Abstract
The pregnane X receptor (PXR, NR1I2) regulates the expression of genes that encode drug-metabolizing enzymes and drug transporter proteins in liver and intestine. Understanding the molecular mechanisms that modulate PXR activity is therefore critical for the development of effective therapeutic strategies. Several recent studies have implicated the activation of kinase signaling pathways in the regulation of PXR biological activity, although direct evidence and molecular mechanisms are currently lacking. We therefore sought to characterize potential phosphorylation sites within the PXR protein by use of a rational, comprehensive, and systematic site-directed mutagenesis approach to generate phosphomimetic mutations (Ser/Thr → Asp) and phospho-deficient mutations (Ser/Thr → Ala) at 18 predicted consensus kinase recognition sequences in the human PXR protein. Here, we identify amino acid residues Ser8, Thr57, Ser208, Ser305, Ser350, and Thr408 as being critical for biological activity of the PXR protein. Mutations at positions 57 and 408 abolish ligand-inducible PXR activity. Mutations in the extreme N terminus and in the PXR ligand-binding domain at positions Ser8, Ser305, Ser350, and Thr408 decrease the ability of PXR to form heterodimers with retinoid X receptor α. Mutations at positions Ser208, Ser305, Ser350, and Thr408 alter PXR-protein cofactor interactions. Finally, the subcellular localization of the PXR protein is profoundly affected by mutations at position Thr408. These data suggest that PXR activity can potentially be regulated by phosphorylation at specific amino acid residues within several predicted consensus kinase recognition sequences to differentially affect PXR biological activity.
Nuclear receptor (NR) proteins constitute a large group of transcription factors, with 48 members present in the human genome that control diverse biological functions including metabolism, homeostasis, reproduction, and development. The C-terminal region of NR proteins contains a ligand-binding domain (LBD) and a ligand-dependent activation function called AF-2. The LBD is connected to the DNA-binding domain (DBD) and an N-terminal activation function called AF-1 by the hinge region (Kumar et al., 2004). Most NR proteins are conventionally activated by the binding of small lipophilic ligands such as hormones, fatty acids, oxysterols, bile acids, and xenobiotics (Maglich et al., 2001). In addition to conventional activation by ligand binding, numerous studies have implicated kinase signaling cascades in the activation of NR biological activity. Several liver-enriched NR proteins are targets of phosphorylation to regulate critical NR function and enable cross-talk between diverse signaling pathways (Rochette-Egly, 2003; Staudinger and Lichti, 2008). Compared with our knowledge other NR superfamily members, we have only a meager understanding of the extent to which pregnane X receptor (PXR, NR1I2) is regulated by phosphorylation.
The PXR transcription factor is a promiscuous NR family member that is activated by a wide range of compounds including steroids, bile acids, and a wide variety of drugs and naturally occurring compounds. PXR has been characterized as a master regulator of xenobiotic-inducible cytochrome P450 (P450) gene expression in liver. It is now clear that activation of PXR by xenobiotic compounds regulates expression of a group of genes that encode drug-metabolizing enzymes and several key drug transporter proteins in liver and intestine (Kliewer et al., 1998; Lehmann et al., 1998; Kast et al., 2002; Maglich et al., 2002; Staudinger et al., 2003). In this manner, PXR activation in liver and intestine increases metabolism, transport, and elimination of potentially toxic compounds from the body, but also represents the molecular basis for an important class of drug-drug interactions. In addition, recent evidence suggests a role for PXR in hepatic glucose and lipid metabolism (Bhalla et al., 2004; Kodama et al., 2004), endocrine homeostasis (Zhai et al., 2007; Lim and Huang, 2008), inflammation (Gu et al., 2006; Zhou et al., 2006; Shah et al., 2007), and drug resistance (Chen et al., 2007; Zhou et al., 2008).
It is well established that hepatic drug-inducible P450 gene expression is responsive to kinase signaling pathways (Sidhu and Omiecinski, 1995; Marc et al., 2000). The exact molecular mechanisms by which the various signaling pathways interface with PXR biological activity is a topic of current investigation by several laboratories. The cyclic AMP-dependent protein kinase (PKA) signaling pathway has been shown to modulate PXR activity in a species-specific manner (Ding and Staudinger, 2005a; Lichti-Kaiser et al., 2009). Paradoxically, although activation of the PKA signaling pathway has a potentiating effect on PXR-mediated gene activation in mouse hepatocytes, it serves as a repressive signal in both human and rat hepatocytes. Kinase assays show that the human PXR protein can serve as an effective substrate for PKA in vitro (Ding and Staudinger, 2005b; Lichti-Kaiser et al., 2009). It has also been shown that PXR exists as a phosphoprotein in vivo and that its phosphothreonine status is modulated by the activation of PKA signaling (Lichti-Kaiser et al., 2009). This evidence suggests one potential mechanism for PKA-mediated modulation of CYP3A gene expression. In addition, activation of protein kinase C (PKC) signaling has been shown to repress PXR activity by increasing the strength of interaction between PXR and the corepressor NCoR, and by abolishing the ligand-dependent interaction between PXR and SRC-1 (Ding and Staudinger, 2005b).
Cyclin-dependent kinase 2 (Cdk2) also attenuates the activation of CYP3A4 gene expression. The PXR protein is a suitable substrate for the Cdk2 enzyme in vitro, and a phosphomimetic mutation at a putative Cdk phosphorylation site at position Ser350 (S350D) seems to impair the function of hPXR, whereas a phosphorylation-deficient mutation (S350A) conferred resistance to the repressive effects of Cdk2 on a reporter gene in HepG2 cells (Lin et al., 2008). An additional study has identified a phosphomimetic mutation within the DBD (T57D) that is associated with the loss of function of hPXR. Furthermore, PXR was identified as a substrate for p70 S6 kinase in vitro, and the phosphorylation-deficient mutation (T57A) conferred resistance to the inhibitory effect of p70 S6K ((Pondugula et al., 2009). The results of these studies suggest that the activity of PXR is modulated by changes in its phosphorylation status. However, the direct phosphorylation and subsequent modulation of PXR activity has not been demonstrated in vivo.
In this study, we systematically mutate 18 serine and threonine amino acid residues within the human PXR protein based n either “in silico” consensus kinase site prediction, or by comparison with other known phosphorylation sites within closely related NR proteins. Using biochemical approaches we identify six critical residues at positions Ser8, Thr57, Ser208, Ser305, Ser350, and Thr408 that significantly modulate PXR activity. The serine and threonine residues identified here selectively affect distinct biological functions of the PXR protein including 1) DNA binding capacity, 2) retinoid X receptor α (RXRα) heterodimerization capacity, 3) interaction with protein cofactors, and 4) alterations in PXR subcellular localization. Taken together, these data suggest that PXR activity is probably regulated by phosphorylation at several key amino acid residues.
Materials and Methods
Compounds and Plasmids.
Unless otherwise stated, all chemical compounds were purchased from Sigma-Aldrich (St. Louis, MO). The pSG5-hPXR, GAL4-SRC2, and GAL4-NCoR1 expression vectors were described previously (Ding and Staudinger, 2005b). The pFR-LUC reporter gene, which is responsive to GAL4-fusion proteins, is commercially available (BD Biosciences, San Jose, CA). The pCMX-FLAG RXRα expression vector was a kind gift from Dr. Koren Mann. PXR wild-type and mutant constructs were fused to the VP16 transcriptional activation domain by subcloning into the pVP16 expression vector (Clontech, Mountain View, CA) at EcoRI and BamHI restriction sites. PXR wild-type and mutant constructs were fused to the GFP by subcloning into the pEGFP-C2 expression vector (Clontech) at EcoRI and BamHI restriction sites.
Site-directed Mutagenesis.
