Abstract
Background and Aims
Leaf venation in many C4 species is characterized by high vein density, essential in facilitating rapid intercellular diffusion of C4 photosynthetic metabolites between different tissues (mesophyll, bundle sheath). Greater vein density has been hypothesized to be an early step in C4 photosynthesis evolution. Development of C4 vein patterning is thought to occur from either accelerated or prolonged procambium formation, relative to ground tissue development.
Methods
Cleared and sectioned tissues of phylogenetically basal C3 Flaveria robusta and more derived C4 Flaveria bidentis were compared for vein pattern in mature leaves and vein pattern formation in developing leaves.
Key Results
In mature leaves, major vein density did not differ between C3 and C4 Flaveria species, whereas minor veins were denser in C4 species than in C3 species. The developmental study showed that both major and minor vein patterning in leaves of C3 and C4 species were initiated at comparable stages (based on leaf length). An additional vein order in the C4 species was observed during initiation of the higher order minor veins compared with the C3 species. In the two species, expansion of bundle sheath and mesophyll cells occurred after vein pattern was complete and xylem differentiation was continuous in minor veins. In addition, mesophyll cells ceased dividing sooner and enlarged less in C4 species than in C3 species.
Conclusions
Leaf vein pattern characteristic to C4 Flaveria was achieved primarily through accelerated and earlier offset of higher order vein formation, rather than other modifications in the timing of vein pattern formation, as compared with C3 species. Earlier cessation of mesophyll cell division and reduced expansion also contributed to greater vein density in the C4 species. The relatively late expansion of bundle sheath and mesophyll cells shows that vein patterning precedes ground tissue development in C4 species.
Key words: Bundle sheath, C4 photosynthesis evolution, Flaveria, heterochrony, leaf development, mesophyll, vein density, vein pattern formation
INTRODUCTION
The majority of terrestrial C4 angiosperms are characterized by distinctive anatomical features that function to separate the carbon assimilation and reduction functions of photosynthesis. These features are collectively termed Kranz anatomy, and include specialized photosynthetic tissues, distinct tissue arrangement and close vein spacing (Dengler and Nelson, 1999; Dengler and Taylor, 2000; Muhaidat et al., 2007). In combination, these aspects of Kranz anatomy facilitate rapid cycling of C4 metabolites to concentrate CO2 near the site of Rubisco, thereby suppressing photorespiration and operating photosynthesis at a maximal rate. Anatomically distinct photosynthetic tissues mesophyll (M) and bundle sheath (BS) physically separate different biochemical processes of the C4 photosynthetic pathway and the ratio of these tissues must be low (near 1 : 1 in some species) to enable rapid intercellular diffusion of C4 metabolites (Dengler and Nelson, 1999). Vein patterning in many C4 species achieves this low ratio of photosynthetic tissues by increasing the relative volume of vein-associated BS and lowering the relative volume of M through greater vein density (Hattersley, 1984; Dengler et al., 1994; Dengler and Taylor, 2000; McKown and Dengler, 2007).
The vascular system is an integral and critical component of leaf structure and physiology, functioning both in transport and in mechanical support (Esau, 1977). Vascular tissue is composed of two conductive tissues (xylem and phloem), forms a structural reticulum within the plane of the leaf, and maintains a close spatial and functional relationship with the photosynthetic tissues. Leaf venation in advanced eudicotyledonous plants is a continuous branching pattern, often with a hierarchy of vein sizes (Esau, 1977; Hickey, 1979). In bifacial leaves, the largest veins are major veins (1st and 2nd size orders, abbreviated to 1°, 2°, etc. hereafter) and are responsible for long-distance bulk flow and mechanical reinforcement of the leaf (Esau, 1977; Hickey, 1979; Roth-Nebelsick et al., 2001; Sack and Holbrook, 2006; A. D. McKown et al., unpubl. res.). Minor veins (3° size order and higher) are smaller in diameter than 1° and 2° veins, and form areoles or end freely within ground tissue. The minor veins also comprise the bulk of the total vein length within the leaf (Sack and Holbrook, 2006). Vein orders of major and minor veins are generally identifiable by patterning within the leaf and vein diameter at the point of branching where veins tend to be thickest (Hickey, 1979; Leaf Architecture Working Group, 1999).
The formation of leaf venation from ground meristem occurs early in leaf ontogeny and simultaneously with leaf expansion (Esau, 1977; reviewed in Nelson and Dengler, 1997). Vein formation has been well documented in studies of leaf development in Arabidopsis using molecular markers (Kang and Dengler, 2002, 2004; Mattsson et al., 2003; Scarpella et al., 2004, 2006; Sieburth and Deyholos, 2006; Kang et al., 2007; Sawchuk et al., 2007; Wenzel et al., 2007; Rolland-Lagan et al., 2009). These studies trace early procambial formation in both juvenile- and adult-phase leaves. In Arabidopsis, veins of all orders are initiated through a common mechanism involving polar auxin transport, which becomes channelled in certain files of ground meristem cells through an increase in auxin efflux carriers within the cell (Scarpella et al., 2006; Berleth et al., 2007). These cells subsequently acquire characteristics diagnostic for procambial cells, such as an elongated cell shape and dense cytoplasm (Esau, 1977; Scarpella et al., 2006).
Studies of vein pattern development show the discrete timing, placement and development of different vein orders (reviewed in Nelson and Dengler, 1997; Scarpella et al., 2006; Kang et al., 2007; Sawchuk et al., 2007; Wenzel et al., 2007). Vein orders are temporally separated by different initiation times during leaf expansion, and are spatially separated by developing in distinct locations within the leaf. In many eudicots, procambium of the mid- or central vein (1° order) is first morphologically recognizable in the leaf primordium in continuity with the stem vasculature (Nelson and Dengler, 1997; Sawchuk et al., 2007; Bayer et al., 2009). The 2° veins are formed in continuity with the 1° vein and appear sequentially in a basipetal direction. Minor vein orders are established later within the developing leaf, form in continuity with previously formed procambium, and appear in a basipetal direction as a reiterating pattern between major veins. In most species, vein pattern development ends with a final stage of freely ending veinlet (FEV) formation. In addition to differences in temporal and spatial formation, vein orders differ in anatomical composition and establish recognizable size classes through different maturation periods. In Arabidopsis, cell cycling and vein enlargement are evident in the major veins after cell cycling has ceased in minor veins, and result in measurable size differences between 1°, 2° and 3° veins (Kang and Dengler, 2002; Kang et al., 2007).
Vein pattern development has been well described in many C3 species; however, despite its importance in C4 function, this has only been examined in a few C4 grasses (Nelson and Dengler, 1992; Dengler et al., 1997; Sud and Dengler, 2000). The greater vein density observed in C4 species compared with C3 species probably involves modifications to minor vein patterning rather than major veins (Ueno et al., 2006; McKown and Dengler, 2007; but see Muhaidat et al., 2007). Development of C4 vein patterning could result solely from limited expansion of tissue between veins; however, changes in the number of veins formed in C4 plants are more likely, despite the higher energetic ‘cost’ of producing more lignin (Sage and McKown, 2006).
A greater number of veins would require an alteration to vein pattern development in the C4 leaf (descendant condition) compared with a C3 leaf (ancestral condition). As vein formation is a continuous and progressive process (see Kang et al., 2007; Sawchuk et al., 2007), this could involve a shift in the timing of vein formation, or the period in which cells of the ground meristem in the developing leaf maintain competency to perceive a vascular-forming signal (e.g. auxin) and form procambial strands (Dengler and Taylor, 2000). In comparisons of C3 and C4 plants, the C4 descendant condition shows ‘greater’ morphological change through higher leaf vein density than the C3 ancestral condition. Rudall and Bateman (2004) outline that potential developmental timing shifts (heterochrony), resulting in plants in which the descendant condition shows greater morphological change, may involve an early or precocious onset of growth (pre-displacement), a delayed offset of growth (hypermorphosis) or an increased rate of growth (acceleration). Thus, increasing vein density in leaves of C4 plants would result from a heterochronic shift in vein formation in relation to ground tissue development compared with a C3 ancestor through: (1) early onset of procambium formation, (2) prolongation of procambium formation by late offset or (3) increased or accelerated rate of procambium formation.
