Abstract
Background and Aims
Stable isotopes have proved a valuable phenotyping tool when breeding for yield potential and drought adaptation; however, the cost and technical skills involved in isotope analysis limit its large-scale application in breeding programmes. This is particularly so for Δ18O despite the potential relevance of this trait in C4 crops. The accumulation of minerals (measured as ash content) has been proposed as an inexpensive way to evaluate drought adaptation and yield in C3 cereals, but little is known of the usefulness of this measure in C4 cereals such as maize (Zea mays). The present study investigates how yield relates to ash content, Δ13C and Δ18O, and evaluates the use of ash content as an alternative or complementary criterion to stable isotopes in assessing yield potential and drought resistance in maize.
Methods
A set of tropical maize hybrids developed by CIMMYT were subjected to different water availabilities, in order to induce water stress during the reproductive stages under field conditions. Ash content and Δ13C were determined in leaves and kernels. In addition, Δ18O was measured in kernels.
Key Results
Water regime significantly affected yield, ash content and stable isotopes. The results revealed a close relationship between ash content in leaves and the traits informing about plant water status. Ash content in kernels appeared to reflect differences in sink–source balance. Genotypic variation in grain yield was mainly explained by the combination of ash content and Δ18O, whilst Δ13C did not explain a significant percentage of such variation.
Conclusions
Ash content in leaves and kernels proved a useful alternative or complementary criterion to Δ18O in kernels for assessing yield performance in maize grown under drought conditions.
Keywords: Ash content, Δ13C, Δ18O, Zea mays, water stress, drought resistance, grain yield
INTRODUCTION
Drought is the major factor constraining world maize (Zea mays) production (Bänziger and Araus, 2009), particularly in tropical areas (Edmeades et al., 1989) where rain-fed maize is a staple food for millions of people. Thus, improving yield potential and drought adaptation are among the main targets in most maize breeding programmes. Yet the evaluation of appropriate phenotyping traits remains one of the bottlenecks in cereal breeding for yield potential and drought adaptation (Araus et al., 2008). In the case of maize, a short anthesis-to-silking interval has proved a useful trait (Bänziger et al., 2000), but recurrent use over breeding cycles leads to a reduction in their genetic variation and further advances in this regard remain uncertain (Monneveux et al., 2008).
Carbon isotope discrimination (Δ13C) has been used in C3 cereals as a surrogate method for screening genotypes with higher water-use efficiency (WUE; the ratio of biomass accumulation to water consumed) (Farquhar and Richards, 1984; Rebetzke et al., 2002); and some drought-tolerant cultivars have been bred using low Δ13C (i.e. high WUE) as a selecting trait (Rebetzke et al., 2002). However, few reports exploring the use of Δ13C in maize are available (but see Heng et al., 2005; Dercon et al., 2006; Monneveux et al., 2007) and the results are not encouraging, mainly due to the C4 metabolism of this species, given that C4 plants are characterized by lower Δ13C than C3 plants (Farquhar, 1983; Bowman et al., 1989). Nevertheless, Δ13C has been successfully used in other species with C4 metabolism, such as sorghum (Sorghum bicolor), to track genotypic differences in transpiration efficiency (i.e. instantaneous WUE at the leaf level) and yield (Hubick et al., 1990; Hammer et al., 1997; Henderson et al., 1998). Therefore, further investigation is justified in maize plants grown under a wide range of growing conditions. Moreover, although much attention has focused on improving WUE when breeding for drought adaptation, it seems that, except for very severe drought conditions, water use (WU, i.e. the total water absorbed and further transpired by the plant) is a more important adaptive trait than WUE (Araus et al., 2002b, 2008; Blum, 2005, 2009; Slafer and Araus, 2007). This is related to the genotypic capacity to use available water and therefore to sustain transpiration under unfavourable conditions (Blum, 1993; Morgan et al., 1993; Slafer et al., 1999). Thus, when analysed in kernels (or other organs developed late during the crop cycle) of C3 cereals, high Δ13C seems to reflect genotypic differences in WU (Araus et al., 2003). This in turn may explain the positive relationship between high Δ13C (and thus high WU) and yield in a wide range of growing conditions, ranging from fully watered to drought stresses decreasing yield by up to approx. 70 % (Araus et al., 2002b, 2003; Slafer and Araus, 2007).
Alternatively, oxygen isotope enrichment (Δ18O) measured in plant tissue can be used either in C3 or in C4 grasses (Helliker and Ehleringer, 2002; Ogée et al., 2007; Araus et al., 2008), owing to its independence of photosynthetic processes, and has therefore been proposed as an indirect measure of transpiration (Sheshshayee et al., 2005; Cernusak et al., 2007, 2008) and WU (Cabrera-Bosquet et al., 2009a), as well as an integrative indicator of genetic differences in stomatal conductance (gs) and yield in cereals such as wheat (Barbour et al., 2000; Ferrio et al., 2007; Cabrera-Bosquet et al., 2009a) and maize (Cabrera-Bosquet et al., 2009b). However, given the cost (over US $15 per sample, twice that for Δ13C) and technical skills and facilities involved in oxygen isotope analysis, its large-scale application to breeding programmes is at present unfeasible.
The accumulation of mineral or ash content in both vegetative tissues (Masle et al., 1992; Mayland et al., 1993; Araus et al., 1998; Monneveux et al., 2004) and kernels (Febrero et al., 1994; Voltas et al., 1998; Araus et al., 1998; Merah et al., 1999, 2001) have been proposed as inexpensive (approx. US $2 per sample) and simple ways to predict yield and genotypic adaptation to drought in different C3 cereals. The mechanism of mineral accumulation in vegetative tissues appears to be explained through the passive transport of minerals via xylem driven by transpiration (Masle et al., 1992; Mayland et al., 1993; Araus et al., 2002a, b). Conversely, mineral accumulation in mature kernels takes place via phloem (Nonogaki et al., 2007). Thus, whereas ash content measured in vegetative tissues provides an indicator of transpirative gas-exchange activity and therefore of the total water transpired (Araus et al., 1998, 2001), ash content in mature kernels could provide information on the integrated photosynthetic and retranslocation processes during grain filling (Febrero et al., 1994; Voltas et al., 1998; Araus et al., 1998, 2001). In such a way, leaf and kernel ash content have been correlated with yield in barley (Febrero et al., 1994; Voltas et al., 1998) and wheat (Araus et al., 1998; Merah et al., 1999, 2001; Monneveux et al., 2004) grown under different water regimes. Interestingly, such an approach can be used in both C3 and C4 crops (Masle et al., 1992; Araus et al., 2001). However, the only existing report in maize (Tanner and Beevers, 1990) investigated vegetative tissues in plants grown in both hydroculture and soil in pots, and the relationships between ash content and genotypic differences in grain yield were not explored.
