Abstract
Novel therapies are needed to address the vascular endothelial cell (EC) barrier disruption that occurs in inflammatory diseases such as acute lung injury (ALI). We previously demonstrated the potent barrier-enhancing effects of both sphingosine 1-phosphate (S1P) and the structurally similar compound FTY720 [2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol] in inflammatory lung injury. In this study, we examined the therapeutic potential of several novel FTY720 analogs to reduce vascular leak. Similar to S1P and FTY720, the (R)- and (S)-enantiomers of FTY720 phosphonate and enephosphonate analogs produce sustained EC barrier enhancement in vitro, as seen by increases in transendothelial electrical resistance (TER). In contrast, the (R)- and (S)-enantiomers of FTY720-regioisomeric analogs disrupt EC barrier integrity in a dose-dependent manner. Barrier-enhancing FTY720 analogs demonstrate a wider protective concentration range in vitro (1–50 μM) and greater potency than either S1P or FTY720. In contrast to FTY720-induced EC barrier enhancement, S1P and the FTY720 analogs dramatically increase TER within minutes in association with cortical actin ring formation. Unlike S1P, these FTY720 analogs exhibit differential phosphorylation effects without altering the intracellular calcium level. Inhibitor studies indicate that barrier enhancement by these analogs involves signaling via Gi-coupled receptors, tyrosine kinases, and lipid rafts. Consistent with these in vitro responses, the (S)-phosphonate analog of FTY720 significantly reduces multiple indices of alveolar and vascular permeability in a lipopolysaccharide-mediated murine model of ALI (without significant alterations in leukocyte counts). These results demonstrate the capacity for FTY720 analogs to significantly decrease pulmonary vascular leakage and inflammation in vitro and in vivo.
Sustained vascular barrier leak, a marked characteristic of acute inflammatory diseases, such as acute lung injury (ALI) and sepsis, contributes to the high mortality of these conditions. Disruption of the pulmonary vascular endothelial cell (EC) monolayer in the lung microcirculation results in flooding of interstitial and alveolar compartments with fluid, protein, and inflammatory cells, resulting in respiratory failure (Dudek and Garcia, 2001). Specific therapies that prevent or reverse inflammation-mediated vascular barrier leak are lacking (Wheeler and Bernard, 2007). We have previously demonstrated the potent barrier-enhancing properties of sphingosine 1-phosphate (S1P), a platelet-derived sphingolipid that rapidly induces EC cytoskeletal rearrangements, leading to augmented EC monolayer integrity (Garcia et al., 2001). Through the ligation of the Gi-coupled S1P1 receptor (S1P1R), S1P initiates a series of downstream events, including Rac activation, cortactin translocation, peripheral myosin light chain (MLC) phosphorylation, and focal adhesion rearrangement, culminating in enhancement of the EC cortical actin ring, improved cell-cell and cell-matrix interactions, and increased barrier function in vitro (Garcia et al., 2001; Shikata et al., 2003; Dudek et al., 2004). We have also shown the in vivo capacity for S1P to attenuate lipopolysaccharide (LPS)-induced murine and canine models of sepsis and ALI (McVerry et al., 2004; Peng et al., 2004), supporting the potential therapeutic utility of this compound in inflammatory states.
Despite this impressive potential, S1P is an endogenous compound that produces a myriad of effects, including several that will limit its usefulness in patients. For example, although intravascular administration of S1P protects against ALI, intratracheal administration can conversely produce pulmonary edema through disruption of the epithelial barrier via ligation of the S1P3 receptor (S1P3R) (Gon et al., 2005). Even in the vasculature, high concentrations of S1P (>10 μM) can disrupt EC monolayer integrity in vitro through ligation of the S1P3R and subsequent Rho activation, suggesting a limited therapeutic window for barrier enhancing properties of S1P. S1P also exhibits well described cardiac toxicity (primarily bradycardia) through activation of S1P3R in the heart (Forrest et al., 2004; Hale et al., 2004a). Finally, S1P can stimulate contraction of human airway smooth muscle cells (Rosenfeldt et al., 2003) and worsens airway hyper-responsiveness in mice (Roviezzo et al., 2007), suggesting a potential for S1P to exacerbate airway obstruction in asthmatics.
Given these limitations, there has been considerable interest in the biologic effects of the structurally similar compound, FTY720, which exhibits potent barrier-enhancing properties both in vitro and in vivo (Sanchez et al., 2003; Peng et al., 2004; Dudek et al., 2007). FTY720 has significant clinical interest as an immunosuppressive agent and has demonstrated efficacy in patients with relapsing multiple sclerosis (Kappos et al., 2006) and in models of leukemia (Neviani et al., 2007). It is currently being evaluated in phase III clinical trials (Brinkmann et al., 2004; Mansoor and Melendez, 2008) and is, therefore, a potential future therapeutic option for inflammatory lung disease. Our prior in vitro studies demonstrate that FTY720 potently enhances EC barrier function, at least in part, via a novel S1P1R-independent mechanism that involves an alternative Gi-coupled receptor (Dudek et al., 2007). We have also reported that a single intraperitoneal injection of FTY720 significantly attenuated murine pulmonary injury measured 24 h after LPS administration (Peng et al., 2004).
However, similar to S1P, FTY720 has properties that may limit its therapeutic utility in patients with ALI. Its effectiveness as an immunosuppressant is related to its ability to induce lymphopenia via down-regulation of lymphocyte S1P1R signaling (Kovarik et al., 2004; Matloubian et al., 2004), but this effect may be detrimental in patients with ALI, many of whom have sepsis or infection as a triggering event (Wheeler and Bernard, 2007). Moreover, FTY720 induces bradycardia through S1P3R-related mechanisms similar to S1P in both animals and patients (Brown et al., 2007), which may worsen the hemodynamic instability present in many ALI patients. Finally, in a recent multiple sclerosis clinical trial (Kappos et al., 2006), FTY720 significantly increased rates of dyspnea and decreased lung function (lower forced expiratory volume in 1 s), perhaps via mechanisms similar to S1P-induced airway hyper-responsiveness (Roviezzo et al., 2007).
Given these observations of S1P and FTY720, we explored the barrier-regulatory capacity of several novel, synthetic analogs of FTY720. We now demonstrate the barrier-regulatory mechanisms of these analogs similar, but not identical, to S1P and FTY720, with one class of analogs producing significant barrier disruption despite structural similarities. Finally, our in vivo data demonstrate that the representative (S)-phosphonate analog of FTY720 significantly reduces LPS-induced vascular leak in a murine model of inflammatory lung injury. These studies advance our understanding of pulmonary vascular permeability and characterize four novel FTY720 analogs that may potentially act as improved therapeutic tools for prevention and reversal of vascular leak.
Materials and Methods
Reagents.
