participants from the subject panel of the MRC Cognition and Brain Sciences Unit, Cambridge provided age and education matched control data (Bozeat et al., 2002).
Non-semantic background tests
Background neuropsychological tests included forward and backward digit span (Wechsler, 1987); verbal fluency, i.e. the number of words starting with F, A and S that could be produced in one minute; the Raven's Coloured Progressive Matrices test of non-verbal reasoning (Raven, 1962); the Wisconsin Card Sort test (WCST; Milner, 1964; Stuss et al., 2000); the Elevator Counting tasks with and without distraction from the Test of Everyday Attention (Robertson et al., 1994) and four subtests from the Visual Object and Space Perception battery (VOSP; dot counting, position discrimination, number location and cube analysis; Warrington and James, 1991).
Semantic background tests
The patients completed the word and picture versions of the PPT test of semantic association (PPT; Howard and Patterson, 1992). In addition, we used the Cambridge 64-item semantic battery to assess knowledge of the same 64 items across a range of input and output modalities (Bozeat et al., 2000). This included the following tests: (i) picture naming (using items from Snodgrass et al., 1980); (ii) spoken word-to-picture matching with nine semantically related distracters; (iii) the Camel and Cactus test, which like the PPT examined judgements of semantic association for words and pictures; and (iv) category fluency that required participants to generate as many exemplars in one minute from each of six different categories.
General praxis
Meaningless gesture imitation
Participants were asked to imitate 10 meaningless gestures that were demonstrated by the examiner (Goldenberg, 1996). Two marks were awarded if the gesture was performed correctly on the first attempt and no points were awarded if the gesture was not correctly produced within two attempts.
Pantomime to verbal command
In order to further rule out the possibility of ideomotor apraxia in SA, these patients were also assessed on their ability to pantomime eight gestures to simple verbal command (e.g. ‘salute’; taken from Strub and Black, 1987). One point was awarded if the gesture was produced correctly on first attempt.
Mechanical puzzles
This task required a combination of problem solving and motor control. Participants were asked to remove a wooden block from a Perspex cylinder using the most appropriate tool from a choice of four implements (Ochipa et al., 1992). Nine different cylinders were presented on separate trials. Each trial was scored for both tool selection and application. One point was awarded if the correct tool was selected on the participant's first attempt (total possible selection score of nine). If an incorrect selection was made, the examiner provided the correct tool for the participant. Tool application was scored out of two, with full marks awarded if the wooden block was removed from the cylinder at once. One point was awarded if the block was removed after a period of hesitation and/or trial and error (total possible application score of 18).
Object-use battery
This battery of tasks tapped object use and knowledge for the same 36 items drawn from three categories (tools, items of stationery and kitchen implements; Bozeat et al., 2002).
- Attribute-matching tasks: three picture–picture matching tests were used to assess knowledge of specific attributes of the 36 items (their recipients, functions and actions). Colour digital photographs were used to present the probe item and four possible responses (the target and three visually/semantically related foils).
- Matching to recipient: participants had to match the probe object with its canonical recipient. For example, the garlic press would be matched with a garlic clove as opposed to the foil responses (cheese, onion and pepper).
- Matching to function: this task required participants to match a probe item with an object with the same function. For example, the correct response for garlic press would be pestle and mortar instead of corkscrew, scissors or pliers.
- Matching-to-action: participants matched the probe object to an item that is moved or manipulated in the same way (although the objects might be held differently). For example, garlic press would be matched to the secateurs as both objects require a common cutting action not shared by the foils (corkscrew, bottle opener and compass).
- Cross-modal item-matching tasks
- Word-picture matching: participants had to select the item from an array of eight pictures when given the spoken name of the target. The foils were drawn from the same category as the target (i.e. tools, stationery items or kitchen implements).
- Action-picture matching: participants were shown the picture arrays from the word-picture matching task but instead of being given the name of the target item, they selected the picture that matched a mime of the object's use.
Naming: objects were presented one at a time for the participants to name.
Single object use: Patients were given each of the 36 items one at a time and asked to demonstrate how they should be used. The demonstrations were videotaped and later scored for accuracy against a set of target features determined from control participants’ object demonstrations (Bozeat et al., 2002). Object use scores comprised three components: hold (including grasp and position on the object), movement and orientation. Points were lost from the total accuracy score if an essential feature of the object's use was omitted from a demonstration. There were a small number of instances in which a participant clearly indicated how an object would be used but could not demonstrate its action due to hemiplegia; these were not scored as errors.
This scoring system was developed by Bozeatet al.. (2002) to capture the performance of patients with SD. In previous studies, Bozeat et al.. had found that SD patients either demonstrated aspects of object use correctly (in line with the degree of remaining semantic knowledge for that object) or omitted such features. They produced very few intrusive features, and thus there was no need to capture such errors within the scoring system.
From our first explorations of object use in SA, it was clear that the SD pattern was not entirely replicated; object use in the SA group was characterized by the intrusion of erroneous information that was not integral to the object's correct use (in addition to the omission of features as in SD). Hence, as well as scoring for the presence of target features using the Bozeat feature scoring method, each component of object use (hold, movement and orientation) was scored for the occurrence of an erroneous intrusion in the SA group (see Table 6 for examples).
Table 6.
Examples of intrusion errors
| Object | Action description | Intrusion error present? |
||
|---|---|---|---|---|
| Hold | Movement | Orientation | ||
| Apple corer | Object picked up in both hands with head orientated towards the floor. Orientation of object changed so that head points upwards. Left hand used to examine the head of the tool whilst right hand grasps handle. Orientation changed so that tool is horizontal. Tool is held at cusp between handle and head, with head pointing upwards, and rocked backwards and forwards in a seesaw motion. Index finger of left hand placed into tool head and removed. Object rocked back and forth again. | Yes | Yes | Yes |
| Wallpaper scraper | Tool grasped in left hand with head orientated upwards. Tool is pushed up and pulled back down repetitively as if scraping a wall. Head of scraper is rocked towards and away from the body. | No | Yes | No |
| Hammer | Hammer gripped on the handle and moved in a seesaw motion. Claw points downwards and hammering surface points towards the ceiling. | No | Yes | Yes |
| Watering can | Handle of object gripped in the left hand. Watering can is tipped towards the floor and the object is moved backwards and forwards. | No | No | No |
Descriptions are given for the object use demonstrations of K.A.
Because some of the patients in this study had profound comprehension impairments (e.g. K.A.), we used practice trials and multimodal instructions to ensure that the purpose of the task was understood. The experimenter was vigilant for evidence of misunderstanding. Some tasks, such as object use demonstration, could be explained easily through verbal instructions and demonstrations. Attribute matching tasks were arguably the most difficult to comprehend but above chance performance suggests that all patients understood the purpose of these tasks.