In a recent study, the physiological basis of variation on Δ18O measured in leaves and kernels was examined, as well as its feasibility to track differences in growth, yield and water status of a set of tropical maize hybrids grown under three contrasting water regimes (Cabrera-Bosquet et al., 2009b). Following this research, as a first objective, the present investigation was developed to study the effect of inducing water stress during the reproductive stages on mineral accumulation in leaves and kernels (measured as ash content) and Δ13C in comparison with Δ18O in kernels. Secondly, the potential use of ash content and Δ13C as alternative or complementary criteria to stable Δ18O in the evaluation of differences in yield potential and drought resistance in maize were studied.
MATERIALS AND METHODS
Germplasm and growth conditions
A set of 16 maize (Zea mays L.) single hybrids were used, together with one commercial hybrid (‘Puma’) used as a check. Briefly, single hybrids were generated by crossing drought-tolerant lines derived from a La Posta Sequía (LPS) population with the tropical testers CML-449 and CML-495. The LPS population is a white dent (Tuxpeño-related synthetic) with improved drought tolerance (International Maize and Wheat Improvement Center, CIMMYT). Selection schemes are detailed elsewhere (see Pandey et al., 1986; Monneveux et al., 2008). Trials took place at the CIMMYT's experimental station in Tlaltizapán, Mexico (18°41′N, 99°07′W, 940 m asl) during the 2007 dry season. The soil of the experimental field is a calcareous vertisol (1·3–1·8 m deep), defined as Isothermic Udic Pellustert according to USDA soil taxonomy, with a pH of 7·6. Climate conditions during the maize-growing season are detailed in Fig. 1. Entries were planted on 14 December, 2006 in one-row plots (5-m rows with 0·25-m spacing within plants and 0·75 m between rows), with a final plant density of 6·67 m−2. Full irrigation (well-watered, WW) and two different levels of water stress were assayed. Each of the three water regimes was set up as a randomized complete block design with three replications per genotype, resulting in a total of 153 rows. Sprinkler irrigation was applied in all treatments after sowing, to ensure homogeneous germination. The WW trials were also irrigated by furrow irrigation every 2 weeks during the entire cycle. Intermediate (IS) and severe (SS) water stress treatments were also irrigated every 2 weeks until water stress was imposed by deficit irrigation of the trials from 1 month after sowing and withholding irrigation around 2 and 4 weeks before anthesis (i.e. tassel flowering), respectively, and irrigating once again 1 week after anthesis. Trials under WW, IS and SS conditions received 1510·7, 685·7 and 335·7 mm of water input (i.e. irrigation plus precipitation), respectively, making their averaged ratio between total water input and reference evapotranspiration (WI/ETP) 1·5, 1·0 and 0·5, respectively. Nitrogen fertilizer was applied before and 35 d after sowing (V6 stage, Ritchie et al., 1993), using a dose of 80 kg ha−1 of urea on both occasions. All trials received 80 kg ha−1 of phosphorous as a calcium superphosphate triple [Ca (H2PO4)2.H2O], applied prior to sowing. No potassium was applied, as previous tests showed no response to this element in these soils. Experiment plots were kept free of weeds, insect pests and diseases by recommended chemical measures as described in Monneveux et al. (2008).
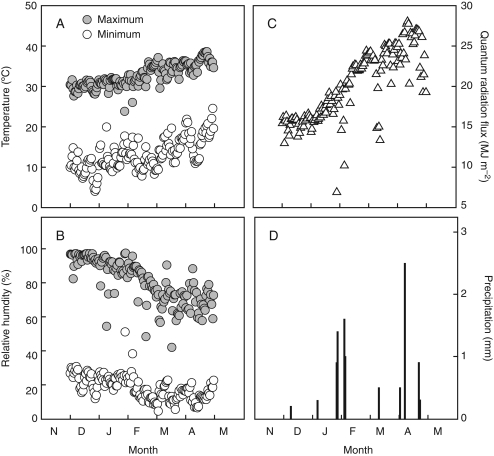
Fig. 1.
Maximum and minimum daily air temperatures (A), maximum and minimum daily relative air humidity (B), total daily quantum radiation flux (C) and daily precipitation (D) during the maize-growing season at the Tlaltizapán CIMMYT's Experimental Station, Mexico (18°41′N, 99°07′W, 940 m asl) from December, 2006 to April, 2007. Total precipitation throughout the maize-growing season was 10·7 mm.
Plant water status and yield
Leaf stomatal conductance (gs) and leaf temperature were measured in the abaxial surface of sun-exposed leaves from the upper part of the plant by using a Decagon SC-1 Leaf Porometer (Decagon Device Inc., Pullman, WA, USA) during the period covering pre-anthesis and grain filling. Measurements were performed in the 4 h around solar noon on sunny and windless days. gs measurements were expressed as crop mean values. Leaf evaporative conditions were further calculated. Thus, leaf and air temperatures and air relative humidity were used to calculate the ratios of atmospheric to intercellular space vapour pressure (ea/ei). In addition, green leaves from one entire plant per plot (sampled 2 weeks after anthesis) were used for measuring leaf water content (LWC) as LWC (%) = 100 × (f. wt – d. wt)/f. wt, where f. wt and d. wt refer to the fresh and dry weight of leaves, respectively. gs, leaf temperature and LWC measurements were performed in three plants per genotype and water treatment. At maturity, grain yield (GY) and its main agronomical components, such as kernel number per hectare and kernel mass, were determined over the entire plots. Kernel mass was calculated as the average of 300 kernels randomly selected from each plot.
Leaf and kernel ash content and carbon and oxygen isotope analyses
The same leaves used for LWC determination and mature kernels were used for ash content analysis. Samples were oven-dried at 60 °C for 48 h and ground. Approximately 2 g of dry mass (either leaves or kernels) was placed in pre-weighed porcelain crucibles. Samples were burnt in a muffle furnace for 6–8 h at 600 °C. The mineral residue was then weighed. Results are expressed as percentage of dry mass.
The 13C/12C ratios (R) of leaves and kernels were analysed using an elemental analyser (Carlo Erba 2100, Milan, Italy) interfaced to an isotope ratio mass spectrometer (IRMS; Thermo-Finnigan Deltaplus Advantage, Bremen, Germany) at the Colorado Plateau Stable Isotope Laboratory (CPSIL). Results are expressed as δ13C values, using a secondary standard calibrated against Vienna Pee Dee Belemnite calcium carbonate (VPDB) and the analytical precision was about 0·1 ‰:
| 1 |
The carbon isotope discrimination (Δ13C) of plant parts was then calculated from δ13Ca and δ13Cp (Farquhar et al., 1989) as:
| 2 |
where δ13Ca and δ13Cp refer to air and plant carbon isotope compositions, respectively. δ13C of free atmospheric CO2 was taken as −8 ‰ (Farquhar et al., 1989).