S1P was purchased from Sigma-Aldrich (St. Louis, MO), and FTY720 was generously provided by Novartis (Basel, Switzerland). All other reagents were obtained from Sigma-Aldrich, unless otherwise noted. Immunofluorescent and Western blotting reagents were obtained as follows: Texas Red phalloidin (Invitrogen, Carlsbad, CA); and rabbit anti-diphosphorylated MLC, rabbit anti-pan-MLC, rabbit anti-phosphorylated ERK, rabbit anti-pan-ERK (Cell Signaling, Beverly, MA). The labeled dextran vascular permeability assay kit was purchased from Millipore Corporation (Bedford, MA). Fura-2/acetoxymethyl ester was obtained through Invitrogen. Pertussis toxin and genistein were purchased from EMD Biosciences (San Diego, CA).
FTY720 Analog Synthesis.
Analogs were synthesized as described elsewhere (Lu et al., 2009).
Cell Culture.
Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza Walkersville, Inc. (Walkersville, MD) and were cultured as described previously (Dudek et al., 2004) in the manufacturer’s recommended endothelial growth medium-2 (EGM-2). Cells were grown at 37°C in a 5% CO2 incubator, and passages 6 to 9 were used for experiments. Media were changed 1 day before experimentation.
Transendothelial Monolayer Electrical Resistance.
EC were grown to confluence in polycarbonate wells containing evaporated gold microelectrodes, and TER measurements were performed using an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) as described previously in detail (Garcia et al., 2001). TER values from each microelectrode were pooled as discrete time points and plotted versus time as the mean ± S.E.M.
Vascular Permeability Assay.
A transendothelial permeability assay was performed as we described previously (Garcia et al., 1986) using labeled tracer flux across confluent EC grown on confluent polycarbonate filters (Vascular Permeability Assay Kit; Millipore Corporation). In brief, EC grown to confluence on Transwell inserts were exposed to agonist stimulation for 1 h. After stimulation, FITC-labeled dextran was added to the luminal compartment for 2 h, and then FITC-dextran clearance across the filter to the abluminal compartment was measured by relative fluorescence excitation at 485 nm and emission at 530 nm.
Immunofluorescence.
EC were grown on gelatinized coverslips before exposure to various conditions as described for individual experiments. EC were then fixed in 3.7% formaldehyde for 10 min, permeabilized with 0.25% Triton X-100 for 5 min, washed in PBS, blocked with 2% bovine serum albumin in Tris-buffered saline with Tween 20 for 1 h, and then incubated for 1 h at room temperature with the primary antibody of interest. After washing, EC were incubated with the appropriate secondary antibody conjugated to immunofluorescent dyes (or Texas Red-conjugated phalloidin for actin staining) for 1 h at room temperature. After further washing with Tris-buffered saline with Tween 20, coverslips were mounted using Prolong Anti-Fade Reagent (Invitrogen) and analyzed using a Nikon Eclipse TE2000 inverted microscope (Nikon, Melville, NY).
Western Blotting.
After treatment as outlined for individual experiments, EC were subsequently washed with cold (4° C) Ca2+/Mg-free PBS and lysed with 0.3% SDS lysis buffer containing protease inhibitors (1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 0.2 trypsin inhibitor unit/ml aprotinin, 10 μM leupeptin, and 5 μM pepstatin A). Sample proteins were separated with 4 to 15% SDS-PAGE gels (Bio-Rad, Hercules, CA) and transferred onto Immobilon-P polyvinylidene difluoride membranes (Millipore Corporation). Membranes were then immunoblotted with primary antibodies (1:500–1000, 4°C, overnight) followed by secondary antibodies conjugated to horseradish peroxidase (1:5000, room temperature, 30 min) and detected with enhanced chemiluminescence (Pierce ECL or SuperSignal West Dura; Pierce Biotechnology, Rockford, IL) on Biomax MR film (Carestream Health, Rochester, NY).
Measurement of Intracellular Calcium.
Measurements of [Ca2+]c using Fura-2 were performed as described previously (Harbeck et al., 2006). HPAEC plated on 25-mm glass coverslips were loaded with 1 μM Fura-2/acetoxymethyl ester ( Invitrogen, Carlsbad, CA) for 20 min at 37°C in KRBH5 buffer (Krebs-Ringer-bicarbonate solution containing 119 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 1 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES-NaOH (pH 7.4), and 5 mM glucose). After replacement of the Fura-2 loading buffer with fresh KRBH5, coverslips were placed into the specimen stage of an inverted fluorescence microscope (Nikon TE-2000U). A Nikon Super Fluor 10× objective was used for these studies. Filters (340- and 380-nm excitation and 530-nm emission) were used for Fura-2 dual excitation ratio imaging. Imaging data acquisition and analysis were accomplished using MetaMorph/MetaFluor software (Molecular Devices, Sunnyvale, CA) and OriginPro 7E (OriginLab Corp, Northampton, MA). Fura-2 340/380 dual excitation ratios were converted to [Ca2+] by in situ calibration. To calibrate Fura-2 ratios, Rmax was obtained by treating cells with 10 μM ionomycin and 2.5 mM Ca2+, and Rmin was obtained by treating cells with EGTA to a final concentration of 10 mM. Fura-2 ratios were converted to [Ca2+] as described by Grynkiewicz et al. (1985) using the equation: [Ca2+] = (Kd′[(R − Rmin)/(Rmax − R)] × Sf/Sb), where Kd′ is the dissociation constant for Fura-2 in the cytosol (225 nM) and Sf and Sb are the measured emission intensities at 380 nm for Ca2+-free and Ca2+-bound Fura-2, respectively. Data summaries for all Ca2+ measurements are expressed as the means ± S.E.
Animals Housing and Procedures.
All experiments and animal care procedures were approved by the Chicago University Animal Resource Center and were handled according to the Animal Care and Use Committee Guidelines at the University of Chicago. C57BL/6 (20–25 g) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed with access to food and water in a temperature-controlled room with a 12-h dark/light cycle. For experiments performed in the intact animal, male C57BL/6 mice (8–10 weeks) were anesthetized with intraperitoneal ketamine and acetylpromazine mixture according to the approved protocol. Escherichia coli LPS solution (2.5 mg/kg) or sterile saline was instilled intratracheally via a 20-gauge catheter. Simultaneously, mice received either FTY720 or analogs (in doses: 0.01, 0.1, 0.5, 1, and 5 mg/kg i.p.) or PBS as vehicle. The animals were allowed to recover for 18 h. BAL and lungs were collected and stored at −70°C for evaluation of lung injury.
Quantification of Total Protein and Leukocytes Analysis in BAL.
Collected BAL was centrifuged (1500g, 15 min, 4°C), and after a second centrifugation of the supernatant (5000g, 20 min, 4°C), pure BAL fluid was used to measure total protein according to the manufacturer’s bicinchoninic acid protein assay kit manual (Bio-Rad). Cell pellets were suspended in Hanks’ solution, and red blood cells were lysed by hypotonic shock (0.2% NaCl) for 5 min. After the cell suspensions were centrifuged and diluted in Hanks’ solution, an aliquot of the cell suspension was examined for the total number of white blood cells using a hemocytometer. Cytospin slides were prepared from cell suspensions. After Diff-Quik staining, the differential cell count of neutrophils and macrophages was determined by counting 300 cells under a microscope.