Results
Non-semantic background tests
In comparison to controls, all of the SA patients showed some evidence of executive/attentional dysfunction (Table 4). Out of seven, three SA patients were impaired on forward digit span and 2/7 on backward digit span tasks. Letter fluency was impaired for all cases except S.C. Two cases (M.E. and K.A.) obtained scores outside the normal range on the Coloured Progressive Matrices test and the others scored at or below the 50th percentile. Three SA participants (P.G., M.E. and L.S.) were impaired on the WCST. The elevator-counting task without distraction was performed below the normal cut-off by four participants (N.Y., P.G., B.B. and L.S.; K.A. not tested) and most participants were impaired when tested with distraction. In addition, all of the SA patients, except N.Y., were impaired on at least one subtest of the VOSP.
Table 4.
Background neuropsychological assessments
| Task | Max | Control mean (SD) | Normal cut-off | SD mean | SA mean | SA |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N.Y. | S.C. | P.G. | B.B. | M.E. | L.S. | K.A. | ||||||
| Digit span | ||||||||||||
| Forward | – | 6.8 (0.9) | 5 | 5.88 | 4.29 | 3a | 6 | 6 | 5 | 6 | 4a | 0a |
| Backward | – | 4.7 (1.2) | 2 | 4.13 | 1.67 | 2 | 2 | 2 | 0a | 3 | 1a | NT |
| Letter fluency (total FAS) | – | 44.2 (11.2) | – | 13.13 | 7.57 | 5a | 24 | 2a | 0a | 14a | 8a | 0a |
| Raven's coloured progressive matrices (percentile score) | – | – | All cases ≥75 | 5 cases ≥10, 2 cases ≤5 | 50 | 50 | 50 | 50 | <5a | 10 | 5a | |
| WCST (number of categories) | 6 | – | 1b | – | 1.4 | 2 | 6 | 0a | 1 | 0a | 0a | 1 |
| TEA: counting without distraction | 7 | – | 6 | – | 4.5 | 3a | 7 | 3a | 4a | 7 | 3a | NT |
| TEA: counting with distraction | 10 | – | 3 | – | 2.3 | 2a | 1a | 0a | 0a | 9 | 2a | NT |
| VOSP | ||||||||||||
| Dot counting | 10 | 9.9 (0.3) | 8 | 9.88 | 6.29 | 10 | 10 | 5a | 10 | 3a | 6a | 0a |
| Position discrimination | 20 | 19.8 (0.6) | 18 | 19.5 | 17.14 | 20 | 17a | 20 | 18a | 15a | 16a | 14a |
| Cube analysis | 10 | 9.7 (2.5) | 6 | 9.88 | 5.67 | 5 | 9 | 10 | 2a | 4a | 4a | NT |
| Number location | 10 | 8.9 (2.8) | 7 | 9.83 | 7.57 | 10 | 10 | 9 | 8 | 2a | 8 | 6 |
SA patients arranged in order of their word–picture matching task (WPM) scores from the Cambridge 64 set battery.
a Denotes impaired performance (at least 2 standard deviations below the control mean).
b Cut-off for 50- to 74-year olds (regardless of educational level).
NT = not tested.
The SD patients performed equivalently to the SA group on the forward digit span test [t(13) = 1.7, NS] but had significantly larger backward digit spans [t(12) = 2.7, P < 0.019]. Letter fluency was equally impoverished for both groups [t(13) < 1]. Unlike the SA group, the SD patients were intact on measures of visuo-spatial processing and non-verbal reasoning: they obtained significantly higher scores on three VOSP subtests [excluding number location; t(11–13) = 2.6–3.3, P < 0.05; Table 4] and scored at or above the 75th percentile on the Coloured Progressive Matrices test (for individual SD patient data, see Bozeat et al. 2002).
Semantic background tests
Every patient was impaired on word and picture versions of the PPT task, except S.C. The SA group were universally impaired relative to controls, however, on both word and picture versions of the Camel and Cactus test, which is more difficult than the PPT because four rather than two response options are presented. Category fluency, picture naming and word-picture matching tests were impaired in every case, with patient K.A. obtaining particularly low scores on all of these measures. The SD and SA patient groups were equally impaired on these standard measures of semantic function [Table 5; t(12) < 1.04, NS].
General praxis testing
Meaningless gesture imitation
Five of the SA cases obtained accuracy scores of 85% or over. The remaining two SA patients (L.S. and K.A.) made less precise imitations of the gestures, and some vague irrelevant movements, which meant that their scores were impaired (10 and 30% accuracy, respectively). The SD group were as good as controls at imitating meaningless gestures [t(16) = 1.5, NS; Bozeat et al., 2002].
Pantomime to verbal command
All SA patients produced at least 7/8 correct gestures on the first attempt except B.B. and K.A. who produced five and four gestures, respectively.
Mechanical puzzles
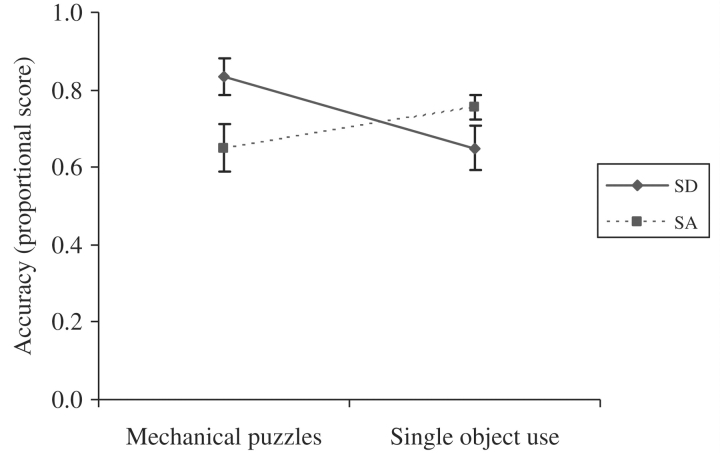
The SA patients’ ability to select the correct tool for the task was substantially worse than their use of this tool when it was provided for them [t(6) = 2.7, P < 0.03]. This pattern was also seen for the SD patients [t(7) = 3.6, P < 0.009] and controls (Fig. 2). In addition, the SA patients performed more poorly than the SD group [t(13) = 2.4, P = 0.03]. This finding might reflect poor motor control in the SA group or, alternatively, an inability to manage the executive demands of the mechanical puzzles task. The latter interpretation is supported by the observation that the SA patients were relatively good at demonstrating the correct actions for single meaningful objects (see below); in addition their performance on the mechanical puzzles task did not correlate with either tool use or general praxis scores (r < 0.1, NS) but did correlate with the Raven's coloured progressive matrices test of non-verbal reasoning (r = 0.72, P = 0.033 one-tailed).
