Abstract
Backgrounds and Aims
Shoot demography affects the growth of the tree crown and the number of leaves on a tree. Masting may cause inter-annual and spatial variation in shoot demography of mature trees, which may in turn affect the resource budget of the tree. The aim of this study was to evaluate the effect of masting on the temporal and spatial variations in shoot demography of mature Betula grossa.
Methods
The shoot demography was analysed in the upper and lower parts of the tree crown in mature trees and saplings over 7 years. Mature trees and saplings were compared to differentiate the effect of masting from the effect of exogenous environment on shoot demography. The fate of different shoot types (reproductive, vegetative, short, long), shoot length and leaf area were investigated by monitoring and by retrospective survey using morphological markers on branches. The effects of year and branch position on demographic parameters were evaluated.
Key Results
Shoot increase rate, production of long shoots, bud mortality, length of long shoots and leaf area of a branch fluctuated periodically from year to year in mature trees over 7 years, in which two masting events occurred. Branches within a crown showed synchronized annual variation, and the extent of fluctuation was larger in the upper branches than the lower branches. Vegetative shoots varied in their bud differentiation each year and contributed to the dynamic shoot demography as much as did reproductive shoots, suggesting physiological integration in shoot demography through hormonal regulation and resource allocation.
Conclusions
Masting caused periodic annual variation in shoot demography of the mature trees and the effect was spatially variable within a tree crown. Since masting is a common phenomenon among tree species, annual variation in shoot demography and leaf area should be incorporated into resource allocation models of mature masting trees.
Keywords: Masting, shoot demography, short shoot, long shoot, temporal variation, spatial variation, leaf area, shoot length, resource allocation, Betula grossa
INTRODUCTION
Masting, or synchronized intermittent reproduction, is a common phenomenon among tree species (Silvertown, 1980). A plant's resource level is regarded as an important proximate factor in masting, together with weather cues (Kelly and Sork, 2002). Because trees require abundant resources to produce large seed crops, they have to wait several years until the amount of resources exceeds a threshold level (Isagi et al., 1997). Although reduction in shoot growth and stem diameter increment associated with masting (e.g. Tappeiner II, 1969) has been regarded as supporting this hypothesis rather than a simple resource matching hypothesis (Kelly and Sork, 2002), it has never been incorporated in the resource budget models (Isagi et al., 1997; Satake and Bjørnstad, 2008). However, shoots are source as well as sink of carbon resources. Shoot production affects tree crown growth, leaf display (Maillette, 1982a; Sterck et al., 2003), carbon resource acquisition and reproduction (Wünsche et al., 1996). If masting causes yearly change in shoot production, it may affect the carbon budget of masting trees. However, previous studies were based on short-term comparison of shoot growth between pre-mast years and mast years or post-mast years with spatially limited sampling (Tappeiner II, 1969; Gross, 1972; Innes, 1994). Demographic analysis of modules such as buds and shoots has never been conducted in the context of masting, although it is an effective way to analyse shoot production and tree growth (Maillette, 1982a; Lehtilä et al., 1994; Sterck et al., 2003). Quantifying the long-term temporal variation in shoot demography and leaf production is necessary for understanding the resource budget of mature masting trees.
Temporal variation in shoot demography might differ between branches within a crown. Shoot demography is influenced by the shoot's light availability and position within the crown (Maillette, 1982a, b; Jones and Harper, 1987). Furthermore, the effect of masting on shoot demography may vary between branches. Shoots carrying reproductive organs (reproductive shoots) and shoots that are not directly involved in the formation of reproductive organs (vegetative shoots) differ in growth and branching pattern (e.g. Newell, 1991; Karlsson et al., 1996; Ishihara and Kikuzawa, 2004). Reproductive shoots are typically produced more abundantly in the upper part of a tree's crown (Waller and Steingraeber, 1995). Therefore, branches in the upper crown may show larger temporal variation in shoot demography than the lower branches. Few studies have explored spatial variation in shoot demography of mature trees (Sterck and Bongers, 2001; Kaitaniemi and Ruohomäki, 2003). Because spatial organization of shoot demography represents adaptive strategy of tree growth, light capture and resource allocation (Maillette, 1982a, b; Takenaka, 2000), quantification of spatial organization together with temporal variation is necessary for understanding the resource allocation of the masting tree.
Temporal and spatial variation in shoot demography may be affected by physiological mechanisms through which growth and reproduction are regulated. If shoots behave as physiologically independent units (Sprugel et al., 1991), then reproductive shoots would differ in shoot production from vegetative shoots, and shoot production from vegetative shoots would not change from the pre-mast year to the post-mast year. On the other hand, if shoots are integrated physiologically with other shoots, then vegetative shoots would also be affected by masting. Vegetative shoots may show reduced production in the mast year, or they may change production yearly in order to offset the constraints imposed by reproductive shoots (Waller and Steingraeber, 1995). Furthermore, if branches are integrated with other branches in a crown, annual variation in shoot demography may be synchronized between branches in different positions within a crown.
Birches (Betula spp.) are known to show masting habits (Silvertown, 1980). They have four types of shoot: vegetative long shoot, reproductive long shoot with male catkins, vegetative short shoot and reproductive short shoot with female catkins (Macdonald and Mothersill, 1983; Ishihara and Kikuzawa, 2004). Shoot demography of young trees (Maillette, 1982a, b; Jones and Harper, 1987), the timing of differentiation of short and long shoots (Kumada, 1979; Macdonald and Mothersill, 1983; Macdonald et al., 1984) and the resource allocation for shoot elongation (Kozlowski and Clausen, 1966) have been studied. Betula grossa has a unique property which enables us to test whether masting affects shoot demography through physiological integration. In most birch species, both short and long vegetative shoots can produce reproductive short shoots, while vegetative short shoots of B. grossa never produce reproductive short shoots (Ishihara and Kikuzawa, 2004). When vegetative short shoots produce fewer long shoots in a mast year than in the previous year, it may result from a trade-off for buds between reproductive short shoots and long shoots as reported for Alnus hirsuta var. sibirica (Hasegawa and Takeda, 2004), whereas in B. grossa such trade-off does not exist (Ishihara and Kikuzawa, 2004). Thus, if in B. grossa vegetative short shoots produce short shoots instead of long shoots in a mast year, it is because these vegetative short shoots are affected by other parts within the crown.