Consensus serine and threonine phosphorylation sites within the human PXR protein were identified by use the NetPhos 2.0 server. Eighteen potential phosphorylation sites were mutated to an aspartic acid, a phosphomimetic mutation, and an alanine, a nonphosphomimetic mutation. The mutant pSG5-hPXR expression vectors were generated by site-directed mutagenesis with use of the QuikChange Mutagenesis system (Stratagene, La Jolla, CA). Primer sequences used for site-directed mutagenesis are shown in Table 1.
TABLE 1.
Oligonucleotide sequences for site-directed mutagenesis of the human PXR protein
| Amino Acid | Oligos for Mutagenesis to A | Oligos for Mutagenesis to D |
|---|---|---|
| Ser8 | 5′-ggaggtgagacccaaagaagcctggaaccatgctg-3′ | 5′-ggaggtgagacccaaagaagactggaaccatgctg-3′ |
| 3′-cagcatggttccaggcttctttgggtctcacctcc-5′ | 3′-cagcatggttccagtcttctttgggtctcacctcc-5′ | |
| Thr20 | 5′-tgtacactgtgaggacgcagagtctgttcctgg-3′ | 5′-gactttgtacactgtgaggacgatgagtctgttcctggaaagccc-3′ |
| 3′-ccaggaacagactctgcgtcctcacagtgtaca-5′ | 3′-gggctttccaggaacagactcatcgtcctcacagtgtacaaagtc-5′ | |
| Thr57 | 5′-ctatcacttcaatgtcatggcatgtgaaggatgcaaggg-3′ | 5′-ctggctatcacttcaatgtcatggattgtgaaggatgcaagggcttttt-3′ |
| 3′-cccttgcatccttcacatgccatgacattgaagtgatag-5′ | 3′-aaaaagcccttgcatccttcacaatccatgacattgaagtgatagccag-5′ | |
| Thr90 | 5′-gatcacccggaaggcccggcgacagtg-3′ | 5′-agatcacccggaaggaccggcgacagtgcc-3′ |
| 3′-cactgtcgccgggccttccgggtgatc-5′ | 3′-ggcactgtcgccggtccttccgggtgatct-5′ | |
| Ser105 | 5′-cgcaagtgcctggaggccggcatgaagaagga-3′ | 5′-gcgcaagtgcctggaggacggcatgaagaaggag-3′ |
| 3′-tccttcttcatgccggcctccaggcacttgcg-5′ | 3′-ctccttcttcatgccgtcctccaggcacttgcgc-5′ | |
| Ser114 | 5′-gaaggagatgatcatggccgacgaggccgtg-3′ | 5′-gaaggagatgatcatggacgacgaggccgtggag-3′ |
| 3′-cacggcctcgtcggccatgatcatctccttc | 3′-ctccacggcctcgtcgtccatgatcatctccttc-5′ | |
| Ser130 | 5′-cttgatcaagcggaagaaagctgaacggacagggactcagc-3′ | 5′-cttgatcaagcggaagaaagacgaacggacagggactcagc-3′ |
| 3′-gctgagtccctgtccgttcagctttcttccgcttgatcaag-5′ | 3′-gctgagtccctgtccgttcgtctttcttccgcttgatcaag-5′ | |
| Thr133 | 5′-gaagaaaagtgaacgggcagggactcagccact-3′ | 5′-cggaagaaaagtgaacgggatgggactcagccactggga-3′ |
| 3′-agtggctgagtccctgcccgttcacttttcttc-5′ | 3′-tcccagtggctgagtcccatcccgttcacttttcttccg-5′ | |
| Thr135 | 5′-tgaacggacaggggctcagccactggg-3′ | 5′-aagtgaacggacaggggatcagccactgggagtg-3′ |
| 3′-cccagtggctgagcccctgtccgttca-5′ | 3′-cactcccagtggctgatcccctgtccgttcactt-5′ | |
| Ser180 | 5′-caggggtgcttagcgctggctgcgagttgc-3′ | 5′-ccaggggtgcttagcgatggctgcgagttgcc-3′ |
| 3′-gcaactcgcagccagcgctaagcacccctg-5′ | 3′-ggcaactcgcagccatcgctaagcacccctgg-5′ | |
| Ser192 | 5′-ctctgcaggccccagcgagggaagaag-3′ | 5′-cagagtctctgcaggccccagatagggaagaagctgcc-3′ |
| 3′-cttcttccctcgctggggcctgcagag-5′ | 3′-ggcagcttcttccctatctggggcctgcagagactctg-5′ | |
| Ser208 | 5′-tccggaaagatctgtgcgctttgaaggtctctctg-3′ | 5′-ggtccggaaagatctgtgcgatttgaaggtctctctgcag-3′ |
| 3′-cagagagaccttcaaagcgcacagatctttccgga-5′ | 3′-ctgcagagagaccttcaaatcgcacagatctttccggacc-5′ | |
| Ser231 | 5′-acccccagccgacgctggcgggaaagag-3′ | 5′-caaacccccagccgacgatggcgggaaagagatc-3′ |
| 3′-ctctttcccgccagcgtcggctgggggt-5′ | 3′-gatctctttcccgccatcgtcggctgggggtttg-5′ | |
| Ser274 | 5′-cgaggaccagatcgccctgctgaaggg-3′ | 5′-atcgaggaccagatcgacctgctgaagggggc-3′ |
| 3′-cccttcagcagggcgatctggtcctcg-5′ | 3′-gcccccttcagcaggtcgatctggtcctcgat-5′ | |
| Thr290 | 5′-gtcaactgagattcaacgcagtgttcaacgcggag-3′ | 5′-ctgtgtcaactgagattcaacgatgtgttcaacgcggagactgga-3′ |
| 3′-ctccgcgttgaacactgcgttgaatctcagttgac-5′ | 3′-tccagtctccgcgttgaacacatcgttgaatctcagttgacacag-5′ | |
| Ser305 | 5′-gtgtggccggctggcctactgcttgga-3′ | 5′-ggagtgtggccggctggactactgcttggaagac-3′ |
| 3′-tccaagcagtaggccagccggccacac-5′ | 3′-gtcttccaagcagtagtccagccggccacactcc-5′ | |
| Ser350 | 5′-catctccctcttcgccccagaccgccc-3′ | 5′-ccatctccctcttcgacccagaccgcccag-3′ |
| 3′-gggcggtctggggcgaagagggagatg-5′ | 3′-ctgggcggtctgggtcgaagagggagatgg-5′ | |
| Thr408 | 5′-caatgctcagcacgcccagcggctgct-3′ | 5′-atcaatgctcagcacgaccagcggctgctgcg-3′ |
| 3′-agcagccgctgggcgtgctgagcattg-5′ | 3′-cgcagcagccgctggtcgtgctgagcattgat-5′ |
Oligos, oligonucleotide sequences.
Transient Transfection and Reporter Gene Analysis.
The XREM-LUC reporter gene assays and the mammalian two-hybrid system assays were performed as described previously (Brobst et al., 2004; Ding and Staudinger, 2005b). In brief, CV-1 cells were plated in 96-well plates at a density of 7000 cells per well. After 24 h the cells were transfected by use of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The PXR transactivation assays were transfected with 110 ng of DNA per well containing SV40-β-gal (40 ng), XREM-Luc (20 ng), pSG5-hPXR (5 ng), and pBluescript (45 ng). The mammalian two-hybrid assays were transfected with 110 ng of DNA per well containing SV40-β-gal (40 ng), pFR-Luc (20 ng), Gal4-Cofactor (20 ng), VP16-hPXR (10 ng), and pBluescript (20 ng). The next day the cells were drug-treated for 24 h with vehicle or 10 μM rifampicin. Luciferase and β-galactosidase activities were determined by use of a standard luciferase assay system (Promega, Madison, WI).
Electrophoretic Mobility Shift Assay.
