Abstract
Murine CMV (MCMV) establishes a systemic, low-level persistent infection resulting in the accumulation of CD8+ T cells specific for a subset of viral epitopes, a process called memory inflation. Although replicating virus is rarely detected in chronically infected C57BL/6 mice, these inflationary cells display a phenotype suggestive of repeated Ag stimulation, and they remain functional. CD4+ T cells have been implicated in maintaining the function and/or number of CD8+ T cells in other chronic infections. Moreover, CD4+ T cells are essential for complete control of MCMV. Thus, we wondered whether CD4+ T cell deficiency would result in impaired MCMV-specific CD8+ T cell responses. Here we show that CD4+ T cell deficiency had an epitope-specific impact on CD8+ T cell memory inflation. Of the three codominant T cell responses during chronic infection, only accumulation of the late-appearing IE3-specific CD8+ T cells was substantially impaired in CD4+ T cell-deficient mice. Moreover, the increased viral activity did not drive increased CD8+ T cell division or substantial dysfunction in any MCMV-specific population that we studied. These data show that CD4+ T cell help is needed for inflation of a response that develops only during chronic infection but is otherwise dispensable for the steady state maintenance and function of MCMV-specific CD8+ T cells.
Murine CMV (MCMV)3 is a β-herpesvirus that establishes a low-level systemic, persistent infection. A defining feature of the immune response to MCMV is the large numbers of functional, virus-specific CD8+ T cells that accumulate over time, a process termed memory inflation (1–5). In C57BL/6 (B6) mice, inflationary T cells are primarily specific for epitopes from three viral proteins, M38, m139, and IE3. Whereas responses to M38 and m139 are present during the acute infection, IE3-specific cells become readily detectable in the blood only ~3 wk into the infection. At late times postinfection, inflationary T cells specific for all three Ags bear a differentiated effector phenotype (CD27lowIL-7RαlowKLRG-1+) and fail to produce IL-2, indicative of repeated Ag stimulation (4, 5). However, these cells remain functional. We found that most of these inflationary T cells can be stimulated to secrete effector cytokines and were responsive to viral challenge despite their differentiation. Interestingly, the chronic infection did not drive most inflationary T cells to divide during chronic infection. Instead, most differentiated inflationary T cells were short lived in the circulation of chronically infected hosts and had to be constantly replaced by new effector T cells in order for the population to be maintained (5). The phenotype of these inflationary T cells along with the fact that they are constantly being replaced, likely suggest that inflationary T cell populations are maintained in an Ag-dependent manner. Indeed, because MCMV persists below the threshold of detection in C57BL/6 mice, the accumulation, differentiation and maintenance of virus-specific CD8+ T cells is the strongest evidence of persistent viral activity.
However, not all virus-specific T cells accumulate or differentiate over time. CD8+ T cells specific for other viral peptides, such as epitopes in M45 and M57, respond robustly during acute infection but contract afterwards and persist in low, stable numbers. These cells resemble central memory T cells in phenotype (CD27highIL-7RαhighKLRG-1−) and recall capacity. Moreover, the cells appear to be quiescent and maintained by homeostatic division, as if they are ignorant of the persistent viral infection (5). Thus, MCMV infection seems to produce both Ag-dependent (inflationary) and Ag-independent (stable-memory) CD8+ T cells during infection of a single animal.
Much of our understanding of how CD8+ T cells deal with chronic viral infections is derived from work with variants of lymphocytic choriomeningitis virus (LCMV) that produce chronic infections (6–13). These LCMV strains replicate to high titers for prolonged periods of time and drive substantial CD8+ T cell dysfunction. Importantly, CD4+ T cell deficiency both accelerates and exacerbates the CD8+ T cell dysfunction during chronic LCMV infection (9, 10). However, the impact of CD4+ T cell deficiency on CD8+ T cells in other models of chronic viral infection (polyomavirus, murine γ-herpesvirus-68, and HSV), has been somewhat variable (14–23). The combined evidence has led to a model proposing that the antigenic load and the degree of CD4+ T cell impairment are proportional to the extent of CD8+ T cell dysfunction (7). According to the model, CD8+ T cells remain functional in chronic CMV infection because the viral Ag load is kept very low and/or because CD4+ T cell help is available. In consequence, we would expect that increased viral load and/or impaired CD4+ T cell help might lead to CD8+ T cell dysfunction.
In MCMV infection, CD4+ T cells are absolutely required for control of replicating virus in the salivary gland (24) and probably play a direct role in preventing viral recrudescence (25). Thus, CD4+ T cell-deficient mice have increased levels of viral replication and also lack CD4+ T cell help for CD8+ T cells. In wild-type mice, inflationary CD8+ T cells appear to be repeatedly stimulated with viral Ag, even though replicating virus is kept below the level of detection. We wondered whether the combination of increased viral replication and an absence of CD4+ T cell help would tip the balance and result in progressive CD8+ T cell dysfunction during chronic MCMV infection.
Here we show that CD4+ T cell deficiency has an epitope-specific impact on memory inflation of CD8+ T cells during MCMV infection. IE3-specific CD8+ T cells, which in wild-type mice appear in significant numbers only during chronic infection, were essentially absent from CD4+ T cell-deficient mice. However, despite high titer persistent infection in the salivary glands and the absence of CD4+ T cell help, CD8+ T cells responses to the two other inflationary epitopes, m139 and M38, developed in near normal numbers and displayed only limited evidence of increased stimulation and dysfunction. Finally, the increased virus replication did not alter the dichotomy between stable and inflationary CD8+ T cells.
Materials and Methods
Mice
C57BL/6, MHC class II (MHC II)−/− (H2dlAb1-Ea/J), CD4−/−, B6.SJL-Ptprca Pepcb/BoyJ (B6.SJL-CD45.1 congenic), B6.PL-Thy1<a>/CyJ (Thy1.1), CD40L−/−, and B6.129S2-Igh-6tm1Cg/J (μMT) mice were purchased from The Jackson Laboratory and bred at Oregon Health and Sciences University (Portland, OR) thereafter. MHC II−/− mice (ABBN12) were purchased from Taconic. OX40−/− mice were bred in house at the La Jolla Institute for Allergy and Immunology (La Jolla, CA), and experiments using these mice were primarily performed there. Additional OX40−/− mice were kindly provided by Dr. Nigel Killeen and maintained at Oregon Health and Sciences University. Mice were between the ages of 6 and 16 wk at the start of the experiments. In all cases, chronically infected mice had been infected for at least 3 mo and typically 4–5 mo before analysis.
Virus strains and infections
Mice were infected i.p. with 2 × 105 PFU of MCMV strain MW97.01, which is derived from a bacterial artificial chromosome of the Smith strain (26). In all cases, virus stocks were grown on mouse embryo fibroblasts and prepared as described (5, 27). Salivary gland tissue was disrupted via dounce and sonication, and viral titers were measured by plaque assay on BALB-3T3 fibroblasts.
Abs, tetramer staining, intracellular cytokine stimulation assay, BrdU and FACS analysis
T cells were harvested from the spleen and blood as previously described (5). Lungs were perfused with 15–20 ml of PBS containing 1U/ml heparin. Tissue was ground through a wire mesh strainer, washed once, resuspended in 40% Percoll, and spun at 500 × g for 25 min. Pellets were washed and resuspended for staining. Tetramers were synthesized by the National Institutes of Health tetramer core facility (http://www.niaid.nih.gov/reposit/tetramer/overview.html). Tetramer staining and measurement of intracellular cytokines were performed as described (4, 5, 27) using the following Abs: CD8α (53-6.7); CD27 (LG.7F9); NKG2A/C/E (20d5); PD-1 (RMP1-30); IFN-γ (XMG.1.2); TNF-α (MP6-XT22) CD45.1 (A20); Thy1.2 (30-H12). For CD4+ T cell depletion, mice were injected i.p. with 100 μg of anti-CD4+ Ab GK1.5 on days −3, −1, 0 (day of infection), and weekly thereafter as indicated. Successful depletion was measured with the CD4-specific Ab RM4–4. For BrdU incorporation, mice were injected with 1 mg of BrdU on the first day of the pulse and treated with 0.8 mg/ml BrdU in the drinking water for the next 2 wk, changing the water every other day. BrdU incorporation was determined with the BrdU detection kit (BD Biosciences). In all cases, samples were analyzed on an LSR II or FACSCalibur flow cytometer (both from BD Biosciences), and data were analyzed using FlowJo software (TreeStar).
