Abstract
Background and Aims
Oils are an unusual floral reward in Orchidaceae, being produced by specialized glands called elaiophores. Such glands have been described in subtribe Oncidiinae for a few species. The aims of the present study were to identify the presence of elaiophores in Gomesa bifolia, to study their structure and to understand how the oil is secreted. Additionally, elaiophores of G. bifolia were compared with those of related taxa within the Oncidiinae.
Methods
Elaiophores were identified using Sudan III. Their structure was examined by using light, scanning electron and transmission electron microscopy.
Key Results
Secretion of oils was from the tips of callus protrusions. The secretory cells each had a large, centrally located nucleus, highly dense cytoplasm, abundant plastids containing lipid globules associated with starch grains, numerous mitochondria, an extensive system of rough and smooth endoplasmatic reticulum, and electron-dense dictyosomes. The outer tangential walls were thick, with a loose cellulose matrix and a few, sparsely distributed inconspicuous cavities. Electron-dense structures were observed in the cell wall and formed a lipid layer that covered the cuticle of the epidermal cells. The cuticle as viewed under the scanning electron microscope was irregularly rugose.
Conclusions
The elaiophores of G. bifolia are of the epithelial type. The general structure of the secretory cells resembles that described for other species of Oncidiinae, but some unique features were encountered for this species. The oil appears to pass through the outer tangential wall and the cuticle, covering the latter without forming cuticular blisters.
Key words: Elaiophore, Gomesa bifolia, Orchidaceae, Oncidiinae, oil secretion, anatomy, micromorphology, ultrastructure
INTRODUCTION
Orchids display many unsurpassed floral specializations in their interaction with animal pollinators (Nilsson, 1992). They can present floral rewards such as nectar and oil, although both are relatively infrequent as pollinator attraction by mimicry and deceit tends to predominate in the family (van der Pijl and Dodson, 1969; Ackerman, 1984; van der Cingel, 2001). Oil as a reward has great nutritional value (Buchmann, 1987) and is produced by specialized secretory glands called elaiophores (Vogel, 1974).
The presence of elaiophores in Orchidaceae is mainly represented in members of the subtribe Oncidiinae (Singer & Cocucci, 1999; Silvera, 2002; Stpiczyńska et al., 2007; Davies and Stpiczyńska, 2008). The first report of this feature was for Oncidium ornithorrhynchum, with a brief description of the nature of the lipids (Vogel, 1974). Reis et al. (2000, 2003) analysed the floral oils for two species of Oncidiinae and reported that their major components were asymmetrically substituted diacylglycerols. Silvera (2002) mapped the presence of oil secretion within a phylogenetic context, and concluded that this character is a polymorphy within the subtribe.
Several anatomical studies of elaiophores in the subtribe Oncidiinae have recently been published (Singer and Cocucci, 1999; Pacek and Stpiczyńska, 2007; Stpiczyńska et al., 2007; Stpiczyńska and Davies, 2008). The diversity of the elaiophore structures reported suggests that they developed along a number of evolutionary lines within the subtribe Oncidiinae (Davies and Stpiczyńska, 2008).
The genus Gomesa R. Br. belongs to the tribe Cymbidieae, subtribe Oncidiinae (Chase et al., 2003), one of the most variable in terms of floral diversity and biology of pollination (Dressler, 1993). Gomesa bifolia (Sims) M.W. Chase & N.H. Williams is an ephitytic plant distributed from Bolivia, Brazil, Paraguay and Uruguay to forested environments of north-west and north-east Argentina. It is characterized by having bifoliate pseudobulbs and yellow, scentless flowers with brown marks. These floral colours and the absence of fragrance are characters usually present in Neotropical orchids, along with secretion of oils that are gathered by bees (Stpiczyńska and Davies, 2008).
This species was traditionally considered a member of the genus Oncidium Sw., one of the most conspicuous of the Neotropical orchids. Chase and Palmer (1992) revealed the polyphyletic nature of this genus and subsequent molecular phylogenies have clarified its evolution (Williams et al., 2001; Chase et al., 2009).