The aim of this study was to evaluate the effect of masting on the inter-annual and spatial variations in shoot demography of mature B. grossa. This species shows masting every 2–3 years (Shibata et al., 2002). Shoot demography was compared over 7 years between branches in the upper and lower parts of the tree crown. Shoot demography of mature trees was compared with those of saplings to distinguish the effect of masting from the exogenous environmental factors such as severe weather. The following questions were asked. Does masting cause annual variation in shoot demography and does it result in spatial variation due to different responses of the upper and lower branches in the crown? If masting affects shoot demography temporally and/or spatially, how is it regulated? Additionally, the effect of annual variation in shoot demography was assessed on leaf area, which may in turn affect carbon resource budget of a tree.
MATERIALS AND METHODS
Study site and materials
Field work was carried out at the Ashiu Experimental Forest of Kyoto University, Kyoto Prefecture, central Japan (35°20′N, 135°44′E). Mean annual temperatures and mean annual precipitation during 1996–2002 were 12·5 °C and 2237 mm, respectively. Cryptomeria japonica D. Don var. radicans Nakai and Fagus crenata Blume are dominant overstorey species, and Quercus crispula Blume and Betula grossa are subdominant species (Ando et al., 1993).
Three mature trees of B. grossa [19·2 ± 0·8 m (mean ± s.d.) tall and 62·8 ± 3·4 cm diameter at breast height (dbh)] were chosen in 2000, and three saplings (3·4 ± 0·1 m tall and 4·3 ± 0·3 cm dbh) in 2001. The mature trees were part of the forest canopy and the saplings were growing along logging roads. Both mature trees and saplings were under full sunlight. These saplings did not reproduce during the period of the survey. A scaffolding system was constructed in 1999 around one of the mature trees.
Census scheme
Shoot demography of the selected or harvested branches from the mature trees and saplings was investigated either by monitoring annually or by reconstructing past growth for 7 years from 1996 to 2002. Each branch contained more than ten shoots that had been produced in 1996, but one lower branch of one of the saplings contained only three shoots that had elongated in 1996.
From the mature tree with a scaffold, 22 upper branches (15·5–18·5 m above the ground) and eight lower branches (9·5–15 m) were selected in the spring of 2000 and monitored until 2002. The ambient photosynthetic photon flux densities (PPFDs) were measured around noon (1000–1300 h) on an overcast day in the summer of 2000 with a quantum sensor (IKS-27/101; Koito Industries, Yokohama, Japan). Relative PPFDs of the upper and lower branches were 65·2 ± 23·3 % (mean ± s.d.) and 21·8 ± 13·4 %, respectively. From three saplings, one upper branch (leader branch) and one lower branch from each sapling were selected in the spring of 2001 and their shoot demography were monitored until 2002. For these monitored branches, shoots that had elongated each year were classified into one of four shoot types: vegetative long shoot (Vl), reproductive long shoot (Rl), vegetative short shoot (Vs) and reproductive short shoot (Rs). Male catkins form on the tip of a long shoot and flower in the following year, while female catkins on a short shoot flower in the current year. The fate of each bud on the shoots was recorded as producing either one of the four shoot types or dying. Some shoots did not form buds and died in the following year. Such shoots were recorded as producing one dead bud. Dormant buds were excluded from the analysis because bud dormancy and shoot production from dormant buds were rare in the surveyed trees.
From the other two mature trees without scaffolds, branches were harvested from the upper crowns in 2000 and from the upper and lower crowns in 2001 and 2002. On average, 5·9 branches were harvested each year from each position in each tree. Shoot demography of these harvested branches was reconstructed for the years before the harvest back to 1996. Shoot types were identified from morphological markers (scars of bud scales, leaves and catkins) [‘retrospective reconstruction’ in Heuret et al. (2006); for detail, see Ishihara and Kikuzawa (2004)]. By using this method, shoot demography of the monitored branches was also reconstructed for the years before the monitoring back to 1996. Although saplings produced sylleptic shoots occasionally, the year when these shoots were produced could easily be determined by cross-dating with other shoots. For the years from 1996 to 1998, some long shoots on the monitored and harvested branches of mature trees had broken tips. Such long shoots were abundant, especially in 1996. It was not possible to identify whether each of these shoots was reproductive or vegetative by retrospective reconstruction. Therefore, the fate of each bud on the shoots was reconstructed only for 1998 and after.
The lengths of long shoots on the monitored and harvested branches of mature trees were measured to the nearest millimetre.
Analysis of annual variation in flowering
As an index of masting, the proportion of reproductive shoots to all shoots was calculated as the number of Rl shoots or the number of Rs shoots divided by the number of all shoots for the years 1996–2002 by pooling the branches for each position and each mature tree. The number of Rl shoots for 1996–1998 was estimated from long shoots with broken tips on the monitored and harvested branches. Most of these long shoots with broken tips had been reproductive because shoot tips of Rl shoots, where male catkins form, break a year or more after flowering. Only 17 % of Vl shoots that were produced in 1999 had broken tips 4 years later in the continuously monitored mature tree. Therefore, the number of Vl shoots with broken tips was estimated as 0·17/(1–0·17) times the number of Vl shoots with unbroken tips. The remainder of long shoots with broken tips were assumed to be reproductive. Adding the estimated number of Rl shoots to the observed number of Rl shoots with unbroken tips gave the estimated number of Rl shoots.
The variation between years in proportion of reproductive shoots was analysed by a generalized linear mixed effect model (GLMM) which used the Laplace approximation and maximum likelihood via the lmer function in the lme4 package of R 2·8·1 (R Development Core Team, 2008). All statistical tests hereafter were performed by using this software. The mixed effect model contains fixed and random effects and accounts for non-independent error due to repeated and nested measurements (Faraway, 2006). The proportions of reproductive shoots were fitted as binomial response variables with a logit-link function. The model included year as a categorical fixed effect and the variance between trees as a random effect. The effect of year was tested by the likelihood ratio test between the full model and the model without year as a fixed effect (Crawley, 2002). The effect of position within the crown was tested for the mast year with position as a fixed effect and tree as a random effect.
