Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) evokes tachycardia followed by a larger cholinergic bradycardia in isolated guinea pig hearts. We used the selective PAC1 receptor agonist maxadilan and vasoactive intestinal polypeptide (VIP) to test the hypothesis that PACAP27-evoked tachycardia and bradycardia are mediated by VPAC and PAC1 receptors, respectively. Chronotropic actions of these peptides were evaluated in isolated perfused hearts. Direct neuronal actions were determined by intracellular voltage recordings from cholinergic neurons in atrial ganglion whole mounts. Administration of 1 nmol of PACAP27 to isolated hearts evoked typical biphasic rate responses, whereas 1 nmol of maxadilan caused only a minor rate decrease. Desensitization with VIP eliminated the positive chronotropic effect of PACAP27 selectively. Local application of PACAP27 to cardiac neurons frequently evoked slow depolarization and caused prolonged increase of neuronal excitability. Maxadilan rarely affected membrane potential but consistently increased excitability. VIP had no effect on excitability and evoked depolarization in only a few neurons. Because maxadilan increased neuronal excitability but did not trigger action potentials as PACAP often does, we evaluated the interaction of maxadilan with substance P (SP) in isolated hearts. SP depolarizes cardiac neurons more consistently than PACAP, often triggers neuronal action potentials, and causes bradycardia but does not increase neuronal excitability. Maxadilan had a persistent effect to augment negative chronotropic responses to SP. These findings support our hypothesis that PACAP evokes tachycardia and bradycardia through VPAC and PAC1 receptors, respectively. They also suggest that maxadilan and PACAP27 differ in activating PAC1 receptors on cardiac neurons and/or stimulating downstream signaling mechanisms.
Pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal polypeptide (VIP) are neuropeptides that belong to the same peptide family, have a wide distribution in the autonomic nervous system, and exert prominent pharmacological effects on cardiovascular function (Vaudry et al., 2000; Laburthe and Couvineau, 2002). Endogenous PACAP occurs as a full-length peptide composed of 38 amino acids (PACAP38) and as a truncated peptide with 27 amino acids (PACAP27). Both forms of PACAP have substantial sequence homology with VIP, and as a consequence, there is some overlap of the pharmacological profiles of these neuropeptides. Shared pharmacological actions of PACAP and VIP can be attributed, in large part, to both peptides having comparable high affinities for the VPAC1 and VPAC2 subtypes of the VIP receptor. In contrast, PACAP receptors (i.e., PAC1) have a very low affinity for VIP but exhibit a high affinity for PACAP27 and PACAP38. Structural analogs of VIP have been developed as selective agonists for VPAC1 and VPAC2 receptors, whereas the only selective PAC1 receptor agonist is a peptide named maxadilan, which was isolated from the sand fly and has no sequence homology with PACAP (Vaudry et al., 2000; Laburthe and Couvineau, 2002; Lerner et al., 2007).
Previous experiments with isolated heart preparations from dog and guinea pig have shown that PACAP produces biphasic changes in heart rate, whereas VIP causes only tachycardia (Hoover, 1989; Rigel and Lathrop, 1991; Seebeck et al., 1996; Hirose et al., 1997; Pintér et al., 1998; Chang et al., 2005). The negative chronotropic responses to PACAP were blocked by atropine in both species, suggesting that they occurred through stimulation of intrinsic cholinergic neurons. This conclusion is supported by electrophysiological evidence that PACAP causes slow depolarization of most cholinergic neurons in whole mount preparations of guinea pig atrial ganglia and often triggers the generation of action potentials by these cells (Braas et al., 1998; Tompkins et al., 2007). PACAP-evoked bradycardia in guinea pigs is probably mediated by the PAC1 receptor, because specific PAC1 immunoreactivity has been localized to the plasma membrane of dissociated intrinsic cardiac neurons (Braas et al., 1998). Furthermore, analysis of mRNA from atrial ganglion preparations confirmed the presence of PAC1 transcripts (Braas et al., 1998). Positive chronotropic responses to PACAP are probably mediated at least in part by VPAC receptors because VIP has positive chronotropic and inotropic actions that are mediated by atrial receptors. Nevertheless, expression of PAC1 receptors has been detected in human, rat, and mouse heart tissue (Baron et al., 2001; Saetrum et al., 2001; Ushiyama et al., 2006), so it is possible that these receptors might also have a role in PACAP-evoked tachycardia.