Figure 2.
Performance on the mechanical puzzles task. SA patients are arranged in order of their word–picture matching scores.
Object use battery
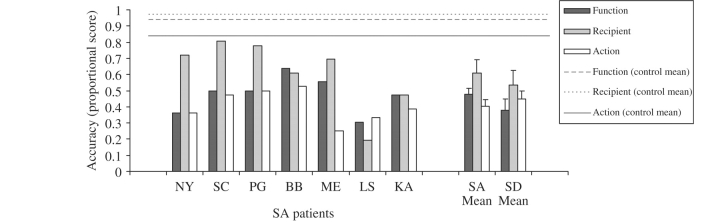
Attribute-matching tests: both patient groups were impaired on each of the attribute-matching subtests (Fig. 3). When the SD and SA groups were compared using a repeated measures ANOVA, a significant effect of matching task was found [i.e. function/action/recipient, F(2,20) = 11.3, P < 0.001] but there was no effect of patient group [F(1,10) < 1] and no interaction [F(2,20) < 1]. Recipient matching was better than function and action matching for both groups.
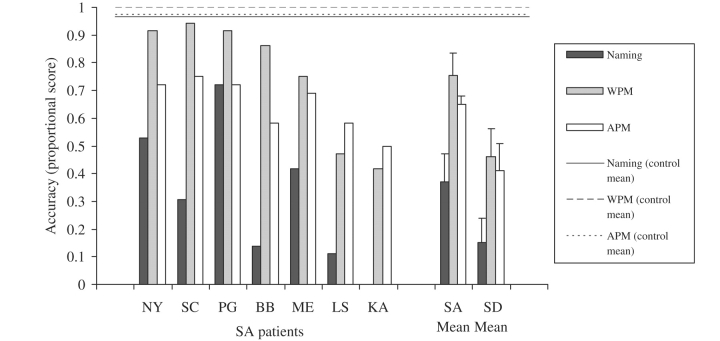
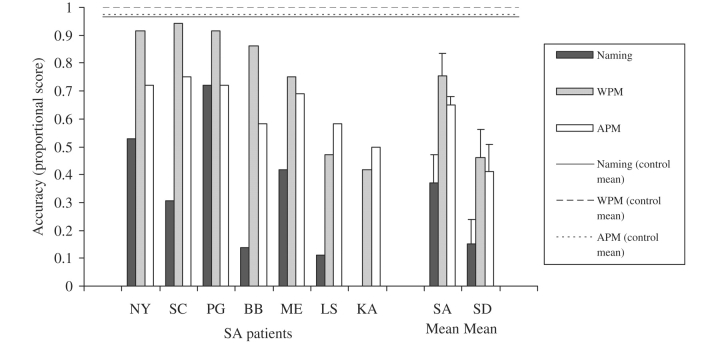
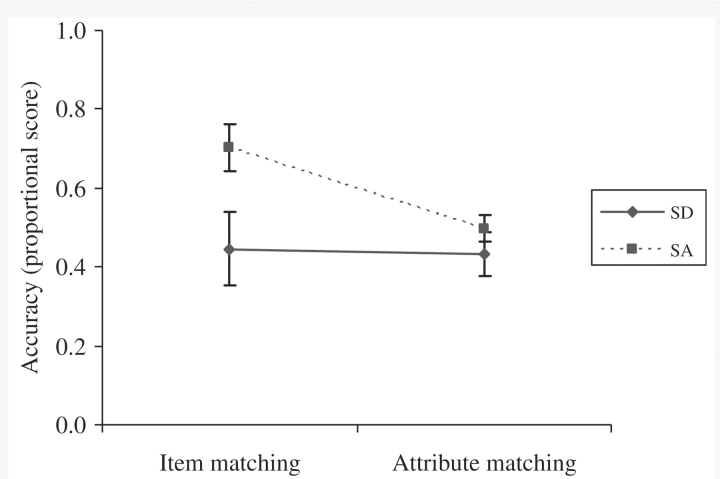
Cross-modal item-matching tests: the SA patients showed somewhat better performance than the SD patients on both word-picture matching [t(13) = 2.1, P = 0.06] and action-picture matching [t(8) = 1.9, P = 0.09], even though the two patient groups had a comparable degree of semantic impairment on the background assessments (Fig. 4). A repeated measures ANOVA found no significant difference between these two tasks [F(1,12) = 3.0, P = 0.1], an effect of patient group that approached significance [F(1,12) = 3.6, P = 0.08] and no interaction [F(1,12) < 1].
Object naming: the SD group showed severely impaired object naming, with four cases failing to name any of the items. The SA patients were somewhat less impaired although the difference between the two groups did not reach significance [t(12) = 1.7, NS; Fig. 4].
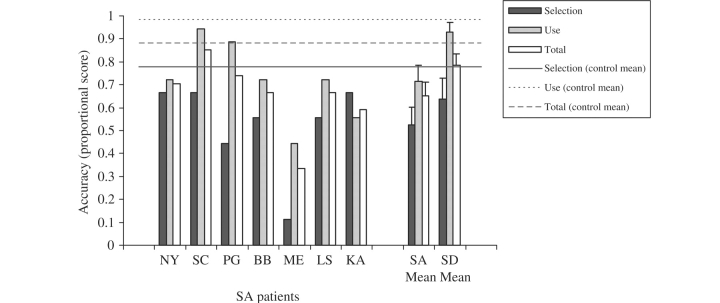
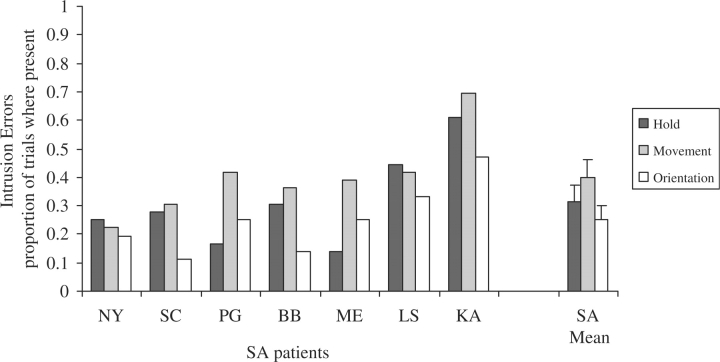
Object use: as detailed in the Method section, object use was scored on three dimensions (hold, movement and orientation) for both accuracy and erroneous intrusion errors. Accuracy: the SA and SD groups performed less well than the controls on all aspects of object use (Fig. 5). A repeated-measures ANOVA comparing the two patient groups revealed a significant effect of component of object use [i.e. hold/movement/orientation, F(2,26) = 31.0, P < 0.001], no main effect of patient group [F(1,13) = 2.5, NS] and a significant interaction between these two factors [F(2,26) = 4.6, P = 0.02]. The SA group obtained significantly poorer scores for object movement compared with both hold [Bonferroni t(6) = 7.3, P = 0.001] and orientation [Bonferroni t(6) = 4.7, P = 0.02], which did not differ. The SD group also obtained significantly lower scores on movement than hold [Bonferroni t(7) = 6.0, P = 0.006] and the difference between movement and orientation approached significance [Bonferroni t(7) = 3.5, P = 0.06]. Scores for orientation were worse than for hold in the SD group although this difference did not reach significance when corrected for multiple comparisons [Bonferroni t(7) = 2.7, P = 0.2]. Intrusion errors: the SA patients made frequent intrusion errors in all three components of object use (see Fig. 6 for the proportion of trials in which at least one intrusion error was made and Table 6 for examples of intrusion errors). Intrusion errors were significantly more frequent for the movement versus orientation component [t(6) = 5.5, P = 0.002], while hold scores did not differ significantly from either of these.
