Abstract
Background and Aims
Cucumis melo subsp. agrestis (Cucurbitaceae) is cultivated in many African regions for its edible kernels used as a soup thickener. The plant, an annual, andromonoecious, trailing-vine species, is of high social, cultural and economic value for local communities. In order to improve the yield of this crop, the first step and our aim were to elucidate its breeding system.
Methods
Eight experimental pollination treatments were performed during three growing seasons to assess spontaneous selfing, self-compatibility and effects of pollen source (hermaphroditic vs. male flowers). Pollination success was determined by pollen tube growth and reproductive success was assessed by fruit, seed and seedling numbers and characteristics. The pollinator guild was surveyed and the pollination distance determined both by direct observations and by indirect fluorescent dye dispersal.
Key Results
The species is probably pollinated by several Hymenoptera, principally by Hypotrigona para. Pollinator flight distances varied from 25 to 69 cm. No evidence for apomixis or spontaneous self-pollination in the absence of insect visitors was found. The self-fertility index (SFI = 0) indicated a total dependence on pollinators for reproductive success. The effects of hand pollination on fruit set, seed number and seedling fitness differed among years. Pollen tube growth and reproductive success did not differ between self- and cross-pollinations. Accordingly, a high self-compatibility index for the fruit set (SCI = 1·00) and the seed number (SCI = 0·98) and a low inbreeding depression at all developmental stages (cumulative δ = 0·126) suggest a high selfing ability. Finally, pollen origin had no effect on fruit and seed sets.
Conclusions
This andromonoecious species has the potential for a mixed mating system with high dependence on insect-mediated pollination. The selfing rate through geitonogamy should be important.
Keywords: Cucumis melo subsp. agrestis, andromonoecy, breeding system, hand pollination, pollinators, self-compatibility, inbreeding depression
INTRODUCTION
The tremendous variety of reproductive systems exhibited by plants is one of the main focuses of evolutionary biologists (Barrett, 2002). One particular reproductive system is andromonoecy, in which both perfect (hermaphroditic) and staminate (i.e. female-sterile or male) flowers are produced on the same individual. Andromonoecy has evolved independently numerous times (Miller and Diggle, 2003) and is found in approx. 4000 species (i.e. 1·7 %) in 33 angiosperm families (Yampolsky and Yampolsky, 1922; Bertin, 1982; Miller and Diggle, 2003). It has been suggested that andromonoecy evolved from hermaphroditism by loss of the female reproductive structures, which is the first step in the evolution of a plant breeding system towards monoecy, androdioecy and dioecy (Bertin, 1982; Zhang and Tan, 2009). Consequently, evolution and maintenance of the male flowers in andromonoecious species have attracted considerable attention (Cuevas and Polito, 2004; Vallejo-Marin and Rausher, 2007).
Three, not necessarily mutually exclusive, hypotheses have been proposed regarding the evolution of male flowers and andromonoecy. First, the resource reallocation hypothesis posits that the production of staminate flowers reduces resource investment and permits the resources saved to be re-allocated toward other fitness-enhancing traits (Bertin, 1982; Emms, 1993; Liao et al., 2006; Vallejo-Marin and Rausher, 2007). This hypothesis assumes that because female reproductive success is limited by resources rather than pollen, individual fitness will be enhanced by the existence of staminate flowers once female reproductive success is maximized since the production of staminate flowers should be less costly (usually smaller than perfect flowers) and enhance male function (Emms, 1993; Spalik and Woodell, 1994; Liao et al., 2006). The second hypothesis, increased pollen donation, in contrast, posits that staminate flowers are more efficient at donating pollen than hermaphroditic flowers for any of several reasons: more or larger pollen, higher pollen viability or greater attractiveness to pollinators (Charlesworth and Morgan, 1991; Harder and Barrett, 1996; Barrett, 2002). Finally, the third hypothesis posits an increased pollen reception on hermaphroditic flowers which also predicts a greater seed production by plants producing some staminate flowers (Vallejo-Marin and Rausher, 2007). This last hypothesis could mainly occur in pollen-limited species.
Another important feature of andromonoecy is its role in cross-pollination. If male flowers increase floral display and consequently affect cross-pollination by attracting more pollinators that bring cross-pollen to the stigmas of hermaphroditic flowers, their presence can increase the outcrossing rate and female fitness of individuals. In general, outcrossed progeny have higher levels of genetic diversity than those produced by selfing, which are expected to have lower individual fitness than outcrossers due to the effects of inbreeding depression and the expression of deleterious, recessive alleles (Schemske and Lande, 1985; Charlesworth and Charlesworth, 1987). On the contrary, selfing provides a selective advantage by increasing reproductive success when pollinators are scarce or limited pollen transfer reduces reproductive output (Holsinger, 2000). Furthermore, through selfing an individual has a transmission advantage of two over outcrossing individuals, and alleles promoting selfing are likely to spread (Fisher, 1941; Holsinger, 2000). This spread can be counterbalanced by inbreeding depression in selfed progeny (Porcher and Lande, 2005). Inbreeding depression can play an important role in the evolution of mating systems, and this reduction in fitness of inbred progeny relative to outbred progeny is common in flowering plants (Charlesworth and Charlesworth, 1987; Husband and Schemske, 1995; Porcher and Lande, 2005). Estimating the mating system of a species (its tendency to self and/or to cross) is thus necessary to assess the role of male flowers in andromonoecious species.
The Cucurbitaceae family, including major food plants such as cucumbers, melons, pumpkins and marrows, comprises about 700 species with a distribution centred in the tropics. Members of the family are typically climbing plants with unisexual flowers on the same or different plants (Heywood, 1993). Most species of Cucurbitaceae are either monoecious (both sexes on the same plant) or dioecious (all flowers of only one sex on the same plant), with one known androdioecious species (Akimoto et al., 1999) and several andromonoecious species (Boualem et al., 2008). Most cultivars of muskmelons (Cucumis melo) are andromonoecious whereas cucumbers (Cucumis sativus) are normally monoecious (McGregor, 1976). In the melon species (C. melo) sex determination is governed by an andromonoecious (a) and a gynoecious (g) gene, and the interplay of these two genes results in a range of sexual types. Andromonoecious individuals bear the aaG- genotype (Kenigsbuch and Cohen, 1990; Boualem et al., 2008).
The African melon C. melo subsp. agrestis Naudin is an andromonoecious, annual, trailing-vine plant. This subspecies is cultivated in many African regions for its edible kernels that can be used as a soup thickener preferentially during popular fetes and prestigious ceremonies (van Epenhuijsen, 1974; Akobundu et al., 1982; Badifu, 2001; Zoro Bi et al., 2003, 2006; Achu et al., 2005; Djè et al., 2006; Mariod and Matthäus, 2007). Seeds present good nutritional value (Loukou et al., 2006). The plant is of high social, cultural and economic value for African communities.