Adoptive transfers
In one experiment (Fig. 10), splenic CD8+ T cells from chronically infected CD45.1 (wild-type) or MHC II−/− mice were enriched by magnetic sorting using the EasySep CD8+ T cell enrichment kit and following the manufacturer’s protocol (Stem Cell Technologies). In our hands, this typically produces cells that are 90–95% CD8+. An equal number of CD8+ T cells from both sources were mixed and transferred into naive Thy1.1 recipients. Wild-type CD8+ T cells (Thy1.2+ CD45.1+) and CD8+ T cells from MHC II−/− mice (Thy1.2+CD45.1−) were distinguished by FACS. The percent of CD8+ T cells from both sources that were Ag specific was analyzed in the donor population by tetramer staining on the day of transfer. Two days after transfer, chimerism in the recipients was verified by FACS, and recipients were infected as above. Splenocytes and peripheral blood were examined 7 days after infection by tetramer staining. For analysis of donor cell expansion, we used a recently described protocol (28). The number of tetramer+CD8+ T cells in the spleen 7 days after viral challenge was compared with the number of tetramer+ T cells that were transferred. Because the total number of transferred Ag-specific cells was used for the day 0 value, rather than the actual number that survived transfer and colonized the spleen, the amount of expansion calculated each animal is almost certainly a substantial underestimate for both donors. For BrdU incorporation in these experiments, mice were injected with 1.25 mg of BrdU (BD Biosciences) 24 h before harvest. BrdU incorporation was assessed as above.
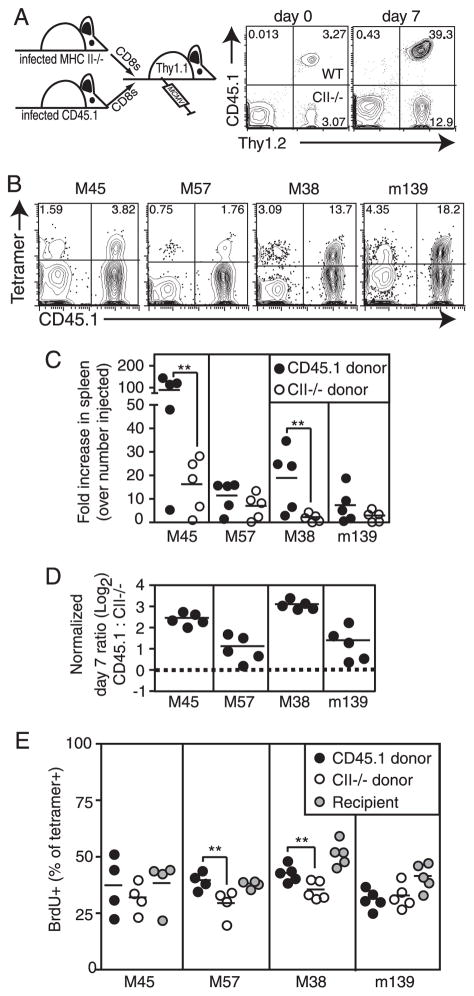
FIGURE 10.
MCMV-specific CD8+ T cells from MHC II−/− mice expand poorly after viral challenge compared with CD8+ T cells from wild-type animals. CD8+ T cells from wild-type mice (Thy1.2+CD45.1+) or MHC II−/− mice (Thy1.2+CD45.2+) were transferred into naive Thy1.1-congenic mice. Recipients were challenged with MCMV 2 days after cell transfer (day 0), and donor CD8+ T cells were analyzed 7 days later. A, Representative FACS plots show that wild-type cells (Thy1.2+CD45.1+, upper right quadrant) and MHC II−/− cells (Thy1.2+CD45.1−, lower right quadrant) were present in equal numbers on day 0, but not on day 7. Numbers indicate the percentage of all CD8+ T cells in the peripheral blood. B, Representative FACS plots of donor T cells from wild-type mice (CD45.1+, right quadrants), or MHC II−/− mice (CD45.1−, left quadrants) are shown with the indicated tetramer staining. Numbers indicate the percent of all donor (Thy1.2+) CD8+ T cells that are tetramer+. C, MCMV-specific CD8+ T cells from wild-type and MHC II−/− mice undergo unequal expansion after viral challenge. The number of transferred Ag-specific cells (day 0 values) and the number of Ag-specific CD8+ T cells present in the spleen on day 7 were calculated for each donor population in each recipient mouse. Shown is the fold increase in tetramer+ donor populations between the adoptive transfer and day 7 postchallenge. Statistical significance was determined as in Fig. 1. D, Wild-type T cells expand more than CD8+ T cells from MHC II−/− mice in each animal. The ratio of wild-type to MHC II−/− Ag-specific T cells in the spleen 7 days after viral challenge was normalized to the ratio of cells that were injected (based on tetramer stains of the donor populations on the day of injection). Shown is the normalized data plotted on a log2 scale. The dotted line indicates a value of 1, which would result from equal expansion of the CD8+ T cells from wild-type and MHC II−/− mice. The fact that all values are above this line indicates that the wild-type donor CD8+ T cells expanded more than the CD8+ T cells from MHC II−/− mice in each animal. E, CD8+ T cells from wild-type and MHC II−/− mice incorporate similar amounts of BrdU after viral challenge. Mice were pulsed with BrdU 24 h before sacrifice. Shown is the percent of tetramer+ T cells in the spleen that incorporated BrdU. Statistical significance was calculated as in Fig. 1.
Results
CD4+ T cell deficiency impacts CD8+ T cell memory inflation in an epitope-dependent manner
The peak of the T cell response (day 7) during acute MCMV infection of C57BL/6 (B6) mice is characterized by a consistent immunodominance hierarchy (4, 27). CD8+ T cells specific for the viral protein M45 dominate the response followed by m139-specific T cells and M38-specific T cells. IE3-specific T cells are barely detectable at these early times. Subsequently, the transition from acute to chronic infection in B6 mice is characterized by a contraction of the M45-specific T cell population and accumulation of M38-, m139-, and IE3-specific T cells (4, 5). To assess the impact of CD4+ T cells on this process, we infected CD4−/− or MHC II−/− (both the H2-Ab1 knockout and the H2-Ab1 through H2-Ea knockout) mice with MCMV. During acute infection (day 7), a slight increase in the magnitude of the CD8+ T cell responses was seen in MHC II-deficient mice, but there was no change in the overall immunodominance hierarchy (Fig. 1). M45-specific T cells still dominated the response followed by m139- and M38-specific T cells. IE3-specific T cells, which are normally only barely detectible in the peripheral blood 7 days postinfection, were not increased in CD4+ T cell-deficient mice at this time.
FIGURE 1.
Memory inflation is inhibited by CD4+ T cell deficiency in an epitope-dependent manner. Mice were infected, and the percent of CD8+ T cells specific for the indicated peptides was measured in the blood over time by intracellular cytokine staining for IFN-γ. For each sample, responses were compared with unstimulated CD8+ T cells from the same animal (not shown), and gates were typically set to include <0.1% of these unstimulated CD8+ T cells. Each symbol represents an individual mouse. Significance was measured by Student’s t test. **, p < 0.05; ***, p < 0.001.