Measurement of Albumin Concentration by Enzyme-Linked Immunosorbent Assay.
Pure BAL fluids prepared for protein measurement or myeloperoxidase activity (MPO) lung homogenates were used to test albumin concentration. The assay was performed in 96-well plastic plates (Nalge Nunc A/S, Roskilde, Denmark). Plates were coated with mouse albumin (Bethyl Lab, Montgomery, TX), washed, and blocked. Aliquots (100-μl) of the sample or standard and 100 μl of goat anti-mouse albumin antibody (horseradish peroxidase-conjugated) (1:50,000) were then added, followed by incubation at 37°C for 1 h. Finally, the substrate 3,3′,5,5′-tetramethylbenzidine was added for 10 min, and the reaction was stopped by adding 100 μl of 2 M H2SO4. The absorbance at 450 nm was read on a kinetic microplate reader (Molecular Devices).
Determination of MPO.
MPO was isolated and measured from snap-frozen right lungs as described previously (Remick et al., 1990). The right lung was homogenized in 1 ml of 50 mM potassium phosphate, pH 6.0, with 0.5% hexadecyltrimethylammonium bromide. The resulting homogenate was sonicated and then centrifuged at 12,000g for 15 min. The supernatant was mixed 1:30 with assay buffer (100 mM potassium phosphate, pH 6.0, 0.005% H2O2, 0.168 mg/ml o-dianisidine hydrochloride), and the absorbance was read at 490 nm. MPO units were calculated as the change in absorbance with respect to time.
Peripheral Blood Analysis.
The peripheral blood was examined by the Missouri University Research Animal Diagnostic Laboratory (Columbia, MO) for determination of total blood cell counts and differentials in blood samples.
Statistical Analysis.
Values are shown as the mean ± S.E. Data were analyzed using a standard Student’s t test or one-way analysis of variance, groups were compared by Newman-Keuls test, and significance in all cases was defined at p < 0.05.
Results
Differential Effects of FTY720 Analogs on Endothelial Cell Barrier Function in Vitro.
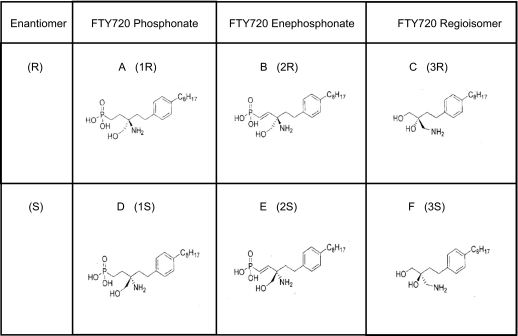
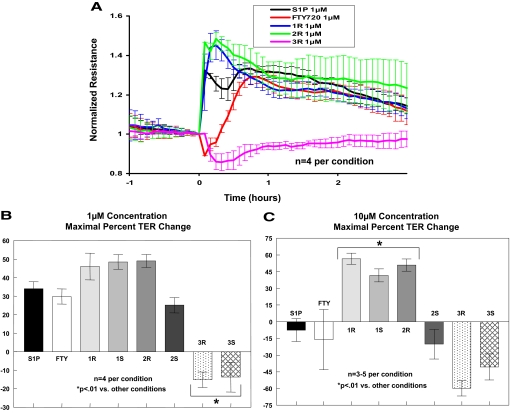
Novel (R)- and (S)-enantiomers of three FTY720 analogs (1 = phosphonate, 2 = enephosphonate, and 3 = regioisomer) were synthesized as described previously (Lu et al., 2009) (Fig. 1 for the structures of the FTY720 analogs used in this study). Our initial studies examined the effects of these six compounds on EC barrier integrity as measured by TER, a highly sensitive in vitro measure of permeability. The (R)- and (S)-enantiomers of 1 and 2 are similar to S1P in that they produce rapid and sustained increases in TER (indicative of enhanced EC barrier function), whereas FTY720 itself induced a delayed onset of barrier enhancement as we have reported previously (Dudek et al., 2007) that was slower to rise in TER relative to S1P and the FTY720 analogs [Fig. 2A; note that only (R)-enantiomer TER data are shown. (S)-Enantiomer results are similar and, therefore, not shown for simplicity]. Interestingly, the FTY720 regioisomers 3R and 3S (in which the positions of the amino groups and one of the hydroxymethyl groups are interchanged) were barrier-disruptive at similar concentrations despite being structurally very similar to the parent FTY720 compound (Fig. 1), indicating the sensitivity of this response to minor structural alterations. Although similar to S1P in the rapid induction of increased TER, the barrier-enhancing FTY720 analogs 1R, 1S, and 2R have a greater maximal percentage TER change at 1 μM compared with both S1P and FTY720 (Fig. 2B). Moreover, when the concentration of these compounds is increased to 10 μM, analogs 1R, 1S, and 2R exhibit even greater maximal TER elevation, whereas S1P, FTY720, and 2S are now somewhat barrier-disruptive at this dose (Fig. 2C), indicating that the barrier-enhancing effects of analogs 1R, 1S, and 2R are sustained over a wider concentration range than those of either S1P or FTY720. In fact, dose-response titrations of 1S, 1R, and 2R demonstrate that these analogs retain near maximal barrier-promoting effects over a range from 1 to 50 μM, suggesting a potential broader therapeutic index for these compounds compared with S1P or FTY720 (data not shown). The results also highlight the importance of enantiomer-specific effects as the enephosphonate analogs (2R and 2S) have diametrically opposing effects on EC barrier function at higher concentrations (≥10 μM).
Fig. 1.
Structures of FTY720 analogs. Enantiomers of FTY720 analogs include 1R (A), 2R (B), 3R (C), 1S (D), 2S (E), and 3S (F).
Fig. 2.
FTY720 analogs promote TER barrier enhancement. A, HPAEC plated on gold electrodes were stimulated with 1 μM S1P (black line), FTY720 (red), 1R (blue), 2R (green), or 3R (purple) at time = 0. The TER tracing represents pooled data (±S.E.M.) from four independent experiments. Bar graphs depict pooled TER data from HPAEC stimulated at 1 (B) or 10 μM (C) with S1P, FTY720, 1R, 1S, 2R, 2S, 3R, or 3S as indicated. The data are expressed as maximal percentage TER change (±S.E.M.) obtained within 60 min. Positive values indicate barrier enhancement. Negative values indicate barrier disruption. n = 3–5 independent experiments per condition; ∗, p < 0.01 versus other conditions.