The reproduction of C. melo subsp. agrestis takes place only by seeds. The fruits are berries, spherical to ovoid in shape, 3–7 cm in diameter and 3–10 cm in length; their mean fresh weight reaches 41 g and they contain a mean of 180 seeds (Kouonon, 2003; Djè et al., 2006). Seeds are small (3–8 mm length × 3–5 mm width, Kouonon, 2003). Hermaphroditic flowers are only present at the two first nodes of ramification of order ≥2, while male flowers are present at all nodes of all stems. Male flowers are grouped in small cymes bearing 2–4 flowers whereas hermaphroditic flowers are solitary (Kouonon, 2003). A higher proportion of male than hermaphroditic flowers is observed per plant (82·9 ± 11·9 %, n = 100). Male flower number varies between eight and 125 per individual, whereas hermaphroditic flower number only reaches 2–15. The African melon is protandrous, with male flower anthesis preceding that of hermaphroditic flowers by between 7 and 10 d (Djè et al., 2006). Male flowers are smaller (16·5 ± 2·3 mm corolla width) than hermaphroditic flowers (20·7 ± 3·4 mm). All flowers are pentamerous, with five yellow petals and five stamens. The hermaphroditic flowers present a trilobulate stigma on a short style. Stigma receptivity is concomitant with flower anthesis. Pollen viability is high and does not differ between male and hermaphroditic flowers (97·6 ± 0·7 vs. 97·4 ± 0·5 %, respectively). Pollen number per flower also does not differ between the two flower types (5580 ± 669 vs. 4985 ± 821 in male and hermaphroditic flowers, respectively; Djè et al., 2006). Ovule number reaches 173·4 ± 56·4 (Kouonon, 2003).
Although floral morphology has been well documented, the breeding system remains unknown, as well as the role of male flowers for pollination success. The aim of our study was to document the pollination and reproductive biology of the species in the field in the Ivory Coast. This research is a first step in a project aiming to increase yield in this traditional crop.
We focused on three questions. (1) Is the species self-compatible and self-fertile? (2) Does the species attract efficient pollinators? (3) Does pollen from male flowers present higher reproductive performance?
MATERIALS AND METHODS
Study area and plant material
The study sites were located at the experimental station of Abobo-Adjame University, in the region of Abidjan, southern Ivory Coast (4°41′N, 4°00′W). The sandy and clayey soils are rich in organic matter. The climate of the region is characterized by four seasons including two rainy (a long period from April to July and a short one from October to November) and two dry seasons (August to September and December to March). The period from November to February is marked by short days, in contrast to those from April to August. This region of wet climate was originally covered by a dense tropical forest. The south of the Ivory Coast has a high annual precipitation (1400–2400 mm) while average temperatures range from 25 to 32 °C.
This study was performed on Cucumis melo subsp. agrestis L. during four experimental growing seasons: season 1, October 2004 to January 2005; season 2, March to June 2006; season 3, September to December 2006; and season 4, February to May 2007. Seeds sown came from a stock collection at Abobo-Adjame University. For the first season, seeds (NI 022) had been collected in 2002 at the village of Ananda (7°17′N; 4°11′W) in central-eastern Ivory Coast, whereas seeds (NI 159) used for the other trials were collected in 2005 at the village of Assie-Assasso (6°39′N; 4°11′W) in eastern Ivory Coast. They were stored at room temperature before sowing.
Insect visitors
Insect visitors were recorded over 45 d during growing seasons 1 and 4 (rainy seasons in 2004 and 2007). The visitation rate and behaviour of floral visitors were monitored by 20 min censuses on 15 flowers. Flight distances between flowers and visit duration per flower were recorded for 30 individuals for each insect species. The visitors were collected, killed with ethyl acetate and identified (Zahradnik and Chvala, 1991; Chinery, 1992; Bolton, 1994; Michener, 2007). For each visitor, pollen identity and quantity on the insect body were assessed under a binocular microscope (in categories from +, 1–10 pollen grains; to ++ + , >100 pollen grains). The efficiency of the different pollinators was indirectly estimated by their relative abundance, their fidelity (proportion of pollen of C. melo subsp. agrestis vs. other species) and their capacity for carrying pollen (categories of pollen grain numbers on their body).
Pollen flow within populations
In 2007, pollen dispersal distances were measured with pollen analogues (Waser and Price, 1982; Irwin, 2003; Gaudeul and Till-Bottraud, 2004). The dispersion was followed on two adjacent experimental plots of 46 × 24 m (66 plants) and 15 × 45 m (96 plants). Five fluorescent powders were used (chartreuse, red, orange, green and blue from Radiant Color™). Dye particles were applied at 0600 h with a toothpick on all anthers of all male flowers (n = 4–10) on two plants (with different dyes per plant) located close to the middle of the plot. To avoid confusion between fluorescent dyes, only two dye colours were used simultaneously per day. Each afternoon, at the end of pollinator activity, all hermaphroditic flowers found in the vicinity (up to 20 m from the pollen donor flowers) were observed under an ultraviolet lamp. Afterwards, the flowers were collected and observed by fluorescence microscopy (Nikon Optiphot-2/LH-M100C-1). The application of dye particles and observation of recipient flowers was repeated 45 times.
Pollination trials
To assess the breeding system, a total of 189 plants were marked and their flowers were allocated to one of eight pollination treatments (Table 1).
Table 1.
Description of the eight treatments used in the pollination trials on Cucumis melo subsp. agrestis
| Treatment code | Treatments | Effect measured | Specific type of selfing | Specific type of crossing | Season | Total no. of plants | Total no. of flowers |
|---|---|---|---|---|---|---|---|
| T1 | Free exposure | Natural fruit set | Natural | Natural | S1, S2, S3 | 23 | 579 |
| T2 | Bagged, no emasculation, self-pollination with pollen from the same hermaphroditic flowers | Autogamy + pollen source | Artificial | No | S2, S3 | 19 | 412 |
| T3 | Bagged, emasculation, self-pollination with pollen from hermaphroditic flowers | Geitonogamy + emasculation + pollen source | Artificial | No | S1, S2, S3 | 21 | 375 |
| T4 | Bagged, emasculation, self-pollination with pollen from male flowers | Geitonogamy + emasculation + pollen source | Artificial | No | S1, S2, S3 | 25 | 455 |
| T5 | Bagged, emasculation, cross-pollination with pollen from hermaphroditic flowers | Allogamy + pollen source | No | Artificial | S1, S2, S3 | 22 | 350 |
| T6 | Bagged, emasculation, cross-pollination with pollen from male flowers | Allogamy + pollen source | No | Artificial | S1, S2, S3 | 25 | 579 |
| T7 | Bagged, emasculation, no pollination | Apomixis | No | No | S1, S2, S3 | 31 | 503 |
| T8 | Bagged, no emasculation, no pollination | Spontaneous self-pollination | Natural | No | S1, S2, S3 | 23 | 578 |
One treatment was carried out in the presence of pollinators: T1, free exposure (control or open pollination). All other treatments involved the use of cotton mesh bags to exclude pollinators (Table 1); T2, no emasculation, self-pollination (with pollen from the hermaphroditic flower itself) to test for self-compatibility (autogamy); T3, emasculation, self-pollination with pollen from hermaphroditic flowers to test whether emasculation has an effect; T4, emasculation, self-pollination with pollen from male flowers, to test if pollen source has an effect (vs. T3); T5, emasculation, cross-pollination, with pollen from hermaphroditic flowers; T6, emasculation, cross-pollination with pollen from male flowers, to test if pollen source has an effect (vs. T5); T7, emasculation, no pollination to test whether apomixis may occur; and T8, no emasculation, no pollination to quantify spontaneous self-pollination (compared with T2).
