Abstract
The γ-aminobutyric acid (GABA)A receptors mediating the discriminative stimulus effects of ethanol were studied by comparing the potency of ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazol(1,5-a)benzodiazepine-3-carboxylate (Ro15-4513) and ethyl 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazol(1,5-a)-benzodiazepine-3-carboxylate (flumazenil, Ro15-1788) to antagonize ethanol, pentobarbital (PB), and midazolam substitution for ethanol. Ro15-4513 has high affinity for receptors containing α4/6 and α5 subunits and lower affinity for α1, α2, and α3 subunits. Flumazenil is nonselective for GABAA receptors containing α1, α2, α3, and α5 subunits and has low affinity for α4/6-containing receptors. Male (n = 9) and female (n = 8) cynomolgus monkeys (Macaca fascicularis) were trained to discriminate ethanol (1.0 or 2.0 g/kg i.g., 30-min pretreatment) from water. Ethanol, PB, and midazolam dose-dependently substituted for ethanol (80% ethanol-appropriate responding). Ro15-4513 (0.003–0.56 mg/kg i.m., 5-min pretreatment) shifted the ethanol, PB, and midazolam dose-response functions rightward in a vast majority of monkeys tested (15/15, 16/17, and 11/12, respectively). In contrast, flumazenil (0.30–10.0 mg/kg i.m., 5-min pretreatment) shifted the ethanol, PB, and midazolam dose-response functions rightward in 9 of 16, 12 of 16, and 7 of 9 monkeys tested, respectively. In the monkeys showing antagonism with both Ro15-4513 and flumazenil, ethanol and PB substitution were antagonized more potently by Ro15-4513 than by flumazenil, whereas midazolam substitution was antagonized with similar potency. There were no sex or training dose differences, with the exception that flumazenil failed to antagonize ethanol substitution in males trained to discriminate 2.0 g/kg ethanol. GABAA receptors with high affinity for Ro15-4513 (i.e., containing α4/6 and α5 subunits) may be particularly important mediators of the multiple discriminative stimulus effects of ethanol through GABAA receptor systems.
The GABA A ionotropic complex is a pentameric configuration of subunits forming a Cl−-conducting channel. The pharmacologic response of GABA A receptors varies with subunit composition, which varies across brain regions (Sieghart, 1995). More than 60 years of research indicates that GABA A receptors mediate the discriminative stimulus effects of ethanol (Drug Comprehensive Bibliography of Drug Discrimination Research; www.dd-database.org). Benzodiazepines, barbiturates, ring-A-reduced 3α-hydroxy neuroactive steroids, and ethanol, which positively modulate GABA A receptors (Sieghart, 1995), produce ethanol-like discriminative stimulus effects (Grant et al., 2000). Research with subunit-preferring ligands further suggests that specific GABA A receptor subtypes mediate the discriminative stimulus effects of ethanol in non-human primates. In particular, α 1 subunit-containing receptors seem to mediate discrimination of higher (i.e., 2.0 g/kg) doses of ethanol (Helms et al., 2008b), whereas α 5 - but not α 1 -containing GABA A receptors mediate discrimination of lower (i.e., 1.0 g/kg) doses of ethanol (intravenous) in squirrel monkeys (Platt et al., 2005).
Antagonism studies also suggest that GABA A receptors are involved in the discriminative stimulus effects of ethanol. The discriminative stimulus effects of ethanol are antagonized by Ro15-4513. In male CD-1 mice, Ro15-4513 (0.10–1.0 mg/kg) antagonized discrimination of 1.0 g/kg i.p. ethanol (Rees and Balster, 1988). In male rats, the discriminative stimulus effects of 1.0 and 1.5, but not 2.0 g/kg i.g. ethanol were antagonized by Ro15-4513 (Gatto and Grant, 1997). However, in female rats and female C57BL/6cr mice, discrimination of 1.2 g/kg i.p. (Hiltunen and Järbe, 1988) and 1.0 g/kg i.p. ethanol (Middaugh et al., 1991) was not antagonized by Ro15-4513. Thus, sex and training dose may affect antagonism of the discriminative stimulus effects of ethanol by Ro15-4513, factors that were investigated in the current study in non-human primates.
In contrast to antagonism by Ro15-4513, few acute effects of ethanol are blocked by Ro15-1788 (flumazenil). In rats, flumazenil does not block ethanol self-administration (Samson et al., 1989), consumption (McBride et al., 1988), sedative effects (Suzdak et al., 1986), or anticonflict effects (Glowa et al., 1988). However, flumazenil blocks tolerance to the ataxic effects of ethanol and behaviors associated with ethanol withdrawal in mice (seizures; Buck et al., 1991) and rats (anxiety; Knapp et al., 2004). Although it did not antagonize acute ethanol effects, flumazenil clearly antagonized the acute effects of other GABA A -positive modulators including benzodiazepines (Herling and Shannon, 1982; Rowlett and Woolverton, 1996, 1998; Paronis and Bergman, 1999; Lelas et al., 2000), and the neuroactive steroid allopregnanolone (Fernández-Guasti and Picazo, 1995), but not the barbiturate PB (Herling and Shannon, 1982; Woolverton and Nader, 1995). However, PB antagonized flumazenil discrimination in diazepam-dependent rhesus monkeys (McMahon et al., 2006), suggesting that barbiturates and flumazenil may act at common receptors in vivo. Flumazenil increased locomotion in female but not male rats (2.5–10.0 mg/kg; Holtman et al., 2003) and antagonized discrimination of high (15 and 20 mg/kg) but now low doses (2.5 and 3.0 mg/kg) of the benzodiazepine chlordiazepoxide (De Vry and Slangen, 1986). The current study therefore assessed sex and training dose effects on flumazenil antagonism of ethanol-like discriminative stimulus effects in cynomolgus monkeys.
Flumazenil and Ro15-4513 act at common GABA A receptor subtypes as flumazenil blocks antagonism by Ro15-4513 (Suzdak et al., 1986) and displaces Ro15-4513 binding at α 4 β 3 δ receptors (Wallner and Olsen, 2008). Studies in recombinant receptor expression systems suggest that flumazenil has very low affinity for “benzodiazepine-insensitive” GABA A receptors containing α 4 and α 6 subunits (K i = 90–107 nM), but equal affinity for “benzodiazepine-sensitive” GABA A receptors containing α 1 , α 2 , α 3 , or α 5 subunits (K i = 0.40–1.5 nM). In contrast, Ro15-4513 has similar affinity for all α subunit variants except α 5 , for which it has 10-fold greater affinity (K i = 0.50 nM) (Sieghart, 1995). Positive modulation of GABA A receptors by midazolam and PB occurs by binding to distinct receptor sites (Sieghart, 1995) and subunit configurations. Midazolam is inactive at benzodiazepine-insensitive GABA A receptors containing α 4/6 subunits (Möhler et al., 2002). Pentobarbital has greater activity at human GABA A α 6 -containing compared with α 1–3 - or α 5 -containing receptors, although positive modulation occurred at all receptor subtypes with similar potency (Xenopus oocytes: α x β 2 γ 2s ) (Thompson et al., 1996). Despite these differences, benzodiazepines and barbiturates substitute for the discriminative stimulus effects of ethanol (Grant et al., 2000). The current research is the first to investigate GABA A receptor mediation of ethanol-like discriminative stimulus effects by comparing the efficacy and potency of Ro15-4513 and flumazenil to antagonize the substitution of ethanol, PB, and midazolam in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol.
Materials and Methods
Subjects
Male (n = 9, 4.9–7.1 kg) and female (n = 8, 2.9–5.1 kg) adult cynomolgus monkeys (Macaca fascicularis) served as subjects. Temperature (23 ± 1°C), humidity (40–60%), and light (12-h light/dark cycle; lights on at 6:00 AM) were controlled in the colony room housing the monkeys. The monkeys were housed in groups of 2, 3, or 4 in stainless steel cages (76 × 60 × 70 cm), except during training and feeding (3–4 h), when the monkeys were housed individually. The monkeys were fed a nutritionally complete diet (Purina, St Louis, MO) that included daily fruit supplements and food obtained during experimental sessions. Water was freely available except during experimental sessions. Details regarding the origin and housing conditions of the monkeys have been published (Grant et al., 2000; Helms et al., 2008b). The studies were conducted in accordance with the Wake Forest University Animal Care and Use Committee and the Guidelines of the Committee on the Care and Use of Laboratory Animal Resources (National Research Council, 1996).
Apparatus
The monkeys were trained in ventilated and sound-attenuating chambers (1.50 × 0.74 × 0.76 m; Med Associates, Inc., St. Albans, VT) that accommodated a primate chair (1.17 × 0.61 × 0.61 m; Plas Labs, Lansing, MI). The front panel (0.48 × 0.69 m) contained two retractable levers within arm’s reach of a monkey seated in a primate chair. Three stimulus lights (amber, green, and red) were set above each lever. A central white stimulus light was positioned 0.72 m from the bottom of the chamber. At the rear of the chamber were two house lights. A food cup was attached to the primate chair to collect 1-g banana-flavored pellets (P. J. Noyes, Lancaster, NH) delivered through vinyl tubing. The tubing was connected to a feeder set outside of the chamber. Data acquisition and the scheduling of events were controlled by a PC- or Macintosh-compatible computer connected to an interface (Med Associates) programmed with LabView software (National Instruments, Austin, TX).