Those species of Oncidium restricted to eastern Brazil and north-eastern Argentina were considered to comprise the ‘Gomesa-clade’ of the genus, forming a monophyletic group in which oil secretion was well represented (Reis et al., 2000; Singer et al., 2006). Chase et al. (2009) decided to transfer the species of the ‘Gomesa-clade’ to the genus Gomesa based upon the results of a robust molecular phylogeny.
Ongoing studies of the reproductive biology in G. bifolia are being developed to investigate the potential for self- and cross-pollination (J. P. Torretta et al., unpubl. res.). These authors presumed the presence of elaiophores in G. bifolia based on field observation. The present study was initiated to test that assumption and to provide additional information about how this particular structure functions in attracting and rewarding pollinators.
The goals were to identify the presence of elaiophores in G. bifolia, to determine their anatomical, micromorphological and ultrastructural characteristics, and to investigate how oil is secreted. Furthermore, the internal structure of the elaiophores and the secretion process in G. bifolia was compared with other species of this genus and related taxa. Additional knowledge about how this character varies within the Oncidiinae will help in the resolution of taxonomic and evolutionary questions in this large and complex subtribe.
MATERIALS AND METHODS
Fresh material was obtained from plants cultivated in the Botanical Garden ‘Lucien Hauman’ of the Faculty of Agronomy, University of Buenos Aires, originating from natural populations from the Dpto. Oberá, Misiones, Argentina. The reference voucher was deposited in the Herbario ‘Gaspar Xuarez’ (BAA 26015) of the Faculty of Agronomy, University of Buenos Aires.
Fresh, intact flowers were stained with a saturated ethanolic solution of Sudan III, and were observed by using a Wild M5 stereomicroscope to locate the secretory area. Fragments of the labellum were subsequently sectioned and the position of elaiophores was confirmed from hand-cut sections stained with Sudan III.
Transverse and longitudinal sections were prepared for light microscopy (LM). Fresh material was fixed in FAA (formalin–acetic-alcohol mixture) for 48 h and stored in 70 % ethanol. The samples were dehydrated in an ethanol series, transferred to xylene, embedded in paraffin, and sectioned on a rotary microtome at 10-μm thickness, according to standard methods. Histological samples were stained with Safranin-Fast Green and mounted in Canada balsam (D'Ambrogio de Argüeso, 1986). Observations were made using a Wild M20 optical microscope.
For scanning electron microscopy (SEM), portions of the labellum were dehydrated and critical-point dried using liquid CO2. The material was then sputter-coated with gold and examined by using a Philips XL 30 microscope.
For transmission electron microscopy (TEM), pieces of the labellum, approx. 1 mm thick, were fixed in 2·5 % glutaraldehyde in 0·1 m phosphate buffer for 3 h at room temperature, washed in buffer, post-fixed in 1·5 % osmium tetroxide with the same buffer for 2 h, dehydrated in an ethanol series and embedded in Spurr's resin. Sections 1 µm thick were stained with toluidine blue 0·1 % for LM observations. Ultrathin sections of 75–90 nm were obtained with glass knives, stained with uranyl acetate followed by lead citrate, and examined and photographed in a JEOL- JEM 1200 EX II transmission electron microscope at 85·0 kV.
RESULTS
Morphology
Gomesa bifolia presents loosely paniculate inflorescences (20–50 cm), laxly flowered (7–15 flowers) and arching at maturity (Fig. 1A). The flowers are yellow (2–3 cm wide and 4–5·5 cm long) with brown marks in sepals and petals which lack fragrance (Fig. 1B). The labellum is three-lobed, with a large, emarginated apical lobe and two small sub-erect and auriculated lateral lobes (Fig. 1D). The callus is formed by several protrusions located on the base of the labellum, between lateral lobes. Three regions can be distinguished in the callus: central keel, proximal crest and lateral protuberances (Fig. 1C).
Fig. 1.
General view of Gomesa bifolia. (A) Inflorescence. (B) Flower showing sepal (s), petal (p) and labellum (lab). (C, D) Flower stained with Sudan III: (C) detail of the callus showing central keel (ck), proximal crest (pc) and lateral protuberances (lp); (D) labellum showing apical lobe (al), lateral lobes (ll) and callus (c). Scale bars: (A) = 1·5 cm, (B) = 0·5 cm, (C) = 0·2 cm, (D) = 0·6 cm.