All eight treatments were performed during three seasons (2, 3 and 4) except T8 that was not carried out in season 2. All flower buds were bagged before anthesis and all bags were removed at the start of the fruit setting. Pollination was carried out by brushing stamen of the donor flower on the stigma of the recipient flower. Hand pollinations were performed daily from 0600 to 1100 h.
Mature fruits were harvested 4–7 weeks after the pollination period. Fruit set, fruit fresh weight, seed number, weight of 100 seeds, germination rate, fresh weight of cotyledon leaves, hypocotyl length and seedling dry weight were measured. All weights were measured with a balance to the precision of 0·001 g (OHAUS Adventurer Balance). For seedling dry weight, the seedlings were dried at 25 °C for 5 d. The last three parameters were quantified on 14-d-old seedlings. Germination was performed on 20 samples of 50 seeds per treatment, in Petri dishes at 25 °C. Germination rate was estimated by the percentage of germinated seeds after 12 d (Zoro Bi et al., 2003).
Pollen germination and pollen tube growth
In 2007, pollen germination on stigmas and pollen tube growth in the styles were examined by using a fluorescence microscope (Nikon Optiphot-2/LH-M100C-1) with aniline blue dye (Kearns and Inouye, 1993; Jacquemart, 2007). Five treatments were compared: spontaneous self-pollination, and four hand-pollination treatments, i.e. self- and cross-pollinations, with both hermaphroditic and male pollen sources. For each treatment, 20 pistils were removed at different times after pollination: just after pollination, 15 and 30 min, 1, 2, 4 and 6 h after pollination (or anther dehiscence for spontaneous self-pollination). Pistils were fixed in FAA (40 % formaldehyde; acetic acid: 95 % alcohol; 1:1:8) and conserved at 4 °C. Before observation, the pistils were rinsed with distilled water, softened and clarified in NaOH (0·8 m) for 75 min at 60 °C. The pistils were rinsed again and coloured during 14 h in 0·1 % aniline blue solution in KH2PO4 (0·1 m). Thereafter, pistils were cut longitudinally. Pollen germination on the stigma and the extent of growth of pollen tubes into styles were recorded.
Data analysis
Fruit set, fruit characteristics, seed number, germination rate and seedling characteristics were compared statistically by two-way analysis of variance [ANOVA; general linear model (GLM)] using SAS Enterprise Guide version 4·1 (SAS Institute, 2006). Percentages (fruit set and germination rate) were arcsin transformed to achieve normality. The other data were left untransformed as they were normally distributed and variances were homogeneous.
Pearson correlations were performed to compare fruit weight and seed numbers.
Comparisons among specific treatments were performed by contrast statements (Student's t-test): contrast 1, effect of emasculation (T2 vs. T3); contrast 2, self-compatibility (self- vs. cross-pollinations – T3 vs. T5 and T4 vs. T6); contrast 3, effect of pollen source (T3 vs. T4 and T5 vs. T6); and contrast 4, pollen limitation (T1 vs. T6).
The self-fertility index (SFI) and self-compatibility index (SCI) were calculated according to Lloyd and Schoen (1992). SFI gives an estimation of the capacity of a plant to produce fruits and seeds in the absence of pollen vectors. It is calculated as the fruit set (or seeds per fruit) of spontaneous self-pollination relative to that of hand cross-pollination (Lloyd and Schoen, 1992; Jacquemart and Thompson, 1996). SCI determines the capacity of a plant to produce zygotes following self-pollination relative to that of outcrossing. It is calculated as the ratio between the fruit set (or seed number) produced after hand self-pollination and the fruit set (or seed number) produced after hand cross-pollination (Lloyd and Schoen, 1992). SCI values <0·2 are considered as indicators of self-incompatibility whereas values >0·2 indicate self-compatibility (Lloyd and Schoen, 1992).
Levels of inbreeding depression at each developmental stage were determined as the ratio between relative performance of selfed progeny (ws) and outcrossed progeny (wc) [δ = 1 − (ws/wc); Charlesworth and Charlesworth (1987)].
Means are given with their standard errors.
RESULTS
Pollinators
Insect visitors
Five orders of insects visiting the flowers were determined in the field: Thysanoptera, Hymenoptera, Coleoptera, Diptera and Lepidoptera (Table 2). A total of 114 Thysanoptera, 145 Hymenoptera, 15 Lepidoptera, eight Diptera and three Coleoptera were caught. The visitors differed among growing seasons. Thysanoptera (86·8 %) were the most abundant during season 1 and Hymenoptera (62·4 %) were prevalent during season 4. Hypotrigona para was the most abundant Apidae. This species also spent the longest time per flower (39·4 s, Table 2) and individuals were all covered with African melon pollen. No movement between different plants was observed for ants of the genus Pheidole and no pollen was found on their bodies. Thrips were observed only remaining inside flowers; their abundance was low in the faded flowers as only four male flowers out of 53 contained one thrip, and no thrips at all were observed in hermaphroditic flowers (out of 18).
Table 2.
Insect visitors on Cucumis melo subsp. agrestis in seasons 1 and 4: relative abundance, time per flower, presence of pollen on insect bodies, mean observed flight distance and estimated pollinator potential
| Order | Family | Subfamily | Genus | Abundance (%) | Number caught | Time per flower (s) | Presence of pollen | Pollinators with pollen (%) | Mean flight distance (cm) | Potential pollinators |
|---|---|---|---|---|---|---|---|---|---|---|
| Season 1 | ||||||||||
| Hymenoptera | ||||||||||
| Formicidae | Myrmicinae | Tetramorium sp. | 0·3 | 3 | − | − | 0 | − | N | |
| Pheidole sp. | 3·8 | 10 | − | − | 0 | − | N | |||
| Formicinae | Lepisiota sp. | 0·1 | 3 | − | − | 0 | − | N | ||
| Camponotus sp. | 0·2 | 2 | − | − | 0 | − | N | |||
| Halictidae | 6·0 | 66 | − | ++ + | 81·6 | − | Y | |||
| Megachilidae | Callomegachile torida | 0·03 | 1 | − | 0 | − | N | |||
| Pseudomegachile lanata | 0·07 | 4 | − | 0 | − | N | ||||
| Andrenidae | 0·1 | 4 | − | − | 0 | − | − | |||
| Coleoptera | Chrysomelidae | 0·07 | 2 | − | − | 0 | − | − | ||
| Tenebrionidae | 0·03 | 1 | − | − | 0 | − | N | |||
| Diptera | Tephritidae | 0·03 | 1 | − | − | 0 | − | N | ||
| Syrphidae | 0·3 | 7 | − | + | 20·0 | − | N | |||
| Lepidoptera | 2·1 | 10 | − | − | 0 | − | N | |||
| Thysanoptera | Thripidae | 86·8 | 81 | − | + | 12·1 | − | N | ||
| Season 4 | ||||||||||
| Formicidae | Mirmicina | Pheidole sp. | 11·7 | − | − | − | − | − | − | |
| Apidae | Apinae | Apis mellifera | 4·6 | 15 | 4·2 ± 3·7 | ++ + | 100 | 25·3 ± 43·2 | Y | |
| Anthophorini (Tribe) | 1·4 | 3 | + | 100 | − | N | ||||
| Trigona carbonaria | 0·5 | 3 | ++ + | 100 | − | Y | ||||
| Meliponinae | Hypotrigona para | 37 | 17 | 39·4 ± 33·6 | ++ + | 100 | 40·1 ± 39·5 | Y | ||
| Hypotrigona sp. | 6·6 | 6 | 34·2 ± 34·1 | ++ + | 100 | 43·0 ± 40·5 | Y | |||
| Sphecidae | 8·8 | 5 | − | − | − | − | − | |||
| Xylocopinae | Ceratinini (Tribe) | 0·6 | 3 | 3·8 ± 3·2 | + | 100 | 69·0 ± 73·3 | N | ||
| Diptera | Tephritidae | 2·4 | − | − | − | − | − | − | ||
| Lepidoptera | 15·3 | 5 | − | 0 | 86·2 ± 67·63 | N | ||||
| Thysanoptera | Thripidae | 10·9 | 33 | + | 12·1 | − | N |
N, no; Y, yes.