Close vein spacing in leaves is speculated to be a critical step in the evolution of the C4 pathway in many C4 angiosperms (Sinha and Kellogg, 1996); however, this has only been demonstrated in the eudicot genus Flaveria (McKown and Dengler, 2007). Phylogenetic and anatomical studies of Flaveria provide an appropriate platform for an examination of leaf vein pattern development between related C3 and C4 species within an evolutionary context (McKown et al., 2005; McKown and Dengler, 2007; Kocacinar et al., 2008). In the genus Flaveria, species are classified as having C3 photosynthesis, C4 photosynthesis (NADP–ME type) or intermediate photosynthesis (C3–C4 and C4-like); however, the ancestral condition is unequivocally C3 photosynthesis, and both intermediate and C4 photosynthesis are derived conditions (McKown et al., 2005). Comparisons of C3, intermediate and C4 Flaveria species suggest that close vein spacing evolved earlier than the specialization of M and BS tissues and is an important component in the evolution of C4 photosynthesis (McKown and Dengler, 2007). The present study compares major and minor vein patterning of the derived species C4 F. bidentis with C3 F. robusta (representing an approximation to the ancestral C3 condition). In addition, the development of vein pattern and ground tissue are followed in adult-phase leaves from these C3 and C4 Flaveria species to elucidate the mechanisms responsible for increased vein density in C4 Flaveria species.
MATERIALS AND METHODS
Plant material and growth analysis
C3 Flaveria robusta Rose and C4 Flaveria bidentis (L.) Kuntze were studied for mature venation characteristics and development of vein pattern. The two species were positively identified by morphology and gene marker analysis (cptrnL–F and nrITS) from representative plants (following McKown et al., 2005). Leaf material for comparison of mature vein patterning was obtained from plants grown either from seeds or from cuttings, and cultivated in an open-air rooftop setting at the University of Toronto (F. Kocacinar, pers. comm.). Comparative experiments following vein pattern development in C3 F. robusta and C4 F. bidentis used plants grown in a growth chamber, or in the University of Toronto greenhouse during summer months. Plants were grown from seed obtained from the University of Toronto Flaveria research collection (R. Sage).
Seed for plants used in the developmental portion of the study were surface sterilized in 10 % bleach solution for 10 min with gentle agitation, rinsed well with distilled water and planted in trays with SunGro Sunshine Mix soil (SunGro Horticulture, Vancouver, BC, Canada). Trays were placed in a high-light growth chamber (Model PGR15, Conviron, Winnipeg, MB, Canada) and seeds were germinated in 12 h of light [500 µmol m−2 s−1 photosynthetic photon flux density (PPFD)] at 24 °C. Seedlings were planted in small pots following the establishment of the first two leaf pairs and transplanted seedlings were grown for a further week in the same light and temperature conditions before commencement of each experiment. Replicate plants were grown in individual pots, watered every 48 h, fertilized every 2 weeks and rotated throughout the duration of each experiment. Plants in the growth chamber were exposed to 16 h of full light (500 µmol m−2 s−1 PPFD) at 24 °C and 8 h of darkness at 20 °C.
All Flaveria species have opposite and decussate phyllotaxis, and the appearance and expansion of each leaf pair was recorded to track the growth of each individual plant. The first two leaf pairs produced in young plants of Flaveria (leaf pairs 1 and 2) are collectively referred to as juvenile-phase leaves, as these do not achieve the same size as following leaf pairs. All other leaves formed after (e.g. leaf pairs 3 and higher) are referred to as adult-phase leaves. Leaf growth curves based on leaf length were constructed for the adult-phase leaves of each plant to determine the typical development of each species, compare plant growth between experiments and assess the suitability of using the leaf plastochron index (LPI). LPI is a numerical indicator of morphological leaf development, and has been a useful method for comparing the growth and development of leaves between different plants, species or experiments. Criteria for use of LPI are: (1) constant leaf production rate, (2) similar growth rate of leaves and (3) early exponential growth between initiation of leaves (Erickson and Michelini, 1957). LPI for each leaf of interest (the nth leaf) is related to the length of a leaf (e.g. n + 1) that is equal or just above a reference length:
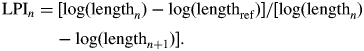 |
In this study, LPI was calculated for adult-phase leaves in the two Flaveria species examined using a reference length of 10 mm. If the nth leaf was under 10 mm or the ‘n + 1’ leaf was non-existent, the LPI was calculated in reference to the leaf below (e.g. n − 1):
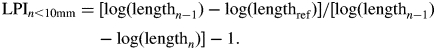 |
Mature vein pattern analysis
Mature leaves from rooftop-grown replicate plants were harvested and fixed in 70 % FAA (formaldehyde–acetic acid–alcohol). Leaves were initially cleared in 2·5 % aqueous NaOH and opaque leaves were rinsed briefly in distilled H2O for several minutes, bleached in 10 % diluted bleach (v/v) for 30 s, and allowed to clear completely in distilled H2O. Leaves were then brought to 100 % EtOH in a dilution series, stained for several minutes with 1 % safranin (1 g per 100 mL EtOH), briefly rinsed with alcohol, counterstained with 1 % fast green (1 g per 100 mL EtOH) and rinsed with alcohol. Stained leaves were rinsed in distilled water, mounted in 100 % glycerol on transparency sheets and placed on a DuoScan T1200 flatbed scanner (AGFAphoto, Cologne, Germany). Major veins were distinguished by vein width at the base of each vein, and the pattern of each size class within the leaf. The pattern of the 1° veins was described using the classification system of Hickey (1979) and the Leaf Architecture Working Group (1999). Leaf length, width, area and length of vein orders (1°, 2°, 3° and higher orders taken together) were measured using ImageJ 1·40 (Rasband, 2007): 1° veins were measured throughout the entire leaf, and 2° veins were measured in half of the cleared leaf; 3° order and higher order veins were measured from subsampled areas in the central portion of the leaf.
Vein pattern development
The development study was based on leaf pair 5, although additional leaf materials from other adult-phase leaf pairs were also studied in the two Flaveria species. Numerous leaves from pair 5 were excised at various lengths (0·5–60 mm) representing different stages of leaf development and fixed in 70 % ethanol. Prior to embedding or clearing, the length and the presence/absence of a petiole were recorded for each leaf. Small leaves (0·5–5 mm) were embedded in Spurr's resin, serially sectioned in the medial plane and stained with toluidine blue O following McKown and Dengler (2007). All sections were observed with bright-field microscopy using a Reichert Polyvar microscope (Reichert-Jung, Vienna, Austria) or Zeiss Axiophot (Carl Zeiss MicroImaging, Thornwood, NY, USA). Images were photographed on the Reichert microscope with a Nikon DXM 1200 digital camera (Nikon Instruments, Melville, NY, USA) or with an Olympus C5050Z digital camera (Olympus Imaging America, Center Valley, PA, USA) on the Zeiss microscope. Larger leaves were divided lengthwise into quarters (not including the petiole). For leaves longer than 10 mm, small squares of leaf tissue (approx. 9–25 mm2) were excised in each leaf quarter between the central and lateral 1° veins and cleared following McKown and Dengler (2007). Clearings were observed with differential interference contrast microscopy using the Reichert microscope and photographed with a Nikon DXM 1200 digital camera.
Sections and clearings were analysed qualitatively for evidence of the formation of procambium in each vein order, or continuity of differentiated protoxylem in each vein order. Vein orders were identified by position of the vein within the leaf, and by the diameter measured at the base of the vein in a leaf clearing. Procambium was anatomically identifiable as strands of elongated cells compared with surrounding ground tissue. Leaf vein pattern was identified as a combination of veins in the procambial stage only, or veins with both procambium and differentiated xylem. In minor vein orders, FEVs were distinguished from ‘connected’ minor vein orders. Within the scope of this study, vein initiation refers to the stage at which procambium of the vein order was first morphologically recognizable within the developing leaf (usually at the apical portion of the leaf), and vein order completion refers to the stage at which the pattern of a vein order was observed throughout the entire developing leaf.
Vein densities, and ground tissue development from the mid-portion of cleared leaves of the Flaveria species were observed with differential interference contrast microscopy using a Reichert Polyvar microscope, and photographed with a Nikon DXM 1200 digital camera. All images from approx. 20 leaves of each species representing a range of developmental stages were measured for vein, branching and FEV densities using Image Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). Veins were identified as described above, and developing ground tissues were identified either as bundle sheath (BS; cells surrounding each vein) or as mesophyll (M; cells between veins with isodiametric shape in the medial plane). Average individual BS cell enlargement in relation to leaf length and vein density was determined by measuring the parallel diameter (parallel to vein axis) and perpendicular diameter (perpendicular to vein axis) of 10–20 BS cells per sample. In addition, the diameters (medial plane of the leaf) of 20 M cells from both palisade and spongy M layers were measured per sample to determine average palisade and spongy M cell growth in relation to vein density. Images of M tissues were also analysed for qualitative evidence of cell divisions based on cell-wall configuration indicating recent divisions.
