Abstract
Cocaine addiction is a worldwide public health problem for which there are no established treatments. The dopamine transporter (DAT) is suspected as the primary target mediating cocaine's abuse-related effects based on numerous pharmacological studies. However, in a previous study, DAT knockout mice were reported to self-administer cocaine, generating much debate regarding the importance of the DAT in cocaine's abuse-related effects. Here, we show that mice expressing a “knockin” of a cocaine-insensitive but functional DAT did not self-administer cocaine intravenously despite normal food-maintained responding and normal intravenous self-administration of amphetamine and a direct dopamine agonist. Our results have three implications. First, they imply a crucial role for high-affinity DAT binding of cocaine in mediating its reinforcing effects, reconciling mouse genetic engineering approaches with data from classic pharmacological studies. Second, they demonstrate the usefulness of knockin strategies that modify specific amino acid sequences within a protein. Third, they show that it is possible to alter the DAT protein sequence in such a way as to selectively target its interaction with cocaine, while sparing other behaviors dependent on DAT function. Thus, molecular engineering technology could advance the development of highly specialized compounds such as a dopamine-sparing “cocaine antagonist.”
Cocaine abuse and dependence are a considerable public health problem, for which there are no established treatments (Gorelick et al., 2004; Sofuoglu and Kosten, 2005). Evidence suggests that cocaine's reinforcing effects depend on its ability to rapidly block the dopamine transporter (DAT). In animal studies, dopamine reuptake inhibitors other than cocaine are also self-administered, with a relative potency that generally correlates positively with their potency in inhibiting the DAT, but not the serotonin or norepinephrine transporters (SERT, NET) (Ritz et al., 1987; Bergman et al., 1989; Howell and Byrd, 1995; Roberts et al., 1999; Wee et al., 2005). Animals trained to self-administer cocaine will also self-administer direct dopamine agonists (Woolverton et al., 1984; Wise et al., 1990; Caine and Koob, 1993). In addition, destruction of dopamine nerve terminals can lead to extinction of cocaine self-administration behavior (Roberts et al., 1977, 1980), and these effects have been observed even when responding maintained by other reinforcers was preserved (Pettit et al., 1984; Caine and Koob, 1994). In humans, cocaine-induced “high” correlates with DAT occupancy in the brain (Volkow et al., 1997).
Therefore, it was surprising that knockout mice lacking the DAT (DAT−/− mice) were reported to self-administer cocaine, and to show cocaine-conditioned place preferences (Rocha et al., 1998; Sora et al., 1998). However, constitutive knockout of the DAT leads to extensive compensatory changes in monoamine systems, which can complicate the interpretation of results obtained with use of constitutive DAT−/− mice (Giros et al., 1996; Jones et al., 1998, 1999). We recently showed that a different line of DAT−/− mice generally failed to self-administer cocaine (Thomsen et al., 2009). Those seemingly inconsistent findings illustrate the potential complications that can be associated with classic gene knockout strategies. To further test the hypothesis that DAT is essential to cocaine's reinforcing effects, a functional but “cocaine-insensitive” DAT was generated and expressed in mice (Chen et al., 2005, 2006). This mutant DAT demonstrated an 89-fold lower affinity for cocaine relative to wild-type DAT, and cocaine failed to increase extracellular dopamine in the nucleus accumbens, or to induce increases in locomotor activity, stereotypies, or conditioned place preferences, in knockin (DATki) mice expressing this mutant DAT (Chen et al., 2006; Tilley and Gu, 2008; Tilley et al., 2009).
In the present study we tested DATki mice along with their wild-type and heterozygous littermates in chronic intravenous cocaine self-administration to test the hypothesis that the reinforcing effects of cocaine would be diminished or lacking in the mutant mice. We also assessed whether any effect on cocaine self-administration was specific, or whether food-maintained operant behavior and amphetamine self-administration were similarly affected. In one group of mice, acquisition of cocaine self-administration was evaluated in drug- and experimentally naive mice. In another group of mice, operant responding was established with liquid food and evaluated over a range of food reinforcer magnitudes, followed by substitution of intravenous cocaine as the reinforcer. Dose-effect functions for cocaine self-administration were then determined under a fixed ratio (FR) 1 schedule of reinforcement. Wild-type and heterozygous mice were also compared under increasing response requirements, including progressive ratio (PR) schedules of reinforcement. Intravenous self-administration of the DAT ligand and dopamine releaser amphetamine, and of the direct dopamine agonist SKF 82958, were also assessed to further verify that operant behavior generally was not impaired by the mutation, and that relevant dopamine systems were functional.
Materials and Methods
Animals and Housing.
Mutant mice expressing a cocaine-insensitive DAT were generated as described previously (Chen et al., 2006). Littermate male and female DATki, heterozygous, and wild-type mice were used. Mice were acclimated to the housing facilities at least 7 days before experiments were initiated. During this time they were also handled, and they were anesthetized briefly once while a microchip was inserted subcutaneously for unambiguous identification during experiments. Animals were kept in a 12-h light/dark cycle at ∼22°C and ∼55% humidity, group housed up to five per cage. Tap water was accessible ad libitum, and standard rodent chow was provided ad libitum. For enrichment purposes, variously flavored rodent “treats,” nesting material, and hiding/nesting devices were provided. Exercise devices (running wheels) were available before catheter implantation only to avoid injuries caused by the protruding catheter base. All testing was conducted during the light phase of the circadian cycle. All procedures were performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were randomly assigned to one of two protocols: one cohort was trained and evaluated in the operant behavior with a food reinforcer before cocaine self-administration, and a separate cohort was allowed to acquire cocaine self-administration with no prior training.
Operant-Conditioning Apparatus.