Cross-task comparisons: the SA group were significantly more impaired than the SD group on some assessments, while the opposite pattern occurred for other tests. Repeated-measures ANOVAs were used to examine this variation across tasks (averaging across different subtests when appropriate; see Figs 7 and 8). First, there was a highly significant interaction between task and patient group when item-matching and attribute-matching tests were contrasted [F(1,13) = 9.3, P = 0.009]. The item-matching tasks (word–picture matching and action–picture matching) required participants to choose the picture on each trial that corresponded to the same item presented in a different modality (spoken word or mime). The attribute-matching tasks (by recipient, function and action) also required participants to select a target from a set of pictures using a pointing response. However, the target on each trial was a different object that shared a specific attribute with the probe object. Therefore, participants had to focus on a particular aspect of the objects, whilst ignoring other features. The SD group performed equally on the two types of matching task, which tapped the same set of 36 items [t(7) < 1]. In contrast, the SA patients were much more impaired at attribute- than item-matching [Bonferroni t(6) = 5.2, P = 0.004], supporting the view that SA patients are impaired at flexibly shaping activation within the semantic system in a task-appropriate fashion.
Second, there was a task by group interaction when performance on the mechanical puzzles was compared with single object use [F(1,13) = 16.0, P = 0.002]. The object use task required access to semantic representations of the objects, adequate motor control and, arguably, a degree of cognitive control given that the object use demonstrations were not supported by an appropriate context (i.e. patients were asked to demonstrate how to use a garlic press without a clove of garlic). The mechanical puzzles task, in contrast, required complex problem-solving as well as motor control: it required participants to decide offline which tool would be most effective in a novel situation. The SD patients performed more poorly on the object use task than the mechanical puzzles, in line with their substantial semantic impairment and intact executive skills [Bonferroni t(7) = 5.1, P = 0.002]. In contrast, the SA patients did not show this difference [Bonferroni t(6) = 1.6, NS].
Figure 3.
Performance on the object-knowledge matching tasks. SA patients are arranged in order of their word–picture matching scores.
Figure 4.
Performance on object naming, word–picture matching and action–picture matching. SA patients are arranged in order of their word–picture matching scores. Patient K.A. was not tested on picture naming due to his extremely poor spoken output.
Figure 5.
Hold, movement and orientation scores on single object use. SA patients are arranged in order of their word–picture matching scores.
Figure 6.
Rate of erroneous intrusions in object use. SA patients are arranged in order of their word–picture matching scores.
Figure 7.
Matching by item and attribute.
Figure 8.
Mechanical puzzles and single object use.
Object use battery summary
The SA and SD groups both showed marked impairment on non-verbal tests of conceptual knowledge for 36 everyday objects. They had difficulty demonstrating their correct use, were poor at selecting typical recipients and objects with similar functions/actions, and they were also impaired at more standard semantic tests, such as naming and word-picture matching for these items. We can therefore conclude that patients with SA do not have deficits restricted to verbal comprehension—instead they have substantial problems on non-verbal receptive and expressive tasks, like patients with SD. Moreover, even though the SD and SA patients were impaired at the same range of verbal and non-verbal semantic tasks, their performance varied across the tests within the object use battery. The SA group were better than the SD group at item-matching tasks such as word–picture matching (SA > SD) but were equally impaired at matching on the basis of a particular semantic attribute (recipient, function and action matching tasks; SA = SD), suggesting that they had difficulty focusing on particular aspects of knowledge in a task-appropriate fashion. In addition, the SA patients were poor at solving mechanical puzzles (SA < SD), while the SA and SD patients showed equivalent levels of difficulty on the single object use task. Object use in the SA group featured a high frequency of erroneous intrusions; in contrast, the SD patients produced omissions of actions for semantically degraded objects. These findings are consistent with the hypothesis that patients with SD suffer from a gradual degradation of the core semantic representations, whilst a deficit of cognitive control produces multimodal semantic impairment in SA. The following analyses explored this possibility further.
Item frequency and object use
Jefferies and Lambon Ralph (2006) found that while SD patients showed considerable effects of item frequency in a range of semantic tasks, SA patients failed to show this effect. Therefore, we explored the influence of frequency on the patients’ object use. Control participants rated each object on a six point scale depending on how often they used each item, with a higher score indicating more frequent use/greater familiarity. Total accuracy scores for object use did not correlate with frequency ratings for the SA group (r = 0.12, NS) but there was a familiarity/accuracy correlation for the SD group (r = 0.39, P < 0.01; both correlations one-tailed). There was also no correlation between erroneous intrusions scores and item frequency for any of the SA patients individually (r = −0.24 to −0.02, P > 0.11) or when considering the group as a whole (r = −0.095, NS).
Inter-task correlations
Correlations between semantic tasks
If the retrieval of relevant semantic information is highly dependent on task demands in SA, these patients should show small or non-existent correlations between different semantic tasks. In contrast, SD patients with a loss of central semantic knowledge should show strong inter-task correlations. We assessed the degree of association between six semantic tests used with all participants in this study. These were (i) picture naming (mean score derived from the 36 objects and 64-set picture naming tasks); (ii) word-picture matching (average score also derived from the object-use and 64-item semantic batteries); (iii) PPT (word/picture mean score); (iv) category fluency; (v) action-picture matching; and (vi) an attribute-matching score including the recipient/function/action matching tasks. Of 15 pair-wise comparisons, only three significant associations were found for the SA group: between word–picture matching and both action–picture matching (r = 0.86, one-tailed P = 0.006) and attribute matching (r = 0.75, one tailed P = 0.026), and between naming and action–picture matching (r = 0.74, one tailed P = 0.047). Conversely, the SD group showed significant correlations between all 15 semantic task comparisons (r > 0.69, one-tailed P < 0.03).