Wild-type and mutant human PXR and RXRα were synthesized in vitro by use of the TNT reticulocyte lysate system (Promega) according to the manufacturer’s instructions. Each 20-μl gel mobility shift reaction contained 10 mM Tris, pH 8, 60 mM KCl, 0.1%; Nonidet P-40, 6%; glycerol, 2 mM dithiothreitol, 2 μg of poly(dI·dC), and 5 μl total in vitro translated protein. Competitor oligonucleotides were added in 5- and 50-fold molar excess. A monoclonal antibody for hPXR (Santa Cruz Biotechnology Inc., Santa Cruz, CA) was added to visualize a supershift. After incubation on ice for 10 min, 4 ng of 32P-labeled oligonucleotide was added. After an additional 10-min incubation on ice, the DNA-protein complexes were resolved on a 4%; polyacrylamide gel. The gel was dried and subjected to autoradiography. The following double-stranded oligonucleotides were used as radio-labeled probes or cold competitors as indicated: CYP3A4 ER6 (5′-GATCAATATGAACTCAAAGGAGGTCAGTG-3′) and mutated CYP3A4 ER6 (5′-GATCAATATGTTCTCAAAGGAGAACAG TG-3′).
PXR-RXRα Heterodimerization Assay.
Wild-type and mutant human PXR and FLAG-RXRα were synthesized in vitro by use of the TNT reticulocyte lysate system (Promega). Ten microliters of each in vitro translated protein was diluted to 500 μl in immunoprecipitation buffer (phosphate-buffered saline, 0.5%; NP-40, and protease inhibitors). The lysates were precleared with 20 μl of immobilized protein A (Repligen Corporation, Waltham, MA). FLAG-tagged RXRα was immunoprecipitated by use of anti-FLAG M2 affinity gel (Sigma-Aldrich) or nonimmune IgG as indicated. Free immune complexes were captured and washed three times with lysis buffer. Immunoprecipitated protein complexes were subjected to SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore Corporation, Billerica, MA). Western blot analysis was performed with use of a polyclonal antibody generated to detect the human PXR protein.
Fluorescence Microscopy.
Twenty-four hours after plating in a 12-well format, CV-1 cells were transfected with expression vectors encoding GFP-wild-type and GFP-mutant PXR fusion proteins. The next day the cells were drug-treated with vehicle or 10 μM rifampicin. Twenty-four hours after drug treatment the subcellular localization of wild-type and mutant PXR was visualized and documented by use of fluorescence microscopy.
Statistical Analysis.
Statistical differences between treatment groups were determined by use of either a Student’s t test, or one-way analysis of variance followed by Duncan’s multiple-range post hoc test.
Results
Identification and Mutation of Predicted Phosphorylation Sites within the Human PXR Protein.
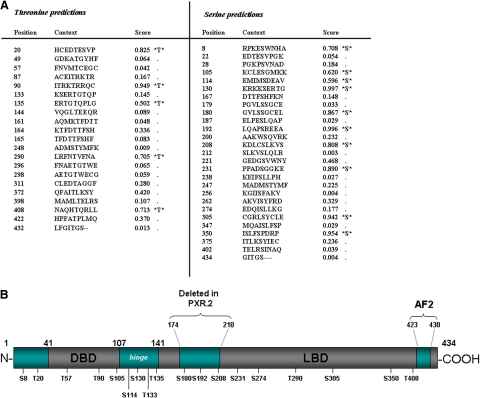
Potential serine and threonine phosphorylation sites within the human PXR protein were identified with use of the NetPhos 2.0 Server (Technical University of Denmark). Forty-eight potential serine and threonine phosphorylation sites were identified and scored from 0.000 to 1.000 based on the likelihood of phosphorylation (Fig. 1A). The serine and threonine residues that have higher scores are indicated as likely phosphorylation sites. In this study, serine and threonine residues that were scored above the threshold of 0.500 were selected for mutagenesis. In addition to the residues that were scored above 0.500, we also selected Thr57, Thr133, and Ser274 for mutagenesis. The Thr57 residue has been previously characterized as a potential phosphorylation site by p70 S6K (Pondugula et al., 2009). Preliminary mass spectroscopy data from our laboratory has indicated Thr133 as a potential PXR phosphorylation site (data not shown). Finally, Ser274 was chosen because of its location within a conserved nuclear localization signal. Therefore, 18 residues in total were mutated to either a negatively charged phosphomimetic residue (Asp) or a phospho-deficient residue containing a hydrophobic side chain (Ala). These 18 residues are located throughout the conserved NR domains of the PXR protein. It is noteworthy that three of the residues Ser180, Ser192, and Ser208 are located in a portion of the PXR protein that is deleted in the alternative splice variant hPXR.2 (Fig. 1B).
Fig. 1.
Identification of potential serine-threonine phosphorylation sites within the conserved human PXR protein domains. A, forty-eight potential serine and threonine phosphorylation sites within the human PXR protein were identified and scored by use of the NetPhos 2.0 server. Those residues scoring above the 0.500 threshold are shown in bold print. B, eighteen total serine and threonine residues that are located throughout the PXR protein conserved DNA-binding, hinge, and ligand-binding domains were selected for site-directed mutagenesis.
Phosphomimetic Mutations Alter the Basal Transcriptional Activity of PXR.
We next sought to determine the effect that the mutations would have on PXR basal and ligand-inducible transcriptional activity in cell-based reporter gene assays. In general, the activation of kinase signaling pathways has been shown to attenuate PXR-target gene expression in primary hepatocytes (Sidhu and Omiecinski, 1995; Marc et al., 2000; Ding and Staudinger, 2005b; Lichti-Kaiser et al., 2009). Therefore, we expected that phosphomimetic mutation at potential PXR phosphorylation sites would probably repress the activity of PXR on the XREM-Luc reporter gene. Furthermore, we expected that the phospho-deficient mutation would either increase or have no effect on PXR-basal transcriptional activity. CV-1 cells were selected to be used to detect changes in basal and ligand-inducible PXR activity based on mutations at 18 phosphorylation sites because they support a robust ligand-mediated PXR response and are easy to culture and transfect in a 96-well format. Expression vectors encoding wild-type and mutant PXR proteins were cotransfected together with the XREM-Luc reporter gene. The basal transcription activity of the PXR-dependent reporter gene was monitored for 48 h after transfection. The fold-induction of each mutant compared with wild-type PXR was recorded (Table 2). Phosphomimetic mutations at four sites, including S8D, T57D, S305D, and S350D, displayed significantly attenuated basal transcriptional activity (p < 0.001). Phospho-deficient mutations at the same four sites displayed no significant change in PXR basal transcription activity. The S208D mutation exhibited attenuated PXR activity (p < 0.001), whereas the phospho-deficient mutation significantly increased PXR activity (p < 0.001). The Thr408 position is also noteworthy because of the extent to which both the phosphomimetic mutation and the phospho-deficient mutation significantly attenuated PXR basal transcriptional activity (p < 0.001). Western blot analysis with a polyclonal antibody generated against the entire LBD of the human PXR protein determined that the observed differences in basal transcriptional activity of the mutant PXR proteins were not due to differences in overall protein expression levels in CV-1 cells (Fig. 1). The Thr90 position has the highest phosphorylation potential with a score of 0.949 and, like Thr57, is located within the conserved zinc-finger motifs of the PXR-DBD. We therefore selected these seven amino acid residues for further analysis to determine their potential for phospho-specific regulation of PXR biological activity. The Ser192 site was omitted from further analysis because of its relatively modest fold-repression of basal activity by both the phosphomimetic and phospho-deficient mutations (Table 2) and wild-type ligand response (data not shown). The repression of PXR activity by mutations that mimic phosphorylation suggest that phosphorylation at those specific sites could in principle confer a measurable functional biological impact by negatively regulating PXR-basal transcriptional activity. Multiple mechanisms could contribute to the impaired transactivation function displayed by these mutant proteins including protein stability, protein cofactor interactions, DNA binding, heterodimerization with RXRα, and alteration of subcellular localization. Therefore, we sought to further elucidate the mechanisms responsible for the observed repression of PXR transactivation capacity at these seven sites.