The selective PAC1 agonist maxadilan and VIP were used in the present study to clarify the roles of PAC1 and VPAC receptors in mediation of cardiac chronotropic responses to PACAP27 in the guinea pig. We hypothesized that maxadilan would 1) replicate the negative chronotropic action of PACAP27 in isolated hearts by stimulating PAC1 receptors on cholinergic neurons and 2) not replicate PACAP-evoked tachycardia if that response is mediated by VPAC receptors. Although the second prediction was true, maxadilan evoked only a minor bradycardia in isolated hearts. However, infusion of maxadilan attenuated PACAP-evoked bradycardia without affecting the positive chronotropic response to PACAP. Tachycardic responses to PACAP were selectively abolished by desensitization with VIP, thereby implicating VPAC receptors in that response. Direct evaluation of neuronal responses showed that maxadilan and PACAP27 increased the excitability of intrinsic cardiac neurons but VIP did not. PACAP27 also caused slow depolarization of many intrinsic cardiac neurons and often triggered action potentials, whereas maxadilan rarely caused slow depolarization. Collectively, these findings suggest that maxadilan and PACAP27 are both agonists at PAC1 receptors on intrinsic cardiac neurons but exhibit response-dependent divergence in efficacy.
Materials and Methods
Animals.
Experiments were performed in vitro using isolated perfused hearts and atrial ganglion whole mount preparations from Hartley guinea pigs (either sex; 250–400 g). Animals were pretreated with 1000 units of heparin (isolated heart studies only) and euthanized by exsanguination while deeply anesthetized with isoflurane. Hearts were removed quickly and washed in ice-cold Krebs-Ringer buffer (gassed with 95% O2, 5% CO2, pH 7.35–7.4). This was followed by either 1) cannulation of the ascending aorta for functional studies of isolated perfused hearts or 2) dissection of the atrial ganglion whole mount preparation for electrophysiological studies of cardiac cholinergic neurons. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Isolated Heart Procedure.
Hearts were perfused at 8 ml/min with a modified Krebs-Ringer buffer (37°C) as described previously (Hoover, 1990; Chang et al., 2005). Cardiac contractions were measured by attaching one end of a silk suture to the apex of the heart and the other end to an isometric force transducer. Perfusion pressure was monitored proximal to the aortic cannula. Data were recorded using a PowerLab 8SP (ADInstruments, Colorado Springs, CO) and a PowerBook computer (Apple Computer, Cupertino, CA) running Chart software (ADInstruments). A ratemeter was used to determine heart rate from the ventricular contraction signal. Drugs or peptides were given by bolus injection (100 μl over 5-s interval) or infusion (100 μl/min) at a site proximal to the aortic cannula. Peptides were diluted with saline containing 0.1% bovine serum albumin. Acetylcholine (ACh) and bethanechol were dissolved in saline. Infusions were performed using a syringe infusion pump (Harvard Apparatus Inc., Holliston, MA). The concentration of infused maxadilan was calculated to achieve a final value of 100 nM at the heart based on infusion at 100 μl/min and constant perfusion at 8 ml/min.
Electrophysiology.
Experiments were performed on atrial whole mount preparations containing the intrinsic cardiac ganglia as described previously (Braas et al., 1998; Tompkins et al., 2006, 2007). Atrial whole mount preparations were dissected from the heart in ice-cold, standard Krebs’ solution (121 mM NaCl, 5.9 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 1.2 mM NaH2PO4, and 8 mM glucose), with the pH maintained at 7.4 by aeration with 95% O2, 5% CO2.
For intracellular recordings, the whole mount preparations were pinned in a 2.5-ml Sylgard-lined chamber and superfused continuously (2–3 ml/min) with a modified Krebs’ solution containing 10 mM HEPES buffer at 30–32°C (Hardwick et al., 1995; Braas et al., 1998; Tompkins et al., 2006, 2007). Cardiac ganglia were visualized with an inverted microscope equipped with Hoffman optics (Modulation Optics Inc., Greenvale, NY), and individual cardiac neurons were impaled using high-impedance borosilicate microelectrodes (2 M KCl-filled; 60–100 MΩ). Membrane potential and action potentials were recorded from the impaled neurons using an Axoclamp-2A amplifier coupled with a Digidata 1322A data acquisition system and pCLAMP 8 (Axon Instruments, Foster City, CA). If required, hyperpolarizing current was injected through the recording electrode to electrotonically maintain the resting membrane potential between −55 and −65 mV. This ensured that action potential generation was tested at the same potential before and after peptide application (Tompkins et al., 2006, 2007). For brief local application, PACAP27, maxadilan, or VIP (each at 50 μM) was applied by pressure application (Picospritzer; General Valve, Fairfield, NJ) through ∼5-μm-diameter “puffer” pipettes positioned 50 to 100 μm from the neuron. To test for peptide-induced changes in neuronal excitability, 1-s depolarizing current steps (0.1–0.4 nA) were given before and 2 to 3 min after peptide application.
Drugs and Peptides.