The 18O/16O ratios (R) of irrigation water were determined by the CO2/H2O equilibration technique and using an isotope ratio mass spectrometer (Delta S Finnigan MAT, Bremen, Germany) at the Scientific Facilities of the University of Barcelona. The 18O/16O ratios of kernel samples were analysed at the CPSIL via pyrolysis over glassy carbon at 1350 °C using a Thermo-Electron thermo-chemical elemental analyser interfaced via a CONFLO-II to a Thermo-Electron Delta Plus XL gas IRMS. Results were expressed as δ18O values, using two secondary standards (IAEA 601 and IAEA 602) calibrated against to the Vienna Standard Mean Oceanic Water (VSMOW), and the analytical precision was about 0·3 ‰ for dry matter and 0·2 ‰ for irrigation water:
| 3 |
Then, the 18O enrichment in kernels (Δ18O) was calculated as follows:
| 4 |
where δ18Op and δ18Oiw refer to the oxygen isotope compositions of plant sample and irrigation water, respectively (δ18Oiw was approx. −10·78 ‰).
Ash content as well as oxygen and carbon isotope analyses of leaves and kernels were performed for each plot individually.
Statistical analysis
Analysis of variance (ANOVA) was performed to calculate the effects of water treatment and genotype. Means were compared using a Duncan's multiple comparison test (P < 0·05), and a bivariate correlation procedure was used to analyse the correlation between measured traits. Data were analysed using the SPSS statistical package (SPSS Inc., Chicago, IL, USA).
RESULTS
Effects of water regime and genotype on growth traits, ash content and stable isotopes
Water regime and genotype significantly affected yield, kernel number per hectare and kernel mass, with the water regime exerting the greater effect as revealed by ANOVA (Table 1). Among hybrids, yield ranged from 5294 to 8877, 580 to 2606 and 139 to 845 kg ha−1, in the WW, IS and SS water treatments, respectively. Crop mean gs was significantly different for the three water treatments. LWC values were the same for the WW and IS treatments, but significantly lower in the most water-stressed (SS) plants (Table 1). Furthermore, genotypic variation for these traits was also found. Leaf and kernel ash were greatly affected by water regime (82 and 78 % variability, respectively), but genotypic differences were also found (Table 1). Leaf ash content decreased significantly: by 26 and 33 % from WW to IS and from WW to SS water stress treatments, respectively. However, kernel ash content followed the opposite trend, with increases of 30 and 54 % in the IS and SS treatments over the WW plants. Kernel Δ18O was also affected by water regime (71·7 %) and genotype (9·1 %), with significant increases of 1·1 and 2·3‰ in the IS and SS treatments, respectively, with respect to WW plants (Table 1). Furthermore, both leaf and kernel Δ13C significantly increased with water stress: by 0·34 and 0·49‰ in leaves, and 0·77 and 0·81‰ in kernels in the IS and SS treatments compared with the WW plants, respectively. Genotypic differences in kernel and leaf Δ13C were also found. However, in contrast to kernel Δ18O, leaf Δ13C and ash contents, when the check (commercial ‘Puma’) was removed and only the 16 genotypes derived from the LPS population were considered, no genotypic differences in kernel Δ13C were found (data not shown). In addition, regardless of the water treatment, higher Δ13C values were found in leaves than in kernels.
Table 1.
Grain yield (GY) and related agronomical yield components, gs, LWC, leaf and kernel ash content, leaf and kernel Δ13C, and kernel Δ18O of a set of maize hybrids grown under three different water regimes (WW, IS and SS)
| Source of variation |
|||||||
|---|---|---|---|---|---|---|---|
| WW | IS | SS | E | G | G × E | Error | |
| GY (kg ha−1) | 7487·2 ± 208·1a | 1631·4 ± 95·6b | 434·2 ± 31·5c | 91·9*** | 2·1*** | 1·8n.s. | 4·1 |
| Kernels per ha (×105) | 248·0 ± 6·0a | 107·9 ± 6·4b | 29·7 ± 2·3c | 84·8*** | 4·5*** | 3·8** | 5·4 |
| Kernel mass (mg) | 301·3 ± 3·4a | 151·8 ± 3·1b | 148·9 ± 5·2b | 89·0*** | 2·1*** | 2·4n.s. | 6·3 |
| gs (mmol m−2 s−1) | 201·4 ± 5·7a | 136·8 ± 4·3b | 116·7 ± 2·9c | 56·6*** | 9·7** | 9·1n.s. | 24·6 |
| LWC (%) | 83·2 ± 0·1a | 82·7 ± 0·2a | 79·3 ± 0·3b | 45·5*** | 14·4*** | 12·7n.s. | 27·4 |
| Leaf ash (%) | 18·6 ± 0·2a | 13·7 ± 0·2b | 12·4 ± 0·2c | 82·0*** | 3·7** | 4·2n.s. | 8·9 |
| Kernel ash (%) | 1·11 ± 0·01c | 1·44 ± 0·03b | 1·71 ± 0·01a | 78·4*** | 4·2* | 3·1n.s. | 14·1 |
| Leaf Δ13C (‰) | 5·29 ± 0·03c | 5·63 ± 0·03b | 5·78 ± 0·03a | 48·8*** | 11·2** | 8·9n.s. | 31·1 |
| Kernel Δ13C (‰) | 3·81 ± 0·02b | 4·58 ± 0·03a | 4·62 ± 0·04a | 84·2*** | 6·9*** | 2·8n.s. | 6·1 |
| Kernel Δ18O (‰) | 40·4 ± 0·1c | 41·5 ± 0·1b | 42·7 ± 0·1a | 71·7*** | 9·1*** | 5·3n.s. | 14·2 |
WW, well-watered plants; IS, intermediate water stress; SS, severe water stress. n.s., not significant; *P < 0·05; **P < 0·01; ***P < 0·001.
Data are the mean ± s.e. of 51 values (corresponding to 17 genotypes and three replications per genotype). Values with different superscript letters are significantly different according to Duncan's multiple range test, P < 0·05. Analysis of variance for the same variables is shown for the water regime environment (E), genotype (G) and interaction (G × E) effects. Results are expressed as the associated percentages of the sum of squares.
