Abstract
Five phenotypically distinct subtypes have been identified in sporadic Creutzfeldt–Jakob disease (sCJD), based on the methionine/valine polymorphic genotype of codon 129 of the prion protein (PrP) gene and the presence of either one of the two protease K-resistant scrapie prion protein (PrPSc) types identified as 1 and 2. The infrequent co-existence of both PrPSc types in the same case has been known for a long time. Recently, it has been reported, using type-specific antibodies, that the PrPSc type 1 is present in all cases of sCJD carrying PrPSc type 2. The consistent co-occurrence of both PrPSc types complicates the diagnosis and the current classification of sCJD, and has implications for the pathogenesis of naturally occurring prion diseases. In the present study, we investigated the prevalence of PrPSc types 1 and 2 co-occurrence, along with its effects on the disease phenotype and PrPSc strain characteristics, comparatively analysing 34 cases of sCJD, all methionine homozygous at codon 129 of the PrP gene (sCJDMM). To minimize overestimating the prevalence of the sCJDMM cases carrying PrPSc types 1 and 2 (sCJDMM1-2), we used proteinase K concentrations designed to hydrolyse all fragments resulting from an incomplete digestion, while preserving the protease-resistant PrPSc core. Furthermore, we used several antibodies to maximize the detection of both PrPSc types. Our data show that sCJDMM cases associated exclusively with either PrPSc type 1 (sCJDMM1) or PrPSc type 2 (sCJDMM2) do exist; we estimate that they account for approximately 56% and 5% of all the sCJDMM cases, respectively; while in 39% of the cases, both PrPSc types 1 and 2 are present together (sCJDMM1-2) either mixed in the same anatomical region or separate in different regions. Clinically, sCJDMM1-2 had an average disease duration intermediate between the other two sCJDMM subtypes. The histopathology was also intermediate, except for the cerebellum where it resembled that of sCJDMM1. These features, along with the PrP immunostaining pattern, offer a diagnostic clue. We also observed a correlation between the disease duration and the prevalence of PrPSc type 2 and sCJDMM2 phenotypes. The use of different antibodies and of the conformational stability immunoassay indicated that the co-existence of types 1 and 2 in the same anatomical region may confer special conformational characteristics to PrPSc types 1 and 2. All of these findings indicate that sCJDMM1-2 should be considered as a separate entity at this time.
Keywords: prion protein, prion disease, co-existence, conformation, sporadic Creutzfeldt–Jakob disease
Introduction
One of the major characteristics of human prion diseases is the heterogeneity of the clinical and pathological phenotype (Monari et al., 1994; Parchi et al., 1996, 1999; Gambetti et al., 2003; Kong et al., 2004). There is a general consensus that in sporadic prion diseases the genotype at codon 129 of the prion protein (PrP) gene and the presence of distinct types of the disease-associated PrP that is generally protease-resistant (hereafter identified as PrPSc) are the major determinants of the disease phenotype (Gambetti et al., 2003). Several years ago, we proposed a classification of human sporadic prion diseases based on the codon 129 genotype and the PrPSc type that distinguishes five subtypes of sporadic Creutzfeldt–Jakob disease (sCJD) (Parchi et al., 1996, 1999, 2000; Gambetti et al., 2003). The genotype of codon 129 is determined by the methionine/valine polymorphism, which results in three patient populations: homozygous methionine (MM), homozygous valine (VV) and heterozygous methionine/valine (MV). The PrPSc present in the majority of the sCJD patients can be distinguished in two groups according to the electrophoretic mobility of the fragments resistant to proteinase K (PK) digestion. In PrPSc type 1, the unglycosylated isoform migrates to the 21 kDa region of the gel, while the corresponding type 2 isoform migrates to 19 kDa (Parchi et al., 1996, 1997; Gambetti et al., 2003). The combination of the 129 genotype and the PrPSc type has allowed for the distinction of five subtypes of sCJD with different clinical and histopathological features that are identified as: (i) sCJDMM1 and sCJDMV1; (ii) sCJDVV1; (iii) sCJDMV2; (iv) sCJDVV2; and (v) the sCJDMM2 (Parchi et al., 1999).
When we originally proposed the sCJD classification, we observed that 14 out of the 300 cases examined (about 5%) had both PrPSc types 1 and 2 (Parchi et al., 1999). Since then, the co-occurrence of PrPSc types 1 and 2 in a relatively small percentage of sCJD cases has been confirmed by a number of subsequent studies (Puoti et al., 1999; Kovács et al., 2002; Haïk et al., 2004; Head et al., 2004; Lewis et al., 2005; Schoch et al., 2006; Uro-Coste et al., 2008). Recently, however, two studies using antibodies to PrP that specifically recognize PrPSc type 1 have suggested that all cases of sCJD associated with PrPSc type 2 also invariably contain PrPSc type 1 (Polymenidou et al., 2005; Yull et al., 2006). This finding has raised a number of questions: (i) Is the sCJD classification that we proposed, which is largely based on the presence of either PrPSc type 1 or 2, still valid or should it be changed? (ii) Does the co-occurrence of both PrPSc types generate a distinct phenotype? (iii) Are the co-occurring PrPSc types different from the corresponding type occurring alone, if this condition exists? (iv) What are the mechanisms of type co-occurrence?
We have decided to address these questions separately in three groups of sCJD determined according to the MM, VV and MV 129 genotypes with the purpose of keeping the issue of PrPSc type co-occurrence relatively simple by reducing heterogeneity. In this initial study, we selected 34 cases of sCJD and followed a complex strategy to analyse in detail the presence, distribution and characteristics of PrPSc types 1 and 2 occurring independently or combined. We then correlated these findings with the clinical and histopathological phenotypes of these cases.
Materials and Methods
Reagents and antibodies
PK, phenylmethylsulphonyl fluoride (PMSF), 10% sodium dodecyl sulphate solution (10% SDS) and guanidine hydrochloride (GdnHCl) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tris–HCl buffers, 30% Acrylamide/Bis, Tetramethylethylenediamine (TEMED) and ammonium persulphate were purchased from Bio-Rad Laboratories (Richmond, CA, USA). Reagents for enhanced chemiluminescence (ECL plus) and the horseradish peroxidase-conjugated antibodies were produced by Amersham Biosciences (Piscataway, NJ, USA). The following anti-human PrP monoclonal antibodies (mAb) were used: 3F4 to PrP residues 109–112 (Kascsak et al., 1987), 12B2 to PrP residues 89–93 (Langeveld et al., 2006), 1E4 to PrP residues 97–108 (Cell Sciences, Canton, MA, USA; Yuan et al., 2008), 9A2 to PrP residues 99–101 (Langeveld et al., 2006), 6H4 to PrP residues 144–152 (Korth et al., 1997; Prionics AG, Schlieren-Zurich, Switzerland) and 8H4 to PrP residues 175–185 (Zanusso et al., 1998). The rabbit polyclonal antibody 2301 to PrP residues 220–231 was also used (Chen et al., 1995; Zou et al., 2003).
Subjects
We selected 34 subjects, all of whom were methionine homozygous at codon 129 (MM) of the human PrP gene (PRNP), and had a definitive diagnosis of sCJD (sCJDMM) at the National Prion Disease Pathology Surveillance Centre (NPDPSC) in Cleveland, OH, USA. Based on routine western blot (WB) examination of two or three brain regions (including frontal, occipital and cerebellum cortices) with the mAb 3F4, the scrapie PrP (PrPSc) type had been classified (i) PrPSc type 1 only (sCJDMM1) (n = 13); (ii) PrPSc type 2 only (sCJDMM2) (n = 9); or (iii) PrPSc types 1 and 2 (sCJDMM1-2) (n = 12). Patients lacked pathogenic mutations in the PRNP and had no history of familial diseases or known exposure to prion agents. These cases underwent detailed analyses of the PrPSc to ascertain the accuracy of their original classification as sCJDMM1, sCJDMM2 or sCJDMM1-2.
Clinical evaluations
Retrospective chart review was carried out for all subjects paying particular attention to cardinal clinical signs of sCJD such as dementia, ataxia and myoclonus. The findings were also recorded on electroencephalography and brain magnetic resonance imaging when available. Chi-square or Fisher exact tests were used to compare the proportions and Wilcoxon rank sum test was used to compare median of age and disease duration between the groups. We omitted the missing observations during analysis.
Brain samples
Coronal sections of human brain tissues were obtained at autopsy and stored at −80°C. The entire cortical gyrus, large amounts of cerebellar and sub-cortical tissues were taken from each brain and used for molecular analyses. The other symmetric cerebral hemisphere was fixed in formalin and used for histological and immunohistochemical purposes. For all cases of sCJDMM1-2 (according to the revised-type classification), six brain regions were sampled for western blotting: frontal (middle gyrus) and occipital (visual) neocortices, subiculum and entorhinal cortex, basal ganglia (putamen), thalamus and cerebellum. Additional brain regions were investigated from the frontal [superior (n = 8), inferior (n = 1) and more posterior middle (n = 3) gyrus] and occipital [superior (n = 1) and inferior (n = 8)] neocortices. For sCJDMM1 and sCJDMM2 cases, three samples of frontal (superior and more posterior middle gyri) and occipital cortex (inferior gyrus) were obtained, in addition to the above six brain regions. Additional samples including the gyrus rectus and the superior gyrus of the occipital cortex were investigated in one sCJDMM1 and one sCJDMM2 case, respectively. Finally, the tectum and periaqueductal grey of the midbrain, along with the periventricular grey and inferior olive were sampled in two sCJDMM2 cases in which the brainstem was available.
Molecular genetics
DNA was extracted from frozen brain tissues in all the cases, and genotypic analysis of PRNP coding region was performed as described (Parchi et al., 1996, 2000).
Histopathology and PrP immunohistochemistry
The percent distribution of the large vacuole spongiosis throughout the brain and fine spongiform degeneration in the molecular layer of the cerebellum was determined by comparing haematoxylin and eosin-stained sections in nine sCJDMM1, five sCJDMM2 and 19 sCJDMM1-2 cases (according to the revised classification). Ten brain regions were examined: frontal, temporal, parietal, entorhinal and visual cortices, hippocampus, basal ganglia (putamen), thalamus (anterior and mediodorsal nuclei), substantia nigra and cerebellum. The sCJDMM1-2 cases were divided into two groups according to the disease duration being either <8 months (1–7 months) or ≥8 months (8–13 months). Immunohistochemistry, according to Parchi et al. (1996), was performed to determine: (i) the percent areas of the cortical (frontal, temporal, parietal, entorhinal, visual and hippocampus) and subcortical regions (basal ganglia and thalamus) occupied by perivacuolar and coarse PrP immunostaining patterns; (ii) the type of PrP immunostaining pattern in the cerebellum (i.e. the diffuse PrP immunostaining pattern described for the sCJDMM1 or the coarse PrP immunostaining pattern characteristic of the sCJDMM2 subtype); and (iii) positive or negative PrP immunostaining of the hippocampus.
