Abstract
Elevated pulmonary arterial systolic pressure is strongly associated with mortality in patients with sickle cell disease (SCD). A tricuspid regurgitant velocity (TRV) of 2.5 m/s or greater by trans-thoracic echocardiogram is a key marker of risk [1–3]. The pathophysiologic mechanism involves release from the red cell during intravascular hemolysis of cell-free plasma hemoglobin and arginase [4]. Hydroxyurea is the only drug approved by the Food and Drug Administrations specifically for SCD. It acts by increasing levels of fetal hemoglobin, which inhibits sickling, and has been shown to reduce the incidence of vaso-occlusive crisis (VOC), and prolong survival in patients with sickle cell disease [5,6]. Because fetal hemoglobin also reduces the rate of hemolysis in SCD, hypothetically, hydroxyurea might also reduce the severity of hemolysis-linked vascular dysfunction and pulmonary hypertension. Herein, we describe five patients with sickle cell disease having elevated pulmonary arterial systolic pressure who exhibited improvement in their baseline laboratory parameters of hemolysis, accompanied by reduced TRV, during treatment with hydroxyurea. Hydroxyurea may have a role in the management of selected patients with elevated TRV.
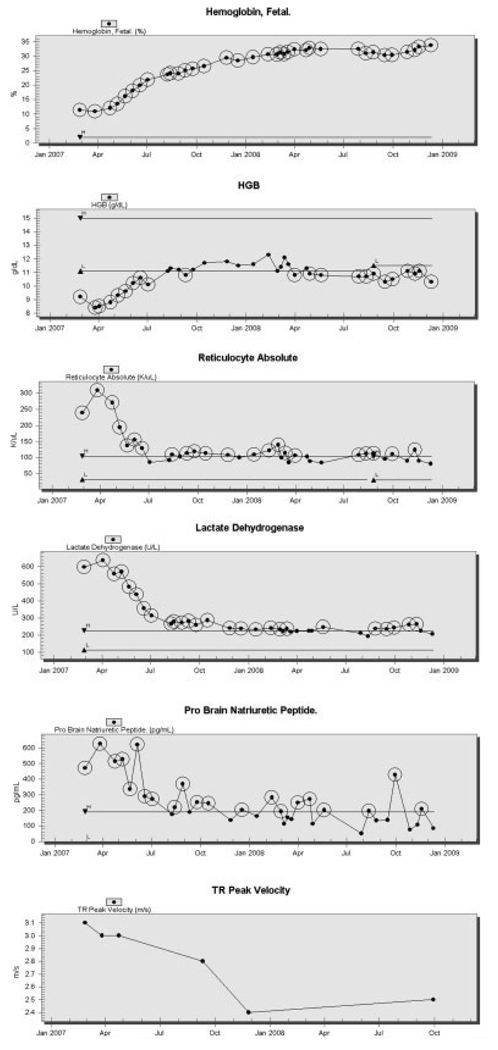
Patient A is a 46-year-old woman with homozygous SCD, prior episodes of acute chest syndrome, cholelithiasis, and elevated pulmonary artery systolic pressure indicated by a peak tricuspid regurgitant velocity (TRV) 3.4 m/s, unchanged by treatment with bosentan for 8 months. Her steady-state laboratory values demonstrated greater than average hemolysis with an absolute reticulocyte count of 431,000/uL, and serum lactate dehydrogenase (LDH) of 650 U/L prior to hydroxyurea therapy. Her baseline hemoglobin F was 12%, suggesting a component of persistent fetal hemoglobin. She was started on hydroxyurea, and the dose was titrated to 17.4 mg/kg/day. After treatment with hydroxyurea (Fig. 1), her fetal hemoglobin increased to 33%, and her untransfused hemoglobin increased from 9.0 to 10.8 g/dL with a corresponding increase in her mean corpuscular volume from 91 to 117 fL. Her hemolytic parameters improved, with her LDH declining steadily to 246 U/L, and her reticulocyte count declining to 84,000/uL. During this same time frame, her pulmonary arterial pressure improved, with her TRV declining to the normal range value of 2.4 m/s.