ACh chloride and bethanechol were from Sigma-Aldrich (St. Louis, MO). PACAP27, guinea pig VIP, and substance P (SP) were from Bachem Biosciences (King of Prussia, PA). Recombinant maxadilan was provided by Dr. Ethan Lerner (Department of Dermatology, Massachusetts General Hospital, Boston, MA) (Lerner and Shoemaker, 1992).
Statistical Analysis.
Statistical comparisons and graphing of data were accomplished using Prism, version 4.01 (GraphPad Software Inc., San Diego, CA). Group data are presented as the arithmetic mean ± S.E.M. Statistical comparisons were made using either a paired or unpaired t test or one-way ANOVA (with or without repeated measures). The Newman-Keuls procedure was used for post hoc comparisons after ANOVA. A probability level of 0.05 or smaller was used to indicate statistical significance.
Results
Maxadilan and PACAP27 Have Distinct Effects on Heart Rate.
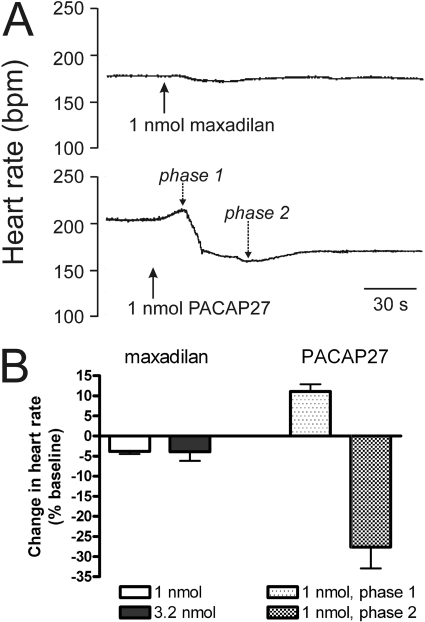
Bolus administration of maxadilan to isolated hearts produced only minor decreases in heart rate at doses of 1 and 3.2 nmol (Fig. 1). In marked contrast, administration of 1 nmol of PACAP27 to separate hearts caused tachycardia initially, and this was followed by a larger, more prolonged bradycardia (Fig. 1), as reported previously (Chang et al., 2005).
Fig. 1.
Chronotropic responses of isolated guinea pig heart to maxadilan and PACAP27. A, recorder tracings showing changes in heart rate evoked by bolus injections of 1 nmol of maxadilan and 1 nmol of PACAP27 in separate hearts. Maxadilan caused only a minor decrease in rate, but PACAP27 produced a biphasic response (phase 1, tachycardia; phase 2, bradycardia). B, quantification of chronotropic responses. Values are means ± S.E.M. (1 nmol of maxadilan, n = 10; 3.2 nmol of maxadilan, n = 3; and 1 nmol of PACAP27, n = 9). Responses to each dose of maxadilan and PACAP27 were determined in separate hearts.
Effect of Maxadilan on Chronotropic Responses to PACAP27.
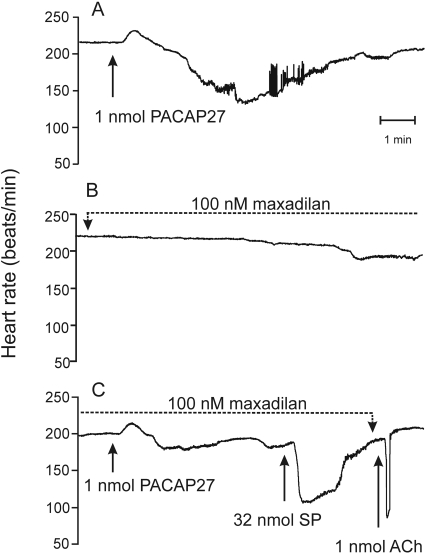
Maxadilan infusion was performed with two isolated hearts to achieve a final concentration of 100 nM in the tissue. Maxadilan had no effect on baseline heart rate in one preparation and decreased the rate by approximately 10% in the other preparation. The negative chronotropic response to 1 nmol of PACAP27 was attenuated (Fig. 2) or blocked when evaluated 15 min after commencing the maxadilan infusion, but the positive chronotropic response was preserved. Maxadilan affected the negative chronotropic response to PACAP27 selectively because bolus administration of 32 nmol of SP or 1 nmol of ACh still caused a prominent bradycardia (Fig. 2). Negative chronotropic responses to SP occur through stimulation of tachykinin receptors on intrinsic cardiac neurons (Hardwick et al., 1997), whereas ACh-evoked bradycardia is mediated by postjunctional muscarinic receptors. Some recovery of the negative chronotropic response to PACAP occurred in both cases when evaluated at >30 min after stopping the maxadilan infusion. Similar infusion of PACAP27 (100 nM peptide at the heart) caused a biphasic heart rate change analogous to that evoked by bolus administration of 1 nmol of PACAP27, but heart rate returned to baseline in spite of continued infusion. Bolus injection of 1 nmol of PACAP27 had no effect on heart rate under this condition, suggesting that desensitization had occurred.