Proteinase K digestions
Brain homogenates (10% wt/vol) were prepared in lysis buffer with 100 mM Tris–HCl (LB100) (100 mM NaCl, 10 mM EDTA, 0.5% NP-40, 0.5% sodium deoxycholate, 100 mM Tris–HCl, pH 8.0) (Notari et al., 2004) and then centrifuged at 1000 g for 10 min to collect the supernatant (S1). Homogenates were incubated with different PK concentrations [48 U/mg specific activity at 37°C, with 1 U/ml equal to 20.8 µg/ml PK]. In an initial study, PK concentrations ranging from 0 to 160 U/ml were used to separate the bona fide PrPSc fragments from those that were incompletely digested. As a result of this preliminary study, a concentration of 5 U/ml PK was adopted to determine the ratio of PrPSc types 1 and 2 when the two types co-occurred in the same brain region, while resistance of PrPSc to digestion with 20 U/ml PK was the requirement for identifying the bona fide PrPSc core fragments (Fig. 1). When needed, P2 fractions were prepared as previously described (Zou et al., 2003). All PK digestions were carried out at 37°C for 1 h, and then stopped by the addition of 2 mM PMSF. Samples were mixed in an equal volume of 2× sample buffer (6% SDS, 5% β-mercaptoethanol, 20% glycerol, 4 mM EDTA, 125 mM Tris–HCl, pH 6.8) and boiled for 10 min.
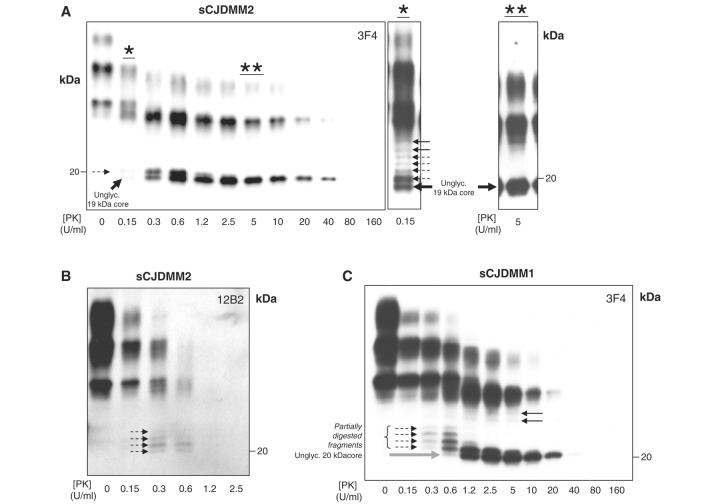
Figure 1.
Validation of PrPSc typing by protease digestion in sCJDMM2 and sCJDMM1. S1 fractions from the frontal cortex were incubated with several amounts of PK and probed with 3F4 (A, C) or 12B2 (B). (A) The core of the unglycosylated PrPSc type 2 (large black solid arrow) is detectable after treatment with up to 40 U/ml PK (up to 160 U/ml in long exposure films). The partially digested PrP fragments (dashed arrows) are visible between 0.15 (denoted by asterisk) and 0.6 U/ml of PK treatment but are completely hydrolyzed at relatively low PK concentrations ranging from 2.5 to 5 U/ml (denoted by double asterisk). The bands indicated by the black solid arrows in A* presumably represent monoglycosylated PK-resistant PrPSc fragments. (B) The mAb 12B2, which binds to the PrPSc type 1 but not to type 2 PK-resistant fragments, immunodetects the four partially digested PrP fragments but not the unglycosylated PrPSc type 2 core confirming that the fragments are incompletely digested, while the ‘core’ belongs to type 2. (C) Four partially cleaved PrP fragments (dashed arrows) are detectable between 0.15 and 0.6 U/ml PK in sCJDMM1 preparations. The core of the unglycosylated PrPSc type 1 (solid grey arrow) is resistant up to 40 U/ml (or up to 80 U/ml PK in long exposure films). For the small black solid arrows see (A*).
Western blot analyses
Western Blots were performed as previously described with minor modifications (Cali et al., 2006). Briefly, proteins were separated by non-commercial 15% Tris–HCl, 20-cm-long SDS–polyacrylamide gel electrophoresis (SDS–PAGE) gels then transferred to Polyvinylidene Fluoride (PVDF) membrane (Immobilon-P; Millipore, Bedford, MA, USA) for 2 h at 60 V. Each antibody was incubated for 2 h at room temperature: 3F4 (0.1 µg/ml), 12B2 (0.05 µg/ml), 1E4 (1 µg/ml), 9A2 (2 µg/ml), 6H4 (1.1 µg/ml), 8H4 (1 µg/ml) and 2301 (1:2000).
The experiments of 3F4 or 1E4 immunoreactivity with PrPSc type 2 that was either artificially mixed or naturally co-existing with PrPSc type 1 (see below and Fig. 2B), were performed at the optimum concentration of 2 µg/ml for the two antibodies. In the sCJDMM1-2 cases, the ratio of the two unglycosylated PrPSc types (T1:T2) co-existing in the same anatomical region was calculated according to 3F4 and 1E4 immunoreactivity to PrPSc type 2. When 3F4 immunoreacted with PrPSc type 2, the T1:T2 ratio (which was identical to the one obtained with 6H4, 8H4 and 2301) was calculated by using the same antibody. When 3F4 did not immunoreact with PrPSc type 2, 1E4 was employed. 1E4 could either fail to detect PrPSc type 2 (therefore, the T1:T2 ratio was equal to 100:0) or recognize a PK-resistant fragment of 19 kDa matching the core of PrPSc type 2. In this latter case, the T1:T2 ratio was calculated as follows: (i) since 1E4 had, on average, ∼12 times higher immunoreactivity to PrPSc type 2 than PrPSc type 1, the densitometric value of PrPSc type 2 was divided by 12 (3F4 shows similar immunoreactivity for both PrPSc types); and (ii) the obtained value was then multiplied by two because 3F4 has ∼2 times more immunoreactivity to PrPSc type 2 than 1E4 (as determined after antigen/antibody saturation curve studies). Immunoreactivity of 3F4 and 1E4 to PrPSc types 1 and 2 was also investigated by artificially mixing low speed brain homogenates (S1) from the sCJDMM1 and sCJDMM2 subtypes. PrPSc types 1 and 2 were mixed in ratios of 100:0, 95:5, 90:10, 60:40, 45:55, 30:70, 15:85 and 0:100. Immunoreactivity of 3F4 and 1E4 antibodies to both PrPSc types did not change when PrPSc types 1 and 2 were denatured in the same or in different experimental tubes.
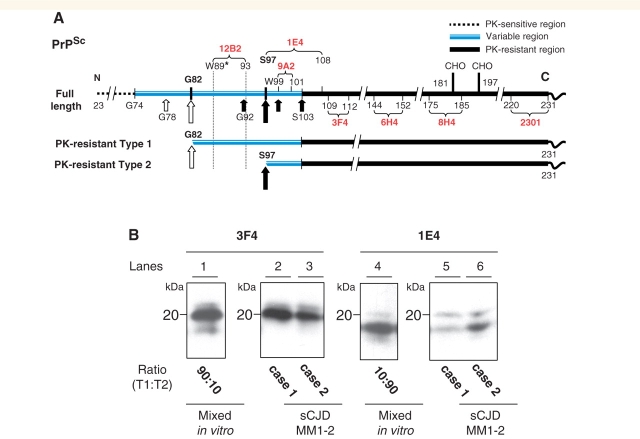
Figure 2.
Diagram of epitopes of various anti-PrP antibodies and western blotting of PrPSc mixed naturally and artificially. (A) Representation of PK cleavage sites, PK-sensitive and PK-resistant regions and epitope location in human PrPSc. Long and short arrows identify the primary and secondary PK cleavage sites located along the variable region G74-S103. This region is depicted out of proportion. The locations of the relevant residues for PrPSc types 1 and 2 are indicated with white and black arrows, respectively. Tryptophan 89 (W89*) represents the expected PK cleavage site (see PeptideCutter Program) generating the PrPSc type 1 fragment of ∼20 kDa when samples are homogenized at pH ∼8.0. The curly brackets indicate the PrP epitopes recognized by the antibodies used in this study. The basis for the reactivity of mAb 12B2 with PrPSc type 1 but not type 2 is illustrated with the dotted lines. (B) Both mAb 3F4 and 1E4 immunoreacted with PrPSc type 2 that had been artificially mixed with PrPSc type 1 (lanes 1 and 4). In contrast, PrPSc type 2 from sCJDMM1-2, where types 1 and 2 co-occurred in the same brain region, was detected by 1E4 only (lanes 5 and 6). Lanes 1 and 4 were generated with tissue from the frontal cortex of sCJDMM1 and sCJDMM2; lanes 2 and 5 with thalamic tissue and lanes 3 and 6 with the cerebellum of two sCJDMM1-2 cases, respectively (T1 = PrPSc type 1; T2 = PrPSc type 2).
To exclude the co-existence of PrPSc types when PrPSc type 1 appeared to be present alone after probing with 3F4, tissue was further probed with 1E4. Similarly, if only PrPSc type 2 was suspected, the co-existence of PrPSc type 1 was excluded with 12B2 which selectively binds to PrPSc type 1. To maximize PrPSc-type immunoreactivity, gels were loaded with up to three times the amount of tissue homogenate loaded with 3F4, and PrPSc-enriched P2 fractions were used when the total amount of PK-resistant PrPSc was low.
Conformational stability immunoassay
The conformational stability immunoassay (CSI) was performed as described, with minor modifications (Zou et al., 2004; Pastore et al., 2005). Briefly, aliquots of 20 µl S1 were mixed with 20 µl of GdnHCl stock solution to give a final concentration of GdnHCl ranging from 0 to 4.0 M. After 1.5 h of incubation at room temperature, samples were precipitated with 5-fold volume excess of pre-chilled methanol overnight at −20°C. Samples were centrifuged at 16 000g for 30 min at 4°C, pellets were re-suspended in 20 µl of LB100 (pH 8.0) and sonicated. Each aliquot was digested with 5 U/ml PK for 1 h at 37°C. The reaction was stopped with 2 mM of PMSF, denatured and loaded onto 15% Tris–HCl pre-cast gels (Bio-Rad) for WB analyses. A total of 31 brain regions were examined: frontal [sCJDMM1 (n = 1), sCJDMM1-2 (n = 4)], visual [sCJDMM1 (n = 7), sCJDMM2 (n = 4) and sCJDMM1-2 (n = 5)], entorhinal [sCJDMM1-2 (n = 2)] cortices, striatum [sCJDMM1-2 (n = 3)] and thalamus [sCJDMM1-2 (n = 5)]. Of the 19 sCJDMM1-2 samples (13 cases), six had PrPSc type 1 [frontal cortex (n = 2) and striatum (n = 1)] and type 2 [frontal (n = 1) and visual (n = 2) cortices] present separately. In the remaining 13 sCJDMM1-2 samples, both PrPSc types co-occurred in the same anatomical brain area, i.e. in the frontal (n = 1), visual (n = 3) and entorhinal (n = 2) cortices, striatum (n = 2) and thalamus (n = 5). CSI study of the unglycosylated PrPSc types 1 and 2 co-existing in the same brain region was performed in 11 out of the 13 sCJDMM1-2 samples that had the best resolution of the two unglycosylated PrPSc bands. In 4 out of 11 sCJDMM1-2 cases, PrPSc type 2 was detectable only by 1E4 mAb. For CSI analysis of the in vitro mixed PrPSc types 1 and 2, S1 homogenates from sCJDMM1 (n = 3) and sCJDMM2 (n = 3) were mixed to generate T1:T2 in ratios of 50:50 and incubated with GdnHCl in the same experimental tube. Since the [GdnHCl]1/2 values vary according to the intensity of the PrP bands, we used the exposure of the films at which the starting amount of PrPSc (as determined at GdnHCl = 0 M) was similar for each sCJDMM case. Densitometric analysis was performed with UN-SCAN-IT gel 5.1 software. Statistical analysis was assessed by analysis of variance (ANOVA).