Figure 1.
Hematological and pulmonary response to hydroxyurea therapy in a representative patient. Baseline measurements were obtained, and then following the commencement of hydroxyurea therapy in this 46-year-old woman with sickle cell disease, who was started on bosentan 8 months before beginning hydroxyurea. Following the induction of fetal hemoglobin, linked rises were seen in total blood hemoglobin, (Hgb), and associated fall in absolute reticulocyte count and serum lactate dehydrogenase. An improvement in pulmonary arterial pressure was suggested by a fall in serum probrain natriuretic peptide and tricuspid regurgitant peak velocity (TR peak velocity).
Four other patients were observed to have similar improvements in their laboratory markers of hemolysis and TRV after treatment with hydroxyurea (Table I). Expected improvements were observed in the hematological parameters. Statistically significant increases were observed in mean corpuscular volume and levels of fetal hemoglobin and F-cells. A significant drop was observed for serum lactate dehydrogenase level, a marker of intravascular hemolysis that is a surrogate marker for diminished nitric oxide bioavailability, vascular dysfunction, and risk for pulmonary hypertension [7]. A statistically significant drop in TRV occurred during hydroxyurea treatment, suggesting a hydroxyurea-induced improvement in pulmonary pressures. The magnitude of decline in the TRV in these five patients was significantly related on linear regression to the hydroxyurea-induced increase in the percentage of fetal hemoglobin (r2 = 0.87, P < 0.05) and rise in mean corpuscular volume (r2 = 0.82, P < 0.05). Similar trends with TRV were seen with LDH, reticulocyte count, F-cell, F-reticulocyte, white blood cell count, and NT-proBNP level. Interestingly, only two of the patients had at baseline an abnormally high level of N-terminal probrain-type natriuretic peptide above 160 pg/mL, a marker linked to elevated pulmonary arterial systolic pressure in SCD [8], and levels did not change significantly during hydroxyurea treatment (Table I).
TABLE I.
Patient Characteristics and Results of Hydroxyurea Treatment in Five Patients with Sickle Cell Anemia and Elevated Pulmonary Pressure at Baseline
| Characteristic | Mean (SD) | ||
|---|---|---|---|
| Age (Years) | 38 (6) | ||
| Male/female | 1/4 | ||
| Hydroxyurea dose | |||
| (mg/day) | 1350 (720) | ||
| (mg/kg/day) | 18 (6) | ||
| Duration (months) | 14 (7) | ||
| Variable | Pre-Hydroxyurea | On Hydroxyurea | P |
| Hospitalizations per year | 4.6 (3.4) | 2.0 (3.5) | n.s. |
| Leukocyte Count (× 1,000/mL) | 10.3 (3.3) | 6.2 (1.2) | n.s. |
| Hemoglobin (g/dL) | 8.3 (1.9) | 8.7 (1.7) | n.s. |
| Mean Corpuscular Volume (fL) | 95 (7) | 109 (13) | 0.03 |
| Platelet Count (× 1,000/mL) | 497 (197) | 343 (49) | n.s. |
| Reticulocyte Count (× 1,000/mL) | 250 (157) | 129 (77) | n.s. |
| Fetal hemoglobin (%) | 8.4 (5.1) | 22.9 (9.4) | 0.008 |
| F-cells (%) | 57 (5) | 71 (22) | 0.05 |
| F-reticulocytes (%) | 32 (9) | 46 (24) | n.s. |
| Lactate dehydrogenase (IU/L) | 329 (61) | 259 (37) | 0.02 |
| NT-proBNP (pg/mL) | 176 (182) | 148 (74) | n.s. |
| TRV (m/sec) | 3.3 (0.3) | 2.5 (0.3) | 0.0004 |
NT-proBNP, N-terminal probrain type natriuretic peptide; TRV, tricuspid regurgitant jet velocity; P values computed by paired t-test.
