Abstract
Background and Aims
The rate of plant decomposition depends on both the decomposition environment and the functional traits of the individual species (e.g. leaf and litter quality), but their relative importance in determining interspecific differences in litter decomposition remains unclear. The aims of this study were to: (a) determine if species from different successional stages grown on soils with low and high nitrogen levels produce leaf and litter traits that decompose differently under identical conditions; and (b) assess which trait of living leaves best relates to litter quality and litter decomposability
Methods
The study was conducted on 17 herbaceous species representative of three stages of a Mediterranean successional sere of Southern France. Plants were grown in monocultures in a common garden under two nitrogen levels. To elucidate how different leaf traits affected litter decomposition a microcosm experiment was conducted to determine decomposability under standard conditions. Tests were also carried out to determine how successional stage and nitrogen supply affected functional traits of living leaves and how these traits then modified litter quality and subsequent litter decomposability.
Key Results
The results demonstrated that leaf traits and litter decomposability varied according to species and successional stage. It was also demonstrated that while nitrogen addition affected leaf and litter traits, it had no effect on decomposition rates. Finally, leaf dry matter content stood out as the leaf trait best related to litter quality and litter decomposability
Conclusions
In this study, species litter decomposability was affected by some leaf and litter traits but not by soil nitrogen supply. The results demonstrated the strength of a trait-based approach to predict changes in ecosystem processes as a result of species shifts in ecosystems.
Key words: Leaf traits, litter quality, litter decomposability, nitrogen addition, secondary succession
INTRODUCTION
Decomposition of dead plant material is a key component in carbon and nutrient cycling in most terrestrial ecosystems (Swift et al., 1979; Couteaux et al., 1995; Chapin et al., 2002). The multiple drivers of decomposition include the effects of decomposition environment, at both regional and microsite scales, the substrate quality of litter and the composition of the decomposer community (Swift et al., 1979), with the relative importance of these three factors varying across ecosystems (Lavelle et al., 1993; Pérez-Harguindeguy et al., 2000).
In a meta-analysis involving 818 species from 66 decomposition experiments on six continents, Cornwell et al. (2008) showed that the degree to which interspecific variations in leaf structure and composition affect their decomposition rate could be as large as the effect of global climatic variation. Several traits of green leaves related to physiological and protective features persist through senescence, and have been shown to affect litter decomposition. This is the case for, for example, the physical strength of leaves (Gallardo and Merino, 1993; Cornelissen, 1996; Cornelissen and Thompson, 1997) or their nitrogen and/or phosphorus concentrations (Cornelissen and Thompson, 1997; Cornwell et al., 2008). In their meta-analysis, Cornwell et al. (2008) used leaf mass per area (LMA; the ratio of leaf mass to its area) and leaf nitrogen concentration (LNC) as the two generic traits describing leaf structure and chemical composition, respectively. Although this is justified by the number of studies in which these traits have been studied, the case of LMA (or its inverse, specific leaf area, SLA) deserves further attention. SLA is a robust index of sclerophylly as a surrogate for more rigorous mechanical properties used in herbivory studies (Hanley et al., 2007) and depends on both leaf thickness and density (Witkowski and Lamont, 1991). Limited evidence suggests that litter decomposition actually depends mainly on the latter: in studies where leaf dry matter content (LDMC; the ratio of leaf dry mass to saturated fresh mass), used as a surrogate for leaf tissue density (see Garnier and Laurent, 1994; Shipley and Vu, 2002), was measured in addition to SLA, relationships with decomposition rates were always found to be stronger with LDMC, be it at the species (Kazakou et al., 2006) or the community (Cortez et al., 2007; Quested et al., 2007; Fortunel et al., 2009) level. Here the hypothesis that LDMC is the better predictor of species-level leaf decomposition rates is tested by explicitly relating plant leaf traits to several indices of litter quality known to affect decomposition. This was done for a set of plant species characterizing the different successional stages associated with the abandonment of Mediterranean arable fields.
A previous study in these successional fields had shown that litters produced by early successional species tend to decompose more rapidly than those produced by species from more advanced stages (Kazakou et al., 2006). This study was conducted on leaf and litters collected in species grown in the field however, which implies that differences in traits and decomposition rates may be due to differences in environmental conditions prevailing in the old fields from different successional stages. Such differences include soil nitrogen and carbon concentrations, litter accumulation and light interception (Garnier et al., 2004, 2007; Kazakou and Navas, 2004). Nitrogen was found to be a strong limiting factor for plant growth in this successional sere, as indicated by the estimation of nitrogen nutrition index [(NNI) approx. 50 %; see Garnier et al., 2007]. Numerous studies have shown that in an initially low nutrient environment, fertilization often causes an increase in nutrient concentration in plants, whether accompanied by an increase in growth (i.e. a limiting nutrient) or leading to luxury uptake (Chapin, 1980; Chapin et al., 1986). However, evidence supporting a link between increased endogenous leaf nutrient concentrations inducing faster decomposition rates is contradictory, sometimes showing no effects (Hobbie and Vitousek, 2000; Bridgham and Richardson, 2003; Güsewell and Verhoeven, 2006) or a stimulatory effect (Coulson and Butterfield, 1978; Pastor et al., 1987; Taylor et al., 1989; Aerts, 1997). Bridgham and Richardson (2003) proposed a conceptual model assuming that as plants from low nutrient environments have low carbon quality litter, decomposition rates will be unaffected by the increase in endogenous nutrient concentrations. If greater nutrient availability leads to better litter quality, for example through increased amino acid or soluble carbohydrate concentrations or decreased lignin or phenolic concentrations (Northrup et al., 1995), then there should be an increase in decay rates. Here we tested if nitrogen fertilization of species grown in monocultures would affect traits and thus whether there is any subsequent effect on decomposition rates.
The main objectives of the present study were therefore to: (a) test whether changes in decomposability and associated traits previously recorded in situ along the successional sere are also apparent when species were grown under identical environmental conditions under low and high nitrogen availabilities (we hypothesized that species effects would be stronger than environmental effects on litter decomposability); (b) assess whether differences in soil nitrogen might affect leaf traits and decomposition rates; and (c) assess which trait of living leaves best relates to litter quality and litter decomposability. Here, we hypothesize that LDMC relates better to the litter quality, hence to the decomposition rate, than to other traits screened for so far such as SLA or leaf nitrogen concentration.