Operant-conditioning chambers and the training and evaluation of food-maintained behavior under a fixed-ratio schedule have been described in detail (Caine et al., 1999; Thomsen et al., 2005). In brief, each operant-conditioning chamber contained two nose-poke holes 10 mm above the grid floor, both equipped with photocells and a yellow discriminative light cue, positioned on either side of a plate for liquid-food delivery. Responding in the right hole resulted in delivery of a reinforcer and the turning on of the cue light for a 20-s time-out period during which no reinforcer could be earned. Responses in the left hole were counted but had no scheduled consequences. The house light was turned on after delivery of a single noncontingent reinforcer (with the exception of acquisition sessions for cocaine self-administration, in which noncontingent reinforcers were never delivered) and stayed on until the session ended.
Training and Evaluation of Food-Maintained Behavior under the FR Schedule.
Experimentally naive mice were placed in the operant-conditioning chamber with one reinforced (right) and one nonreinforced (left) nose-poke hole, for one 2-h session daily, 5 days per week. During the acquisition phase, liquid food (Ensure protein drink, vanilla flavor; Abbott Laboratories, Abbott Park, IL) was presented according to a FR 1 (time-out) 20-s schedule. Each food reinforcer was 25 μl. The mice were allowed at least five consecutive sessions to acquire responding, and as long as needed until the criteria were met (criteria: two consecutive sessions with a minimum of 20 reinforcers earned per session, no more than 20% variation in number of reinforcers earned between the two sessions, and at least 70% responses in the active hole). After acquisition criteria were met, water was substituted for at least three sessions and until responding was extinguished to <80% of food responding, and mice were required to show nose-poking levels comparable with baseline levels when 100% food was again made available to meet criteria for stable acquisition (all mice met criteria). A range of liquid-food dilutions (water, 3, 10, 32, and 100%) was then presented within-subjects according to a Latin square design. All mice tested with food completed the experiment (no attrition): 20 DATki mice (10 male, 10 female), 15 heterozygous mice (5 male, 10 female), and 14 wild-type mice (9 female, 5 male). This relatively large number of animals was trained to allow for attrition in the subsequent intravenous chronic self-administration studies.
Catheter Implantation Surgery and Maintenance.
An indwelling catheter was implanted into the right or left external jugular vein under oxygen/sevoflurane vapor anesthesia. The surgical procedure was based on techniques established in rats (Emmett-Oglesby et al., 1993) and has been described in great detail elsewhere (Thomsen and Caine, 2005). In brief, a catheter was inserted 1.2 cm into the jugular vein and delicately anchored to the vein. The catheter ran subcutaneously to the base located above the midscapular region. The mice were allowed 7 days to recover, during which 0.02 ml of 0.9% saline containing heparin (30 USP units/ml) and antibiotic (cefazolin, 67 mg/ml) was infused daily through the catheter to forestall clotting and infection. Outside self-administration sessions, the free end of the cannula guide was kept closed at all times. Catheter patency was confirmed after completion of an experimental phase by the infusion of 0.02 to 0.03 ml of 15 mg/ml ketamine + 0.75 mg/ml midazolam in saline. Loss of muscle tone and clear signs of anesthesia within 3 s of infusion indicated catheter patency.
Cocaine Self-Administration Behavior under the FR Schedule.
After jugular catheter implantation, intravenous cocaine was available as the reinforcer (1.0 mg/kg i.v. per infusion of cocaine HCl in 0.9% saline) until baseline criteria were met (two consecutive sessions with a minimum of 20 reinforcers earned per sessions, no more than 20% variation in number of reinforcers earned between the two sessions, and at least 70% responses in the active hole). Thereafter, saline was substituted for cocaine until extinction to criteria (<80% of baseline self-administration level), then baseline behavior was re-established with 1.0 mg/kg per infusion, followed by dose-effect functions determined within-subjects (saline, 0.032, 0.1, 0.32, 1.0, and 3.2 mg/kg per infusion, tested according to a Latin-square design). Cocaine solutions or saline were delivered in 0.56 ml/kg, e.g., for a 32-g mouse, 18 μl infused over 3.2 s. To prevent overdose, the total drug intake was limited to 30 mg/kg/session. Compared with the food-maintained behavior, session length was extended to 3 h after surgery (primarily to facilitate extinction when saline was available), whereas all other parameters (time-out, etc.) remained the same. Cocaine dose-effect functions were obtained from 13 DATki mice (4 female, 9 male), 14 heterozygous mice (8 female, 6 male), and 14 wild-type mice (6 female, 8 male).
Acquisition of Cocaine Self-Administration Behavior in Experimentally Naive Mice.
After jugular catheter implantation and recovery, experimentally naive mice were introduced to the operant chamber with 1.0 mg/kg i.v. per infusion of cocaine as the reinforcer. Sessions were identical to the FR 1 schedule described above, with the following two exceptions. First, the sessions were started immediately before introducing the animal into the chamber to ensure that the first nose pokes were reinforced. Second, there was no noncontingent infusion at the start of the sessions. Mice were allowed to self-administer cocaine for at least five and up to seven sessions (if criteria were not met within five sessions). Criteria for acquisition of self-administration were: 1) a minimum of 15 reinforcers earned per session for at least two consecutive sessions (“acquisition level”); 2) at least 70% responses in the active hole on the second day; 3) extinction of responding when saline was substituted for cocaine (i.e., less than 80% of the acquisition level); and 4) mice were required to show nose-poking levels comparable with baseline levels when cocaine was again made available after extinction. Thus, criteria were slightly more lenient than for acquisition of food-maintained behavior or baseline in food-trained mice, to allow for the possibility that the DATki mice might acquire cocaine self-administration at lower and/or less stable levels than wild-type mice. To prevent overdose, the total drug intake was limited to 30 mg/kg/session. Catheter patency was verified at the end of the acquisition phase and again after the extinction and “rebaseline” phases; animals in which catheter patency could not be demonstrated were removed from the data set. Complete acquisition data were collected from 11 DATki mice (6 female, 5 male), 14 heterozygous mice (5 female, 9 male), and 19 wild-type mice (6 female, 13 male). This relatively large number of animals was trained to allow for attrition in the subsequent drug self-administration studies.
Increased Response Requirement.