Correlations with object use
In the SD group, the same semantic tasks (as above) were also highly correlated with the total object use score and specific components of the object use task (i.e. hold, movement and orientation). Of 24 task combinations, 23 reached significance (r > 0.64, P < 0.045) and one approached significance (between naming and object hold; r = 0.55, P = 0.079; all correlations are one-tailed). For the SA group, 10 combinations showed a significant correlation. These occurred between category fluency and total object use (r = 0.85, P < 0.016), object hold (r = 0.81, P < 0.025) and object movement (r = 0.79, P < 0.03), as well as between word–picture matching and total object use (r = 0.75, P < 0.025), object hold (r = 0.7, P < 0.04) and object orientation (r = 0.97, P < 0.001). The action–picture matching task correlated with total object use (r = 0.8, P < 0.015), object movement (r = 0.72, P < 0.032) and object orientation (r = 0.83, P < 0.01). Finally, the function/action/recipient combined score correlated significantly with object orientation (r = 0.69, P < 0.04).
Correlations with executive tests
The Raven's Coloured Progressive Matrices test, completed by all of the patients in the study, correlated with semantic and object use performance in the SA group but not the SD group (r < 0.33, P > 0.3). This test of non-verbal reasoning predicted SA patients’ performance on the following elements of the object use battery: word-picture matching (r = 0.82, P = 0.01); overall object use (r = 0.58, P = 0.08); orientation component of object use (r = 0.69, P = 0.04); action-matching (r = 0.63, P = 0.08) and mechanical puzzles (r = .072, P = 0.03; all correlations are one-tailed). These findings are consistent with our hypothesis that verbal and non-verbal semantic deficits in SA are associated with executive dysfunction.
Item consistency
In previous studies, SD patients have been found to be highly consistent when the same items are probed using different tasks, whereas SA patients only perform consistently when the control demands of a task are kept constant (Jefferies and Lambon Ralph, 2006). Simultaneous logistic regression was used to determine if performance in one task would predict performance for the same items in another test. Familiarity was also included as a predictor as this factor can produce moderate levels of consistency if performance is modulated by this variable (Bozeat et al., 2000).
Consistency was examined for six tasks from the object use battery, including word–picture matching, action-picture matching, the three attribute matching tasks (function/action/recipient) and object use. For the purpose of this analysis, object use demonstrations were considered ‘correct’ if they contained 75% or more of the features of a complete demonstration. The SD group demonstrated consistency of performance for 20 of 30 pair-wise comparisons (Wald > 3.41, P < 0.065). The only task combinations for which patients did not demonstrate consistent performance occurred when matching by action was included in the comparison. This is probably due to floor effects in the SD group as three participants were unable to complete the action matching task.
For the SA participants only six task combinations showed item consistency, they were between word-picture matching and the following tasks: action matching (Wald > 5.7, P < 0.017), recipient matching (Wald > 13.08, P < 0.001) and object use (Wald > 10.1, P < 0.001).
When the groups were compared using an interactive term, the SD group performed more consistently than the SA group on 10 task combinations, including word-picture matching and all other tasks (Wald > 2.93, P < 0.087), action-picture matching with recipient matching and object use (recipient matching: Wald > 6.44, P < 0.011 object use: Wald > 3.9, P < 0.048) and function and action matching (Wald > 4.22, P < 0.04). Overall, the SD group were more consistent than the SA patients across tasks that tapped the same items but required different responses, such as matching by different features or demonstrating an object's use.
Factors affecting performance on semantic tasks
In their case-series comparison of SD and SA, Jefferies and Lambon Ralph (2006) used simultaneous logistic regression to explore which of three factors influenced accuracy in the picture/word versions of the CCT. Control participants rated each trial on a scale of 1–5 according to how difficult each judgement of semantic association was. Two elements of executive demand were considered separately: (i) ease of determining the relevant semantic relationship; and (ii) ease of rejecting distracters. Compared to the SD group, the SA participants showed a greater effect on accuracy of both of these factors. These results suggested that the SA group were more dependent than the SD group on the executive demands made by a task such as the CCT.
The same two rating scales were also used in the present study to assess the difficulty of the function, action and recipient matching tasks as well as the action-picture and word-picture matching tasks. Ratings were collected from seven healthy participants. A global difficulty score was generated from an average of these two ratings. When the global executive difficulty scores for the attribute-matching tasks were compared using paired samples t-tests, action matching was rated as significantly harder than function matching [t(6) = 2.82, P = 0.03] and recipient matching was found to be the easiest task [t(6) > 4.87, P < 0.003]. When the item-matching tasks were compared, action–picture matching was rated as significantly harder than word-picture matching [t(6) = 5.3, P = 0.002]. Thus, both groups exhibited patterns of performance that reflected how executively demanding the tasks were rated to be. When the average difficulty scores for the item and attribute matching tasks were compared, however, item-matching was rated as significantly easier than attribute-matching [t(6) = 5.29, P = 0.002]. Hence, whilst the SD patients performed equivalently on item- and attribute-matching, the SA group showed poorer performance on the more executively demanding attribute-matching tasks.
Simultaneous logistic regression was used to determine the extent to which the items passed or failed in the matching task battery were predicted by the degree of executive demand on each trial (from average of two ratings) and task type (i.e. matching by item/attribute) for the two patient groups (SA/SD). Task difficulty and patient group were found to be significant predictors of performance (Wald = 61.31, P < 0.001; Wald = 9.64, P = 0.002, respectively) but there were no main effects of individual task identity (action/function/recipient matching, action–picture/word-picture matching), patient identity or task group (Wald < 1). There was a significant interaction between patient group and task type (Wald = 19.03, P < 0.001) as well as between patient group and task difficulty (Wald = 6.9, P = 0.009). Analysis of each group separately revealed that while task type (item/attribute matching) and difficulty were significant predictors for both SA and SD (Wald > 3.97, P < 0.05), these effects were larger for the SA patients (SA: Exp B = 1.68 and 2.12; SD: Exp B = 0.5 and 1.7).
Discussion
This study assessed the impact of multi-modal semantic impairment in the context of two neuropsychological conditions, namely SD and SA. This was achieved using a battery of object use tests that tapped conceptual knowledge relating to 36 common objects and the ability to use the same objects. The nature of the deficits in the two patient groups was compared in order to establish if the qualitative differences observed by Jefferies and Lambon Ralph (2006) would extend to a highly non-verbal domain—that of using single objects. The two patient groups were equally impaired on tests of general semantic function, including tests from the object-use battery (object naming and matching tasks) and other background semantic tests (category fluency, word-picture matching, picture and word PPT and picture naming). Moreover, both groups of patients showed impaired knowledge of object use when compared to control participants. Not only were the SA patients unable to complete picture selection tasks on the basis of object use, they were also poorer than controls at demonstrating the correct actions for objects. Therefore the SA patients, like those with SD, had genuinely multimodal semantic problems affecting non-verbal receptive and expressive tasks. However, there were numerous differences in the way that SD and SA patients failed these non-verbal semantic tasks. This differential behavioural pattern supported the hypothesis that SD produces degradation of the core amodal semantic representations themselves, while patients with SA have a deficit of cognitive control that affects both verbal and non-verbal semantic tasks.