These cases represent to our knowledge the first published observation in adults with SCD for hydroxyurea reducing pulmonary arterial systolic pressure, an important predictor of mortality. Our observation indicates another potential clinical benefit of hydroxyurea therapy in SCD. The pathophysiology of SCD is multifactorial, with components of vaso-occlusion due to decreased erythrocyte compliance, abnormal endothelial adhesion, and hemolysis-linked endothelial dysfunction promoting proliferative vasculopathy [4]. The latter process is associated epidemiologically with an increased steady state level of hemolytic markers such as LDH and reticulocyte count, as well as cutaneous leg ulceration, priapism, and pulmonary hypertension [7]. Hydroxyurea may reduce the steady state of sickle-related hemolysis, which may contribute to its beneficial effects on vascular dysfunction. Consistent with this possibility, we observed a decrease in baseline hemolytic parameters of LDH and reticulocyte count in our patients after hydroxyurea therapy.
In published results of two registry studies, hydroxyurea use was not clearly associated with a lower prevalence of elevated pulmonary pressures [1,3]. This has seemed paradoxical, because part of the pathobiological basis of pulmonary hypertension in SCD is believed to be related to hemolysis, and hemolysis is known to be attenuated by the antisickling effects of the fetal hemoglobin induced by hydroxyurea [9]. In contrast, other cross-sectional studies have reported a lower prevalence of pulmonary hypertension in patients on hydroxyurea therapy compared with those not taking the drug [2,10], and a recent pediatric series suggests that TRV may fall during hydroxyurea therapy [11]. However, confounding by indication is an issue in nonrandomized registry studies in cases where drug intervention is involved. Similar prevalence of high TRV in the hydroxyurea and nonhydroxyurea groups in a nonrandomized study does not rule out the possibility that the hydroxyurea group might have had a high initial prevalence of elevated TRV that fell during treatment to a similar level as the untreated group. The patients in our series had a modest improvement in their hemoglobin after starting hydroxyurea, in concordance with the level of improvement seen on the Multicenter Study of Hydroxyurea study [5]. Factors other than decreased hemolysis may also contribute to the observed improvement in TRV, such as decreased sickling, decreases in endothelin-1 levels [12,13], or a nitric oxide donor effect of hydroxyurea [14–16]. A prospective, randomized controlled trial with correlative studies would more directly address these issues.
It is noteworthy that while patient A had only two VOC hospitalizations per year before commencing hydroxyurea, she had moderately elevated pulmonary pressure that persisted on a pulmonary vasodilator for several months and improved significantly only after the addition of hydroxyurea therapy. Similarly, another of the patients was treated with hydroxyurea despite having no prior VOC admissions in the previous year, and his pulmonary pressure declined to normal after treatment. Thus, hydroxyurea may be worth considering in SCD patients with elevated pulmonary arterial pressure even in the absence the usual indication of frequent hospitalizations for acute pain episodes. We did not systematically search our database for patients whose TRV did not fall with commencement of hydroxyurea, though anecdotally some such counterexamples were seen. Compared with the prototypical SCD patient with high TRV [1], the responsive patients in this case series on the average are younger, with more mild TRV elevation, and with very robust fetal hemoglobin induction by hydroxyurea. As seen in pulmonary hypertension caused by other etiologies, treatment at a younger age with less longstanding disease may be more successful in reversal. A larger, prospective clinical trial is needed to validate these findings and investigate the predictors of reduced TRV after hydroxyurea treatment.