Fig. 2.
Infusion of maxadilan attenuates the negative chronotropic response to PACAP27 without affecting the positive chronotropic response. A, control heart rate response to bolus injection of 1 nmol of PACAP27. B, chronotropic response to maxadilan infusion (final concentration, 100 nM) started 15 min after PACAP27 administration. C, chronotropic responses to a second bolus of 1 nmol of PACAP27 given after 15 min of maxadilan infusion. The positive chronotropic response to PACAP27 was preserved, but its negative chronotropic effect was attenuated. Negative chronotropic responses to SP and ACh were preserved. Data are representative of two experiments.
Exposure to VIP Prevents the Tachycardic Response to PACAP27.
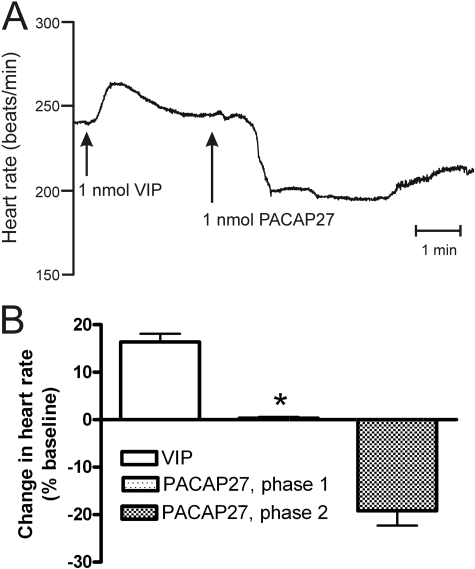
Bolus injection of 1 nmol of guinea pig VIP caused a positive chronotropic response in isolated guinea pig hearts as reported previously (Hoover, 1989). Desensitization was evident when another bolus of VIP was given immediately after recovery from the first exposure to VIP. Under this condition, injection of 1 nmol of PACAP27 caused bradycardia only instead of the normal biphasic heart rate response (Fig. 3).
Fig. 3.
Desensitization with VIP eliminates the positive chronotropic (phase 1) response to PACAP27. A, bolus injection of 1 nmol of VIP causes tachycardia that is accompanied by desensitization in the isolated perfused guinea pig heart. Under this condition, bolus injection of PACAP27 causes bradycardia only instead of the typical biphasic change in heart rate. B, summary data showing the loss of phase 1 response to PACAP27 after desensitization with VIP. ∗, significantly different from control response to PACAP27 shown in Fig. 1 (P = 0.003; n = 4 for VIP desensitization group).
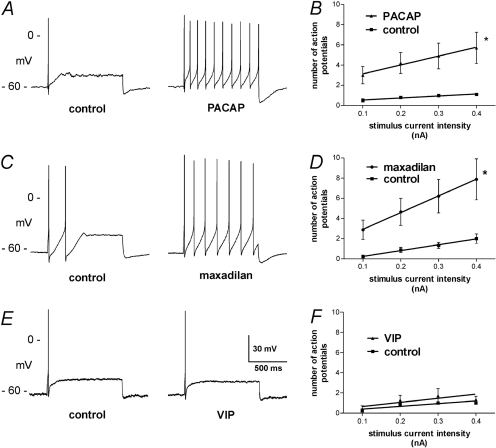
PACAP27 Evoked a Higher Incidence and Magnitude of Membrane Depolarization.
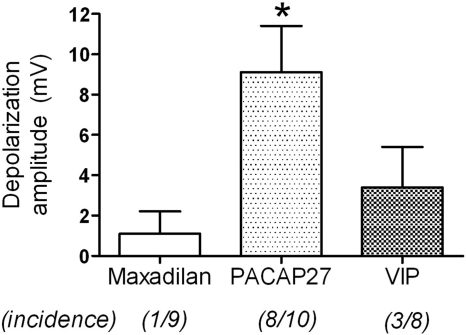
To evaluate directly the effects of maxadilan on intrinsic cardiac neurons, intracellular recordings were obtained from these cells in whole mount preparations of guinea pig intrinsic cardiac ganglia. Pressure application of PACAP27 to intrinsic cardiac neurons usually caused slow depolarization, and this effect was sometimes accompanied by the generation of action potentials as reported previously (Tompkins et al., 2007). In marked contrast, application of maxadilan at the same concentration caused slow depolarization of only one neuron of nine evaluated, and none of these cells fired action potentials in response to maxadilan. Pressure application of VIP likewise caused slow depolarization of only a few intrinsic cardiac neurons and never evoked action potentials. The average magnitude of depolarization was significantly higher for PACAP27, and average values for maxadilan and VIP did not differ significantly (Fig. 4).