Statistical analysis
We investigated the effect of the following demographic, clinical and laboratory variables on survival: race, sex, age at onset, electrophoretic type of PrPSc and stability of PrPSc in GdnHCl (Safar et al., 1998; Peretz et al., 2002). Cumulative survival curves were constructed by the Kaplan–Meier method, both overall, and by stratifying for each of the above variables. The 20 sCJDMM1-2 were compared with the 9 sCJDMM1 and 5 sCJDMM2 cases of the present study or with a larger population of 166 sCJDMM1 and 19 sCJDMM2 received at the NPDPSC from 2005 to 2007. For each type of PrPSc, we report as descriptive statistics the survival times overall and stratified for each variable; the comparisons of survival curves between groups were carried out by the log rank (Mantel–Cox) and generalized Wilcoxon test. To obtain estimates of the dependency of duration of the disease on stability of PrPSc, the [GdnHCl]1/2 values were analysed by non-linear regression. Statistical analyses were performed using SPSS 16 software (SPSS Inc., Chicago, IL, USA).
Percentage distribution of the sCJDMM types
The original PrPSc-type distribution of the sCJDMM cases examined by the NPDPSC was estimated based on the corrections of the type classification made in this study, as the result of the detailed analyses of the PrPSc type. The NPDPSC has received and classified 234 sCJDMM consecutive cases during 2005–2007, a three-year period. Based only on routine western blotting examination, without any special study, 188 (80%) of these cases were classified as sCJDMM1, 23 (10%) as sCJDMM2 and 23 (10%) as sCJDMM1-2. In the present study, 4 of the 13 cases originally classified as sCJDMM1 and four of the nine classified as sCJDMM2 were reclassified as sCJDMM1-2. The original NPDPSC percent distribution of the 234 sCJDMM cases was then corrected according to the type changes resulting from the detailed analyses used in this study.
Results
Validation of PrPSc typing
Optimal conditions of PK digestion
A major challenge in PrPSc-type determination is avoiding incomplete protein digestion, most often caused by hydrolysis with an insufficient amount of PK or performed at an unsuitable pH (Notari et al., 2004; Cali et al., 2006). Inappropriate conditions generate a number of smaller PrPSc fragments representing the product of the partial PrPSc digestion, which, especially in the case of PrPSc type 2, may erroneously be construed as representing the co-occurrence of types 1 and 2 (Notari et al., 2007). Since the major goal of this study is to explore the correlations between the disease phenotype and the co-occurrence of both PrPSc types, it was imperative to distinguish the genuine cases of sCJDMM1-2 from those caused by artefacts. This was done largely according to Notari et al. (2007). Incubation of low speed supernatant (S1) (see Methods section) from sCJDMM1 and sCJDMM2 with PK concentrations between 0 and 160 U/ml (1 U/ml equal to 20.8 µg/ml PK) resulted in as many as seven higher molecular weight (MW) PrPSc fragments which could be detected in long exposure films following treatment with low doses of PK (up to 0.6 U/ml) (Fig. 1). In the sCJDMM2 preparations, of the seven bands only the 19.0 kDa band was still well detectable after digestion with high doses of PK (up to 160 U/ml) in long exposure films (Fig. 1A). The mAb 12B2, which binds to the PrPSc type 1 but not to PrPSc type 2 PK-resistant fragment, immunoreacted with four of the higher MW fragments but not, as expected, with the 19.0 kDa fragment (Fig. 1B) (Yull et al., 2006; Notari et al., 2007; Uro-Coste et al., 2008). This finding indicates that the four higher MW fragments had cleavage sites closer to the N-terminus than the 19.0 kDa fragment, probably due to incomplete PK digestion, while the highly PK-resistant 19.0 kDa fragment represents the PrPSc type 2 original PK-resistant ‘core’, thereafter referred to as type 2 core.
The same experiments carried out on sCJDMM1 preparations revealed the same disparity in PK sensitivity (Fig. 1C) leading to the conclusion that the four fragments migrating in sCJDMM1 above the 20.0 kDa were also probably generated by incomplete PK digestion, while the 20.0 kDa band represents the unglycosylated, highly PK-resistant core of PrPSc type 1 (Fig. 1C).
The two remaining higher MW bands that were still detectable at PK concentrations of 20 U/ml in both sCJDMM2 and sCJDMM1 likely carry the monoglycosylated PrPSc core of types 2 and 1, respectively, since in the sCJDMM2 preparations they did not react with 12B2 (Fig. 1A*–C).
Since 5 U/ml was the PK concentration at which (i) the bona fide PrPSc core was well represented in both sCJDMM1 and sCJDMM2; and (ii) all the PrPSc fragments associated with sCJDMM2 that resulted from PK incomplete digestion were hydrolysed, we adopted this PK concentration for determining the ratio of PrPSc types 1 and 2 when they co-occurred, while the resistance to at least 20 U/ml PK was the requirement for identifying PrPSc as the bona fide PrPSc core fragment in sCJDMM1 and sCJDMM2 (Fig. 1).
Antibody selection
We primarily used 3F4 and 1E4 to maximize our ability to detect both PrPSc types 1 and 2, while 12B2 was used to identify incompletely digested fragments (Figs 1A and 2A). The combined use of mAb 1E4 and 3F4 was especially valuable in establishing the ratios of types 1 and 2: (i) when types 1 and 2 co-existed naturally in sCJDMM1-2; or (ii) in control experiments in which PrPSc types 1 and 2 preparations obtained from the sCJDMM1 and sCJDMM2 were mixed in vitro. These two antibodies bound to both PK-resistant PrPSc types when they were mixed in vitro (Fig. 2B). In contrast, when the PrPSc types 1 and 2 co-existed naturally, mAb 3F4, as well as 6H4, 8H4, 9A2 and 2301 immunoreacted with PrPSc type 2 only in some of the sCJDMM1-2 cases (Fig. 2A and B and data not shown). In contrast, 1E4 reaction was much more consistent; for example, while 3F4 detected the co-occurrence of PrPSc types 1 and 2 in 46 out of 114 sCJDMM1-2 brain regions, 1E4 increased this number to 79, corresponding to 72% more brain regions with co-existing PrPSc types. Surprisingly, the lack of 3F4 immunoreaction, when it occurred, was complete, i.e. no reaction could be detected regardless of the length of exposure or amount of loading (Fig. 2B). Therefore, in some sCJDMM1-2 cases, the co-existence of PrPSc types 1 and 2 in the same anatomical region remained undetected unless 1E4 was used. Furthermore, the complete lack of immunoreaction of PrPSc type 2 with 3F4, only when types 1 and 2 co-existed in the same anatomical region, indicates that in some cases the native PrPSc type 2 in sCJDMM1-2 is different from that of sCJDMM2 cases and that the close co-existence may modify the structural characteristics of PrPSc type 2. The detection of the PK-resistant PrPSc in various brain regions of sCJDMM1, sCJDMM2 and sCJDMM1-2 is exemplified in Fig. 3.
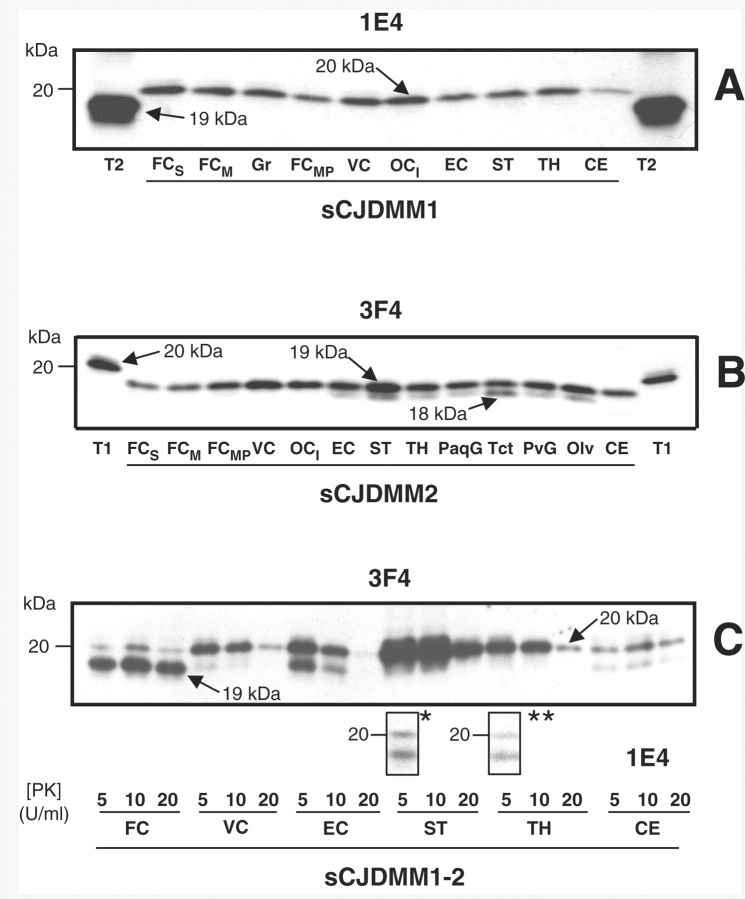
Figure 3.