MATERIALS AND METHODS
Study site, species and experimental design
The common garden experiment was conducted in the experimental field of the Centre d'Ecologie Fonctionnelle et Evolutive located in Montpellier, France (43°59′N, 43°51′E). Soil pH (7·82), soil total average nitrogen (1·38 g kg−1) and carbon (14·54 g kg−1) concentration were close to those recorded in the old-field succession studied by Garnier et al. (2004). Seventeen herbaceous species were selected as representative of plant communities from French Mediterranean old-field successions (Table 1). Three main stages were recognized based on the time since abandonment: early (0–6 years); intermediate (7–15 years); and advanced (16–45 years). Communities of intermediate succession showed the highest number of species (mean number of species = 7) and those of advanced succession the lowest (mean number of species = 2) (see table 2 in Garnier et al., 2004). Five species were chosen for the early stage and six species for the intermediate and advanced stages. These species represented from 57 to 97 % of the community total above-ground biomass (Vile et al., 2006). Among these species, four groups of three species from each successional stage were chosen to form four taxonomic groups: the order Lamiales and the families Asteraceae, Fabaceae and Poaceae (Table 1). In October 2003, four replicated monocultures (1·2 × 1·2 m plots) per species were established at two levels of nitrogen supply by transplanting seedlings or ramets (seeds or ramets of all the species were collected in the field and grown in a glasshouse before transplantation to the experimental plots; for a full description of the site, see Garnier et al., 2004). This ensured better survival and a standard plant density (100 plants m−2). In the fertilized treatment (N+ treatment), 25 g N m−2 in the form of NH4NO3 were applied three times between January and March 2004. No fertilization was added in the N− treatment. Growth limitation by N was assessed by comparing the NNI of the two treatments. The NNI was calculated as the ratio between the actual nitrogen concentration of above-ground biomass and the critical nitrogen concentration (i.e. the concentration allowing potential growth), as proposed by Lemaire and Gastal (1997). The NNI was approx. 50 % in the N− treatment, and between 84 and 132 % in the N+ treatment (Kazakou et al., 2007). This compares with an NNI of approx. 50 % in the successional sere (Garnier et al., 2007). Hence the N− treatment was considered strongly growth limiting, whereas the N+ treatment was non-limiting for plant growth.
Table 1.
List of the species studied
| Species | Successional status | Family/taxonomic group | Abbreviation |
|---|---|---|---|
| Bromus madritensis | Early | Poaceae (1) | BROMMADR |
| Crepis foetida | Early | Asteraceae (2) | CREPFOET |
| Geranium rotundifolium | Early | Geraniaceae | GERAROTU |
| Medicago minima | Early | Fabaceae (3) | MEDIMINI |
| Veronica persica | Early | Plantaginaceae (4) | VEROPERSI |
| Calamintha nepeta | Intermediate | Lamiaceae (4) | CALANEPE |
| Dactylis glomerata | Intermediate | Poaceae (1) | DACTGLOM |
| Daucus carota | Intermediate | Apiaceae | DAUCCARO |
| Picris hieracioides | Intermediate | Asteraceae (2) | PICRHIER |
| Tordylium maximum | Intermediate | Apiaceae | TORDMAXI |
| Trifolium angustifolium | Intermediate | Fabaceae (3) | TRIFANGU |
| Bituminaria bituminosa | Advanced | Fabaceae (3) | BITUBITU |
| Brachypodium phoenicoides | Advanced | Poaceae | BRACPHOE |
| Bromus erectus | Advanced | Poaceae (1) | BROMEREC |
| Inula conyza | Advanced | Asteraceae (2) | INULCONY |
| Rubia peregrina | Advanced | Rubiaceae | RUBIPERE |
| Teucrium chamaedrys | Advanced | Lamiaceae (4) | TEUCCHAM |
Species are representative of three stages of a Mediterranean post-cultural succession (see Garnier et al., 2004 for details) following vineyard abandonment: early (2–6 years); intermediate (7–15 years); advanced (15–45 years). Four taxonomic groups were constructed (taxonomic relationships from Soltis et al., 2000) with one species per successional stage.
Table 2.
List of traits, abbreviations and units
| Abbreviation |
|||
|---|---|---|---|
| Trait | Green leaves | Litter | Unit |
| Specific leaf area | SLAgreen | – | m2 kg−1 |
| Leaf dry matter content | LDMCgreen | – | mg g−1 |
| Leaf tensile strength | LTSgreen | – | MN m−2 |
| Leaf resistance to fracture | LRFgreen | – | J m−2 |
| Leaf nitrogen concentration | LNCgreen | LNClitter | mg g−1 |
| Leaf phosphorus concentration | LPCgreen | LPClitter | mg g−1 |
| Leaf carbon concentration | LCCgreen | LCClitter | mg g−1 |
| Initial lignin concentration | – | LIGlitter | mg g−1 |
| Initial cellulose concentration | – | CELlitter | mg g−1 |
| Initial hemicellulose concentration | – | HEMlitter | mg g−1 |
| Hollocellulose to lignocellulose quotient | – | HLQlitter | – |
| Fibre component | – | LCHlitter | mg g−1 |
| Potential decomposability | – | Kpot | g kg−1 d−1 |
Indications (given as trait abbreviation) of the condition of the leaf material (green leaf or litter) when the trait is measured are given.
Collection of material
Traits of living leaves were measured on 12 replicate samples per species at each level of nitrogen during spring 2004, on the youngest fully expanded, well-lit leaves at their peak period of growth.
Leaf litter was collected at the season of maximum leaf senescence for each species (summer and autumn 2004). For each species litter was collected from the four plots of a given treatment and then mixed to form a composite sample. For some species that shed their leaves once they had senesced (e.g. Veronica persica), dead leaves that dropped after gently shaking the plants were collected. In species that retain dead leaves on the plant (e.g. Brachypodium phoenicoiedes and Dactylis glomerata) or that die completely above-ground (e.g. Bromus madritensis and Bromus erectus), leaves that were dead were cut off from the standing plant. Dead leaves were carefully cleaned, air-dried and stored in the laboratory.
Trait measurements
Thirteen leaf and litter traits were assessed in this study (see Table 2). Leaf traits were measured on each species, using standardized procedures (Cornelissen et al., 2003). Specific leaf area (SLAgreen) and leaf dry matter content (LDMCgreen) were calculated as the ratio between leaf area and leaf dry mass, and between leaf dry mass and saturated fresh mass, respectively. The physical strength of leaves was assessed by measuring leaf tensile strength (LTSgreen) and leaf resistance to fracture (LRFgreen). LTSgreen was calculated as the force needed to tear a leaf divided by its width (e.g. Cornelissen and Thompson, 1997). It was measured with an apparatus comparable in design with that described by Hendry and Grime (1993). In order to calculate LTSgreen, measurements of leaf width and average thickness at the point of rupture were made with a digital calliper and a linear variable displacement transducer, respectively. LRF (also called ‘force to fracture’ or ‘work to shear’), which was calculated as the mean force needed to cut a leaf or a leaf fragment at a constant angle (20 °) and speed (e.g. Wright and Cannon, 2001), was measured with a device adapted from that described by Wright and Cannon (2001). The measurement of LTS and LRF for Daucus carota was not possible due to its particular leaf shape.
Nitrogen and carbon concentrations were determined with an elemental analyser (model EA 1108; Carlo Erba Instruments, Milan, Italy). Phosphorus concentrations were measured colorimetrically with an autoanalyser (Evolution II; Alliance Instruments, Frépillon, France), using the molybdenum blue method following digestion by sulfuric acid (Grimshaw et al., 1989).
A sub-sample of the litter of each species was ground in a cyclone mill (Cyclotec Sample Mill, Tecator, Höganäs, Sweden) and scanned using a near infrared reflectance spectrophotometer (NIRS; NIRSystems 6500, Foss NIRSystems, Raamsdonksveer, The Netherlands). For these samples, lignin (LIGlitter), cellulose (CELlitter) and hemicellulose (HEMlitter) concentrations were determined by NIRS according to the method described by Joffre et al. (1992). The predicted values were obtained with standard errors of calibration of 2·8 % for LIGlitter and 1·7 % for CELlitter and HEMlitter. We also calculated the total fibre content of litter (LCHlitter = LIGlitter + CELlitter + HEMlitter), and the hollocellulose: lignocellulose ratio [HLQlitter = (CELlitter + HEMlitter)/LCHlitter] which indicates the proportion of the less recalcitrant non-labile compounds (McClaugherty and Berg, 1987; Berg et al., 1993; Gillon et al., 1994; Cornelissen et al., 2004; Cortez et al., 2007).