Data are shown as means ± s.e.
On the basis of pollen abundance on their body and their fidelity (proportion of C. melo subsp. agrestis pollen), insects were classified as simple visitors or potential pollinators (Table 2). Seven bee species could be considered as potential pollinators: Apis mellifera, H. para and Trigona carbonaria, and four undetermined species of Hypotrigona, Ceratinini, Anthophorini and Halictidae. These insects contacted the stigma during their visit (L.C.K., pers. obs.). Direct observations of insects revealed that their flight distances varied from 25·3 ± 7·6 cm (A. mellifera) to 69·0 ± 13·4 cm (Ceratinini spp).
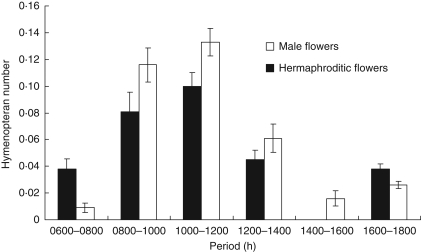
During a total of 336 observation periods, high visitation rates were observed between 0800 and 1400 h (for all insects, 1·9 visits in 20 min per hermaphroditic flower, 2·10 visits per male flower). No differences were observed for Hymenopteran number of visits between male and hermaphroditic flowers (t = 0·4; P = 0·7, Fig. 1).
Fig. 1.
Number of visits of Hymenopteran insects on hermaphroditic and male flowers of Cucumis melo subsp. agrestis. Visits to male flowers and hermaphroditic flowers as indicated. The numbers are expressed as the total number of Apidae visits per flower per 2 h.
Pollen dispersal distances
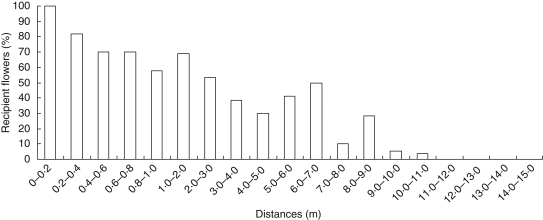
A majority of pollen analogues were dispersed at short distances as 100 % of the flowers received pollen analogues at distances <20 cm but their proportion decreased to 72 % at 1 m. No flower carried fluorescent powder above a distance of 11 m (Fig. 2).
Fig. 2.
Proportion of observed stigmas of Cucumis melo subsp. agrestis covered with fluorescent dye according to distance (n = 989).
Breeding system evaluation
Pollen germination and pollen tube growth
Fluorescent microscopy of stigmas and styles of non-emasculated and bagged flowers (spontaneous selfing treatment, T8) did not show any pollen germination while a high number of pollen grains were present. Pollen germination and pollen tube growth were observed for all remaining treatments, hand self- and hand cross-pollinations, with both pollen sources. Pollen grew on pistils without any sign of inhibition in all hand pollination treatments. Moreover, the pollen germination rate on stigmas and pollen tube growth in the styles were similar among the hand pollination treatments. The growth began 15–30 min after pollen deposition on the stigmas. Four hours later, all pollen tubes were observed at the base of the styles (Fig. 3).
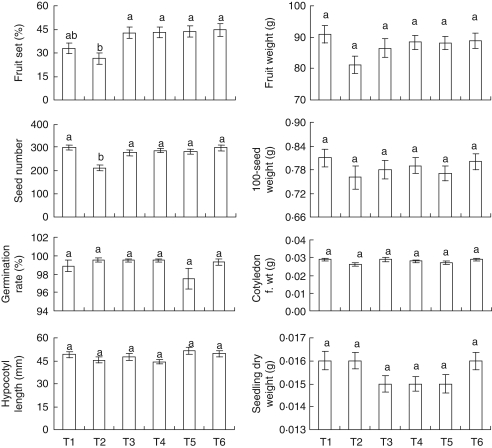
Fig. 3.
Mean reproductive success parameters of Cucumis melo var. agrestis for seasons 2 and 3 for the six pollination treatments. T1, free exposure (control, open pollination); T2, non-emasculation, hand self-pollination with pollen from the same hermaphroditic flower; T3, emasculation, hand self-pollination with pollen from hermaphroditic flowers; T4, emasculation, hand self-pollination with pollen from male flowers; T5, emasculation, hand cross-pollination with pollen from hermaphroditic flowers; T6, emasculation, hand cross-pollination with pollen from male flowers. Significant differences among treatments (P ≤ 0·05) are indicated by different letters above the standard error bars.
Effects of seasons, treatments and interactions
A two-way ANOVA revealed no significant differences among seasons for fruit set, fruit weight, germination rate and seedling biomass (Table 3). On the other hand, seed number, weight of 100 seeds, cotyledon leaf weight and hypocotyl length showed significant differences among seasons.
Table 3.