Methods
The patients provided informed consent for enrollment in a study of the natural history of sickle cell disease approved by the institutional review board of the National Heart, Lung and Blood Institute, ClinicalTrials.gov identifier NCT00081523. Cases were identified with a screening echocardiogram tricuspid regurgitant jet velocity above 2.5 m/sec, who subsequently began hydroxyurea treatment of sickle cell disease. Standard clinical laboratory and echocardiography data were reviewed prior to and following initiation of hydroxyurea for an average of 14 months (range 4–22 months). All the patients had homozygous sickle cell anemia. Electronic chart review was performed for 12 months prior to initiation of hydroxyurea and the follow up period for hospitalization rate, and calculated as yearly rate. Patients were started on hydroxyurea at 15 mg/kg per day, and the dose was titrated up by 5 mg/kg/day every 2 months as tolerated to a maximum dose of 35 mg/kg/day. The indications for initiating hydroxyurea were frequent VOC in three patients, and two patients elected to start hydroxyurea to attenuate the severity of their disease, partly over their concern for their elevated TRV. One patient was on bosentan for 5 months without a significant change in her TRV prior to starting hydroxyurea, while the other four patients were on no pulmonary vasodilators. This is a clinical case series obtained as a convenience sample. Paired data points were compared where appropriate by paired t-test. Linear regression was performed to compare the relationship of change in hematologic variables to decline in TRV. Statistical significance was assumed for P < 0.05.
Acknowledgments
The authors acknowledge protocol management support from Mary Hall, clerical support from Heather Kennedy, and the patients whose participation makes this manuscript possible.
Contract grant sponsors: National Heart, Lung and Blood Institute, Clinical Center of the National Institutes of Health
Footnotes
Conflict of Interest: Nothing to report.
Clinical Trials Registration: http://clinicaltrials.gov/ct2/show/NCT00081523.
References
- 1.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 2.Ataga KI, Moore CG, Jones S, et al. Pulmonary hypertension in patients with sickle cell disease: A longitudinal study. Br J Haematol. 2006;134:109–115. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 3.De Castro LM, Jonassaint JC, Graham FL, et al. Pulmonary hypertension associated with sickle cell disease: Clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2008;83:19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 4.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the multicenter study of hydroxyurea in sickle cell anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: Risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 7.Kato GJ, McGowan VR, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado RF, Anthi A, Steinberg MH, et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296:310–318. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- 9.Rodgers GP, Dover GJ, Noguchi CT, et al. Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N Engl J Med. 1990;322:1037–1045. doi: 10.1056/NEJM199004123221504. [DOI] [PubMed] [Google Scholar]
- 10.Ataga KI, Sood N, De GG, et al. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117:665–669. doi: 10.1016/j.amjmed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Longitudinal follow up of elevated pulmonary artery pressures in children with sickle cell disease. Br J Haematol. 2009;144:736–741. doi: 10.1111/j.1365-2141.2008.07501.x. [DOI] [PubMed] [Google Scholar]
- 12.Lapouméroulie C, Benkerrou M, Odièvre MH, et al. Decreased plasma endothelin-1 levels in children with sickle cell disease treated with hydroxyurea. Haematologica. 2005;90:401–403. [PubMed] [Google Scholar]
- 13.Brun M, Bourdoulous S, Couraud PO, et al. Hydroxyurea downregulates endothelin-1 gene expression and upregulates ICAM-1 gene expression in cultured human endothelial cells. Pharmacogenomics J. 2003;3:215–226. doi: 10.1038/sj.tpj.6500176. [DOI] [PubMed] [Google Scholar]
- 14.Cokic VP, Andric SA, Stojilkovic SS, et al. Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood. 2008;111:1117–1123. doi: 10.1182/blood-2007-05-088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cokic VP, Beleslin-Cokic BB, Tomic M, et al. Hydroxyurea induces the eNOS-cGMP pathway in endothelial cells. Blood. 2006;108:184–191. doi: 10.1182/blood-2005-11-4454. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Kim-Shapiro DB, King SB. Catalase-mediated nitric oxide formation from hydroxyurea. J Med Chem. 2004;47:3495–3501. doi: 10.1021/jm030547z. [DOI] [PubMed] [Google Scholar]