Analysis of shoot demography
Three demographic parameters were calculated for each branch: shoot increase rate, proportion of long shoots, and dead bud production rate. Long shoots have one or more buds, while short shoots normally have only one bud. Therefore, shoot increase rate is determined by the balance between the increase of surplus buds (new meristems) produced by long shoots and the decrease of buds by their death. Shoot increase rate from year t – 1 to t, λ(t), was defined as:
where N(t) is the total number of current year shoots in year t. The proportion of long shoots to all shoots per branch, L(t), was given by
where Nl(t) is the number of long shoots in year t. The proportion of dead buds, D(t), was given by
where Nd(t) is the number of dead buds in year t. The proportion of dead buds for t = 1997–1999 was not calculated because retrospective reconstruction of shoot demography for these years might give an underestimated value.
GLMM was used to evaluate the effects of year and position within the crown on the demographic parameters. λ(t) was analysed by N(t) as a response variable with Poisson error distribution, log-link function and N(t – 1) as an offset term. L(t) and D(t) were fitted as binomial response variables with the logit-link function. The model for saplings included year and position and interaction term as fixed effects, and tree as a random effect. In the model of mature trees, fixed effects were the same as for the model of saplings and random effects were branch nested within trees and tree. To examine the difference between years, all models under 203, 877 or 5 possible groupings of 6 years (1997–2002) were evaluated for λ(t), of 7 years (1996–2002) for L(t) and of 3 years (2000–2002) for D(t), respectively. The model with the smallest AIC value was selected as the best model (Crawley, 2002).
Analysis of length of long shoots
Differences in length of long shoots between years from 1999 to 2002 were analysed by the linear mixed effect model (LMM). Shoot length in 1996–1998 was not included because retrospective reconstruction might give underestimated values. Year was a categorical fixed effect and the variance between trees was a random effect. The effect of year was tested by the likelihood ratio test. Differences in shoot length between the upper and lower crowns or between Rl and Vl shoots were also analysed by LMM and the likelihood ratio test.
Analysis of shoot production by reproductive and vegetative shoots
To examine whether masting affects only reproductive shoots or both reproductive and vegetative shoots, the production rate of each shoot type was calculated by counting the number of each shoot type production (Rl to Vl, Vs to dead, etc.). The values of NX1X2(t), the number of shoot type X2 in year t produced by shoot type X1, were divided by the values of NX1(t – 1), which is the number of shoot type X1 in year t – 1. Production rate was calculated for each tree and each position within the crown for t = 1999–2002. The variation between years was analysed by GLMM with NX1X2(t) as a response variable with Poisson error distribution, log-link function and NX1(t – 1) as an offset term. Since only the effect of year was of interest, the model contained year as a categorical fixed effect and position nested in tree and tree as random effects. The effect of year was tested by the likelihood ratio test and multiple comparisons via the glht function in the multicomp package with adjusted P-values.
Estimation of leaf area
Leaf area of each sampled upper branch of mature trees was estimated for 1999–2002. Leaves of 48 Vs shoots and 14 Rs shoots were sampled in year 2000 and those of 54 Vl shoots and 22 Rl shoots in 2002 from the upper crowns when the leaves were fully expanded. The leaf area of each of the sampled long shoots was estimated by either of two methods: (1) direct measurement of lamina area by the image analysis program Scion Image (Scion Corporation, Frederick, MD, USA); (2) estimation of lamina area (Al) from lamina length (Ll) using a close relationship between the two (early leaves: Al = 0·302Ll2 + 5·577, r2 = 0·83, n = 31; late leaves: Al = 0·317 · Ll2 + 0·159, r2 = 0·98, n = 87). A close relationship was found between shoot length (Ls) and leaf area of a long shoot (As) (Vl shoots: As = 5·55Ls + 33·29, r2 = 0·92; Rl shoots: As = 5·65Ls + 20·71, r2 = 0·87). The leaf area of each long shoot was estimated from this relationship and was summed to give the total leaf area of long shoots on each branch. Since leaf area of a short shoot was not different from the normal distribution (Kolmogorov–Smirnov test, P > 0·29), the total leaf area of short shoots on each branch was estimated by multiplying mean leaf area of a short shoot (Vs shoots, 34·03 cm2; Rs shoots, 22·74 cm2) by the number of short shoots. Total leaf area of each branch was estimated by summing the total leaf area of long shoots and those of short shoots. The effect of year on total leaf area was evaluated by LMM with year as a categorical fixed effect and branch nested within trees and tree as random effects.
RESULTS
Annual variation in flowering
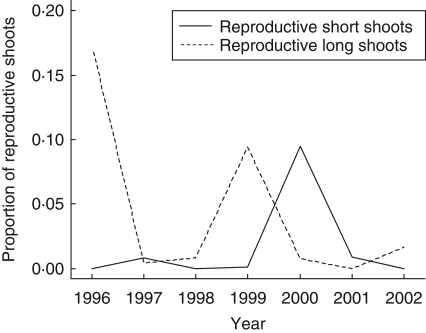
The proportions of reproductive shoots to total shoots varied between years (Fig. 1; Rl shoots: χ26 = 1201·8, P < 0·001; Rs shoots: χ26 = 2380·8, P < 0·001). Proportion of Rl shoots increased in 1996 and 1999, and that of Rs shoots increased in 2000. Therefore, there were two mast years, 1997 and 2000, during the 7 years because male catkins on Rl shoots bloom in the next spring, while female catkins on Rs shoots bloom in the current year (Ishihara and Kikuzawa, 2004). The proportion of Rs shoots in 1997 must have been underestimated because Rs shoots die and fall a year after flowering (Ishihara and Kikuzawa, 2004) and could not be detected by the retrospective survey. The proportions of reproductive shoots were higher in the upper crown than in the lower crown (Rl shoots in 1999: χ21 = 350·18, P < 0·001; Rs shoots in 2000: χ21 = 478·39, P < 0·001).
Fig. 1.
Proportions of reproductive long and short shoots in mature trees in years 1996–2002. Average values for branches in different positions within a crown and for different trees are shown. Reproductive long shoots form male catkins, which flower in the next year, and reproductive short shoots produce female catkins flowering in the current year. Proportions of reproductive long shoots in years 1996–1998 were estimated.