Results of ANOVA for the effects of growing seasons, treatments and interactions between seasons and treatments on the reproductive success parameters of C. melo subsp. agrestis
| Variable | Effect | SS | d.f. | MS | F | P |
|---|---|---|---|---|---|---|
| Fruit set | Season | 0·02 | 1 | 0·02 | 0·76 | 0·38 |
| Treatment | 0·63 | 5 | 0·12 | 4·78 | <0·01 | |
| Interaction | 0·51 | 5 | 0·10 | 3·92 | <0·01 | |
| Fruit weight | Season | 1586 | 1 | 1586 | 4·37 | 0·03 |
| Treatment | 2495 | 5 | 499 | 1·37 | 0·23 | |
| Interaction | 9928 | 5 | 1985 | 5·47 | <0·01 | |
| Number of seeds | Season | 316 290 | 1 | 316 290 | 53·34 | <0·01 |
| Treatment | 23 510 | 5 | 47 030 | 7·93 | <0·01 | |
| Interaction | 148 456 | 5 | 29 691 | 5·01 | <0·01 | |
| Weight of 100 seeds | Season | 0·22 | 1 | 0·22 | 7·78 | <0·01 |
| Treatment | 0·08 | 5 | 0·01 | 0·6 | 0·7 | |
| Interaction | 0·38 | 5 | 0·07 | 2·68 | 0·02 | |
| Germination rate | Season | 0·05 | 1 | 0·049 | 3·08 | 0·08 |
| Treatment | 0·10 | 5 | 0·02 | 1·35 | 0·24 | |
| Interaction | 0·18 | 5 | 0·036 | 2·27 | 0·04 | |
| Cotyledon leaf weight | Season | 610−3 | 1 | 6 × 10−3 | 148·09 | <0·01 |
| Treatment | 410−4 | 5 | 9 × 10−5 | 2·03 | 0·07 | |
| Interaction | 110−3 | 5 | 2 × 10−4 | 6·45 | <0·01 | |
| Hypocotyl length | Season | 105 221 | 1 | 105 221 | 2545·55 | <0·01 |
| Treatment | 2530·6 | 5 | 506·12 | 12·24 | <0·01 | |
| Interaction | 1163 | 5 | 232·79 | 5·63 | <0·01 | |
| Seedling dry mass | Season | 10−5 | 1 | 10−5 | 1·23 | 0·26 |
| Treatment | 10−4 | 5 | 2 × 10−5 | 1·87 | 0·09 | |
| Interaction | 10−4 | 5 | 2 × 10−5 | 2·72 | 0·01 |
Significant differences were detected among treatments for fruit set, seed number and hypocotyl length (Table 3). For all treatments, there was no significant difference in fruit weight, seed weight, germination rate, cotyledon leaf weight and seedling biomass.
There was a significant treatment × season interaction for all dependent variables except germination rate (Table 3).
Apomixis and spontaneous selfing
During all three seasons, no fruit was formed by apomixis (bagged and emasculated flowers) nor by spontaneous self-pollination (bagged non-emasculated non-pollinated flowers). Thus the value of the SFI remained zero.
Natural fruit set and general fruit and seed characteristics
Natural fruit set did not vary significantly among seasons and averaged 36 % (Table 4) whereas other fruit and seed characteristics differed significantly, with several lower values in season 4 (Table 4). In season 3, open pollination presented a higher fruit weight compared with hand cross-pollination (102·3 ± 0·8 g, Table 4). Similarly, seed number and seed weight were also more important (350·1 ± 6·6 seeds per fruit and 0·9 ± 0·01 g per 100 seeds, Table 4). The germination rate was always very high (98–99·8 %) and did not differ among seasons. Seed number was not correlated to fruit weight (r = 0·408, r2 = 0·167).
Table 4.
Differences in open pollination success of C. melo subsp. agrestis during the three growing seasons.
| Season 2 | Season 3 | Season 4 | F | P | |
|---|---|---|---|---|---|
| Fruit set (%) | 36·6 ± 4·4a | 36·6 ± 4·5a | 29·7 ± 4·8a | 0·7 | 0·5 |
| Fruit weight (g) | 86·9 ± 2·2b | 102·3 ± 0·8a | 76·3 ± 3·4c | 19·99 | <0·01 |
| Number of seeds per fruit | 294·7 ± 8·4b | 350·1 ± 6·6a | 227·0 ± 12·7c | 40·81 | <0·01 |
| 100 seeds weight (g) | 0·8 ± 0·01a | 0·9 ± 0·01a | 0·7 ± 0·03b | 8·35 | <0·01 |
| Germination rate (%) | 99·4 ± 0·4a | 98·0 ± 1·2a | 99·8 ± 0·9a | 1·04 | 0·36 |
| Cotyledon leaf weight (g) | 0·030 ± 0·0009a | 0·027 ± 0·001b | 0·030 ± 0·0012a | 9·42 | <0·01 |
| Hypocotyl length (mm) | 26·5 ± 0·4c | 35·4 ± 0·8b | 67·5 ± 1·22a | 477·52 | <0·01 |
| Seedling dry mass (g) | 0·015 ± 0·0004a | 0·017 ± 0·0003a | 0·016 ± 0·0006a | 2·13 | 0·12 |
Different superscript letters indicate significant differences among seasons (P < 0·05).
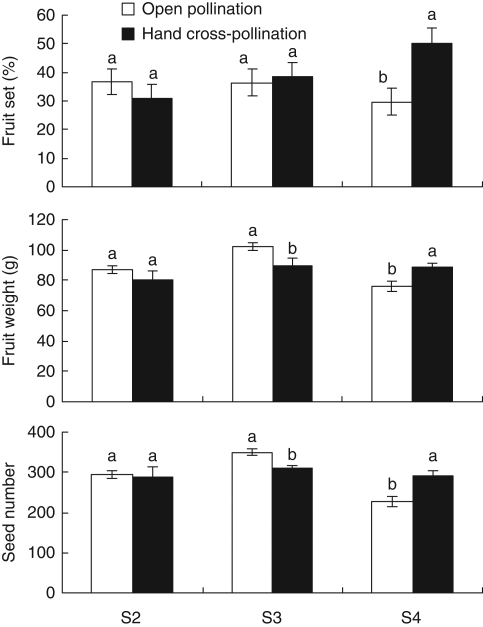
Reproductive success parameters (natural fruit set, fruit weight and seed number) were lower for open pollination than for hand pollinations only in season 4 (Fig. 4).
Fig. 4.
Comparison between open and hand pollination reproductive success in seasons 2, 3 and 4 (S2, S3 and S4) of Cucumis melo subsp. agrestis. Treatments are open pollination (T1) and hand cross-pollination with pollen from male flowers (T6), as indicated. Significant differences among treatments (P ≤ 0·05) are indicated by different letters above the standard error bars.
Effect of emasculation
Emasculation (contrast between T2 and T3) did not decrease fruit set, seed number, cotyledon and leaf weight, and had no effect on the other variables (Fig. 5).
Self-compatibility
On average, cross-pollination had no effect on fruit and seed production, fruit and seed weight, seed germination and offspring vigour compared with self-pollination (T3 vs. T5, T4 vs. T6, Fig. 5) except for cotyledon leaf weight (T3 vs. T5, t = 2·15; P = 0·03) during season 2, hypocotyl length (T4 vs. T6, t = 2·06, P = 0·04) during season 3 and seedling biomass (T3 vs. T5, t = 2·04, P = 0·04 for season 2 and t =2·53, P = 0·01 for season 4). The SCI for fruit set (SCIf) averaged 1·08, 0·92 and 1·01 (mean 1·00) for seasons 2, 3 and 4, respectively. When calculated for seed number, SCIs values were 0·98, 1·03 and 0·93 (mean 0·98), respectively for the three seasons.
Effect of pollen source
Pollen origin (from hermaphroditic or male flowers, T3 vs. T4 and T5 vs. T6) had no effect on any characteristic of reproductive success. No differences were observed between fruits, seeds and seedlings produced after pollination with pollen of hermaphroditic vs. male flowers except for fruit set in season 3 (T3 vs. T4, t = 2·12, P = 0·04), cotyledon leaf weight in season 2 (T5 vs. T6, t = 2·79, P <0·01) and seedling biomass in season 3 (T5 vs. T6, t = 2·27, P = 0·02).