After training and testing under the FR 1 schedule, a subset of heterozygous and wild-type mice was randomly chosen for evaluation of cocaine self-administration behavior under increased response requirement. Each mouse was allowed to self-administer 1.0 mg/kg per infusion of cocaine under four different schedules of reinforcement: FR 1; FR 3; PR 1 schedule in which the starting ratio was 1, and was incremented by 1 after each reinforcer delivery; and a “PRlgt” schedule in which the starting ratio was 3, and was incremented following a logit function as described previously (ratios: 3, 9, 13, 16, 18, 20, 22, 24, etc.; Thomsen and Caine, 2005). The schedules were presented for one or two consecutive sessions, in the following sequence: FR 1, PR 1, PRlgt, FR 3. For the PR schedules, the breaking point was defined as the step value associated with the last completed ratio (i.e., number of reinforcers earned) after a 60-min limited hold (i.e., period with no reinforcer earned). If a breaking point had not been reached within 6 h, the session was terminated to avert health hazard. Data were collected from eight heterozygous mice (two female, six male) and seven wild-type mice (three female, four male).
Self-Administration of d-Amphetamine and SKF 82958.
After testing with cocaine, 0.1 mg/kg per infusion of d-amphetamine was substituted as the reinforcer until baseline criteria were met (same as for cocaine, see above), followed by extinction, baseline again, and finally determination of a dose-effect function in each mouse (0.001, 0.0032, 0.01, 0.032, and 0.1 mg/kg per infusion, presented according to a Latin square design), in the same manner as for cocaine. Finally, the dopamine D1 receptor agonist SKF 82958 was substituted as the reinforcer, 0.01 mg/kg per infusion, followed by extinction again. Amphetamine dose-effect data were collected from 7 DATki mice (2 female, 5 male), 8 heterozygous mice (3 female, 5 male), and 15 wild-type mice (6 female, 9 male). SKF 82958 data were collected from 5 male DATki mice and 11 wild-type mice (4 female, 7 male).
Drugs.
Cocaine hydrochloride was supplied by the National Institute on Drug Abuse (National Institutes of Health, Bethesda, MD), d-amphetamine sulfate was purchased from Sigma-Aldrich (St. Louis, MO), and (±)SKF 82958 hydrobromide was from Sigma/RBI (Natick, MA). All drugs were dissolved in 0.9% saline. All drug doses refer to the weights of the respective salts. Drug doses were selected based on previously published data (Caine et al., 1999, 2007) or pilot studies.
Data Analysis.
The amount of behavior during the acquisition phases (food or cocaine) was compared by use of a mixed-model ANOVA with genotype and sex as between-subjects variables, and with session and nose-poke hole (reinforced/nonreinforced) as within-subjects (repeated measures) variables. Number of sessions to acquisition of operant behaviors (food or cocaine) was compared by use of the log-rank test (survival statistics) with genotypes as groups (sexes combined), and with sexes as groups (genotypes combined). For cocaine, mice that failed to meet acquisition criteria within 10 sessions were assigned a censored 10-session value (there was no time restriction for food). For the baseline-extinction-rebaseline comparison, baseline and rebaseline were compared with extinction by use of Dunnett's multiple comparisons test in each genotype. For food concentration-effect functions and drug dose-effect functions, comparisons were made by use of a mixed-model ANOVA with genotype and sex as between-subjects variables and food concentration or drug dose as within-subjects (repeated measures) variables. For procedures under the FR 1 schedule of reinforcement, the dependent variable was the number of reinforcers earned per hour, and for the effect of increasing response requirement, the total number of reinforcers earned per session was used (i.e., the breaking point for the PR schedules). Significant effects were followed where appropriate by two-sided paired or unpaired t test. Significance level was set at p < 0.05. There were no significant effects or consistent trends for sex differences; therefore, all data are reported as sexes combined.
Results
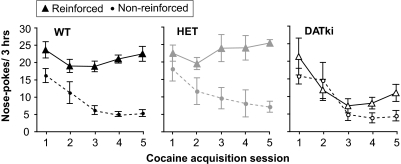
Without prior training or drug exposure, wild-type mice, heterozygous mice, and DATki mice were allowed to acquire intravenous self-administration of 1.0 mg/kg per infusion of cocaine by nose poking, under a FR 1 schedule of reinforcement. Nose-poking behavior is shown in Fig. 1 and was related to genotype (F2,38 = 8.78, p = 0.0007), hole (i.e., active versus inactive; F1,38 = 70.87, p < 0.0001), and training session (F4,152 = 15.47, p < 0.0001). All wild-type and heterozygous mice readily acquired cocaine self-administration, rapidly earning stable numbers of cocaine infusions per session, whereas nose poking in the inactive hole decreased to very low levels, as supported by a significant hole by session interaction (wild-type: F4,68 = 3.81, p = 0.008; heterozygous: F4,48 = 4.72, p = 0.003). In contrast, the DATki mice initially exhibited high levels of nose poking in both holes, but nose poking decreased over time in both the reinforced and inactive hole (no hole by session interaction). Extinction of nose-poking behavior when saline was substituted for cocaine, followed by re-establishment of cocaine self-administration, was then observed in the wild-type mice. Heterozygous mice generally behaved as the wild-type mice with the exception that extinction of nose poking could not be achieved in 4 of the 14 mice. None of the DATki mice showed a pattern of nose poking indicative of self-administration, as saline and cocaine maintained the same low levels of nose poking.
Fig. 1.
Acquisition of cocaine self-administration behavior in experimentally naive wild-type (WT), heterozygous (HET),and DATki mice. The abscissae present the consecutive training session numbers. The ordinates show the reinforced and nonreinforced nose pokes per 3-h session. All wild-type and heterozygous mice met acquisition criteria within seven sessions, whereas the DATki mice as a group failed to maintain nose poking selectively in the reinforced hole. Data are group means; bars represent one S.E.M.