Litter decomposability in microcosms
Litter was incubated in microcosms in the laboratory, under controlled temperature and humidity conditions. Microcosms, as simplified analogues of natural ecosystems, allow the study of litter decomposition by controlling temperature and humidity, with similar soil conditions and decomposer populations, while maintaining a sufficiently natural situation so that results of laboratory tests may be extrapolated to the field situation with confidence (Taylor and Parkinson, 1988). The microcosm type used for this experiment was described by Taylor and Parkinson (1988). Each microcosm chamber, 15 cm high, was made of a 15 cm diameter polyvinylchloride pipe, fitted with a lid and a sealed bottom. The lid could be opened to allow gas exchange and the plug at the bottom could be removed to drain excess water. A grid, 2 cm above the bottom, divided the chamber into two unequal parts: a usable space of 1·5 L capacity and a drainage compartment of 300 mL. One kilogram of soil, of known water-holding capacity, was placed on the grid. The soil was a 3 : 1 mixture of mineral soil and surface organic horizon taken from the common garden where the experiment was conducted (for further details, see Kazakou et al., 2006).
For each of the 17 species, ten litter samples (3 ± 0·01 g each) per nitrogen supply were sealed in a nylon litter bag of 1 mm mesh (Northern Mesh, Oldham, UK). Each litter sample was soaked for 24 h in 0·1 L of water, and then placed on the surface of the microcosm soil. In order to keep all the soluble nutrients in the system, the water used for soaking was poured into the microcosm. The soil was subsequently moistened up to 80 % of field capacity. The microcosms were kept in the dark at 22 °C throughout the experiment and watered once a week to maintain constant soil moisture during incubation. Two litter samples per species and nitrogen supply were removed from the microcosms at the end of 1, 2, 4, 6 and 8 weeks. Soil particles were carefully removed from the litter bags and the litter samples were weighed after drying for 48 h at 55 °C. Prior to the experiment, two litter samples of 2 g from each species were weighed, dried in an oven for 48 h at 55 °C and weighed again in order to correct the initial mass for the water content of the litter.
The percentage of oven-dried litter mass remaining is denoted %MR hereafter. To compare the decomposability of the different species, the single negative exponential model proposed by Olson (1963) was fitted to the %MR of each litter during the course of the experiment: %MR = 100 e−Kpott, where Kpot is the potential decomposition rate constant (litter decomposability) over time t in days; and %MR is expressed as a percentage of the original mass. The Kpot rate constants was multiplied by 103 and expressed in g kg−1 d−1.
Data analyses
For the leaf and litter traits 12 replicates per species (three individuals per plot) and per nitrogen supply were used (the plot effect was not significant for any of the traits measured). The effects of species nested within successional stage, successional stage, N supply and their interaction (successional stage × N supply) on the various variables were tested with a three-way analysis of variance (ANOVA). When the interaction effect was not significant, this was removed and the ANOVA analysis was repeated. If the effect of stage was significant, post hoc tests (Student–Newman–Keuls comparisons) were performed within each N supply in order to identify the variations between successional stages. Two-way ANOVA was carried out for the 12 species belonging to the four taxonomic groups, with successional stage, taxonomic group and their interaction as effects.
Bivariate correlations between green leaf, litter traits and Kpot were evaluated with Pearson product–moment correlation coefficients. In all analyses significance levels were corrected by the improved Bonferroni procedure (Simes, 1986; Sokal and Rohlf, 1995). All variables were log transformed when required. All the analyses were carried out with the Statistical Analysis System (SAS Institute, Cary, NC, USA, version 8).
RESULTS
Effects of successional stage and nitrogen addition on litter decomposability and traits
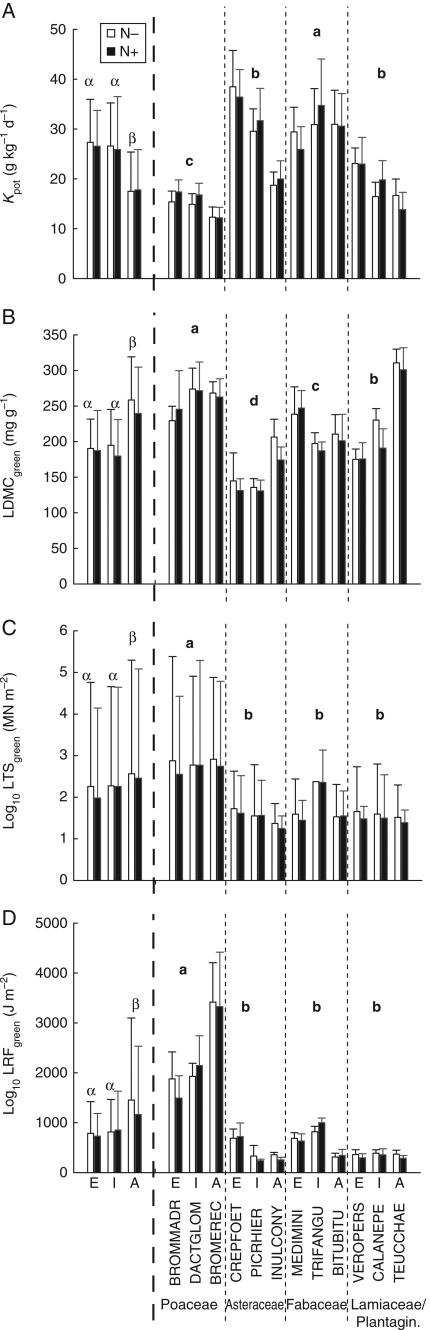
Litter decomposability differed significantly among species from different successional stages (Table 3 and Appendix 1). The litter mass remaining after 8 weeks of litter incubation in the microcosms ranged from 71 % (B. phoenicoides in the N− treatment) to 35 % (T. maximum in the N+ treatment). Mass loss of all litters fitted the single exponential decay model (0·01 < P < 0·1). The ANOVA indicated significant differences in Kpot according to species successional stage, with early and intermediate species decomposing more rapidly than species from advanced successional stages (Fig. 1A). Within taxonomic groups, Poaceae had the slowest decomposition rates while Fabaceae had the highest (Fig. 1A).
Table 3.