Fig. 4.
PACAP27 causes slow depolarization of most intrinsic cardiac neurons. Maxadilan, PACAP27, or VIP was delivered by local puffer application to individual intrinsic cardiac neurons in atrial whole mount preparations while recording membrane potential (Vm). PACAP27 caused membrane depolarization in a majority of neurons, whereas maxadilan and VIP rarely affected Vm. ∗, significantly different from responses to maxadilan and VIP (ANOVA: F2,26 = 5.03, P < 0.02).
Maxadilan and PACAP27 Increase the Excitability of Intrinsic Cardiac Neurons.
Excitability of the predominant phasic neurons was evaluated before and after application of maxadilan, PACAP27, or VIP by pressure ejection from a micropipette (each at 50 μM). Changes in excitability were evaluated after recovery from the initial membrane depolarization evoked by some peptide applications. Maxadilan and PACAP27 both increased the excitability of intrinsic cardiac neurons, as evidenced by an increase in the number of action potentials evoked during 1-s depolarizing current pulses (Fig. 5, A–D). This contrasts with VIP, which had no effect on neuronal excitability (Fig. 5, E and F).
Fig. 5.
PACAP27 and maxadilan increase neuronal excitability. Most intrinsic cardiac neurons fire only one or two action potentials during the injection of depolarizing current. Treatment with PACAP27 or maxadilan increased the number of action potentials evoked by depolarizing current, but treatment with VIP did not. Best-fit linear regression lines were calculated and compared between groups using Prism. Asterisks denote significant difference between lines. Values are the mean ± S.E.M. (PACAP, n = 10; maxadilan, n = 9; and VIP, n = 7).
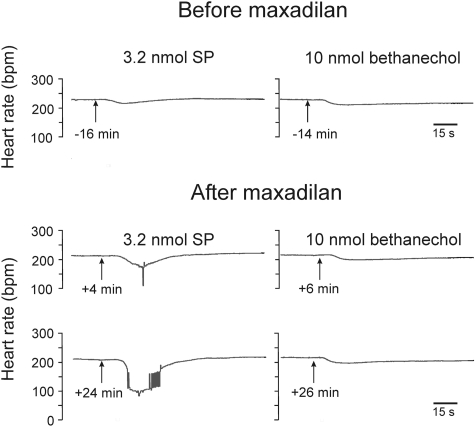
Maxadilan Augments the Negative Chronotropic Response to SP.
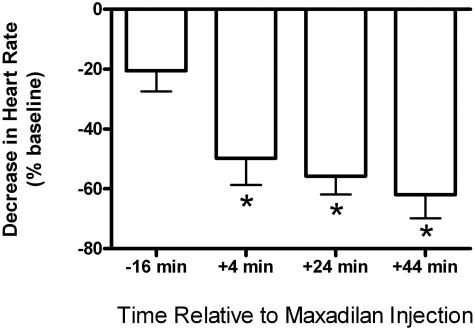
Because phasic exposure to maxadilan increased the excitability of intrinsic cardiac neurons, we hypothesized that treatment with this PAC1 agonist would augment the bradycardia that SP evokes in isolated guinea pig hearts. Previous work established that SP causes concentration-dependent depolarization of intrinsic cardiac neurons in atrial ganglion whole mount preparations (Hardwick et al., 1997), and this effect triggers action potentials if the threshold potential is reached (Hardwick et al., 1995; Zhang et al., 2001). Thus, SP causes bradycardia in isolated guinea pig hearts by stimulating cholinergic neurons of the intrinsic cardiac ganglia (Hoover et al., 2000). We selected a submaximal dose of 3.2 nmol of SP for testing our hypothesis and repeated this dose at 20-min intervals to avoid tachykinin receptor desensitization (Hoover, 1990). Consistent with our previous work, 3.2 nmol of SP caused a small negative chronotropic response when given before exposure to maxadilan (Figs. 6 and 7). This effect of SP was enhanced significantly when the same dose was repeated 4 min after bolus injection of 1 nmol of maxadilan (Figs. 6 and 7). Potentiation of the negative chronotropic response to SP persisted for a surprisingly long duration after the administration of a single bolus dose of maxadilan. Figure 7 shows that the augmenting effect of maxadilan was still strong after 44 min, and we found that it persisted for well over an hour. In the same experiments, maxadilan also decreased perfusion pressure by 39 ± 5% (baseline, 51 ± 2 mm Hg; n = 4), indicating a relaxant effect on coronary resistance vessels. The vasodilator response to bolus injection of maxadilan was likewise quite prolonged, with perfusion pressure still being reduced by 32 ± 5% after an hour (n = 3).