Shoot demography
The most-parsimonious models included year, position and an interaction term as fixed effects in both mature trees and saplings, except for the proportion of dead buds in saplings (Table 1). In mature trees, the model with only year as a fixed effect performed nearly as well as the model with both year and position as fixed effects. In saplings, the model with both position and year as fixed effects performed better than the model with only year as a fixed effect.
Table 1.
Summary of generalized linear mixed effect model (GLMM) for (a) shoot increase rate, (b) proportion of long shoots and (c) proportion of dead buds in mature trees and saplings
| Fixed effects of candidate models | AIC | LL | DF | %DE |
|---|---|---|---|---|
| (a) Shoot increase rate | ||||
| (i) Mature trees | ||||
| Position + year (2001 + 2002) + position × year | 1352 | −664 | 12 | 58·1 |
| Position + year + position × year | 1354 | −663 | 14 | 58·1 |
| Position + year | 1391 | −686 | 9 | 56·6 |
| Year | 1391 | −688 | 8 | 56·6 |
| Position | 3167 | −1579 | 4 | 0·2 |
| Null | 3173 | −1583 | 3 | 0 |
| (ii) Saplings | ||||
| Position + year + position × year | 205 | −89 | 13 | 84·6 |
| Position + year | 205 | −95 | 8 | 83·7 |
| Year | 349 | −167 | 7 | 71·2 |
| Position | 1102 | −548 | 3 | 5·8 |
| Null | 1168 | −582 | 2 | 0 |
| (b) Proportion of long shoots | ||||
| (i) Mature trees | ||||
| Position + year + position × year | 1989 | −979 | 16 | 61·6 |
| Position + year | 1995 | −988 | 10 | 61·3 |
| Year | 2044 | −1013 | 9 | 60·3 |
| Position | 5048 | −2520 | 4 | 1·2 |
| Null | 5106 | −2550 | 3 | 0 |
| (ii) Saplings | ||||
| Position + year + position × year | 180 | −75 | 15 | 75·7 |
| Position + year | 186 | −84 | 9 | 72·8 |
| Year | 258 | −121 | 8 | 60·6 |
| Position | 586 | −290 | 3 | 5·7 |
| Null | 619 | −308 | 2 | 0 |
| (c) Proportion of dead buds | ||||
| (i) Mature trees | ||||
| Position + year + position × year | 807 | −395 | 8 | 52·1 |
| Position + year | 827 | −407 | 6 | 50·7 |
| Year | 837 | −414 | 5 | 50·0 |
| Position | 1656 | −824 | 4 | 0·3 |
| Null | 1659 | −826 | 3 | 0 |
| (ii) Saplings | ||||
| Position + year | 53 | −21 | 5 | 67·7 |
| Position + year + position × year | 55 | −20 | 7 | 69·0 |
| Year | 70 | −31 | 4 | 53·1 |
| Position | 123 | −59 | 3 | 10·9 |
| Null | 136 | −66 | 2 | 0 |
Shown are AIC, log-likelihood (LL), degree of freedom (DF), the percentage deviance explained (%DE) of the models. The model with lowest AIC values are selected. Random effects are not shown.
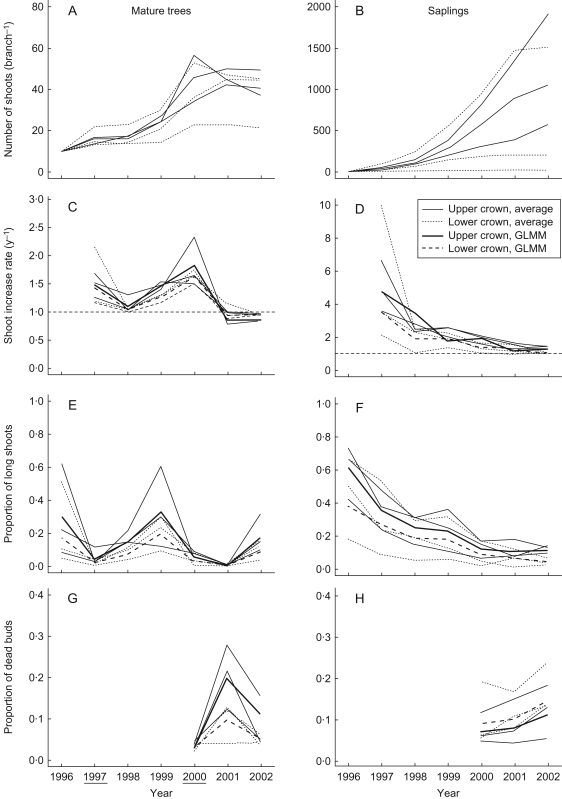
The number of shoots of the mature trees increased more gradually than those of the saplings (Fig. 2A, B). The shoot number increase rate in mature trees fluctuated from year to year (Fig. 2C). The annual variation was synchronized between branches even in different positions. The increase rate was high in the mast year (1997 and 2000) and lower in the post-mast years (1998 and 2001). Increase rate was higher in the upper branches than in the lower branches in the years when the rate was high. However, in 2001 and 2002, the rate for the upper branches was lower than that for the lower branches. In saplings, the increase rate decreased from 1997 to 2002 in both the upper and lower branches (Fig. 2D). The rate was higher in the upper branches than in the lower branches in some years.
Fig. 2.
Shoot demography in mature trees (A, C, E, G) and saplings (B, D, F, H) in years 1996–2002: (A, B) number of shoots; (C, D) shoot increase rate; (E, F) proportion of long shoots to all shoots; and (G, H) proportion of dead buds to all buds of branches. Each thin continuous and dotted line represents the average value of the branches in the upper and lower crowns of each tree, respectively. Continuous thick and dashed lines show the estimated values by GLMM for the upper and lower branch, respectively. Years underlined were mast years. Because the lower branch of one sapling contained only three shoots that had elongated in 1996, the number of shoots was multiplied by 10/3 for comparison with other branches.
The proportion of long shoots in the mature trees was high in the pre-mast years (1996 and 1999), and lower in the mast and post-mast years (Fig. 2E). The pattern of fluctuation was repeated the year after by the shoot increase rate. The proportion of long shoots in the upper branches was higher than that of the lower branches only in years when the proportion was high. In saplings, the proportion of long shoots decreased from 1997 to 2002 in both the upper and lower branches (Fig. 2F). The proportion in the upper branches was higher than that in the lower branches throughout the years.