Procedure
Experimental Design.
Four groups of monkeys constituted a 2 × 2 experimental design with sex and ethanol training dose as group factors. Discrimination of 1.0 g/kg ethanol (20% w/v, 5 ml/kg i.g.) from water was trained in six male (4864, 4865, 4867, 4892, 4889, 4995) and four female (2830, 2852, 2913, 3123) monkeys. Discrimination of 2.0 g/kg ethanol (20% w/v, 10 ml/kg) from water was trained in three male (4890, 4891, 5496) and four female (2835, 3220, 5400, 5999) monkeys.
Discrimination Training.
The monkeys were first trained to respond on both levers with a fixed ratio (FR)-1 reinforcement schedule which was increased to a final FR between 15 and 100, i.e., one reinforcer was delivered after 15 to 100 consecutive responses on the lever. The FR was chosen for each subject to limit session duration to approximately 5 min and to equalize the number of pellets obtained in a session. During each session, males and females received a maximum of 20 to 25 and 10 to 15 pellets, respectively. Each session ended after 30 min or after the maximum number of pellets was delivered. Response training was complete when a monkey made at least 0.25 responses/s for three consecutive sessions. To habituate the monkeys to nasogastric (i.g.) gavage, an infant feeding tube (5 French, 1.7 × 381 mm) was passed down one nostril, through the esophagus, and into the stomach. For five consecutive sessions, the monkeys were given tap water (5 ml/kg i.g.) and after a 30-min pretreatment, only the “water-appropriate” lever extended. Responding was reinforced according to the final FR schedule. For the next five sessions, the monkeys received a training dose of ethanol (1.0 or 2.0 g/kg i.g.) and 30 min later the “ethanol-appropriate” lever was presented and responding was reinforced according to the final FR schedule. Discrimination training began after these 10 sessions. Whether the first training session was an ethanol or water training session was randomly determined. Thirty minutes later, both levers extended and responding on the substance-appropriate lever was reinforced on each individual’s FR schedule. If the monkey responded on the incorrect lever, the FR for the correct lever was reset to zero. Tap water (5 ml i.g.) was administered after each ethanol or water dose and the monkeys were given a sugar pellet to mask any taste differences between water and ethanol.
Ethanol and water training sessions occurred in a double-alternating pattern (e.g., ethanol, ethanol, water, water). The training condition changed for the next session if ≥70% of responses in the first FR and ≥90% in the entire session were on the substance-appropriate lever. Discrimination training was completed and substitution testing began if responding was accurate according to these criteria for five consecutive sessions.
Substitution and Antagonism Testing.
For substitution tests, a dose of ethanol (0.25–2.5 g/kg i.g.), PB (0.017–17.0 mg/kg i.g.), or midazolam (0.017–30.0 mg/kg i.g.) was administered. A maximum of two tests were conducted per week. After a 30-min pretreatment, both levers extended into the chamber. During test sessions, responding on either lever was reinforced so that feedback from reinforcer delivery could not bias choice. Training sessions occurred in between test sessions. If discrimination responding did not meet criteria, training sessions were continued until responding achieved the discrimination criteria for three consecutive sessions. For each monkey, the doses tested typically included a minimal dose that did not substitute for ethanol and for which rates of responding did not decrease and a maximal dose that decreased response rates by ≥50% from the monkey’s average baseline response rate. The first dose tested was usually midrange, with subsequent doses equally distributed among higher and lower doses. Most (87.5%) doses were tested once per monkey. Doses were tested twice in 18 of 184 (9.8%) of cases and three times in 5 of 184 (2.7%) cases. Doses were retested if a monkey responded more slowly than during training or the percentage of ethanol-appropriate responding was inconsistent with the monkey’s previous test results. All tests were included in the analyses.
Antagonism of stimulus substitution was tested with single doses of Ro15-4513 (0.003–3.0 mg/kg i.m.) or flumazenil (0.017–17.0 mg/kg i.m.) administered 5 min before the levers extended into the chamber. The 5-min pretreatment was chosen to ensure pharmacologically active doses during the test session. Positron-emission tomography imaging showed peak Ro15-4513 binding at 15 min with nearly complete elimination by 90 min in monkeys (Maeda et al., 2003). In addition, flumazenil binding peaked 12 to 20 min after injection in humans with reduced binding beginning approximately 30 min after injection (Shinotoh et al., 1986). Antagonist doses were chosen individually for each monkey. The first antagonist dose tested was midrange, followed by decreasing doses, if ethanol-appropriate responding was completely blocked, or increasing doses, if ethanol-appropriate responding was unaffected. The maximum dose tested was limited by suppression of rates of responding.
Drugs
Anhydrous ethanol (1.0 or 2.0 g/kg; Warner-Graham Co., Cockeysville, MD) was diluted to 20% (w/v) with tap water and was administered in a 5 to 10 ml/kg i.g. volume. Ro15-4513 (Sigma/RBI, Natick, MA) and flumazenil (Ro15-1788; Sigma-Aldrich, St. Louis, MO) were dissolved in 20% intralipid (Kabi Pharmacia Inc., Clayton, NC) to a concentration of 10 mg/ml and sonicated until injected. Multiple injections (maximum of six) spread out over the quadriceps and hamstrings were administered for large doses with equivalent volumes administered to matched injection sites during vehicle tests. Pentobarbital (Sigma-Aldrich) was dissolved in saline in concentrations <10 mg/ml. Midazolam HCl (Hoffman-La Roche, Inc., Nutley, NJ) was dissolved in saline in concentrations <10 mg/ml. Midazolam and PB were administered in injection volumes of 5 to 10 ml/kg. All drugs were prepared fresh daily.
Blood-Ethanol Concentration
Blood samples (20 μl) for measurement of blood ethanol concentration (BEC) were collected by needle-stick of the saphenous vein after 54%, 83%, and 94% of tests with vehicle, Ro15-4513, or flumazenil pretreatment, respectively, with samples obtained 52.6 ± 1.5 (range, 37–68), 51.9 ± 0.7 (range, 33–85), and 51.1 ± 1.0 (range, 35–87) min after ethanol administration. The blood was stored at −4°C. Analysis of blood-ethanol concentration was accomplished by use of gas chromatography (Hewlett-Packard 5890 Series II) with a head space autosampler, flame-ionization detector and Hewlett-Packard integrator (3392A) (Hewlett Packard, Palo Alto, CA).
Data Analysis
The percentage of total responding on the ethanol-appropriate lever (total responses on the ethanol-appropriate lever/total responses) and response rate (total responses/session time) were calculated for each monkey and test session. For each monkey, baseline response rate was the average response rate across all ethanol and water training sessions preceding each test session. Complete and partial substitution for ethanol by a test drug dose were operationally defined, respectively, as ≥80% and ≥20 to 79% mean responses on the ethanol-appropriate lever, with all session percentages contributing to the mean (Grant et al., 2000).
Antagonism was defined as a decrease in ethanol-appropriate responding with any dose of Ro15-4513 or flumazenil relative to sessions with vehicle pretreatment in which the same dose or a lower dose of ethanol, PB, or midazolam completely substituted. Furthermore, in cases of antagonism, decreased ethanol-appropriate responding was maintained in tests with higher doses of the antagonist. All the cases counted as antagonism are presented in Supplemental Figs. 1 to 10. When two doses were tested that encompassed 50% ethanol-appropriate responding, an ED 50 was computed via linear interpolation. Rightward shifts in the dose-response curve indicative of antagonism occurred when the ED 50 for substitution with Ro15-4513 or flumazenil pretreatment was greater than the ED 50 with vehicle pretreatment. In this case, a dose ratio was computed: ED 50 with the antagonist to ED 50 with vehicle.
For most monkeys, a vehicle pretreatment dose-response curve was collected once. For these monkeys, the ED 50 values from vehicle tests were identical for Ro15-4513 and flumazenil. The ED 50 values were double-determined for the ethanol and midazolam vehicle pretreatment dose-response curves for monkey 4865. For one male trained to discriminate 2.0 g/kg ethanol (5496), only one ethanol test (2.0 g/kg) preceded by vehicle injection was available. The control dose-response curve for this monkey was from ethanol tests without vehicle pretreatment in addition to the single test with vehicle pretreatment. For one male trained to discriminate 1.0 g/kg ethanol (4892), the lowest dose of midazolam tested (0.56 mg/kg) resulted in 59% ethanol-appropriate responding. The midazolam ED 50 for this monkey was estimated by linear interpolation between 0 and 0.56 mg/kg midazolam by assuming 0% ethanol-appropriate responding after 0 mg/kg midazolam, as obtained in an earlier vehicle substitution test (Grant et al., 2000). Antagonism tests were not conducted if the test drug did not completely substitute (e.g., midazolam tests: 4865, 4964, 4867, 4995, 4891, and 5496).