Statistical analyses
Relationships between quantitative vein pattern variables in C3 and C4 Flaveria species were tested with Student's t-test using SigmaStat software (Systat Software, Richmond, CA, USA). Variables studied throughout leaf development were tested for significant correlations with Pearson product moment correlation tests or linear regressions using SigmaStat software.
RESULTS
Leaf shape and mature vein patterning
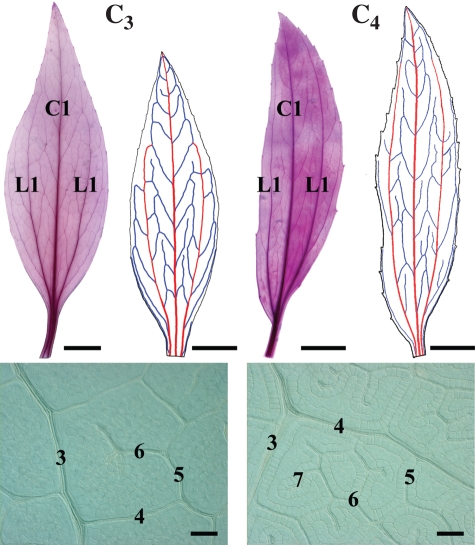
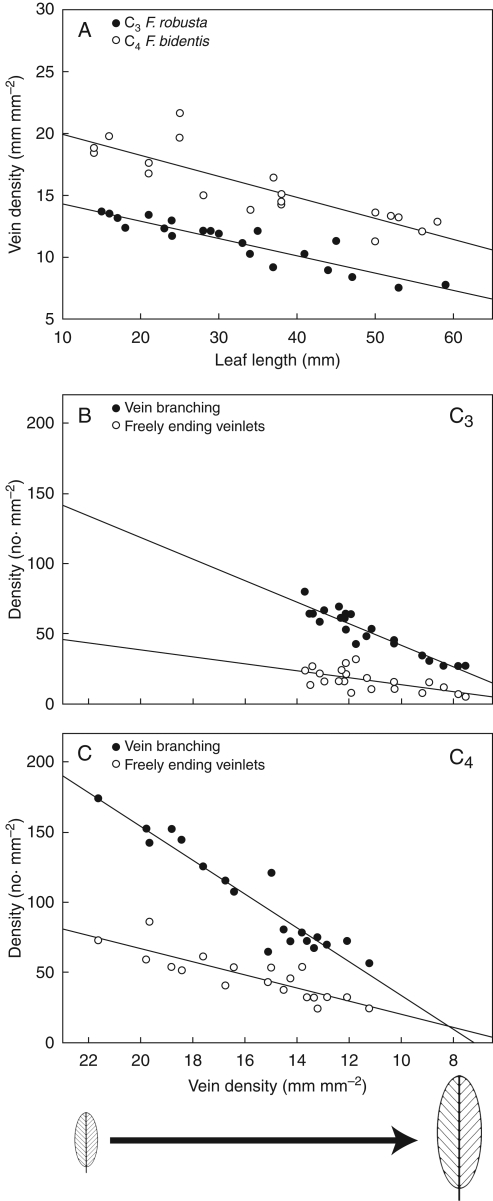
Mature leaves of C3 F. robusta and C4 F. bidentis are elliptical in shape with cuneate apices and bases, and the two species have distinct petioles (Fig. 1). Leaves of the two species have three 1° veins (a central 1° vein and two lateral 1° veins), classifying the venation as basal acrodromous. There is also an additional hierarchical order of minor veins in C4 F. bidentis (seven orders) compared with C3 F. robusta (six orders). This greater number of vein orders was also observed in C4 F. trinervia (our unpubl. res.). Mature leaves of both species have equivalent length to width dimensions and whole leaf areas (Table 1). In addition, there are no significant differences in the densities of 1° and 2° major veins or 3° minor veins between C3 F. robusta and C4 F. bidentis. By contrast, the density of higher order minor veins (4° and higher orders together), the branching of higher order veins and density of FEVs are significantly greater in C4 F. bidentis than in C3 F. robusta.
Fig. 1.
Adult leaves of C3 Flaveria robusta and C4 F. bidentis showing mature vein patterning. Cleared and stained leaves on left show overall leaf vein pattern. Traced leaf images on right show 1° veins in red (central C1 vein, lateral L1 veins), and 2° veins in blue. Higher magnifications of cleared leaves show the patterning and numbers of minor vein orders. Scale bars: whole-leaf clearings = 1 cm; higher-magnification clearings = 100 µm.
Table 1.
Mature leaf and vein pattern traits for C3 and C4 Flaveria species (mean values are given for measured traits ± s.e.)
| Leaf† and vein pattern‡ traits | C3F. robusta | C4F. bidentis |
|---|---|---|
| Leaf area (cm2)NS | 11·9 ± 1·3 | 12·8 ± 3·3 |
| Length (cm)NS | 7·76 ± 0·37 | 7·50 ± 0·49 |
| Length/widthNS | 3·49 ± 0·19 | 3·37 ± 0·41 |
| 1° vein density (cm cm−2)NS | 1·81 ± 0·19 | 1·84 ± 0·35 |
| 2° vein density (cm cm−2)NS | 7·54 ± 0·40 | 7·95 ± 0·42 |
| 3° vein density (cm cm−2)NS | 11·3 ± 0·091 | 11·1 ± 0·30 |
| Minor vein density§ (mm mm−2)* | 7·44 ± 0·48 | 10·8 ± 0·40 |
| Minor vein branching density§ (no. mm−2)* | 24·7 ± 3·5 | 44·9 ± 2·8 |
| Freely ending veinlet density (no. mm−2)** | 9·52 ± 0·78 | 24·0 ± 1·4 |
| Number of vein orders | 6 | 7 |
† n = 4 replicate leaves for whole leaf traits.
‡ n = 3 replicate leaves for venation traits.
§ 4° and higher orders.
NS, not significant; *P < 0·01; **P < 0·001.
Leaf initiation and expansion
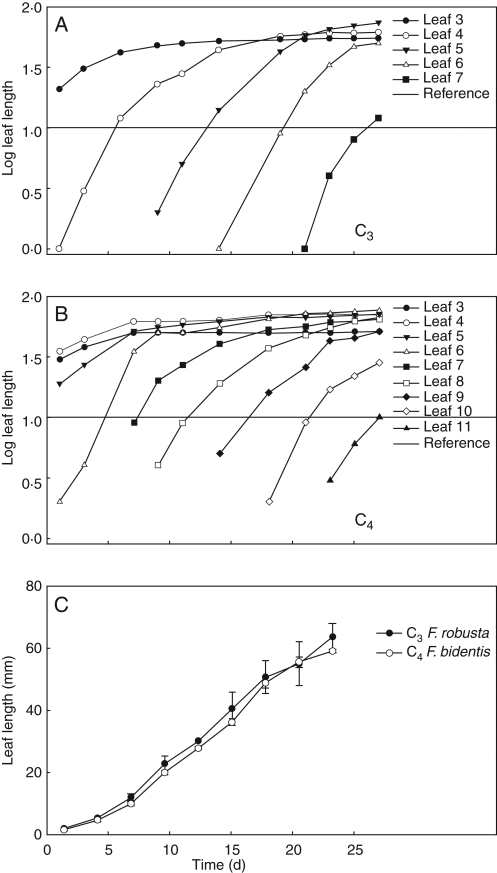
In the two species, successive adult-phase leaves are initiated at relatively constant intervals, show early exponential growth and expand at similar rates (Fig. 2). In C4 F. bidentis, leaves are initiated at a slightly faster rate over time, and the resulting plastochron length is shorter than in C3 F. robusta (Fig. 2A, B). Based on these data, use of LPI is appropriate for comparisons of leaf growth and development within each species, but shows a shift between species as the plastochron is shorter in C4 F. bidentis. A comparison of growth in adult-phase leaf 5 shows that expansion in length occurs at relatively equivalent rates between C3 F. robusta and C4 F. bidentis (Fig. 2C) and suggests that leaves of similar length may be at a similar developmental stage.
Fig. 2.
Leaf growth curves showing adult-phase leaf length change over time for representative growth chamber grown C3 and C4 Flaveria species. (A) C3 F. robusta. (B) C4 F. bidentis. (C) Growth of adult-phase leaf 5 in C3 and C4 Flaveria. Horizontal lines in (A) and (B) indicate the reference length for leaf plastochron index.