The presence of secreted oils was detected upon the labellar callus; the tips of the central keel, proximal crest and lateral protuberances stained intensely with Sudan III, indicating the presence of elaiophores (Fig. 1C, D; note the darker tips of the callus protrusions).
Anatomy (LM)
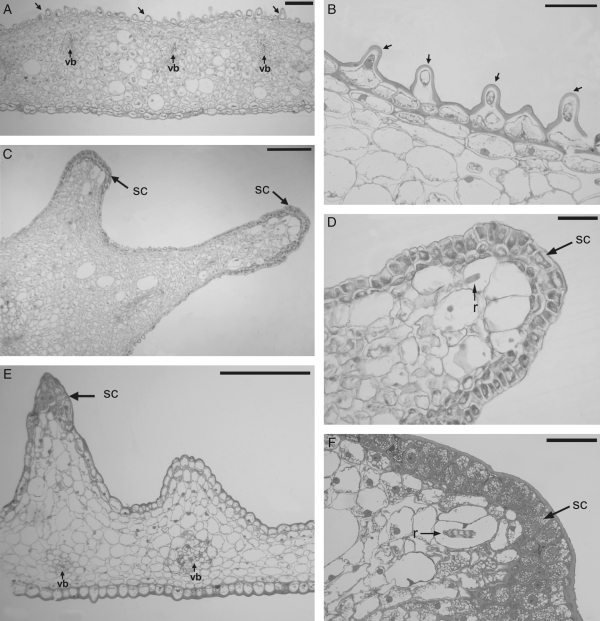
Epidermal and sub-epidermal cells on the tips of the callus protrusions showed large, centrally located nuclei and highly dense cytoplasm (Fig. 2C–F). Differences between cells of the tips of the callus protrusions and remaining epidermal cells of the callus and lateral lobes were observed. Epidermal cells in the areas among callus protrusions and lateral lobes were more vacuolated, and the outer tangential wall was slightly convex to strongly papillose (Fig. 2A, B).
Fig. 2.
Light micrographs of transverse sections of the lateral lobe and callus of Gomesa bifolia. (A, B) Lateral lobe: (A) section with vascular bundles (vb) and papillose cells (arrows); (B) detail of the papillose cells (arrows). (C, D) Lateral protuberances of the callus: (C) section showing two protrusions with secretory cells on the tips (sc); (D) detail of the tip of the protuberance showing secretory cells (sc) and raphides in the parenchyma (r). (E, F) Proximal crest of the callus: (E) section showing vascular bundles (vb) and secretory cells on the tip of a protrusion (sc); (F) detail of the tip of protrusion showing secretory cells (sc) and raphides in the parenchyma (r). Scale bars: (A) = 120 µm; (B) = 60 µm; (C) = 280 µm; (D, F) = 70 µm; (E) = 250 µm.
The parenchyma of the ground tissue revealed intercellular spaces and cells were rounded to isodiametric, with large vacuoles and nuclei in parietal position. Elliptical idioblasts with raphides and vascular bundles consisting of both xylem and phloem were observed in this region (Fig. 2D–F).
Micromorphology (SEM)
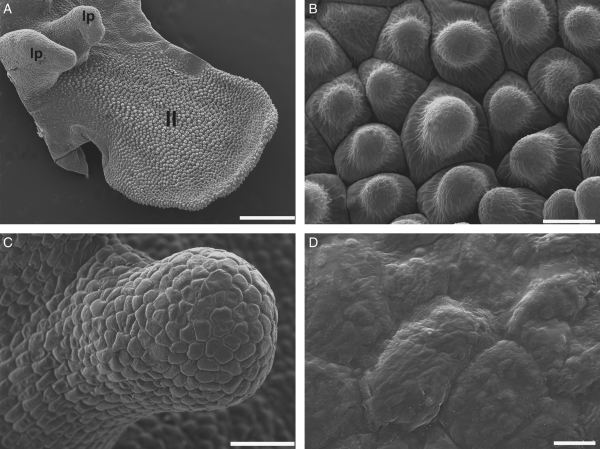
The callus protrusions had a finger-like shape (Fig. 3A, C). Their epidermal cells in surface view were of equal length and breadth, and the cuticle was irregularly rugose (Fig. 3D). The remaining areas of the callus and lateral lobes revealed epidermal cells bearing papillae with shortly conic with rounded tips, covered by a striate cuticle (Fig. 3B).