When fewer than three shifts in the dose-response function were available, the potency of Ro15-4513 or flumazenil to increase the ED 50 2-fold was quantified with apparent pK B : −log[B/(dose ratio − 1)], where B is the antagonist dose in moles per kilogram. Apparent pK B quantifies shifts in each dose-response function after Ro15-4513 or flumazenil pretreatment and estimates the affinity of Ro15-4513 and flumazenil for the receptor population mediating the effects of the test drug (e.g., Rowlett and Woolverton, 1996). When three or more shifts in the dose-response function were obtained, apparent pA 2 was calculated to more accurately assess Ro15-4513 and flumazenil affinity. To calculate apparent pA 2 for each monkey, nonlinear regression analyses were conducted with GraphPad Prism software (GraphPad Software, San Diego, CA) with log (dose ratio − 1) as the dependent variable and the antagonist dose [−log(mol/kg)] as the independent variable (i.e., Schild plots). When the slope of the Schild plot is −1, the antagonist dose at which log (dose ratio − 1) is 0 indicates apparent pA 2 (Arunlakshana and Schild, 1959). For each monkey, regression slopes between −0.4 and −1.6 were constrained to −1, and the antagonist affinity indicated apparent pK B (Tallarida et al., 1979). Mean slopes were considered to conform to unity if the 95% confidence interval for the slope included −1 but excluded 0 (Paronis and Bergman, 1999).
Percentage of ethanol-appropriate responding and the percentage of baseline response rate were analyzed with 2 (sex) × 2 (training dose) repeated measures ANOVAs with dose as a within-subjects factor. Because not all doses were tested in every subject, the doses tested in the greatest number of subjects were selected for the repeated measures ANOVAs. Because too few pA 2 values were available for statistical analysis, mean apparent pK B and pA 2 were combined and evaluated with a 2 (sex) × 2 (training dose) × 2 (antagonist: flumazenil, Ro15-4513) ANOVA for each test drug. Comparison across test drugs was accomplished with a 2 (sex) × 2 (training dose) × 2 (antagonist: flumazenil, Ro15-4513) × 3 (test drug: ethanol, PB, midazolam) ANOVA. Bonferroni-corrected pairwise comparisons were used for post hoc tests. For all tests, α was 0.05.
Results
Substitution.
As expected, ethanol completely substituted in all monkeys tested. Ethanol-appropriate responding increased with ethanol dose according to a 2 (sex) × 2 (training dose) × 4 (dose: 0.50, 1.0, 1.5, 2.0 g/kg) mixed-factor repeated measures ANOVA, F(3,12) = 22.6, p < 0.001. The ED 50 for ethanol substitution was lower in monkeys trained to discriminate 1.0 g/kg (mean ± S.E.M. g/kg, 0.49 ± 0.05) compared with monkeys trained to discriminate 2.0 g/kg ethanol (0.94 ± 0.14 g/kg), F(1,13) = 10.01, p = 0.007, but did not vary by sex (Table 1).
TABLE 1.
Mean (± S.E.M.) potency (ED50, mg/kg) for test drugs to produce 50% ethanol-appropriate responding in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol
The number of monkeys for whom it was possible to calculate an ED50 is shown in parentheses.
| Test Drug | 1.0 g/kg Ethanol
Training |
2.0 g/kg Ethanol
Training |
||
|---|---|---|---|---|
| Females, n = 4 | Males, n = 6 | Females, n = 4 | Males, n = 3 | |
| Ethanol a | 455 ± 48 (4) | 511 ± 79 (6) | 919 ± 24 (4) | 964 ± 18 (3) |
| Pentobarbital a | 2.66 ± 0.19 (4) | 3.12 ± 0.34 (6) | 3.88 ± 0.50 (4) | 4.70 ± 1.07 (3) |
| Midazolam | 2.10 ± 0.82 (4) | 1.25 ± 0.44 (2) | 3.11 ± 1.18 (4) | 1.49 (1) |
Main effect of training dose.
Like ethanol, PB completely substituted in all monkeys tested. Ethanol-appropriate responding increased with the dose of PB (dose: 1.0, 10.0, 17.0 mg/kg), F(2,6) = 61.2, p < 0.001. Pentobarbital substituted more potently in monkeys trained to discriminate 1.0 g/kg ethanol as indicated by lower mean ED 50 (2.94 ± 0.22 mg/kg) compared with monkeys trained to discriminate 2.0 g/kg ethanol (4.22 ± 0.51 mg/kg), F(1,13) = 7.39, p = 0.018. Mean ED 50 for PB substitution did not differ between males and females (Table 1).
Midazolam completely substituted for ethanol in all monkeys except two males (4891, 5496) trained to discriminate 2.0 g/kg ethanol and four males trained to discriminate 1.0 g/kg ethanol (4865, 4995, 4864, 4867). Ethanol-appropriate responding increased dose-dependently after midazolam administration (1.0, 1.7, 3 mg/kg), F(2,6) = 26.14, p = 0.001. For midazolam substitution, mean ED 50 did not vary by sex or training dose, although the mean ED 50 for midazolam substitution was numerically greater for females trained to discriminate 2.0 g/kg ethanol compared with every other group (Table 1). Assessment of sex, training dose, and interactions on ethanol-appropriate responding after midazolam administration was underpowered because midazolam completely substituted in only one male monkey trained to discriminate 2.0 g/kg ethanol and two male monkeys trained to discriminate 1.0 g/kg ethanol.
Antagonism of Ethanol Substitution.
Ethanol substitution was antagonized by Ro15-4513 in all monkeys tested (Supplemental Figs. 1 and 2). An example of rightward shifts in the ethanol dose-response function after pretreatment with Ro15-4513 is shown in Fig. 1. In all female monkeys trained to discriminate 1.0 g/kg ethanol, Ro15-4513 pretreatment resulted in three or more shifts in the ethanol dose-response function. Schild analysis yielded a mean slope that conformed to unity for this group (Table 2), with apparent pA 2 of 6.11 (95% confidence interval, 5.20, 7.02). For three of four males trained to discriminate 1.0 g/kg ethanol, Ro15-4513 pretreatment resulted in three or more shifts in the dose-response function, but only 4867 had a slope close to −1.0 and the apparent pA 2 was 6.42. The mean slope of the Schild function for males trained to discriminate 1.0 g/kg ethanol did not conform to unity (Table 2), so apparent pA 2 was not calculated. For monkeys trained to discriminate 2.0 g/kg ethanol, two of four females showed three or more shifts in the dose-response function, and the Schild analysis mean slope conformed to unity (Table 2). Last, for one of three males trained to discriminate 2.0 g/kg ethanol, three or more shifts in the dose-response function were obtained, but the slope deviated from unity (Table 2). Increasing doses of ethanol overcame antagonism of the discriminative stimulus effects of ethanol by Ro15-4513 in every monkey tested.
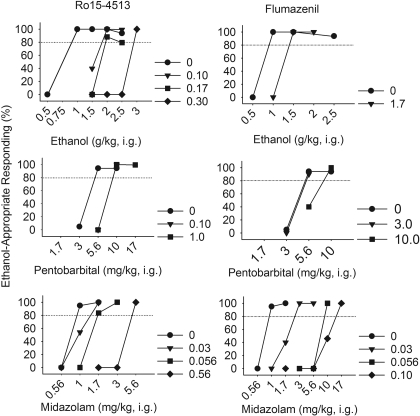
Fig. 1.
Rightward shifts in the ethanol (top), pentobarbital (middle), and midazolam (bottom) dose-response curves for ethanol-appropriate responding in a female cynomolgus monkey (5999) trained to discriminate 2.0 g/kg i.g. ethanol. Ro15-4513 (left) or flumazenil (right) (●, ▼, ■, ♦, mg/kg i.m.) were administered 5 min before the test session.
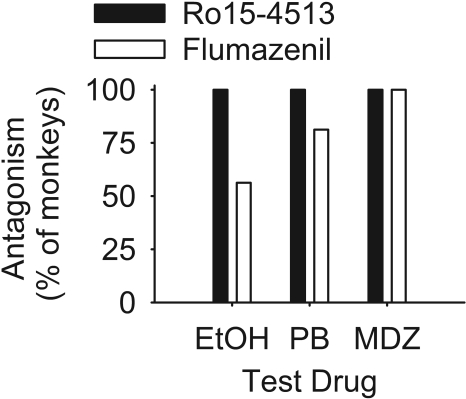
Fig. 2.
Percentage of monkeys tested showing antagonism of ethanol-appropriate responding during tests with ethanol (EtOH), pentobarbital (PB), and midazolam (MDZ) when pretreated with Ro15-4513 or flumazenil.