A finding common to SD and SA patients was a lack of association between scores on non-semantic general praxis testing and semantically demanding object use tasks, suggesting that the observed deficits in object use were not the result of a general action production disorder. Although the groups were equivalent in the extent of their semantic impairments, a number of differences were observed in the qualitative nature of their object use impairment, which are concordant with the four hypotheses we identified in the Introduction. First, item familiarity correlated with performance on the object use task for the SD group but not the SA group. Second, while the SD patients performed consistently across tasks that tapped different aspects of knowledge and object use for the same items, the SA participants were dependent on the control requirements of the task. Performance across different tests within the object use battery was significantly more consistent for the SD than the SA patients, even when item familiarity was included as an independent predictor. Essentially, if one test suggested that knowledge of a particular object was semantically degraded for an individual SD patient, the same item was likely to be impaired when probed by other tests. This finding was supported by significant correlations for both word- and picture-based semantic tasks and between these tasks and object use demonstrations for the SD group. In contrast, the SA patients showed very little item consistency across tasks and few significant correlations between different tests in the object use battery, even though these tests assessed knowledge of the same items. Instead, the SA patients’ performance reflected the control requirements of each task: they performed relatively well on simple item-matching tasks (such as word–picture matching) but more poorly when they were required to match different objects on the basis of a particular attribute, such as a common function or action. In line with our third hypothesis, a similar pattern was true of expressive tasks; whilst single object use was relatively preserved in SA, at least in comparison to patients with SD, this group showed much poorer performance on a mechanical puzzles task with substantial problem solving demands. Ratings of executive difficulty were better predictors of performance for the SA than the SD patients. Finally, consistent with our fourth hypothesis, the two groups of patients made different errors in object use. Patients with SA made many erroneous intrusions in their demonstrations, such as inappropriate object movements. In contrast, response omissions were more common in SD.
These findings indicate that semantic cognition breaks down in qualitatively different ways in SA and SD—and that this pattern is maintained across verbal, pictorial and object-use tasks. We propose that SD patients have damage to core amodal semantic representations, whereas patients with SA have a more general executive impairment that leads to difficulty controlling activation within the semantic system in a flexible, task-appropriate fashion, giving rise to problems on both verbal and non-verbal semantic tasks (Jefferies and Lambon Ralph, 2006). It follows that semantic cognition is underpinned by at least two interacting principal components: amodal semantic representations in the ATL, degraded in patients with SD, and semantic control processes reliant on left prefrontal and temporoparietal regions—areas that are frequently damaged in SA. It should be noted, however, that stroke-related neural damage can be more widespread than that observed in SD, making it harder to localize impairments. Relatively diffuse disruption due to SAH, for example, has been associated with a range of cognitive deficits, including executive dysfunction (e.g. Bellebaum et al., 2004; Orbo et al., 2008). Although some of the SA patients in the present study exhibited SAH, which could have contributed toward their control impairment, this was not true of all cases. Hence, we discuss our results in the context of the focal lesions common amongst the SA group.
As described in the Introduction section, the neuroimaging literature is largely consistent with neuropsychological studies of semantic cognition. Although the exact peaks vary from study to study, activation is commonly observed in the ATL, temporoparietal areas and PFC when healthy people engage in semantically demanding tasks (provided that both PET and fMRI are considered, Devlin et al., 2000; Visser et al., 2009a, b). Furthermore, the left prefrontal and temporoparietal regions show sensitivity to the cognitive control demands of semantic tasks (Thompson-Schill et al., 1997; Wagner et al., 2001; Gold and Buckner, 2002; Noppeney et al., 2004). Although this literature has primarily focussed on the role of PFC, temporoparietal regions can show similar responses. For example, greater activation is found when participants are required to activate different semantic associations flexibly from the same items, when the target is only weakly associated with the probe, and/or when the target is embedded in a larger array of distracters (Thompson-Schill et al., 1997; Wagner et al., 2001; Gold and Buckner, 2002; Noppeney et al., 2004). Therefore, it appears that left prefrontal and temporoparietal regions may work in tandem to underpin semantic control, which is consistent with the finding that these regions show coupled activation during other executive tasks (Garavan et al., 2000; Collette et al., 2005). Substantial white matter connections between the two regions via the arcuate and superior longitudinal fasciculi reinforce this notion (Gloor, 1997; Parker et al., 2005). Furthermore, damage to these disparate cortical regions can result in virtually indistinguishable patterns of semantic impairment. Berthier (2001) reported that patients with TSA who had damage to either temporoparietal or frontal regions showed almost identical neuropsychological and language profiles (see also Jefferies and Lambon Ralph, 2006).
According to this view of semantic cognition, gradual degradation of amodal semantic representations within the ATL in patients with SD explains the strong degree of association between different tests that tap the same concepts in different ways. The same core semantic representations are engaged across tasks regardless of whether the input involves words, pictures or real objects, and irrespective of whether spoken names, matching responses, judgements of semantic association or object use are required as outputs (Bozeat et al., 2000, 2002; Jefferies and Lambon Ralph, 2006). As a consequence, in this study, the SD patients’ ability to demonstrate the use of everyday objects correlated with general semantic testing for the same items; moreover, knowledge of these items was consistently impaired across different tasks from the object-use battery. The strong effects of item familiarity/frequency in SD can also be explained in terms of gradual damage to core semantic representations: objects that are encountered infrequently are thought to form weaker representations within the semantic system, making this information more vulnerable to damage in SD.