Fig. 3.
Scanning electron micrographs of the lateral lobe and callus of Gomesa bifolia. (A) Surface of a lateral lobe (ll) and two lateral protuberances of the callus (lp). (B) Detail of the papillae in the lateral lobe. (C, D) Lateral protuberance of the callus: (C) general view of a lateral protuberance; (D) epidermal cells on the tip. Scale bars: (A) = 500 µm, (B) = 20 µm, (C) = 100 µm, (D) = 10 µm.
Ultrastructure (TEM)
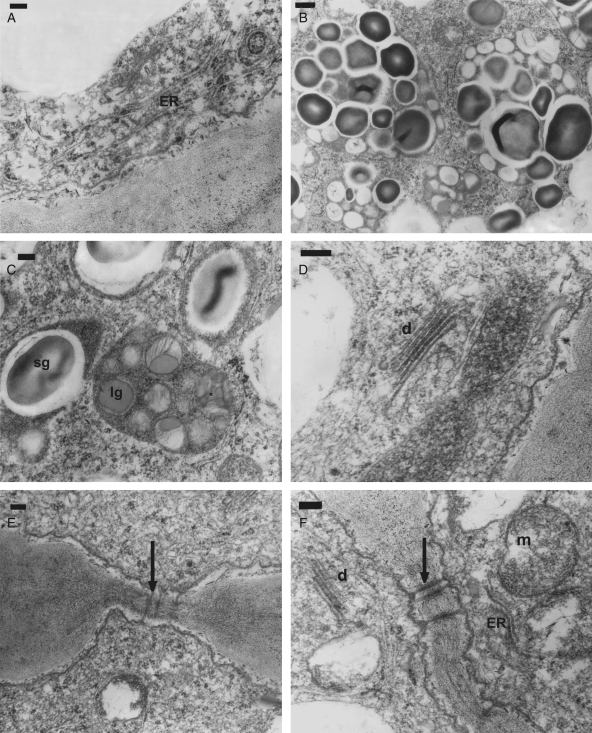
The epidermal cells at the tips of the callus protrusions showed abundant plastids containing lipid globules in groups associated with starch grains (Fig. 4B, C). Also observed were numerous mitochondria with well-developed cristae (Fig. 4F), an extensive internal membrane system of rough and smooth endoplasmatic reticulum (Fig. 4A, F), and dictyosomes with electron-dense cisternae (Fig. 4D, F). The sub-epidermal parenchyma cells attached to the epidermis at the tips of the callus protrusions had ultrastructural characteristics similar to those of the epidermal cells.
Fig. 4.
TEM sections through an elaiophore of Gomesa bifolia. (A–F) Section of secretory cells on the tip of the callus protrusions and their complement of organelles. (A) Detail of cytoplasm with abundant endoplasmic reticulum (ER). (B, C) Detail of plastids with starch grains (sg) and lipid globules (lg). (D) Detail of cytoplasm with dictyosomes (d). (E) Detail of the connections (arrow) between epidermal and subepidermal cells. (F) Detail of the connections (arrow) between radial walls of the secretory epidermal cells; cytoplasm showing mitochondria (m), dictyosomes (d) and endoplasmic reticulum (ER). Scale bars: (A, C–F) = 0·2 µm; (B) = 0·5 µm.
The epidermal cells in the remaining areas of the callus and lateral lobes differed in that the cytoplasm was less dense and had a smaller number of plastids.