TABLE 2.
Results of Schild analyses of antagonism of ethanol substitution for the discriminative stimulus effects of 1.0 and 2.0 g/kg ethanol by Ro15-4513 or flumazenil in cynomolgus monkeys
Apparent pA2 for individual monkeys was calculated when three or more shifts in the dose-response function (n) were obtained and the Schild slope was between −0.4 and −1.6. Apparent pKB was calculated when fewer than three shifts in the dose-response function (n) were obtained.
| Females |
Males |
||||||
|---|---|---|---|---|---|---|---|
| n | Slope | pA2 a (pKB) | n | Slope | pA2 (pKB) | ||
| Ro15-4513 (1.0 g/kg ethanol training) | |||||||
| 3123 | 3 | −0.38 | NC | 4865 | 6 | −0.24 | NC |
| 2830 | 3 | −1.14 | 5.75 | 4864 | 4 | −0.26 | NC |
| 2852 | 4 | −1.11 | 5.95 | 4867 | 4 | −0.90 | 6.42 |
| 2913 | 4 | −0.61 | 6.64 | 4995 | 2 | NC | (5.83) |
| Mean | −0.81 | 6.11 | Mean | 0.58 | 6.42 (5.83) | ||
| 95% CI | −0.07, −1.55 | 5.20, 7.02 | 95% CI | 0.30, −1.46 | NC (5.18, 6.47) | ||
| Ro15-4513 (2.0 g/kg ethanol training) | |||||||
| 2835 | 3 | −0.43 | 6.89 | 4891 | 3 | −0.16 | NC |
| 5999 | 3 | −0.80 | 6.48 | 4890 | 1 | NC | (6.51) |
| 5400 | 1 | NC | (5.81) | 5496 | 1 | NC | (6.45) |
| 3220 | 1 | NC | (6.94) | ||||
| Mean | −0.61 | 6.68 (6.38) | Mean | −0.16 | NC (6.48) | ||
| 95% CI | −0.10, −1.12 | 6.12, 7.25 (4.82, 7.94) | 95% CI | NC | NC (6.39, 6.57) | ||
| Flumazenil (1.0 g/kg ethanol training) | |||||||
| 2830 | 2 | NC | (5.16) | 4867 | 1 | NC | (5.48) |
| 4865 | 1 | NC | (5.75) | ||||
| 4889 | 1 | NC | (4.47) | ||||
| 4995 | 1 | NC | (5.05) | ||||
| 4864 | 1 | NC | (4.69) | ||||
| Mean | NC | (5.16) | Mean | NC | (5.09) | ||
| 95% CI | NC | (4.46, 5.87) | 95% CI | NC | (4.05, 6.13) | ||
| Flumazenil (2.0 g/kg ethanol training) | |||||||
| 2835 | 4 | −0.21 | NC | 4890 | 0 | ||
| 3220 | 1 | NC | (4.84) | 4891 | 0 | ||
| 5999 | 1 | NC | (5.08) | 5496 | 0 | ||
| Mean | −0.21 | NC (4.96) | Mean | NC | NC | ||
| 95% CI | NC | NC (4.65, 5.28) | 95% CI | NC | NC | ||
For all pA2 analyses slopes were constrained to −1; NC, not calculable.
In contrast to antagonism by Ro15-4513, flumazenil antagonized ethanol substitution in one of three female monkeys trained to discriminate 1.0 g/kg ethanol and zero of three male monkeys trained to discriminate 2.0 g/kg ethanol. Flumazenil antagonized ethanol substitution in three of four female monkeys trained to discriminate 2.0 g/kg ethanol and five of six male monkeys trained to discriminate 1.0 g/kg ethanol (for an example, see Fig. 1; Supplemental Fig. 3). For some monkeys showing antagonism by flumazenil, rightward shifts in the ethanol dose-response function were not detected. Ethanol dose-response functions were shifted rightward by flumazenil in one of three and three of four female monkeys, and in five of six and zero of three male monkeys, trained to discriminate 1.0 or 2.0 g/kg ethanol, respectively. Antagonism of the discriminative stimulus effects of ethanol by flumazenil was surmountable by increasing doses of ethanol in every monkey showing shifts in the dose-response function. Nonetheless, three or more shifts in the dose-response function were obtained for only one monkey (2835). The slope of the Schild function for this monkey did not conform to unity, so the apparent pA 2 was not calculated (Table 2).
A 2 (sex) × 2 (training dose) × 2 (antagonist) ANOVA for the apparent pA 2 and pK B values from Table 2 indicated that ethanol substitution was antagonized more potently by Ro15-4513 (mean ± S.E.M. potency, 6.33 ± 0.13) compared with flumazenil (5.06 ± 0.15), F(1,12) = 24.56, p < 0.001. The potency of Ro15-4513 and flumazenil to antagonize ethanol substitution did not vary between the groups because there were no main effects or interactions involving sex or training dose. Thus, Ro15-4513 antagonized the discriminative stimulus effects of ethanol more efficaciously and potently than flumazenil.
Antagonism of Pentobarbital Substitution.
The substitution of PB for ethanol was antagonized by Ro15-4513 in all monkeys tested (e.g., Fig. 1; Supplemental Figs. 4 and 5). Furthermore, rightward shifts in the pentobarbital dose-response functions were observed in all monkeys tested, with the exception of one male trained to discriminate 2.0 g/kg ethanol (5496), for which antagonism was apparent. In general, shifts in the dose-response function were parallel and surmountable by increasing doses of PB. Schild analyses were conducted for four of four and one of four females, and three of four and one of three males, trained to discriminate 1.0 or 2.0 g/kg ethanol, respectively, as three or more shifts in the dose-response function were obtained for these monkeys. Results of the Schild analysis revealed that mean slope conformed to unity only for females discriminating 1.0 g/kg ethanol. For monkeys showing fewer than three shifts in the dose-response function, apparent pK B was calculated for each shift (Table 3).
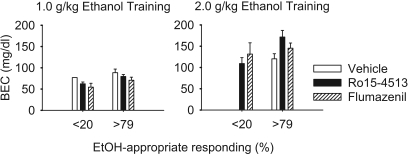
Fig. 4.
Postsession mean (± S.E.M.) BEC in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg i.g. ethanol from water after a 30-min pretreatment interval for sessions with no substitution (<20% ethanol-appropriate responding) and sessions with complete substitution (>79% ethanol-appropriate responding). Vehicle, Ro15-4513, or flumazenil were administered 5 min before the onset of test sessions with the training dose of ethanol.
TABLE 3.
Results of Schild analyses of antagonism of pentobarbital substitution for the discriminative stimulus effects of 1.0 and 2.0 g/kg ethanol by Ro15-4513 or flumazenil in cynomolgus monkeys
Apparent pA2 for individual monkeys was calculated when three or more shifts in the dose-response function (n) were obtained and the Schild slope was between −0.4 and −1.6. Apparent pKB was calculated when less than three shifts in the dose-response function (n) were obtained.
| Females |
Males |
||||||
|---|---|---|---|---|---|---|---|
| n | Slope | pA2 a (pKB) | n | Slope | pA2 (pKB) | ||
| Ro15-4513 (1.0 g/kg ethanol training) | |||||||
| 3123 | 5 | −1.53 | 5.40 | 4865 | 3 | −1.39 | 7.46 |
| 2830 | 4 | −1.20 | 6.55 | 4889 | 4 | −0.43 | 6.54 |
| 2852 | 4 | −0.63 | 6.20 | 4867 | 4 | −>0.38 | NC |
| 2913 | 4 | −1.13 | 6.92 | 4995 | 1 | NC | (6.68) |
| 4892 | 2 | NC | (6.68) | ||||
| 4864 | 1 | NC | (6.46) | ||||
| Mean | −1.12 | 6.27 | Mean | −0.48 | 7.00 (6.63) | ||
| 95% CI | −0.40, −1.85 | 5.00, 7.53 | 95% CI | 1.26, −2.22 | 5.73, 8.27 (6.39, 6.87) | ||
| Ro15-4513 (2.0 g/kg ethanol training) | |||||||
| 3220 | 3 | −0.80 | 6.48 | 4891 | 3 | −1.36 | 6.35 |
| 5400 | 1 | NC | (5.52) | 4890 | 2 | NC | (5.88) |
| 2835 | 1 | NC | (7.21) | 5496 | 0 | ||
| 5999 | 2 | NC | (5.90) | ||||
| Mean | −0.80 | 6.48 (6.13) | Mean | −1.36 | 6.35 (5.88) | ||
| 95% CI | NC | NC (4.48, 7.79) | 95% CI | NC | NC (5.59, 6.17) | ||
| Flumazenil (1.0 g/kg ethanol training) | |||||||
| 3123 | 3 | −0.74 | 4.57 | 4865 | 3 | 0.00004 | NC |
| 2830 | 2 | NC | (5.00) | 4892 | 1 | NC | (5.33) |
| 2852 | 2 | NC | (4.79) | 4867 | 2 | NC | (4.77) |
| 2913 | 1 | NC | (4.76) | 4864 | 2 | NC | (4.49) |
| Mean | −0.74 | 4.57 (4.86) | Mean | 0.00004 | NC (4.77) | ||
| 95% CI | NC | NC (4.41, 5.32) |
95% CI | NC | NC (4.05, 5.49) | ||
| Flumazenil (2.0 g/kg ethanol training) | |||||||
| 3220 | 2 | NC | (4.77) | 4891 | 3 | −0.81 | 5.10 |
| 5999 | 2 | NC | (3.63) | 4890 | 1 | NC | (4.64) |
| Mean | NC | (4.20) | Mean | −0.81 | 5.10 (4.64) | ||
| 95% CI | NC | (2.66, 5.74) | 95% CI | NC | NC | ||
For all pA2 analyses slopes were constrained to −1; NC, not calculable.