Examples of PK-resistant PrPSc distribution in various brain regions in sCJDMM1, sCJDMM2 and sCJDMM1-2 according to the criteria applied in this study. (A–C) Only the unglycosylated PrPSc isoform is shown in each box. (A) In sCJDMM1, PrPSc type 1 (20 kDa, arrow) but not PrPSc type 2 is detected in several brain regions after probing with mAb 1E4; PrPSc type 2 (T2) is shown at each edge of the gel as a control. (B) The PrPSc associated with sCJDMM2 (19 kDa, arrow) immunoreact with mAb 3F4. A PK-resistant PrPSc fragment of 18 kDa is often associated with PrPSc type 2 (18 kDa, arrow) (Pan et al., 2005); PrPSc type 1 (T1) is shown at each edge of the gel as control. (C) A case of sCJDMM1-2 with PrPSc types 1 and 2 co-occurring (20 and 19 kDa, arrows) in four brain regions (FC, VC, EC and CE) after incubation with 3F4. However, with 1E4 a 19 kDa band matching PrPSc type 2 was detected in the striatum (denoted by asterisk) and thalamus (denoted by double asterisk) even after loading one-half of the original amount used for PrPSc incubation with 3F4. FCS = frontal cortex (cx), superior gyrus; FCM = frontal cx, middle gyrus; FCMP = frontal cx, more posterior middle gyrus; VC = visual cx; OCI = occipital cx, inferior gyrus; EC = entorhinal cx; ST = striatum; TH = thalamus; PaqG = periaqueductal gray; Tct = tectum; PvG = periventricular grey; Olv = inferior olive; CE = cerebellum; T2 = PrPSc type 2; T1 = PrPSc type 1.
Case re-classification
Based on the aforementioned analyses, the original PrPSc type classification of the 34 cases initially selected for this study had to be changed. Four of the 13 cases classified as sCJDMM1 following routine examination and four of the nine classified as sCJDMM2 were reclassified as sCJDMM1-2. Therefore, 8 of the 22 sCJDMM cases originally thought to be associated with only one type of PrPSc were found to have both types. Age and gender were similar in these three groups (P > 0.05). The newly formed groups were then comparatively examined in detail with regard to the PrPSc characteristics, histopathological and clinical phenotype and correlation between PrPSc type and phenotype.
PrPSc levels and type distributions in sCJDMM1, sCJDMM2 and sCJDMM1-2
In the 20 sCJDMM1-2 subjects, both types were present in 69% of all brain regions (79 out of 114 samples); PrPSc type 1 was found in 21% and PrPSc type 2 in 10% (Table 1). All regions could contain PrPSc types 1 and 2, but the combined sub-cortical regions, which accounted for 82%, and above all, the thalamus accounting for 90% were best represented (Table 1). In contrast, the cerebellum had the lowest occurrence of PrPSc types 1 and 2 (56%); type 1 alone was observed in the remaining 44% of cases, about three times those of the cortical and sub-cortical regions. Type 2 alone was not observed in the cerebellum and was rare (2%) in the sub-cortical regions, while it had the same prevalence as type 1 alone in the cerebral cortex (Table 1).
Table 1.
Topographic representation of PrPSc types 1 and 2, type 1, and type 2 in 20 sCJDMM1-2 expressed as percentage of the regions containing each of the three PrPSc types
| Brain region | PrPSc types 1 and 2 (%) | PrPSc type 1 (%) | PrPSc type 2 (%) |
|---|---|---|---|
| Cerebral cortexa (n = 58)b | 66 (n = 38)c | 17 (n = 10) | 17 (n = 10) |
| Subcortical regionsd (n = 38) | 82 (n = 31) | 16 (n = 6) | 2 (n = 1) |
| Cerebellum (n = 18) | 56 (n = 10) | 44 (n = 8) | 0 |
a Mean of frontal, occipital and entorhinal cortex.
b Total samples examined.
c Detected PrPSc type.
d Mean of striatum and thalamus.
The immunoreactivity characteristics of PrPSc types 1 and 2 differed also in gyri of the same lobe, i.e. the superior and middle frontal gyri or visual and non-visual occipital cortices. For example, 50% of the pairs of samples from two adjacent gyri differed on whether the PrPSc type 2 component was detected when only 3F4 was used but this discrepancy was reduced to <10% after also probing with 1E4. It indicates that adjacent brain regions could be populated by distinct type 2 species: one recognized by both 3F4 and 1E4 and the other by only 1E4. In addition, a difference in the ratio (but not in the number) of the two PrPSc types was often seen in sample pairs obtained from the same gyrus at different anterior–posterior locations.
The study of the quantitative distribution after probing with both 3F4 and 1E4 showed that the mean ratios of PrPSc types 1 and 2, regardless of being found in the same or different brain locations and expressed as percentage of the total PrPSc types 1 and 2 (1:2), was 54%:46% in the cortical regions combined, 67%:33% in the thalamus and striatum and 84%:16% in the cerebellum, indicating that PrPSc type 1 dominated in all the regions examined, but especially in the sub-cortical regions and in the cerebellum.
Characterization of PrPSc types 1 and 2 by conformational stability immunoassay
The CSI is based on the concept that slight differences in protein structure can be determined by the measurement of the conformational stability when the protein is exposed to a denaturant such as GdnHCl in an appropriate range of concentrations (Shirley, 1995). In the case of PrPSc, with increasing concentrations of GdnHCl, PrPSc dissociates and unfolds from native β-sheet-structured aggregates, becoming protease sensitive.
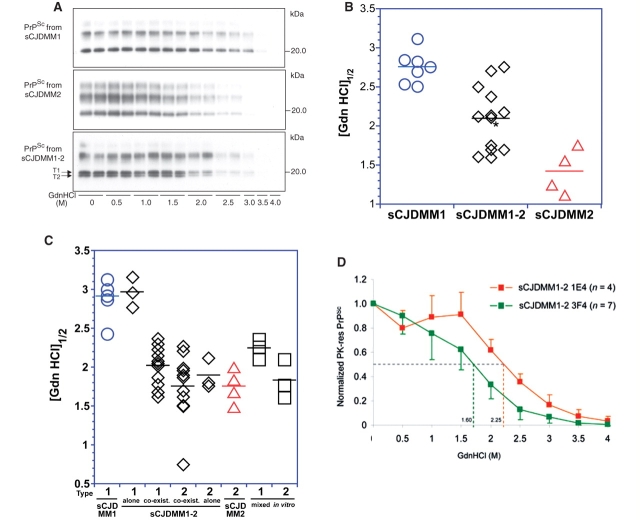
In the present study, the conformational characteristics of PrPSc associated with the sCJDMM1-2 were analysed with the aim of determining whether they (i) were similar to those of type 1 PrPSc; (ii) were similar to those of type 2 PrPSc; (iii) matched those of PrPSc types 1 and 2 mixture; or (iv) were different from those previously reported. As expected, the PrPSc associated with sCJDMM1 and sCJDMM2 had significantly different stability characteristics as revealed by CSI (Fig. 4A and B). The guanidine concentrations needed to render half of the PrPSc sensitive to PK, the so-called [GdnHCl]1/2, were 2.76 M for sCJDMM1 and 1.42 M in sCJDMM2 indicating that the PrPSc associated with sCJDMM1 is ∼1.9-fold more stable than that of sCJDMM2 (P < 0.0001) (Fig. 4A and B). The average [GdnHCl]1/2 in the sCJDMM1-2 examined was 2.05 M, i.e. roughly in between the [GdnHCl]1/2 of sCJDMM2 and sCJDMM1 (sCJDMM1-2 versus sCJDMM1, P = 0.00086; sCJDMM1-2 versus sCJDMM2, P = 0.008) (Fig. 4A and B). PrPSc from the sCJDMM1-2 cases could be divided into three groups according to whether (i) PrPSc types 1 and 2 were present separately; (ii) both PrPSc types co-occurred in the same brain region after probing with 3F4; and (iii) both PrPSc types were detected together in the same brain region after incubation with only 1E4. To determine the CSI characteristics of the PrPSc types 1 and 2 present together in the same brain region, it was necessary to analyse the unglycosylated forms, which can be monitored individually with ease. This approach allowed for the CSI monitoring of the PrPSc types 1 and 2, individually but in the same preparation (Fig. 4A). Preliminary analyses with PrPSc types 1 and 2 present separately confirmed that the [GdnHCl]1/2 data generated by CSI using the unglycosylated forms were representative of those generated from the analyses of all the bands (Supplementary Fig. S1) (P > 0.05). As expected, the [GdnHCl]1/2 of PrPSc from sCJDMM1-2 cases with PrPSc types 1 and 2 present separately were similar to those of the sCJDMM1 and sCJDMM2 subtypes, respectively (Fig. 4C). Surprisingly, when the two PrPSc types were present together in the same anatomical region, PrPSc type 1 showed a mean [GdnHCl]1/2 value similar to that of PrPSc type 2 (2.01 and 1.75 M, respectively) (Fig. 4C) suggesting that when they occur jointly, PrPSc type 2 maintains its original stability characteristics while PrPSc type 1 adopts those of PrPSc type 2. To assess the specificity of this finding, we examined the [GdnHCl]1/2 of PrPSc types 1 and 2 obtained from sCJDMM1 and sCJDMM2, respectively, after mixing these two species in vitro (Fig. 4C). We observed a similar result: the [GdnHCl]1/2 of the unglycosylated PrPSc type 1 from the in vitro mixture shifted similarly towards that of the type 2 present in the same mixture (Fig. 4C). This finding makes it impossible to determine whether the stability shift of PrPSc type 1 towards PrPSc type 2 takes place only in vitro or also in intact tissue when the two types occur together. Finally, a difference in [GdnHCl]1/2 values was observed between the PrPSc type 2 species immunoreacting only with the mAb 1E4 and the PrPSc type 2 recognized by both 1E4 and 3F4 mAb (2.25 and 1.60 M, respectively), but the difference did not reach statistical significance (Fig. 4D).
Figure 4.