Relationships between ash content, stable isotopes and water status
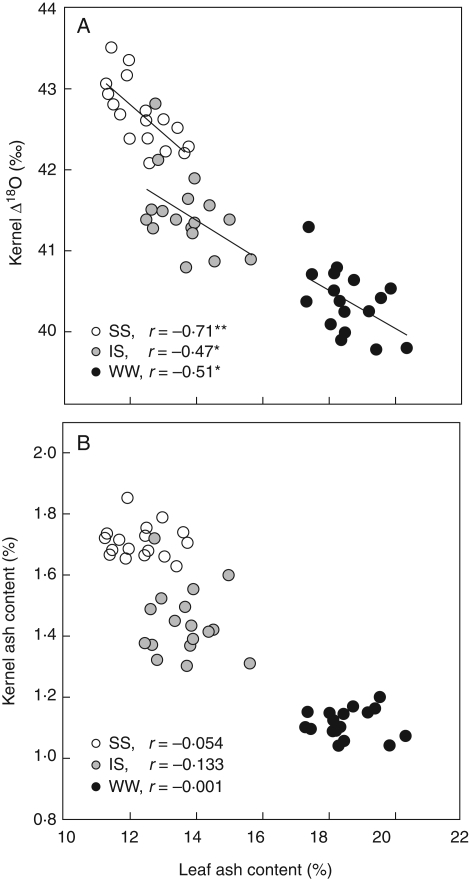
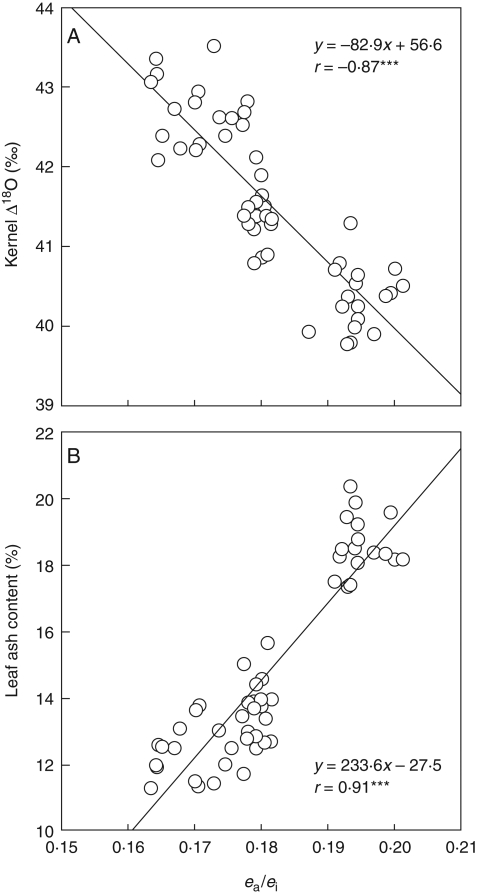
Significant negative correlations were observed between kernel Δ18O and leaf ash content across genotypes within each water treatment (Fig. 2A), whereas no relationship was found between kernel and leaf ash content (Fig. 2B). No relationship was found between kernel Δ18O and kernel ash content (data not shown). In addition, leaf ash content correlated positively with the ratio of atmospheric to intercellular space vapour pressure (ea/ei) (r = 0·91, P < 0·001) across genotypes and water treatments, whereas kernel Δ18O correlated negatively with ea/ei (r = −0·87, P < 0·001; Fig. 3). Significant positive correlations were observed between leaf ash content and mean gs (r = 0·81, P < 0·001), when all the genotypes and water treatments were plotted together (data not shown). However, no genotypic correlations (i.e. within each water treatment) were found between leaf ash and gs measurements (Fig. 4). In addition, leaf ash correlated positively with LWC across water treatments (r = 0·60, P < 0·001, data not shown), and also weakly within the SS (r = 0·40, P < 0·1) and the IS (r = 0·53, P < 0·05) treatments (Fig. 4). No correlation was found in the WW treatment.
Fig. 2.
The relationships between leaf ash content and (A) oxygen isotope enrichment (Δ18O) in kernels and (B) kernel ash. The three water treatments (WW, IS and SS) were plotted together, each point representing the mean of three replicates (n = 51).
Fig. 3.
The relationships between the ratio of atmospheric to intercellular space vapour pressure (ea/ei) and (A) oxygen isotope enrichment (Δ18O) in kernels, and (B) leaf ash content. The three water treatments (WW, IS and SS) were plotted together, each point representing the mean of three replicates (n = 51).
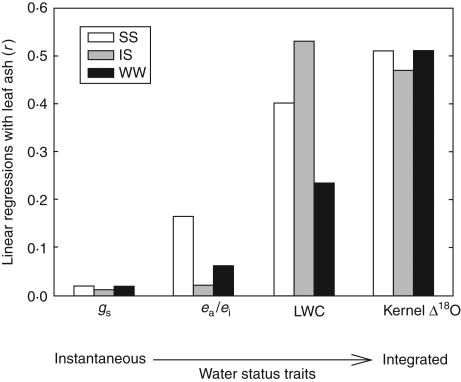
Fig. 4.
Correlation coefficients (r) of the linear regressions across genotypes within each water treatment between leaf ash and the water status traits gs, ea/ei, LWC and kernel Δ18O. The x-axis shows the water status traits evaluated, with the more instantaneous (gs and ea/ei) on the left and the more integrative in time and plant level (LWC and kernel Δ18O) on the right. For the 15 degrees of freedom available, Pearson correlation coefficients greater than 0·33, 0·41 and 0·56 are statistically significant at the 10, 5 and 1 % levels, respectively. Correlation coefficients are shown in absolute values.
Relationships between grain yield, stable isotopes and ash content
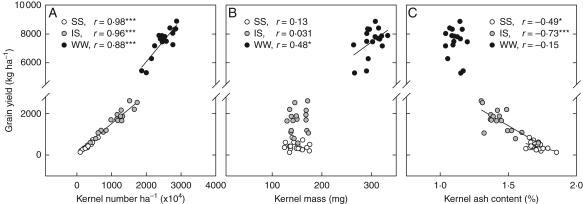
GY strongly and positively correlated with kernel number per hectare in the WW (r = 0·88, P < 0·001), IS (r = 0·96, P < 0·001) and SS (r = 0·98, P < 0·001) treatments (Fig. 5A). GY also positively correlated with kernel mass in WW plants (r = 0·48, P < 0·05), but no correlations were found in the two other water regimes (Fig. 5B). Leaf ash correlated negatively with GY (r = −0·50, P < 0·05) and kernel number per hectare (r = −0·51, P < 0·05) in the SS treatment, whereas no correlations were found in plants grown under IS and WW conditions (Table 2). Kernel ash correlated negatively with GY in the IS (r = −0·73, P < 0·001) and SS (r = −0·49, P < 0·05) treatments (Table 3, Fig. 5C), and also correlated negatively with kernel number per hectare (r = −0·64, P < 0·01 and r = −0·52, P < 0·05 for the IS and SS treatments, respectively), whereas no correlations were found in WW plants (Table 2). No correlations were found between either leaf or kernel ash content and kernel mass in any of the three water treatments. Kernel and leaf Δ13C did not correlate with any of the studied yield parameters within any of the three water treatments assayed (Table 2). Kernel Δ18O correlated negatively with GY (r = −0·48, P < 0·05) and kernel mass (r = −0·53, P < 0·05) in the WW treatments, and with GY (r = −0·58, P < 0·01) and kernel number per hectare (r = −0·58, P < 0·01) in the IS plants. Conversely, a positive but not significant trend was observed between kernel Δ18O with GY and kernel number per hectare in the SS treatment (Table 2). In addition, highly significant correlations between leaf ash, kernel ash, leaf and kernel Δ13C and kernel Δ18O in relation to GY, kernel per hectare and kernel mass were found when the three water treatment were combined (Table 2).
Fig. 5.
The relationships between grain yield and (A) kernel number per hectare, (B) kernel mass and (C) kernel ash content. The three water treatments (WW, IS and SS) were plotted together, each point representing the mean of three replicates (n = 51).
Table 2.