Fig. 6.
Recorder tracings showing that maxadilan augments the negative chronotropic response to SP in isolated guinea pig hearts. Bolus injections of 3.2 nmol of SP were given every 20 min beginning 16 min before a single injection of 1 nmol of maxadilan. Bolus injections of 10 nmol of bethanechol were given 2 min after SP. Maxadilan had no effect on postjunctional sensitivity to the muscarinic agonist bethanechol.
Fig. 7.
A single bolus injection of 1 nmol of maxadilan caused prolonged potentiation of the negative chronotropic response to 3.2 nmol of SP. SP and maxadilan were given as described in the previous legend. ∗, significantly different from control response at −16 min (repeated measures ANOVA: F3,18 = 28.8, P < 0.0001; n = 4).
Discussion
Results of this study improve our understanding of the mechanisms that mediate chronotropic responses to PACAP27 in the isolated guinea pig heart and reveal unique differences in PAC1 receptor-mediated responses evoked by PACAP27 and the selective PAC1 agonist maxadilan. First, our findings show that positive chronotropic responses to PACAP27 are mediated by VPAC receptors. This conclusion is supported by the lack of tachycardic response to maxadilan and the continued ability of PACAP to evoke tachycardia during infusion of maxadilan. Furthermore, desensitization of VPAC receptors with VIP eliminated the positive chronotropic component of the biphasic heart rate response to PACAP27, leaving only the negative chronotropic effect. Collectively, this evidence supports the conclusion that PAC1 receptors mediate the negative chronotropic response to PACAP27. Further support for the latter point comes from the observation that the magnitude of PACAP-evoked bradycardia was reduced during infusion of maxadilan. However, in marked contrast to PACAP27, bolus injections of maxadilan had negligible effect on heart rate. This discrepancy can be explained by differences in the response pattern of intrinsic cardiac neurons to these peptides. Although PACAP27 and maxadilan both increased the excitability of intrinsic cardiac neurons, only PACAP27 also consistently caused membrane depolarization. Further studies with isolated heart preparations showed that maxadilan has a synergistic interaction with SP to augment the negative chronotropic response that this tachykinin peptide causes through depolarization of cardiac cholinergic neurons (Hardwick et al., 1995, 1997; Zhang et al., 2001). This augmenting effect of maxadilan persisted for a surprisingly long duration.
Maxadilan is a unique natural peptide that is present in the salivary gland of the sand fly Lutzomyia longipalpis, and transfer of the peptide to humans during bloodfeeding causes a “persistent vasodilator” response of the skin (Moro and Lerner, 1997; Lerner et al., 2007). Although maxadilan has no sequence homology with PACAP, it binds selectively to mammalian PAC1 receptors and functions as a potent PAC1 agonist in many systems. In fact, maxadilan is regarded as the only selective PAC1 agonist available at present. Accordingly, we were quite surprised to find that bolus injections of maxadilan did not cause a prominent cholinergic neuron-mediated bradycardia in the isolated guinea pig heart. Previous studies established that PAC1 receptors were localized to cholinergic neurons in atrial ganglia of the guinea pig and that local application of PACAP to these neurons caused slow depolarization and increased excitability in almost 90% of the neurons studied (Braas et al., 1998). Approximately a third of the intrinsic cardiac neurons had a burst of action potentials during the slow depolarization. The latter effect might be attributed to the combined effects of slow depolarization and increased excitability, and such action potentials probably underlie the prominent cholinergic bradycardia that PACAP27 causes in isolated guinea pig hearts (Chang et al., 2005). In the present study, we found that maxadilan increased the excitability of all intrinsic cardiac neurons studied but rarely caused slow depolarization. Thus, the agonist action of maxadilan is more restricted compared with that of PACAP27 at PAC1 receptors on guinea pig cardiac neurons.