Blade expansion in leaves of both Flaveria species begins as an extension of the initially linear–lanceolate-shaped primordia in the medial plane, but leaves of C3 F. robusta do not begin to substantially expand laterally until leaves are fairly long (30 mm, LPI = 0·5). By contrast, developing leaves in C4 F. bidentis begin to expand laterally and have a broader lamina earlier in ontogeny after a few millimetres of growth (4 mm, LPI = −0·4). Adult-phase leaves in the two Flaveria species form a petiole after the leaf lamina is expanded in the distal half of the leaf, but not in the proximal portion. The onset of petiole development is evident much earlier in leaf ontogeny in C4 F. bidentis (14 mm, LPI = 0·1) than in C3 F. robusta (25 mm, LPI = 0·4).
Progressive vein pattern development
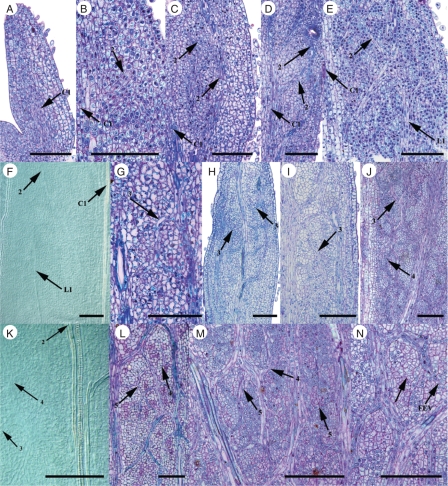
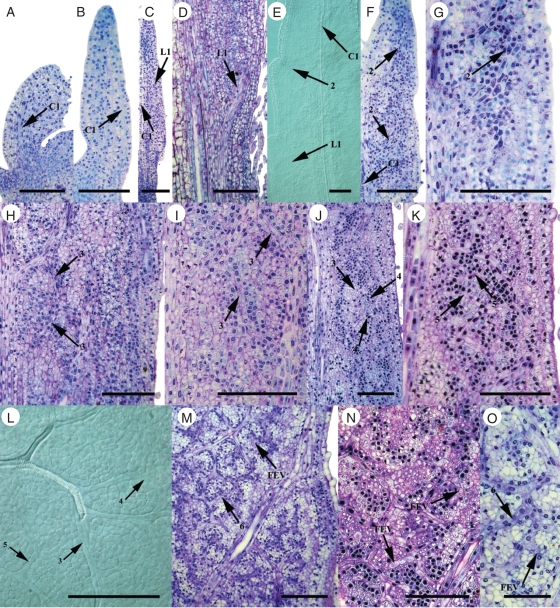
Procambium of the central 1° vein is present in both Flaveria species at a very early stage in leaf ontogeny regardless of photosynthetic type, and a prominent central 1° vein continuous with stem vasculature was observed in the smallest leaves sampled from C3 and C4 Flaveria species (0·5 and 0·4 mm, respectively, Figs 3A and 4A, B, Table 2). The lateral 1° veins are initiated in smaller leaves earlier in development in C4 F. bidentis than in C3 F. robusta based on leaf length and LPI (Figs 3E, F and 4C–E, Table 2). In addition, when lateral 1° veins were first detected in C3 F. robusta leaves, they occurred in the basal portion of developing leaves only and did not extend apically as far as those of the C4 species. This did not affect ultimate 1° vein density in mature leaves of the C3 species, however, as 1° vein density was not significantly different from the C4 species (see above). C3 F. robusta and C4 F. bidentis begin to initiate 2° veins in the apical portion of the leaf at similar leaf lengths, although this represents an earlier LPI stage in the C4 species (Figs 3B–D and 4E–G, Table 2).
Fig. 3.
Onset and early development of vein orders in paradermal sections and clearings of leaves of C3 Flaveria robusta. (A) Young leaf showing central 1° vein procambium (1 mm). (B) Leaf showing onset of 2° vein procambium (1 mm). (C) Developing 2° vein procambium loops (1·5 mm). (D) Connection of 2° vein loops (2 mm). (E) Basal portion of leaf with lateral 1° vein procambium (2 mm). (F) Mid-portion of leaf with lateral 1° and 2° vein procambium (4 mm). (G) Onset of 3° veins branching from 2° veins (2 mm). (H) 3° vein mesh in mid-portion of leaf (4 mm). (I) Developing 3° vein procambium within a 2° vein loop (4 mm). (J) 4° minor veins branching from 3° veins in basal portion of leaf (8 mm). (K) Mid-portion of leaf with 3° and 4° minor vein procambium (4 mm). (L) 4° and 5° minor vein orders in mid-portion of leaf (5 mm). (M) Minor vein reticulum in mid-portion of leaf (8 mm). (N) Appearance of freely ending veinlets compared with connected minor veins (5 mm). Abbreviations: C1, central 1° vein; FEV, freely ending veinlet; L1, lateral 1° vein. Scale bars: all images = 100 µm.
Fig. 4.
Onset and early development of vein orders in paradermal sections and clearings of leaves of C4 Flaveria bidentis. (A) Basal portion of young leaf showing central 1° vein procambium (0·5 mm). (B) Young leaf showing central 1° vein procambium (1 mm). (C) Mid-portion of leaf from central 1° vein to margin with lateral 1° vein procambium (1 mm). (D) Basal portion of leaf with lateral 1° vein procambium (3 mm). (E) Mid-portion of leaf with lateral 1° and 2° vein procambium (2 mm). (F) Leaf showing onset of 2° vein procambium (1 mm). (G) Connection of 2° vein loops (1 mm). (H) Appearance of 3° vein mesh (2 mm). (I) Developing 3° vein procambium within a 2° vein loop (2 mm). (J) 4° minor veins branching from 3° veins in basal portion of leaf (4·5 mm). (K) Serial section of leaf in J showing 5° minor veins. (L) Mid-portion of leaf with minor vein procambium (4·5 mm). (M) Mid-portion of the leaf with developing minor vein reticulum (7 mm). (N) Appearance of freely ending veinlets connected to minor veins in mid-portion of leaf (5 mm). (O) Mid-portion of leaf with freely ending veinlets compared with connected minor veins (7 mm). Abbreviations: C1, central 1° vein; L1, lateral 1° vein. Scale bars: all images = 100 µm, except (O) = 50 µm.
Table 2.
Timing of vein initiation and xylem differentiation in developing leaves of C3 and C4 Flaveria species
| Vein development phase | Vein order | C3F. robusta† | C4F. bidentis‡ |
|---|---|---|---|
| Vein initiation | Central 1° vein | <0·5 (<− 2·0)* | <0·4 (<− 2·5)* |
| Lateral 1° veins | 2 (−0·8)** | 0·6 (−1·9) | |
| 2° veins | 1 (−0·9) | 1 (−1·3) | |
| 3° veins | 2 (−0·8) | 2 (−1·1) | |
| Minor veins | 4 (−0·5) | 4 (−0·9) | |
| FEVs | 5 (−0·4) | 4·5 (−0·8) | |
| Xylem differentiation | Central 1° vein | 1 (−0·9) | 0·6 (−1·9) |
| Major veins (1°, 2°) | 25 (0·4) | 18 (0·3) | |
| Minor veins (3° +) | 50 (1·0) | 35 (1·1) | |
| FEVs*** | 53 (1·3) | 45 (1·3) |
Vein initiation is first appearance of procambium in apical quarter of leaf. Xylem differentiation is presence of continuous xylem within a vein order throughout the leaf. Ontogeny of leaves is indicated in average length (mm; unbracketed numbers) and leaf plastochron units (LPI; numbers in parentheses).
† n = 39 for vein initiation, n = 83 for xylem differentiation.
‡ n = 51 for vein initiation, n = 123 for xylem differentiation.
FEV, freely ending veinlet. * Stage inferred but not directly observed. ** Lateral 1° veins observed in basal sectors of the leaf only. *** Does not include late-formed FEVs.
In both species, the onset of 3° order vein pattern formation occurs prior to other higher orders of minor veins, and begins to form the characteristic 3° vein mesh in the apical portion of the leaf (Figs 3G–I and 4H, I, Table 2). These veins were observed to form in leaves of similar length between the C3 and C4 species, although this is an earlier LPI stage for C4 F. bidentis. Subsequently, the reticulum of connected minor veins (4° and higher orders) is initiated at the apical portion of the leaf, and is formed rapidly within the leaves of both C3 F. robusta and C4 F. bidentis. The timing of the onset of these higher vein orders occurred at similar leaf lengths for both species, but at an earlier LPI stage in C4 F. bidentis (Figs 3J–M and 4J–M, Table 2). The last minor veins initiated are the FEVs in both Flaveria species; however, in C3 F. robusta, this is the 6° vein order whereas in C4 F. bidentis, this is the 7° vein order (Figs 3N and 4N, O, Table 2). The initiation of FEVs occurs after the formation of the reticulum of ‘connected’ minor veins in the two species. The connected minor veins were visually distinctive as long procambial strands, whereas the shape of adjacent FEV procambium cells were modified only slightly in comparison with surrounding ground tissue (e.g. Fig. 3M). Like other vein orders, this final iteration of vein formation occurs at relatively similar leaf lengths between C3 F. robusta and C4 F. bidentis, but represents an earlier LPI stage in the C4 species.