The proportion of dead buds in mature trees increased in the post-mast year (2001) (Fig. 2G). The proportion in the upper branches was higher than that in the lower branches in 2001 and 2002. In saplings, the proportion of dead buds increased from 2000 to 2002 (Fig. 2H). The proportion in the upper branches was lower than that in the lower branches in all three years.
Shoot length of long shoots
Annual variation in shoot length was only testable for Vl shoots in the upper branches because Vl shoots in the lower branches or Rl shoots were few in some years. The length of Vl shoots in the upper branches was significantly different between years (Table 2; χ22 = 59·3, P < 0·001) and was shorter in 2000 (mast year) than in the pre-mast year. The comparisons of shoot length between the upper and lower crown and between Vl and Rl shoots were possible only for 1999, when long shoots were abundant. Vl shoots in the upper crown were shorter than those in the lower crown (χ21 = 36·8, P < 0·001). The length of Rl shoots was not significantly different from Vl shoots (χ21 = 3·6, P = 0·06).
Table 2.
Effect of years, positions (upper and lower crown) and shoot types (vegetative and reproductive shoots) on long shoot length in mature trees
| Type of long shoot | Position | Year† | Estimated shoot length (cm) |
|---|---|---|---|
| (a) Effect of years (***) | |||
| Vegetative | Upper | 1999 (pre-mast year) | 8·8 (346) |
| Vegetative | Upper | 2000 (mast year) | 7·0 (164) |
| Vegetative | Upper | 2002 | 8·4 (398) |
| (b) Effect of positions (***) | |||
| Vegetative | Upper | 1999 | 9·1 (346) |
| Vegetative | Lower | 1999 | 10·7 (122) |
| (c) Effect of shoot types (n.s.) | |||
| Reproductive | Upper | 1999 | 9·3 (341) |
| Vegetative | Upper | 1999 | 8·9 (346) |
Estimated mean lengths from linear mixed effect models (LMM) are shown.
Numbers in parentheses are the number of replicates.
*** Significant difference between years or positions at P < 0·001; n.s., not significant.
†Effects of years and position were not testable for reproductive shoots. Shoot length in year 2001 was not obtained because few long shoots were produced in that year (see Fig. 2).
Shoot production by vegetative and reproductive shoots
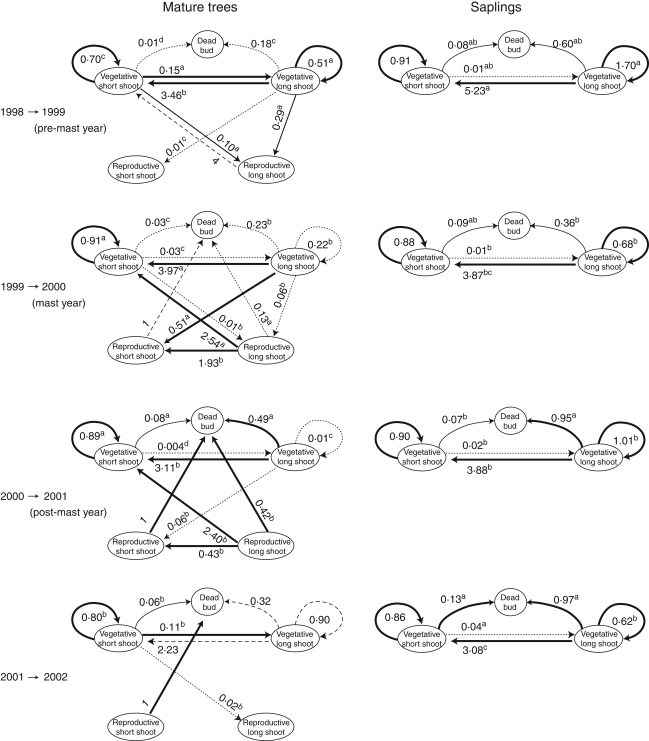
The shoot production rate differed qualitatively between shoot types in mature trees (Fig. 3). Rl shoots never produced long shoots regardless of years, while Vl shoots did. Rs shoots did not form buds, never produced any shoots and died in the following year. Vs shoots produced any one of the shoot types except Rs shoots.
Fig. 3.
Shoot production pathways in mature trees and saplings in years 1998--2002. Thick and dotted arrows indicate production rate higher than 10% or lower than 5%, respectively, of the total production rates of each shoot type. Values along arrows are production rates (numbers of new shoots or dead buds produced from each of four shoot types from year t – 1 to t) averaged for the branches in different positions within a crown and for different trees. Different letters indicate significant difference between years at P = 0·001 from multiple comparison. When the number of shoots of a shoot type was ≤10 in any crown positions of any trees, the pathways from that shoot type (dashed arrows) are omitted from the statistical test.
Vegetative shoots changed shoot production from year to year. In the mast year (2000), Vl shoots produced fewer long shoots than in the pre-mast year and fewest in the post-mast year (from Vl to Vl: χ22 = 126·1, P < 0·001; from Vl to Rl: χ22 = 220·5, P < 0·001). Vl shoots produced more short shoots in the mast year than in the pre-mast year (from Vl to Vs: χ22 = 33·5, P < 0·001; from Vl to Rs shoots: χ22 = 168·6, P < 0·001) and more dead buds in the post-mast year than other years (χ22 = 70·1, P < 0·001).
Vs shoots showed similar annual variation to Vl shoots. Vs shoots produced fewer long shoots and more Vs shoots in the mast year and the post-mast year than in the pre-mast year (from Vs to Vl: χ23 = 964·3, P < 0·001; from Vs to Rl: χ23 = 704·8, P < 0·001; from Vs to Vs: χ23 = 120·2, P < 0·001). Production of long shoots by Vs shoots recovered in 2002 to some extent. Vs shoots produced more dead buds in the post-mast year (χ23 = 206·6, P < 0·001) than other years.
Saplings showed only quantitative difference between years. Vl shoots produced both Vl shoot and Vs shoots. Vs shoots scarcely produced Vl shoots, regardless of years.