TABLE 2.
Mutations created within the human PXR protein
Changes in basal hPXR transcriptional activity are represented as fold induction of hPXR mutants compared with wild-type basal transcription activity on the XREM-Luc reporter gene.
| Site | D | A |
|---|---|---|
| Ser8 | 0.31** | 1.17 |
| Thr20 | 0.67 | 0.89 |
| Thr57 | 0.16** | 0.93 |
| Thr90 | 0.81 | 0.75 |
| Ser105 | 0.40* | 0.76 |
| Ser114 | 0.70* | 1.07 |
| Ser130 | 1.55 | 1.27 |
| Thr133 | 0.78 | 0.19** |
| Thr135 | 0.68 | 0.75 |
| Ser180 | 0.79 | 0.56* |
| Ser192 | 0.41** | 0.34** |
| Ser208 | 0.44** | 2.67** |
| Ser230 | 0.48* | 0.81 |
| Ser274 | 0.46* | 0.70* |
| Thr290 | 0.23** | 0.35* |
| Ser305 | 0.07** | 0.83 |
| Ser350 | 0.22** | 1.52 |
| Thr408 | 0.06** | 0.23** |
P < 0.01
P < 0.001.
Phosphomimetic Mutations at Thr57 and Thr408 Attenuate the Ligand-inducible Transactivation Capacity of PXR.
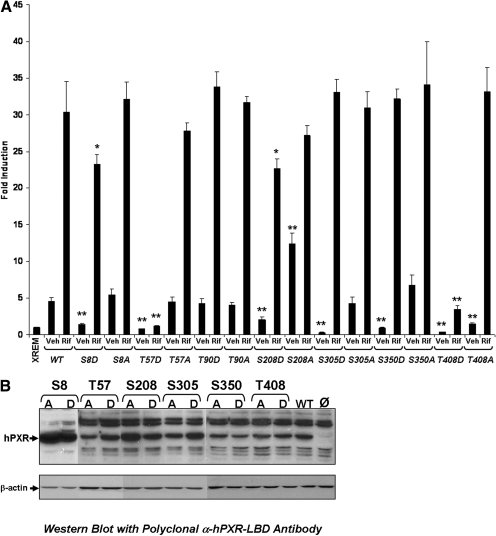
To determine the effect that phosphomimetic mutations at the seven selected sites of interest have on the inducible transactivation capacity of PXR, CV-1 cells were transiently transfected with the XREM-Luc reporter gene construct and expression vectors encoding wild-type or the indicated mutant PXR proteins. Twenty-four hours after transfection, cells were treated with either vehicle or 10 μM rifampicin, a prototypical human PXR ligand. Luciferase activity was observed an additional 24 h after drug treatment. As expected, wild-type PXR significantly enhanced the XREM-Luc reporter gene activity in the presence of rifampicin by approximately 30-fold over the reporter gene alone. The T57D mutation exhibited no ligand-inducible activation of the XREM-Luc reporter gene. In contrast, the T57A mutant PXR protein retained all of its ligand-inducible activation capacity. The T408D mutant PXR protein exhibited a significantly diminished level of ligand-dependent reporter gene activity compared with wild-type PXR protein. Similar to the T57A mutant PXR protein, the T408A mutant PXR protein retained ligand-dependent XREM-Luc reporter gene activity. In contrast to the phosphomimetic mutations at positions Thr57 and Thr408, which reduced rifampicin-dependent induction of XREM-Luc reporter gene activity from nearly 30-fold to approximately 0.8- and 3.5-fold, respectively (P < 0.001), the phosphomimetic and/or phospho-deficient mutations at positions Ser8, Thr90, Ser208, Ser305, and Ser350 either failed to have an effect (Thr90, Ser305, and Ser350) or resulted in a modest reduction in rifampin-induced reporter gene expression from approximately 30- to 23-fold (P < 0.01) (Fig. 2A). Western blot analysis using a polyclonal antibody generated against the entire LBD of the human PXR protein determined that the observed differences in transcriptional activity of the mutant PXR proteins were not due to differences in overall protein expression levels in CV-1 cells (Fig. 2B).
Fig. 2.
The basal transactivation capacity of mutant PXR proteins. A, CV-1 cells were transfected with the XREM-Luc reporter gene construct and expression vectors encoding wild-type or mutant PXR. Twenty-four hours after transfection, cells were treated with either vehicle or 10 μM rifampicin. Luciferase activity was observed for an additional 24 h after drug treatment. PXR proteins containing phosphomimetic mutations at Thr57 and Thr408 were not activated by rifampicin treatment. The data are normalized to β-galactosidase activity and represented as fold induction ± S.D. (n = 4). Statistically significant differences compared with vehicle- and rifampicin-treated wild-type PXR values are denoted with an asterisk (∗, P < 0.01; ∗∗P, < 0.001). B, Western blot analysis using a polyclonal antibody that recognizes the entire LBD of human PXR was performed on extracts isolated from CV-1 cells that express wild-type or the indicated mutant PXR proteins.
Phosphomimetic Mutation at Thr57 Impairs the Ability of PXR to Bind to its DNA Response Element.
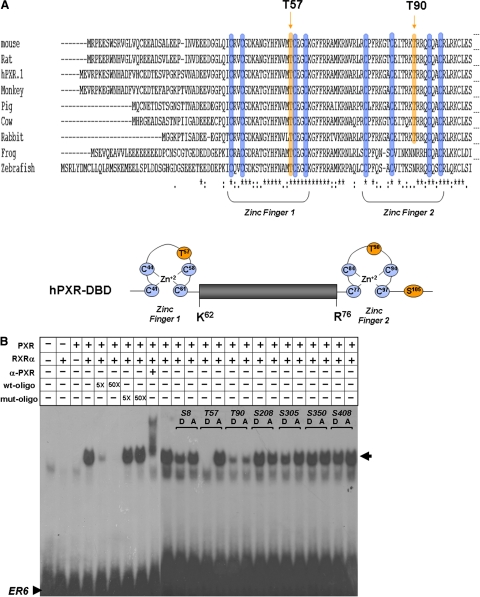
PXR-mediated gene activation requires direct binding of the PXR-RXRα heterodimeric protein to response elements on the respective gene promoter sequences. The proximal PXR-response element in the human CYP3A4 promoter exists as an everted repeat spaced by six nucleotides (ER6). The PXR-DBD contains two zinc-finger motifs that are essential for DNA binding. Threonine residues at positions 57 and 90 are located within the first and second zinc-finger motifs, respectively (Fig. 3A). Thus, phosphorylation of the PXR protein at these residues may be expected to alter the DNA-binding capacity of PXR toward the ER6 response element sequence. We therefore hypothesize that the lack of the inducible transactivation capacity exhibited by the T57D mutant PXR protein may be due to its lack of ability to bind to PXR-responsive ER6 promoter element.
Fig. 3.
DNA-binding capacity of mutant PXR proteins. A, Thr57 and Thr90 are located within evolutionarily conserved zinc-finger motifs within the DBD of the PXR protein. B, in vitro transcribed and translated RXRα together with wild-type and mutant PXR proteins were coincubated with a radiolabeled oligonucleotide corresponding to the ER6 PXR response element derived from the CYP3A4 enhancer element. Complexes were resolved on a nondenaturing polyacrylamide gel. The gel was dried and exposed to X-ray film. Specificity of binding was determined by use of nonradiolabeled wild-type and mutant ER6 oligonucleotides. The α-hPXR antibody was used to verify that the PXR protein was present in the shifted complex.