The dual effects of PACAP27 to cause slow depolarization and increased excitability of intrinsic cardiac neurons cannot be attributed to the stimulation of both PAC1 and VPAC receptors because VIP had no influence on neuronal excitability, and, like the PAC1 agonist maxadilan, showed little effect on membrane potential. Previous work established that guinea pig cardiac neurons express predominantly one isoform of the PAC1 receptor (Braas et al., 1998), so it is unlikely that dual effects of PACAP27 are mediated by different PAC1 receptors. As an alternative explanation for the broader agonist actions of PACAP27 compared with maxadilan, it is possible that PACAP27 might activate more PAC1 receptor-coupled signaling mechanisms. This scenario is supported by recent work, which demonstrated that different isoforms of the PAC1 receptor can vary in their affinity for different agonists and in ligand-mediated signaling (Ushiyama et al., 2007). Many isoforms of the PAC1 receptor occur due to alternate splicing in the first extracellular domain and the third intracellular loop. In this regard, the N/HOP1 variant is noteworthy because it exhibited a phenomenon analogous to our observation for PAC1 receptors on guinea pig intrinsic cardiac neurons. PACAP38 and maxadilan had similar high potencies for stimulation of cAMP production in Chinese hamster ovary cells expressing the N/HOP1 isoform but only PACAP38 caused an elevation of [Ca2+]i. These findings with transfected Chinese hamster ovary cells, and our results with intrinsic cardiac neurons expressing native PAC1 isoforms suggest that different agonists acting through the same receptor can evoke different cellular responses.
Although maxadilan failed to cause a prominent bradycardia in isolated guinea pig hearts, infusion of this peptide (100 nM at tissue) suppressed negative chronotropic responses to PACAP27. Nonspecific inhibition of neuronal or cardiac nodal responsiveness are unlikely causes because maxadilan did not suppress the cholinergic bradycardia due to stimulation of intrinsic cardiac neurons by SP or direct stimulation of postjunctional muscarinic receptors by ACh or bethanechol. Although our results do not establish the underlying mechanism, it is likely that continued exposure to maxadilan either occluded or desensitized the PAC1 receptors so that PACAP was less effective.
The interaction of maxadilan and SP in affecting heart rate was evaluated in this study because the combined effects of these peptides on intrinsic cardiac neurons (i.e., increased excitability and slow depolarization, respectively) seemed equivalent to the PAC1 receptor-mediated effects of PACAP27 alone. In their case, maxadilan causes increased excitability by stimulation of PAC1 receptors and SP causes slow depolarization and bradycardia by stimulating neurokinin receptors (Hardwick et al., 1997; Chang et al., 2000). This prediction was correct because maxadilan treatment substantially increased the negative chronotropic response of isolated hearts to a submaximal dose of SP. Exposure to maxadilan had no effect on the negative chronotropic response to bethanechol, which acts by stimulating cardiac muscarinic receptors. The prolonged duration of the synergistic effect of maxadilan was unexpected because only a single bolus injection was administered to hearts, and the buffer was not recirculated. Accordingly, PAC1 receptors in the heart would be exposed to a single wave of maxadilan, and continued perfusion with buffer should wash out any unbound peptide. It is noteworthy that the prolonged action of maxadilan was not restricted to intrinsic cardiac neurons because a single bolus of maxadilan also caused a persistent lowering of perfusion pressure, indicating vasodilation of coronary resistance vessels. Both of these actions of maxadilan remained at or near peak effect for the duration of the experiments. These findings clearly demonstrate that maxadilan has long-acting effects on the intrinsic cardiac neurons and coronary vessels, but additional work is required to elucidate the underlying mechanism.
The unique pharmacological actions of maxadilan might be beneficial in the context of cardiovascular disease, where impaired vagal control of heart rate is a recognized risk factor for increased morbidity and mortality. In this context, maxadilan or a maxadilan-like drug might improve vagal control of heart rate by augmenting the response of intrinsic cardiac neurons to preganglionic vagal input without affecting resting heart rate. At the same time, prolonged relaxation of coronary resistance vessels by maxadilan might be of further benefit to patients with ischemic heart disease.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL54633, HL65481] (to D.B.H. and R.L.P., respectively); the National Institutes of Health National Center for Research Resources [Grant P20-RR16435] (to R.L.P.); the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant K01-DK081444] (to J.D.T.); and by a grant-in-aid from the American Heart Association Greater Southeast Affiliate (to D.B.H.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- PACAP
- pituitary adenylate cyclase-activating polypeptide
- VIP
- vasoactive intestinal polypeptide
- ACh
- acetylcholine
- SP
- substance P
- ANOVA
- analysis of variance.