CSIs of PrPSc from each sCJDMM group of cases. (A) Representative WB of PK-resistant PrPSc from sCJDMM1, sCJDMM2 and sCJDMM1-2 probed with 3F4 at increasing molar (M) concentrations of GdnHCl and used for the CSI shown in (B). The case of sCJDMM1-2 illustrated here is case 6 of Table 3, and is identified with an asterisk in (B). The two arrows indicate the unglycosylated bands belonging to PrPSc type 1 (T1) and type 2 (T2) analysed for the sCJDMM1-2 of C. (B) GdnHCl molar amounts needed to render PK-sensitive one-half of the PrPSc, [GdnHCl]1/2, as determined with CSI performed with the whole PrPSc from sCJDMM1, sCJDMM2 and sCJDMM1-2 subtypes. PrPSc from sCJDMM1 (blue circles), sCJDMM1-2 (black diamonds) and sCJDMM2 (red triangles) show distinct [GdnHCl]1/2 values. The sCJDMM1-2 group appears to include two populations with a mean [GdnHCl]1/2 of 2.4 ± 0.2 and 1.7 ± 0.1, respectively. The [GdnHCl]1/2 correlated with the disease duration, which was 4.4 ± 2.8 months in the cases of the upper cluster and 7.8 ± 3.2 months in the lower. The lower cluster includes cases 4, 12, 13, 14 and 15 of Table 3. In 1 of the 7 sCJDMM1 cases and in patients 3 and 1 of the 13 cases of sCJDMM1-2, [GdnHCl]1/2 represents the mean of 2 or 3 brain regions. The disease duration of the seven sCJDMM1 cases spans from 1 month [(GdnHCl)1/2 = 2.54 M] to 24 months [(GdnHCl)1/2 = 2.51 M]. (C) [GdnHCl]1/2 of the unglycosylated PrPSc isoform from sCJDMM1 (blue circles), sCJDMM1-2 (black diamonds), sCJDMM2 (red triangles) and PrPSc types 1 and 2 from sCJDMM1 and sCJDMM2 mixed in vitro (black squares). The 17 tests from the sCJDMM1-2 cases are grouped as follows: tests of PrPSc types 1 or 2 present separately (alone), and of PrPSc types 1 and 2 co-existing in the same preparation but quantified separately by densitometric analysis of the corresponding unglycosylated band [see arrows in (A)] (co-exist). (D) CSI curves of the unglycosylated PrPSc isoform from the sCJDMM1-2 regions in which PrPSc type 2, co-existing with PrPSc type 1 in the same brain region, was recognized either by both mAb 3F4 and 1E4 (green line) (n = 7 regions) or exclusively by 1E4 (orange line) (n = 4 regions). The values of [GdnHCl]1/2 for each curve are indicated on the X-axis; (M) = molarity.
Phenotype characterization: histopathology and immunohistochemistry in sCJDMM1, sCJDMM2 and sCJDMM1-2
Type and topography of the lesions in sCJDMM1 and sCJDMM2 were similar to those previously described (Parchi et al., 1996, 1999). Briefly, in sCJDMM1, the spongiform degeneration was characterized by fine vacuoles homogeneously distributed within the affected brain regions (Fig. 5A and B). None of the nine sCJDMM1 cases, including three cases having disease duration of 8, 11 and 24 months, respectively, showed the large vacuoles typical of sCJDMM2. The cortical and sub-cortical distributions of the spongiform degeneration were similar to those previously reported (Fig. 5A) (Parchi et al., 1999). The cerebellum showed moderate vacuolization, often focal, except for one case of 24-month duration, where the spongiosis was ubiquitous (Fig. 5A).
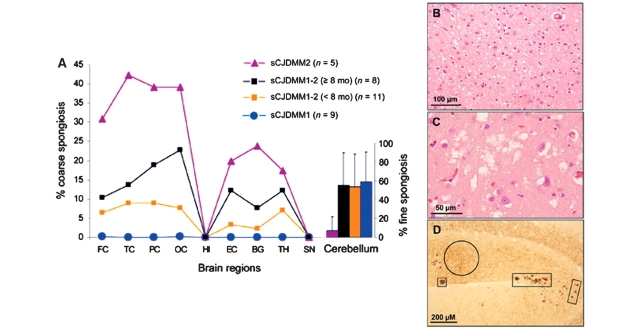
Figure 5.
Spongiform profile of the sCJDMM patients. (A) Percent distribution of large vacuole spongiform degeneration in sCJDMM1, sCJDMM2 and sCJDMM1-2. Individual data points are the mean percentage of the surfaces occupied by large vacuole spongiform degeneration characteristic of sCJDMM2. Standard deviations are omitted for clarity. In the cerebellum, the bars refer to the percentage of the surface of the molecular layer occupied by the ‘fine’ type of spongiform degeneration characteristic of sCJDMM1. On average of all regions, sCJDMM1-2 had 3.2 times and statistically significant less large vacuole spongiform degeneration than sCJDMM2 (P < 0.05). No large vacuole spongiform degeneration was seen in sCJDMM1. The profiles of sCJDMM1-2 of long (≥8 months) and short (<8 months) durations were not significantly different (P > 0.05). The fine spongiform degeneration in the cerebellum of sCJDMM2 cases was significantly less (P < 0.05) than in the other subtypes. Statistical analyses were performed by ANOVA followed by the Dunn's multiple comparison post-test. (B) sCJDMM1 fine spongiform degeneration. (C) sCJDMM2 large vacuole spongiform degeneration. (D) Fine, punctuate (circle) and plaque-like (squares) patterns of PrP immunoreactivity in the cerebellum of the sCJDMM1-2. FC = frontal cortex; TC = temporal cortex; PC = parietal cortex; OC = occipital (visual) cortex; HI = hippocampus; EC = entorhinal cortex; BG = basal ganglia (putamen); TH = thalamus (anterior and mediodorsal nuclei); SN = substantia nigra.
In sCJDMM2, the spongiform degeneration consisted mostly of large vacuoles, the hallmark of this subtype, often mixed with fine spongiform degeneration indistinguishable from that of sCJDMM1 (Fig. 5C) (Parchi et al., 1996). Overall, spongiosis and astrogliosis were more pervasive than in sCJDMM1. The lesion distribution was as previously reported (Fig. 5A) (Parchi et al., 1999). As in sCJDMM1, no spongiform degeneration was associated with the hippocampal gyrus, but contrary to sCJDMM1, the cerebellum was virtually spared (Fig. 5A).
In sCJDMM1-2, most of the cortical sections had both large and fine vacuoles as sCJDMM2, but in sCJDMM1-2 the spongiform degeneration was more circumscribed. Overall, the sCJDMM1-2 spongiform profile (as determined by the relative regional area occupied by the ‘grape-like’ type of spongiform degeneration) ran approximately in between that of sCJDMM1 where the large vacuoles were absent and that of sCJDMM2 where ∼40% of the cortex was occupied by large vacuoles (Fig. 5A). In contrast, the cerebellum showed a degree of fine spongiform degeneration in the molecular layer, comparable with that of sCJDMM1 and only in the cerebellum were the lesions significantly more severe (P < 0.002) than those of sCJDMM2, matching in severity with those of sCJDMM1 (Fig. 5A).
The lesion profiles determined separately in sCJDMM1-2 cases with <8-month and ≥8-month durations revealed no significant difference between the two groups although lesions were consistently more severe in the ≥8-month group; both groups closely paralleled the lesion profile of sCJDMM2 with the exception of the cerebellum (P < 0.03) (Fig. 5A).
The immunohistochemistry of the sCJDMM1 subtype demonstrated the classical punctate or ‘synaptic’ pattern of staining with the intensity distribution previously reported (Parchi et al., 1996). In sCJDMM2, both the perivacuolar and coarse patterns of PrP deposition were clearly distinguishable from the PrP staining pattern of sCJDMM1. Semi-quantitative analysis revealed that the temporal neocortex, with on average 95% of the surface immunostained, was the most affected cortical region followed by the parietal and occipital (80%), entorhinal (74%) and frontal (70%) cortices (Table 3). The coarse immunostaining pattern was invariably present in the molecular layer of the cerebellum. The coarse pattern of PrP immunostaining in the five cases examined occupied 70% of the averaged surfaces of the cortical (frontal, occipital and entorhinal) and sub-cortical (basal ganglia and thalamus) regions (Table 3). In the cerebellum, the coarse immunostaining pattern occupied ∼10% of the molecular layer.
Table 3.
Percentage of surface occupied by the PrP sCJDMM2 immunostaining pattern (IP), representation of type 1 or 2 immunostaining pattern in the hippocampus and relative amount of PrPSc type 2 in sCJDMM1-2 cases grouped according to disease duration
| Case | Duration | sCJDMM2 PrP IP (%)a | PrP IHC pattern |
PrPSc type 2 (%)b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cortexc | ST | TH | CEd | HI | Cortexc | ST | TH | CE | ||
| 1 | 1 | 11 | 0 | 15 | 0 | T1 | 0 | 0 | 1 | 0 |
| 2 | 1.5 | 37 | 13 | 35 | 0 | T1 | 3 | 0 | 9 | 0 |
| 3 | 2 | 1 | 1 | 1 | 0 | T1 | 21 | 10 | 1 | 0 |
| 4 | 2 | 24 | 1 | 15 | 0 | T1 | 51 | 10 | 95 | 7 |
| 5 | 3 | 9 | 1 | 21 | 0 | T1 | 28 | 15 | 42 | 0 |
| 6 | 3 | 36 | 2 | 20 | 85 | T1 | 83 | 79 | 86 | 2 |
| 7 | 4 | 0 | 0 | 0 | 0 | T1 | 2 | 0 | 0 | 0 |
| 8 | 4 | 24 | 12 | 55 | 60 | T2 | 4 | 79 | 31 | 6 |
| 9 | 5 | 30 | 13 | 50 | 10 | T2 | 90 | 92 | 60 | 1 |
| 10 | 5.5 | 18 | 1 | 5 | 1 | T1 | 21 | 1 | 12 | 0 |
| 11 | 6 | 8 | 2 | 1 | 80 | NA | 66 | 1 | 1 | NF |
| 12 | 7 | 0 | 0 | 0 | 5 | T1 | 37 | 9 | 8 | 30 |
| Mean ± SD | 3.7 ± 2 | 16.5 ± 13 | 4 ± 5 | 18 ± 18 | 20 ± 32 | T2/T1 ratio 2/9 | 34 ± 31 | 25 ± 34 | 29 ± 33 | 4 ± 8 |
| 13 | 8 | 25 | 1 | 7 | 60 | T2 | 14 | 1 | 5 | 0 |
| 14 | 8 | 30 | 7 | 30 | 70 | T1 | 85 | 11 | 55 | 7 |
| 15 | 9 | 54 | 3 | 50 | 60 | T2 | 75 | 63 | 35 | 5 |
| 16 | 9 | 25 | 12 | 1 | 55 | NA | 45 | NA | NA | >95 < 100 |
| 17 | 11 | 63 | 0 | 50 | 99 | T2 | 100 | 100 | >95 < 100 | 70 |
| 18 | 11 | 8 | 62 | 2 | 20 | T1 | 2 | 0 | 0 | 0 |
| 19 | 12 | 16 | 1 | 25 | 95 | T1 | 100 | 55 | >95 < 100 | 68 |
| 20 | 13 | 35 | 2 | 25 | 50 | 97 | 88 | 1 | NF | |
| Mean ± SD | 10.1 ± 2 | 32 ± 17 | 14 ± 21 | 27 ± 20 | 70 ± 26 | T2/T1 ratio 4/3 | 65 ± 37 | 45 ± 39 | 42 ± 40 | 36 ± 38 |
| Cases with single PrPSc type 1 or 2 | ||||||||||
| MM1 (n = 9) | 6.1 ± 7 (mean ± SD) | 0 | 0 | 0 | 0 | T1 | 0 | 0 | 0 | 0 |
| MM2 (n = 5) | 11.8 ± 5 (mean ± SD) | 75 | 60 | 65 | 100 | T2 | 100 | 100 | 100 | 100 |
a Data obtained with 3F4 and expressed as percentage of the total surface examined.
b Data obtained from PrPSc immunoblots probed with 3F4 and 1E4 and expressed as percentage of total PrPSc present.
c Average of frontal, occipital and entorhinal cortices.
d Data expressed as the percentage of the total immunostained area with 3F4 occupied by aggregates of coarse PrP immunostaining pattern (IP).