Means of leaf and litter traits and decomposition decay at each successional stage and nitrogen supply
| N− |
N+ |
|||||
|---|---|---|---|---|---|---|
| Traits | Early | Intermediate | Advanced | Early | Intermediate | Advanced |
| Green leaves | ||||||
| SLAgreen (m2 kg−1) | 26·6 (7·5) | 20·8 (3·0) | 13·8 (2·8) | 29·0 (9·5) | 21·9 (2·3) | 15·9 (3·4) |
| LDMCgreen (mg g−1) | 190 (41·3) | 195 (50·3) | 258 (60·8) | 187 (56·4) | 180 (51·3) | 240 (65·2) |
| LTSgreen (MN m−2) | 181 (320) | 187 (243) | 365 (541) | 96 (145) | 182 (243) | 287 (423) |
| LRFgreen (J m−2) | 787 (635) | 816 (651) | 1455 (1644) | 731 (455) | 854 (779) | 1166 (1366) |
| LNCgreen (mg g−1) | 24·4 (7·7) | 26·3 (7·2) | 21·1 (3·6) | 35·7 (6·1) | 39·3 (6·0) | 35·4 (8·6) |
| LPCgreen (mg g−1) | 2·8 (0·4) | 3·9 (1·2) | 2·3 (0·4) | 2·8 (0·7) | 3·2 (0·8) | 2·4 (0·5) |
| LCCgreen (mg g−1) | 409 (8·97) | 410 (26·5) | 415 (15·6) | 420 (13·4) | 426 (14·6) | 427 (18·6) |
| Litter | ||||||
| LNClitter (mg g−1) | 12·6 (4·1) | 10·1 (6·3) | 7·7 (2·8) | 21·0 (5·4) | 14·5 (4·3) | 10·7 (4·2) |
| LPClitter (mg g−1) | 2·1 (1·0) | 2·4 (1·2) | 0·9 (0·4) | 1·6 (0·6) | 1·2 (0·7) | 0·7 (0·3) |
| LCClitter (mg g−1) | 384 (10·5) | 362 (30·0) | 372 (47·0) | 389 (13·7) | 381 (21·2) | 383 (43·5) |
| LIG litter (mg g−1) | 167 (50·6) | 15·3 (73·6) | 149 (59·2) | 158 (44·8) | 165 (91·9) | 134 (57) |
| CEL litter (mg g−1) | 198 (65) | 157 (84·5) | 165 (70·9) | 153 (62·8) | 161 (45·1) | 120 (30·5) |
| HEM litter (mg g−1) | 138 (5·05) | 128 (51·0) | 134·4 (64·9) | 163 (33·4) | 179 (91·4) | 156 (82·5) |
| LCH litter (mg g−1) | 503 (13·3) | 438 (185) | 485 (73) | 548 (94) | 434 (109) | 553 (136) |
| HLQ litter | 0·66 (0·08) | 0·65 (0·13) | 0·68 (0·15) | 0·65 (0·10) | 0·65 (0·09) | 0·68 (0·13) |
| Kpot (g kg−1 d−1) | 22·7 (7·6) | 23·4 (8·4) | 17·4 (10·8) | 23·4 (8·1) | 27·1 (9·6) | 16·6 (8·2) |
Standard errors are given in parentheses (n = 5 for the early stage and n = 6 for the intermediate and advanced stage).
Fig. 1.
Means and s.e. of (A) decomposition rate Kpot, (B) leaf dry mass content (LDMCgreen), (C) leaf tensile strength (LTSgreen) and (D) resistance to fracture (LRFgreen) for species from different successional stages (E, early; I, intermediate; A, advanced succession) at each nitrogen supply (N− and N+ treatments, as indicated). In the first part of each histogram, the mean and s.e. for all species combined (n = 17 for each N supply) are indicated. Results of post hoc tests (Student–Newman–Keuls comparisons) with successional stage as factor are given with Greek letters (F- and P-values are given in Table 3). Means and s.e. in the 12 species from each taxonomic group are shown in the second part of the histograms (separated by the dotted line). Results of post hoc tests with taxonomic group as factor are given with Roman letters (F- and P-values are given in Appendix 2).
Leaf and litter traits varied according to species successional stage. SLAgreen decreased in advanced successional species. Species from advanced successional stages also had higher LDMCgreen, LTSgreen and LRFgreen than species from earlier stages in both N supply treatments (Table 3 and Fig. 1B–D). Poaceae showed higher values of structural traits (LDMCgreen, LTSgreen and LRFgreen) than species from other taxonomic groups (Table 3 and Fig. 1B–D). Species from the intermediate stage had higher LNCgreen and LPCgreen values than species typical of other stages (Table 3), with Fabaceae species showing the highest LNCgreen values and Asteraceae showing the highest LPCgreen values (Appendix 2). Regarding litter traits, species from early successional stages had higher LNClitter values and species from early and intermediate stages had higher LPClitter values (Table 3). LCC (green and litter), LIGlitter, CELlitter, HEMlitter, non-labile compounds and HLQlitter did not vary with species successional status (Table 3). Poaceae species had higher HEMlitter and LCHlitter values than species from other taxonomic groups (Appendix 2).
N addition had no effect on the litter decomposition rate but affected leaf and litter traits (Table 3). With increased N supply, SLAgreen, LNCgreen and LNClitter increased (9, 33 and 36 %, respectively), LDMCgreen, LTSgreen and LPClitter decreased (6·5, 6 and 28 %, respectively) whereas all other traits were not affected by N supply (Table 3).
Relationships between litter decomposability, leaf and litter traits
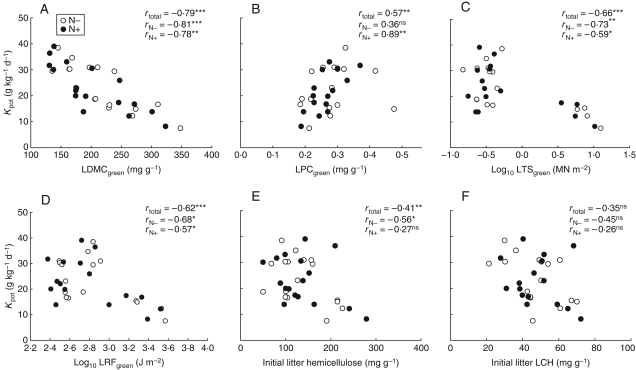
There were clear links between litter decomposability and leaf structural traits. LDMCgreen, for instance, was most closely related to litter decomposability: species with low LDMCgreen tend to decompose more rapidly than species with high LDMCgreen (Table 4 and Fig. 2A). A negative relationship was found between LTSgreen, LRFgreen and Kpot (Table 4 and Fig. 2C, D). The relationship between Kpot and the two traits related to leaf physical strength (LTSgreen and LRFgreen) is mainly due to the leaves of Poaceae species which have higher values than all the other species. LPCgreen was positively correlated to litter decomposability under the N+ treatment (Table 4, Fig. 2B). The non-significant relationship between Kpot and LPCgreen in the N− treatment is due to D. glomerata: when this species is removed from the analysis, the relationship becomes significant (rN− = 0·54, P = 0·03). This relationship could be attributed to the negative correlation between LPCgreen and LDMCgreen (Table 3). No significant relationship was found between Kpot and SLAgreen, LNC and LCC of green leaves and litter.
Table 4.