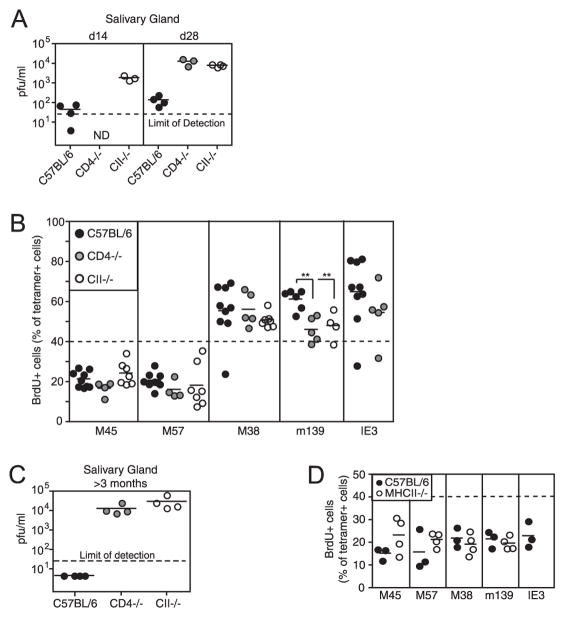
During the chronic phase of infection, which is associated with persistent viral replication in the salivary gland in CD4+ T cell-deficient animals (Refs. 24 and 29 and Fig. 5C), CD4+ T cell deficiency had a different impact on responses to different epitopes. M38-specific T cell memory inflation was largely unaffected in CD4+ T cell-deficient mice. However, accumulation of both m139- and IE3-specific T cells, when measured as a percent of CD8+ T cells in the blood, was almost completely inhibited (Fig. 1). Thus, m139-specific cells failed to increase in number after appearing in normal (CD4 −/−) or increased (MHC II−/−) numbers at wk 1, and IE3-specific responses were low (CD4 −/−) or undetectable (MHC II−/−) in most mice. The impact of CD4+ T cell deficiency was generally more profound in MHC II−/− mice than in CD4−/− mice, perhaps because CD4−/− mice still contain class II-restricted T cells (30).
FIGURE 5.
Inflationary T cells proliferate early but not late during MCMV infection regardless of the presence of CD4+ T cell help. A, The replicating virus in the salivary gland of 14- and 28-day-infected wild-type B6, CD4−/− and MHC II−/− mice was measured by plaque assay. Dotted line, limit of detection in this assay. B, MCMV-infected mice were treated with BrdU from days 14–28, and the percent of peripheral blood, tetramer+ T cells that incorporated BrdU is shown. The dotted line was arbitrarily drawn at 40% to illustrate the difference between stable memory (M45- and M57-specific T cells) and inflationary (M38-, m139-, and IE3-specific T cells). There were insufficient numbers of IE3-specific T cells in MHC II−/− mice to accurately assess their BrdU incorporation. C, The replicating virus in the salivary gland of chronically infected (>5 mo) wild-type B6, CD4−/− and MHC II−/− mice was measured by plaque assay as in A. D, Chronically infected mice were treated with BrdU for 2 wk, and the percent of peripheral blood, tetramer+ T cells that incorporated BrdU is shown. Again, there were too few IE3-specific CD8+ T cells in MHC II−/− mice to accurately determine their BrdU incorporation. The dotted line was drawn at 40% BrdU incorporation for comparison with B.
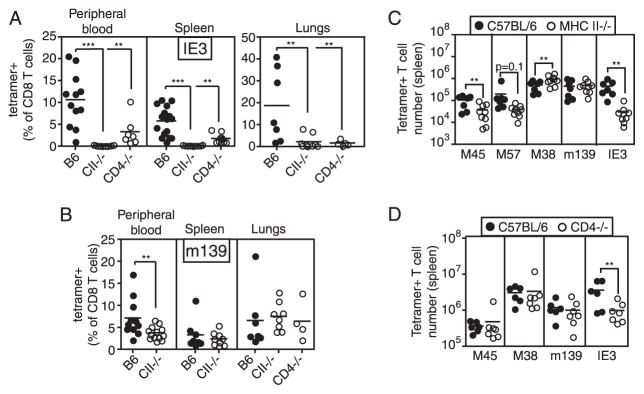
A similar impact of CD4+ T cell deficiency on both m139- and IE3-specific T cells in the peripheral blood was seen by tetramer staining (Fig. 2), excluding the possibility that these responses had developed, but did not secrete IFN-γ in response to Ag. However, the epitope-specific impact of CD4+ T cell deficiency was slightly different in the tissues. Mirroring the results in the blood, there were substantially reduced percentages of IE3-specific T cells in both the spleen and lungs of CD4+ T cell-deficient mice (Fig. 2A), and M38-specific T cell percentages were normal in both organs (not shown). However, whereas the percent of m139-specific CD8 T cells was reduced in the blood of MHC II−/− mice, they were the same as in wild-type mice in both lung and spleen (Fig. 2B). In addition, the absolute number of IE3-specific T cells was substantially reduced in the spleens of CD4+ T cell-deficient mice, especially MHC II−/− mice in which the numbers of IE3-specific cells approached our limit of detection with the IE3 peptide-loaded tetramer staining reagent (Fig. 2, C and D). In both MHC II−/− mice and CD4−/− mice, m139-specific T cells were present in normal numbers; whereas M38-specific T cells were slightly elevated in MHC II−/− mice (Fig. 2, C and D).
FIGURE 2.
The reduction of IE3- and m139-specific T cells can be measured by tetramer staining. A, IE3-specific T cells in the peripheral blood, spleen, and lungs were measured by tetramer staining. Data are combined from several experiments, and mice were infected for 3–10 mo. B, m139-specific T cells were measured by tetramer staining as in A. C and D, The number of Ag-specific T cells was measured in the spleens of B6, MHC II−/−, and CD4−/− mice by tetramer staining. Data are combined from two independent experiments (MHC II−/−) or a single experiment (CD4−/−). Significance was measured as in Fig. 1.
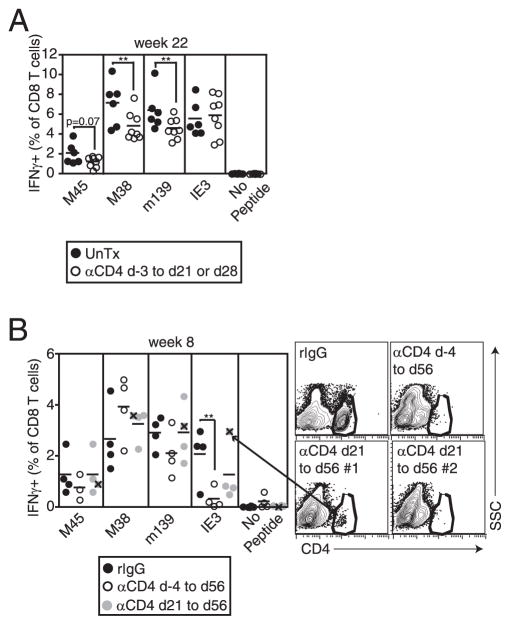
To test whether IE3-specific CD8+ T cells needed CD4+ T cell help before memory inflation begins (Ref. 4 and Fig. 1), CD4+ T cells were depleted from B6 mice during the first 3–4 wk of infection. As shown in Fig. 3A, IE3-specific T cells accumulated normally in the peripheral blood, whereas m139- and M38-specific T cells were slightly reduced by this treatment. This result contrasted with the absent IE3 inflation we had seen in mice genetically deficient in CD4+ T cells (Figs. 1 and 2) and suggested that IE3 inflation was not dependent on programming during the acute infection. However, to validate the comparison between the results with genetic and Ab depletion, we wanted to confirm that chronic CD4+ T cell depletion would give the same results that we had seen with the genetically deficient mice (i.e., reduce IE3-specific T cell inflation). For this purpose, we used μMT (B cell-deficient) mice. Because μMT mice cannot reject the depleting Ab, long-term CD4+ T cell depletion is significantly easier. Indeed, depletion of CD4+ T cells throughout the time course (in this case, 8 wk) eliminated the accumulation of IE3-specific T cells, confirming our results with genetically deficient mice (Fig. 3B). These CD4+ T cell-depleted μMT mice had substantial viral replication in their lungs and spleens as well as salivary glands (101–104 PFU/organ, not shown), perhaps because the lack of Abs allowed the virus to spread (31). In addition, depletion of CD4+ T cells only from wk 3 to wk 8 resulted in reduced IE3-specific T cell accumulation in three of four mice (Fig. 3B). The one mouse in which IE3-specific T cells inflated normally in this experiment also had incomplete CD4+ T cell depletion (Fig. 3B, FACS plot, lower left).