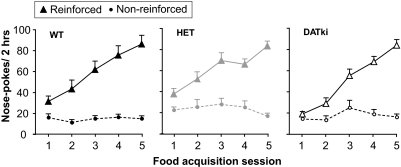
A different group of mice was allowed to perform the same operant procedure, but reinforced with a palatable liquid food instead of cocaine (Fig. 2). All wild-type mice, heterozygous mice, and DATki mice readily acquired nose poking, including extinction of the behavior when water was substituted, and earned comparable numbers of food reinforcers across genotypes. Nose-poking behavior was related to nose-poke hole (i.e., active versus inactive; F1,43 = 150.69, p < 0.0001) and training session (F4,172 = 55.72, p < 0.0001), with a significant hole by session interaction (F4,172 = 62.65, p < 0.0001), but no significant effect of genotype. The food-trained mice were then implanted with catheters to examine cocaine self-administration under conditions that facilitate acquisition. Even after prior operant training and demonstration of stable responding when reinforced with food, DATki mice did not self-administer cocaine (below).
Fig. 2.
Acquisition of food-maintained behavior in experimentally naive wild-type (WT), heterozygous (HET), and DATki mice. The abscissae present the consecutive training session numbers. The ordinates show the reinforced and nonreinforced nose pokes per 2-h session. Mice of all three genotypes acquired nose-poke behavior reinforced by food within five sessions, regardless of DAT genotype. Data are group means; bars represent one S.E.M.
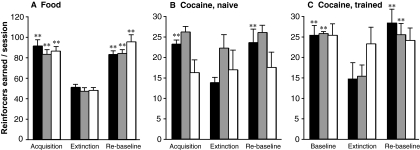
Figure 3 shows reinforcers earned when criteria were met for the acquisition/baseline phase, the extinction phase, and the re-establishment of behavior phase for the acquisition of food-maintained behavior or cocaine self-administration, and for food-trained mice in which cocaine was substituted as the reinforcer. For food-maintained behavior (Fig. 3A), mice of all three genotypes showed extinction and re-establishment of the behavior (“rebaseline”). In contrast for the cocaine acquisition (Fig. 3B), in agreement with the acquisition data (Fig. 1), wild-type mice showed extinction and rebaseline, whereas DATki mice showed consistent low levels of nose poking. Although most heterozygous mice (10 of 14) extinguished and re-established their behavior, a few showed persistent and very high levels of responding for at least 15 sessions of extinction, and cocaine self-administration did not reach significance relative to extinction in this group. For cocaine self-administration in the food-trained mice (Fig. 3C), both wild-type and heterozygous mice extinguished and then re-established self-administration, and nose poking was not related the cocaine availability in the DATki mice.
Fig. 3.
Response levels at criteria for acquisition (baseline), extinction, and rebaseline in wild-type (WT), heterozygous (HET), and DATki mice. The abscissae show the training phase, and the ordinates show the reinforcers earned per session. A, acquisition, extinction, and rebaseline of food-maintained behavior. B, acquisition, extinction, and rebaseline of cocaine self-administration in naive animals. C, initial baseline, extinction, and rebaseline of cocaine self-administration in food-trained animals. Group sizes are as indicated in Materials and Methods with the following exceptions: B, rebaseline data are missing in 7 DATki and 4 heterozygous mice; C, wild-type, n = 6; heterozygous, n = 9; DATki, n = 13. ∗∗, p < 0.01 versus the extinction condition, Dunnett's multiple comparison test. Data are group means; bars represent one S.E.M.
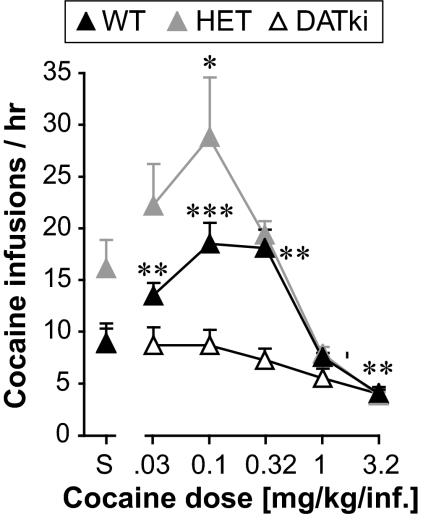
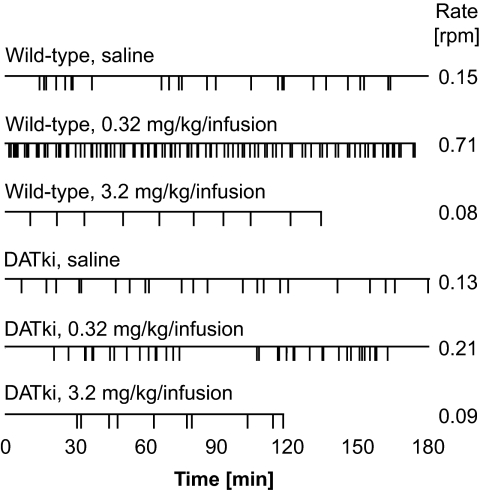
After baseline and extinction, with or without preceding food training, cocaine dose-effect functions were determined and are shown in Fig. 4. Mice from the food-trained and the naive acquisition groups yielded indistinguishable results, which were therefore pooled for presentation and analysis. Wild-type mice and heterozygous mice exhibited inverted U-shaped functions typical of cocaine self-administration under a FR 1 schedule. The DATki mice did not self-administer any dose of cocaine; instead, cocaine mildly suppressed response rates relative to saline in the DATki mice. ANOVA confirmed that nose-poking behavior was related to cocaine dose (F5,190 = 25.71, p < 0.0001) and genotype (F2,190 = 11.67, p = 0.0001), with a dose by genotype interaction (F10,190 = 4.75, p < 0.0001). Additional ANOVAs comparing each mutant genotype with wild-type showed an effect of both the homozygous DATki (F1,125 = 12.42, p = 0.002) and the heterozygous mutation (F1,130 = 5.26, p = 0.03). The DATki mice earned fewer infusions than wild-type mice at doses of 0.032, 0.1, and 0.32 mg/kg per infusion (p = 0.03 to p < 0.0001). The heterozygous mice showed an apparent upward shift of the ascending limb of the dose-effect function, but this included higher responding under the saline condition. This upward shift reached significance post hoc only for saline (p = 0.03), with a trend at the 0.032 mg/kg per infusion dose (p = 0.052). The cocaine effect was significant in all genotypes (p = 0.0004 or lower), with significantly suppressed rates at the highest cocaine dose in the DATki mice (3.2 mg/kg per infusion, p = 0.009 versus saline). In the wild-type mice, cocaine doses from 0.032 to 0.32 mg/kg per infusion maintained higher rates of nose poking compared with saline (p = 0.003 or lower), whereas 3.2 mg/kg per infusion engendered very low but stable response rates (p = 0.005 versus saline). In the heterozygous mice, only 0.10 mg/kg per infusion of cocaine maintained a higher rate than saline (p = 0.04), whereas 1.0 and 3.2 mg/kg per infusion engendered low, stable response rates (p = 0.009 and p = 0.0008 versus saline). High levels of responding in the heterozygous mice when saline was available, rather than low responding maintained by cocaine, seem to explain this difference between wild-type and heterozygous mice (Fig. 4). Figure 5 illustrates response patterns in a representative wild-type mouse and a DATki mouse, when saline, 0.32 mg/kg per infusion of cocaine (i.e., a peak dose), and the highest dose of cocaine were available. The wild-type mouse shows a typical irregular pattern of nose poking during the saline session, whereas 0.32 mg/kg per infusion of cocaine engendered a consistently high rate of responding and 3.2 mg/kg per infusion of cocaine engendered regularly spaced responses until the session was terminated. In contrast, the DATki mouse shows irregular patterns of nose poking in all sessions.