In vitro transcribed and translated wild-type PXR and mutant PXR proteins were combined with RXRα protein and incubated with a radiolabeled double-stranded oligonucleotide corresponding to the ER6 PXR-response element in the CYP3A4 promoter and resolved on a nondenaturing gel. The specificity of PXR-RXRα binding is demonstrated by the addition of increasing molar concentrations of nonlabeled double-stranded ER6 oligonucleotide that effectively competes with the binding of the labeled ER6 sequence, whereas the addition of a mutant ER6 oligonucleotide does not. Furthermore, incubation with α-hPXR antibody supershifted the PXR-RXRα-oligonucleotide complex. The T57A mutant PXR protein bound to the labeled ER6 sequence similarly to the wild-type PXR protein, whereas the T57D mutation completely abolished binding (Fig. 3B). Both the T90D and T90A mutant PXR proteins bound to the ER6 sequence, although to a lesser extent than wild-type PXR. Phosphomimetic and phospho-deficient mutant PXR proteins at positions Ser8, Ser208, Ser305, and Ser350 had no effect on DNA binding compared with wild-type (Fig. 3B). Taken together, these observations demonstrate that phosphorylation at position Thr57 within the PXR protein is likely to inhibit the ability of PXR to bind to its response elements within the promoter sequences of its target genes.
Phosphomimetic Mutations at Ser8, Ser208, Ser305, Ser350, and Thr408 Impair the Ability of PXR to Heterodimerize with RXRα.
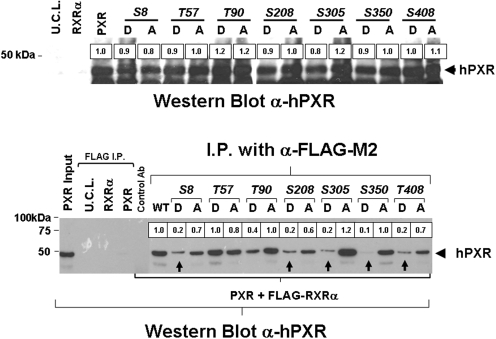
Although some NR proteins function as monomers, most NR proteins are active as dimers; either as homodimers, or as heterodimers with RXR. PXR-mediated transactivation of its target genes requires heterodimerization with RXRα. On ligand binding, the PXR-RXR heterodimer binds to multiple sites on the PXR-target gene promoters and activates gene expression. Because PXR and RXRα form a single type of heterodimeric complex, the regions that connect proteins must allow considerable flexibility to account for variations in response elements. Dimerization surfaces are located within the LBD of both PXR and RXRα. Therefore, we hypothesize that phosphomimetic mutations contained within the PXR LBD may interfere with PXR-RXR heterodimerization. Coimmunoprecipitation studies show that phosphomimetic mutations at Ser305, Ser350, and Thr408, contained within the PXR-LBD, do, in fact, disturb PXR-RXR heterodimerization.
Expression constructs encoding FLAG-tagged RXRα, wild-type PXR, and mutant PXR proteins were in vitro transcribed and translated. The expression of the wild-type and mutant PXR proteins was approximately equivalent as demonstrated by Western blot analysis using an anti-hPXR antibody (Fig. 4, top). The ability of wild-type and mutant PXR proteins to form heterodimeric complexes with FLAG-RXRα were assessed by use of the anti-FLAG antibody. The immunoprecipitated protein complexes were washed three times, and the presence of PXR was detected by use of an anti-hPXR antibody in Western blot analysis. As expected, wild-type PXR protein was coimmunoprecipitated with the FLAG-RXRα protein. To verify the specificity of the coimmunoprecipitation in detecting PXR-RXR heterodimerization, we immunoprecipitated lysates containing RXRα alone, PXR alone, or unprogrammed cell lysate by use of the anti-FLAG antibody. We also performed immunoprecipitation by use of lysates containing both FLAG-RXRα and wild-type PXR by use of a nonimmune antibody as a negative control. As expected, PXR was not coimmunoprecipitated or detected by Western blot analysis in any of the control reactions. In this study, heterodimerization and coimmunoprecipitation of PXR proteins containing phosphomimetic mutations within the LBD at Ser8, Ser208, Ser305, Ser350, and Thr408 was disturbed as evidenced by the decreased detection via Western blot and subsequent image analysis using scanning densitometry. Phospho-deficient mutation at the same sites did not seem to appreciably affect heterodimerization. Phosphomimetic mutation at Thr57 and Thr90 (located in the DBD) did not affect PXR-RXR heterodimerization. These data indicate that potential phosphorylation at Ser8, Ser208, Ser305, Ser350, and Thr408 within the PXR protein may decrease PXR-RXR heterodimerization and contribute to decreased PXR activity. Although Ser8, S305D, S350D, and T408D mutant PXR proteins did display decreased basal PXR activity, these mutants were still functional and able to induce the full or near-full expression of reporter gene activity in response to rifampicin, perhaps, in part, because of decreased PXR-RXR heterodimerization.
Fig. 4.
Analysis of mutant PXR proteins ability to heterodimerize with RXRα. FLAG-tagged RXRα, wild-type, and mutant PXR were in vitro transcribed and translated. The expression of the wild-type and mutant PXR proteins was analyzed by Western blot using a α-hPXR antibody (top). The PXR proteins were coimmunoprecipitated with FLAG-tagged RXRα using the α-FLAG antibody. The presence of PXR was detected by use of the α-hPXR antibody. Wild-type PXR protein was coimmunoprecipitated together with FLAG-RXRα protein when using α-FLAG, but not a nonimmune control antibody. The PXR protein was not detected by use of the α-PXR antibody in the α-FLAG-immunoprecipitates of lysates containing RXRα alone, PXR alone, or unprogrammed cell lysate (U.C.L.). Mutant PXR proteins containing phosphomimetic mutations at positions Ser8, Ser208, Ser305, Ser350, and Thr408 were not effectively immunoprecipitated with RXRα as measured by scanning densitometry, whereas phospho-deficient mutations at the same sites did not seem to appreciably affect RXRα-PXR heterodimerization capacity.
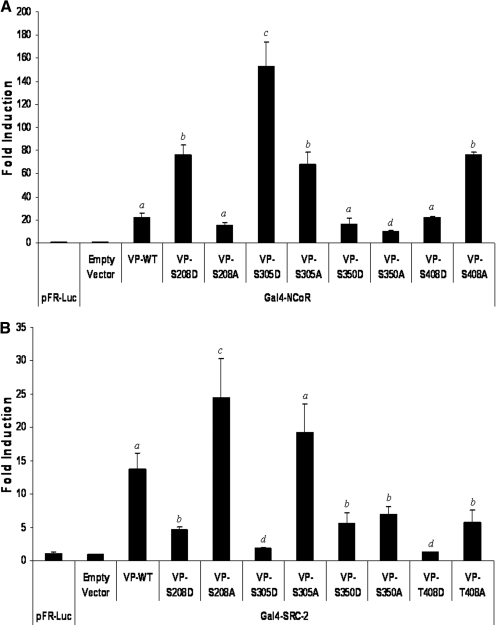
Phosphomimetic and Phospho-deficient Mutations at Ser208 and Ser305 Differentially Modulate PXR-Protein Cofactor Interaction.
The complete function of many NR proteins, including PXR, depends on their ability to interact with protein cofactors. For example, in the absence of ligand, PXR is associated with corepressor proteins such as NCoR. However, ligand-binding disrupts the PXR-corepressor association and induces the association of PXR with coactivator proteins such as SRC1 and SRC2 (Kliewer et al., 1998). Phosphorylation is known to regulate the ability of NR proteins to bind to cofactor proteins (reviewed in Staudinger and Lichti, 2008). Given that PXR interacts with protein cofactors primarily through the LBD, we hypothesized that phosphomimetic mutations contained within the PXR-LBD may interfere PXR-protein cofactor interactions. Therefore, we used the mammalian two-hybrid system to determine whether the loss of PXR function observed in the phosphomimetic mutations contained within the LBD could be attributed to alterations in PXR-protein cofactor interactions.