Differentiation of continuous xylem
The 1° and 2° major veins have continuous strands of differentiated xylem early in leaf ontogeny, regardless of photosynthetic type (Fig. 5A–F, Table 2). The xylem of these major veins is continuous throughout the leaf in smaller leaves at an earlier LPI in C4 F. bidentis compared with C3 F. robusta (Table 2). In minor vein orders, the differentiation of xylem in 3° and higher order veins (including FEVs) occurs basipetally, and is hierarchical in both Flaveria species, where 3° veins differentiate first, followed by 4° and higher order veins, with FEVs differentiating last (Fig. 5G–J, Table 2). Continuous strands of differentiated xylem in the minor vein orders is observed in smaller leaves of the same LPI in C4 F. bidentis compared with C3 F. robusta (Table 2). In both species, observed continuous xylem in all minor veins throughout the entirety of the leaf occurs when leaves reach approximately two-thirds of their mature length (Tables 1 and 2).
Fig. 5.
Early xylem differentiation in hierarchical vein orders in clearings of C3 and C4 Flaveria leaves. (A) Distal half of young leaf showing the central 1° vein and 2° vein loops in C3 F. robusta (3 mm). (B) Distal half of young leaf between central 1° vein and the leaf margin showing a lateral 1° vein and 2° vein loops in C4 F. bidentis (3 mm). (C) Discontinuous xylem differentiation of a lateral 1° vein in C3 F. robusta (6 mm). (D) Discontinuous xylem differentiation of a lateral 1° vein in C4 F. bidentis (4 mm). (E) Basipetal xylem differentiation of 2° veins in C3 F. robusta (2 mm). (F) Basipetal xylem differentiation of 2° veins in C4 F. bidentis (2 mm). (G) Basipetal xylem differentiation of 3° veins in C3 F. robusta (7 mm). (H) Basipetal xylem differentiation of 3° veins in C4 F. bidentis (5 mm). (I) Minor vein network in C3 F. robusta. (J) Minor vein network in C4 F. bidentis. (K) Freely ending veinlet ending in enlarged cells in C3 F. robusta. Arrow indicates tracheary element with helical thickenings. (L) Procambial-shaped cells joining a FEV to a mature higher order vein in C3 F. robusta. Abbreviations: C1, central 1° vein; L1, lateral 1° vein. Scale bars: all images = 100 µm.
In both C3 and C4 Flaveria species, strands of cells lacking mesophyll characteristics are sometimes observed in a full sized leaf after xylem has fully differentiated. These strands occur either as short branches from minor veins with differentiated xylem or as extensions of pre-existing FEVs with differentiated xylem that often connect with an adjacent vein. Cells of these FEVs or FEV ‘extensions’ either resemble elongated procambium cells or are rounded, but show helical thickenings (Fig. 5K). Most of these procambial strands end freely within the mesophyll; however, some procambial strands appear to connect a mature FEV to mature minor veins in both C3 and C4 Flaveria species, thereby losing the ‘freely ending’ condition (Fig. 5L).
Vein density expansion and ground tissue (BS and M) enlargement
Offset of vein pattern formation occurs earlier in the C4 species, so that vein pattern is complete in smaller leaves at an earlier LPI in C4 F. bidentis (7 mm, LPI = −0·3) compared with C3 F. robusta (15 mm, LPI = 0·15). The mature vein pattern observed is more complex in C4 F. bidentis than in C3 F. robusta through an additional vein order (see above), and is reflected in greater minor vein branching, and numbers of FEVs in C4 F. bidentis (Fig. 1, Table 1). These results were also observed in developing leaves of C4 F. trinervia (our unpubl. res.). In both C3 and C4 Flaveria species, vein density decreases with lengthwise expansion of the leaf lamina (Fig. 6A). Decreasing vein density for both species occurs after xylem is continuous in higher order minor veins of the mid-portion of the leaf (but not necessarily in basal portions of the leaf or in FEVs, see above). Using leaf expansion as a proxy for rate of change, the linear relationships between vein density, branching density and FEV density are comparable between the C3 and C4 Flaveria species (Fig. 6B, C). The overall decrease in FEV density is less than branching density in the two Flaveria species, and may reflect late FEV formation in the leaf (see above).
Fig. 6.
Relationships between leaf length and vein density, and between vein density, vein branching density and freely ending veinlet density in multiple samples of adult-phase leaves harvested at different stages of ontogeny from C3 and C4 Flaveria plants. Trendlines indicate the relative changes in densities as leaves expand. (A) Vein density vs. leaf length in C3 F. robusta and C4 F. bidentis. (B) Branching and freely ending veinlet densities vs. vein density in C3 F. robusta. (C) Branching and freely ending veinlet densities vs. vein density in C4 F. bidentis.
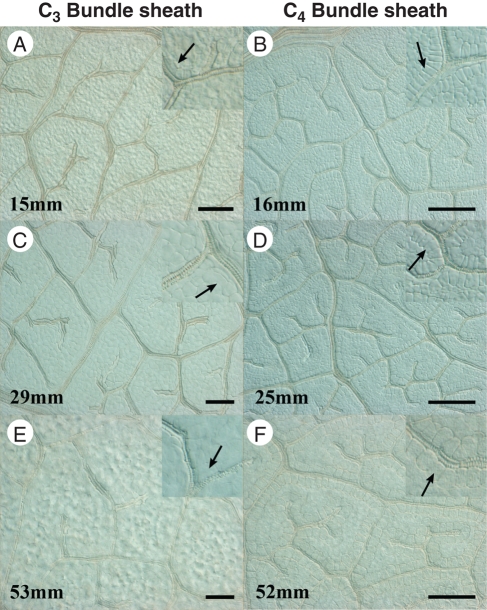
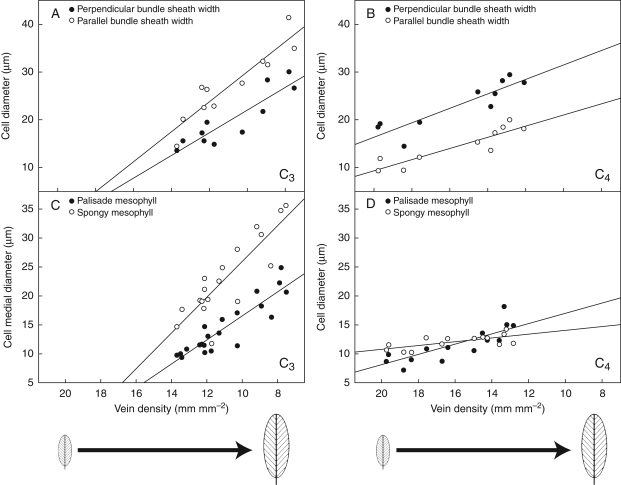
The BS cells in both C3 and C4 Flaveria species do not enlarge until xylem differentiation is continuous in all minor vein orders (but not necessarily in FEVs). In C3 F. robusta, BS cells expand as the veins elongate (Fig. 7A, C), and mature BS cells are not morphologically distinct from M cells (Fig. 7E). The mature BS cells in C3 F. robusta are wider in parallel diameter (in relation to the vein axis) than in perpendicular diameter (see Fig. 9A below). By contrast, the enlargement of BS cells in C4 F. bidentis occurs first by expansion in perpendicular diameter (in relation to the vein axis; Fig. 7B), and then in parallel diameter as vein spacing increases and veins elongate (Fig. 7D, F). Mature BS cells in C4 F. bidentis are structurally distinctive from surrounding cells, and are ultimately wider in perpendicular diameter than in parallel diameter (see Fig. 9B below).
Fig. 7.
Bundle sheath development from clearings of C3 and C4 Flaveria leaves. (A, C, E) Bundle sheath cells are indistinct from mesophyll in developing leaves of C3 F. robusta. Insets with arrows show enlargement of bundle sheath cells and lack of distinguishing morphological features. (B, D, F) Bundle sheath cells are distinct from mesophyll in developing leaves of C4 F. bidentis. Insets with arrows demonstrate bundle sheath cells enlarging in perpendicular diameter in younger leaves, and in parallel diameter in older leaves. Scale bar: all images = 100 µm; insets are 2×.
Fig. 9.