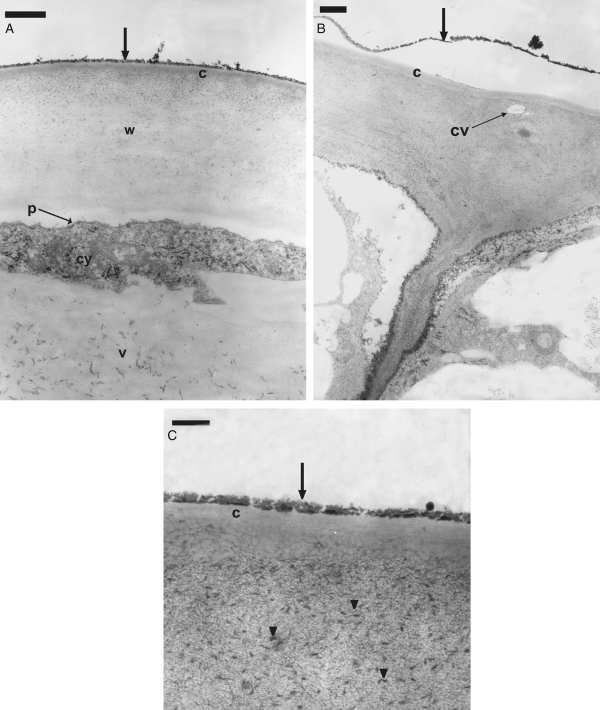
Connections among epidermal secretory cells were observed as plasmodesmata in primary pit fields located on the inner tangential and radial walls (Fig. 4E, F). The outer tangential walls were thick, and the cellulose microfibrils of the matrix appeared to be loosely arranged with a few inconspicuous, sparsely distributed cavities (Fig. 5B). The cuticle covering these cells was stratified but not distended (Fig. 5C). Abundant electron-dense structures were embedded among the microfibrils of the outer tangential wall, between this and the plasmalemma, and upon the cuticle (Fig. 5A, C). These corpuscles formed a thin, continuous layer covering the epidermal cells, sometimes detached at a few points (Fig. 5B).
Fig. 5.
TEM sections through an elaiophore of Gomesa bifolia. (A–C) Section of the outer tangencial wall of the secretory cells on the tips of the callus protrusions. (A) Detail of outer tangential wall covered by an electron-dense layer (arrow); cuticle (c), cell wall (w), plasmalemma (p), cytoplasm (cy) and vacuole (v) are indicated. (B) Outer tangential wall showing a small cavity (cv) and detached electron-dense layer (arrow). (C) Detail of the outer tangential wall showing the cuticle (c), electron-dense corpuscles (arrowheads) and lipid layer (arrow). Scale bars: (A, B) = 1 µm; (C) = 0·2 µm.
DISCUSSION
Anatomical and ultrastructural features
This paper represents the first report of elaiophores in G. bifolia based on anatomical evidence and supports the previous assumption of J. P. Torretta et al. (unpubl. res.) for their presence based upon field observations. These authors detected bees over the flowers of G. bifolia and suggested that they were collecting oils. The bees that gather oils from New World orchids belong to the genera Centris, Paratetrapedia and Tetrapedia (Buchmann, 1987; Singer and Cocucci, 1999; Machado, 2004). They have specialized leg structures that enable them to collect the oil (Neff and Simpson, 1981; Cocucci et al., 2000). The floral lipids seem to be used by bees principally as a constituent of the food of their larvae (Machado, 2004).
Based on the classification of Vogel (1974), the elaiophores of G. bifolia belong to the epithelial type and the secretory cells are located in the tips of callus protrusions. Ultrastructural observations of the epidermal and sub-epidermal cells in these areas reveal large, centrally located nuclei, a dense cytoplasm, numerous mitochondria, extensive endoplasmic reticulum and dictyosomes, indicating that these cells are secretory. We assume that the principal component of the secretion is oil as it reacted positively with Sudan III. Additionally, TEM observations revealed plastids containing lipid globules.
The plastids of the secretory cells conatined abundant starch grains, a feature frequently related to the emission of volatile material (Fahn, 1979). This characteristic was also observed in elaiophores of Ornithocephalus kruegeri Rchb. f. and Oncidium cheirophorum Rchb. f. (Pacek and Stpiczyńska, 2007). Despite the fact that starch prevails in nectaries and osmophores, it can exist as a source of energy for intensive metabolic cellular processes (Pacek and Stipiczyńska, 2007). This is probably the role of the starch in G. bifolia, as no fragrance was detected.