CV-1 cells were transfected with the GAL4-dependent reporter gene construct (pFR-Luc) and expression vectors encoding GAL4-cofactor fusion proteins and VP16-fused wild-type or mutant PXR proteins. Cells were treated with rifampicin 24 h after transfection. Luciferase activity was observed 48 h after transfection. Ligand-dependent association of either NCoR or SRC2 protein cofactors with PXR were not disrupted by any of the selected mutations (data not shown). Ligand-dependent association of either NCoR or SRC2 protein cofactors with PXR were not disrupted by any of the selected mutations (data not shown). In the absence of ligand, the S208D mutant PXR protein exhibited significantly increased strength of association with the corepressor NCoR, whereas the S208A mutant PXR protein exhibited a significant decrease in the strength of association with NCoR compared with that of the wild-type PXR protein (Fig. 5A). Symmetrically, the S208D mutation exhibited a significant decrease in the strength of association with the coactivator protein SRC2, whereas the S208A mutation significantly increased their association (Fig. 5B). This differential modulation of cofactor binding to S208D and S208A hPXR is consistent with the modulation of basal transcriptional activity exhibited by these mutant PXR proteins in the reporter gene assay. Alterations in the phosphorylation status of PXR at Ser208 may contribute to the modulation of basal PXR transcriptional activity by disrupting receptor-cofactor protein interactions. Furthermore, the phosphomimetic mutation at position Ser305 had a similar effect compared with Ser208. The S305D mutant PXR protein associated more strongly with NCoR, and also exhibited decreased strength of association with SRC2, compared with wild-type PXR protein, respectively. Phosphomimetic mutations at positions Ser350 and Thr408 resulted in no change in NCoR strength of association, and a general decrease in the strength of SRC2 cofactor binding (Fig. 5, A and B). The phospho-deficient mutations at positions Ser350 and Thr408 exhibited no change in strength of association, and an increase in strength of association with NCoR, respectively, whereas the strength of association with SRC2 was decreased by phospho-deficient mutation at positions Ser350 and Thr408.
Fig. 5.
Analysis of mutant PXR proteins ability to interact with protein cofactors. CV-1 cells were transfected with the pFR-Luc reporter gene construct and expression vectors encoding GAL4-cofactor fusion proteins and VP16-wild-type or -mutant PXR fusion proteins. Luciferase activity was observed 48 h after transfection. A, phosphomimetic mutations at Ser208 and Ser305 increase the strength of interaction between PXR and the corepressor NCoR. Phospho-deficient mutations at positions Ser305 and Ser408 also increase the strength of interaction between PXR and the corepressor NCoR. B, phosphomimetic mutations at positions Ser208, Ser305, Ser350, and Thr408 decrease the strength of interaction between PXR and the coactivator protein SRC2. Phosphodeficient mutation at position Ser208 increases interaction with SRC2, whereas the S350A and T408A mutations decrease strength of association with SRC2. The data are normalized to β-galactosidase activity and represented as fold induction above reporter gene alone ± S.D. (n = 4). Letters different from each other represent statistically different groups (p < 0.05).
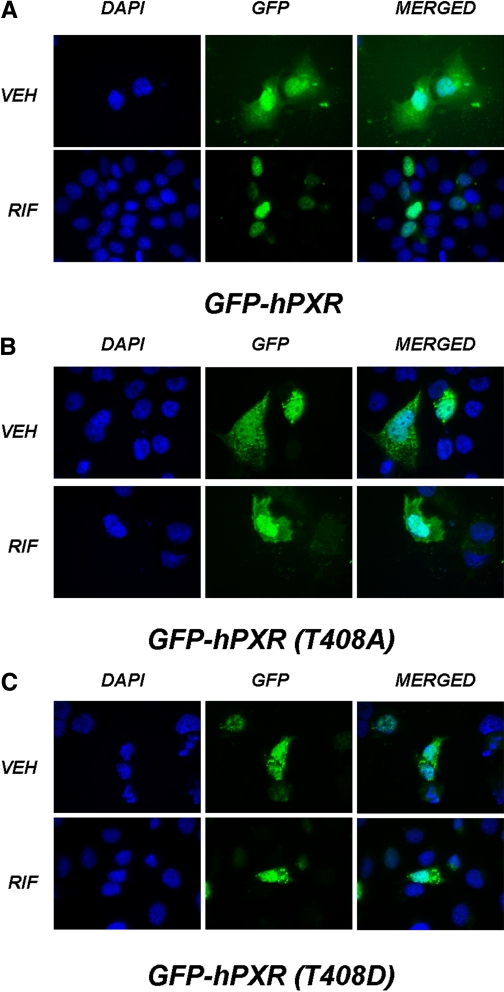
Phosphomimetic Mutation at Thr408 Alters the Subcellular Localization of PXR.
Like many NR proteins, upon ligand-mediated activation, PXR translocates to the nuclear compartment where it mediates target gene activation. Ligand-binding disrupts the association of PXR from Hsp90 and CAR cytoplasmic retention protein in the cytoplasm and allows the NR to translocate to the nucleus to activate transcription (Squires et al., 2004). Alterations in the phosphorylation status at position 202 have been shown to alter the subcellular localization of the constitutive androgen receptor protein (Hosseinpour et al., 2006). We therefore sought to determine the extent to which phosphomimetic and phospho-deficient mutations within the PXR-LBD affected the subcellular compartmentalization of the GFP-PXR protein. Therefore, CV-1 cells were transfected with constructs expressing wild-type and mutant GFP-PXR fusion proteins at positions Ser208, Ser305, Ser350, and Thr408 within the PXR-LBD. Twenty-four hours after transfection, cells were treated with either vehicle or 10 μM rifampicin. The subcellular localization of the expressed GFP-PXR fusion proteins were observed using fluorescence microscopy an additional 24 h after drug treatment. As expected, the wild-type GFP-PXR fusion protein was located in both the cytoplasmic and nuclear compartments in cells treated with vehicle (0.1%; dimethyl sulfoxide). Treatment of cells expressing wild-type GFP-PXR fusion protein with rifampicin resulted in the exclusion of GFP-hPXR from the cytoplasm, and concentration of the protein within the nuclear compartment (Fig. 6A). The phosphomimetic mutation, T408D, resulted in a punctate distribution in the cytoplasmic and nuclear compartments, irrespective of the presence of either vehicle or rifampicin (Fig. 6B). Although the phospho-deficient mutant PXR protein, T408A, did not display a punctate distribution, it failed to concentrate to the nuclear compartment upon treatment with rifampicin (Fig. 6C). Mutations at positions Ser208, Ser305, and Ser350 did not produce alterations in subcellular localization (data not shown). Pondugula et al. reported that a phosphomimetic mutation at PXR-T57D exhibited a punctate nuclear localization pattern, whereas wild-type and PXR-T57A exhibited homogenous nuclear distribution (Pondugula et al., 2009). It is likely, therefore, that phosphorylation of PXR at both Thr57 and Thr408 would impair the biological function of PXR by altering its ability to translocate to the nucleus.
Fig. 6.
Subcellular localization of Thr408 mutant PXR proteins. CV-1 cells were cotransfected with constructs expressing wild-type and mutant GFP-PXR fusion proteins. Twenty-four hours after transfection, cells were treated with either vehicle or 10 μM rifampicin. The subcellular localization of GFP-PXR protein was observed by fluorescence microscopy for an additional 24 h after drug treatment. A, treatment with rifampicin resulted in the complete translocation of wild-type GFP-hPXR to the nuclear compartment. B, phosphomimetic mutation at Thr408 resulted in a punctate distribution of GFP-T408D in the cytoplasm and nucleus both in the presence of vehicle or rifampicin treatment. C, the phosphodeficient GFP-T408A PXR protein was not excluded from the cytoplasmic compartment upon rifampicin treatment. DAPI, 4,6-diamidino-2-phenylindole-2-HCl; VEH, vehicle; RIF, rifampicin.