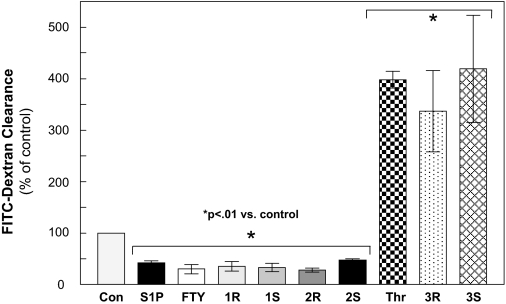
As a complementary approach to further characterize the barrier-protective effects of these FTY720 analogs in vitro, we next assayed permeability of FITC-labeled dextran across the pulmonary EC monolayer (Garcia et al., 1986). Whereas TER measurements are an assessment of EC permeability in terms of resistance to an electrical current, this assay allows for characterization of changes in EC permeability to higher molecular weight molecules. Compared with control EC, those treated with S1P, FTY720, or FTY720 analogs 1 and 2 all demonstrate significantly decreased permeability in this assay, consistent with the TER data shown above (Fig. 3). In contrast, the regioisomers (3R and 3S) increase EC permeability to a degree similar to thrombin, a well described and potent barrier-disrupting agent (Dudek and Garcia, 2001).
Fig. 3.
FTY720 analogs reduce Transwell endothelial cell permeability. HPAEC plated on Transwell inserts were stimulated with S1P, FTY720, 1R, 1S, 2R, 2S (each at 1 μM), thrombin (1 unit/ml), 3R, or 3S (both 25 μM; lower concentrations did not alter permeability) for 1 h before addition of FITC-dextran. After a 2-h incubation, FITC-dextran clearance relative fluorescence was measured by excitation at 485 nm and emission at 530 nm. Data were normalized to unstimulated control. n = 3 independent experiments per condition; ∗, p < 0.01 versus unstimulated condition..
Differential Cytoskeletal Rearrangement and Intracellular Signaling of FTY720 Analogs.
S1P generates dramatic EC cytoskeletal rearrangements such as enhanced cortical actin accumulation and peripheral MLC phosphorylation (Garcia et al., 2001), which are not observed during FTY720-induced barrier enhancement (Dudek et al., 2007). Because the barrier enhancing analogs 1 and 2 produce immediate TER elevation similar to S1P (Fig. 2A), we next evaluated whether these compounds elicited rapid F-actin cytoskeletal rearrangements similar to exposure to S1P (Fig. 4A). Immunofluorescent analysis reveals that compounds 1 and 2 rapidly induce (within 5 min) increased cortical actin ring formation in the periphery of pulmonary EC characteristic of S1P-induced barrier enhancement (Fig. 4A, arrows) (Garcia et al., 2001). In contrast, as we have reported previously (Dudek et al., 2007), FTY720 fails to elicit cortical actin ring formation early at 5 min (data not shown) or at data time points (30 min) associated with peak TER elevation (Fig. 2A). Interestingly, the barrier-disrupting FTY720 analog 3 does not produce dramatic F-actin rearrangements.
Fig. 4.
FTY720 analogs induce cytoskeletal rearrangement. A, confluent HPAEC were stimulated with vehicle control or 1 μM S1P, 1R, 2R, or 3R for 5 min or with FTY720 (1 μM) for 30 min. Cells were fixed using formaldehyde and stained with Texas Red phalloidin for F-actin. Arrows indicate increased cortical actin. B, confluent HPAEC were stimulated with S1P, FTY720, and FTY720 analogs at 1 μM for 5 min and then lysed for Western blotting with phospho-MLC, pan-MLC, phospho-ERK, or pan-ERK antibodies as indicated. Note that all wells represent equal loading of total proteins. Experiments were independently performed in triplicate with representative blots shown.
Whereas the barrier-enhancing FTY analogs exhibit similarities to S1P in cortical actin ring formation, their effects on intracellular signaling events are varied (Fig. 4B). Evaluation of EC lysates for MLC and ERK phosphorylation demonstrate increased MLC and ERK phosphorylation at 5 min in response to S1P, whereas analogs 1R and 2R cause increased phosphorylation of ERK at 5 min. Neither FTY720 nor any of its analogs induces significant MLC phosphorylation over this time frame. Interestingly, the enantiomers 1S and 2S differ from 1R and 2R in terms of ERK signaling because the former fail to induce phosphorylation of this kinase. Thus, these closely related compounds are not equivalent in terms of their downstream signaling effects on cultured pulmonary EC. The barrier-disruptive FTY regioisomers 3R and 3S do not increase ERK or MLC phosphorylation (5 min), unlike the well described barrier-disruptive agent thrombin (Dudek and Garcia, 2001).
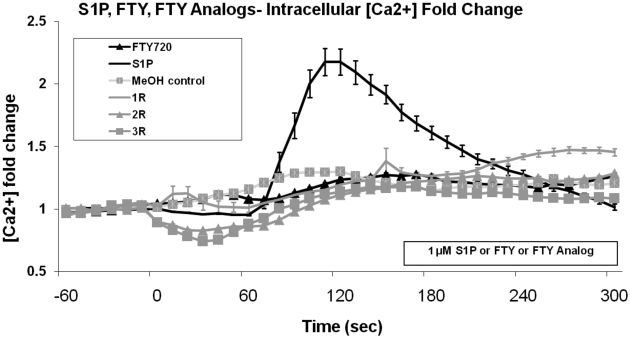
To further explore the mechanistic differences in barrier regulation, intracellular calcium responses to the FTY720 analogs, S1P, and FTY720 were examined. Previous studies have described a brief but substantial increase in intracellular calcium (Ca2+) following S1P exposure in pulmonary endothelial cells (Garcia et al., 2001), whereas FTY720 fails to increase intracellular Ca2+ (Dudek et al., 2007). Changes in HPAEC [Ca2+]i after treatment with FTY720 analogs, S1P, FTY720, and vehicle (all at 1 μM concentration) revealed that only S1P produced a transient Ca2+ spike (Fig. 5), demonstrating that the FTY720 analog-induced barrier enhancement does not require the calcium signaling observed in association with S1P.
Fig. 5.
FTY720 analogs do not stimulate intracellular calcium release. Cultured HPAEC were stimulated with methanol vehicle or 1 μM S1P, FTY720, 1R, 2R, or 3R at time 0, and intracellular calcium levels were measured as fold change in [Ca2+] relative to 60-s average before treatment, as determined by Fura-2 as described under Materials and Methods. n = 3 independent experiments per condition..
Mechanistic Components of FTY720 Analog-Induced Barrier Enhancement.
We next pursued a series of experiments designed to mechanistically explore the manner in which these FTY720 analogs produce barrier enhancement. Similar to S1P and FTY720 (Dudek et al., 2007), TER elevation induced by all four barrier-enhancing compounds (1R, 1S, 2R, and 2S) is significantly inhibited by preincubation with either pertussis toxin (PTX) or genistein, a nonspecific tyrosine kinase inhibitor (Table 1), indicating essential involvement of Gi-coupled signaling and tyrosine phosphorylation events in these barrier-enhancing responses. We have also previously reported that signaling pathways initiated in membrane lipid rafts are essential to S1P- and FTY720-induced barrier enhancement (Singleton et al., 2005; Dudek et al., 2007). Consistent with the involvement of lipid rafts in FTY720 analog barrier enhancement, the lipid raft-disrupting agent, methyl-β-cyclodextrin (MβCD), significantly attenuates their TER elevation (Table 1). Overall, these in vitro data support a barrier-enhancing pathway induced by FTY720 analogs 1R, 1S, 2R, and 2S that probably includes lipid raft signaling and Gi-linked receptor coupling to downstream tyrosine phosphorylation events.