Relationships (Pearson's correlation coefficients) between measured green living traits and initial litter traits (two levels of N supply are considered together) (n = 34 for each correlation)
| Kpot | SLAgreen | LDMCgreen | LTSgreen | LRFgreen | LNCgreen | LPCgreen | LCCgreen | LNClitter | LPClitter | LCClitter | LIGlitter | CELlitter | HEMlitter | LCHlitter | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLAgreen | ns | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| LDMCgreen | −0·79*** | ns | – | – | – | – | – | – | – | – | – | – | – | – | – |
| LTSgreen | −0·66*** | ns | 0·69*** | – | – | – | – | – | – | – | – | – | – | – | – |
| LRFgreen | −0·62*** | ns | 0·64*** | 0·92*** | – | – | – | – | – | – | – | – | – | – | – |
| LNCgreen | ns | ns | ns | ns | ns | – | – | – | – | – | – | – | – | – | – |
| LPCgreen | 0·57** | ns | −0·51* | ns | ns | ns | – | – | – | – | – | – | – | – | – |
| LCCgreen | ns | ns | 0·4* | ns | ns | ns | ns | – | – | – | – | – | – | – | – |
| LNClitter | ns | 0·37* | ns | −0·42* | –0·35 | 0·34* | ns | ns | – | – | – | – | – | – | – |
| LPClitter | ns | 0·45* | ns | ns | ns | −0·42* | 0·7*** | ns | ns | – | – | – | – | – | – |
| LCClitter | ns | ns | 0·38* | ns | ns | −0·45* | ns | 0·86*** | ns | ns | – | – | – | – | – |
| LIGlitter | ns | ns | ns | –0·36 | −0·39* | ns | ns | 0·44* | ns | ns | ns | – | – | – | – |
| CELlitter | ns | ns | 0·38* | 0·57** | 0·64*** | ns | ns | ns | ns | ns | ns | ns | – | – | – |
| HEMlitter | −0·41** | ns | 0·47** | 0·71*** | 0·75*** | ns | ns | ns | ns | ns | ns | ns | 0·93*** | – | – |
| LCHlitter | –0·35 | ns | 0·39* | 0·45* | 0·49** | ns | ns | ns | ns | ns | ns | 0·43* | 0·95*** | 0·87*** | – |
| HLQlitter | ns | ns | ns | 0·68*** | 0·76*** | ns | ns | ns | ns | ns | ns | −0·56** | 0·70*** | 0·77*** | 0·47** |
*P < 0·05; **P < 0·01; ***P < 0·001 (significance levels corrected by the improved Bonferroni procedure. Marginally significant results (0·05 < P < 0·10) are shown in italics.
Fig. 2.
Relationships between potential decomposition decay (Kpot) and (A) leaf dry matter content (LDMCgreen), (B) leaf phosphorus content (LPCgreen), (C) leaf tensile strength (LTSgreen), (D) resistance to fracture of green leaves (LRFgreen), (E) initial litter hemicellulose (HEMlitter) and (F) initial litter non-labile compounds (LCHlitter). Each point represent each species (n = 17 for each N supply); N− and N+ treatments as indicated. Pearson's correlation coefficients between all variables (rtotal) and with each of the fertilization treatments are given (rN− and rN+).
Among the chemical traits of litter, HEMlitter and LCHlitter (marginally) were most closely negatively related to the decay decomposition rate (Table 4 and Fig. 2E, F). These relationships were conserved at both N levels. Leaf structural traits (LDMCgreen, LTSgreen and LRFgreen) were closely correlated all together (Table 4).
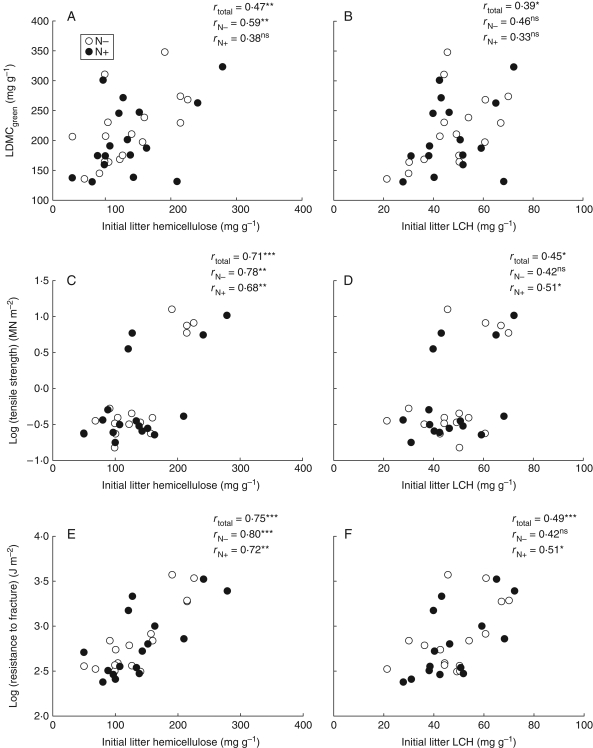
Traits of living leaves are related well to litter quality. Strong positive correlations were found between LDMCgreen and HEMlitter or LCHlitter (Fig. 3A, B). The same positive correlation was found between LTSgreen and LRFgreen, and HEMlitter and LCHlitter (Fig. 3C–F) with the Poaceae species having a determinant role in the relationship between LTSgreen and litter traits.
Fig. 3.
Relationships between initial litter chemistry (hemicelluloses and non-labile compounds LCH) and (A, B) leaf dry matter content (LDMCgreen), (C, D) tensile strength and (E, F) resistance to fracture of green leaves; N− and N+ treatments as indicated. Pearson's correlation coefficients between all variables (rtotal) and with each of the fertilization treatment are given (rN− and rN+).
DISCUSSION
Leaf traits, litter quality, litter decomposability and successional stage
The first objective of the present study was to test whether successional changes in litter decomposability and associated traits were also apparent when species were grown under identical environmental conditions. Our common garden experiment demonstrated that species typical of later successional stages had tougher leaves (higher LDMCgreen, LTSgreen and LRFgreen), producing litter that decomposed more slowly than species from earlier successional stages. The patterns observed here were consistent with studies conducted on individuals growing in the field (Kazakou et al., 2006) and also on ‘average’ community litters from the same old-field succession (Garnier et al., 2004; Cortez et al., 2007), and in successional seres from other sites in Europe (Quested et al., 2007; Fortunel et al., 2009). These studies demonstrated that litter from early successional stages or regularly disturbed sites tended to decompose more rapidly than litter from later stages or more stable sites. When we compared the Kpot of species grown in the common garden experiment (present study) with those grown in situ (Kazakou et al., 2006), a strong positive relationship was found (Pearson's r = 0·91; P < 0·001 for seven species common in both studies). Combining these results with the finding of the present study, we can advocate that the differences in litter decay observed during succession could be attributed to the differences in species composition rather than to changes in environmental conditions. Moreover, when a common litter was incubated for decomposition in the three successional stages no significant effect of the successional stage was found (data not shown). Based on these conclusions, we suggest that the patterns of litter decay rate observed along our successional gradient are robust enough to scale up from species to community level, in spite of potential mixing effects in the plurispecific litters when decomposition is assessed at the community level (see Hättenschwiler et al., 2005).
Does nitrogen supply affect leaf traits and litter decomposability?
Despite the recognition that N availability is an important factor controlling decomposition (Swift et al., 1979), the relative effects of exogenous (N in the environment, external to the decaying material) vs. endogenous N (N concentration of the litter itself) on decomposition remain unclear (Bridgham and Richardson, 2003). Several studies have reported significantly faster decay rates in response to increased external N availability (Hunt et al., 1988; Hobbie, 2000). Many others have reported either no significant change (Pastor et al., 1987; Hunt et al., 1988; Prescott, 1995; Bryant et al., 1998; Carreiro et al., 2000; Hobbie and Vitousek, 2000) or a decrease in decay rates (Magill and Aber, 1998; Carreiro et al., 2000).