Discussion
In general, modulation of the phosphorylation status of liver-enriched NR and NR-associated proteins is a critical determinant of the responsiveness of these proteins to changes in environmental stimuli and pathological conditions (reviewed in Rochette-Egly, 2003; Staudinger and Lichti, 2008). It is well documented that pathological conditions such as inflammation, diabetes, obesity, malnutrition, and alcohol consumption alter the expression and activity of hepatic drug-metabolizing enzymes (Aitken et al., 2006; Kim and Novak, 2007). Although ligand binding is the primary mechanism of PXR activation in hepatocytes, post-translational modification of PXR and PXR-associated proteins probably is involved in fine-tuning its activity in response to environmental stimuli and alterations in disease status.
In the current study, we identify 18 potential phosphorylation sites in the PXR protein by use of in silico consensus site prediction methods. Of the 18 sites identified, seven were chosen for further characterization based on their ability to modulate PXR activation function in reporter gene analysis. Phosphomimetic mutations at six of seven sites resulted in the repression of PXR basal transcriptional activity, whereas phospho-deficient mutations both increased (S208A) and decreased (T408A) the basal activity of human PXR in reporter gene assays. We show that phosphomimetic mutations at positions Thr57 and Thr408 completely ablate ligand-inducible transactivation capacity of the human PXR protein, whereas phospho-deficient mutations at these sites do not alter PXR ligand-inducible transactivation capacity at all compared with wild-type PXR. We observed that a phosphomimetic mutation at Ser350 resulted in the decreased basal expression of PXR in cell-based reporter gene assays; however, we did not observe an attenuation of ligand-induced PXR activity as reported by Lin et al. (2008). The different results could be due to the use of different cell lines for reporter gene analysis. The same study indicates that CDK2 can phosphorylate human PXR in vitro at more the one site. Another recent study from the same group sought to characterize the effect of a phosphomimetic mutation at Thr57 on the activity of human PXR (Pondugula et al., 2009). The Thr57 residue is highly conserved across the NR superfamily, and it is located in the first zinc-finger motif of the DBD. The Thr57 phosphomimetic mutant PXR protein loses its transactivation function and displays a punctate nuclear distribution. Gel shift assays suggest that this may be because of the impaired ability of the mutant PXR to bind to its DNA response elements (Pondugula et al., 2009). The same study identified Thr57 as a potential phosphorylation site for p70 S6K and showed that p70 S6K can phosphorylate hPXR in vitro (Pondugula et al., 2009).
There are multiple mechanisms by which phosphorylation of the PXR protein at a specific site could result in decreased transcriptional activity, including the impairment of the activation function through loss of DNA binding, modulation of RXRα heterodimerization, alterations in protein cofactor interactions, and induced alterations in subcellular localization. The lack of PXR transactivation capacity displayed by the T57D PXR mutant seems to be due to its inability to bind to DNA. Given that both Thr57 and Thr90 are highly conserved residues located within the first and second zinc fingers of the PXR-DBD, respectively, it is tempting to speculate that phosphorylation at those sites would disrupt DNA binding in vivo. However, both T90D and T90A mutant proteins retained their ability to bind to DNA in vitro, albeit to a lesser extent compared with wild-type PXR. Although the Thr90 residue seems to be a critical residue for efficient DNA binding, our reporter gene data indicate that phosphorylation at this site may not have a profound impact on PXR transcriptional activity. Phosphomimetic mutations at Ser305, Ser350, and Thr408 inhibit PXR-RXRα heterodimerization, thus providing a potential mechanism by which phosphorylation at those sites could result in the observed decreased in PXR basal transcriptional activity. Mammalian two-hybrid experiments suggest that phosphorylation of PXR at Ser208 or Ser305 could also result in modulation of PXR-protein cofactor interactions such that recruitment of NCoR is favored over interaction with coactivator proteins. Finally, both phosphomimetic and phospho-deficient mutations at position Thr408 resulted in impaired subcellular localization of the GFP-PXR protein. Although CV-1 cells are not known to express CCRP, the aberrant nuclear accumulation of the Thr408 mutant PXR proteins mirrors altered association with RXRα and protein cofactors. These data therefore suggest that protein-protein interactions between PXR, RXRα, NCoR, and SRC2 contribute to receptor subcellular localization independent of CCRP function.
There are, however, some inconsistencies present in this data set. For example, a phosphomimetic mutation at position Ser350 inhibits the ability of PXR to heterodimerize with RXRα in coimmunoprecipitation assays, but it does not affect its ability to bind to its response element together with the RXRα protein in gel shift analysis. In addition, both phosphomimetic and phosphodeficient mutations at position Thr90 slightly impair the ability of PXR to bind to its response element in gel shift analysis but do not effect PXR transactivation capacity in reporter gene assays. Thus, it is difficult to interpret the extent to which the alterations in the parameters of PXR activity, as measured in this study, are functionally significant when using overexpression methods and cell-based analysis. Furthermore, it is currently unknown if phosphorylation at any of the characterized sites is physiologically significant. The generation of antibodies against phosphoserine or phosphothreonine residues identified in this study in the human PXR protein will probably be a useful approach to examine this question more closely.
Given the high potential for disease-drug interaction in patients receiving combination therapy, it is critical to understand the likely role of PXR in mediating transcriptional repression of its target genes in response to environmental stimuli and pathological conditions. In silico computer-based analysis reveals that the seven serine and threonine residues identified in this study are good potential substrates for an array of kinases (Table 3). However, preliminary experiments performed in our laboratory by use of constitutively active kinase expression systems have thus far been unsuccessful in identifying single amino acid residues in the PXR protein that are responsible for modulation of its transcriptional activity (data not shown). Further studies are required to determine the physiological connection between the activation of kinase signaling pathways and altered PXR activity, and to determine the extent to which the direct phosphorylation of serine and threonine residues in PXR or PXR-associated proteins is involved.
TABLE 3.
In silico identification of consensus phosphorylation sites for protein kinases in the human PXR protein
| Phosphorylation Site | Predicted Kinase |
|---|---|
| Ser8 | PKA |
| PKG | |
| Thr57 | CK1 |
| p70 S6K | |
| Thr90 | PKA |
| PKC | |
| Ser208 | PKC |
| Ser305 | CK2 |
| MAPK | |
| PKA | |
| Ser350 | CDK2 |
| CDK5 | |
| CK1 | |
| MAPK | |
| Ser408 | PKC |
MAPK, mitogen-activated protein kinase.
It is now known that the PKA, PKC, and CDK2 signaling pathways are involved in the regulation of PXR activity (Ding and Staudinger, 2005a,b; Lin et al., 2008; Lichti-Kaiser et al., 2009). In primary cultures of mouse hepatocytes, treatment with phorbol ester dramatically represses the expression of Cyp3a11 gene expression (Ding and Staudinger, 2005b). However, treatment of mouse hepatocytes with 8-bromo-cyclic AMP, a cell-permeable PKA activator, increases the drug-inducible PXR-mediated expression of the Cyp3a11 gene in a synergistic manner (Ding and Staudinger, 2005a). Cell-based mammalian two-hybrid experiments suggest that the modulation of Cyp3a11 expression by PKC and PKA is due, in part, to the increased association of PXR with the cofactors NCoR and SRC-1, respectively (Ding and Staudinger, 2005a,b).
Although activation of PKA signaling increases PXR-mediated drug-inducible expression of the Cyp3a11 gene in mouse hepatocytes, it suppresses rifampicin-mediated induction of CYP3A4 in human hepatocytes (Lichti-Kaiser et al., 2009). Cotreatment of primary cultures of hepatocytes isolated from a PXR humanized-transgenic mouse model with rifampicin and 8-bromo-cyclic AMP resulted in increased levels of Cyp3a11 expression. However, it is noteworthy that the activation of PKA signaling increased the expression of Cyp3a11 in wild-type mouse hepatocytes in a synergistic manner, whereas the effect in humanized-transgenic mouse hepatocytes was merely additive (Lichti-Kaiser et al., 2009). These data suggest that the species-specific interface between PXR activity and PKA signaling is not entirely contained within the PXR protein. Nonetheless, it is known that human PXR exists as a phosphoprotein in vivo and is a good substrate for PKA in vitro (Lichti-Kaiser et al., 2009). Therefore, we can speculate that the modulation of PXR activity by PKA and other kinase signaling pathways is probably due to a combination of events that includes alterations in the phosphorylation status of PXR and PXR-interacting proteins.