FIGURE 3.
IE3-specific T cell inflation occurs when CD4+ T cells are depleted during chronic infection. A, CD4+ T cells were depleted (αCD4) from B6 mice beginning on day (d) −3 and depletion was continued to day 21 (experiment #1) or 28 (experiment #2). Control mice were left untreated (UnTx). Analysis of the T cells in the peripheral blood was performed as in Fig. 1 after 22 wk, and data were combined from the two experiments. B, CD4+ T cells were continuously depleted from μMT mice beginning on day −4 or day 21, and peripheral blood T cells were measured by intracellular cytokine analysis after 8 wk. In one mouse, depletion of CD4+ T cells was incomplete. Responses from this mouse are indicated by an X within the gray circles (e.g., arrow), and the FACS plot of CD4+ T cells from this mouse, along with representative FACS plots from the other groups are shown. If this animal is not included in the group, the reduction in IE3-specific T cells between rIgG-treated and day 21 to day 56-depleted approaches significance (p = 0.0869). Statistical significance was measured as in Fig. 1.
By comparison, noninflationary T cells behaved like central memory T cells in CD4+ T cell-deficient mice. The absolute numbers of M45-specific T cells, and a similar population specific for M57, were both reduced in the spleens of MHC II−/− mice (Fig. 2C), consistent with previous evidence suggesting that the number of central memory CD8+ T cells can be reduced in CD4+ T cell-deficient mice (32–34). It is still unknown what distinguishes the epitopes that normally undergo memory inflation from those that dominate the acute response (4, 27).
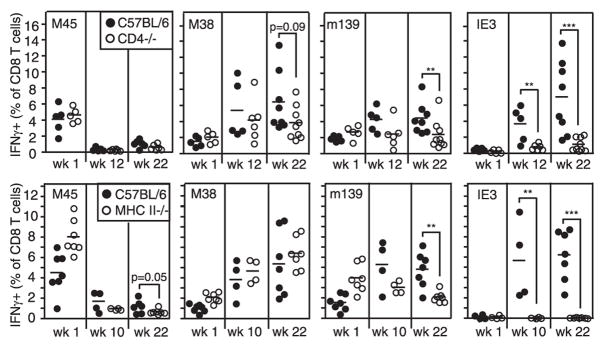
IE3-specific T cell inflation is inhibited in CD40L−/− and OX40−/− mice
Although CD40-CD40L-mediated costimulation has been implicated in CD4+ T cell help for CD8+ T cell memory (35–38), the impact of CD40 stimulation on MCMV-specific CD8+ T cell responses has not been studied. To determine whether the accumulation of IE3-specific CD8+ T cells was also dependent on CD40-mediated costimulation, we infected CD40L−/− mice with MCMV. As shown in Fig. 4, CD40L−/− mice had a significant reduction in the accumulation of IE3-specific CD8+ T cells. In contrast, M38- and m139-specific CD8+ T cells accumulated normally (not shown). There was no evidence of viral replication in the salivary glands of CD40L−/− mice at sacrifice (24 wk postinfection; data not shown), which suggests that continued viral replication was not the sole cause of the deficiency in IE3-specific T cell accumulation.
FIGURE 4.
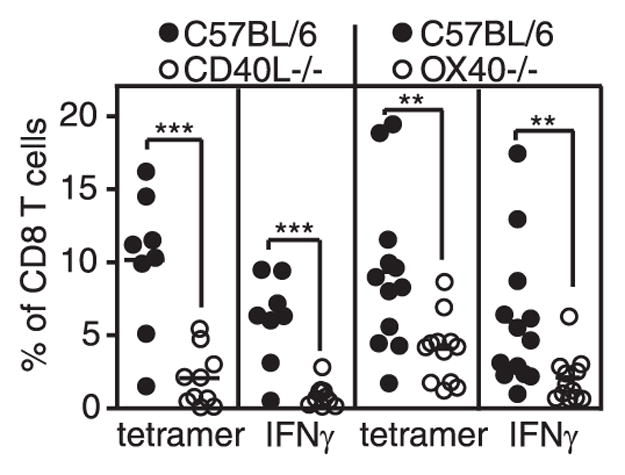
CD40L and OX40 molecules impact inflation of IE3-specific T cells. IE3-specific T cells in the peripheral blood of the indicated mice were measured by tetramer staining and IFN-γ production. CD40L−/− mice and controls were analyzed 24 wk postinfection. OX40−/− mice and controls were analyzed 14 wk postinfection. Statistical significance was measured as in Fig. 1.
Other TNF family members have also been shown to impair MCMV-specific T cell inflation. 4 –1BB/4 –1BBL appears to contribute to memory inflation (I. Humphreys and M. Croft, submitted for publication), and OX40 expression, likely by CD4+ T cells, is important for the normal kinetics of memory inflation after MCMV infection (39). In these previous experiments, OX40−/− mice exhibited delayed inflation of m139- and M38-specific CD8+ T cells and the overall amount of M38-specific T cell accumulation was reduced ~2-fold. However, the impact of OX40 deficiency on IE3-specific CD8+ T cells was not addressed. As shown in Fig. 4, loss of OX40 also had a significant impact on the inflation of IE3-specific CD8+ T cells, although the effect was less extensive than in CD40L−/− mice. In these OX40−/− mice, whereas there was no significant reduction in the accumulation of m139-specific T cells, M38-specific T cells were significantly reduced (~1.5 fold; data not shown), agreeing with previous results (39). Three of five OX40−/− mice tested contained a small amount (~102–103 PFU/organ) of replicating virus in their salivary gland 14 wk postinfection, suggesting that OX40 deficiency has impaired the ability of CD4+ T cells to control salivary gland replication.
Together, the data in Figs. 1–4 show that memory inflation in MCMV infection can occur in the absence of CD4+ T cell help, given that M38-specific T cells displayed largely normal or even increased inflation in CD4+ T cell-deficient mice. Inflation of m139-specific cells seemed to require CD4+ T cells for normal accumulation in the blood but not the tissues. Finally, inflation of IE3-specific T cells was exquisitely dependent on CD4+ T cell help, perhaps via costimulatory molecules CD40L and OX40, in both blood and tissues.