Fig. 4.
Cocaine dose-effect functions under a FR 1 schedule of reinforcement in wild-type (WT), heterozygous (HET), and DATki mice. Abscissa shows the unit dose of cocaine in saline (milligrams per kilograms per infusion); S designates saline. Ordinate shows the cocaine reinforcers earned per hour. Wild-type mice and heterozygous mice self-administered cocaine showing a typical inverted U-shaped dose-effect curve. The DATki mice never self-administered cocaine, but only showed suppression of behavior at high doses. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001 versus saline, two-sided paired-sample t test. Data are group means; bars represent one S.E.M.
Fig. 5.
Ester lines from saline and cocaine self-administration sessions under the FR 1 schedule of reinforcement in a wild-type mouse and a DATki mouse. Sessions lasted 3 h or until 100 reinforcers were earned, or, for 3.2 mg/kg per infusion of cocaine, until 10 reinforcers were earned, to prevent overdose. Cocaine maintained typical patterns of responding in the wild-type mice, but more erratic patterns in the DATki mice. Note also the high rate of responding maintained by a peak cocaine dose in the wild-type mouse (0.32 mg/kg per infusion).
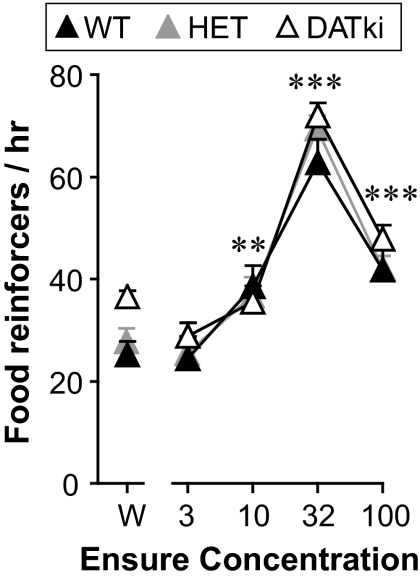
Food concentration-effect functions, determined under a FR 1 schedule of reinforcement, are shown in Fig. 6 . DATki mice exhibited food-maintained behavior comparable with the wild-type mice and heterozygous mice. Thus, nose-poking behavior was related to food concentration (F4,172 = 70.49, p < 0.0001), but not to genotype, and with no significant interaction. Food concentrations of 10 to 100% maintained nose poking at levels above water (p = 0.009 to p < 0.0001, genotypes combined).
Fig. 6.
Food concentration-effect functions under a FR 1 schedule of reinforcement in wild-type (WT), heterozygous (HET), and DATki mice. The abscissa shows the percentage of liquid food in water; W designates water. The ordinate shows the food reinforcers earned per hour. Food maintained comparable rates of nose poking in wild-type, heterozygous, and DATki mice across a range of liquid-food dilutions. ∗∗, p < 0.01; ∗∗∗, p < 0.001 versus water, two-sided paired-sample t test. Data are group means; bars represent one S.E.M.
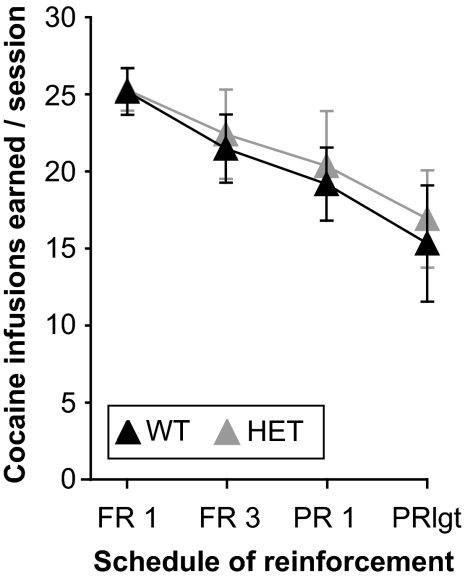
For further evaluation of the heterozygous mice, self-administration of 1.0 mg/kg per infusion of cocaine was assessed under conditions of increased response requirement. As shown in Fig. 7 , the number of cocaine infusions earned per session decreased moderately as response requirement increased (F3,39 = 9.60, p = 0.0001), but did not differ between wild-type and heterozygous mice (no effect of genotype or interaction). Data are combined from food-trained and naive acquisition mice.
Fig. 7.
Self-administration of 1.0 mg/kg per infusion of cocaine under increasing response requirement in wild-type (WT) and heterozygous (HET) mice. The abscissa shows the schedule of reinforcement. The ordinates show the total cocaine reinforcers earned during the session (i.e., for the PR schedules, breaking point). Increased response requirement moderately decreased the number of reinforcers earned regardless of genotype.