In contrast, the lack of consistency and frequency/familiarity effects for the SA patients suggests that the semantic impairment in this condition is not underpinned by a frequency-graded loss of central semantic knowledge. The SA patients showed little consistency across semantic tests with differing executive control requirements, even when these tasks had matching surface characteristics. For example, their ability to select the typical recipients of tools did not predict their ability to select objects with related functions, even though these tasks involved the same items, used pictures as stimuli and required a pointing response. Instead, the SA group performed more poorly on tasks that required greater executive control. For example, they were more impaired than SD patients on a mechanical puzzles task that required a degree of problem solving, (i.e. the selection and use of a novel tool), even though they showed equivalent ability to demonstrate the use of single objects. Moreover, the SA patients were good at picture selection tasks when they required item matching (i.e. word-picture matching and action-picture matching), even though they showed substantial impairment at selecting objects on the basis of specific attributes such as function/action. This might be because the attribute-matching tests required the patients to focus in a flexible fashion on a particular aspect of the meaning of the items—global similarity is not sufficient to match scissors with pliers (similar action) or scissors with saw (similar function). Moreover, ratings of the executive difficulty of each trial on the various matching tests were a significantly better predictor of performance for the SA than SD patients. Therefore, in both the object use and picture selection domains, the SA patients’ performance showed sensitivity to the degree of executive control required. Finally, semantic control problems were also evident in the types of errors made by SA patients. In picture naming, they showed associative semantic errors (e.g. potato peeler → ‘chips’), suggesting they were led astray by strong but irrelevant semantic associations. They also made perseverations, which might have reflected a failure to overcome competition from previously activated responses (see Jefferies and Lambon Ralph, 2006). Similarly, in object use, the patients produced many incorrect features rather than showing a loss of information about the correct actions: this suggests they may have had difficulty overcoming competition from previously produced actions or stereotypical movements.
These findings confirm that the ATL, damaged in SD, and the prefrontal and temporoparietal region, the areas mostly commonly damaged in SA, make distinctive contributions to semantic cognition in both the verbal and non-verbal domains. Many of the qualitative differences between SD and SA found by Jefferies and Lambon Ralph (2006) in standard semantic tests were reproduced here, indicating that these two dissociable components of the semantic network make a parallel contribution to verbal and non-verbal semantic tasks. Damage to the ATL is associated with degradation of amodal semantic representations in SD. In contrast, it seems likely that damage to the network underpinning cognitive control causes semantic processing to become deregulated in SA, which affects the flexible application of semantic knowledge in object use tasks as well as verbal and picture-based semantic assessments.
The purpose of this study was to examine the processes supporting semantic cognition with particular emphasis on the non-verbal consequences of deregulation in SA. The results could also make a contribution to the apraxia literature. An impaired ability to use everyday objects due to a deficit at the level of conceptual knowledge, either due to a lack of access to the semantic store (De Renzi and Lucchelli, 1988) or through disturbance of the sequential organization of actions (Poeck and Lehmkul, 1980), has previously been referred to as ‘ideational apraxia’ (IA). Ideational apraxia has been associated with temporoparietal lesions—an important region of damage in our SA cases (De Renzi et al., 1988). A similar disorder, known as action disorganization syndrome (ADS) arises in the context of frontal damage (Schwartz et al., 1991, 1995, 1998; Duncan, 1986). Despite the very different sites of neural damage underlying IA and ADS, the two conditions cannot be separated behaviourally or through computational modelling (Cooper et al., 2005). The SA patients examined in this study all exhibited characteristics of semantic deregulation that impaired their ability to use objects. Based on this evidence, as well as convergent data from neuroimaging (described above), we have concluded that these two regions work collaboratively as part of a neural network underpinning control. It is possible, therefore, that there is no real distinction between IA and ADS, but both arise as a result of semantic deregulation. Because semantic memory has not been assessed more broadly in previous reports of IA and ADS, however, it is difficult to be sure about how these disorders relate to SA.
Funding
MRC-ESRC studentship (to F.C.); RCUK fellowship (to E.J.); NIMH (MH64445); MRC (G0501632).
Acknowledgements
We would like to thank all of the patients and their carers for their continued support of our studies. We are also indebted to Prof. Karalyn Patterson for making the semantic dementia data available to us for re-analysis.
Glossary
Abbreviations
- ATL
anterior temporal lobe
- CCT
Camel and Cactus Task
- PFC
prefrontal cortex
- PPT
Pyramids and Palm Trees
- SA
semantic aphasia
- SD
semantic dementia
References
- Bellebaum C, Schafers L, Schoch B, Wanke I, Stolke D, Forsting M, et al. Clipping versus coiling: neuropsychological follow up after aneurysmal subarachnoid haemorrhage (SAH) J Clin Exp Neuropsychol. 2004;26:1081–92. doi: 10.1080/13803390490515342. [DOI] [PubMed] [Google Scholar]
- Berthier ML. Unexpected brain-language relationships in aphasia: evidence from transcortical sensory aphasia associated with frontal lobe lesions. Aphasiology. 2001;15:99–130. [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–15. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Hodges JR. When objects lose their meaning: what happens to their use? Cogn Affect Behav Neurosci. 2002;2:236–51. doi: 10.3758/cabn.2.3.236. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss H, Tyler LK. Unitary vs multiple semantics: PET studies of word and picture processing. Brain Lang. 2004;89:417–32. doi: 10.1016/j.bandl.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Weekes B, Lee KM, Soon CS, Schreiber A, Hoon JJ, et al. Overlap and dissociation of semantic processing of Chinese characters, English words, and pictures: evidence from fMRI. Neuroimage. 2000;12:392–403. doi: 10.1006/nimg.2000.0631. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D, Deaudon C, Whitehead V. On the status of object concepts in aphasia. Brain Lang. 1997;58:203–32. doi: 10.1006/brln.1997.1771. [DOI] [PubMed] [Google Scholar]
- Coccia M, Bartolini M, Luzzi S, Provinciali L, Lambon Ralph MA. Semantic memory is an amodal, dynamic system: evidence from the interaction of naming and object use in semantic dementia. Cogn Neuropsychol. 2004;21:513–27. doi: 10.1080/02643290342000113. [DOI] [PubMed] [Google Scholar]
- Collette F, Olivier L, Van der Linden M, Laureys S, Delfiore G, Luxen A, et al. Involvement of both prefrontal and inferior parietal cortex in dual task performance. Cogn Brain Res. 2005;24:237–51. doi: 10.1016/j.cogbrainres.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Cooper RP, Schwartz MF, Yule P, Shallice T. The simulation of action disorganisation in complex activities of daily living. Cogn Neuropsychol. 2005;22:959–1004. doi: 10.1080/02643290442000419. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- De Renzi E, Lucchelli F. Ideational apraxia. Brain. 1988;111:1173–85. doi: 10.1093/brain/111.5.1173. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex - a functional MRI study of task-difficulty and process specificity. J Neurosci. 1995;15:5870–8. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, et al. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Duncan J. Disorganization of behavior after frontal-lobe damage. Cogn Neuropsychol. 1986;3:271–90. [Google Scholar]