Relationships between vein density and size of bundle sheath, palisade and spongy mesophyll cells from multiple samples of adult-phase leaves harvested at different stages of ontogeny in C3 and C4 Flaveria plants. Bundle-sheath cell enlargement is represented both in the perpendicular diameter to the vein axis (perpendicular width) and in the parallel diameter to the vein axis (parallel width). Trendlines indicate the relative change in mesophyll cell size as vein density decreases through leaf expansion. (A) C3 F. robusta bundle-sheath cell expansion. (B) C4 F. bidentis bundle-sheath cell expansion. (C) C3 F. robusta mesophyll cell expansion. (D) C4 F. bidentis mesophyll cell expansion.
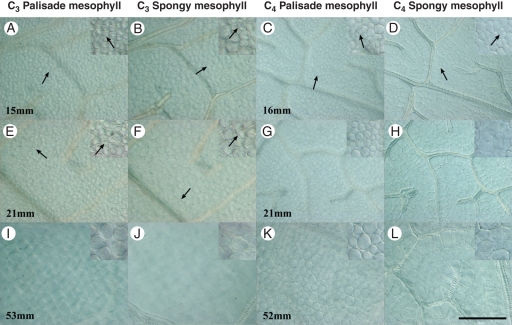
The development of M cells highlights further differences in ground tissue development between C3 and C4 Flaveria species. In C3 F. robusta, cell-wall configuration indicating recent divisions in M cells was observed for a slightly longer period in leaf expansion (up to 25 mm, Fig. 8A, B, E, F) compared with C4 F. bidentis (up to 20 mm, Fig. 8C, D, G, H). Both palisade and spongy M cells in the C3 species are larger in medial diameter than M cells of the C4 species (Fig. 8I–L). In the C3 species, mature M cells are not different in diameter from adjacent BS cells, whereas in C4 F. bidentis both palisade and spongy M cells are substantially smaller than BS cells. In addition, there is a size distinction between palisade and spongy M cell diameters in C3 F. robusta, which is not apparent in C4 F. bidentis (Figs 8I–L and 9C, D).
Fig. 8.
Palisade and spongy mesophyll development from clearings of C3 and C4 Flaveria leaves. Insets show enlargement and recent divisions of palisade and spongy mesophyll. (A, B, E, F, I, J) Mesophyll cells enlarge showing morphological and size differences between palisade and spongy mesophyll in C3 F. robusta. Arrows indicate recently divided cells. (C, D, G, H, K, L) Mesophyll cells enlarge showing little morphological and size differences between palisade and spongy mesophyll in C4 F. bidentis. Arrows indicate recently divided cells. Scale bar: all images = 100 µm; insets are 2×.
Correlation analyses of vein pattern characters throughout leaf development show that minor vein density correlates significantly with leaf length, vein branching and FEV densities in both C3 and C4 Flaveria species (Table 3, Fig. 9). In addition, vein density is highly correlated with BS, palisade and spongy M cell sizes. Ground tissues show strong correlations in both C3 and C4 species, and palisade and spongy M cell sizes are positively correlated. In C3 F. robusta, the size of BS cells is also positively correlated with both M cell types. In C4 F. bidentis, whereas there is a strong correlation between BS cell size and palisade M cell size, this relationship is much weaker with spongy M cell size. These correlative relationships were also observed in C4 F. trinervia (our unpubl. res.).
Table 3.
Correlation analyses between measured vein pattern and ground tissue traits in mid-portion of leaf in C3 and C4 Flaveria species
| Correlated variables† | C3F. robusta | C4F. bidentis |
|---|---|---|
| Vein density vs. leaf length | − *** | − *** |
| Vein density vs. branching | + *** | + *** |
| Vein density vs. freely ending veinlets | + *** | + *** |
| Vein density vs. bundle sheath | − *** | − *** |
| Vein density vs. palisade mesophyll | − *** | − *** |
| Vein density vs. spongy mesophyll | − *** | − ** |
| Palisade mesophyll vs. spongy mesophyll | + *** | + ** |
| Bundle sheath vs. palisade mesophyll | + *** | + *** |
| Bundle sheath vs. spongy mesophyll | + *** | + * |
† Size based on medial cell diameter; *P < 0·05; **P < 0·005; ***P < 0·001.
DISCUSSION
C3 vs. C4 vein pattern development
This comparative analysis of leaf development shows that vein pattern differences between C3 and C4 species arise from modifications to the highest orders of veins, and in C4 Flaveria species this occurs through formation of an additional minor vein order. The comparable densities of the first three orders of veins (1°, 2° and 3° vein orders) between C3 F. robusta and C4 F. bidentis are in contrast to significantly greater densities of higher order minor veins (4° and higher orders) observed in C4 Flaveria species compared with C3 Flaveria species. These results correspond with previous studies of mature vein pattern in C3 and C4 Flaveria species (McKown and Dengler, 2007). The similarities in 1°, 2° and 3° vein densities between the C3 and C4 species suggest these veins in Flaveria probably have roles that are not necessarily related to photosynthetic type, but rather perform similar physiological functions, such as leaf structural support, and bulk flow and dispersal of water. This indicates that evolutionary modifications to vein patterning resulting in greater vein densities observed in C4 species are largely related to the smallest veins within the leaf (Ueno et al., 2006; McKown and Dengler, 2007).
The initiation of major vein pattern (1°, 2° veins) throughout the leaf is comparable between C3 and C4 Flaveria leaves that are similar in length, although these are not at the same LPI stage (Fig. 10). The lateral 1° veins are an exception, as these are initiated earlier in C4 F. bidentis; however, this does not change ultimate 1° vein densities between C3 and C4 Flaveria species. Length of the lateral 1° veins may relate to structural support, as leaves from C4 F. bidentis have earlier distal lamina growth, and are generally wider in the apical portion of the leaf compared with C3 F. robusta. In accordance with observations of major vein development, the onset of minor vein pattern formation begins in leaves of the same size in both C3 and C4 Flaveria species, although this is also an earlier LPI stage in C4 F. bidentis. The C4 higher order minor vein pattern, including FEVs, is more complex than the C3 pattern and thus vein density (including branching and FEV density) is greater throughout leaf expansion in C4 F. bidentis compared with C3 F. robusta.
Fig. 10.
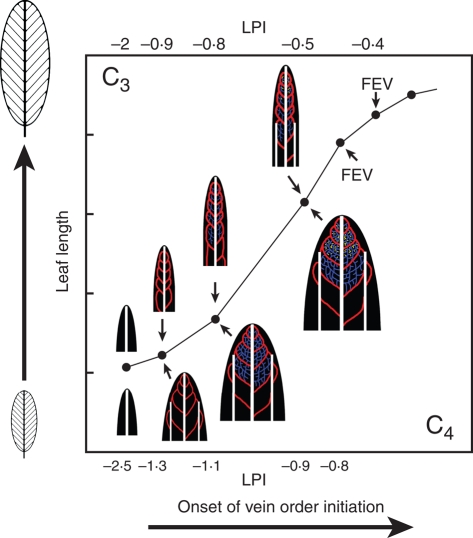
Diagrammatic representation of vein pattern formation in leaves of C3 and C4 Flaveria species. Leaf developmental stages relate formation of vein orders to leaf length and leaf plastochron index (LPI) for each species. The onset of vein orders (major and minor) occurs in leaves that are equivalent in length for the two species, and leaf expansion is relatively similar. Lateral lamina expansion occurs earlier in C4 Flaveria and at later stages in C3 Flaveria. LPI values differ, as C4 Flaveria initiate leaves at a faster rate. The earlier establishment of the minor vein network and freely ending veinlets (FEV) throughout the C4 leaf and earlier offset of vein formation indicate an accelerated rate of higher order vein formation in C3 Flaveria compared with C3 Flaveria. Between the two species, ultimate leaf shape and size are equivalent but minor vein density is not (see Fig. 1, Table 1). White = central and lateral 1° veins, red = 2° veins, blue = 3° veins, yellow = 4° and higher order minor veins. Abbreviations: FEV = freely ending veinlets; LPI = leaf plastochron index.
The equivalency of leaf size observed during very early events in leaf ontogeny strongly suggests that initiation of vein orders occurs at a very similar developmental point for C3 and C4 Flaveria species. In addition, the rate of expansion of adult leaves was similar between C3 and C4 Flaveria species (e.g. leaf 5, Fig. 2C). The discrepancy between leaf length and LPI stage corresponds with faster leaf initiation and corresponding shorter plastochron in C4 F. bidentis (Fig. 2B). Although LPI is a commonly used metric for developmental studies of leaves, its utility within this study was more limited to highlighting comparisons of leaf development within species, rather than between species. Thus, relating the earliest ontogenetic events to leaf size between Flaveria species proposes that there is little developmental shift in the timing of onset of vein order formation (except for lateral 1° veins, Fig. 10).