Many elaiophores of Oncidiinae show blisters in the epidermis produced by a distended cuticle that accumulates oils beneath its surface (Singer and Cocucci, 1999; Pacek and Stpiczyńska, 2007; Stpiczyńska et al., 2007; Stpiczyńska and Davies, 2008). Full oil discharge is achieved when the cuticle is ruptured by a visiting insect (Stpiczyńska and Davies, 2008). In contrast, SEM and TEM observations revealed here that a distended cuticle was absent in G. bifolia.
The occurrence of cavities in thick outer tangential cell walls has been documented for several elaiophores in Oncidiinae (Pacek and Stpiczyńska, 2007; Stpiczyńska et al., 2007; Stpiczyńska and Davies, 2008). These cavities may aid in the transport of hydrophobic components across the hydrated cell wall, similar to the formation of the cuticle (Kunst and Samuels, 2003). However, as these cavities are very small and sparsely distributed, we consider that they are not essential in oil secretion, at least for G. bifolia.
Idioblasts containing raphides in elaiophores have been observed in other species of Oncidiinae (Pacek and Stpiczyńska, 2007; Stpiczyńska et al., 2007; Stpiczyńska and Davies, 2008). Although no roles have been suggested for this structure in those species, we believe that a potential function is to add mechanical strength and protection against the activity of the pollinator when oil is removed.
In G. bifolia, small electron-dense corpuscles immersed on the outer tangential wall and upon the cuticle were observed, and these form a continuous lipid layer covering the epidermis. In addition, the cuticle is not distended, the wall cavities are very small and the cellulose matrix of the wall contains sparse, electron-dense corpuscles. Based on these features, we suggest that the lipid passes through the wall as small lipid moieties that then reassemble to form larger globules on the surface of the labellum. Davies et al. (2003) mentioned the same secretory process in Maxillaria Ruiz & Pav. (Cymbidieae: Maxillariinae), but the secretion in this genus is a resin-like substance composed of lipids and aromatic amino acids.
The remaining areas among the callus protrusion and lateral lobes in G. bifolia are covered by papillose cells that have a striated cuticle. These traits could facilitate the accumulation and retention of oils in these portions of the labellum.
Taxonomic implications
Broadly, the internal structure of the elaioiphores in G. bifolia is similar to that most commonly described for the subtribe Oncidiinae. Elaiophores of eight species of four genera of the Oncidiinae (sensu Chase et al., 2009) have been described anatomically (Table 1). Among these species, G. bifolia is most similar to G. loefgrenii (Cogn.) M.W. Chase & N.H. Williams and G. venusta (Drapiez) M.W. Chase & N.H. Williams. These species share the presence of epithelial elaiophores located in the callus (the lateral lobes are also secretory in G. venusta), lack a distended cuticle and the outer tangential walls show cavities (although inconspicuous in G. bifolia).
Table 1.
Comparison of selected characteristics in elaiophores of Gomesa bifolia with other published species of the subtribe Oncidiinae
| Gomesa bifolia | Gomesa loefgrenii (ex Oncidium loefgrenii) | Gomesa paranaensis (ex Oncidium paranaense) | Gomesa radicans (ex Ornithophora radicans) | Gomesa recurva | Gomesa venusta (ex Oncidium trulliferum) | Oncidium cheirophorum | Ornithocephalus kruegeri | Trichocentrum cavendishianum | |
|---|---|---|---|---|---|---|---|---|---|
| Reference | Stpiczyńska et al. (2007) | Singer and Cocucci (1999) | Stpiczyńska and Davies (2008) | Stpiczyńska et al. (2007) | Stpiczyńska and Davies (2008) | Pacek and Stpiczyńska (2007) | Pacek and Stpiczyńska (2007) | Stpiczyńska et al. (2007) | |
| Elaiophore position | Callus | Callus | Lateral lobes | Callus | Callus | Callus + Lateral lobes | Lateral lobes | Callus | Lateral lobes |
| Elaiophore type | Epithelial | Epithelial | Epithelial | Epithelial | Not obvious | Epithelial | Epithelial | Unicellular trichomes | Epithelial |
| Distended cuticle | − | − | X | X | − | − | X | X | X |
| Wall cavities | X | X | n.d. | − | − | X | X | − | X |
| Vascular tissue | X | n.d. | n.d. | X | n.d. | X | X | n.d. | X |
| Idioblast | X | X | n.d. | X | n.d. | − | n.d. | X | X |
| Nuclei centrally placed | X | n.d. | n.d. | X | n.d. | X | X | X | X |
| Mitochondria | Abundant | Abundant | n.d. | n.d. | n.d. | Abundant | n.d. | n.d. | Abundant |
| Myelin-like membranes | − | X | n.d. | − | n.d. | X | n.d. | n.d. | P |
| Cytoplasm | Electron-dense | n.d. | n.d. | Granular | n.d. | Electron − dense | n.d. | n.d. | n.d. |
| Endoplasmic reticulum | Smooth and rough | Principally smooth | n.d. | Principally smooth | Principally smooth | n.d. | n.d. | n.d. | Principally smooth |
| Lipid globules | X | X | n.d. | X | X | X | X | X | X |
| Starch | X | − | n.d. | − | − | n.d. | X | X | − |
X, present; −, absent; n.d., not described.