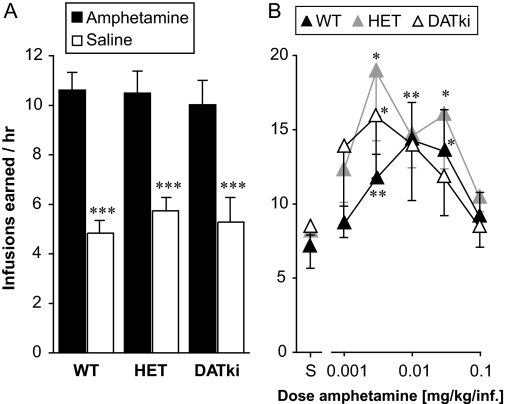
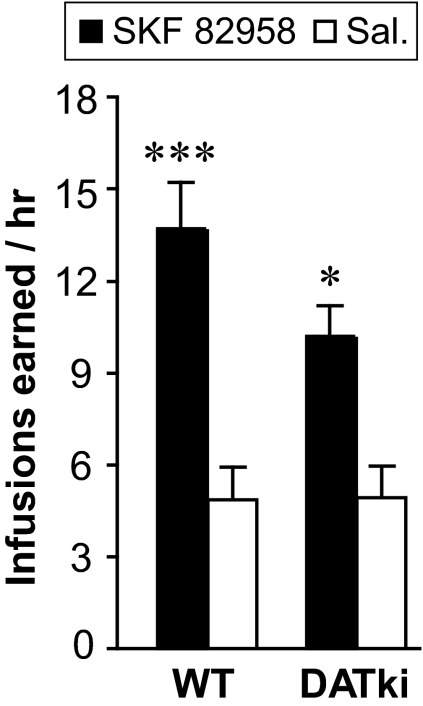
Substitution of d-amphetamine for cocaine as the reinforcer (0.1 mg/kg per infusion for baseline behavior, then saline to extinction, then 0.1 mg/kg per infusion again followed by a range of doses) was also assessed to determine whether DATki mice could show normal self-administration behavior reinforced by a DAT ligand other than cocaine. d-Amphetamine maintained robust and dose-dependent self-administration in the DATki mice, at levels comparable with the wild-type mice and heterozygous mice, as shown in Fig. 8. At baseline, numbers of reinforced nose pokes were related to amphetamine availability (F1,46 = 147.0, p < 0.0001), with no significant effect of genotype or interaction, and amphetamine maintained responding above saline levels in all three genotypes (Fig. 8A). Similarly for the dose-effect functions there was an effect of amphetamine dose (F5,130 = 7.92, p < 0.0001), but no effect of genotype or interaction (Fig. 8B). Amphetamine doses of 0.0032, 0.01, and 0.032 mg/kg per infusion reached significance relative to saline in the wild-type mice and the heterozygous mice (p = 0.02 to p = 0.004), and doses of 0032 and 0.01 mg/kg per infusion reached significance in the DATki mice (p = 0.02, p = 0.007). Finally, wild-type mice and DATki mice were allowed to self-administer a single dose of the direct dopamine D1 receptor agonist SKF 82958 (0.01 mg/kg per infusion) or saline, as shown in Fig. 9. SKF 82958 maintained nose poking above saline levels (F1,10 = 18.70, p = 0.0004) with no significant effect of genotype or interaction. For both amphetamine and SKF 82958, mice from food-trained and naive acquisition groups yielded indistinguishable results, which were therefore pooled for presentation and analysis.
Fig. 8.
Self-administration of intravenous amphetamine under a FR 1 schedule of reinforcement in wild-type (WT), heterozygous (HET), and DATki mice. The ordinates show the reinforcers earned per hour. A, baseline and extinction conditions in each genotype. B, d-amphetamine dose-effect functions. The abscissa shows the unit dose of d-amphetamine in saline (milligrams per kilograms per infusion), S designates saline. ∗p, < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001 versus saline, two-sided paired-sample t test. Data are group means; bars represent one S.E.M.
Fig. 9.
Self-administration of intravenous SKF 82958 under a FR 1 schedule of reinforcement in wild-type (WT) and DATki mice. The dopamine D1 agonist SKF 82958 maintained self-administration at higher rates than saline in both wild-type mice and DATki mice. ■, 0.01 mg/kg per infusion of SKF 82958; □, saline. ∗, p < 0.05; ∗∗∗, p < 0.001 versus saline, two-sided paired-sample t test. Data are group means, bars represent one S.E.M.
Discussion
We evaluated the reinforcing effect of cocaine in mice expressing a mutant cocaine-insensitive but functional DAT, under various conditions. We found that the reinforcing effects of cocaine were completely lacking in these DATki mice, despite normal operant responses to food, the DAT ligand amphetamine, and a direct dopamine agonist. These findings indicate three major conclusions. First, they provide strong evidence that blockade of the DAT is necessary for cocaine to produce reinforcing effects, at least in mice with a functional dopamine system. Second, our results illustrate the usefulness of knockin strategies that allow investigators to examine the effects of specific amino acid mutations as opposed to whole-protein deletions. Third, they show that it is possible to alter the DAT in such a way as to selectively target its interaction with cocaine, while sparing other behaviors dependent on DAT binding and dopamine systems.