Similarly to Ro15-4513, flumazenil antagonized PB substitution for ethanol in all monkeys tested, with the exception of one male trained to discriminate 1.0 g/kg ethanol (4889) and two females (5400, 2835) trained to discriminate 2.0 g/kg ethanol (e.g., Fig. 1; Supplemental Figs. 6 and 7). Monkey 2835 did not, however, show complete substitution of PB for ethanol, precluding measurement of antagonism. Among the monkeys showing antagonism, rightward shifts in the PB dose-response function with flumazenil were obtained in four of four and two of four females, and four of five and two of three males, trained to discriminate 1.0 or 2.0 g/kg ethanol, respectively. One monkey (4891) showed three or more shifts in the dose-response function. Schild analysis revealed a slope that deviated from unity, however, so apparent pA 2 was not calculated.
Similarly to ethanol, Ro15-4513 shifted the PB dose-response functions to the right more potently (mean ± S.E.M. potency, 6.41 ± 0.15) than flumazenil (4.71 ± 0.13), F(1,17) = 57.28, p < 0.001. The potency of Ro15-4513 to antagonize PB substitution did not differ between the sexes, or between the training doses of ethanol.
Antagonism of Midazolam Substitution.
In all monkeys tested, pretreatment with either Ro15-4513 or flumazenil antagonized the substitution of midazolam for 1.0 or 2.0 g/kg ethanol (e.g., Fig. 1; Supplemental Figs. 8–10). Among the monkeys tested with midazolam and pretreated with Ro15-4513, rightward shifts in the dose-response function were obtained for four of four female monkeys trained to discriminate 1.0 g/kg and four of four females trained to discriminate 2.0 g/kg ethanol, and two of three and one of one male monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol, respectively. Among the monkeys tested, flumazenil shifted the midazolam dose-response function for two of three females and two of two males trained to discriminate 1.0 g/kg ethanol, and two of three females and one of one males trained to discriminate 2.0 g/kg ethanol. For females trained to discriminate 1.0 or 2.0 g/kg ethanol, respectively, four of four and two of four monkeys showed three or more shifts in the midazolam dose-response function with Ro15-4513. Mean slope conformed to unity only for the 1.0 g/kg female group (Table 4). For males trained to discriminate 1.0 or 2.0 g/kg ethanol, respectively, one of three and one of one monkeys tested showed three or more shifts in the midazolam dose-response function with Ro15-4513 pretreatment. Schild analyses revealed that none of the slopes for these groups conformed to unity. Among the monkeys pretreated with flumazenil and tested with midazolam, three or more shifts in the dose-response function were obtained with flumazenil in one of three and two of two females and in one of two and one of one males trained to discriminate 1.0 or 2.0 g/kg ethanol, respectively. Overall, rightward shifts in the midazolam dose-response function by flumazenil and Ro15-4513 were parallel and antagonism was surmountable with increasing doses of midazolam.
TABLE 4.
Results of Schild analyses of antagonism of midazolam substitution for the discriminative stimulus effects of 1.0 and 2.0 g/kg ethanol by Ro15-4513 or flumazenil in cynomolgus monkeys
Apparent pA2 for individual monkeys was calculated when three or more shifts in the dose-response function (n) were obtained and the Schild slope was between −0.4 and −1.6. Apparent pKB was calculated when less than three shifts in the dose-response function (n) were obtained.
| Females |
Males |
||||||
|---|---|---|---|---|---|---|---|
| n | Slope | pA2 a (pKB) | n | Slope | pA2 (pKB) | ||
| Ro15-4513 (1.0 g/kg ethanol training) | |||||||
| 3123 | 3 | −1.02 | 7.23 | 4889 | 3 | −1.89 | NC |
| 2830 | 4 | −1.07 | 7.19 | 4892 | 2 | NC | (7.57) |
| 2852 | 3 | −0.60 | 7.19 | 4865 | 0 | ||
| 2913 | 3 | −0.95 | 7.15 | ||||
| Mean | −0.91 | 7.19 | Mean | −1.89 | NC (7.57) | ||
| 95% CI | −0.50, −1.32 | 7.13, 7.26 | 95% CI | NC | NC (7.47, 7.66) | ||
| Ro15-4513 (2.0 g/kg ethanol training) | |||||||
| 3220 | 3 | −0.36 | NC | 4890 | 3 | −0.08 | NC |
| 5400 | 2 | NC | (6.67) | ||||
| 2835 | 2 | NC | (7.85) | ||||
| 5999 | 3 | −0.87 | 6.58 | ||||
| Mean | −0.62 | 6.58 (7.26) | Mean | −0.08 | NC | ||
| 95% CI | 0.09, −1.32 | NC (5.89, 8.64) | 95% CI | NC | NC | ||
| Flumazenil (1.0 g/kg ethanol training) | |||||||
| 3123 | 0 | 4892 | 3 | NC | (7.22) | ||
| 2830 | 1 | NC | (7.36) | 4889 | 2 | NC | (6.13) |
| 2852 | 3 | −0.44 | 7.18 | ||||
| Mean | −0.44 | 7.18 (7.36) | Mean | NC | (6.68) | ||
| 95% CI | NC | NC | 95% CI | NC | (5.42, 7.94) | ||
| Flumazenil (2.0 g/kg ethanol training) | |||||||
| 3220 | 5 | −0.74 | 6.34 | 4890 | 3 | −1.31 | 7.09 |
| 5999 | 3 | −1.81 | NC | ||||
| Mean | −1.27 | 6.34 | Mean | −1.31 | 7.09 | ||
| 95% CI | −0.21, −2.76 | NC | 95% CI | NC | NC | ||
For all pA2 analyses slopes were constrained to −1; NC, not calculable.
In contrast to ethanol and PB substitution, midazolam substitution for ethanol was antagonized with equal potency by Ro15-4513 (mean ± S.E.M. potency, 7.26 ± 0.20) and flumazenil (6.84 ± 0.21), F(1,7) = 3.44, p = 0.11. The potency of Ro15-4513 or flumazenil to antagonize the substitution of midazolam for ethanol did not vary by sex or training dose (Table 4).
Comparison of Antagonist Efficacy and Potency.
Figure 2 shows that Ro15-4513 antagonized the substitution of ethanol and PB in a greater percentage of monkeys compared with flumazenil, although midazolam substitution was antagonized by both drugs in all monkeys tested. The potency of Ro15-4513 or flumazenil also differed depending on the test drug. A 2 (sex) × 2 (training dose) × 2 (antagonist) × 3 (test drug) ANOVA for apparent pK B and pA 2 revealed a main effect of test drug, F(2,37) = 32.19, p < 0.001, a main effect of antagonist, F(1,37) = 45.87, p < 0.001, and an interaction between the two, F(2,37) = 5.10, p < 0.001. Midazolam substitution for ethanol was more potently antagonized by Ro15-4513 (mean ± S.E.M., 7.18 ± 0.15) compared with ethanol (6.31 ± 0.12) and PB (6.41 ± 0.16) substitution for ethanol. Likewise, flumazenil more potently antagonized the substitution of midazolam (6.89 ± 0.21) compared with ethanol (5.06 ± 0.15) and PB (4.71 ± 0.13). Overall, Ro15-4513 antagonized ethanol-appropriate responding across all training conditions and test drugs more potently (6.59 ± 0.09) than flumazenil (5.57 ± 0.11). Potency differences between Ro15-4513 and flumazenil were, however, greatest between the test drugs ethanol and PB, with negligible potency differences in tests with midazolam.
Response Rate Effects.