Inbreeding depression
Even if some variation in inbreeding depression was observed among stages, the level remained null to very low. For the first three parameters (fruit set, fruit weight and seed number) inbreeding depression values were 0·02. Seed weight level presented an inbreeding depression value of 0·01. Mean inbreeding depression for the germination rate reached −0·01. A negative value of inbreeding depression was also obtained for cotyledon leaf weight (−0·03). The value of inbreeding depression, measured at the level of hypocotyl length, was a little bit higher (0·09), but for dry biomass δ was null. In consequence, the value for cumulative inbreeding depression calculated for all stages reached 0·126.
DISCUSSION
Breeding system
In our experiments neither apomixis nor spontaneous self-pollination was found. Similar to the American muskmelon, the isolation from pollinating insects has proven that hermaphroditic flowers are incapable of performing spontaneous self-pollination, even if its flowers are self-fertile. The pollen must be transferred from the anthers to the stigma by insects (McGregor, 1976; Kato et al., 1998). The absence of spontaneous self-pollination may result from the presence of mechanistic barriers on the stigma surface (Nair et al., 2004). Without external intervention, pollen from hermaphroditic flowers failed to grow onto stigmas. Friction occurring during hand pollinations (or by the pollinator) probably breaks down these barriers and could explain the success of hand pollinations. It has already been shown for several species that friction on the surface of the stigma breaks the stigmatic cuticle and liberates lipid secretions that can moisturize pollen and favour its germination (Nair et al., 2004; Sigrist and Sazima, 2004). Such a barrier to fertility (inhibiting the spontaneous self-pollination) could operate in C. melo subsp. agrestis but needs to be examined further.
According to our experiments, C. melo subsp. agrestis appears highly self-compatible and autogamous. There was no difference between self- and cross-pollen tube growth in pistils, and no difference could be found for any reproductive parameter (fruit set, seed number, germination rate, etc.). In consequence, the SCI is very high (mean SCI = 0·97). This high selfing ability is in accordance with our previous results regarding pollen/ovule (P/O) ratios (31·6 ± 3·4 and 28·2 ± 3·0 when pollen originated from male and hermaphroditic flowers, respectively; Djè et al., 2006). These low P/O ratios can be considered as an indicator for a facultative selfed mating system (Cruden, 1977; Jacquemart, 2003).
Moreover, the level of inbreeding depression is low (δ = 0·126). Other Cucurbitaceae species, such as the common melon, also exhibit such a low inbreeding depression (Rubino and Wehner, 1986; Sari and Yetesir, 2002). Several hypotheses have been suggested to explain this lack of vigour loss, e.g. the purging of recessive deleterious alleles. For cucurbit species, this assertion is supported by the fact that several generations of inbreeding improve offspring vigour (Rubino and Wehner, 1986; Jenkins, 1942; Oviedo et al., 2008).
Pollinators
The failure of spontaneous self-pollination proves that C. melo subsp. agrestis is unable to reproduce in the absence of pollinators. Pollinator availability and efficiency are thus important. We found seven bee species acting as putative pollinators including A. mellifera, H. para and T. carbonaria. In the USA, bees, primarily honey bees, are the major pollinating agents of the muskmelon C. melo and of the cucumber C. sativus (Mc Gregor, 1976; Kato et al., 1998). Moreover, Hypotrigona and Trigona bees are known to be efficient pollinators (Lobreau-Callen et al., 1990; White et al., 2001). The stingless bees Hypotrigona collect both pollen and nectar. They are social, generally opportunistic but fairly selective, showing a preference for a small number of plant species (Lobreau-Callen et al., 1990). Similarly, T. carbonaria presents high constancy and fidelity with only one or two pollen species, even for several days (White et al., 2001). This constancy enhances its efficiency as a pollinator by increasing the chances of pollen being transferred to stigmas of the same plant species. These stingless bees can thus be of ecological importance and valuable for crop pollination such as that of C. melo.
The proportion of flowers receiving fluorescent dye was high over short distances (<1 m) but rapidly decreased (1–11 m). In the same way, direct observation of insects revealed that flight distances did not exceed 1 m. In consequence, as individuals of C. melo subsp. agrestis usually extend up to 5 m length, pollen flow would mainly occur between flowers of the same plant, thus the selfing rate through geitonogamy should be considerable in the population.
Natural fruit set varied from 29·7 to 36·6 % across the different seasons. Only during season 4 were fruit set, fruit weight and seed number lower after open natural pollination than after hand pollination. This reduced pollination success could be due to pollen transfer limitation: during this wet season plants produced flowers hidden under abundant foliage. Insect visits were less abundant, suggesting reduced pollination.
Pollen origin
Pollen origin (hermaphroditic vs. male flowers) had no effect on fruit and seed production for our subspecies. Andromonoecious reproductive systems can be maintained in a population if plants allocate resources to male and female functions in ways that increase their fitness relative to plants producing only hermaphroditic flowers (Bertin, 1982). Male or staminate flowers are less costly in terms of resource needs than hermaphroditic perfect flowers as they lack pistils and ovaries. As such, male flowers could provide a cheap way to increase the size of the floral display. Male flowers of C. melo subsp. agrestis are smaller than hermaphroditic ones, but in this study we did not test for a resource reallocation process. Thus, we can neither reject nor accept the first hypothesis on male floral evolution (the ‘resource reallocation’ hypothesis, Bertin, 1982).
A larger floral display could enhance attractiveness to pollinators, which could in turn increase the quantity or quality of the produced seeds (Janzen, 1977; Bertin, 1982). The production of numerous male flowers in an andromonoecious system increases the conspicuousness of the flowers and thus improves the pollination efficiency of the hermaphroditic flowers. The high number of male flowers can attract more pollinators and increase reproductive success in our subspecies. In contrast to the findings of other studies (Huang et al., 2000; Huang, 2003; Zhang and Tan, 2009), male flowers did not produce either more or larger grains, male pollen viability was not higher and male flowers did not attract more insect visitors than hermaphroditic flowers (Fig. 1). Therefore, we probably can reject the ‘increased pollen donation’ hypothesis (Barrett, 2002). This implies that in our case, male flowers do not have a larger potential male function than hermaphroditic flowers (Elle and Meagher, 2000). Other floral traits need to be studied to elucidate the pollinator attraction for both flower types.
Even if the results of several studies on andromonoecious species also fail to support the pollinator attraction hypothesis for the maintenance of andromonoecy (Bertin, 1982; Solomon, 1986; Spalik, 1991), this breeding system could be particularly effective in increasing male fitness in pollen-limited populations by increasing pollen receipt on hermaphroditic flowers (Steven et al., 1999; Huang, 2003; Vallejo-Marin and Rausher, 2007). As we did not perform hand supplemental pollination, pollen transfer limitation remains to be tested in C. melo subsp. agrestis. Nevertheless, it was observed that natural fruit set was sometimes reduced compared with hand-pollinated flowers. Thus, the third hypothesis, ‘the increased pollen reception’ (Vallejo-Marin and Rausher, 2007), can be accepted as our results suggest pollen transfer limitation.