The full activity of the PXR signaling pathway depends on both cross-talk with other signaling pathways and on PXR-protein cofactor interactions. For example, it is known that PXR activation suppresses the hepatic immunological response. However, inflammation is known to decrease PXR-mediated gene activation. The evidence suggests that the activation of the nuclear factor κB signaling pathway during inflammation interferes with PXR heterodimerization with RXRα (Zhou et al., 2006). Furthermore, the activation of PXR has recently been shown to decrease energy metabolism and increase hepatic triglyceride levels. The cross-talk between metabolic signaling pathways and PXR is thought to be caused by direct interactions between PXR and the transcriptional regulators CREB, PGC-1α, and FOXO family members (Bhalla et al., 2004; Miao et al., 2006; Kodama et al., 2007; Nakamura et al., 2007). The molecular basis for the increased interaction between the PXR protein and these proteins is unknown, although kinase signaling and phosphorylation events are probably involved. It is also well known that the activation of kinase signaling disrupts NR-protein cofactor interactions (reviewed in Rochette-Egly, 2003; Staudinger and Lichti, 2008). We therefore feel that it is most likely that the phosphorylation of PXR-interacting proteins, in addition to phosphorylation of PXR itself, contributes to the observed alterations in PXR-biological activity. Understanding the molecular mechanisms by which environmental stimuli and signal transduction pathways modulate PXR-mediated gene activation is critical for the development of safe and effective therapeutic strategies.
This work was supported by the National Institutes of Health [Grant DK068443].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- NR
- nuclear receptor
- PXR
- pregnane X receptor
- hPXR
- human PXR
- SRC2
- steroid receptor coactivator 2
- NCoR
- nuclear receptor corepressor
- P450
- cytochrome P450
- LBD
- ligand-binding domain
- DBD
- DNA-binding domain
- PKA
- protein kinase A
- PKC
- protein kinase C
- Cdk2
- cyclin-dependent kinase 2
- RXR
- retinoid X receptor
- GFP
- green fluorescent protein
- CCRP
- CAR cytoplasmic retention protein.
References
- Aitken AE, Richardson TA, Morgan ET. ( 2006) Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol 46: 123–149 [DOI] [PubMed] [Google Scholar]
- Bhalla S, Ozalp C, Fang S, Xiang L, Kemper JK. ( 2004) Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1alpha.Functional implications in hepatic cholesterol and glucose metabolism. J Biol Chem 279: 45139–45147 [DOI] [PubMed] [Google Scholar]
- Brobst DE, Ding X, Creech KL, Goodwin B, Kelley B, Staudinger JL. ( 2004) Guggulsterone activates multiple nuclear receptors and induces CYP3A gene expression through the pregnane X receptor. J Pharmacol Exp Ther 310: 528–535 [DOI] [PubMed] [Google Scholar]
- Chen Y, Tang Y, Wang MT, Zeng S, Nie D. ( 2007) Human pregnane X receptor and resistance to chemotherapy in prostate cancer. Cancer Res 67: 10361–10367 [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. ( 2005a) Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther 312: 849–856 [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. ( 2005b) Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem Pharmacol 69: 867–873 [DOI] [PubMed] [Google Scholar]
- Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. ( 2006) Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem 281: 17882–17889 [DOI] [PubMed] [Google Scholar]
- Hosseinpour F, Moore R, Negishi M, Sueyoshi T. ( 2006) Serine 202 regulates the nuclear translocation of constitutive active/androstane receptor. Mol Pharmacol 69: 1095–1102 [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. ( 2002) Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 277: 2908–2915 [DOI] [PubMed] [Google Scholar]
- Kim SK, Novak RF. ( 2007) The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther 113: 88–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, et al. ( 1998) An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92: 73–82 [DOI] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M, Yamamoto Y. ( 2004) Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol 24: 7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Moore R, Yamamoto Y, Negishi M. ( 2007) Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem J 407: 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Johnson BH, Thompson EB. ( 2004) Overview of the structural basis for transcription regulation by nuclear hormone receptors. Essays Biochem 40: 27–39 [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. ( 1998) The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102: 1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti-Kaiser K, Xu C, Staudinger JL. ( 2009) Cyclic AMP-dependent protein kinase signaling modulates pregnane x receptor activity in a species-specific manner. J Biol Chem 284: 6639–6649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YP, Huang JD. ( 2008) Interplay of pregnane X receptor with other nuclear receptors on gene regulation. Drug Metab Pharmacokinet 23: 14–21 [DOI] [PubMed] [Google Scholar]
- Lin W, Wu J, Dong H, Bouck D, Zeng FY, Chen T. ( 2008) Cyclin-dependent kinase 2 negatively regulates human pregnane X receptor-mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. J Biol Chem 283: 30650–30657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Sluder A, Guan X, Shi Y, McKee DD, Carrick K, Kamdar K, Willson TM, Moore JT. ( 2001) Comparison of complete nuclear receptor sets from the human, Caenorhabditis elegans and Drosophila genomes. Genome Biol 2: RESEARCH0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. ( 2002) Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol 62: 638–646 [DOI] [PubMed] [Google Scholar]
- Marc N, Galisteo M, Lagadic-Gossmann D, Fautrel A, Joannard F, Guillouzo A, Corcos L. ( 2000) Regulation of phenobarbital induction of the cytochrome P450 2b9/10 genes in primary mouse hepatocyte culture.Involvement of calcium- and cAMP-dependent pathways. Eur J Biochem 267: 963–970 [DOI] [PubMed] [Google Scholar]
- Miao J, Fang S, Bae Y, Kemper JK. ( 2006) Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J Biol Chem 281: 14537–14546 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Moore R, Negishi M, Sueyoshi T. ( 2007) Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem 282: 9768–9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondugula SR, Brimer-Cline C, Wu J, Schuetz EG, Tyagi RK, Chen T. ( 2009) A phosphomimetic mutation at threonine-57 abolishes transactivation activity and alters nuclear localization pattern of human pregnane x receptor. Drug Metab Dispos 37: 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette-Egly C. ( 2003) Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal 15: 355–366 [DOI] [PubMed] [Google Scholar]
- Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ. ( 2007) Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol 292: G1114–G1122 [DOI] [PubMed] [Google Scholar]
- Sidhu JS, Omiecinski CJ. ( 1995) cAMP-associated inhibition of phenobarbital-inducible cytochrome P450 gene expression in primary rat hepatocyte cultures. J Biol Chem 270: 12762–12773 [DOI] [PubMed] [Google Scholar]
- Squires EJ, Sueyoshi T, Negishi M. ( 2004) Cytoplasmic localization of pregnane X receptor and ligand-dependent nuclear translocation in mouse liver. J Biol Chem 279: 49307–49314 [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Lichti K. ( 2008) Cell signaling and nuclear receptors: new opportunities for molecular pharmaceuticals in liver disease. Mol Pharm 5: 17–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Madan A, Carol KM, Parkinson A. ( 2003) Regulation of drug transporter gene expression by nuclear receptors. Drug Metab Dispos 31: 523–527 [DOI] [PubMed] [Google Scholar]
- Zhai Y, Pai HV, Zhou J, Amico JA, Vollmer RR, Xie W. ( 2007) Activation of pregnane X receptor disrupts glucocorticoid and mineralocorticoid homeostasis. Mol Endocrinol 21: 138–147 [DOI] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, et al. ( 2006) Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest 116: 2280–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Liu M, Zhai Y, Xie W. ( 2008) The antiapoptotic role of pregnane X receptor in human colon cancer cells. Mol Endocrinol 22: 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]