Leaf area
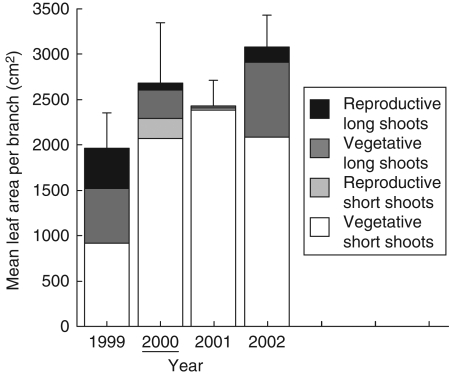
The total leaf area of an upper branch differed between years from 1999 to 2002 (Fig. 4, χ23 = 48·38, P < 0·001). Leaf area increased from the pre-mast year (1999) to the mast year (2000) (χ21 = 48·15, P < 0·001). In 2000, total leaf area of long shoots decreased because the number of long shoots decreased (Fig. 2); however, the total leaf area of short shoots increased > 2-fold because many short shoots were produced in that year.
Fig. 4.
Leaf area per upper branch on four shoot types in mature trees. Columns and vertical lines represent the mean values (+s.d.) for different branches and different trees. Year 2000 was a mast year.
DISCUSSION
Annual variation in shoot demography
Shoot number increase rate, proportion of long shoots, bud mortality, and length of long shoots fluctuated markedly from year to year in mature trees over 7 years (Fig. 2). The fluctuation was caused by two masting events. Exogenous environmental factors such as severe weather cannot be the cause of fluctuation because the saplings did not show such fluctuation in shoot demography. The saplings showed a gradual yearly change in shoot demography, which is a typical growth pattern of saplings and young trees (Maillette, 1982b; Jones and Harper, 1987). As branches grow, they have more shaded and high-order shoots, which produce only short shoots and experience high mortality. In mature trees, such an effect of branch development on shoot demography was much weaker than the effect of masting.
The present results concur with previous studies. Shoot length was shorter in the mast year as reported for Douglas-fir (Tappeiner II, 1969) and for Betula alleghaniensis and B. papyrifera (Gross, 1972). Shoot mortality in the post-mast year was high as reported for other birches (Gross, 1972) and for beech (Innes, 1994). However, present long-term demographic analysis found that the number of shoots increased at a higher rate in the mast years than in other years. Masting caused periodic annual variation in shoot demography of masting trees. This means that any short-term study should be carefully interpreted, otherwise it might lead to wrong conclusions about shoot production in mature masting trees.
Spatial variation in shoot demography between branches
Annual variation in shoot demography was synchronized even between branches in different positions to some extent and the temporal variation was larger than the spatial variation in mature trees (Table 1). This is not an artefact due to sampling design because the effect of branch position on shoot demography was apparent in saplings. The extent of fluctuation was larger in the upper branches than in the lower branches.
The spatial variation was apparent only in particular years. The proportion of long shoots and shoot increase rate were higher in the upper branches than those in the lower branches in the pre-mast and mast years, respectively (Fig. 2). Such spatial pattern was found in saplings in most years (Fig. 2), in young B. pendula (Maillette, 1982b; Jones and Harper, 1987) and in other species (Takenaka, 2000). It has been regarded as an effective strategy for growth and light capture of saplings and young trees (Takenaka, 1994; Sterck and Schieving, 2007). However, other spatial variations in mature trees contradicted those of saplings. Shoot increase rate was lower and bud mortality was higher in the upper branches than in the lower branches in one of two post-mast years in the mature trees (Fig. 2). This is because reproductive short shoots, which die in post-mast years, were more abundant in the upper crown and increased bud mortality. Furthermore, vegetative long shoots were shorter in the upper crown than in the lower crown in the pre-mast year (Table 2). This result is the opposite of the pattern found in young B. pendula (Maillette, 1982b; Jones and Harper, 1987) and may reflect water deficit in the upper crown or the etiolation in the dark lower crown in mature trees. These results suggest that spatial organization of shoot demography in mature masting trees is reproductive oriented rather than growth oriented. All branches within a crown are affected by masting with the effect dependent on position or light availability of a branch.
Regulation of annual variation in shoot demography associated with masting
Both reproductive and vegetative shoots contributed to the shoot dynamics in mature trees. Reproductive shoots produced shoots differently from vegetative shoots (Fig. 3), although the lengths of these two shoot types were same (Table 2). The difference between reproductive and vegetative shoots may be due to both morphological constraints and resource or meristem limitation (Ishihara and Kikuzawa, 2004). Because the number of reproductive shoots increased as masting occurred, shoot production patterns specific to reproductive shoots caused dynamic shoot demography. Vegetative shoots did not offset such constraints imposed by reproductive shoots.
Vegetative shoots changed the fate of buds between dead buds, long shoots and short shoots from year to year (Fig. 3). Buds on vegetative long shoots that were to differentiate into long shoots were used instead for reproductive short shoots in the mast year, and there was a trade-off for buds between long shoots and reproductive short shoots. However, vegetative short shoots have no such trade-off because they never produced reproductive short shoots (Fig. 3; Ishihara and Kikuzawa, 2004). Thus, the change of bud fate found in vegetative short shoots suggests that these shoots were influenced not only by their own internal or environmental factors but also by factors from other parts within a tree through hormonal regulation or resource allocation.
Reduced shoot growth associated with masting has been understood as a switching of resource allocation from vegetative growth to reproduction, and assumed as a key evidence for the resource allocation mechanisms exaggerating the annual variation in crop size (Kelly and Sork, 2002). However, the present study suggests that resource depletion due to masting is insufficient to explain all aspects of the dynamic shoot demography associated with masting. Resource depletion due to mass flowering and seeding cannot affect the fate of buds on vegetative short shoots in the mast year. The fate of buds is determined in the summer of the previous year in B. platyphylla (Kumada, 1979) and in B. papyrifera (Macdonald et al., 1984). If B. grossa has a similar schedule of bud differentiation, the shift of bud fate on vegetative short shoots from long shoots to vegetative short shoots in the mast year is already determined in the pre-mast year and should be related to hormonal regulation rather than resource depletion due to flowering and fruit production. Resource depletion due to mast seeding might have changed the fate of a bud from long shoots to short shoots or dead buds in the post-mast year. It might have also reduced the shoot length of long shoots in the mast year (Table 2) because shoot elongation is assumed to be affected by the amount of current-year assimilates in other birch species (Kozlowski and Clausen, 1966; Henriksson and Ruohomäki, 2000). By using B. grossa, it was found that masting affects shoot demography in a physiologically integrated manner through hormonal regulation and resource depletion.