Correlation coefficients of the linear regressions between leaf and kernel ash content, leaf and kernel Δ13C, and kernel Δ18O in relation to GY, kernel number per hecare and kernel mass across genotypes within each water treatment (WW, IS and SS) and across water treatments
| GY |
Kernels per hectare |
Kernel mass |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | IS | SS | Across | WW | IS | SS | Across | WW | IS | SS | Across | |
| Leaf ash | 0·36n.s. | 0·27n.s. | −0·50* | 0·95*** | 0·23n.s. | 0·34n.s. | −0·51* | 0·95*** | 0·31n.s. | 0·40n.s. | 0·05n.s. | 0·92*** |
| Kernel ash | −0·15n.s. | −0·73*** | −0·49* | −0·92*** | −0·14n.s. | −0·64** | −0·52* | −0·91*** | −0·12n.s. | −0·33n.s. | 0·24n.s. | −0·85*** |
| Leaf Δ13C | −0·13n.s. | 0·01n.s. | 0·02n.s. | −0·80*** | −0·07n.s. | 0·07n.s. | −0·02n.s. | −0·78*** | −0·10n.s. | −0·17n.s. | 0·01n.s. | −0·78*** |
| Kernel Δ13C | 0·26n.s. | −0·30n.s. | 0·17n.s. | −0·90*** | 0·31n.s. | −0·23n.s. | 0·16n.s. | −0·82*** | 0·02n.s. | −0·18n.s. | −0·40n.s. | −0·92*** |
| Kernel Δ18O | −0·48* | −0·58** | 0·23n.s. | −0·86*** | −0·28n.s. | −0·58** | 0·26n.s. | −0·89*** | −0·53* | 0·12n.s. | −0·17n.s. | −0·78*** |
WW, well-watered plants; IS, intermediate water stress; SS, severe water stress. n.s., not significant; *P < 0·05; **P < 0·01; ***P < 0·001.
Table 3.
Percentage of grain yield (GY) variation across genotypes within each water treatment (WW, IS and SS) explained by the combination of kernel Δ18O, leaf and kernel ash content
| Combination of traits | WW (% GY) | IS (% GY) | SS (% GY) |
|---|---|---|---|
| Leaf ash + kernel ash | 15·2 | 56·3 | 52·2 |
| Leaf ash + kernel Δ18O | 25·0 | 33·5 | 28·2 |
| kernel ash + kernel Δ18O | 24·4 | 60·4 | 35·0 |
| Leaf ash + kernel ash + kernel Δ18O | 26·3 | 60·8 | 52·6 |
The percentage of variation in GY explained by the combination of leaf ash, kernel ash and kernel Δ18O was studied with a multiple correlation analysis (Table 3). Different results were found, depending on the water treatment. In the IS and SS treatments, over 50 % of GY variability was explained by the combination of leaf and kernel ash content. Alternatively, the combination of leaf ash plus kernel Δ18O explained about 30 % of GY variability in both IS and SS plants, while the combination of kernel ash plus kernel Δ18O explained 60 and 35 % in IS and SS treatments, respectively. The combination of the three parameters (leaf ash, kernel ash plus kernel Δ18O) slightly increased the percentage of variation in GY over the best combination of two traits. Conversely, in the WW plants, only 15 % of the variation in GY was explained by the combination of leaf and kernel ash, while the combination of Δ18O with any of the other two traits explained about 26 %. Further addition of leaf or kernel Δ13C in combination with ash content and kernel Δ18O values did not improve the percentage of GY variability explained (data not shown).
DISCUSSION
Although the use of secondary traits such as mineral accumulation and stable isotopes when breeding for yield potential and drought adaptation in C3 cereals has been tackled, little is known about its application in C4 cereals such as maize. We suggest mineral accumulation (measured as ash content) and Δ13C (despite limitations in maize owing to its C4 photosynthetic metabolism) in combination with or alternative to Δ18O as potential secondary phenotypic traits for maize breeding under drought conditions.
Source of variation in ash content and plant isotope signatures
Increasing the level of water stress significantly decreased leaf ash content, as previously reported in wheat and barley plants exposed to different water regimes (Araus et al., 1998; Voltas et al., 1998). As mineral accumulation in vegetative tissues appears to take place via xylem through the transpirational stream, it is closely related to the amount of water transpired by the plant (Masle et al., 1992). Thus, differences in mineral accumulation between water treatments could probably be explained by differences in total water input. In addition, within each water treatment, genotypes able to maintain a better water status through higher stomatal conductance and transpiration rates (i.e. higher WU) will accumulate more minerals in vegetative parts. The results here support such a hypothesis, as leaf ash content was found to be positively correlated with gs and ea/ei ratio when all treatments were plotted together. However, despite the relationships found between leaf ash and ‘time-instantaneous’ traits such as gs and ea/ei, leaf ash content should be more closely related to time-integrated traits such as LWC (performed at the whole plant level) and kernel Δ18O than to instantaneous measurements performed in single leaves. In such a way, there was a clear increase in the correlations between leaf ash and traits informing about plant water status (gs, ea/ei, LWC and Δ18O) when moving from instantaneous (gs and ea/ei) to time-integrated (LWC and Δ18O) measurements.
The negative relationships observed between leaf ash and kernel Δ18O support the fact that mineral accumulation in vegetative tissues may reflect differences in transpiration and then water use. In fact, Δ18O in dry matter is known to integrate leaf evaporative conditions throughout the crop cycle (Barbour et al., 2000; Barbour, 2007; Cabrera-Bosquet et al., 2009a). Thus, Δ18O has been proposed as a selection criterion to track genotypic differences in stomatal conductance in bread wheat grown under well-watered conditions (Barbour et al., 2000), as well as an indirect measurement of transpiration and WU (Sheshshayee et al., 2005; Cernusak et al., 2007, 2008; Cabrera-Bosquet et al., 2009a,b). Increases in kernel Δ18O with decreasing water input have been reported in wheat plants grown under different water regimes (Ferrio et al., 2007; Cabrera-Bosquet et al., 2009a). In addition, kernel Δ18O correlated negatively with the ratio of atmospheric to intercellular space vapour pressure (ea/ei), so reflecting the close relationship between Δ18O and leaf evaporative conditions.
By contrast, the significant increase in kernel ash content from WW to SS plants is consistent with previous studies in C3 cereals (Febrero et al., 1994; Araus et al., 1998; Voltas et al., 1998). Such an increase is explained by the mechanisms involved in mineral accumulation in kernels. In maize and other cereals, the main proportions of phosphorous and other cations in the grain are found together in the form of phytin (i.e. myo-inositol hexaphosphoric acid) (Lott, 1984). In addition, in maize nearly 90 % of the total grain phytin is in the embryo, constituting from 1 % to several per cent of the seed's total dry weight (O'Dell et al., 1972). Minerals are retranslocated to the grains from the photosynthetic tissues during plant senescence (Rajcan and Tollenaar, 1999). However, increasing the proportion of carbohydrates in the starchy endosperm, due to an enhanced supply of photo-assimilates during grain filling (such as in the WW treatments), lowers the proportion of minerals in the grain. Therefore, whereas leaf ash reflects differences in WU or transpiratory gas exchange, kernel ash reflects photosynthetic and translocation processes during grain filling. This may explain the lack of genotypic correlations between kernel ash in relation to both leaf ash content and Δ18O in all three water treatments.