Using leaf length as an indicator of developmental age, the onset of major and minor vein pattern begins at an equivalent developmental period for individual leaves of C3 F. robusta and C4 F. bidentis. The appearance of the last iteration of minor veins (FEVs) occurs in slightly smaller leaves in C4 F. bidentis than in C3 F. robusta (Table 2) and is a higher order of veins being formed in the minor vein network compared with C3 F. robusta (seven orders vs. six orders). This suggests that formation of a more complex minor vein pattern in the C4 species is offset from the C3 species as this occurs in a slightly shorter developmental time period (Fig. 10). The similarity in onset of minor vein pattern formation and earlier offset indicates that the denser vein network observed in the C4 species than in the C3 species relates to an increase or acceleration in the formation and establishment of higher order veins during leaf development, not a precocious onset of vein formation or a prolongation of this period. Furthermore, this shift does not appear to affect the major vein orders. Thus, vein pattern acceleration is specifically the initiation of an additional minor vein order in C4 F. bidentis compared with C3 F. robusta, and establishment of the minor vein network occurring within a slightly shorter ontogenetic timeframe.
Ground tissue development also shows differences between C3 and C4 Flaveria species; however, in the two species, differential expansion of ground tissues occurs after the vein pattern has been established in the leaf. The duration of apparent M cell divisions, M cell enlargement patterns and directions of BS cell expansion are different between C3 F. robusta and C4 F. bidentis, but it is likely that only the development of M tissue plays a role in vein density at leaf maturity. This is related to earlier cessation of M cell divisions, and smaller sizes of individual M cells (palisade and spongy) in the C4 species than the C3 species. The expansion of M cells has been previously suggested as a component of the mechanism in which C4 vein patterning and C4 photosynthetic tissue proportions are maintained (McKown and Dengler, 2007). This could also provide the leaf with a plastic means to modify vein spacing somewhat to acclimate the leaf to different environments as necessary (Sage and McKown, 2006).
Vein pattern formation in relation to ground tissue development
This study suggests that the change from a C3 to a C4 vein density in the developing leaf may involve a small number of important modifications (Fig. 10). The developmental timing in the onset of major and minor vein pattern initiation in C3 and C4 Flaveria species based on leaf size indicates that signalling in the leaf primordium for the induction of vein procambium must occur at a relatively similar developmental stage. Furthermore, the results of this study suggest that C4 Flaveria species have an acceleration of procambium formation, not a prolongation of procambium formation after ground tissue has begun to mature.
In Arabidopsis, Scarpella et al. (2004) suggest that leaf vein pattern formation may be halted through the gain of M characteristics in the surrounding ground tissue. Loss or decrease of a procambium-forming signal (such as auxin), or the perception of cells to procambium formation signals may also functionally terminate vein formation. Higher order vein procambium formation in Arabidopsis is halted by general suppression of cell cycling, indicating that vein pattern formation requires sustained cell proliferation (Kang et al., 2007). In both C3 and C4 Flaveria species, divisions appear to continue in immature M cells after vein pattern has been formed, indicating that procambium formation ceases while ground tissue is still meristematic. In the C4 species, BS cells do not appear to gain any mature features such as chloroplasts, large size and rounded shape before vein patterning is fully established, and both BS and M cells were not observed to enlarge in size until minor vein pattern was complete, and xylem had begun to differentiate in these veins.
Although factors influencing vein formation (such as cell proliferation and M differentiation) were not implicitly examined in this study, the observations suggest that photosynthetic tissue development may have a minimal role in terminating vein formation in Flaveria, regardless of photosynthetic type. Primarily, direct observations of procambial strands (minor vein reconnections and FEV extensions) after surrounding veins had continuous differentiated xylem were not uncommon in either C3 or C4 Flaveria species. In C3 F. robusta, FEVs also appeared directly to ‘co- opt’ adjacent M cells to form vein extensions late in leaf ontogeny. Secondly, during leaf expansion, FEV density did not decrease at the same rate as branching density, and is consistent with the cytological evidence. Late connection of FEVs has also been observed in Arabidopsis, supporting the hypothesis that minor veins are not predisposed to be either connecting or freely ending, and that FEV formation is not a genetically determined programme (Scarpella et al., 2004; Kang et al., 2007). The observations of late FEV formation or minor vein ‘re-connection’ in this study and in Scarpella et al. (2004) show that the leaf maintains a capacity to modify vein density after initial vein pattern is already established. In turn, this may provide the leaf with some plasticity depending on hydraulic or transport needs.
The overall greater vein density observed in C4 Flaveria species compared with C3 Flaveria species could be explained by increased auxin production, modified leaf ground meristem cell competency to becoming procambium, or a combination of these developmental parameters. The role of auxin as a signal in vein pattern development is well supported by a large body of evidence from studies examining auxin reporter expression and procambial pattern development in Arabidopsis, in addition to auxin mutant characterization, auxin inhibition experiments and numerous theoretical models (reviewed in Sieburth and Deyholos, 2006; Rolland-Lagan, 2008). External application of indole-acetic-acid (IAA) to mimic the hormone auxin on developing leaves of Arabidopsis resulted in an increase in the number of vascular strands and dramatically upregulated auxin efflux carriers in ground meristem (Scarpella et al., 2006). Model simulations increasing the rate of auxin production also resulted in more numerous, densely packed veins (Feugier and Iwasa, 2006). Comparable timings in formation of major and minor veins in both C3 and C4 Flaveria species based on leaf length suggest that there may be little difference in initial competency of leaf meristematic tissue to forming procambium. The difference in developmental timing of a complete minor vein pattern network in C3 vs. C4 Flaveria species is relatively small but the offset was observed sooner in the C4 species, suggesting that ground meristem cells in C4 leaves may lose competency to form new vascular strands at a slightly earlier stage. The more complex vein pattern of the C4 species compared with the C3 species (seven vs. six vein orders) could also arise from an increase in the amount of auxin produced during the same developmental period. Further study with external application of auxin to C3 plants or auxin inhibitors to C4 plants could test hypotheses relating auxin to modifying vein pattern as part of the mechanism through which higher vein density is achieved.
Evolution of high vein density in C4 Flaveria
In the evolution of C4 photosynthesis, increased vein density is hypothesized as one of the earliest anatomical changes, and may represent a ‘precondition’ for evolving intermediate or C4 photosynthetic function (Sage, 2001, 2004). Greater vein density is intimately linked with Kranz anatomy, and the evolution of both intermediate and C4 photosynthesis from C3 progenitors (McKown and Dengler, 2007; Vogan et al., 2007). The C3 species in this study (F. robusta) is known to have slightly denser venation compared with other C3 Flaveria species, and based on phylogenetic analyses, F. robusta is probably more similar to the C3 ancestor of the derived intermediate and C4 Flaveria species than the other C3 Flaveria species. Some intermediate photosynthetic species of Flaveria with few physiological or anatomical changes from the C3 condition show an increase in vein density. In combination, these lines of evidence support the hypothesis that increased vein density may occur early in the transition from C3 to C4 photosynthesis (McKown et al., 2005; McKown and Dengler, 2007).
The development of increased vein density in C3 plants has a relative adaptive value in arid environments with high irradiance (Roth-Nebelsick et al., 2001; Sage, 2004), and increasing vein density is cited as an adaptation adding structural integrity to a C3 leaf in windy environments (Sage, 2004). C3 plants found in xeric or high-irradiance conditions tend to have high vein densities (Roth-Nebelsick et al., 2001). Higher numbers of veins may confer greater water movement through the photosynthetic tissues and less water stress by shortening the water pathway from xylem to mesophyll, and by providing more numerous, ‘parallel’ exits for water into ground tissues (A. D. McKown et al., unpubl. res.), thereby alleviating photorespiration by allowing stomata to remain open longer (Roth-Nebelsick et al., 2001; Sage, 2004; Sack and Holbrook, 2006; Muhaidat et al., 2007). In addition, Sage (2004) suggests that a shift in minor vein density could improve water status by increasing the water supply relative to the evaporative surface of the leaf, despite the higher energetic ‘cost’ of lignin. Sack and Frole (2006) further this idea as a ‘cost’ that could be quickly repaid in high-light environments.
Thus, if increased vein density has a high adaptive and functional value in C3 plants, it may be relatively easy to achieve this ‘precondition’ through alterations to leaf development as outlined in this study. The retention of a C3-adaptive vein patterning in C4 plants, however, may be more closely linked to photosynthetic tissue proportioning and physical C4 functioning. Thus, during the evolution of Kranz anatomy in C4 species where vein densities are increased, such as in C4 Flaveria, vein patterning is altered from the C3 condition through an acceleration of procambium formation during leaf development. Further study of the mechanisms for shifting vein density, such as modifications to auxin production and/or cell competency to auxin, is necessary to understand fully this important developmental shift and its broad-reaching significance both in C4 photosynthesis and in leaf evolution in general.