Baseline rates of responding were higher for monkeys trained to discriminate 1.0 g/kg (mean ± S.E.M., 2.38 ± 0.37 responses/s) compared with monkeys trained to discriminate 2.0 g/kg (1.53 ± 0.25 responses/s) ethanol, F(1,13) = 5.22, p = 0.04. Rates of responding did not vary between the sexes. The test drugs did not systematically decrease rates of responding relative to control rates (Table 5). A 2 (sex) × 2 (training dose) × 8 (ethanol dose: 0, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5 g/kg) ANOVA indicated that the percentage of baseline response rates did not vary with sex, training dose, or ethanol test dose. The same analysis with PB dose as a factor (1.0, 1.70, 3.0, 5.60, 10.0, 17.0 mg/kg) revealed a main effect of training dose, F(1,51) = 5.06, p = 0.029, with PB decreasing response rates more in monkeys trained to discriminate 1.0 g/kg (mean ± S.E.M., 78.0 ± 5.27% baseline response rate) compared with monkeys trained to discriminate 2.0 g/kg ethanol (102.86 ± 10.53%). This analysis did not indicate a significant dose-dependent decrease in rates of responding or an effect of sex. A main effect of dose, F(6,43) = 4.63, p = 0.001, indicated dose-dependent decreases in rates of responding relative to baseline with midazolam administration. Bonferroni-corrected pairwise comparisons showed that mean percent of baseline response rate was lower after 10 mg/kg midazolam (12.38 ± 20.52%) compared with 1.0 mg/kg (116.68 ± 13.44%) and 1.7 mg/kg (94.40 ± 12.15%) midazolam. In addition, the percentage of baseline response rate was lower after 3.0 mg/kg (52.42 ± 10.69%) compared with 1.0 mg/kg midazolam. Midazolam decreased rates of responding more in males (mean ± S.E.M., 56.79 ± 9.03% baseline response rate) compared with females (83.72 ± 8.77%) according to a main effect of sex, F(1,43) = 5.24, p = 0.027.
TABLE 5.
Mean (± S.E.M.) potency (ED50, mg/kg) for test drugs to decrease rates of responding by 50% in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol
The number of monkeys showing dose-dependent decreases in response rate during vehicle tests to the number of monkeys tested is shown in parentheses.
| Test Drug | 1.0 g/kg Ethanol
Training |
2.0 g/kg Ethanol
Training |
||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Ethanol | (0/4) | 843 (1/6) | (0/4) | (0/3) |
| Pentobarbital | 8.52 (1/4) | (0/6) | (0/4) | NC a (1/3) |
| Midazolam | 4.71 (1/4) | 4.73 ± 1.75 (3/6) | 5.98 ± 2.71 (2/4) | 6.89 (1/2) |
NC, not calculable because the maximum percentage of the baseline response rate is <50%.
Antagonism of Response Rate Effects.
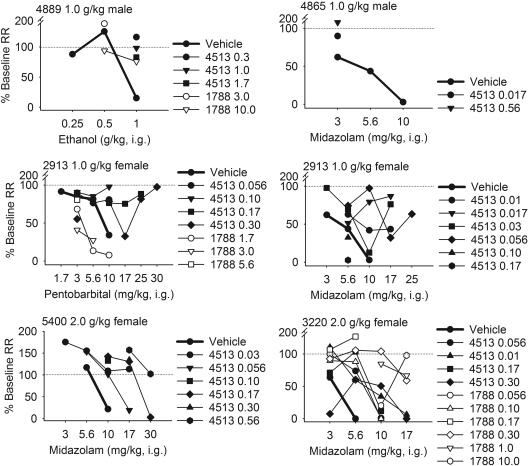
Among the monkeys sensitive to rate-decreasing effects, Ro15-4513 and flumazenil antagonized the rate-decreasing effects of ethanol, PB, and midazolam in six of nine cases (Fig. 3). Antagonism was surmountable by increasing doses of PB and midazolam, but too few tests were available with ethanol to assess whether the antagonism was competitive. The rate-decreasing effects of 1.0 g/kg ethanol were antagonized by 0.30 to 1.7 mg/kg Ro15-4513 and 10 mg/kg flumazenil for monkey 4889. For monkey 2913, the response rate decrease after 10 mg/kg pentobarbital was antagonized by 0.056 mg/kg Ro15-4513. Apparent pKB from the single dose-response shift for this monkey was 6.16. All doses of flumazenil seemed to potentiate the rate-decreasing effects of pentobarbital in the single monkey tested (2913). Among the monkeys tested for which midazolam decreased response rate, the rate-decreasing effects of midazolam were antagonized by Ro15-4513. For monkeys 2913, 5400, and 3220, respectively, mean (±95% confidence interval) apparent pKB for Ro15-4513 antagonism of midazolam-induced response rate decrease was 7.08 (7.72, 6.44), 6.52 (6.68, 6.35), and 6.60 (7.13, 6.07). Apparent pK B for flumazenil antagonism of the response rate-decreasing effects of midazolam in monkey 3220 was 5.56 (7.27, 3.85).
Fig. 3.
Effects of Ro15-4513 (4513: ●,
▲, ■, ♦,
▲,  , mg/kg)
and flumazenil (1788: ○,
▽, □, ◇,
△, ⬡, mg/kg) on the
response rate-decreasing effects of ethanol,
pentobarbital and midazolam (bold). RR,
response rate.
, mg/kg)
and flumazenil (1788: ○,
▽, □, ◇,
△, ⬡, mg/kg) on the
response rate-decreasing effects of ethanol,
pentobarbital and midazolam (bold). RR,
response rate.
Blood-Ethanol Concentration.
Pretreatment with Ro15-4513 or flumazenil did not affect ethanol pharmacokinetics as measured by postsession BEC (Fig. 4). In tests with the training dose of ethanol, BEC was greater after 2.0 g/kg (mean ± S.E.M.: 141.91 ± 11.21 mg/dl) compared with 1.0 g/kg (73.41 ± 3.18 mg/dl) ethanol according to a main effect of training dose in a 2 (training dose) × 2 (test category: vehicle, Ro15-4513) × 2 (substitution category: no substitution, complete substitution) ANOVA, F(1,64) = 25.42, p < 0.001. This analysis also revealed a main effect of substitution category, F(1,64) = 5.10, p = 0.02, because BECs were significantly higher during sessions for which the training dose of ethanol completely substituted (108.78 ± 7.91 mg/dl) compared with sessions with no substitution (75.30 ± 5.99 mg/dl). Interactions were observed between training dose and test category, F(1,64) = 6.56, p = 0.01, and training dose and substitution category, F(1,64) = 7.22, p = 0.009. Bonferroni-corrected pairwise comparisons indicated, however, that there were no differences between test category (i.e., vehicle versus Ro15-4513) conditions within any training dose and substitution category. The same analysis using BECs from flumazenil test sessions revealed only a main effect of training dose, F(1,30) = 22.07, p < 0.001, with higher BECs after 2.0 g/kg (138 ± 8.91 mg/dl) compared with 1.0 g/kg (73.57 ± 5.07 mg/dl) ethanol. A sex × training dose × test category ANOVA revealed a main effect of sex, F(1,67) = 5.19, p = 0.03, an interaction between sex and training dose, F(1,67) = 6.53, p = 0.01, but no interactions involving test category. Females had higher BECs than males particularly after 2.0 g/kg ethanol (1.0 g/kg: males, 71.84 ± 3.75 mg/dl; females, 74.58 ± 5.73 mg/dl; 2.0 g/kg: males, 122.29 ± 10.32 mg/dl; females, 168.30 ± 17.26 mg/dl).
Discussion
This is the first study to investigate GABAA receptor mediation of the ethanol-like effects of pentobarbital and midazolam. The current study showed rightward, generally parallel shifts in the ethanol, pentobarbital, and midazolam dose-response functions after pretreatment with Ro15-4513 and flumazenil (Fig. 1; Supplemental Figs. 1–10), suggesting competitive antagonism of the discriminative stimulus effects of ethanol and the ethanol-like effects of pentobarbital and midazolam in non-human primates. The absence of tremors or seizures suggested that Ro15-4513 and flumazenil were behaviorally inactive for gross motor functions. Minor and nonsystematic differences in substitution ED 50 between Grant et al. (2000) and vehicle sessions of the current study show that an intramuscular injection 5 min before the beginning of a test session did not affect substitution potency. Pretreatment with Ro15-4513 or flumazenil did not affect postsession BECs, suggesting that shifts in the dose-response function have a pharmacodynamic explanation.
Apparent pA 2 was calculated in 24 of 36 dose-response curves for which three or more shifts were obtained because the slope of the Schild function met our unity criterion. However, the mean slope and 95% confidence intervals of the Schild function conformed to unity in two of eight, one of eight, and two of eight groups during tests with ethanol, pentobarbital, and midazolam, respectively, for dose-response curve shifts produced by both antagonists. Slopes that did not conform to unity could reflect nonequilibrium concentrations of the test drugs or antagonists (Arunlakshana and Schild, 1959). Pretreatment times for Ro15-4513 and flumazenil were chosen to capture peak brain concentrations. In monkeys, BEC peaks 60 min after administration of 1.0 g/kg i.g. ethanol (Green et al., 1999). Although pretreatment times in the current study were 30 min, the proportion of slopes that conformed to unity across all testing conditions was similar between 1.0 g/kg (15/21) and 2.0 g/kg (8/13) groups. Thus, any differences in BEC time course between the training dose conditions seem not to influence the correlation between the magnitude of the ED 50 shift and antagonist dose. To our knowledge, the time course of ethanol, pentobarbital, and midazolam brain concentrations has not been studied in monkeys. Drug concentrations are dynamic in vivo, but at receptors, equilibrium seems to be constant during drug elimination as Schild slope unity is maintained across a wide range of pretreatment times (Gerak and France, 2007). Alternatively, lack of unit slope could also indicate the involvement of multiple GABA A receptor subtypes, suggesting individual differences in receptor subtypes mediating the discriminative stimulus effects of ethanol.