Relationship of ash content and of stable isotopes to GY
The negative correlations between leaf ash in relation to GY and kernel number per hectare in the SS treatment, and the positive correlations (although not reaching statistical significance) in the WW and IS treatments, are comparable with the results of Voltas et al. (1998) in barley grown under different water regimes. They reported negative relationships between ash content in straw and GY in plants growing in the poorest yield conditions. This is explained by the fact that under severe water stress conditions (here, plants showed a decrease of 94 % in GY compared with WW plants), those genotypes that are more able to save water (i.e. with reduced WU) are not penalized in terms of productivity. This hypothesis was supported by the significant positive correlations of kernel Δ18O to GY and kernels per hectare in the SS treatments (when removing the check, ‘Puma’), and the negative correlations between kernel Δ18O and GY in the WW and IS treatments. Δ18O may record the transpirative status (and then WU) during the weeks before flowering, when the total number of kernels (the main agronomic component) is defined. Therefore, under WW to IS conditions, those genotypes showing high leaf ash or low kernel Δ18O (i.e. increased ability to sustain transpiration) are the most productive. Conversely, under severe water stress conditions, the highest yields were achieved by those genotypes that have the lowest leaf ash and the highest kernel Δ18O values. Such a change in the sign of the correlation between leaf ash or between kernel Δ18O and GY is comparable with that reported when using Δ13C for breeding in C3 cereals under Mediterranean conditions, where drought develops steadily from flowering onwards. Under such conditions, the genotypic relationship between Δ13C and GY moves from positive, under moderate water stress and well-watered conditions, to negative (or absent) under severe water stress conditions (Voltas et al., 1999; Condon et al., 2004; Royo et al., 2005; Araus et al., 2003, 2008). A highly significant negative correlation of either leaf or kernel Δ13C with GY and the yield components studied was observed when all treatments were combined. However, no significant relationships across genotypes within each water treatment were found between either leaf or kernel Δ13C and GY, kernel per hectare or kernel mass. The few previous studies that report the relationship between yield and Δ13C in maize have shown contradictory results. Whereas Monneveux et al. (2007) reported positive correlations between Δ13C measured in leaves, ears and silks in relation to ear dry weight measured at female flowering, but not to yield, Heng et al. (2005) reported positive, negative or non-existent relationships between Δ13C measured in different plant parts and yield. The lack of correlation between either leaf or kernel Δ13C and GY in the present study can be explained by the very low range of variation in Δ13C between genotypes within each water treatment. Thus, Δ13C only ranged from 4·98 to 5·53, from 5·37 to 5·83 and from 5·53 to 5·95 ‰ in leaves and from 3·59 to 4·01, from 4·22 to 4·74 and from 4·07 to 4·78 ‰ in kernels for the WW, IS and SS treatments, respectively. The low genotypic and even environmental variation in Δ13C observed here is far lower than that reported in C3 plants such as wheat grown under different water conditions (e.g. 13·0–17·7 and 14·59–18·36 ‰, see Araus et al., 1997, 2003; 14·21–17·64 ‰, see Ferrio et al., 2007; 13·68–18·58 ‰, see Cabrera-Bosquet et al., 2007), because in C4 plants the potentially large effect of fractionation by Rubisco is suppressed in the semi-closed bundle sheath (Bowman et al., 1989). Nevertheless, the increases in Δ13C under drought conditions, and the higher Δ13C values in vegetative tissues (i.e. leaves) than in reproductive organs (i.e. kernels) are consistent with those reported previously in maize hybrids and lines grown under different water regimes (Heng et al., 2005; Dercon et al., 2006; Monneveux et al., 2007). Monneveux et al. (2007) reported the usefulness of Δ13C for preliminary screening of maize lines and hybrids that have a high contrast for drought tolerance. However, overall, the lack of genotypic differences in kernel Δ13C between the set of genotypes derived from the LPS population, together with the lack of correlation of either kernel or leaf Δ13C with yield, prevents the use of Δ13C in maize for breeding purposes.
The negative correlation of kernel ash with GY under drought conditions and the lack of relationship under WW conditions is in line with previous studies in barley (Febrero et al., 2004; Voltas et al., 1998). In addition, no relationship was found between kernel ash and kernel mass in any of the three water treatments. Therefore, differences in kernel ash seem to be sustained by factors other than differences in kernel mass (Araus et al., 1998). The negative relationships between kernel ash and kernel number per hectare in the IS and SS treatments support such a hypothesis. In fact, within each water treatment, and particularly in the IS and SS treatments, differences in GY were largely explained by increases in the kernel number per unit area, rather than an increase in kernel mass (Bolaños and Edmeades, 1996; Bruce et al., 2002). However, in plants grown under WW conditions, differences in GY were also determined by changes in kernel mass. Such differences could also explain the lack of correlation of kernel ash in relation to GY and kernel number per hectare in the WW treatments. Therefore, kernel ash seems to reflect the balance between the reproductive sink and the photosynthetic source. Thus, as minerals in maize accumulate mainly in the embryo rather than in the endosperm (O'Dell et al., 1972), ash concentration in kernels indirectly combines information on the number of kernels per plant, the main agronomic component determining grain yield under drought (Bruce et al., 2002). This corroborates the negative relationship between ash content in kernels and the number of kernels per hectare, and the lack of a correlation with kernel mass.
Implications for plant breeding
Under drought conditions the combination of leaf and kernel ash accounted for more than 50 % of the genotypic variability in GY, far higher than Δ18O alone. However, under WW conditions, leaf ash combined with kernel ash explained only 15 % of the variation in GY, and the addition of kernel Δ18O explained up to 26 % of GY variation. In fact, under WW conditions, the only trait that correlated significantly with GY was Δ18O. Araus et al. (1998) reported that in durum wheat growing under contrasting water conditions, about 40 % of the variation in GY was explained by the combination of leaf and kernel ash. In the present study, the combination of ash content and Δ18O in kernels provided a reasonable prediction of GY, regardless of the water regime under consideration. In conclusion, there is a close relationship between mineral accumulation in vegetative tissues (leaves) and the plant's water status (gs, ea/ei, LWC, Δ18O), together with a correlation between yield and ash content in leaves and kernels. This supports the combined use of mineral content in leaves and kernels as a useful alternative to Δ18O in kernels for assessing yield performance and selecting maize genotypes that are better suited to drought conditions. In addition, when complemented with Δ18O in kernels, combined leaf and kernel mineral content also explained differences in yield under irrigated conditions. This approach could be particularly interesting if it were coupled with techniques such as near-infrared reflectance spectroscopy, which would allow a fast, cheap (US $0·5 per sample) and reliable estimation of ash content (see Araus, 1996), to be routinely applied in grain quality tests.