ACKNOWLEDGEMENTS
We thank Petra Donnelly for help with sectioning, Laurel Christie with cell counting, Dr Ann Hirsch with use of the Zeiss Axiophot microscope, and the helpful advice and encouragement from two anonymous reviewers. This work was supported by NSERC grant no.A5720.
LITERATURE CITED
- Bayer EM, Smith RS, Mandel T, et al. Integration of transport-based models for phyllotaxis and midvein formation. Genes & Development. 2009;23:373–384. doi: 10.1101/gad.497009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleth T, Scarpella E, Prusinkiewicz P. Towards the systems biology of auxin-transport-mediated patterning. Trends in Plant Science. 2007;12:151–159. doi: 10.1016/j.tplants.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Dengler NG, Nelson T. Leaf structure and development in C4 plants. In: Sage RF, Monson RK, editors. C4 plant biology. San Diego: Academic Press; 1999. pp. 133–172. [Google Scholar]
- Dengler NG, Taylor WC. Developmental aspects of C4 photosynthesis. In: Leegood RC, Sharkey TD, von Caemmerer S, editors. Photosynthesis: physiology and metabolism. Dordrecht: Kluwer Academic Publishers; 2000. pp. 471–495. [Google Scholar]
- Dengler NG, Dengler RE, Donnelly PM, Hattersley PW. Quantitative leaf anatomy of C3 and C4 grasses (Poaceae): bundle sheath and mesophyll surface area relationships. Annals of Botany. 1994;73:241–255. [Google Scholar]
- Dengler NG, Woodvine MA, Donnelly PM, Dengler RE. Formation of vascular pattern in developing leaves of the C4 grass Arundinella hirta. International Journal of Plant Sciences. 1997;158:1–12. [Google Scholar]
- Erickson RO, Michelini FJ. The plastochron index. American Journal of Botany. 1957;44:297–305. [Google Scholar]
- Esau K. Anatomy of the seed plants. 2nd edn. New York: John Wiley and Sons; 1977. [Google Scholar]
- Feugier FG, Iwasa Y. How canalization can make loops: a new model of reticulated leaf vascular pattern formation. Journal of Theoretical Biology. 2006;243:235–244. doi: 10.1016/j.jtbi.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Hattersley PW. Characterization of C4 type leaf anatomy in grasses (Poaceae). Mesophyll: bundle sheath area ratios. Annals of Botany. 1984;53:163–179. [Google Scholar]
- Hickey LJ. A revised classification of the architecture of dicotyledonous leaves. In: Metcalfe CR, Chalk L, editors. Systematic anatomy of leaf and stem, with a brief history of the subject. Anatomy of the dicotyledons. 2nd edn, Vol. Vol. 1. New York: Oxford University Press; 1979. pp. 25–39. [Google Scholar]
- Kang J, Dengler NG. Cell cycling frequency and expression of the homeobox gene AtHB-8 during leaf vein development in Arabidopsis. Planta. 2002;216:212–219. doi: 10.1007/s00425-002-0847-9. [DOI] [PubMed] [Google Scholar]
- Kang J, Dengler NG. Vein pattern development in adult leaves of Arabidopsis thaliana. International Journal of Plant Sciences. 2004;165:231–242. [Google Scholar]
- Kang J, Mizukami Y, Wang H, Fowke L, Dengler NG. Modification of cell proliferation patterns alters leaf vein architecture in Arabidopsis thaliana. Planta. 2007;226:1207–1218. doi: 10.1007/s00425-007-0567-2. [DOI] [PubMed] [Google Scholar]
- Kocacinar F, McKown AD, Sage TL, Sage RF. Photosynthetic pathway influences xylem structure and function in the genus Flaveria. Plant, Cell and Environment. 2008;31:1363–1376. doi: 10.1111/j.1365-3040.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- Leaf Architecture Working Group. Manual of Leaf Architecture – morphological description and categorization of dicotyledonous and net-veined monocotyledonous angiosperms. Washington, DC: Smithsonian Institution; 1999. [Google Scholar]
- Mattsson J, Ckurshumova W, Berleth T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiology. 2003;131:1327–1339. doi: 10.1104/pp.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Dengler NG. Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae) American Journal of Botany. 2007;94:382–399. doi: 10.3732/ajb.94.3.382. [DOI] [PubMed] [Google Scholar]
- McKown AD, Moncalvo JM, Dengler NG. Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. American Journal of Botany. 2005;92:1911–1928. doi: 10.3732/ajb.92.11.1911. [DOI] [PubMed] [Google Scholar]
- Muhaidat RM, Sage RF, Dengler NG. Diversity of Kranz anatomy and biochemistry in C4 eudicots. American Journal of Botany. 2007;94:362–381. doi: 10.3732/ajb.94.3.362. [DOI] [PubMed] [Google Scholar]
- Nelson T, Dengler NG. Photosynthetic tissue differentiation in C4 plants. International Journal of Plant Sciences. 1992;(Supplement):S93–S105. [Google Scholar]
- Nelson T, Dengler NG. Leaf vascular pattern formation. The Plant Cell. 1997;9:1121–1135. doi: 10.1105/tpc.9.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. Bethesda, MD: US National Institutes of Health; 2007. pp. 1997–2008. http://rsb.info.nih.gov/ij/ [Google Scholar]
- Rolland-Lagan A. Vein patterning in growing leaves: aces and polarities. Current Opinion in Genetics and Development. 2008;18:348–353. doi: 10.1016/j.gde.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Rolland-Lagan A, Amin M, Pakulska M. Quantifying leaf venation patterns: two-dimensional maps. The Plant Journal. 2009;57:195–205. doi: 10.1111/j.1365-313X.2008.03678.x. [DOI] [PubMed] [Google Scholar]
- Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H. Evolution and function of leaf venation architecture: a review. Annals of Botany. 2001;87:553–566. [Google Scholar]
- Rudall PJ, Bateman RM. Evolution of zygomorphy in monocot flowers: iterative patterns and developmental constraints. New Phytologist. 2004;162:25–44. [Google Scholar]
- Sack L, Frole K. Leaf structural diversity is related to hydraulic capacity in tropical rainforest trees. Ecology. 2006;87:483–491. doi: 10.1890/05-0710. [DOI] [PubMed] [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Sage RF. Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthetic syndrome. Plant Biology. 2001;3:202–213. [Google Scholar]
- Sage RF. The evolution of C4 photosynthesis. New Phytologist. 2004;161:341–370. doi: 10.1111/j.1469-8137.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- Sage RF, McKown AD. Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? Journal of Experimental Botany. 2006;57:303–317. doi: 10.1093/jxb/erj040. [DOI] [PubMed] [Google Scholar]
- Sawchuk MG, Head P, Donner TJ, Scarpella E. Time-lapse imaging of Arabidopsis leaf development shows dynamic patterns of procambium formation. New Phytologist. 2007;176:560–571. doi: 10.1111/j.1469-8137.2007.02193.x. [DOI] [PubMed] [Google Scholar]
- Scarpella E, Francis P, Berleth T. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development. 2004;131:3445–3455. doi: 10.1242/dev.01182. [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes and Development. 2006;20:1015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE, Deyholos MK. Vascular development: the long and winding road. Current Opinion in Plant Biology. 2006;9:48–54. doi: 10.1016/j.pbi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Sinha NR, Kellogg EA. Parallelism and diversity in multiple origins of C4 photosynthesis in grasses. American Journal of Botany. 1996;83:1458–1470. [Google Scholar]
- Sud R, Dengler NG. Cell lineage of vein formation in variegated leaves of the C4 grass Stenotaphrum secundatum. Annals of Botany. 2000;86:99–112. [Google Scholar]
- Ueno O, Kawano Y, Wakayama M, Takeda T. Leaf vascular systems in C3 and C4 grasses: a two-dimensional analysis. Annals of Botany. 2006;97:611–621. doi: 10.1093/aob/mcl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan PJ, Frolich MW, Sage RF. The functional significance of C3–C4 intermediate traits in Heliotropium L. (Boraginaceae): gas exchange perspectives. Plant, Cell and Environment. 2007;30:1337–1345. doi: 10.1111/j.1365-3040.2007.01706.x. [DOI] [PubMed] [Google Scholar]
- Wenzel CL, Schuetz M, Yu Q, Mattsson J. Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. The Plant Journal. 2007;49:387–398. doi: 10.1111/j.1365-313X.2006.02977.x. [DOI] [PubMed] [Google Scholar]