Conclusions
Our results on pollen germination and pollen tube growth in both hand self- and cross-pollinations, as well as those on fruit and seed production showed that C. melo subsp. agrestis is self-compatible. No apoximis and no spontaneous self-pollination were recorded. Fruit and seed characteristics resulting from geitonogamy and allogamy (open natural as well as hand cross-pollinated flowers) did not differ, suggesting that pollination in C. melo subsp. agrestis may include both self and cross pollen.
Our data indicate that while C. melo subsp. agrestis appears to be self-compatible, insect-mediated pollination is critical to reproduction. Several insect species belonging to the family Apidae (Hypotrigona and Trigona stingless bees as well as A. mellifera) can act as pollinators even if their flight distances remain short (<11 m). Given the production of multiple flowering axes, insect behaviour and the absence of spontaneous self-pollination, geitonogamy potentially accounts for a substantial proportion of the fertilization events.
We detected no effect of pollen source (male vs. hermaphroditic flowers) on reproductive success and male flowers did not present higher male fitness. The role of these male flowers in the maintenance of andromonoecy in this species remains to be elucidated.
ACKNOWLEDGEMENTS
The work was supported by the Direction Générale de la Coopération au Développement, Belgium (Projet interuniversitaire PIC 2004); and by the Université catholique de Louvain (bourse de doctorat d'aide à la coopération, 2008–2009, fellowship to L.C.K.). We are grateful to M. J. Abi, E. Koffi and A. E. Goba who provided valuable help in the field. We thank L. Baeyens and M. Leloup for making Belgian stay infrastructure available for L.C.K. We also thank I. K. Kouassi, K. K. Koffi, V. Cawoy, C. Mayer, A. Vervoort, V. Vanparys and two anonymous reviewers whose comments have greatly improved the manuscript.
LITERATURE CITED
- Achu MB, Fokou E, Tchiegang C, Fotso M, Tchouanguep MF. Nutritive value of some Cucurbitaceae oilseeds from different regions in Cameroon. African Journal of Biotechnology. 2005;4:1329–1334. [Google Scholar]
- Akimoto J, Fukuhara T, Kikuzawa K. Sex expression and genetic variation in a functionally androdioecious species, Schizopepon bryoniaefolius (Cucurbitaceae) Annals of Botany. 1999;86:880–886. [PubMed] [Google Scholar]
- Akobundu ENT, Cherry JP, Simmons JG. Chemical, functional and nutritional properties of Egussi (Colocynthis citrullus) seed protein products. Journal of Food Science. 1982;47:829–835. [Google Scholar]
- Badifu GIO. Effect of processing on proximate composition, antinutritional and toxic contents of kernels from Cucurbitaceae species grown in Nigeria. Journal of Food Composition and Analysis. 2001;14:153–161. [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews Genetics. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Bertin RI. The evolution and maintenance of andromonoecy. Evolutionary Theory. 1982;6:25–32. [Google Scholar]
- Bolton B. Identification guide to ant genera of the world. Cambridge, MA: Harvard University Press; 1994. [Google Scholar]
- Boualem A, Fergany M, Fernandez R, et al. A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science. 2008;321:836–838. doi: 10.1126/science.1159023. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Charlesworth D, Morgan MT. Allocation of resources to sex functions in flowering plants. Philosophical Transactions of the Royal Society B: Biological Sciences. 1991;332:91–102. [Google Scholar]
- Chinery M. Insectes d'Europe. Paris: Bordas; 1995. [Google Scholar]
- Cruden RW. Pollen–ovule ratios: a conservation indicator of breeding systems in flowering plants. Evolution. 1977;31:32–46. doi: 10.1111/j.1558-5646.1977.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Cuevas J, Polito VS. The role of staminate flowers in the breeding systems of Olea europaea (Oleaceae): an andromonecious, wind-pollinated taxon. Annals of Botany. 2004;93:547–553. doi: 10.1093/aob/mch079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djè Y, Kouonon LC, Zoro Bi IA, Gnamien GY, Baudoin JP. Etude des caractéristiques botaniques, agronomiques et de la biologie florale du melon africain (Cucumis melo L. subsp. agrestis Naudin, Cucurbitaceae) Biotechnologie, Agronomie, Société et Environnement. 2006;10:109–119. [Google Scholar]
- Elle E, Meagher TR. Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). II. Paternity and functional gender. American Naturalist. 2000;156:622–636. doi: 10.1086/316997. [DOI] [PubMed] [Google Scholar]
- Emms SK. Andromonoecy in Zigadenus paniculatus (Liliaceae): spatial and temporal patterns of sex allocation. American Journal of Botany. 1993;80:914–923. [Google Scholar]
- van Epenhuijsen CW. Growing native vegetables in Nigeria. Rome: FAO; 1974. [Google Scholar]
- Fisher RA. Average excess and average effect of a gene substitution. Annals of Eugenics. 1941;11:53–63. [Google Scholar]
- Gaudeul M, Till-Bottraud I. Reproductive ecology of the endangered alpine species Eryngium alpinum L. (Apiaceae): phenology, gene dispersal and reproductive success. Annals of Botany. 2004;93:711–721. doi: 10.1093/aob/mch098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman & Hall; 1996. pp. 140–190. [Google Scholar]
- Heywood VH. Flowering plants of the world. New York: Oxford University Press; 1993. [Google Scholar]
- Holsinger KE. Reproductive systems and evolution in vascular plants. Proceedings of the National Academy of Sciences, USA. 2000;97:7037–7042. doi: 10.1073/pnas.97.13.7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-Q. Flower dimorphism and the maintenance of andromonoecy in Sagittaria guyanensis subsp. lappula (Alismataceae) New Phytologist. 2003;157:357–364. doi: 10.1046/j.1469-8137.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- Huang S-Q, Song N, Wang Q, Tang L-L, Wang X-F. Sex expression and the evolutionary advantages of male flowers in an andromonoecious species, Sagittaria guyanensis subsp. lappula (Alismataceae) Acta Botanica Sinica. 2000;42:1108–1114. [Google Scholar]
- Husband BC, Schemske DW. Magnitude and timing of inbreeding depression in a diploid population of Epiobium angustifollum (Onagraceae) Heredity. 1995;75:206–215. [Google Scholar]
- Irwin RE. Impact of nectar robbing on estimates of pollen flow: conceptual predictions and empirical outcomes. Ecology. 2003;84:485–895. [Google Scholar]
- Jacquemart A-L. Floral traits of Belgian Ericaceae species: are they good indicators to assess the breeding systems? Belgian Journal of Botany. 2003;136:154–164. [Google Scholar]