In sCJDMM1-2, the brain surface showing the coarse and perivacuolar immunostaining pattern was, on average, smaller in all of the combined cases than that of sCJDMM2 cases; the highest percentage was detected in frontal, occipital and entorhinal cortices combined (23%), thalamus (22%) and striatum (8%) (Table 3). Only 2% of the area of the molecular layer of the cerebellum was occupied by the coarse PrP immunostaining characteristic of sCJDMM2, while ∼18% was associated with the punctuate pattern of the sCJDMM1 (Fig. 5D).
Correlations of disease duration with clinical features, PrPSc type, immunostaining pattern and PrPSc representation in sCJDMM1-2
There was no prevalence of any of the clinical signs and tests examined among the three groups which would significantly distinguish sCJDMM1-2 from sCJDMM1 and sCJDMM2, probably because the number of cases examined was too small (Table 2). The mean ages at onset were also similar: 61 years in sCJDMM1-2, 68 years in sCJDMM1 and 64 years in sCJDMM2. However, sCJDMM1-2 subjects presented with progressive cognitive decline in almost all the cases (95%) and had a relatively high prevalence of MRI abnormalities (58%), like the subjects with sCJDMM2. Other signs such as ataxia and myoclonus, along with typical EEG changes, had a more similar prevalence to those of sCJDMM1.
Table 2.
Clinical features of sCJDMM1-2, sCJDMM1 and sCJDMM2
| Presentation | MM1-2 (n = 20) | MM1 (n = 9) | Statistical testa | MM2 (n = 5) | Statistical testa |
|---|---|---|---|---|---|
| Age at onset (mean ± SD) (years) | 61.4 ± 9.5 | 68.3 ± 10.7 | NS | 64.4 ± 19.7 | NS |
| Duration (mean ± SD) (months) | 6.3 ± 3.6 | 6.1 ± 7 | NS | 11.8 ± 5 | P < 0.05 |
| Range | (1–13) | (1–24) | (6–20) | ||
| Male gender | 11/20b (55) | 5/9 (56) | NS | 2/5 (40) | NS |
| Dementia | 19/20 (95) | 6/9 (67) | NS | 5/5 (100) | NS |
| Ataxia | 12/19 (63) | 5/8 (62) | NS | 3/5 (60) | NS |
| Myoclonus | 6/19 (32) | 3/9 (33) | NS | 2/5 (40) | NS |
| Focal weakness | 0/19 | 1/8 (12) | NS | 0/5 | NS |
| Visual symptoms | 4/19 (21) | 1/8 (12) | NS | 1/4 (20) | NS |
| Psychosis | 4/19 (21) | 1/8 (12) | NS | 2/5 (40) | NS |
| Seizure | 2/19 (11) | 1/9 (11) | NS | 0/5 | NS |
| PSWC on EEG | 9/17 (53) | 4/7 (57) | NS | 0/3 | NS |
| Abnormal MRIc | 11/19 (58) | 2/8 (25) | NS | 3/5 (60) | NS |
All values are given as n (%) unless otherwise mentioned.
a Chi-squared or Fisher exact tests were used to compare the proportions and Wilcoxon rank sum test used to compare median of age and disease duration between the groups.
b Cases with the feature listed/total cases examined.
c As determined with diffusion-weighted MR images.
NS = not significant.
A major and readily measurable clinical feature that clearly distinguishes sCJDMM1 and sCJDMM2 is the disease duration (Parchi et al., 1999). The mean disease durations in sCJDMM1, sCJDMM2 and sCJDMM1-2 were 6.1 ± 7, 11.8 ± 5 and 6.3 ± 3.6 months, respectively. Therefore, the disease duration was the longest in sCJDMM2, while it was similar in sCJDMM1 and sCJDMM1-2 (P > 0.05). However, if the sCJDMM1 case with the atypical 24-month duration is excluded, the Kaplan–Meier analyses showed that patients with PrPSc type 1 had a significantly shorter survival (P = 0.029) than patients with PrPSc type 2, despite the same codon 129 MM polymorphism, age and sex distribution (data not shown; Table 2). There is an apparent trend for longer survival in sCJDMM1-2 when compared with those with pure type 1. However, this trend was not statistically significant (P = 0.124), while the survival in sCJDMM1-2 is significantly shorter than that of sCJDMM2 (P = 0.034). When the Kaplan–Meier analyses were performed on 166 sCJDMM1 and 19 sCJDMM2 patients, consecutively received by the NPDPSC from 2005 to 2007, and compared with our group of sCJDMM1-2 cases (Supplementary Fig. S2), the sCJDMM1 cases had a shorter survival than sCJDMM2 (P < 0.0001), whereas the disease duration of the sCJDMM1-2 was intermediate but significantly different from both sCJDMM1 (P = 0.004) and sCJDMM2 (P = 0.001). These findings support the hypothesis that in sCJDMM, the type of PrPSc deposited in the brain influences the disease duration. Therefore, we searched for the correlation of the sCJDMM1-2 disease duration (which ranges between 1 and 13 months) with (i) the extent of the immunochemical features mimicking those of sCJDMM2; and (ii) the relative amount of PrPSc type 2 after separating the sCJDMM1-2 cases into two arbitrary groups according to whether the disease duration was <8 months or ≥8 months (Table 3). The first group of 12 cases had a mean disease duration of 3.7 ± 1.8 months (range: 1–7 months); in the second group of eight cases the mean disease duration was 10.1 ± 1.7 months (range: 8–13 months). On average, the extent of the sCJDMM2-like PrP immunostaining pattern and the amount of PrPSc type 2 in cases with disease duration ≥8 months were ∼2.0 and 1.8 times higher than that associated with <8-month duration cases (P < 0.02–0.05). Furthermore, the analysis of the relationship between disease duration and the extent of PrPSc type 2 immunostaining pattern along with percent representation of the PrPSc type 2 revealed good correlations among these variables (r = 0.83 and 0.81, respectively), further strengthening the conclusion that the disease duration is directly related to both PrPSc type 2 representation and the extent of sCJDMM2-like immunostaining pattern (Supplementary Fig. S3).
Discussion
Previous studies have shown that the co-occurrence of PrPSc types 1 and 2 in sCJDMM subjects may be over-diagnosed unless stringent experimental conditions are followed, allowing the distinction of the bona fide PrPSc types 1 and 2 fragments, referred to as core fragments, from the partially digested fragments (Notari et al., 2004, 2007). On the other hand, the co-occurrence of PrPSc types 1 and 2 may be underestimated if appropriate antibodies are not used or a sufficient number of brain regions are not examined. To navigate these Scylla and Charybdis of PrPSc typing, we used differential digestion to separate the PrPSc core fragments that were highly resistant to PK hydrolysis from the fragments generated during the stepwise digestion of PrPSc as previously shown by Notari et al. (2007). Since the partially digested fragments are present in all cases regardless of the PrPSc type and whether PrPSc types occur independently or combined, we inferred that they do not act as component of the prion strain and, therefore, do not play a role in phenotype determination.
The previous reports on high prevalence of co-occurrence of both PrPSc types in sCJDMM cases are based on the use of antibodies that specifically recognize PK-resistant fragments of PrPSc type 1, increasing the chances of detecting type 1 in sCJDMM2 cases. We have also used one of these antibodies, the mAb 12B2 (Langeveld et al., 2006) (Fig. 2A). To minimize the chances of missing PrPSc type 2, we used mAb 1E4 which has been demonstrated to have higher immunoreactivity to PrPSc type 2 and lower to type 1, than 3F4 (Yuan et al., 2005, 2006, 2008; Gambetti et al., 2008).
The identification of the types 1 and 2 core fragments, based on the degree of protease resistance and the careful typing of the core fragments with auxiliary antibodies, have in our opinion maximized the detectability of ‘true’ PrPSc types 1 and 2 in the present study. The detailed search and analyses of PrPSc types 1 and 2 in 277 brain regions from 34 sCJDMM cases, the correlation between the PrPSc type with the disease phenotype and the PrPSc characterization have lead to several conclusions that deserve separate discussion.
Existence of sCJDMM cases carrying solely PrPSc Type 1 or 2, along with cases carrying both PrPSc types 1 and 2
Contrary to the notion put forward previously that all cases associated with PrPSc type 2 invariably also carried type 1, our analysis shows that along with sCJDMM cases carrying both PrPSc types (sCJDMM1-2) there are cases in which only either type 1 (sCJDMM1) or type 2 (sCJDMM2) can be detected (Parchi et al., 1999; Puoti et al., 1999; Head et al., 2004; Schoch et al., 2006; Uro-Coste et al., 2008). The precise prevalence of the type ‘pure’ and ‘mixed’ cases cannot be established in the present sCJDMM cases because they were not collected randomly but based on the criteria of adequate representation of the three sCJDMM case groups. Therefore, to obtain an estimate of the sCJDMM-type distribution, we applied the same PrPSc type corrections, which were prompted by the extensive analyses carried out in this study, to the percent distributions of the sCJDMM1, sCJDMM2 and sCJDMM1-2 cases received and examined at the NPDPSC from 2005 to 2007 (see Methods section for details). Using this approach, we estimate that of the 234 consecutive sCJDMM cases, 39% were sCJDMM1-2 while the sCJDMM1 and sCJDMM2 cases accounted for 56% and 5%, respectively. Although showing conspicuous percentage of sCJDMM1-2 cases, our data—along with those by Puoti et al. (1999), Schoch et al. (2006), Collins et al. (2006) and Uro-Coste et al. (2008)—support the existence of sCJDMM cases solely associated with PrPSc of one type. The consideration that more extensive sampling would have eventually uncovered small amounts of the other PrPSc type making all cases types 1 and 2 cannot be dismissed in this and previous studies (Head et al., 2004). Such consideration seems to be of theoretical importance, but likely to be less relevant to the purpose of assessing the effect that the co-occurrence of both PrPSc types has on the sCJDMM phenotype. Therefore, according to our findings, the current classification of sCJDMM into the sCJDMM1 and sCJDMM2 subtypes should be maintained. Whether sCJDMM1-2 deserves to be considered as a distinct subtype of sCJD will be considered below.