- Garavan H, Ross TJ, Li SJ, Stein EA. A parametric manipulation of central executive functioning. Cerebral Cortex. 2000;10:585–92. doi: 10.1093/cercor/10.6.585. [DOI] [PubMed] [Google Scholar]
- Gloor P. The temporal lobe and the limbic system. Oxford: Oxford University Press; 1997. [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;34:803–12. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Defective imitation of gestures in patients with left and right hemisphere damage. J Neurol. 1996;61:176–80. doi: 10.1136/jnnp.61.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H. The assessment of aphasia and related disorders. 2nd. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Gotts SJ, Plaut DC. The impact of synaptic depression following brain damage: a connectionist account of ‘access/refractory’ and ‘degraded-store’ semantic impairments. Cogn Affect Behav Neurosci. 2002;2:187–213. doi: 10.3758/cabn.2.3.187. [DOI] [PubMed] [Google Scholar]
- Hart J, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia with anatomical correlation. Ann Neurol. 1990;27:226–31. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patterson K. Charting the progression in semantic dementia - implications for the organization of semantic memory. Memory. 1995;3:463–95. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal-lobe atrophy. Brain. 1992;115:1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramid and palm trees: a test of semantic access from pictures and words. Bury St Edmunds: Thames Valley Test Company; 1992. [Google Scholar]
- Jefferies E, Baker SS, Doran M, Lambon Ralph MA. Refractory effects in stroke aphasia: a consequence of poor semantic control. Neuropsychologia. 2007;45:1065–79. doi: 10.1016/j.neuropsychologia.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–47. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph MA. Deficits of knowledge versus executive control in semantic cognition: insights from cued naming. Neuropsychologia. 2008;46:649–58. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J, Lesser R, Coltheart M. Psycholinguistic assessments of language processing in aphasia (PALPA) Hove, UK: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Lambon Ralph MA, Graham KS, Ellis AW, Hodges JR. Naming in semantic dementia: what matters? Neuropsychologia. 1998;36:775–84. doi: 10.1016/s0028-3932(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Graham KS, Patterson K, Hodges JR. Is a picture worth a thousand words? Evidence from concept definitions by patients with semantic dementia. Brain Lang. 1999;70:309–35. doi: 10.1006/brln.1999.2143. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Lowe C, Rogers T. Both type and distribution of damage are critical for category-specific semantic deficits: evidence from semantic dementia, herpes simplex virus encephalitis and a neural network model of conceptual knowledge. Brain. 2007;130:1127–37. doi: 10.1093/brain/awm025. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- McLeod JG, Lance JW. Introductory neurology. 2nd. Oxford: Blackwell Scientific Publications; 1989. [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting: the role of the frontal lobes. Arch Neurol. 1964;9:100–10. [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Hodges JR. Declarative memory impairments in Alzheimer's disease and semantic dementia. Neuroimage. 2006;30:1010–20. doi: 10.1016/j.neuroimage.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Corbett F, Lambon Ralph MA. Elucidating the nature of deregulated semantic cognition in semantic aphasia: evidence for the roles of prefrontal and temporoparietal cortices. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21289. (in press) [DOI] [PubMed] [Google Scholar]
- Noppeney U, Phillips J, Price C. The neural areas that control the retrieval and selection of semantics. Neuropsychologia. 2004;42:1269–80. doi: 10.1016/j.neuropsychologia.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Ochipa C, Rothi LJG, Heilman KM. Conceptual Apraxia in Alzheimer's disease. Brain. 1992;115:1061–71. doi: 10.1093/brain/115.4.1061. [DOI] [PubMed] [Google Scholar]
- Orbo M, Waterloo K, Egge A, Isaksen J, Ingebrigtsen T, Romner B. Predictors for cognitive impairment one year after surgery for aneurysmal subarachnoid hemorrhage. J Neurol. 2008;255:1770–6. doi: 10.1007/s00415-008-0047-z. [DOI] [PubMed] [Google Scholar]
- Parker GJM, Luzzi S, Alexander DC, Wheeler-Kingshott CAM, Clecarelli O, Lambon Ralph MA. Lateralisation of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–66. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Perani D, Schnur T, Tettamanti C, Gorno-Tempini M, Cappa SF, Fazio F. Word and picture matching: a PET study of semantic category effects. Neuropsychologia. 1999;37:293–306. doi: 10.1016/s0028-3932(98)00073-6. [DOI] [PubMed] [Google Scholar]
- Poeck K, Lehmkuhl G. The syndrome of ideational apraxia and the localization of the underlying brain lesion. Nervenarzt. 1980;51:217–25. [PubMed] [Google Scholar]
- Postler J, De Bleser R, Cholewa J, Glauche V, Hamzei F, Weiller C. Neuroimaging the semantic system(s) Aphasiology. 2003;17:799–814. [Google Scholar]
- Raven JC. Coloured progressive matrices sets A, AB, B. London: H.K. Lewis; 1962. [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, Nimmo-Smith I. The test of everyday attention. Flempton: Thames Valley Test Company; 1994. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–35. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Saffran EM. The organization of semantic memory: in support of a distributed model. Brain Lang. 2000;71:204–12. doi: 10.1006/brln.1999.2251. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Dick F, Wilson SM, Dronkers NF, Bates E. Neural resources for processing language and environmental sounds. Brain. 2003;126:928–45. doi: 10.1093/brain/awg082. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Montgomery MW, Buxbaum LJ, Lee SS, Carew TG, Coslett HB, et al. Naturalistic action impairment in closed head injury. Neuropsychology. 1998;12:13–28. doi: 10.1037//0894-4105.12.1.13. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Montgomery M, Fitzpatrick-DeSalme E, Ochipa C, Coslett HB, Mayer NH. Analysis of a disorder of everyday action. Cogn Neuropsychol. 1995;12:863–92. [Google Scholar]
- Schwartz MF, Reed ES, Montgomery M, Palmer C, Mayer NH. The quantitative description of action disorganization after brain-damage: a case-study. Cogn Neuropsychol. 1991;8:381–414. [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity and visual complexity. J Exp Psychol Hum Learni Mem. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol. 1989;2:167–82. [Google Scholar]
- Strub RL, Black FW. The mental status examination in neurology. Philadelphia: F.A. Davis Company; 1987. [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, et al. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. PNAS. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–6. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–32. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Visser M, Embleton KV, Jefferies E, Parker GJM, Lambon Ralph MA. The anterior temporal lobes and semantic memory clarified: novel evidence from distortion-corrected spin-echo EPI fMRI. 2009a doi: 10.1016/j.neuropsychologia.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: A meta-analysis of the functional neuroimaging literature. Journal of Cognitive Neuroscience. 2009b doi: 10.1162/jocn.2009.21309. (in press) [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–38. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Cipolotti L. Word comprehension: the distinction between refractory and storage impairments. Brain. 1996;119:611–25. doi: 10.1093/brain/119.2.611. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. The visual object and space perception battery: Bury ST. Edmunds, UK: Thames Valley Test Company; 1991. [Google Scholar]
- Warrington EK, McCarthy RA. Category specific access dysphasia. Brain. 1983;106:859–78. doi: 10.1093/brain/106.4.859. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised (WMS-R) New York: Psychological Corporation; 1987. [Google Scholar]