References
- Baron A, Monnier D, Roatti A, Baertschi AJ. ( 2001) Pituitary adenylate cyclase-activating polypeptide activates K(ATP) current in rat atrial myocytes. Am J Physiol Heart Circ Physiol 280: H1058–H1065 [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. ( 1998) Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18: 9766–9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Hoover DB, Hancock JC, Smith FM. ( 2000) Tachykinin receptor subtypes in the isolated guinea pig heart and their role in mediating responses to neurokinin A. J Pharmacol Exp Ther 294: 147–154 [PubMed] [Google Scholar]
- Chang Y, Lawson LJ, Hancock JC, Hoover DB. ( 2005) Pituitary adenylate cyclase-activating polypeptide: localization and differential influence on isolated hearts from rats and guinea pigs. Regul Pept 129: 139–146 [DOI] [PubMed] [Google Scholar]
- Hardwick JC, Mawe GM, Parsons RL. ( 1995) Evidence for afferent fiber innervation of parasympathetic neurons in the guinea-pig cardiac ganglion. J Auton Nerv Syst 53: 166–174 [DOI] [PubMed] [Google Scholar]
- Hardwick JC, Mawe GM, Parsons RL. ( 1997) Tachykinin-induced activation of non-specific cation conductance via NK3 neurokinin receptors in guinea-pig intracardiac neurones. J Physiol 504: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M, Furukawa Y, Nagashima Y, Lakhe M, Miyashita Y, Chiba S. ( 1997) PACAP-27 causes negative and positive dromotropic effects in anesthetized dogs. Eur J Pharmacol 338: 35–42 [DOI] [PubMed] [Google Scholar]
- Hoover DB. ( 1989) Effects of guinea pig vasoactive intestinal peptide on the isolated perfused guinea pig heart. Peptides 10: 343–347 [DOI] [PubMed] [Google Scholar]
- Hoover DB. ( 1990) Effects of substance P on rate and perfusion pressure in the isolated guinea pig heart. J Pharmacol Exp Ther 252: 179–184 [PubMed] [Google Scholar]
- Hoover DB, Chang Y, Hancock JC, Zhang L. ( 2000) Actions of tachykinins within the heart and their relevance to cardiovascular disease. Jpn J Pharmacol 84: 367–373 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources ( 1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Laburthe M, Couvineau A. ( 2002) Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regul Pept 108: 165–173 [DOI] [PubMed] [Google Scholar]
- Lerner EA, Iuga AO, Reddy VB. ( 2007) Maxadilan, a PAC1 receptor agonist from sand flies. Peptides 28: 1651–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner EA, Shoemaker CB. ( 1992) Maxadilan. Cloning and functional expression of the gene encoding this potent vasodilator peptide. J Biol Chem 267: 1062–1066 [PubMed] [Google Scholar]
- Moro O, Lerner EA. ( 1997) Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem 272: 966–970 [DOI] [PubMed] [Google Scholar]
- Pintér A, Nadeau R, Dandan N, Pagé PL. ( 1998) Effect of vasoactive intestinal peptide on pacemaker location and heart rate in the dog atrium. Can J Physiol Pharmacol 76: 457–462 [DOI] [PubMed] [Google Scholar]
- Rigel DF, Lathrop DA. ( 1991) Vasoactive intestinal polypeptide enhances automaticity of supraventricular pacemakers in anesthetized dogs. Am J Physiol 261: H463–H468 [DOI] [PubMed] [Google Scholar]
- Saetrum Opgaard O, Knutsson M, de Vries R, Tom B, Saxena PR, Edvinsson L. ( 2001) Vasoactive intestinal peptide has a direct positive inotropic effect on isolated human myocardial trabeculae. Clin Sci (Lond) 101: 637–643 [DOI] [PubMed] [Google Scholar]
- Seebeck J, Schmidt WE, Kilbinger H, Neumann J, Zimmermann N, Herzig S. ( 1996) PACAP induces bradycardia in guinea-pig heart by stimulation of atrial cholinergic neurones. Naunyn Schmiedebergs Arch Pharmacol 354: 424–430 [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Ardell JL, Hoover DB, Parsons RL. ( 2007) Neurally-released pituitary adenylate cyclase-activating polypeptide (PACAP) enhances guinea pig intrinsic cardiac neurone excitability. J Physiol 582: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL. ( 2006) Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol 95: 2134–2142 [DOI] [PubMed] [Google Scholar]
- Ushiyama M, Ikeda R, Sugawara H, Yoshida M, Mori K, Kangawa K, Inoue K, Yamada K, Miyata A. ( 2007) Differential intracellular signaling through PAC1 isoforms as a result of alternative splicing in the first extracellular domain and the third intracellular loop. Mol Pharmacol 72: 103–111 [DOI] [PubMed] [Google Scholar]
- Ushiyama M, Sugawara H, Inoue K, Kangawa K, Yamada K, Miyata A. ( 2006) Characterization of the PAC1 variants expressed in the mouse heart. Ann N Y Acad Sci 1070: 586–590 [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. ( 2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52: 269–324 [PubMed] [Google Scholar]
- Zhang L, Tompkins JD, Hancock JC, Hoover DB. ( 2001) Substance P modulates nicotinic responses of intracardiac neurons to acetylcholine in the guinea pig. Am J Physiol Regul Integr Comp Physiol 281: R1792–R1800 [DOI] [PubMed] [Google Scholar]