Division of MCMV-specific CD8+ T cells is minimally affected by the absence of CD4+ T cell help
Increased viral Ag during chronic LCMV infection drives increased T cell proliferation with eventual CD8+ T cell dysfunction or exhaustion (7, 10). Because CD4+ T cell-deficient mice have elevated levels of MCMV replication in the salivary gland as early as 2 wk postinfection (Fig. 5A), we asked whether this increased viral activity would lead to increased CD8+ T cell division. To this end, we pulsed wild-type, CD4−/− or MHC II−/− mice with BrdU from day 14 to day 28 and assessed the incorporation of BrdU by the tetramer+, virus-specific T cells. In wild-type mice, inflationary T cells (M38-, m139-, and IE3-specific) incorporated substantially more BrdU than their stable memory (M45- and M57-specific) counterparts (Fig. 5B). Thus as early as 2 wk postinfection, when virus was still evident in the salivary glands of wild-type mice, inflationary T cells were already diverging from the stable memory populations. This observation fits with the previous indication that memory inflation correlates with increased cell division (as assessed by Ki-67 staining) during the first 6 wk of infection (40). However, the elevated viral activity in CD4+ T cell-deficient mice did not translate into increased BrdU incorporation by any of the Ag-specific T cell populations (Fig. 5B). Furthermore, the difference between inflationary and stable memory populations was preserved: M45- and M57-specific T cells still incorporated low amounts of BrdU in CD4+ T cell-deficient mice when compared with inflationary T cells in the same animals. In fact, the only statistically significant difference we found indicated that m139-specific T cells divided less over this time period in the absence of CD4+ T cell help. These data show that inflationary T cells divided more frequently than stable memory T cells at the outset of memory inflation, but that the rate of division was not increased in accordance with the increased salivary gland viral replication in CD4+ T cell-deficient mice.
In chronically infected wild-type mice, the differentiated inflationary MCMV-specific T cells undergo very little Ag-driven cell division at late times after infection, even though these cells retain the ability to proliferate, which they do extensively when transferred into acutely infected animals (5). In addition, stable memory CD8+ T cells undergo homeostatic division in wild-type animals and seem to ignore the ongoing infection (5, 40). Hence, we assumed that the normally low levels of T cell division in wild-type animals reflected the exceedingly low level of viral replication ongoing in these mice. CD4+ T cell-deficient mice never control viral replication in their salivary gland (Fig. 5C). In addition, these mice display sporadic evidence of a low level (~101–102 PFU/organ) viral replication in their lungs and spleens as shown previously (Ref. 24 and data not shown). Thus we wondered whether inflationary CD8+ T cells would maintain their high level of cell division at late times in CD4+ T cell-deficient, but not wild-type, animals. However, despite the ongoing viral replication, Ag-specific CD8+ T cells incorporated a low amount of BrdU in all mice during chronic infection (4 mo postinfection; Fig. 5D). The M45-and M57-specific CD8+ T cells in MHC II−/− mice displayed slightly elevated BrdU incorporation, suggesting that these cells may be stimulated more in CD4+ T cell-deficient mice, although this did not reach statistical significance (p = 0.1632 for M45-specific T cells; p = 0.2887 for M57-specific T cells). Nevertheless, there was no substantial increase in the BrdU incorporation for any Ag-specific CD8+ T cells in MHC II−/− mice.
Together, these data show for the first time that inflationary cells continue to proliferate after the peak of the primary CD8+ T cell response, whereas stable memory cells do not. Strikingly, in CD4+ T cell-deficient animals, in which the virus persists at high levels in the salivary glands and can be found sporadically in other organs, the kinetics of proliferation of both inflationary and stable memory T cells essentially parallels that found in wild-type animals, where virus is well controlled.
MCMV-specific T cells display some evidence of increased Ag stimulation in the absence of CD4+ T cells, but do not develop substantial dysfunction
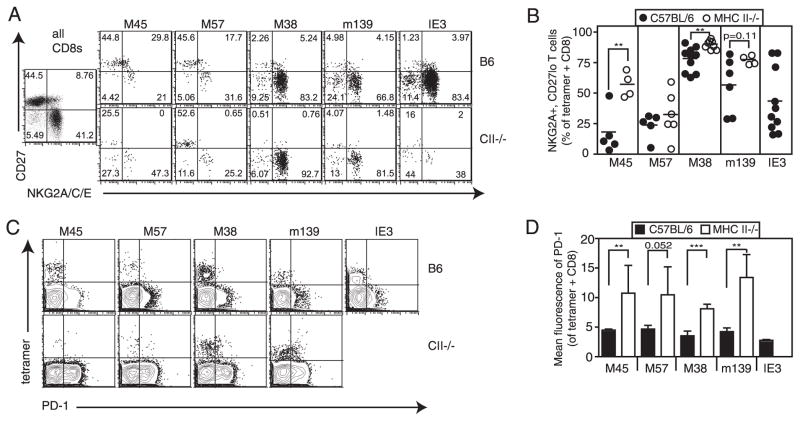
Because both CD4+ T cell deficiency and chronic viral replication have been shown to promote CD8+ T cell exhaustion, we hypothesized that inflationary CD8+ T cells, which show evidence of repeated Ag encounter even in wild-type animals (4, 5, 40), might develop substantial dysfunction in CD4+ T cell-deficient mice. To investigate this, we examined the phenotype and function of inflationary T cells in wild-type and CD4+ T cell-deficient mice. The vast majority of M38- and m139-specific inflationary T cells in both wild-type and CD4+ T cell-deficient mice expressed low levels of the differentiation markers CD127, CD27, and CD28 and high levels of the inhibitory proteins NKG2A and KLRG-1 (Fig. 6, A and B, and data not shown). These populations were more likely to be extensively differentiated in every MHC II−/− animal, whereas wild-type animals showed a wider distribution of phenotypes (Fig. 6B). However, this modest increase in differentiation of the inflationary populations was only evident in MHC II−/− mice; in CD4−/− animals these populations were indistinguishable from their wild-type counterparts (not shown). For reasons that are unclear, IE3-specific T cells in wild-type mice differ a little in phenotype from the other inflationary epitopes; although they are typically CD27low, they are often heterogeneous for NKG2A expression (Fig. 6, A and B, and Ref. 5). In MHC II−/− mice, these IE3-specific T cells were mostly absent. However, in the occasional MHC II−/− animal which had enough IE3-specific T cells to assess their phenotype, they appeared to have a phenotype similar to that of their wild-type counterparts (Fig. 6A). Finally, M45- and M57-specific T cells that do not inflate and appear phenotypically similar to central memory T cells in B6 animals (CD127high CD122highCD27highNKG2AlowKLRG-1low) showed some evidence of increased differentiation in MHC II−/−, but not CD4−/− mice (Fig. 6, A and B, and data not shown). However, even in MHC II−/− mice, in which replicating virus persists in the salivary gland indefinitely, these stable memory populations did not differentiate extensively to resemble inflationary T cells.
FIGURE 6.
There is some phenotypic evidence of persistent viral replication in CD4+ T cell-deficient mice. A, Ag-specific T cells from the peripheral blood of B6 and MHC II−/− mice were measured by tetramer staining were costained with the indicated Abs. Representative FACS plots are shown. Numbers indicate the percentage of gated cells in each quadrant. B, Combined phenotype data from two independent experiments (as in A) are shown. C, Tetramer+ T cells from the spleens of B6 and MHC II−/− mice were costained with PD-1-specific Abs. Representative FACS plots are shown. D, Mean fluorescence intensity of PD-1 staining (as in C) is shown for tetramer+, CD8+ T cells for four mice per group.
In wild-type animals, neither inflationary nor stable memory CD8+ T cells up-regulate PD-1, which is a hallmark of CD8+ T cell exhaustion (11). There was no evidence of increased PD-1 expression by any MCMV-specific CD8+ T cells in the blood of CD4+ T cell-deficient animals (not shown). However, some Ag-specific cells in the spleen displayed increased PD-1 expression in MHC II−/− mice (Fig. 6, C and D), though the difference was slight and again, not seen in CD4−/− animals (not shown). Thus, the increased amount of viral replication in CD4+ T cell-deficient animals does not substantially alter the phenotype of MCMV-specific CD8+ T cells.