- Jacquemart A-L. Methods for determining compatibility and pollinator efficiency in temperate fruit species. Fruit, Vegetable and Cereal Science and Biotechnology. 2007;1:26–38. [Google Scholar]
- Jacquemart A-L, Thompson JD. Floral and pollination biology of three sympatric Vaccinium (Ericaceae) species in the upper Ardennes, Belgium. Canadian Journal of Botany. 1996;74:210–221. [Google Scholar]
- Jenkins JM. Natural self-pollination in cucumber. Proceedings of the National Academy of Sciences, USA. 1942;40:411–412. [Google Scholar]
- Janzen DH. A note on optimal mate selection by plants. American Naturalist. 1977;111:365–371. [Google Scholar]
- Kato K, Yukari A, Okamoto A, Kadoka S, Masuda M. Isozyme polymorphism in melon (Cucumis melo L.), and application to seed purity test of F1 cultivars. Breeding Science. 1998;48:237–242. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Niwot: University Press of Colorado; 1993. [Google Scholar]
- Kenigsbuch D, Cohen Y. The inheritance of gynoecy in muskmelon. Genome. 1990;33:317–320. [Google Scholar]
- Kouonon LC. Ivory Coast: Université d'Abobo-Adjamé; 2003. Contribution à l′étude de la biologie de reproduction d'une espèce de ‘pistache’ Cucumis melo var. agrestis (Naudin) (Cucurbitaceae) Dissertation. [Google Scholar]
- Liao W-J, Song Q-F, Zhang D-Y. Pollen and resource limitation in Veratrum nigrum L. (Liliaceae), an andromonoecious herb. Journal of Integrative Plant Biology. 2006;48:1401–1408. [Google Scholar]
- Lobreau-Callen D, Le Thomas A, Darchen B, Darchen R. Quelques facteurs déterminant le comportement de butinage d'Hypotrigona pothieri (Trigonini) dans la végétation de Côte d'Ivoire. Apidologie. 1990;21:69–83. [Google Scholar]
- Lloyd G, Schoen D. Self- and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences. 1992;153:358–369. [Google Scholar]
- Loukou AL, Gnakri D, Djè Y, et al. Macronutrient composition of three cucurbit species cultivated for seed consumption in Côte d'Ivoire. African Journal of Biotechnology. 2006;6:529–533. [Google Scholar]
- Mariod A, Matthäus B. Fatty acids, tocopherols, sterols, phenolic profiles and oxidative stability of Cucumis melo subsp. agrestis oil. Journal of Food Lipids. 2008;15:56–67. [Google Scholar]
- McGregor SE. Insect pollination of cultivated crop plants. 1976. http//gears.tucson.ars.ag.gov/book. (accessed 13 March 2009)
- Michener CD. The bees of the world. Baltimore: Johns Hopkins University Press; 2007. [Google Scholar]
- Miller JS, Diggle PK. Diversification of andromonoecy in Solanum section Lasiocarpa (Solanaceae): the roles of phenotypic plasticity and architecture. American Journal of Botany. 2003;90:791–793. doi: 10.3732/ajb.90.5.707. [DOI] [PubMed] [Google Scholar]
- Nair RM, Dundas IS, Wallwork M, Verlin DC, Waterhouse L, Dowling K. Breeding system in a population of Trigonella balansae (Leguminosae) Annals of Botany. 2004;94:883–888. doi: 10.1093/aob/mch216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo VRS, Godoy AR, Cardoso AII. Performance of advanced generation from a hybrid Japanese cucumber. Scientia Agricola. 2008;65:553–556. [Google Scholar]
- Porcher E, Lande R. The evolution of self-fertilization and inbreeding depression under pollen limitation and pollen discounting. Journal of Evolutionary Biology. 2005;18:497–508. doi: 10.1111/j.1420-9101.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- Rubino DB, Wehner TC. Effect of inbreeding on horticultural performance of cucumber families developed from a variable population. Cucurbit Genetics Cooperative Report. 1984;7:21–22. [Google Scholar]
- Sari N, Yetisir H. Some agronomical characteristics of doubled haploid lines produced by irradiated pollen technique and parental diploid genotypes in melons. Turkish Journal of Agriculture and Forestry. 2002;26:311–317. [Google Scholar]
- SAS Institute. SAS enterprise guide. Release 4.01 edn. Cary, NC: SAS Institute; 2006. [Google Scholar]
- Schemske DW, Lande R. The evolution of self-fertilization and inbreeding depression in plants. II. Empirical observations. Evolution. 1985;39:41–52. doi: 10.1111/j.1558-5646.1985.tb04078.x. [DOI] [PubMed] [Google Scholar]
- Sigrist MR, Sazima M. Pollination and reproductive biology of twelve species of neotropical Malpighiaceae: stigma morphology and its implications for the breeding system. Annals of Botany. 2004;94:33–41. doi: 10.1093/aob/mch108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BP. Sexual allocation and andromonoecy: resource investment in male and hermaphrodite flowers of Solanum carolinense (Solanaceae) American Journal of Botany. 1986;73:1215–1221. [Google Scholar]
- Spalik K. On evolution of andromonoecy and ‘overproduction’ of flowers: a resource allocation model. Biological Journal of Linnean Society. 1991;42:325–336. [Google Scholar]
- Spalik K, Woodell SRJ. Regulation of pollen production in Anthriscus sylvestris, an andromonoecious species. International Journal of Plant Sciences. 1994;155:750–754. [Google Scholar]
- Steven JC, Peroni PA, Rowell E. The effects of pollen addition on fruit set and sex expression in andromonoecious herb horsenettle (Solanum carolinense) American Midland Naturalist. 1999;141:247–252. [Google Scholar]
- Vallejo-Marin M, Rausher MD. The role of male flowers in andromonoecious species: energetic cost and siring success in Solanum carolinense L. Evolution. 2007;61:404–412. doi: 10.1111/j.1558-5646.2007.00031.x. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. A comparison of pollen and fluorescent dye carry-over by natural pollinators of Ipomopsis aggregata (Polemoniaceae) Ecology. 1982;63:1168–1172. [Google Scholar]
- White D, Cribb BW, Heard TA. Flower constancy of stingless bee Trigona carbonaria Smith (Hymenoptera: Apidaea: Meliponini) Australian Journal of Entomology. 2001;40:61–64. [Google Scholar]
- Yampolsky E, Yampolsky H. Distribution of sex forms in the phanerogamic flora. Bibliographia Genetic. 1922;3:1–62. [Google Scholar]
- Zahradnik J, Chvala M. La grande encyclopédie des insectes. Prague: Aventinum; 1991. [Google Scholar]
- Zhang T, Tan D-Y. An examination of the function of male flowers in an andromonoecious shrub Capparis spinosa. Journal of Integrative Plant Biology. 2009;51:316–324. doi: 10.1111/j.1744-7909.2008.00800.x. [DOI] [PubMed] [Google Scholar]
- Zoro Bi IA, Koffi KK, Djè Y. Caractérisation botanique et agronomique de trois espèces de cucurbites consommées en sauce en Afrique de l'Ouest: Cucumeropsis mannii (Naudin) et Lagenaria siceraria (Molina) Standl. Biotechnologie, Agronomie, Société et Environnement. 2003;7:189–199. [Google Scholar]
- Zoro Bi IA, Koffi KK, Djè Y, Malice M, Baudoin J-P. Indigenous cucurbits of Côte d'Ivoire: a review of their genetic resources. Sciences & Nature. 2006;3:1–9. [Google Scholar]