Despite the similarity among elaiophores of G. bifolia, G. loefgrenii and G. venusta, neither traditional taxonomy (Garay and Stacy, 1974) nor the most current phylogeny (Chase et al., 2009) support affinities among these species. Garay and Stacy (1974), in their synopsis of the genus Oncidium, placed G. bifolia in Section Synsepala, G. loefgrenii in Section Paucituberculata and G. venusta in Section Rostrata (under O. bifolium, O. loefgrenii and O. venustum, respectively). By contrast, even if any currently published phylogeny included G. bifolia or G. loefgrenii, G. bifolia should be close to the clade that includes G. warmingii and the other species (M. W. Chase, Royal Botanic Gardens, Kew, pers. comm.), instead of being placed as allied to G. venusta as depicted in the phylogenetic scheme presented by Chase et al. (2009).
The anatomical and ultrastructural traits of the elaiophores observed in the present study appear to be subject to convergence, as for other floral characteristics, giving additional support to the hypothesis provided by Chase et al. (2009). However, we agree with Stpiczyńska et al. (2007) who expressed the need to extend morphological studies of elaiophores in Oncidiinae, because the evolution of this structure can be interpreted only when a complete and well-resolved phylogeny is available.
ACKNOWLEDGEMENTS
We thank M. W. Chase and anonymous reviewers for their valuable comments, M. Gotelli and J. Saunders for help with revision of the English text, and G. Zarlavsky for her technical assistance.
LITERATURE CITED
- Ackerman JD. Pollination of tropical and temperate orchids. In: Tan KW, editor. Proceedings of the 11th World Orchid Conference; American Orchid Society; Miami, FL. 1984. pp. 98–101. [Google Scholar]
- Buchmann SL. The ecology of oil flowers and their bees. Annual Review of Ecology and Systematics. 1987;18:343–369. [Google Scholar]
- Chase MW, Palmer JD. Floral morphology and chromosome number in subtribe Oncidiinae (Orchidaceae): evolutionary insights from a phylogenetic analysis of chloroplast DNA restriction site variation. In: Soltis PS, Soltis DE, Doyle JJ, editors. Molecular systematics of plants. New York: Chapman and Hall; 1992. pp. 324–339. [Google Scholar]
- Chase MW, Cameron KN, Barrett RL, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KM, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 69–89. [Google Scholar]
- Chase MW, Williams NH, de Faria AD, Neubig KM, Amaral MCE, Whitten WM. Floral convergence in Oncidiinae (Cymbidieae; Orchidaceae): an expanded concept of Gomesa and a new genus Nohawilliamsi. Annals of Botany. 2009;104:387–402. doi: 10.1093/aob/mcp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination: America, Africa, Asia, and Australia. Rotterdam: AA. Balkema; 2001. [Google Scholar]
- Cocucci AA, Sércic A, Roig-Alsina AH. Oil-collecting structures in Tapinotaspidini: their diversity, function, and probable origin. Mitteilungen der Münchner Entomologischen Gesellschaft. 2000;90:51–74. [Google Scholar]
- D'Ambrogio de Argüeso AC. Manual de técnicas en histología vegetal. Buenos Aires: Hemisferio Sur; 1986. [Google Scholar]
- Davies KL, Stpiczyńska M. The anatomical basis of floral, food-reward production in Orchidaceae. In: Teixeira da Silva J, editor. Floriculture, ornamental and biotechnology: advances and topical issues. Vol. V. Isleworth: Global Science Books; 2008. pp. 392–407. [Google Scholar]