Effect of annual variation in shoot demography on the resource budget of trees
Total leaf area per upper branch increased from the pre-mast year (1999) to the mast year (2000; Fig. 4). This is because many long shoots were produced in the pre-mast year, which increased the number of shoots in the mast year (Fig. 2). The number of shoots increased so much that it compensated for the decrease in total leaf area on long shoots. The leaf area of lower branches may also have increased in the mast year because the number of shoots on the lower branches increased in the mast years. No branch death or a flush of epicormic shoots from the pre-mast year to the mast year were observed in the continuously monitored mature tree. Therefore, total leaf area of a tree may have increased in the mast year. Annual variation in leaf production associated with masting may be quite common among masting species, although the pattern may be species specific. Reduction in leaf litter in mast years has been reported in Fagus crenata (Kawada and Maruyama, 1986), which suggests that leaf production is reduced in mast years. In three evergreen oak species, leaf litter in a heavy-crop year decreased to 2–47 % of leaf litter in a low-crop year, suggesting that new shoot and leaf production was reduced in the heavy-crop year (Hirayama et al., 2008).
Annual variation in shoot demography and leaf production may result in the annual fluctuation in the amount of photosynthate produced every year. Although, in some species, carbon resources needed for mass fruiting are supplied by stored assimilates (e.g. Goldschmidt and Golomb, 1982; Miyazaki et al., 2002) and by current photosynthates of reproductive shoots (Hasegawa et al., 2003; Ichie et al., 2005), current photosynthates from other shoots or branches are also used for fruit maturation in some species (Miyazaki et al., 2007) and when reproductive branches were defoliated (Hoch, 2005). A larger leaf area in mast years might result in an increased net production, which to some extent might compensate for the resource depletion by a large crop in B. grossa. However, resource budget models assumed a constant amount of current photosynthate and focused only on fluctuation of stored reserves (Isagi et al., 1997; Satake and Bjørnstad, 2008). Recent studies incorporated annual variation in carbon acquisition due to variation in the external environment such as weather (e.g. Masaka, 2001). Incorporating temporal fluctuation in shoot demography and leaf area to resource budget models may deepen our understanding of the mechanism of masting.
Shoots function as both source and sink of carbon resources. Long shoots are costly but inevitable investments for tree growth and to increase the number of shoots, while short shoots are less costly investments specialized for leaf display (Maillette, 1982a; Jones and Harper, 1987; Karlsson et al., 1996). Furthermore, long shoots are prerequisite for production of male flowers in birches and for production of both male and female flowers in B. grossa (Ishihara and Kikuzawa 2004). Changing resource allocation from a year with relatively more long shoots to a year with a large crop and increased number of short shoots may achieve a temporal division of labour. It might be another type of compensatory mechanism to reduce the cost of reproduction (Obeso, 2002). Comparative studies on long-term shoot demography between different masting species may lead to further understanding of resource acquisition and allocation mechanisms in masting trees.
ACKNOWLEDGEMENTS
We thank Takanobu Yagi, Maki Suzuki, Michimasa Yamasaki, Michinori Sakimoto, Shigeaki Hasegawa, Onno Muller, and all members of the Laboratory of Forest Biology, Kyoto University. The staff of the Ashiu Experimental Forest, Kyoto University, helped with the sampling. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 11213202), and partly by the 21st Century COE Program, Kyoto University, ‘Innovative Food and Environmental Studies Pioneered by Entomomimetic Sciences’.
LITERATURE CITED
- Ando M, Sakai T, Wada S. The dynamics of natural forest on cool temperate deciduous forest zone mixing sugi (Cryptomeria japonica) trees. 1. Change of population and growing stock on Masukami permanent plot of University Forest in Ashiu for 8 years. Bulletin of the Kyoto University Forest. 1993;24:37–44. [Google Scholar]
- Crawley MJ. Statistical computing: an introduction to data analysis using S-Plus. Chichester: John Wiley and Sons; 2002. [Google Scholar]
- Faraway JJ. Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. Boca Raton, FL: Chapman and Hall; 2006. [Google Scholar]
- Goldschmidt EE, Golomb A. The carbohydrate balance of alternate bearing citrus trees and the significance of reserves for flowering and fruiting. Journal of the American Society for Horticultural Science. 1982;107:206–208. [Google Scholar]
- Gross HL. Crown deterioration and reduced growth associated with excessive seed production by birch. Canadian Journal of Botany. 1972;50:2431–2437. [Google Scholar]
- Hasegawa S, Takeda H. Current-year shoot based approach for annual variation in the reproductive output in Siberian alder (Alnus hirsuta var. sibirica) Trees – Structure and Function. 2004;18:436–441. [Google Scholar]
- Hasegawa S, Koba K, Tayasu I, Takeda H, Haga H. Carbon autonomy of reproductive shoots of Siberian alder (Alnus hirsuta var. sibirica) Journal of Plant Research. 2003;116:183–188. doi: 10.1007/s10265-003-0085-7. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Ruohomäki K. Assessing cost of reproduction in mountain birch: the importance of considering the modular level. Annals of Botany. 2000;86:503–510. [Google Scholar]
- Heuret P, Meredieu C, Coudurier T, Courdier F, Barthélémy D. Ontogenetic trends in the morphological features of main stem annual shoots of Pinus pinaster (Pinaceae) American Journal of Botany. 2006;93:1577–1587. doi: 10.3732/ajb.93.11.1577. [DOI] [PubMed] [Google Scholar]
- Hirayama D, Nanami S, Itoh A, Yamakura T. Individual resource allocation to vegetative growth and reproduction in subgenus Cyclobalanopsis (Quercus, Fagaceae) trees. Ecological Research. 2008;23:451–458. [Google Scholar]