Experimentally naive DATki mice failed to acquire cocaine self-administration behavior despite showing initial nose poking in the operant chamber, comparable with their wild-type littermates. DATki mice readily acquired the same operant procedure when reinforced by food instead of cocaine. Therefore, the failure of the DATki mice to acquire cocaine self-administration cannot easily be attributed to nonspecific impairments in the ability to acquire or perform the operant task, or to respond to positive reinforcers in general. When the food-trained mice were allowed to self-administer intravenous cocaine, the DATki mice again failed to self-administer cocaine, lacking sustained levels of nose poking over several sessions at higher rates than when saline was substituted. When various doses of cocaine were presented, wild-type mice showed inverted U-shaped dose-effect functions and patterns of responding typical of cocaine self-administration under a FR 1 schedule of reinforcement (e.g., a regular patterns of evenly spaced drug infusions at high doses of cocaine). In contrast, DATki mice did not self-administer cocaine above saline levels at any dose. Moreover, response patterns in the DATki mice were generally not indicative of self-administration, showing low rates of more or less erratic patterns of infusions, similar to patterns of responding observed in wild-type mice under saline substitution conditions. Thus, even under permissive conditions (prior operant training with food, wide range of cocaine doses), cocaine self-administration could not be established in the DATki mice. When tested with a range of liquid-food dilutions under the same schedule of reinforcement, DATki mice did not differ from their wild-type and heterozygous littermates in levels of responding, further suggesting that the mutation did not impair operant performance, but rather specifically eliminated the reinforcing effects of cocaine.
Although cocaine did not produce reinforcing effects in the DATki mice, it did significantly affect nose-poking behavior. Indeed, cocaine seemed to dose-dependently suppress responding, and did so significantly at the highest dose. Although similarly low rates were seen in wild-type and heterozygous mice at the highest cocaine dose, we speculate that different mechanisms may contribute to this effect between genotypes. In the absence of DAT blockade, cocaine presumably acted as a SERT/NET reuptake inhibitor in the DATki mice. Increased SERT blockade has been associated with decreased reinforcing value and/or a rate-decreasing effect on operant behavior in general (Peltier and Schenk, 1993; Roberts et al., 1999; Czoty et al., 2002; Wee et al., 2005). It was suggested that SERT blockade contributes to aversive effects of cocaine based on increased cocaine-conditioned place preferences in SERT knockout mice (Sora et al., 1998), although we found no difference in cocaine self-administration between SERT knockout and wild-type mice with use of a wide range of conditions (Thomsen et al., 2009). Conditioned taste aversion studies using relatively selective monoamine transporters in rats supported a critical involvement of NET blockade in cocaine's aversive effects (Freeman et al., 2005; Serafine and Riley, 2009). Thus, decreased poking in the DATki mice could reflect an unmasking of general inhibitory effects or aversive effects, probably associated with SERT and/or NET blockade. Both nonspecific behavioral inhibition and aversion are consistent with previously reported behavioral effects of cocaine in DATki mice: decreased locomotor activity, and induction of conditioned place aversion (Chen et al., 2006). Therefore, we speculate that this inhibitory effect of cocaine in the DATki mice makes it unlikely that the abolition of cocaine's reinforcing effects could be surmountable, had higher cocaine doses been made available.
In contrast to the homozygous DATki mutation, our findings do not support any appreciable effect of a heterozygous DATki mutation on cocaine's reinforcing effects. An apparent upward shift of the ascending portion of the cocaine dose-effect function under the FR 1 schedule of reinforcement might suggest a moderately increased reinforcing effect of cocaine. However, this upward shift did not reach significance post hoc, except when saline was available, suggesting that the high levels of nose poking were attributable to increased perseveration (resistance to extinction) in the heterozygous mice. A perseverative phenotype might be associated with a moderate hyperdopaminergic state induced by the DATki mutation, although dopamine levels were not measured in the heterozygous mice (Chen et al., 2006). Consistent with this hypothesis, enhanced resistance to extinction was reported in DAT−/− mice trained to nose poke for a food reinforcer, relative to wild-type mice (Hironaka et al., 2004). Furthermore, heterozygous mice and wild-type mice earned comparable numbers of cocaine reinforcers of a moderately high dose (1.0 mg/kg per infusion) over a range of schedules of reinforcement with increasing response requirements, consistent with the interpretation that the reinforcing effects of cocaine were unaltered in the heterozygous mice.
For an even more focused investigation of the specific nature of the deficit in cocaine's reinforcing effects than comparisons with food-maintained behavior, we tested whether DATki mice could acquire and maintain intravenous self-administration of a psychostimulant drug other than cocaine. To this end, we substituted d-amphetamine for cocaine as the reinforcer in mice of all three genotypes. Although the reinforcing effects of d-amphetamine are also believed to depend on the DAT, its binding to the DAT molecule differs from cocaine (Wayment et al., 1998; Chen et al., 2005). Indeed, d-amphetamine increased extracellular dopamine levels in the nucleus accumbens and induced locomotor hyperactivity and conditioned place preferences in the DATki mice (Chen et al., 2006). Consistent with those findings, d-amphetamine maintained robust and dose-dependent self-administration in the DATki mice, at levels comparable with wild-type mice. Furthermore, both DATki mice and wild-type mice self-administered the direct dopamine D1 receptor agonist SKF 82958, consistent with earlier findings in wild-type mice (Caine et al., 2007). Complementing the data for food reinforcers, these data with amphetamine and SKF 82958 clearly show that the DATki mice were fully capable of acquiring and maintaining self-administration under conditions identical to those used to test cocaine. In addition, the self-administration of amphetamine and SKF 82958 suggests that dopamine systems were functional and largely intact in the DATki mice, and is consistent with the amphetamine-conditioned place preference previously observed in DATki mice (Chen et al., 2006).
In conclusion, cocaine failed to serve as a positive reinforcer in DATki mice, whereas food, d-amphetamine, and a direct dopamine agonist reliably maintained operant behavior in these mice, at levels comparable with wild-type mice. These data show that removing cocaine's ability to block the DAT is sufficient to abolish its reinforcing effects in mice. This finding is in apparent contrast with a previous report that DAT−/− mice self-administered cocaine (Rocha et al., 1998). However, the DAT−/− mice have profound compensatory changes in dopamine homeostasis (e.g., delayed clearance of extracellular dopamine, reduced total dopamine, decreased dopamine receptor expression; Giros et al., 1996; Jones et al., 1998, 1999), whereas DATki mice show only moderate compensatory changes (Chen et al., 2006). In addition, we found that a separate line of DAT−/− mice generally did not self-administer cocaine despite robust responding when reinforced with food or a direct dopamine agonist (Thomsen et al., 2009). The fact that cocaine was observed to increase extracellular dopamine levels in the nucleus accumbens of the line of DAT−/− mice shown to self-administer cocaine, but not in the DATki mice (Carboni et al., 2001; Chen et al., 2006), also supports the crucial involvement of the nucleus accumbens in cocaine self-administration (Roberts et al., 1977, 1980; Pettit et al., 1984; Caine and Koob, 1994). This study provides strong evidence that DAT blockade is critical for cocaine's reinforcing effects.