Phenotypically, sCJDMM1-2 Falls in between sCJDMM1 and sCJDMM2
Although some differences in clinical features emerge between sCJDMM1-2 and the other two subtypes, they are not sufficient to identify sCJDMM1-2 as a clinically identifiable entity. However, it has been reported that patients with co-occurrence of PrPSc types 1 and 2 presented with a clinical phenotype that was different from those of the sCJDMM1 and sCJDMM2 subtypes (Collins et al., 2006). The histopathological and immunohistochemical characteristics were also intermediate between those of sCJDMM2 and sCJDMM1, except for the cerebellum where the spongiform degeneration matched in type and severity with that of sCJDMM1. Furthermore, in the cerebellum, the sCJDMM1-2 PrP immunostaining pattern included the sCJDMM1 pattern in 19 of 20 sCJDMM1-2 cases (95%), which was absent in the cerebellum of the sCJDMM2 cases. Therefore, if there is a defining histopathological feature characterizing sCJDMM1-2, it is the association of abundant large vacuoles and spongiform degeneration, characteristic of sCJDMM2, in the cerebral cortex and sub-cortical regions along with the fine spongiform degeneration and PrP immunostaining pattern of sCJDMM1 in the molecular layer of the cerebellum.
Characteristics of PrPSc in sCJDMM1-2
The brain distribution of PrPSc types 1 and 2, present either together or separately, appeared to be non-random. Both types combined were found in the thalamus of 90%, and in the cerebellum of 56% of the cases. This diverse distribution suggests that the surrounding tissues in different brain regions have a distinct effect on the formation of types 1 and 2 in sCJDMM1-2 with the thalamus especially favouring the co-occurrence of the two types. However, when occurring in isolation, the cerebral cortex was the most permissive of the formation of type 2, while the cerebellum, where PrPSc type 2 alone was never observed, was the least permissive. Overall PrPSc type 1 was better represented than type 2 when they were present separately or together. When occurring separately, type 1 was observed in 69% of the brain regions examined from the 20 sCJDMM1-2 cases; type 2 in the remaining 31%. When present together, the average ratio of PrPSc types 1 and 2 expressed as a percentage of the total PrPSc types 1 and 2 found in the cortical, sub-cortical regions and cerebellum, was 61% for type 1 and 39% for type 2. Both sets of data argue that in sCJDMM1-2 the PrPC to PrPSc conversion process is skewed in favour of type 1. This finding is not surprising since methionine homozygosity at codon 129, as in sCJDMM, strongly favours the formation of PrPSc type 1 (see below) (Gambetti et al., 2003). We observed a good correlation between the representation of the PrPSc type 2 and the extent of the corresponding histopathological lesions, as well as PrP immunostaining pattern in agreement with the conclusions by Puoti et al. (1999) that the PrPSc type influences the histopathology in sCJDMM cases. Since the frontal cortex is the anatomical region having the highest amount of PrPSc type 2 and the thalamus having the highest co-occurrence of PrPSc type 1 and 2, these two brain regions are the most suitable for the tissue diagnosis of sCJDMM1-2.
There was also a definite correlation between disease duration and representation of PrPSc type 2 over that of PrPSc type 1 as well as of sCJDMM2 histological phenotypic features in every brain region examined. This is of interest since perhaps the least ambiguous clinical difference between sCJDMM1 and sCJDMM2 is the disease duration. The finding that in sCJDMM1-2, PrPSc type 2 is under-represented in the cases of short duration but increases over the type 1 (and the phenotype becomes progressively more similar to that of sCJDMM2) with longer disease duration indicates that the rate of PrPSc type 2 formation exceeds that of PrPSc type 1 and raises the question of whether the mere presence of PrPSc type 2 is the determinant of the disease duration or the longer duration determined by other factors that allows for accruing larger amounts of PrPSc type 2. This chicken-and-egg question is difficult to answer. However, two considerations seem to argue that the presence of PrPSc type 2 plays a role in the determination of the disease duration. The first is that the sCJDMM1-2 disease duration is different from those of sCJDMM1 and of sCJDMM2, especially when the current, extensively scrutinized sCJDMM1-2 cases are compared with large series of cases of sCJDMM1 and sCJDMM2; the second is the aforementioned strong correlation between PrPSc type 2 representation and duration of the disease. On the other hand, duration alone is not sufficient to permit the formation of PrPSc type 2 in all cases since three sCJDMM1 cases with disease duration of 8, 11 and 24 months carried PrPSc type 1 only and, as previously shown (Cali et al., 2006), had the same CSI characteristics as the PrPSc of the short duration cases.
An unexpected finding in sCJDMM1-2 is the physical–chemical features of the PrPSc. Using the two mAb, 3F4 and 1E4, which recognize overlapping PrP epitopes but seemingly have distinct affinities for different PrPSc conformations (Yuan et al., 2006, 2008; Gambetti et al., 2008), we observed that, in sCJDMM1-2, type 2 immunoreacted only with 1E4 and not with 3F4, regardless of the immunoblot loading and film exposure. This 3F4 versus 1E4 immunoreactivity difference was observed only when PrPSc types 1 and 2 shared the same anatomical region and not when they were from different regions or cases or were mixed in vitro. CSI that gauges the PrPSc conformation by determining the resistance to denaturation, showed a difference in stability of ∼0.65 M between the PrPSc type 2, exclusively recognized by 1E4, and that recognized by both but it was not statistically different, probably due to the limited number of cases tested. However, the differential mAb recognition indicates that the co-occurrence is likely to affect the conformation of PrPSc type 2 in sCJDMM1-2. The 1E4 properties that determine this selectivity and differentiate this mAb from other antibodies to PrP are unclear. In a recent study (Yuan et al., 2008) we observed that, although 1E4 and 3F4 have adjacent epitopes along human PrP residues 97–112, their accessibility to these epitopes is different because of different neighbouring N-terminal residues. It is possible that the 1E4 selectively detected PK-resistant PrP fragments have N-terminal starting sites that are different from those of the well-characterized PrPr types 1 and 2, even if the difference is based on only a few amino acids. An alternative or complementary explanation is that 1E4 is a partially conformation-sensitive antibody, which is affected by the residual conformation of PrP maintained in polyacrylamide gels. Additional analyses of 1E4, along the lines of the characterization we carried out for a conformational mAb to PrPSc, are needed to resolve this issue (Zou et al., 2004).
Extending the CSI analyses to all sCJDMM subtypes, we observed (as expected) that when PrPSc types 1 and 2 occur separately, either in different cases (as in sCJDMM1 and sCJDMM2) or in different anatomical regions of the same case (as in some of the sCJDMM1-2 brain regions), the two types have distinct CSI characteristics consistent with different conformations, conferring different resistance to denaturation. However, when PrPSc types 1 and 2 occurred together in the same anatomical region, PrPSc type 1 took on CSI characteristics similar to those of the PrPSc type 2, although it apparently maintained the original PK cleavage site. However, this shift in PrPSc features did not require in vivo conditions, such as the presence of intact tissue or long co-existence time of the two types, since it was also observed in vitro after artificially mixing the two PrPSc types. Therefore, even in sCJDMM1-2 this CSI shift might take place in vitro at the time of homogenization and not be present in the intact tissue. Nevertheless, this finding represents, to our knowledge, the first demonstration of a drastic conformational effect of a naturally occurring PrPSc strain on another when they are mixed. This finding raises challenging questions: does the new PrPSc type 2-like conformation confer phenotype-determining characteristics of the PrPSc type 2 to the re-conformed PrPSc type 1? What is the mechanism by which the protease cleavage site of PrPSc type 1 is maintained in the presence of drastic CSI conformational changes? What is the role of each of the two conformations, one characterized by the protease cleavage site and the other by the CSI features, in determining the disease phenotype? How common is the shift of the PrPSc CSI characteristics in prion diseases? These questions must wait for experiments involving transmission to suitable animal models and protein misfolding cyclical amplification (Bartz et al., 2000; Jones and Surewicz, 2005; Meyerett et al., 2008; Jones et al., 2008).
Does sCJDMM1-2 deserve to be considered as a distinct subtype of sCJD?
Although the clinical features overlap, the duration of sCJDMM1-2 is different from those reported in large sCJDMM1 and sCJDMM2 patient populations (Parchi et al., 1999; Pocchiari et al., 2004; Krasnianski et al., 2006; Collins et al., 2006; Heinemann et al., 2007; this study). Furthermore, the contrasting histopathological features (significant large vacuole spongiform degeneration in the cerebral cortex and fine spongiform degeneration in the cerebellum) as well as PrP immunostaining pattern (mixed sCJDMM1 and sCJDMM2 patterns) allow sCJDMM1-2 to be distinguished from sCJDMM1 and sCJDMM2. Finally, in addition to being associated with both PrPSc types, sCJDMM1-2 also seems to often be characterized by the presence of a unique PrPSc type 2 species that immunoreacts with mAb 1E4, but not with 3F4, and other PrP mAb and might have distinct CSI characteristics. This argues that the PrPSc associated with sCJDMM1-2 is not simply a mixture of the two PrPSc types but it has unique features. Therefore, it seems justified to keep sCJDMM1-2 as a separate subtype of sCJDMM at this time.
Speculations on the mechanisms of PrPSc type co-occurrence in sCJDMM1-2
Considerable evidence indicates that PrPSc-type formation is influenced by the genotype at codon 129 of the PrP gene. However, a considerable number of cases appear to escape this rule. In a series of 172 cases of sCJD carrying either PrPSc type 2 or 1 examined in a previous study, PrPSc type 1 was associated with methionine homozygosity at codon 129 (129MM) in 95% of the cases. In contrast, when one or both 129 valine codons (129MV or 129VV) were present, 86% of the cases had PrPSc type 2 (Parchi et al., 1999; Gambetti et al., 2003; Gambetti unpublished data). In the series of 234 sCJDMM cases corrected for PrPSc type distribution reported in this study (see above), 56% were associated with type 1 only and 5% with type 2 only, while in 39%, both types were detectable. These data argue that although the 129MM genotype favours the deposition of type 1 and the presence of one or two 129 valine codons favours PrPSc type 2 deposition, at least 5–14% of the sCJD cases, including the sCJDMM cases, are insensitive to the influence of codon 129.
We propose that these considerations equally apply to individual brain regions. This suggestion carries several implications, which are in agreement with the present findings. First, it is obvious that the chances to detect cases of sCJDMM1-2 are directly related to the number of areas examined. Second, different brain regions may have different degrees of permissiveness for the formation of the ‘escaped’ PrPSc type. We observed that the thalamus was the most permissive location for the deposition of both PrPSc types and the cerebellum the least permissive, apparently not supporting the formation of type 2 alone. Third, the examination of the relative representation of the two PrPSc types related to disease duration suggests that PrPSc deposition in the cases of sCJDMM1-2 starts with predominantly or exclusively type 1. Then, after it appears, type 2 increases at a faster rate since, relative to the type 1, its amount increases progressively with the disease duration. The possibility that PrPSc augments because of types 1 to 2 conversion cannot be excluded. However, this possibility seems unlikely because of the strong correlation between type and histopathological features. If the conversion of types 1 and 2 occurred, the histopathological features associated with PrPSc type 1 should be over-represented compared to the amount of PrPSc type 1. This is because it is unlikely that these features would disappear or convert into the features associated with the type 2.