- Hoch G. Fruit-bearing branchlets are carbon autonomous in mature broad-leaved temperate forest trees. Plant, Cell & Environment. 2005;28:651–659. [Google Scholar]
- Ichie T, Kenzo T, Kitahashi Y, Koike T, Nakashizuka T. How does Dryobalanops aromatica supply carbohydrate resources for reproduction in a masting year? Trees – Structure and Function. 2005;19:703–710. [Google Scholar]
- Innes JL. The occurrence of flowering and fruiting on individual trees over 3 years and their effects on subsequent crown condition. Trees – Structure and Function. 1994;8:139–150. [Google Scholar]
- Isagi Y, Sugimura K, Sumida A, Ito H. How does masting happen and synchronize? Journal of Theoretical Biology. 1997;187:231–239. [Google Scholar]
- Ishihara M, Kikuzawa K. Species-specific variation in shoot production patterns of five birch species with respect to vegetative and reproductive shoots. Canadian Journal of Botany. 2004;82:1393–1401. [Google Scholar]
- Jones M, Harper JL. The influence of neighbours on the growth of trees. I. The demography of buds in Betula pendula. Proceedings of the Royal Society of London B. 1987;232:1–18. [Google Scholar]
- Kaitaniemi P, Ruohomäki K. Factors controlling resource allocation in mountain birch. Perspectives in Plant Ecology Evolution and Systematics. 2003;5:231–249. [Google Scholar]
- Karlsson PS, Olsson L, Hellström K. Trade-offs among different long-shoot functions: variation among mountain birch individuals. Journal of Ecology. 1996;84:915–921. [Google Scholar]
- Kawada H, Maruyama K. Effect of seed bearing of a natural beech (Fagus crenata Blume) forest on amount of litter fall and its nutrients. Japanese Journal of Ecology. 1986;36:3–10. [Google Scholar]
- Kelly D, Sork VL. Mast seeding in perennial plants: why, how, where? Annual Review of Ecology and Systematics. 2002;33:427–447. [Google Scholar]
- Kozlowski TT, Clausen JJ. Shoot growth characteristics of heterophyllous woody plants. Canadian Journal of Botany. 1966;44:827–843. [Google Scholar]
- Kumada H. Growth and development of long and short shoots in Betula platyphylla. I. Process of differentiation of axillary buds to long and short shoot buds. Transactions of the meeting in Hokkaido Branch of the Japanese Forestry Society. 1979;28:99–102. [in Japanese] [Google Scholar]
- Lehtilä K, Tuomi J, Sulkinoja M. Bud demography of the mountain birch Betula pubescens spp. tortuosa near tree line. Ecology. 1994;75:945–955. [Google Scholar]
- Macdonald AD, Mothersill DH. Shoot development in Betula papyrifera. I. Short-shoot organogenesis. Canadian Journal of Botany. 1983;61:3049–3065. [Google Scholar]
- Macdonald AD, Mothersill DH, Caesar JC. Shoot development in Betula papyrifera. III. Long shoot organogenesis. Canadian Journal of Botany. 1984;62:437–445. [Google Scholar]
- Maillette L. Structural dynamics of silver birch. I. The fate of buds. Journal of Applied Ecology. 1982;a 19:203–218. [Google Scholar]
- Maillette L. Structural dynamics of silver birch. II. A matrix model of the bud population. Journal of Applied Ecology. 1982;b 19:219–238. [Google Scholar]
- Masaka K. Modeling the masting behaviour of Betula platyphylla var. japonica using the resource budget model. Annals of Botany. 2001;88:1049–1055. [Google Scholar]
- Miyazaki Y, Hiura T, Kato E, Funada R. Allocation of resources to reproduction in Styrax obassia in a masting year. Annals of Botany. 2002;89:767–772. doi: 10.1093/aob/mcf107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Hiura T, Funada R. Allocation of photo-assimilated C-13 from reproductive and non-reproductive shoots to fruits in Styrax obassia. Plant Species Biology. 2007;22:53–57. [Google Scholar]
- Newell EA. Direct and delayed costs of reproduction in Aesculus californica. Journal of Ecology. 1991;79:365–378. [Google Scholar]
- Obeso JR. The costs of reproduction in plants. New Phytologist. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. (http://www.R-project.org. ) [Google Scholar]
- Satake A, Bjørnstad ON. A resource budget model to explain intraspecific variation in mast reproductive dynamics. Ecological Research. 2008;23:3–10. [Google Scholar]
- Shibata M, Tanaka H, Iida S, et al. Synchronized annual seed production by 16 principal tree species in a temperate deciduous forest, Japan. Ecology. 2002;83:1727–1742. [Google Scholar]
- Silvertown JW. The evolutionary ecology of mast seeding in trees. Biological Journal of the Linnean Society. 1980;14:235–250. [Google Scholar]
- Sprugel DG, Hinckley TM, Schaap W. The theory and practice of branch autonomy. Annual Review of Ecology and Systematics. 1991;22:309–334. [Google Scholar]
- Sterck FJ, Bongers F. Crown development in tropical rain forest trees: patterns with tree height and light availability. Journal of Ecology. 2001;89:1–13. [Google Scholar]
- Sterck FJ, Schieving F. 3-D growth patterns of trees: effects of carbon economy, meristem activity, and selection. Ecological Monographs. 2007;77:405–420. [Google Scholar]
- Sterck FJ, Bongers F, During HJ, Martinez-Ramos M, De Kroon H. Module responses in a tropical forest tree analyzed with a matrix model. Ecology. 2003;84:2751–2761. [Google Scholar]
- Takenaka A. A simulation-model of tree architecture development based on growth-response to local light environment. Journal of Plant Research. 1994;107:321–330. [Google Scholar]
- Takenaka A. Shoot growth responses to light microenvironment and correlative inhibition in tree seedlings under a forest canopy. Tree Physiology. 2000;20:987–991. doi: 10.1093/treephys/20.14.987. [DOI] [PubMed] [Google Scholar]
- Tappeiner J., II Effect of cone production on branch, needle, and xylem ring growth of Sierra Nevada Douglas-fir. Forest Science. 1969;15:171–174. [Google Scholar]
- Waller DM, Steingraeber DA. Opportunities and constraints in the placement of flowers and fruits. In: Gartner BL, editor. Plant stems: physiology and functional morphology. San Diego, CA: Academic Press; 1995. [Google Scholar]
- Wünsche JN, Lakso AN, Robinson TL, Lenz F, Denning SS. The bases of productivity in apple production systems: the role of light interception by different shoot types. Journal of the American Society for Horticultural Science. 1996;121:886–893. [Google Scholar]