TABLE 1.
Pharmacologic inhibitor effects on FTY720 analog barrier enhancement
Confluent HPAEC were plated on gold microelectrodes and then stimulated with 1 μM S1P, FTY720, 1R, 1S, 2R, or 2S after either a 2-h preincubation with 100 ng/ml PTX, 30-min preincubation with 200 μM genistein (Gen), or 2-h preincubation with 2 mM MßCD or their respective vehicle controls. Data were pooled from multiple TER experiments (4–10 independent experiments per condition) and expressed as percentage inhibition of maximal barrier enhancement at 60 min relative to agonist-only control.
| % Inhibition of Maximal TER Response (of Agonist-Only Control) | |||
|---|---|---|---|
| PTX** | Gen* | MβCD** | |
| S1P | 98.35 (±0.25) | 42.4 (±13.9) | 81.5 (±8.0) |
| FTY | 84.0 (±9.1) | 86.2 (±10.7) | 88.1 (±5.6) |
| 1R | 79.2 (±5.9) | 54.3 (±14.7) | 67.0 (±19.7) |
| 1S | 92.8 (±2.6) | 51.9 (±21.0) | 97.2 (±0.8) |
| 2R | 88.1 (±7.8) | 41.1 (±12.2) | 87.4 (±5.4) |
| 2S | 76.3 (±12.0) | 91.6 (±2.4) | 95.2 (±1.0) |
All EC treated with this inhibitor exhibit P < 0.01 decreased TER compared with agonist-only control.
All EC treated with this inhibitor exhibit P < 0.05 decreased TER compared with agonist-only control.
FTY720 Analog 1S Is Protective in a LPS-Induced Murine Lung Injury Model.
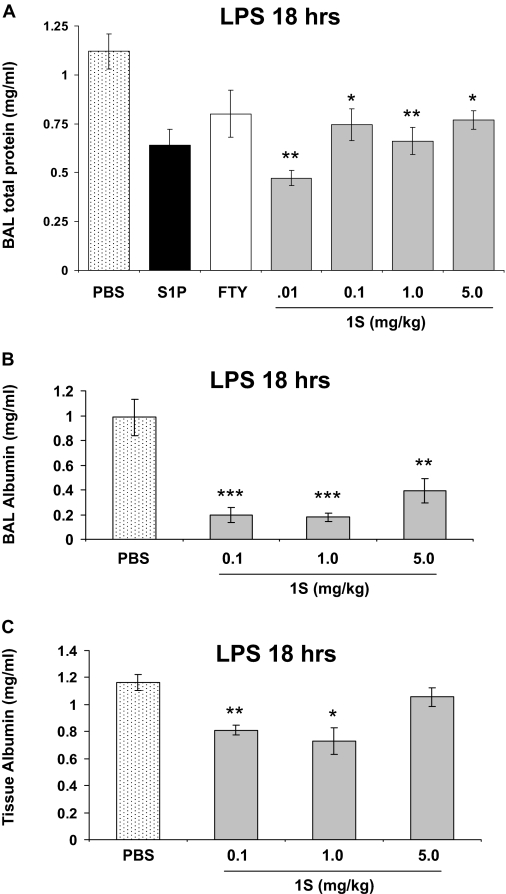
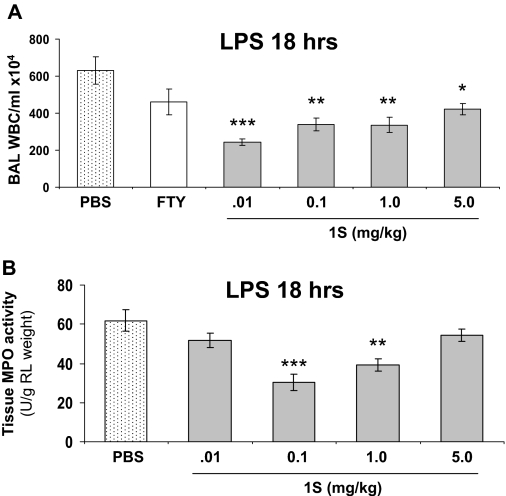
To extend our in vitro findings that the FTY720 analogs promote lung EC integrity, we employed a well characterized murine model of LPS-induced lung injury (Materials and Methods) to examine the in vivo effects of these compounds on pulmonary vascular leak and inflammatory injury. Preliminary studies indicated that 1S was superior to the other barrier-promoting analogs (1R, 2R, and 2S) in this model (data not shown). Therefore, we proceeded to further characterize the representative barrier-enhancing FTY720 analog 1S on pulmonary vascular leak and inflammatory injury in this mouse model. As we have described previously (Peng et al., 2004), intratracheal administration of LPS (2.5 mg/kg) produces significant murine inflammatory lung injury at 18 h as assessed by measurements of BAL total protein and cell count, BAL albumin, and lung tissue albumin. Moreover, LPS increases tissue MPO activity, another reflection of lung parenchymal phagocyte infiltration, compared with control mice (Peng et al., 2004). Intraperitoneal injection of a single dose of FTY720 analog 1S (0.1–5.0 mg/kg) delivered 1 h after LPS exposure significantly reduces capillary leak relative to PBS control at all of the concentrations studied as measured by total BAL protein concentrations (Fig. 6A). This reduction in permeability by 1S is comparable to that achieved by S1P or FTY720. In addition, 1S significantly reduces LPS-induced albumin leakage from the vascular space into both the surrounding lung tissue and BAL (Fig. 6, B and C), as well as BAL WBC accumulation and lung tissue MPO activity (Fig. 7, A and B). These combined data suggest that the optimal protective dose of 1S is 0.1 to 1.0 mg/kg in this model.
Fig. 6.
FTY720 analog 1S reduces LPS-induced vascular permeability in murine lungs. A, male C57BL/6 mice were given LPS (2.5 mg/kg) intratracheally. One hour later, mice received PBS vehicle, FTY720 (0.5 mg/kg), or 1S (doses labeled on the graph, milligram/kilogram) intraperitoneally, or S1P (0.026 mg/kg) via jugular vein injection simultaneous with LPS. The treated mice were allowed to recover for 18 h. BAL fluid was processed to determinate total protein concentrations. n = 3–5 animals per condition. ∗, p < 0.05 and ∗∗, p < 0.01 compared to PBS vehicle treatment. B, BAL albumin levels were also measured in these animals. ∗∗∗, p < 0.001 and ∗∗, p < 0.01 compared to PBS vehicle treatment. C, in similarly treated mice, lung tissue albumin was measured. n = 4–5 animals per condition. ∗, p < 0.05 and ∗∗, p < 0.01 compared to PBS vehicle treatment.
Fig. 7.