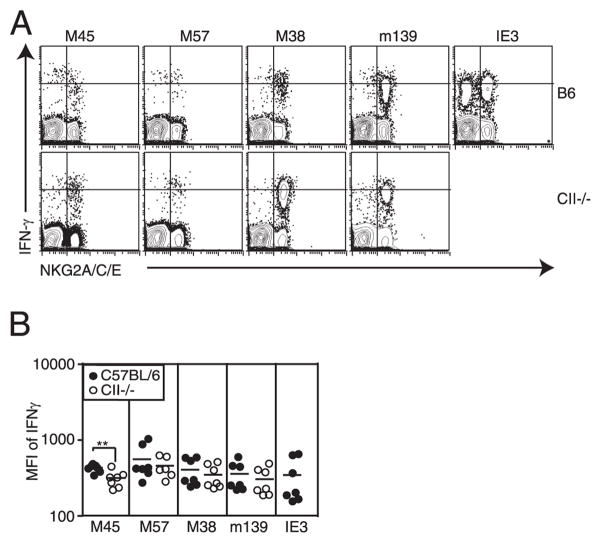
Another hallmark of T cell dysfunction is reduced cytokine expression (7, 10). We saw no evidence of reduced IFN-γ production on a per cell basis from the inflationary T cells in MHC II−/− mice (Fig. 7A) or CD4−/− mice (not shown). However, these cells are already differentiated and express slightly less IFN-γ per cell than their stable memory counterparts even in B6 animals (Fig. 7). The fraction of M45- and M57-specific T cells that differentiated during chronic infection (in both wild-type and MHC II−/− mice) also produced less IFN-γ per cell than their less differentiated counterparts (Fig. 7A). The example in Fig. 7A shows IFN-γ production by NKG2A-positive and -negative cells, although similar results were found for CD27low and CD127low CD8+ T cells (not shown). In MHC II−/− mice, the increased differentiation and reduction in IFN-γ production by M45-specific T cells were substantial enough to result in an overall reduction in the amount of IFN-γ produced per cell within this population (Fig. 7B). Although we also saw an increase in differentiated T cells within the M57-specific population, these cells more frequently seemed to retain their ability to secrete high levels of IFN-γ, and the overall amount of IFN-γ produced per cell was not significantly changed.
FIGURE 7.
Differentiated T cells express lower amounts of IFN-γ per cell after peptide stimulation. A, Representative FACS plots of CD8+ T cells from the peripheral blood stimulated with the indicated peptide and costained with Abs specific for NKG2A/C/E. Quadrants were drawn to illustrate the segregation of low and high IFN-γ expression by NKG2A+ and NKG2A− CD8+ T cells. B, Overall mean fluorescence intensity of IFN-γ staining after stimulation of peripheral blood T cells with the indicated peptides for B6 and MHC II−/− mice.
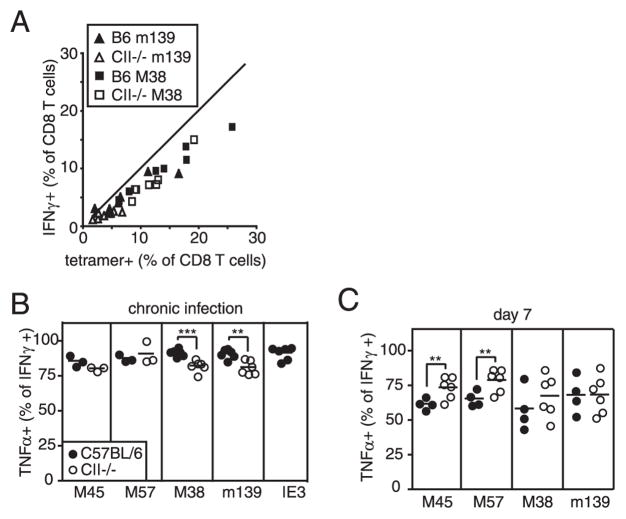
Most tetramer+, inflationary CD8+ T cells from chronically infected wild-type mice retained the ability to produce IFN-γ upon stimulation, and this was unchanged in MHC II−/− and CD4−/− mice (Fig. 8A and data not shown). We did find, however, that in MHC II−/− mice there was a small but significant reduction in the percent of M38- and m139-specific IFN-γ producing T cells that also produced TNF-α (Fig. 8B). Nevertheless, even in the absence of CD4+ T cell help, most MCMV-specific T cells retained the capacity to produce both IFN-γ and TNF-α, at least within the time frame described here (3–5 mo postinfection). The reduced TNF-α expression was not attributable to the MHC II−/− background per se, because no reduction was seen in these mice at day 7 postinfection, the peak of the acute CD8+ T cell response (Fig. 8C). In fact, both M45- and M57-specific T cells exhibited increased TNF-α expression at this early time in MHC II−/− mice. Most inflationary T cells do not produce IL-2, even in wild-type animals (4), and this was also unchanged in CD4+ T cell-deficient mice (not shown).
FIGURE 8.
Inflationary T cells retain the ability to secrete effector cytokines in CD4+ T cell-deficient mice. A, The percent of peripheral blood CD8+ T cells as measured by tetramer staining and IFN-γ production was measured in the same animals at the same time. B and C, The CD8+ T cells from the peripheral blood that produce IFN-γ in response to the indicated peptides were gated and the percentage that also produce TNF-α was measured.
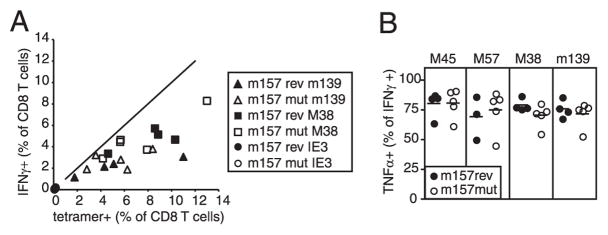
C57BL/6 mice are resistant to wild-type MCMV infection due to viral expression of the m157 protein, which stimulates a robust NK cell response in Ly49H-expressing mouse strains (41, 42). We considered the possibility that this robust NK cell response was compensating for the increased viral replication and preventing CD8+ T cell dysfunction. However, MHC II−/− mice infected with MCMV that either expressed m157 (m157rev) or lacked m157 (m157mut; K. S. Cho and A. B. Hill, manuscript in preparation) gave identical results (Fig. 9) suggesting that the strong NK cell response was not protecting the CD8+ T cells from exhaustion. Together, these data show that CD4+ deficiency does not lead to substantial and rapid CD8+ T cell exhaustion during chronic MCMV infection, although there is some evidence of increased T cell stimulation in MHC II−/− mice. Together, these data show that the effector cytokine production and phenotype of MCMV-specific CD8+ T cells is relatively unaffected by CD4+ T cell deficiency, despite the ongoing viral replication and lack of CD4+ T cell help.
FIGURE 9.
The presence or absence of m157 in the infecting virus does not affect the ability of Ag-specific CD8+ T cells to secrete effector cytokines. MHC II−/− mice were infected with m157rev (wild-type) or m157mut (m157 deficient) virus and analyzed in the peripheral blood after 22 wk of infection. A, The percent of peripheral blood CD8+ T cells as measured by tetramer staining and IFN-γ production was measured in the same animals at the same time. B, The CD8+ T cells that produce IFN-γ in response to the indicated peptides were gated, and the percentage that also produce TNF-α was measured.