This study also demonstrates the utility of mutagenesis combined with mouse gene knockin as a strategy to investigate in vivo the functions not only of proteins, but also of individual amino acids in a protein's structure. Specifically, our findings indicate that it is possible to target the DAT in such a way as to alter its interaction with cocaine, while sparing other components of DAT function. Much effort has gone into the search for a dopamine-sparing DAT ligand that might act as a “cocaine antagonist,” but this approach has been largely unsuccessful to date (Uhl and Lin, 2003; Gorelick et al., 2004). Though speculative, the mutant DAT in the present study, or a similar one, may point to a target region in the DAT for rational drug design by use of computer modeling of DAT structure and DAT-drug interactions, that may aid in the development of a “dopamine-sparing cocaine antagonist.”
Acknowledgments
We thank Joon Ying Boon, Jennifer Dohrmann and Dana Angood for technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R29-DA12142, R01-DA14644, R01-DA17323, T32-DA07232, R01-DA014610, R01-DA20124].
This work was presented in part at the “Dopamine 50 Years” symposium in Göteborg, Sweden, May 30 to June 2, 2007.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- ANOVA
- analysis of variance
- DAT
- dopamine transporter
- DATki
- dopamine transporter knock-in
- DAT−/−
- dopamine transporter knockout
- FR
- fixed ratio
- PR
- progressive ratio
- SERT
- serotonin transporter
- NET
- norepinephrine transporter
- SKF 82958
- (±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine.
References
- Bergman J, Madras BK, Johnson SE, Spealman RD. ( 1989) Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther 251: 150–155 [PubMed] [Google Scholar]
- Caine SB, Koob GF. ( 1993) Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 260: 1814–1816 [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. ( 1994) Effects of mesolimbic dopamine depletion on responding maintained by cocaine and food. J Exp Anal Behav 61: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. ( 1999) Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology (Berl) 147: 22–24 [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. ( 2007) Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci 28: 13140–13150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. ( 2001) Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci 21 (RC141): 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Han DD, Gu HH. ( 2005) A triple mutation in the second transmembrane domain of mouse dopamine transporter markedly decreases sensitivity to cocaine and methylphenidate. J Neurochem 94: 352–359 [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, et al. ( 2006) Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A 103: 9333–9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. ( 2002) Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 300: 831–837 [DOI] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Peltier RL, Depoortere RY, Pickering CL, Hooper ML, Gong YH, Lane JD. ( 1993) Tolerance to self-administration of cocaine in rats: time course and dose-response determination using a multi-dose method. Drug Alcohol Depend 32: 247–256 [DOI] [PubMed] [Google Scholar]
- Freeman KB, Rice KC, Riley AL. ( 2005) Assessment of monoamine transporter inhibition in the mediation of cocaine-induced conditioned taste aversion. Pharmacol Biochem Behav 82: 583–589 [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. ( 1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379: 606–612 [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. ( 2004) Agents in development for the management of cocaine abuse. Drugs 64: 1547–1573 [DOI] [PubMed] [Google Scholar]
- Hironaka N, Ikeda K, Sora I, Uhl GR, Niki H. ( 2004) Food-reinforced operant behavior in dopamine transporter knockout mice: enhanced resistance to extinction. Ann N Y Acad Sci 1025: 140–145 [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. ( 1995) Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther 275: 1551–1559 [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. ( 1998) Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A 95: 4029–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. ( 1999) Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci 2: 649–655 [DOI] [PubMed] [Google Scholar]
- Peltier R, Schenk S. ( 1993) Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacology (Berl) 110: 390–394 [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. ( 1984) Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 84: 167–173 [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. ( 1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237: 1219–1223 [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. ( 1977) On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav 6: 615–620 [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. ( 1980) Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav 12: 781–787 [DOI] [PubMed] [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H. ( 1999) Self-administration of cocaine analogs by rats. Psychopharmacology (Berl) 144: 389–397 [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. ( 1998) Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci 1: 132–137 [DOI] [PubMed] [Google Scholar]
- Serafine KM, Riley AL. ( 2009) Possible role of norepinephrine in cocaine-induced conditioned taste aversions. Pharmacol Biochem Behav 92: 111–116 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. ( 2005) Novel approaches to the treatment of cocaine addiction. CNS Drugs 19: 13–25 [DOI] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. ( 1998) Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A 95: 7699–7704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. ( 2005) Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci Chapter 9: Unit 9.20 [DOI] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wörtwein G, Fink-Jensen A, Wess J, Caine SB. ( 2005) Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci 25: 8141–8149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. ( 2009) Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci 29: 1087–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, Gu HH. ( 2008) Dopamine transporter inhibition is required for cocaine-induced stereotypy. Neuroreport 19: 1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, O'Neill B, Han DD, Gu HH. ( 2009) Cocaine does not produce reward in absence of dopamine transporter inhibition. Neuroreport 20: 9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Lin Z. ( 2003) The top 20 dopamine transporter mutants: structure-function relationships and cocaine actions. Eur J Pharmacol 479: 71–82 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, et al. ( 1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386: 827–830 [DOI] [PubMed] [Google Scholar]
- Wayment H, Meiergerd SM, Schenk JO. ( 1998) Relationships between the catechol substrate binding site and amphetamine, cocaine, and mazindol binding sites in a kinetic model of the striatal transporter of dopamine in vitro. J Neurochem 70: 1941–1949 [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. ( 2005) Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther 313: 848–854 [DOI] [PubMed] [Google Scholar]
- Wise RA, Murray A, Bozarth MA. ( 1990) Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology (Berl) 100: 355–360 [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. ( 1984) Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther 230: 678–683 [PubMed] [Google Scholar]