In the present study, we tested whether species grown on nutrient-rich soils produced leaves and litters that decomposed faster than species grown on nutrient-poor soils. Our results demonstrated that N addition did result in changes of some leaf and litter traits. However, litter decomposability was not affected by these changes in leaf and litter traits. Even if some structural and chemical traits were modified by nitrogen addition, the responses of leaf traits to increased N supply were not strong enough to affect litter decomposability.
Our study therefore provides clear evidence that greater N availability does not result in more rapid litter decomposition for the studied species. These patterns are in agreement with the results of Knorr et al. (2005), Berg et al. (1993), Edmonds (1980) and Prescott (1995) who found that even if litter from fertilized trees contained twice as much N as litter from control trees they decomposed at the same rate.
Relationships between leaf traits and litter decomposability
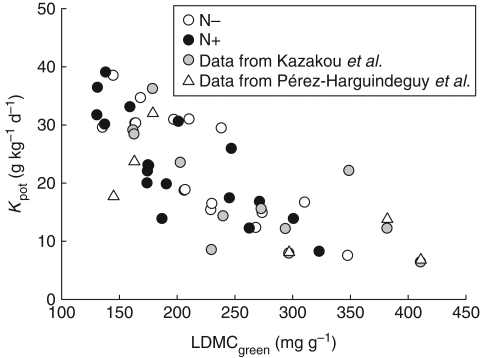
Our second aim was to assess which trait of living leaves best relates to litter quality and litter decomposability. LDMCgreen, LTSgreen and LRFgreen were negatively correlated with litter Kpot (Fig. 2A, C, D). Comparable results were found in species from Argentina and Great Britain for LTSgreen (Cornelissen et al., 1999; Pérez-Harguindeguy et al., 2000) and in the same set of species measured in situ for the LDMCgreen (Kazakou et al., 2006). Our results support the hypothesis of ‘afterlife’ effects of functional traits of living leaves on decomposability (Cornelissen and Thompson, 1997; Wardle et al., 1998; Pérez-Harguindeguy et al., 2000; Cornelissen et al., 2004) for plants grown under identical environmental conditions. Relationships between Kpot and leaf structural traits (LDMCgreen, LTSgreen and LRFgreen) were found under both N supplies, which means that these patterns are independent of variations in soil N availability. In addition, when data from the present study are combined with experiments conducted when species were grown in situ and decomposed in microcosms (Kazakou et al., 2006) or litter beds (N. Pèrez-Harguindeguy et al., UNC-CONICET, Córdoba, Argentina, unpubl. res.), a single relationship between Kpot and LDMC is observed (Fig. 4). We may therefore conclude that the relationships between LDMCgreen and litter decomposability are independent from species responses to environmental factors.
Fig. 4.
Relationships between potential decomposition decay (Kpot) and leaf dry matter content (LDMCgreen). N− and N+ treatments (this study, rN− = −0·81, P < 0·001, rN+ = −0·78, P < 0·001); data from Kazakou et al. (2006, rKaz. = −0·75, P < 0·001), and data from N. Pérez-Harguindeguy et al. (UNC-CONICET, Córdoba, Argentina, unpubl. res.; rP-H. = −0·74, P = 0·04*): rtotal = −0·76***.
The positive relationship between LPCgreen and Kpot in the N+ treatment, (Fig. 2B, rN+ = 0·89, P < 0·001) can result from a higher P demand under high N supply as plants may have suffered from P limitation: P resorption efficiency as well as the N to P ratio were higher in the N+ treatment (see Kazakou et al., 2007). Phosphorus control over decomposition has been reported in regions where P availability is low compared with N availability (Aerts, 1997; Hoorens et al., 2003; Vivanco and Austin, 2006).
Concerning the relationships between traits of living leaves and litter traits, LDMCgreen was positively related to HEMlitter which was the trait of litter best correlated to Kpot (Fig. 3A). Strong correlations were also found between leaf structure-related traits and the LIGlitter, CELlitter, HEMlitter and non-labile compounds. Support for this interpretation is available from independent studies (Choong et al., 1992; Wright and Illius, 1995; Cornelissen et al., 1999), confirming that high concentrations of lignin and other carbon-rich compounds, particularly when invested in fibres, strengthen living leaves considerably.
In conclusion, among the traits quantified here, LDMCgreen stood out as being best related to litter quality and thus Kpot. LDMCgreen reflects the amount of mesophyll vs. structural compounds in a leaf (Garnier and Laurent, 1994; van Arendonk and Poorter, 1994). A high LDMCgreen corresponds to a high proportion of vascular tissues and sclerenchyma (dense tissues) (Dijkstra and Lambers, 1989; Garnier and Laurent, 1994). In terms of chemical composition, this corresponds to leaves rich in (hemi)cellulose, insoluble sugars and lignin (Poorter and Bergkotte, 1992). In our study, high values of LDMCgreen (dense leaf tissues) in leaf result in initial litter rich in HEMlitter with a slow decomposition rate. Moreover, LDMCgreen is an easily measurable trait, much less variable and the best single variable for locating plant species on a resource acquisition–conservation axis (Wilson et al., 1999): species with high LDMCgreen tend to conserve resources more efficiently in resource-poor environments and have lower growth rates than species with low LDMCgreen (Poorter and Bergotte, 1992; Ryser and Aeschlimann, 1999). Additionally, the strong connection between LDMCgreen and litter decomposition was also confirmed in studies measuring litter decomposition at the community level (Garnier et al., 2004; Cortez et al., 2007; Quested et al., 2007; Quetier et al., 2007; Fortunel et al., 2009).
Conclusions
Our results provide evidence that leaf traits and litter decomposability vary according to species successional stage. Nitrogen fertilization affected some leaf and litter traits but these changes were not translated into changes in their decomposability. Leaf dry matter content appears as the single trait of green leaves best related to decomposability, closely related to initial litter quality. This demonstrates the strength of a trait-based approach to predict changes in ecosystem processes as a result of species shifts in ecosystems.
ACKNOWLEDGEMENTS
E.K. was financially supported by the EU project VISTA (Vulnerability of Ecosystem Services to Land Use Change in Traditional Agricultural Landscapes) (contract no. EVK2-2001-15 000356). We thank R. Joffre for the NIRS analysis, G. Laurent, C. Collin, D. Landais and D. Degueldre for help in the field, and B. Buatois for elemental analysis. T. Handa provided helpful comments on various drafts of the manuscript. This is a publication from the GDR 2574 ‘Utiliterres’ (CNRS, France).