- Davies KL, Turner MP, Gregg A. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae) Annals of Botany. 2003;91:439–446. doi: 10.1093/aob/mcg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Fahn A. Secretory tissues in plants. London: Academic Press Inc; 1979. [Google Scholar]
- Garay LA, Stacy JE. Synopsis of the genus Oncidium. Bradea. 1974;1:393–424. [Google Scholar]
- Kunst L, Samuels AL. Biosynthesis and secretion of plant cuticular wax. Progress in Lipid Research. 2003;42:51–80. doi: 10.1016/s0163-7827(02)00045-0. [DOI] [PubMed] [Google Scholar]
- Machado IC. Oil-collecting bees and related plants: a review of the studies in the last twenty years and case histories of plants occurring in NE Brazil. In: Freitas BM, Pereira JOP, editors. Solitary bees. Conservation, rearing and management for pollination. Fortaleza, Brazil: Imprensa Universitária; 2004. pp. 255–280. [Google Scholar]
- Neff J, Simpson B. Oil-collecting structures in Anthophoridae (Hymenoptera): morphology, function, and use in systematics. Journal of the Kansas Entomological Society. 1981;54:95–123. [Google Scholar]
- Nilsson LA. Orchid pollination biology. Trends in Ecology & Evolution. 1992;7:255–259. doi: 10.1016/0169-5347(92)90170-G. [DOI] [PubMed] [Google Scholar]
- Pacek A, Stpiczyńska M. The structure of elaiophores in Oncidium cheirophorum Rchb. f. and Ornithocephalus kruegeri Rchb. f. (Orchidaceae) Acta Agrobotanica. 2007;60:9–14. [Google Scholar]
- van der Pijl L, Dodson CH. Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press; 1969. [Google Scholar]
- Reis MG, de Faria AD, Bittrich V, Amaral MC, Marsaioli AJ. The chemistry of flower rewards – Oncidium (Orchidaceae) Journal of the Brazilian Chemical Society. 2000;11:600–608. [Google Scholar]
- Reis MG, de Faria AD, Amaral MC, Marsaioli AJ. Oncidinol – a novel diacylglycerol from Ornithophora radicans Barb. Rodr. (Orchidaceae) floral oil. Tetrahedron Letters. 2003;44:8519–8523. [Google Scholar]
- Silvera KI. Adaptive radiation of oil-reward compounds among Neotropical orchid species (Oncidiinae). University of Florida; 2002. MSc thesis. [Google Scholar]
- Singer RB, Cocucci AA. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana. 1999;14:47–56. [Google Scholar]
- Singer RB, Marsaioli AJ, Flach A, Reis MG. The ecology and chemistry of pollination in Brazilian orchids: recent advances. In: Teixeira da Silva J, editor. Floriculture, ornamental and biotechnology: advances and topical issues. Vol. IV. Isleworth: Global Science Books; 2006. pp. 570–583. [Google Scholar]
- Stpiczyńska M, Davies K. Elaiophore structure and oil secretion in flowers of Oncidium trulliferum Lindl. and Ornithophora radicans (Rchb.f.) Garay & Pabst (Oncidiinae: Orchidaceae) Annals of Botany. 2008;101:375–384. doi: 10.1093/aob/mcm297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Elaiophore diversity in three contrasting members of Oncidiinae (Orchidaceae) Botanical Journal of the Linnean Society. 2007;155:135–148. [Google Scholar]
- Vogel S. Ölblumen und ölsammelnde Bienen. Abhandlungen Akademie Wissenschaften Mathematisch-Naturwissenschaften Klasse Tropische und Subtropische Pfanzenwelt. 1974;7:1–267. [Google Scholar]
- Williams NH, Chase MW, Fulcher T, Whitten WM. Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscriptions of Cyrtochilum, Erycina, Otoglossum, and Trichocentrum and a new genus (Orchidaceae) Lindleyana. 2001;16:113–139. [Google Scholar]