Finally, in other model systems, CD4+ T cell deficiency is often, but not always, associated with poor proliferation in response to Ag challenge (32–34, 43–46). To test whether MCMV-specific CD8+ T cells primed in CD4+ T cell-deficient mice also exhibit poor recall activity, we purified CD8+ T cells from chronically infected MHC II−/− or wild-type mice, CFSE labeled them, and transferred them separately into naive, congenic recipients. After infection of recipient mice with MCMV, it was apparent that all donor CD8+ T cells were able to divide extensively, fully diluting CFSE within 6 days of the challenge infection (not shown). However, there were some hints that CD8+ T cells from MHC II−/− mice expanded less than their wild-type counterparts. Thus, to directly compare the proliferative capacity of each population, purified CD8+ T cells from wild-type mice (CD45.1+Thy1.2+) or MHC II−/− mice (CD45.1−Thy1.2+) were cotransferred into naive, Thy1.1 (CD45.1−Thy1.2−) recipients. Recipients were then challenged and MCMV-specific donor cells (Thy1.2+CD8+ T cells; Fig. 10A) were analyzed 7 days later. Although Ag-specific CD8+ T cells from both donor populations underwent clonal expansion, the wild-type CD8+ T cells consistently expanded to a greater degree (Fig. 10, B–D). When compared directly within each mouse, the wild-type CD8+ T cells expanded between 2× and 8× better than CD8+ T cells with the same specificity from MHC II−/− mice (Fig. 10D). However, when we analyzed BrdU uptake by Ag-specific T cells over the final 24 h (days 6–7), the CD8+ T cells from MHC II−/− mice were largely unimpaired in BrdU incorporation (Fig. 10E). Thus, although the CD8+ T cells from MHC II−/− mice failed to accumulate to the same degree as their wild-type counterparts, this may not be exclusively due to a lack of T cell division. Rather, it is possible that CD8+ T cells from MHC II−/− mice also survive more poorly than wild-type CD8+ T cells, as has been suggested elsewhere (43).
Together, our data show that whereas one inflationary population (IE3 specific) failed to accumulate, the M38- and m139-specific T cell populations inflated normally and did not become markedly exhausted in CD4+ T cell-deficient mice, despite the elevated levels of viral replication in these animals. These cells were fully differentiated in phenotype and were not dividing extensively, consistent with the notion of a linkage between exhaustion and Ag-driven proliferation (47). However, CD4+ T cell deficiency did affect the ability of MCMV-specific CD8+ T cells to expand in response to acute viral challenge, similar to many experiments with acute, cleared infections (32, 33, 43–46). This impairment was seen in both the inflationary and stable memory populations and is consistent with the notion of altered programming during their initial priming, rather than being related to dysfunction induced by chronic infection. However, this reduced recall capacity failed to substantially affect the steady-state maintenance of inflationary CD8+ T cells that developed during chronic MCMV infection of CD4+ T cell-deficient mice.
Discussion
Although it is clear that CD4+ T cell help is important for the generation or maintenance of memory CD8+ T cells in acute, cleared infections, the role of CD4+ T cells in persistent viral infections has varied with the infectious model. Here we investigated the impact of CD4+ T cell deficiency on the chronic CD8+ T cell response to MCMV in B6 mice. Because CD4+ T cell deficiency results in high-titer MCMV persistence in the salivary gland, we were looking at the combined impact of an increased antigenic burden and the absence of CD4+ T cell help. We expected that this would result in an increased activation state and perhaps inflation of the normally stable memory cells and a shift toward exhaustion in the inflationary cells. Instead, we found that, although recall responses were diminished, the pattern and function of both stable and inflationary memory responses during chronic infection were remarkably similar to those of the wild type. The single exception was the absence of a detectable inflationary response to the normally codominant IE3 epitope.
It is unclear at this time why IE3-specific CD8+ T cells differ from m139- and M38-specific CD8+ T cells in their CD4+ T cell dependency, although it seems likely that the cause is related to the different kinetics of the responses (Fig. 1 and Ref. 4). Because IE3-specific CD8+ T cells are not prominent early in infection, it is tempting to speculate that they must be primed at late times, after the majority of the virus has been controlled. Indeed, we have shown that IE3-specific T cells (as well as m139- and M38-specific T cells) can be primed at late times and during memory inflation in B6 mice (Ref. 5 and data not shown). Although inflammatory signals may be able to license dendritic cells during acute infection, thus priming m139- and M38-specific T cells, CD4+ T cells may be required to license dendritic cells after viral replication has been largely controlled. Analogous results were obtained after infection with polyomavirus (14). In these experiments, CD4+ T cell deficiency resulted in reduced priming of new virus-specific T cells during chronic infection and thus fewer virus-specific T cells at steady state. The reduction of IE3-specific CD8+ T cells in CD40L−/− mice is also consistent with the hypothesis that priming of IE3-specific T cells is impaired in CD4+ T cell-deficient mice. CD40 is important for dendritic cell licensing (36, 37) and could be vital for licensing dendritic cells during chronic MCMV infection.
It is also possible that the survival or maintenance of IE3-specific CD8+ T cells is dependent on CD4+ T cell help. During chronic infection with virulent strains of LCMV, T cells specific for some epitopes are deleted, which is considered to be an extreme stage of exhaustion (6, 7, 9, 10, 13). Hence, one could imagine that IE3-specific T cells are deleted in CD4+ T cell-deficient mice due to severe exhaustion. However, the fact that the other inflationary populations showed no sign of exhaustion makes this seem less likely.
Exhaustion of CD8+ T cells in chronic LCMV infection is characterized by a progressive loss of the ability of cells to produce IL-2, followed by TNF-α and ultimately IFN-γ. During MCMV infection in wild-type animals, inflationary CD8+ T cells fail to make IL-2, but produce both TNF-α and IFN-γ upon peptide stimulation (4, 5). Although CD4+ T cell-deficient mice have increased viral replication and no CD4+ T cell help, the vast majority of M38- and m139-specific CD8+ T cells continued to produce both TNF-α and IFN-γ, suggesting that they were not becoming progressively dysfunctional. In fact, a reduced recall capacity was the only obvious functional defect in the MCMV-specific CD8+ T cells that we measured. However, this is a well-established effect of CD4+ T cell deficiency on CD8+ T cell memory in many models of acute cleared infections and is linked to programming during acute infection rather than exhaustion (32, 33, 43–46).
CD4+ T cell deficiency exacerbates and accelerates T cell exhaustion in the chronic LCMV model of infection (9, 10). However, similar to our results with MCMV, CD4+ T cell deficiency has a much more limited impact on CD8+ T cell function during chronic infections of polyomavirus, HSV, or murine γ-herpesvirus-68 (14–19, 22, 23). Wherry and Ahmed (48) have suggested that repeated antigenic stimulation is the main determinant of T cell exhaustion and that differing viral loads during chronic infection primarily account for the differences seen with these different models. Thus, perhaps, some differences between infection models can be ascribed to the absolute amount of viral Ag. Even in CD4+ T cell-deficient animals, the MCMV burden is markedly lower than in chronic LCMV infection (Ref. 24 and Fig. 5C). MCMV replicates to higher titers in the absence of effective NK cell control (such as in BALB/c mice or Δm157 virus in B6 mice), but even in these cases CD4+ T cell-deficient animals maintain functional antiviral CD8+ T cells (Fig. 9 and Ref. 24). Nevertheless, these manipulations still do not result in the level of virus burden that is seen in chronic LCMV infection, particularly in central organs, and the concept of Ag load as a determinant of T cell exhaustion remains attractive.
MCMV is used as a model for human CMV infection. In general, human CMV-specific CD8+ T cells also remain functional throughout the chronic, lifelong infection. However, in transplant recipients there are periods of viremia, and these have been shown to correlate with CD8+ T cell dysfunction (49–52). These periods of viremia likely represent vastly more systemic antigenic load than is found during MCMV infection, even in CD4+ T cell-deficient mice, which is consistent with antigenic load as a major determinant of T cell dysfunction.