APPENDIX 1
Results of three-way ANOVA (F-values and probabilities) on the effects of species, successional stage and N supply for the 17 species on each measured variable
| Parameter | Species (d.f. = 14) | Successional stage (d.f. = 2) | N supply (d.f. = 1) |
|---|---|---|---|
| SLAgreen | 23·9*** | 212***abc | 12·5** |
| LDMCgreen | 48·7*** | 117***bba | 11·6** |
| LTSgreen | 33·3*** | 17·2***bba | 3·48ns |
| LRFgreen | 37·6*** | 17·1***bba | 1·76ns |
| LNCgreen | 1·86ns | 2·16ns | 47·27*** |
| LPCgreen | 3·02* | 14·9**bac | 1·24ns |
| LCCgreen | 4·05** | 1·21ns | 12·5** |
| LNClitter | 3·12* | 15·5**abc | 19·6** |
| LPClitter | 2·21ns | 10·7**aab | 10·6** |
| LCClitter | 10·5*** | 3·36ns | 7·11* |
| LIGlitter | 1·13ns | 0·99ns | 0·75ns |
| CELlitter | 1·66ns | 0·70ns | 0·16ns |
| HEMlitter | 2·20ns | 1·38ns | 0·43ns |
| LCHlitter | 1·06ns | 0·35ns | 0·29ns |
| HLQlitter | 3·08* | 1·83ns | 0·88ns |
| Kpot | 11·0*** | 25·4***aab | 0·05ns |
Superscript letters indicate the results of post hoc tests (Student–Newman–Keuls comparisons) with successional stage as factor (results were identical for each nitrogen supply).
* P < 0·05; ** P < 0·01; *** P < 0·001; ns, not significant.
APPENDIX 2
Results of two-way ANOVA (F values and probabilities) on the effects of successional stage, taxonomic groups and their interaction for the 12 species (Table 1)
| Variable | Successional stage | Taxonomic group | Suc. Stage × Phylo. group |
|---|---|---|---|
| SLAgreen | 173*** | 63·2*** | 20·1*** |
| LDMCgreen | 77·7*** | 179*** | 40·5*** |
| LTSgreen | ns | 131*** | ns |
| LRFgreen | 10·7*** | 180*** | 18·7*** |
| LNCgreen | ns | 6·80** | ns |
| LPCgreen | 17·7*** | 8·55*** | 6·47*** |
| LCCgreen | 3·79* | ns | ns |
| LNClitter | 9·66** | 8·81*** | 2·94* |
| LPClitter | 8·35** | 3·92* | 2·24* |
| LCClitter | ns | 15·9*** | 16·72*** |
| LIGlitter | ns | ns | ns |
| HEMlitter | ns | 7·15* | ns |
| CELlitter | ns | 10·1** | ns |
| Kpot | 6·58* | 19·2*** | 3·55* |
LITERATURE CITED
- Aerts R. Climate, leaf litter chemistry, and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos. 1997;79:439–449. [Google Scholar]
- Berg B, Berg MP, Bottner P, et al. Litter mass loss rates in pine forests of Europe and eastern United States: some relationships with climate and litter quality. Biogeochemistry. 1993;20:127–159. [Google Scholar]
- Bridgham SD, Richardson CJ. Endogenous versus exogenous nutrient control over decomposition and mineralization in North Carolina peatlands. Biogeochemistry. 2003;65:151–178. [Google Scholar]
- Bryant DM, Holland EA, Seastedt TR, Walker MD. Analysis of litter decomposition in an alpine tundra. Canadian Journal of Botany. 1998;76:1295–1304. [Google Scholar]
- Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF. Microbial enzyme shifts explain litter decay responses to simulated N deposition. Ecology. 2000;81:2359–2365. [Google Scholar]
- Chapin FS. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics. 1980;11:233–260. [Google Scholar]
- Chapin FS, III, McKendrick JD, Johnson DA. Seasonal changes in Alaskan tundra plants differing in growth form: implications for herbivores. Journal of Ecology. 1986;74:707–731. [Google Scholar]
- Chapin FS, III, Matson PA, Mooney HA. Principles of terrestrial ecosystem ecology. New York: Springer-Verlag; 2002. [Google Scholar]
- Choong MF, Lucas PW, Ong JSY, Pereira B, Tan HTW, Turner IM. Leaf fracture toughness and sclerophylly – their correlations and ecological implications. New Phytologist. 1992;121:597–610. [Google Scholar]
- Cornelissen JHC. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. Journal of Ecology. 1996;84:573–582. [Google Scholar]
- Cornelissen JHC, Thompson K. Functional leaf attributes predict litter decomposition rate in herbaceous plants. New Phytologist. 1997;135:109–114. doi: 10.1046/j.1469-8137.1997.00628.x. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, Pèrez-Harguindeguy N, Diaz S, et al. Leaf structure and defense control litter decomposition rate across species and life forms in regional floras on two continents. New Phytologist. 1999;143:191–200. [Google Scholar]
- Cornilessen JHC, Lavorel S, Garnier E, et al. A handbook of protocols for standardised and easy measurements of plant functional traits wordwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Cornelissen JHC, Quested HM, Gwynn-Jones D, et al. Leaf digestibility and litter decomposability are related in a wide range of subarctic plant species and types. Functional Ecology. 2004;18:779–786. [Google Scholar]
- Cornwell WK, Cornelissen JHC, Amatangelo K, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters. 2008;11:1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- Cortez J, Garnier E, Pérez-Harguindeguy N, Debussche M, Gillon D. Plant traits, litter quality and decomposition in a Mediterranean old-field succession. Plant and Soil. 2007;296:19–34. [Google Scholar]
- Coûteaux MM, Bottner P, Berg B. Litter decomposition, climate and litter quality. Trends in Ecology and Evolution. 1995;10:63–66. doi: 10.1016/S0169-5347(00)88978-8. [DOI] [PubMed] [Google Scholar]
- Coulson JC, Butterfield J. An investigation of the biotic factors determining the rates of decomposition on blanket bog. Journal of Ecology. 1978;66:631–650. [Google Scholar]
- Dijkstra P, Lambers H. Analysis of specific leaf area and photosynthesis of two inbred lines of Plantago major differing in relative growth rate. New Phytologist. 1989;113:283–290. doi: 10.1111/j.1469-8137.1989.tb02405.x. [DOI] [PubMed] [Google Scholar]
- Edmonds RL. Litter decomposition and nutrient release in Douglas fir, Red Alder, Western Hemlock and Pacific silver fir ecosystems in western Washington. Canadian Journal of Forest Research. 1980;10:327–337. [Google Scholar]
- Fortunel C, Garnier E, Joffre R, et al. Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology. 2009;90:598–611. doi: 10.1890/08-0418.1. [DOI] [PubMed] [Google Scholar]
- Gallardo A, Merino J. Leaf decomposition in two Mediterranean ecosystems of southern Spain: influence of substrate quality. Ecology. 1993;74:152–161. [Google Scholar]
- Garnier E, Laurent G. Leaf anatomy, specific mass and water content in congenetic annual and perennial grass species. New Phytologist. 1994;128:725–736. [Google Scholar]
- Garnier E, Cortez J, Billes G, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85:2630–2637. [Google Scholar]
- Garnier E, Lavorel S, Ansquer P, et al. Assessing the effects of land use change on plant traits, communities and ecosystem functioning in grasslands: a standardized methodology and lessons from an application to 11 European sites. Annals of Botany. 2007;99:967–985. doi: 10.1093/aob/mcl215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon D, Joffre R, Ibrahima A. Initial litter properties and decay-rate – a microcosm experiment on Mediterranean species. Canadian Journal of Botany-Revue Canadienne de Botanique. 1994;72:946–954. [Google Scholar]
- Grimshaw HM, Allen SE, Parkinson JA. Nutrient elements. In: Allen SE, editor. Chemical analysis of ecological material. Oxford: Blackwell Scientific; 1989. pp. 81–159. [Google Scholar]