1S reduces LPS-induced WBC accumulation in mouse lungs. A, in mice treated as described in Fig. 6, BAL fluid was also analyzed for total WBC count. n = 3–5 animals per condition. ∗, p < 0.05, ∗∗, p < 0.01, and ∗∗∗, p < 0.001 compared to PBS vehicle treatment. B, in similarly treated mice, lung tissue MPO activity was assayed. n = 4–6 animals per condition. ∗∗, p < 0.01 and ∗∗∗, p < 0.001 compared to PBS vehicle treatment..
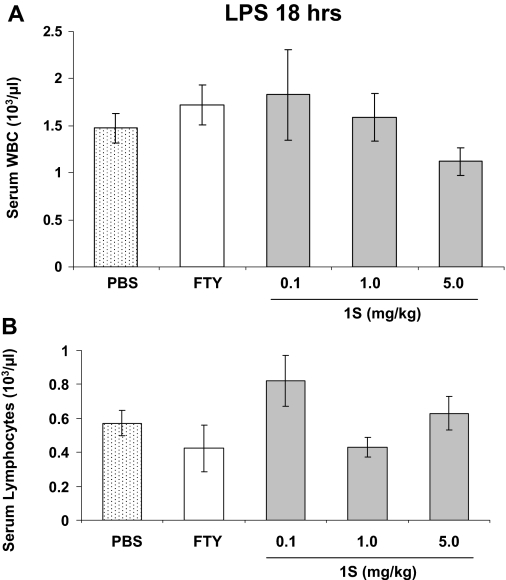
One potential concern when using FTY720 or related compounds in sepsis-related processes such as acute lung injury is the known lymphopenia effect of the parent compound (Kovarik et al., 2004). Therefore, peripheral blood WBC levels were assessed in this mouse model. For comparison, at baseline in control mice (no LPS), total circulating WBC is 4.11 ± 1.58 × 103/μl and the lymphocyte count is 3.57 ± 1.74 × 103/μl (n = 6), so these levels are significantly suppressed (p ≤ 0.001 for both total WBC and lymphocyte count) by LPS alone in this model 18 h after its administration (Fig. 8). However, 1S treatment in these mice does not further alter peripheral blood leukocyte and lymphocyte levels relative to PBS controls (Fig. 8), suggesting that the 1S analog does not produce additional immunosuppression in this LPS model. Interestingly, FTY720 itself also does not suppress circulating WBC levels relative to PBS controls in this model of inflammatory lung injury. In summary, the FTY720 analog 1S decreases multiple indices of LPS-induced pulmonary injury in this murine model without apparent hematologic toxicity.
Fig. 8.
Peripheral blood leukocyte counts in FTY720 analog- and LPS-treated mice. Mice received intratracheal LPS followed 1 h later by PBS, FTY720 (0.5 mg/ml), or 1S (doses labeled on the graph, milligram/kilogram) intraperitoneally as described previously. Blood was collected 18 h after LPS for total WBC (A) and lymphocytes (B) quantification. n = 3–7 animals per condition. There are no statistical differences among any of the conditions shown.
Discussion
In this study, we demonstrate potent pulmonary vascular permeability effects of several novel FTY720 analogs both in vitro and in vivo. These findings have direct therapeutic relevance for the ALI/acute respiratory distress syndrome, a highly morbid condition afflicting an estimated 200,000 people annually and causing 75,000 deaths in the United States (Rubenfeld et al., 2005). To date, there are no effective interventions that target the critical pulmonary vascular leak that underlies this syndrome (Wheeler and Bernard, 2007). Our laboratory group was the first to identify the potential of S1P to serve in a vascular barrier-enhancing capacity in vitro (Garcia et al., 2001); however, our more recent animal work suggests that modulation of S1P-related pathways in lung endothelium also holds promise in vivo with S1P infusion into murine and canine models of inflammatory lung injury highly protective (McVerry et al., 2004; Peng et al., 2004), whereas others have demonstrated that administration of an S1P1R antagonist induces lung capillary leakage (Sanna et al., 2006). Unfortunately, the endogenous compound S1P is a suboptimal therapeutic candidate because of its potential to produce negative effects, including cardiac toxicity and pulmonary edema at higher doses (Forrest et al., 2004; Gon et al., 2005). In fact, multiple agents for inhibiting various components of the S1P pathway are currently under therapeutic investigation for various clinical indications (Takabe et al., 2008). Because the structurally related synthetic compound FTY720 exhibits potent barrier-enhancing properties both in vitro and in vivo (Sanchez et al., 2003; Peng et al., 2004; Dudek et al., 2007) and is in advanced clinical trials for treatment of multiple sclerosis (Brown et al., 2007), it remains a promising alternative to S1P that may soon be available for trials in patients with ALI. However, FTY720 has demonstrated bradycardic and immunosuppressive effects (Kovarik et al., 2004; Brown et al., 2007; Tedesco-Silva et al., 2007) that may be detrimental to critically ill patients with ALI.
Therefore, we generated multiple analogs of FTY720 to further our mechanistic understanding of how these compounds regulate EC barrier regulation in the hopes of designing a more optimal therapeutic agent. Other groups have synthesized multiple derivatives of FTY720, including phosphonates (Mandala et al., 2002; Forrest et al., 2004; Hale et al., 2004b), phosphothioates (Foss et al., 2005), 4(5)-phenylimidazole-containing analogs (Clemens et al., 2005), and conformationally constrained analogs (Hanessian et al., 2007; Zhu et al., 2007), primarily for the purposes of characterizing them in terms of S1P receptor affinity and the ability to induce lymphopenia. Additional analogs have been employed to evaluate the proapoptotic effects of sphingosine and FTY720 (Don et al., 2007) or as possible antiangiogenic agents (Nakayama et al., 2008). However, this report is the first to use this valuable pharmacological approach to explore the potential of FTY720-related compounds to regulate pulmonary vascular permeability.
Our data illustrate the usefulness of this approach as the FTY720 analogs described here exhibit dramatically differential effects on lung EC barrier function. The FTY720 phosphonate (1R and 1S) and enephosphonate (2R and 2S) compounds display in vitro barrier enhancing properties comparable or superior to S1P and FTY720, whereas the FTY720 regioisomers (3R and 3S) are barrier-disruptive despite being structurally very similar to the parent FTY720 compound (Figs. 2B and 3). These results suggest that three of the barrier-enhancing analogs (1R, 1S, and 2R) may be more appealing as potential clinical agents than S1P or FTY720 for blocking ALI-associated pulmonary edema because they exhibit a broader therapeutic index with increased potency in vitro (Fig. 2, B and C). Our preliminary mechanistic studies indicate that Gi-coupled receptor signaling, tyrosine kinases, and lipid raft domains are involved in mediating the enhanced EC barrier function induced by these analogs, as they are in the S1P response (Table 1). Ongoing studies are seeking to determine the signaling events that account for the differential effects of these compounds on EC barrier function. One intriguing possibility is that FTY720 phosphonate and enephosphonate compounds may not be hydrolyzed by lipid phosphatases because a similar mechanism was noted to result in differential intracellular signaling for a S1P phosphonate analog (Zhao et al., 2007).