The efficacy of Ro15-4513 and flumazenil to antagonize the substitution of ethanol, pentobarbital, or midazolam for the discriminative stimulus effects of ethanol generally did not vary by sex. A major exception was the lack of antagonism of ethanol discrimination by flumazenil in male monkeys trained to discriminate 2.0 g/kg ethanol (Table 2). Previous reports suggest that unique receptor populations mediate discrimination of 2.0 g/kg ethanol in male monkeys; however, with only partial substitution of N-methyl-d-aspartate receptor antagonists (Vivian et al., 2002) and greater efficacy of the GABA A receptor α 1 subunit-preferring zolpidem relative to the other sex and training dose groups in the current study (Helms et al., 2008b). If the α 1 subunit mediates discrimination of 2.0 g/kg ethanol in male monkeys, the nonselective antagonist flumazenil would have been expected to block ethanol substitution in these monkeys, but this was not the case. Additional studies are required to identify the GABA A receptor subtypes mediating discrimination of 2.0 g/kg ethanol.
Sex differences were not apparent in the efficacy or potency of Ro15-4513 to antagonize response rate effects, and could not be evaluated for flumazenil. Sex differences have been reported in rodents. In mice, 1 and 2 mg/kg Ro15-4513 disrupted operant responding to a greater degree in males compared with females (Bao et al., 1992). Female but not male rats showed increased locomotor activity after 2.5 to 10 mg/kg flumazenil administration (Holtman et al., 2003). Among the monkeys for whom the test drugs decreased rates of responding, lower doses of Ro15-4513 and flumazenil antagonized the rate-decreasing effects, whereas higher doses tended to further decrease the response rate (Fig. 3). This finding is consistent with studies showing that Ro15-4513 greatly decreased response rate only at higher doses in mice [30 mg/kg (Glowa et al., 1989), 4 mg/kg (Bao et al., 1992)]. In the current study, male monkeys were more sensitive to the rate-decreasing effects of midazolam compared with female monkeys. Differences between the sexes in the response rate-decreasing effects of midazolam may be due to endogenous neuroactive steroids that also decrease rates of responding and produce ethanol-like effects by positively modulating GABA A receptors (Grant et al., 2008).
The training dose of ethanol did not influence the efficacy or potency of Ro15-4513 or flumazenil to antagonize the discriminative stimulus effects of ethanol or the ethanol-like effects of pentobarbital and midazolam. This contrasts with data from rodents. In male CD-1 mice, Ro15-4513 more potently antagonized the discriminative stimulus effects of 1.0 g/kg (0.10–1.0 mg/kg Ro15-4513) compared with 1.5 g/kg ethanol (> 1.0 mg/kg Ro15-4513) (Rees and Balster, 1988). In male rats, Ro15-4513 more efficaciously antagonized the discriminative stimulus effects of 1.0 and 1.5 compared with 2.0 g/kg i.g. ethanol. Only three of eight rats trained to discriminate 2.0 g/kg ethanol showed antagonism, and only with high doses (3.0–17 mg/kg) of Ro15-4513 (Gatto and Grant, 1997). In monkeys, doses ranging from 0.003 to 0.17 mg/kg Ro15-4513 antagonized the discriminative stimulus effects of ethanol in the current study irrespective of training dose.
Discrimination of a range of blood- and brain-ethanol concentrations seems to be antagonized by Ro15-4513 and flumazenil. After tests with 1.0 and 2.0 g/kg ethanol, respectively, BECs were 37 to 128 mg/dl and 71 to 249 mg/dl in the current study. In rats (Quertemont et al., 2003), monkeys (C. Kroenke, personal communication), and humans (Hetherington et al., 1999), brain-ethanol concentration is proportional to ethanol dose. Furthermore, complete substitution of the training dose of ethanol occurs at lower brain-ethanol concentrations in rats trained to discriminate 1.0 g/kg ethanol (12 mM) compared with rats trained to discriminate 2.0 g/kg ethanol (28 mM) (Quertemont et al., 2003). Thus, Ro15-4513 and flumazenil antagonize ethanol stimulus effects encompassing mild to moderate intoxication.
The current study is the first to show flumazenil antagonism of the discriminative stimulus effects of ethanol and the second study to show flumazenil antagonism of pentobarbital effects in vivo. The maximum flumazenil doses tested were approximately 10- to 1000-fold higher compared with other drug discrimination antagonism studies (e.g., Lelas et al., 2000; McMahon and France, 2006). Apparent pA 2 and pK B analyses suggested that the flumazenil concentrations antagonizing ethanol and pentobarbital substitution were in the low to high micromolar range. In mouse forebrain, saturating concentrations of flumazenil (∼240 nM) are reached 10 min after 2.0 mg/kg i.p. flumazenil (Hopkins et al., 2009). The high doses of flumazenil tested in the current study may have allowed flumazenil to act as its low-affinity sites (e.g., α 4/6 -containing receptors). Indeed, high-dose interactions between pentobarbital and flumazenil have been observed previously. In rats, the anticonvulsant effects of 40 mg/kg pentobarbital are antagonized by 100 to 200 mg/kg flumazenil (Rastogi and Ticku, 1985).
In the current study, flumazenil antagonized midazolam substitution for ethanol at nanomolar concentrations, similar to findings from studies of benzodiazepine discriminative stimulus effects. In previous studies, substitution of the benzodiazepine zolpidem for the discriminative stimulus effects of ethanol was antagonized by Ro15-4513 with apparent pK B ranging from 6.34 to 7.89 (Helms et al., 2008b), similar to the apparent pK B for Ro15-4513 and flumazenil antagonism of midazolam substitution for ethanol in the current study (Table 4). In rhesus monkeys trained to discriminate 0.56 mg/kg midazolam, Ro15-4513 antagonized midazolam (apparent pA 2 , 7.53) and triazolam (apparent pA 2 , 6.88) substitution, and flumazenil antagonized triazolam (apparent pA 2 , 7.41) and diazepam (apparent pA 2 , 7.69) substitution (Lelas et al., 2000). Flumazenil antagonized the response rate-decreasing effects of midazolam in squirrel monkeys, with a pA 2 value of 7.18 (Paronis and Bergman, 1999). In rhesus monkeys trained to discriminate 10 mg/kg pentobarbital, flumazenil antagonized the substitution of diazepam (pK B , 6.51) and chlordiazepoxide (pK B , 6.57) (Woolverton and Nader, 1995). Similarities among apparent pK B and pA 2 values across these studies suggest that Ro15-4513 and flumazenil antagonize benzodiazepine effects via common benzodiazepine-sensitive GABA A receptor types. Thus, midazolam seems to produce ethanol-like discriminative stimulus effects via α 1–3/5 -containing GABA A receptors.
The substitution of ethanol and pentobarbital for the discriminative stimulus effects of ethanol was antagonized with greater efficacy and potency by Ro15-4513 compared with flumazenil. Ro15-4513 and flumazenil have similar affinity for α 1–3,5 -containing GABA A receptors, but Ro15-4513 has much greater affinity for α 4/6 -containing receptors compared with flumazenil (Sieghart, 1995). Thus, benzodiazepine-insensitive GABA A receptor subtypes containing α 4/6 subunits may have a greater role in mediating the ethanol-like discriminative stimulus effects of ethanol and pentobarbital compared with benzodiazepine-sensitive subtypes. This finding does not exclude the contribution of other GABA A receptor subtypes to the discriminative stimulus effects of ethanol as has been revealed with use of other antagonists (e.g., α 5 ; Platt et al., 2005). Overall, the data suggest that both benzodiazepine-sensitive and -insensitive GABA A receptors mediate the discriminate stimulus effects of ethanol in non-human primates, but with the latter having a dominant role, especially in males discriminating 2.0 g/kg ethanol. Future studies could identify the factors accounting for group differences in GABA A receptor subtypes mediating ethanol-like discriminative stimulus effects.
Supplementary Material
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health [Grants AA10009, AA13860, AA17040].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
This work was presented at the Annual Meeting of Experimental Biology; 2008 Apr 5–9; San Diego, CA (Helms et al., 2008a).