- Güsewell S, Verhoeven JTA. Nutrient limitation of leaf litter decomposability in wetland graminoids. Plant and Soil. 2006;287:131–143. [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution & Systematatics. 2007;8:157–178. [Google Scholar]
- Hättenschwiler S, Tiunov AV, Scheu S. Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Ecology, Evolution, and Systematics. 2005;36:191–218. [Google Scholar]
- Hendry GA, Grime JP. Methods in comparative plant ecology. London: Chapman & Hall; 1993. [Google Scholar]
- Hobbie SE. Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems. 2000;3:484–494. [Google Scholar]
- Hobbie SE, Vitousek PM. Nutrient limitation of decomposition in Hawaiian forests. Ecology. 2000;81:1867–1877. [Google Scholar]
- Hoorens B, Aerts R, Stroetenga M. Does initial litter chemistry explain litter mixtures effects on decomposition? Oecologia. 2003;442:578–586. doi: 10.1007/s00442-003-1365-6. [DOI] [PubMed] [Google Scholar]
- Hunt HW, Ingham ER, Coleman DC, Elliott ET, Reid CPP. Nitrogen limitation of production and decomposition in prairie, mountain meadow, and pine forest. Ecology. 1988;69:1009–1016. [Google Scholar]
- Joffre R, Gillon D, Dardenne P, Agneessens R, Biston R. The use of near-infrared reflectance spectroscopy in litter decomposition studies. Annales des Sciences Forestières. 1992;49:481–488. [Google Scholar]
- Kazakou E, Navas ML. Variation in intensity of competition along a Mediterranean successional gradient. In: Arianoutsou M, Papanastasis VP, editors. Ecology, Conservation and Management of Mediterranean Climate Ecosystems; Proceedings of the X MEDECOS Conference; 26–30 April 2004; Rhodos, Greece. Rotterdam, The Netherlands: Millpress; 2004. [Google Scholar]
- Kazakou E, Vile D, Shipley B, Gallet C, Garnier E. Co-variations in litter decomposition, leaf traits and plant growth in species from a Mediterranean old-field succession. Functional Ecology. 2006;20:21–30. [Google Scholar]
- Kazakou E, Garnier E, Navas ML, Roumet C, Collin C, Laurent G. Components of nutrient residence time and the leaf economics spectrum in species from Mediterranean old-fields differing in successional status. Functional Ecology. 2007;21:235–245. [Google Scholar]
- Knorr M, Frey SD, Curtis PS. Nitrogen additions and litter decomposition: a meta-analysis. Ecology. 2005;86:3252–3257. [Google Scholar]
- Lavelle P, Blanchart E, Martin A, et al. A hierarchical model for decomposition in terrestrial ecosystems – application to soils of the humid tropics. Biotropica. 1993;25:130–150. [Google Scholar]
- Lemaire G, Gastal F. N uptake and distribution in plant canopies. In: Lemaire G, editor. Diagnosis of the nitrogen status in crops. Berlin: Springer-Verlag; 1997. pp. 3–44. [Google Scholar]
- Magill AH, Aber JD. Long-term effects of experimental nitrogen additions on foliar litter decay and humus formation in forest ecosystems. Plant and Soil. 1998;203:301–311. [Google Scholar]
- McClaugherty C, Berg B. Cellulose, lignin and nitrogen concentrations as rate regulating factors in late stages of forest litter decomposition. Pedobiologia. 1987;30:101–112. [Google Scholar]
- Northrup RR, Yu Z, Dahlgren RA, Vogt KA. Polyphenol control of nitrogen release from pine litter. Nature. 1995;377:227–229. [Google Scholar]
- Olson JS. Energy storage and the balance of producters and the decomposers in ecological systems. Ecology. 1963;14:322–331. [Google Scholar]
- Pastor J, Stillwell MA, Tilman D. Little bluestem litter dynamics in Minnesota old fields. Oecologia. 1987;72:327–330. doi: 10.1007/BF00377559. [DOI] [PubMed] [Google Scholar]
- Pèrez-Harguindeguy N, Diaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A. Chemistry and toughness predict litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant and Soil. 2000;218:21–30. [Google Scholar]
- Poorter H, Bergkotte M. Chemical composition of 24 wild species differing in relative growth rate. Plant, Cell and Environment. 1992;15:221–229. [Google Scholar]
- Prescott CE. Does N availability control rates of litter decomposition in forests? Plant and Soil. 1995;168–169:83–88. [Google Scholar]
- Quested H, Eriksson O, Fortunel C, Garnier E. Plant traits relate to whole community litter quality and decomposition following land use change. Functional Ecology. 2007;21:1003–1183. [Google Scholar]
- Quétier F, Thébault A, Lavorel S. Plant traits in a state and transition framework as markers of ecosystem response to land use change. Ecological Monographs. 2007;77:33–52. [Google Scholar]
- Ryser P, Aeschlimann U. Proportional dry-mass content as an underlying trait for the variation in relative growth rate among 22 Eurasian populations of Dactylis glomerata. Functional Ecology. 1999;13:473–482. [Google Scholar]
- Shipley B, Vu T. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytologist. 2002;153:359–364. [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd edn. San Francisco: W.H. Freeman and Company; 1995. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW. Angiosperm phylogeny inferred from: 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:381–461. [Google Scholar]
- Swift MJ, Heal OW, Anderson JM. Decomposition in terrestrial ecosystems. Oxford: Blackwell Scientific; 1979. [Google Scholar]
- Taylor B, Parkinson D. A new microcosm approach to litter decomposition studies. Canadian Journal of Botany. 1988;66:1933–1939. [Google Scholar]
- Taylor BR, Parkinson D, Parsons WF. Nitrogen and lignin content as predictor of litter decay rates: a microcosm test. Ecology. 1989;70:97–104. [Google Scholar]
- Van Arendonk J, Poorter H. The chemical composition and anatomical structure of leaves of grass species differing in relative growth rate. Plant, Cell and Environment. 1994;17:963–970. [Google Scholar]
- Vile D, Shipley B, Garnier E. Ecosystem productivity can be predicted from potential relative growth rate and species abundance. Ecology Letters. 2006;9:1061–1067. doi: 10.1111/j.1461-0248.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- Vivanco L, Austin AT. Intrinsic effects of species on leaf litter and root decomposition: a comparison of temperate grasses from North and South America. Oecologia. 2006;150:97–107. doi: 10.1007/s00442-006-0495-z. [DOI] [PubMed] [Google Scholar]
- Wardle DA, Barker GM, Bonner KI, Nicholson KS. Can comparative approaches based on plant ecophysiological traits predict the nature of biotic interactions and individual plant species effects in ecosystems? Journal of Ecology. 1998;86:405–420. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist. 1999;143:155–162. [Google Scholar]
- Witkowski ETF, Lamont BB. Leaf specific mass confounds leaf density and thickness. Oecologia. 1991;88:486–493. doi: 10.1007/BF00317710. [DOI] [PubMed] [Google Scholar]
- Wright W, Illius AW. A comparative-study of the fracture properties of grasses. Functional Ecology. 1995;9:269–278. [Google Scholar]
- Wright IJ, Cannon K. Relationships between leaf lifespan and structural defences in a low nutrient, sclerophyll flora. Functional Ecology. 2001;15:351–359. [Google Scholar]