ACKNOWLEDGEMENTS
This study was supported in part by the Drought-Tolerant Maize for Africa (DTMA) project, funded by the Bill and Melinda Gates Foundation; and the Precision phenotyping for improving drought stress tolerant maize in southern Asia and eastern Africa project, funded by the Die Bundesministerium für Wirtschaftliche Zusammenarbeit und Entwicklung (BMZ), Germany. L.C.-B. was the recipient of a research grant (Programa Nacional de Formación de Personal Universitario, AP2005-4965) sponsored by the Spanish Ministry of Education and Science. We are grateful to Pedro Chepetla's team (CIMMYT Tlaltizapán Experimental Station) for their helpful contribution in the field. Natalia Palacios is also acknowledged for ash content analysis.
LITERATURE CITED
- Araus JL. Integrative physiological criteria associated with yield potential. In: Reynolds MP, Rajaram S, McNab A, editors. Increasing yield potential in wheat: breaking the barriers. Mexico, DF: CIMMYT; 1996. pp. 150–167. [Google Scholar]
- Araus JL, Amaro T, Zuhair Y, Nachit MM. Effect of leaf structure and water status on carbon isotope discrimination field-grown durum wheat. Plant Cell and Environment. 1997;20:1484–1494. [Google Scholar]
- Araus JL, Amaro T, Casadesús J, Asbati A, Nachit MM. Relationships between ash content, carbon isotope discrimination and yield in durum wheat. Australian Journal of Plant Physiology. 1998;25:835–842. [Google Scholar]
- Araus JL, Casadesús J, Bort J. Recent tools for the screening of physiological traits determining yield. In: Reynolds MP, Ortiz-Monasterio JI, McNab A, editors. Application of physiology in wheat breeding. Mexico, DF: CIMMYT; 2001. pp. 59–77. [Google Scholar]
- Araus JL, Casadesus J, Asbati A, Nachit MM. Basis of the relationship between ash content in the flag leaf and carbon isotope discriminitation in kernels of durum wheat. Photosynthetica. 2002;a 39:591–596. [Google Scholar]
- Araus JL, Slafer GA, Reynolds MP, Royo C. Plant breeding and drought in C3 cereals: what should we breed for? Annals of Botany. 2002;b 89:925–940. doi: 10.1093/aob/mcf049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Villegas D, Aparicio N, et al. Environmental factors determining carbon isotope discrimination and yield in durum wheat under Mediterranean conditions. Crop Science. 2003;43:170–180. [Google Scholar]
- Araus JL, Slafer GA, Royo C, Serret MD. Breeding for yield potential and stress adaptation in cereals. Critical Reviews in Plant Science. 2008;27:377–412. [Google Scholar]
- Bänziger M, Araus JL. Recent advances in breeding maize for drought and salinity stress tolerance. In: Jenks MA, Hasegawa PM, Mohan S, editors. Advances in molecular breeding toward drought and salt tolerant crops. The Netherlands: Springer; 2009. pp. 587–601. [Google Scholar]
- Bänziger M, Edmeades GO, Beck D, Bellon M. Breeding for drought and nitrogen stress tolerance in maize: from theory to practice. Mexico, D.F: CIMMYT; 2000. [Google Scholar]
- Barbour MM. Stable oxygen isotope composition of plant tissue: a review. Functional Plant Biology. 2007;34:83–94. doi: 10.1071/FP06228. [DOI] [PubMed] [Google Scholar]
- Barbour MM, Fischer RA, Sayre KD, Farquhar GD. Oxygen isotope ratio of leaf and grain material correlates with stomatal conductance and grain yield in irrigated wheat. Australian Journal of Plant Physiology. 2000;27:625–637. [Google Scholar]
- Blum A. Yield potential and drought resistance: are they mutually exclusive? In: Reynolds MP, Rajaram S, McNab A, editors. Increasing yield potential in wheat: breaking the barriers. Madison, WI: CSSA; 1993. pp. 343–347. [Google Scholar]
- Blum A. Drought resistance, water-use efficiency, and yield potential: are they compatible, dissonant, or mutually exclusive? Australian Journal of Agricultural Research. 2005;56:1159–1168. [Google Scholar]
- Blum A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Research. 2009;112:119–123. [Google Scholar]
- Bolaños J, Edmeades GO. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crops Research. 1996;48:65–80. [Google Scholar]
- Bowman WD, Hubick KT, von Caemmerer S, Farquhar GD. Short-term changes in leaf carbon isotope discrimination in salt- and water-stressed C4 grasses. Plant Physiology. 1989;90:162–166. doi: 10.1104/pp.90.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce WB, Edmeades GO, Barker TC. Molecular and physiological approaches to maize improvement for drought tolerance. Journal of Experimental Botany. 2002;53:13–25. [PubMed] [Google Scholar]
- Cabrera-Bosquet L, Molero G, Bort J, Nogués S, Araus JL. The combined effect of constant water deficit and nitrogen supply on WUE, NUE and Δ13C in durum wheat potted plants. Annals of Applied Biology. 2007;151:277–289. [Google Scholar]
- Cabrera-Bosquet L, Molero G, Nogués S, Araus JL. Water and nitrogen conditions affect the relationships of Δ13C and Δ18O to gas exchange and growth in durum wheat. Journal of Experimental Botany. 2009;a 60:1633–1644. doi: 10.1093/jxb/erp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Bosquet L, Sánchez C, Araus JL. Oxygen isotope enrichment (Δ18O) reflects yield potential and drought resistance in maize. Plant Cell and Environment. 2009b doi: 10.1111/j.1365-3040.2009.02013.x. Epub ahead of print 22 June 2009, doi:10.1111/j.1365-3040.2009.02013.x. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Aranda J, Turner BL, Marshall JD. Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. Journal of Experimental Botany. 2007;58:3549–3566. doi: 10.1093/jxb/erm201. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Aranda J, Turner BL. Conifers, angiosperm trees, and lianas: growth, whole-plant water and nitrogen use efficiency, and stable isotope composition (δ13C and δ18O) of seedlings grown in a tropical environment. Plant Physiology. 2008;148:642–659. doi: 10.1104/pp.108.123521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Breeding for high water-use efficiency. Journal of Experimental Botany. 2004;55:2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- Dercon G, Clymans E, Diels J, Merckx R, Deckers J. Differential 13C isotopic discrimination in maize at varying water stress and at low to high nitrogen availability. Plant and Soil. 2006;282:313–326. [Google Scholar]
- Edmeades GO, Bolaños J, Lafitte HR, Rajaram S, Pfeiffer W, Fischer RA. Traditional approaches to breeding for drought resistance in cereals. In: Baker FWG, editor. Drought resistance in cereals. Wallingford: ICSU and CABI; 1989. pp. 27–52. [Google Scholar]
- Farquhar GD. On the nature of carbon isotope discrimination in C4 species. Australian Journal of Plant Physiology. 1983;10:205–226. [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology. 1984;11:539–552. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick K. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:503–537. [Google Scholar]