- Ro15-4513
- ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazol(1,5-a)benzodiazepine-3-carboxylate
- BEC
- blood-ethanol concentration
- Ro15-1788
- flumazenil, ethyl 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazol(1,5-a)-benzodiazepine-3-carboxylate
- FR
- fixed ratio
- PB
- pentobarbital
- ANOVA
- analysis of variance
References
- Arunlakshana O, Schild HO. ( 1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14: 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao K, Middaugh LD, Becker HC, Shepherd CL. ( 1992) Effects of Ro 15-4513 and ethanol on operant behavior of male and female C57BL/6 mice. Alcohol 9: 193–198 [DOI] [PubMed] [Google Scholar]
- Buck KJ, Heim H, Harris RA. ( 1991) Reversal of alcohol dependence and tolerance by a single administration of flumazenil. J Pharmacol Exp Ther 257: 984–989 [PubMed] [Google Scholar]
- De Vry J, Slangen JL. ( 1986) Effects of chlordiazepoxide training dose on the mixed agonist-antagonist properties of benzodiazepine receptor antagonist Ro15-1788, in a drug discrimination procedure. Psychopharmacology 88: 177–183 [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Picazo O. ( 1995) Flumazenil blocks the anxiolytic of allopregnanolone. Eur J Pharmacol 281: 113–115 [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Grant KA. ( 1997) Attenuation of the discriminative stimulus effects of ethanol by the benzodiazepine partial inverse agonist Ro 15-4513. Behav Pharmacol 8: 139–146 [PubMed] [Google Scholar]
- Gerak LR, France CP. ( 2007) Time-dependent decreases in apparent pA 2 values for naltrexone studied in combination with morphine in rhesus monkeys. Psychopharmacology 193: 315–321 [DOI] [PubMed] [Google Scholar]
- Glowa JR, Crawley J, Suzdak PD, Paul SM. ( 1989) Ethanol and the GABA receptor complex: studies with the partial inverse agonist Ro 15-4513. Pharmacol Biochem Behav 31: 767–772 [DOI] [PubMed] [Google Scholar]
- Grant KA, Helms CM, Rogers LS, Purdy RH. ( 2008) Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther 326: 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT. ( 2000) Characterization of the discriminative stimulus effects of GABA A receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology (Berl) 152: 181–188 [DOI] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. ( 1999) Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fascicularis). Alcohol Clin Exp Res 23: 611–616 [PubMed] [Google Scholar]
- Helms CM, Rogers LSM, Grant KA. ( 2008a) Antagonism of the substitution of ethanol, pentobarbital, and midazolam for 1.0 and 2.0 g/kg ethanol by Ro15-4513 and Ro15-1788 in cynomolgus monkeys. FASEB J 22: 711–12 [Google Scholar]
- Helms CM, Rogers LS, Waters CA, Grant KA. ( 2008b) Zolpidem generalization and antagonism in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol. Alcohol Clin Exp Res 32: 1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herling S, Shannon HE. ( 1982) Ro 15-1788 antagonizes the discriminative stimulus effects of diazepam in rats but not similar effects of pentobarbital. Life Sci 31: 2105–2112 [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Telang F, Pan JW, Sammi M, Schuhlein D, Molina P, Volkow ND. ( 1999) Spectroscopic imaging of the uptake kinetics of human brain ethanol. Magn Reson Med 42: 1019–1026 [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ, Järbe TU. ( 1988) Ro 15-4513 does not antagonize the discriminative stimulus- or rate-depressant effects of ethanol in rats. Alcohol 5: 203–207 [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Sloan JW, Jing X, Wala EP. ( 2003) Modification of morphine analgesia and tolerance by flumazenil in male and female rats. Eur J Pharmacol 470: 149–156 [DOI] [PubMed] [Google Scholar]
- Hopkins SC, Brian Nofsinger J, Allen MS, Koch P, Varney MA. ( 2009) In vivo saturation binding of GABA-A receptor ligands to estimate receptor occupancy using liquid chromatography/tandem mass spectrometry. Biopharm Drug Dispos 30: 9–20 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources ( 1996) Guide for the Care and Use of Laboratory Animals, 7th ed . Institute of Laboratory Animal Resources Commission on Life Sciences, National Research Council,, Washington, DC: [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. ( 2004) SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol 32: 101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelas S, Gerak LR, France CP. ( 2000) Antagonism of the discriminative stimulus effects of positive γ-aminobutyric acid A modulators in rhesus monkeys discriminating midazolam. J Pharmacol Exp Ther 294: 902–908 [PubMed] [Google Scholar]
- Maeda J, Suhara T, Kawabe K, Okauchi T, Obayashi S, Hojo J, Suzuki K. ( 2003) Visualization of α5 subunit of GABA A /benzodiazepine receptor by [ 11 C]Ro15-4513 using positron emission tomography. Synapse 47: 200–208 [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li TK. ( 1988) Effects of Ro 15-4513, fluoxetine and desipramine on the intake of ethanol, water and food by the alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav 30: 1045–1050 [DOI] [PubMed] [Google Scholar]
- McMahon LR, Gerak LR, France CP. ( 2006) Efficacy and the discriminative stimulus effects of negative GABA A modulators, or inverse agonists, in diazepam-treated rhesus monkeys. J Pharmacol Exp Ther 318: 907–913 [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Bao K, Becker HC, Daniel SS. ( 1991) Effects of Ro 15-4513 on ethanol discrimination in C57BL/6 mice. Pharmacol Biochem Behav 38: 763–767 [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. ( 2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300: 2–8 [DOI] [PubMed] [Google Scholar]
- Paronis CA, Bergman J. ( 1999) Apparent pA 2 values of benzodiazepine antagonists and partial agonists in monkeys. J Pharmacol Exp Ther 290: 1222–1229 [PubMed] [Google Scholar]
- Platt DM, Duggan A, Spealman RD, Cook JM, Li X, Yin W, Rowlett JK. ( 2005) Contribution of α 1 GABA A and α 5 GABA A receptor subtypes to the discriminative stimulus effects of ethanol in squirrel monkeys. J Pharmacol Exp Ther 313: 658–667 [DOI] [PubMed] [Google Scholar]
- Quertemont E, Green HL, Grant KA. ( 2003) Brain ethanol concentrations and ethanol discrimination in rats: effects of dose and time. Psychopharmacology 168: 262–270 [DOI] [PubMed] [Google Scholar]
- Rastogi SK, Ticku MK. ( 1985) Involvement of a GABAergic mechanism in the anticonvulsant effect of pentobarbital against maximal electroshock-induced seizures in rats. Pharmacol Biochem Behav 22: 141–146 [DOI] [PubMed] [Google Scholar]
- Rees DC, Balster RL. ( 1988) Attenuation of the discriminative stimulus properties of ethanol and oxazepam, but not of pentobarbital, by Ro 15-4513 in mice. J Pharmacol Exp Ther 244: 592–598 [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. ( 1996) Assessment of benzodiazepine receptor heterogeneity in vivo: apparent pA 2 and pK B analyses from behavioral studies. Psychopharmacology 128: 1–16 [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. ( 1998) Discriminative stimulus effects of benzodiazepine agonists and partial agonists in pentobarbital in pentobarbital-trained rhesus monkeys. Behav Pharmacol 9: 81–92 [PubMed] [Google Scholar]
- Samson HH, Haraguchi M, Tolliver GA, Sadeghi KG. ( 1989) Antagonism of ethanol-reinforced behavior by the benzodiazepine inverse agonists Ro15-4513 and FG 7142: relation to sucrose reinforcement. Pharmacol Biochem Behav 33: 601–608 [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Yamasaki T, Inoue O, Itoh T, Suzuki K, Hashimoto K, Tateno Y, Ikehira H. ( 1986) Visualization of specific binding sites of benzodiazepine in human brain. J Nucl Med 27: 1593–1599 [PubMed] [Google Scholar]
- Sieghart W. ( 1995) Structure and pharmacology of γ-amino-butyric acid A receptor subtypes. Pharmacol Rev 47: 181–234 [PubMed] [Google Scholar]
- Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM. ( 1986) A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science 234: 1243–1247 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Cowan A, Adler MW. ( 1979) pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci 25: 637–654 [DOI] [PubMed] [Google Scholar]
- Thompson SA, Whiting PJ, Wafford KA. ( 1996) Barbiturate interactions at the human GABA A receptor: dependence on receptor subunit combination. Br J Pharmacol 117: 521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. ( 2002) Characterization of the discriminative stimulus effects of N-methyl-d-aspartate ligands under different ethanol training conditions in the cynomolgus monkey (Macaca fascicularis). Psychopharmacology 162: 273–281 [DOI] [PubMed] [Google Scholar]
- Wallner M, Olsen RW. ( 2008) Physiology and pharmacology of alcohol: the imidazobenzodiazepine alcohol antagonist site on subtypes of GABA A receptors as an opportunity for drug development? Brit J Pharmacol 154: 288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Nader MA. ( 1995) Effects of several benzodiazepines, alone and in combination with flumazenil, in rhesus monkeys trained to discriminate pentobarbital from saline. Psychopharmacology 122: 230–236 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.