Our data further demonstrate that orientation changes present in the regioisomers compared to FTY720 are sufficient to produce opposite effects on EC permeability. Understanding how these effects are mediated may provide important additional insights into EC barrier regulation. The mechanism through which 3R and 3S disrupt the EC barrier does not appear to involve MLC phosphorylation, actin stress fiber formation, or actomyosin contraction (Fig. 4) as observed after thrombin (Dudek and Garcia, 2001). Our data also highlight the importance of stereoisomeric structure in determining the bioactivity of these compounds in barrier regulation. Although FTY720 phosphonate (compound 1 in this study) has been synthesized previously by others (Mandala et al., 2002; Forrest et al., 2004; Hale et al., 2004b), these prior studies used only a racemic mixture that did not differentiate enantiomeric-specific effects. Such effects are known to be functionally important in the metabolism of the parent compound FTY720 because measurements in rats and humans demonstrate that in vivo phosphorylation of FTY720 results only in formation of the (S)-enantiomer of FTY phosphate, which is a much higher affinity agonist for the S1P receptor family than (R)-FTY phosphate (Albert et al., 2005). In the present study, several interesting properties differentiate the (R)- and (S)-enantiomers of FTY720 phosphonate and enephosphonate. For example, 1R and 2R rapidly activate ERK, whereas the (S)-enantiomers of these compounds do not (Fig. 4B). Although 10 μM 2R potently increases EC barrier function in vitro, this concentration of 2S is barrier-disruptive (Fig. 2C). Finally, the 1S phosphonate compound is more efficacious than 1R in the mouse model of LPS-induced lung injury (data not shown). Thus, further study of the chiral effects of these compounds on EC barrier function is warranted.
An important aspect of mechanistically characterizing these analogs is to determine their relative S1P receptor activities. Most relevant are their effects on S1P1R and S1P3R because these are the best described S1P receptors in terms of their respective barrier-enhancing (S1P1R) (Garcia et al., 2001; Singleton et al., 2005; Sanna et al., 2006) and barrier-disrupting (S1P3R) (Gon et al., 2005) properties. Recently published data characterize the relative activity of the four barrier-enhancing analogs (1S, 2S, 1R, and 2R) on S1P1R (Lu et al., 2009). In this study, HTC4 cells (which do not express endogenous S1P receptors) were stably transfected with S1P1R, and Ca2+ mobilization assays were performed after stimulation with various concentrations of S1P, FTY720 phosphate, and analogs. The 1R (93% of maximal S1P response) and the 2R (73%) analogs exhibited substantial S1P1R activation in these assays similar to that of FTY720 phosphate (76%) (Lu et al., 2009) and consistent with this receptor being the primary transducer of barrier enhancement by these analogs. In contrast, 1S produced only 36% of the maximal S1P response despite exhibiting comparable barrier-enhancing potency to the other compounds (Figs. 2 and 3). These interesting data suggest that barrier enhancement by 1S may be transduced at least in part via another receptor as we have previously suggested for FTY720 itself (Dudek et al., 2007). Another intriguing possibility is that the 1S analog may alter the conformational state of S1P1R leading to differential effector coupling and altered downstream signaling that results in reduced Ca2+ mobilization but comparable barrier enhancement. A similar model of S1P1R signaling involving multiple conformational states has been proposed by others (Pyne and Pyne, 2008). Even more robust evidence for an alternative barrier-enhancing receptor is provided by 2S, which is capable of significantly improving EC barrier function (Figs. 2 and 3) despite a complete inability to activate the S1P1R as measured by this Ca2+ mobilization assay (Lu et al., 2009).
In addition, this same group has characterized the relative activity of 1S, 2S, 1R, and 2R on S1P3R in the Ca2+ mobilization assay (W. J. Valentine and G. Tigyi, personal communication). It is important to note that these barrier-enhancing analogs all exhibit little (1R and 2R, ∼10% of maximal S1P response) or no (1S and 2S, both 0%) S1P3R activity as measured by Ca2+ mobilization. Thus, all of the barrier-enhancing analogs identified in our study may have therapeutic advantages over S1P and FTY720 in terms of decreasing the S1P3R-related negative effects on barrier function and cardiac toxicity (Forrest et al., 2004; Hale et al., 2004a; Gon et al., 2005). The S1P receptor profiles of the barrier-disrupting FTY720 analogs identified in our study (3S and 3R) also merit further study.
In summary, these results provide important mechanistic insights into the regulation of EC barrier function and demonstrate the potential therapeutic utility of several novel FTY720 analogs to reverse the pulmonary vascular leak that characterizes ALI. (S)-FTY720-phosphonate is particularly promising both in vitro and in vivo. Moreover, animal data presented here suggest that, at doses sufficient to protect against lung injury, FTY720 and its derivative 1S do not adversely affect circulating WBC levels during inflammatory states (Fig. 8) and thus may be appropriate to use in critically ill patients with infection-associated ALI. Given the high mortality of this syndrome and lack of specific therapies (Wheeler and Bernard, 2007), clinical trials of these agents in ALI may be warranted in the near future.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant P01-HL58064, R01-HL68071, R01-HL79396, R01-HL88144] (to J.G.N.G., V.N., and S.M.D.); and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [R01-DK07496602] (to M.R.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- ALI
- acute lung injury
- BAL
- bronchoalveolar lavage
- EC
- endothelial cell
- HPAEC
- human pulmonary artery endothelial cells
- LPS
- lipopolysaccharide
- MβCD-cyclodextrin
- MβCD-cyclodextrin
- MPO
- myeloperoxidase
- MLC
- myosin light chain
- PTX
- pertussis toxin
- S1P
- sphingosine 1-phosphate
- S1P1R
- S1P1 receptor
- S1P3R
- S1P3 receptor
- TER
- transendothelial electrical resistance
- ERK
- extracellular signal-regulated kinase
- WBC
- white blood cells
- FTY720 (FTY)
- 2-amino-2-(2-[4-octylphenyl]ethyl)-13-propanediol
- 1R
- (R)-FTY720 phosphonate
- 2R
- (R)-FTY720 enephosphonate
- 3R
- (R)-FTY720 regioisomer
- 1S
- (S)-FTY720 phosphonate
- 2S
- (S)-FTY720 enephosphonate
- 3S
- (S)-FTY720 regioisomer
- FITC
- fluorescein isothiocyanate
- PBS
- phosphate-buffered saline.