- Febrero A, Bort J, Voltas J, Araus JL. Grain yield, carbon isotope discrimination and mineral content in mature kernels of barley, under irrigated and rainfed conditions. Agronomie. 1994;2:127–132. [Google Scholar]
- Ferrio JP, Mateo MA, Bort J, Abdalla O, Voltas J, Araus JL. Relationships of grain δ13C and δ18O with wheat phenology and yield under water-limited conditions. Annals of Applied Biology. 2007;150:207–215. [Google Scholar]
- Hammer GL, Farquhar GD, Broad I. On the extent of genetic variation for transpiration efficiency in Sorghum. Australian Journal of Agricultural Research. 1997;48:649–655. [Google Scholar]
- Helliker BR, Ehleringer JR. Differential 18O enrichment of leaf cellulose in C3 versus C4 grasses. Functional Plant Biology. 2002;29:435–442. doi: 10.1071/PP01122. [DOI] [PubMed] [Google Scholar]
- Henderson S, von Caemmerer S, Farquhar GD, Wade L, Hammer G. Correlations between carbon isotope discrimination and transpiration efficiency in lines of the C4 species Sorghum bicolor in the glasshouse and the field. Australian Journal of Plant Physiology. 1998;25:111–123. [Google Scholar]
- Heng LK, Cai G, Ramana MV, et al. The effect of soil fertility, crop management on carbon-isotope discrimination and their relationships with yield and water-use efficiency of crops in semi-arid and arid environments. Nutrient and water management practices for increasing crop production in rainfed arid/semi-arid areas. 2005:15–41. IAEA-TECDOC-1468, 2005. [Google Scholar]
- Hubick KT, Hammer GL, Farquhar GD, Wade LJ, von Caemmerer S, Henderson SA. Carbon isotope discrimination varies genetically in C4 species. Plant Physiology. 1990;91:534–537. doi: 10.1104/pp.92.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott JNA. Accumulation of seed reserves of phosphorous and other minerals. In: Murray DR, editor. Seed physiology. vol. 1. Sidney: Academic Press; 1984. pp. 139–163. [Google Scholar]
- Masle J, Farquhar GD, Wong SC. Transpiration ratio and plant mineral content are related among genotypes of a range of species. Australian Journal of Plant Physiology. 1992;19:709–721. [Google Scholar]
- Mayland HF, Johnson DA, Asay KH, Read JJ. Ash, carbon isotope discrimination and silicon as estimators of transpiration efficiency in crested wheatgrass. Australian Journal of Plant Physiology. 1993;20:361–369. [Google Scholar]
- Merah O, Deléens E, Monneveux P. Grain yield, carbon isotope discrimination, mineral and silicon content in durum wheat under different precipitation regimes. Physiologia Plantarum. 1999;107:387–394. [Google Scholar]
- Merah O, Deléens E, Souyris I, Monneveux P. Ash content might predict carbon isotope discrimination and grain yield in durum wheat. New Phytologist. 2001;149:275–282. doi: 10.1046/j.1469-8137.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Monneveux P, Reynolds MP, Trethowan R, Peña J, Zapata F. Carbon isotope discrimination, leaf ash content and grain yield in bread and durum wheat grown under full-irrigated conditions. Journal of Agronomy and Crop Science. 2004;190:389–394. [Google Scholar]
- Monneveux P, Sheshshayee MS, Akhter J, Ribaut JM. Using carbon isotope discrimination to select maize (Zea mays L.) inbred lines and hybrids for drought tolerance. Plant Science. 2007;173:390–396. [Google Scholar]
- Monneveux P, Sánchez C, Tiessen A. Future progress in drought tolerance in maize needs new secondary traits and cross combinations. Journal of Agricultural Science. 2008;146:1–14. [Google Scholar]
- Morgan JA, LeCain DR, McCaig TN, Quick JS. Gas exchange, carbon isotope discrimination and productivity in Winter wheat. Crop Science. 1993;33:178–186. [Google Scholar]
- Nonogaki H, Chen F, Bradford KJ. Mechanisms and genes involved in germination sensu stricto. In: Bradford KJ, Nonogaki H, editors. Seed development, dormancy and germination. Annual plant reviews. Volume 27. Oxford: Blackwell Publishing; 2007. pp. 264–304. [Google Scholar]
- O'Dell BL, de Boland AR, Koirtyohann SR. Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. Journal of Agronomy and Food Chemistry. 1972;3:718–721. [Google Scholar]
- Ogée J, Cuntz M, Peylin P, Bariac T. Non-steady-state, non-uniform transpiration rate and leaf anatomy effects on the progressive stable isotope enrichment of leaf water along monocot leaves. Plant Cell and Environment. 2007;30:367–387. doi: 10.1111/j.1365-3040.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- Pandey S, Diallo AO, Islam TMT, Deutsch J. Progress from selection in eight tropical maize populations using international testing. Crop Science. 1986;26:879–884. [Google Scholar]
- Rajcan I, Tollenaar M. Source:sink ratio and leaf senescence in maize: II. Nitrogen metabolism during grain filling. Field Crops Research. 1999;60:255–265. [Google Scholar]
- Rebetzke GJ, Condon AG, Richards RA, Farquhar GD. Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Science. 2002;42:739–745. [Google Scholar]
- Ritchie SW, Hanway JJ, Benson GO. How a corn plant develops. Ames, IA: Iowa State University of Science and technology, Cooperative Extension Service; 1993. Special Report no. 48. [Google Scholar]
- Royo C, García del Moral LF, Slafer G, Nachit MN, Araus JL. Selection tools for improving yield-associated physiological traits. In: Royo C, Nachit MN, Di Fonzo N, Araus JL, Pfeiffer WH, Slafer GA, editors. Durum wheat breeding: current approaches and future strategies. New York: Haworth Press; 2005. pp. 563–598. [Google Scholar]
- Sheshshayee MS, Bindumadhava H, Ramesh R, Prasad TG, Lakshminarayana MR, Udayakumar M. Oxygen isotope enrichment (Delta O-18) as a measure of time-averaged transpiration rate. Journal of Experimental Botany. 2005;56:3033–3039. doi: 10.1093/jxb/eri300. [DOI] [PubMed] [Google Scholar]
- Slafer GA, Araus JL. Physiological traits for improving wheat yield under a wide range of conditions. In: Spiertz JH, Struik PC, van Laar HH, editors. Scale and complexity in plant systems research: gene–plant–crop relations. Dordrecht: Springer; 2007. pp. 145–154. [Google Scholar]
- Slafer GA, Araus JL, Richards RA. Physiological traits to increase the yield potential of wheat. In: Satorre EH, Slafer GA, editors. Wheat: ecology and physiology of yield determination. New York: Food Product Press; 1999. pp. 379–415. [Google Scholar]
- Tanner W, Beevers H. Does transpiration have an essential function in long-distance ion transport in plants? Plant, Cell and Environment. 1990;13:745–750. [Google Scholar]
- Voltas J, Romagosa I, Muñoz P, Araus JL. Mineral accumulation, carbon isotope discrimination and indirect selection for grain yield in two-rowed barley grown under semiarid conditions. European Journal of Agronomy. 1998;9:147–155. [Google Scholar]
- Voltas J, Romagosa I, Lafarga A, Armesto AP, Sombrero A, Araus JL. Genotype by environment interaction for grain yield and carbon isotope discrimination of barley in Mediterranean Spain. Australian Journal of Agricultural Research. 1999;50:1263–1271. [Google Scholar]