Another question driving our experiments was whether ongoing, high-level viral replication would disrupt the normal dichotomy between stable and inflationary cells. The determinants of inflationary and stable memory in MCMV infection are unknown. We show here that inflationary and stable memory T cells diverge as early as 2–4 wk postinfection, before substantial inflation becomes evident, but while the virus is still replicating in the salivary gland of infected animals (Fig. 5). It was striking to us that this divergence in the rate of proliferation was independent of viral control or CD4+ T cell help. We might have expected the stable memory populations (M45 and M57 specific) to continue dividing and begin inflating in response to the elevated viral replication in CD4+ T cell-deficient mice. Instead, M45- and M57-specific CD8+ T cells, which resemble central memory T cells in wild-type mice, were reduced in number at late times in CD4+ T cell-deficient mice (Fig. 2), similarly to central memory T cells generated by acute, cleared infections in the absence of CD4+ T cell help (32, 34, 45). In fact, there was no increase in T cell proliferation by stable or inflationary CD8+ T cells in CD4+ T cell-deficient mice despite the ongoing, elevated viral replication. Together, these data could suggest that salivary gland viral replication is not well recognized by either memory cell population. Instead, abortive reactivation of latent virus in a central compartment could be the main driver of inflationary CD8+ T cell responses in both wild-type and CD4+ T cell-deficient animals.
In summary, our data show that CD4+ T cell help has an epitope-specific impact on memory inflation in B6 mice. A defect in priming of IE3-specific T cells in CD4+ T cell-deficient animals may explain their failure to inflate. However, the steady-state numbers and effector function of the other CD8+ T cells populations were not substantially impaired despite the ongoing high level of viral replication and lack of CD4+ T cell help. Thus, at the level of viral persistence seen in our model, CD4+ T cell help is not a dominant factor in the maintenance of functional MCMV-specific CD8+ T cells.
Footnotes
This work was supported by National Institutes of Health Grant R01 A I47206 and an American Heart Association Grant-in-Aid (to A.B.H.) as well as American Heart Association Postdoctoral Training Grant 0725786Z (to C.M.S.) and Deutsche Forschungsgemeinschaft Grant LO 1421/1-1 (to A.L.).
Abbreviations used in this paper: MCMV, murine CMV; LCMV, lymphocytic choriomeningitis virus; MHC II, MHC class II; ICS, intracellular cytokine staining; μMT, B6.129S2-Igh-6tm1Cg/J.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62Llo memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 3.Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, Freigang S, Koszinowski UH, Phillips RE, Klenerman P. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol. 2004;78:2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 5.Snyder CM, Cho KS, Morrison EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice: role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 12.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 14.Kemball CC, Pack CD, Guay HM, Li ZN, Steinhauer DA, Szomolanyi-Tsuda E, Lukacher AE. The antiviral CD8+ T cell response is differentially dependent on CD4+ T cell help over the course of persistent infection. J Immunol. 2007;179:1113–1121. doi: 10.4049/jimmunol.179.2.1113. [DOI] [PubMed] [Google Scholar]
- 15.Kemball CC, Szomolanyi-Tsuda E, Lukacher AE. Allogeneic differences in the dependence on CD4+ T-cell help for virus-specific CD8+ T-cell differentiation. J Virol. 2007;81:13743–13753. doi: 10.1128/JVI.01778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belz GT, Stevenson PG, Castrucci MR, Altman JD, Doherty PC. Postexposure vaccination massively increases the prevalence of γ-herpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice. Proc Natl Acad Sci USA. 2000;97:2725–2730. doi: 10.1073/pnas.040575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson PG, Belz GT, Altman JD, Doherty PC. Virus-specific CD8+ T cell numbers are maintained during γ-herpesvirus reactivation in CD4-deficient mice. Proc Natl Acad Sci USA. 1998;95:15565–15570. doi: 10.1073/pnas.95.26.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belz GT, Liu H, Andreansky S, Doherty PC, Stevenson PG. Absence of a functional defect in CD8+ T cells during primary murine γherpesvirus-68 infection of I-A(b−/−) mice. J Gen Virol. 2003;84:337–341. doi: 10.1099/vir.0.18821-0. [DOI] [PubMed] [Google Scholar]
- 20.Flano E, Woodland DL, Blackman MA. Requirement for CD4+ T cells in Vβ4+ CD8+ T cell activation associated with latent murine γherpesvirus infection. J Immunol. 1999;163:3403–3408. [PubMed] [Google Scholar]
- 21.Evans AG, Moser JM, Krug LT, Pozharskaya V, Mora AL, Speck SH. A γherpesvirus-secreted activator of Vβ4+CD8+ T cells regulates chronic infection and immunopathology. J Exp Med. 2008;205:669–684. doi: 10.1084/jem.20071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, Belz GT. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 23.Jennings SR, Bonneau RH, Smith PM, Wolcott RM, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991;133:234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- 24.Jonjic S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med. 1989;169:1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Luccaronin P, Jonjic S, Koszinowski UH. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med. 1998;188:1047–1054. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner M, Jonjic S, Koszinowski UH, Messerle M. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J Virol. 1999;73:7056–7060. doi: 10.1128/jvi.73.8.7056-7060.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol. 2006;176:3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- 28.Fuse S, Zhang W, Usherwood EJ. Control of memory CD8+ T cell differentiation by CD80/CD86−CD28 costimulation and restoration by IL-2 during the recall response. J Immunol. 2008;180:1148–1157. doi: 10.4049/jimmunol.180.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton SM, Wyrsch P, Munks MW, Zimmermann A, Hengel H, Hill AB, Oxenius A. The dynamics of mouse cytomegalovirus-specific CD4 T cell responses during acute and latent infection. J Immunol. 2008;181:1128–1134. doi: 10.4049/jimmunol.181.2.1128. [DOI] [PubMed] [Google Scholar]
- 30.Tyznik AJ, Sun JC, Bevan MJ. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med. 2004;199:559–565. doi: 10.1084/jem.20031961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonjic S, Pavic I, Polic B, Crnkovic I, Lucin P, Koszinowski UH. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med. 1994;179:1713–1717. doi: 10.1084/jem.179.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marzo AL, Vezys V, Klonowski KD, Lee SJ, Muralimohan G, Moore M, Tough DF, Lefrancois L. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173:969–975. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 35.Sarawar SR, Lee BJ, Reiter SK, Schoenberger SP. Stimulation via CD40 can substitute for CD4 T cell function in preventing reactivation of a latent herpesvirus. Proc Natl Acad Sci USA. 2001;98:6325–6329. doi: 10.1073/pnas.101136898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 37.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 38.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 39.Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW, Ware CF, Croft M. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T cells: a CD4-dependent mechanism. J Immunol. 2007;179:2195–2202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 40.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 41.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 43.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 44.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 45.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 46.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Widmann T, Sester U, Gartner BC, Schubert J, Pfreundschuh M, Kohler H, Sester M. Levels of CMV specific CD4 T cells are dynamic and correlate with CMV viremia after allogeneic stem cell transplantation. PLoS ONE. 2008;3:e3634. doi: 10.1371/journal.pone.0003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.La Rosa C, Krishnan A, Longmate J, Martinez J, Manchanda P, Lacey SF, Limaye AP, Diamond DJ. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis. 2008;197:25–33. doi: 10.1086/523652. [DOI] [PubMed] [Google Scholar]
- 51.Ozdemir E, St John LS, Gillespie G, Rowland-Jones S, Champlin RE, Molldrem JJ, Komanduri KV. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood. 2002;100:3690–3697. doi: 10.1182/blood-2002-05-1387. [DOI] [PubMed] [Google Scholar]
- 52.Crough T, Fazou C, Weiss J, Campbell S, Davenport MP, Bell SC, Galbraith A, McNeil K, Khanna R. Symptomatic and asymptomatic viral recrudescence in solid-organ transplant recipients and its relationship with the antigen-specific CD8+ T-cell response. J Virol. 2007;81:11538–11542. doi: 10.1128/JVI.00581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]