References
- Albert R, Hinterding K, Brinkmann V, Guerini D, Müller-Hartwieg C, Knecht H, Simeon C, Streiff M, Wagner T, et al. ( 2005) Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J Med Chem 48: 5373–5377 [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Cyster JG, Hla T. ( 2004) FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant 4: 1019–1025 [DOI] [PubMed] [Google Scholar]
- Brown BA, Kantesaria PP, McDevitt LM. ( 2007) Fingolimod: a novel immunosuppressant for multiple sclerosis. Ann Pharmacother 41: 1660–1668 [DOI] [PubMed] [Google Scholar]
- Clemens JJ, Davis MD, Lynch KR, Macdonald TL. ( 2005) Synthesis of 4(5)-phenylimidazole-based analogues of sphingosine-1-phosphate and FTY720: discovery of potent S1P1 receptor agonists. Bioorg Med Chem Lett 15: 3568–3572 [DOI] [PubMed] [Google Scholar]
- Don AS, Martinez-Lamenca C, Webb WR, Proia RL, Roberts E, Rosen H. ( 2007) Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J Biol Chem 282: 15833–15842 [DOI] [PubMed] [Google Scholar]
- Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JG. ( 2007) Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal 19: 1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. ( 2001) Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500 [DOI] [PubMed] [Google Scholar]
- Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. ( 2004) Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 279: 24692–24700 [DOI] [PubMed] [Google Scholar]
- Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, Hale J, Keohane C, Meyers C, Milligan J, et al. ( 2004) Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther 309: 758–768 [DOI] [PubMed] [Google Scholar]
- Foss FW, Jr, Clemens JJ, Davis MD, Snyder AH, Zigler MA, Lynch KR, Macdonald TL. ( 2005) Synthesis, stability, and implications of phosphothioate agonists of sphingosine-1-phosphate receptors. Bioorg Med Chem Lett 15: 4470–4474 [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. ( 2001) Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW, 2nd, Malik AB. ( 1986) Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol 128: 96–104 [DOI] [PubMed] [Google Scholar]
- Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, Rosen H.(2005)S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci U S A 102: 9270–9275 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Grynkiewicz G, Poenie M, Tsien RY. ( 1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450 [PubMed] [Google Scholar]
- Hale JJ, Doherty G, Toth L, Mills SG, Hajdu R, Keohane CA, Rosenbach M, Milligan J, Shei GJ, Chrebet G, et al. ( 2004a) Selecting against S1P3 enhances the acute cardiovascular tolerability of 3-(N-benzyl)aminopropylphosphonic acid S1P receptor agonists. Bioorg Med Chem Lett 14: 3501–3505 [DOI] [PubMed] [Google Scholar]
- Hale JJ, Neway W, Mills SG, Hajdu R, Ann Keohane C, Rosenbach M, Milligan J, Shei GJ, Chrebet G, Bergstrom J, et al. ( 2004b) Potent S1P receptor agonists replicate the pharmacologic actions of the novel immune modulator FTY720. Bioorg Med Chem Lett 14: 3351–3355 [DOI] [PubMed] [Google Scholar]
- Hanessian S, Charron G, Billich A, Guerini D. ( 2007) Constrained azacyclic analogues of the immunomodulatory agent FTY720 as molecular probes for sphingosine 1-phosphate receptors. Bioorg Med Chem Lett 17: 491–494 [DOI] [PubMed] [Google Scholar]
- Harbeck MC, Chepurny O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. ( 2006) Simultaneous optical measurements of cytosolic Ca2+ and cAMP in single cells. Sci STKE 2006: pl6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. ( 2006) Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 355: 1124–1140 [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Schmouder RL, Slade AJ. ( 2004) Overview of FTY720 clinical pharmacokinetics and pharmacology. Ther Drug Monit 26: 585–587 [DOI] [PubMed] [Google Scholar]
- Lu X, Sun C, Valentine WJ, Shuyu E, Liu J, Tigyi G, Bittman R. ( 2009) Chiral vinylphosphonate and phosphonate analogues of the immunosuppressive agent FTY720. J Org Chem 74: 3192–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. ( 2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346–349 [DOI] [PubMed] [Google Scholar]
- Mansoor M, Melendez AJ. ( 2008) Recent trials for FTY720 (fingolimod): a new generation of immunomodulators structurally similar to sphingosine. Rev Recent Clin Trials 3: 62–69 [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. ( 2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360 [DOI] [PubMed] [Google Scholar]
- McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. ( 2004) Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 170: 987–993 [DOI] [PubMed] [Google Scholar]
- Nakayama S, Uto Y, Tanimoto K, Okuno Y, Sasaki Y, Nagasawa H, Nakata E, Arai K, Momose K, Fujita T, et al. ( 2008) TX-2152: a conformationally rigid and electron-rich diyne analogue of FTY720 with in vivo antiangiogenic activity. Bioorg Med Chem 16: 7705–7714 [DOI] [PubMed] [Google Scholar]
- Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, et al. ( 2007) FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest 117: 2408–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. ( 2004) Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245–1251 [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. ( 2008) Sphingosine 1-phosphate, lysophosphatidic acid and growth factor signaling and termination. Biochim Biophys Acta 1781: 467–476 [DOI] [PubMed] [Google Scholar]
- Remick DG, Strieter RM, Eskandari MK, Nguyen DT, Genord MA, Raiford CL, Kunkel SL. ( 1990) Role of tumor necrosis factor-alpha in lipopolysaccharide-induced pathologic alterations. Am J Pathol 136: 49–60 [PMC free article] [PubMed] [Google Scholar]
- Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA Jr, Spiegel S. ( 2003) Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB J 17: 1789–1799 [DOI] [PubMed] [Google Scholar]
- Roviezzo F, Di Lorenzo A, Bucci M, Brancaleone V, Vellecco V, De Nardo M, Orlotti D, De Palma R, Rossi F, D’Agostino B, et al. ( 2007) Sphingosine-1-phosphate/sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. Am J Respir Cell Mol Biol 36: 757–762 [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. ( 2005) Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693 [DOI] [PubMed] [Google Scholar]
- Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. ( 2003) Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem 278: 47281–47290 [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, et al. ( 2006) Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2: 434–441 [DOI] [PubMed] [Google Scholar]
- Shikata Y, Birukov KG, Garcia JG. ( 2003) S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK and paxillin. J Appl Physiol 94: 1193–1203 [DOI] [PubMed] [Google Scholar]
- Singleton PA, Dudek SM, Chiang ET, Garcia JG. ( 2005) Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J 19: 1646–1656 [DOI] [PubMed] [Google Scholar]
- Takabe K, Paugh SW, Milstien S, Spiegel S. ( 2008) “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60: 181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco-Silva H, Szakaly P, Shoker A, Sommerer C, Yoshimura N, Schena FP, Cremer M, Hmissi A, Mayer H, Lang P. ( 2007) FTY720 versus mycophenolate mofetil in de novo renal transplantation: six-month results of a double-blind study. Transplantation 84: 885–892 [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Bernard GR. ( 2007) Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369: 1553–1564 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, et al. ( 2007) Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem 282: 14165–14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Snyder AH, Kharel Y, Schaffter L, Sun Q, Kennedy PC, Lynch KR, Macdonald TL. ( 2007) Asymmetric synthesis of conformationally constrained fingolimod analogues–discovery of an orally active sphingosine 1-phosphate receptor type-1 agonist and receptor type-3 antagonist. J Med Chem 50: 6428–6435 [DOI] [PMC free article] [PubMed] [Google Scholar]