Funding
NIH Award AG-14359; CDC Award UR8/CCU515004; the Charles S. Britton Fund; the CJD Foundation.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors are deeply grateful to Ms Diane Kofskey, Ms Phyllis Scalzo and Ms Kay Edmonds for their invaluable technical help.
Glossary
Abbreviations
- CSI
conformational stability immunoassay
- mAb
monoclonal antibodies
- MM
homozygous methionine
- MV
heterozygous methionine/valine
- NPDPSC
National Prion Disease Pathology Surveillance Centre
- PK
proteinase K
- PrP
prion protein
- sCJD
sporadic Creutzfeldt–Jakob disease
- VV
homozygous valine
References
- Bartz JC, Bessen RA, McKenzie D, Marsh RF, Aiken JM. Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy. J Virol. 2000;74:5542–7. doi: 10.1128/jvi.74.12.5542-5547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali I, Castellani R, Yuan J, Al-Shekhlee A, Cohen ML, Xiao X, et al. Classification of sporadic Creutzfeldt-Jakob disease revisited. Brain. 2006;129(Pt 9):2266–77. doi: 10.1093/brain/awl224. [DOI] [PubMed] [Google Scholar]
- Chen SG, Teplow DB, Parchi P, Teller JK, Gambetti P, Autilio-Gambetti L. Truncated forms of the human prion protein in normal brain and prion diseases. J Biol Chem. 1995;270:19173–80. doi: 10.1074/jbc.270.32.19173. [DOI] [PubMed] [Google Scholar]
- Collins SJ, Sanchez-Juan P, Masters CL, Klug GM, van Duijn C, Poleggi A, et al. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob disease. Brain. 2006;129(Pt 9):2278–87. doi: 10.1093/brain/awl159. [DOI] [PubMed] [Google Scholar]
- Gambetti P, Dong Z, Yuan J, Xiao X, Zheng M, Alshekhlee A, et al. A novel human disease with abnormal prion protein sensitive to protease. Ann Neurol. 2008;63:697–708. doi: 10.1002/ana.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213–39. doi: 10.1093/bmb/66.1.213. [DOI] [PubMed] [Google Scholar]
- Haïk S, Peoc’h K, Brandel JP, Privat N, Laplanche JL, Faucheux BA, et al. Striking PrPSc heterogeneity in inherited prion diseases with the D178N mutation. Ann Neurol. 2004;56:909–10. doi: 10.1002/ana.20327. author reply 910–11. [DOI] [PubMed] [Google Scholar]
- Head MW, Bunn TJ, Bishop MT, McLoughlin V, Lowrie S, McKimmie CS, et al. Prion protein heterogeneity in sporadic but not variant Creutzfeldt-Jakob disease: UK cases 1991-2002. Ann Neurol. 2004;55:851–9. doi: 10.1002/ana.20127. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Krasnianski A, Meissner B, Varges D, Kallenberg K, Schulz-Schaeffer WJ, et al. Creutzfeldt-Jakob disease in Germany: a prospective 12-year surveillance. Brain. 2007;130(Pt 5):1350–9. doi: 10.1093/brain/awm063. [DOI] [PubMed] [Google Scholar]
- Jones EM, Surewicz WK. Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell. 2005;121:63–72. doi: 10.1016/j.cell.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Jones M, Peden AH, Wight D, Prowse C, Macgregor I, Manson J, et al. Effects of human PrPSc type and PRNP genotype in an in-vitro conversion assay. Neuroreport. 2008;19:1783–6. doi: 10.1097/WNR.0b013e328318edfa. [DOI] [PubMed] [Google Scholar]
- Kascsak RJ, Rubenstein R, Merz PA, Tonna-DeMasi M, Fersko R, Carp RI, et al. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987;61:3688–93. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Surewicz WK, Petersen RB, Zou WQ, Chen SG, Parchi P, et al. Inherited prion diseases. In: Prusiner SB, editor. Prion biology and disease. New York: Cold Spring Harbor Laboratory Press; 2004. pp. 673–775. [Google Scholar]
- Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, et al. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997;390:74–7. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- Kovács GG, Head MW, Hegyi I, Bunn TJ, Flicker H, Hainfellner JA, et al. Immunohistochemistry for the prion protein: comparison of different monoclonal antibodies in human prion disease subtypes. Brain Pathol. 2002;12:1–11. doi: 10.1111/j.1750-3639.2002.tb00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnianski A, Schulz-Schaeffer WJ, Kallenberg K, Meissner B, Collie DA, Roeber S, et al. Clinical findings and diagnostic tests in the MV2 subtype of sporadic CJD. Brain. 2006;129(Pt 9):2288–96. doi: 10.1093/brain/awl123. [DOI] [PubMed] [Google Scholar]
- Langeveld JP, Jacobs JG, Erkens JH, Bossers A, van Zijderveld FG, van Keulen LJ. Rapid and discriminatory diagnosis of scrapie and BSE in retro-pharyngeal lymph nodes of sheep. BMC Vet Res. 2006;2:19. doi: 10.1186/1746-6148-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis V, Hill AF, Klug GM, Boyd A, Masters CL, Collins SJ. Australian sporadic CJD analysis supports endogenous determinants of molecular-clinical profiles. Neurology. 2005;65:113–18. doi: 10.1212/01.wnl.0000167188.65787.a0. [DOI] [PubMed] [Google Scholar]
- Meyerett C, Michel B, Pulford B, Spraker TR, Nichols TA, Johnson T, et al. In vitro strain adaptation of CWD prions by serial protein misfolding cyclic amplification. Virology. 2008;382:267–76. doi: 10.1016/j.virol.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Monari L, Chen SG, Brown P, Parchi P, Petersen RB, Mikol J, et al. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: different prion proteins determined by a DNA polymorphism. Proc Natl Acad Sci USA. 1994;91:2839–42. doi: 10.1073/pnas.91.7.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari S, Capellari S, Giese A, Westner I, Baruzzi A, Ghetti B, et al. Effects of different experimental conditions on the PrPSc core generated by protease digestion: implications for strain typing and molecular classification of CJD. J Biol Chem. 2004;279:16797–804. doi: 10.1074/jbc.M313220200. [DOI] [PubMed] [Google Scholar]
- Notari S, Capellari S, Langeveld J, Giese A, Strammiello R, Gambetti P, et al. A refined method for molecular typing reveals that co-occurrence of PrP(Sc) types in Creutzfeldt-Jakob disease is not the rule. Lab Invest. 2007;87:1103–12. doi: 10.1038/labinvest.3700676. [DOI] [PubMed] [Google Scholar]
- Pan T, Li R, Kang SC, Pastore M, Wong BS, Ironside J, et al. Biochemical fingerprints of prion diseases: scrapie prion protein in human prion diseases that share prion genotype and type. J Neurochem. 2005;92:132–42. doi: 10.1111/j.1471-4159.2004.02859.x. [DOI] [PubMed] [Google Scholar]
- Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen SG, et al. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 1996;39:767–78. doi: 10.1002/ana.410390613. [DOI] [PubMed] [Google Scholar]
- Parchi P, Capellari S, Chen SG, Petersen RB, Gambetti P, Kopp N, et al. Typing prion isoforms. Nature. 1997;386:232–4. doi: 10.1038/386232a0. [DOI] [PubMed] [Google Scholar]
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999;46:224–33. [PubMed] [Google Scholar]
- Parchi P, Zou W, Wang W, Brown P, Capellari S, Ghetti B, et al. Genetic influence on the structural variations of the abnormal prion protein. Proc Natl Acad Sci USA. 2000;97:10168–72. doi: 10.1073/pnas.97.18.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore M, Chin SS, Bell KL, Dong Z, Yang Q, Yang L, et al. Creutzfeldt-Jakob disease (CJD) with a mutation at codon 148 of prion protein gene: relationship with sporadic CJD. Am J Pathol. 2005;167:1729–38. doi: 10.1016/S0002-9440(10)61254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz D, Williamson RA, Legname G, Matsunaga Y, Vergara J, Burton DR, et al. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron. 2002;34:921–32. doi: 10.1016/s0896-6273(02)00726-2. [DOI] [PubMed] [Google Scholar]
- Pocchiari M, Puopolo M, Croes EA, Budka H, Gelpi E, Collins S, et al. Predictors of survival in sporadic Creutzfeldt-Jakob disease and other human transmissible spongiform encephalopathies. Brain. 2004;127(Pt 10):2348–59. doi: 10.1093/brain/awh249. [DOI] [PubMed] [Google Scholar]
- Polymenidou M, Stoeck K, Glatzel M, Vey M, Bellon A, Aguzzi A. Coexistence of multiple PrPSc types in individuals with Creutzfeldt-Jakob disease. Lancet Neurol. 2005;4:805–14. doi: 10.1016/S1474-4422(05)70225-8. Erratum in: Lancet Neurol. 2005; 4: 795. [DOI] [PubMed] [Google Scholar]
- Puoti G, Giaccone G, Rossi G, Canciani B, Bugiani O, Tagliavini F. Sporadic Creutzfeldt-Jakob disease: co-occurrence of different types of PrP(Sc) in the same brain. Neurology. 1999;53:2173–6. doi: 10.1212/wnl.53.9.2173. [DOI] [PubMed] [Google Scholar]
- Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, et al. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med. 1998;4:1157–65. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- Schoch G, Seeger H, Bogousslavsky J, Tolnay M, Janzer RC, Aguzzi A, et al. Analysis of prion strains by PrPSc profiling in sporadic Creutzfeldt-Jakob disease. PLoS Med. 2006;3:e14. doi: 10.1371/journal.pmed.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley BA. Urea and guanidine hydrochloride denaturation curves. Methods Mol Biol. 1995;40 doi: 10.1385/0-89603-301-5:177. 177–90 [Review] [DOI] [PubMed] [Google Scholar]
- Uro-Coste E, Cassard H, Simon S, Lugan S, Bilheude JM, Perret-Liaudet A, et al. Beyond PrPres type 1/type 2 dichotomy in Creutzfeldt-Jakob disease. PLoS Pathog. 2008;4:e1000029. doi: 10.1371/journal.ppat.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Dong Z, Guo JP, McGeehan J, Xiao X, Wang J, et al. Accessibility of a critical prion protein region involved in strain recognition and its implications for the early detection of prions. Cell Mol Life Sci. 2008;65:631–43. doi: 10.1007/s00018-007-7478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Kinter M, McGeehan J, Perry G, Kneale G, Gambetti P, et al. Concealment of epitope by reduction and alkylation in prion protein. Biochem Biophys Res Commun. 2005;326:652–9. doi: 10.1016/j.bbrc.2004.11.088. [DOI] [PubMed] [Google Scholar]
- Yuan J, Xiao X, McGeehan J, Dong Z, Cali I, Fujioka H, et al. Insoluble aggregates and protease-resistant conformers of prion protein in uninfected human brains. J Biol Chem. 2006;281:34848–58. doi: 10.1074/jbc.M602238200. [DOI] [PubMed] [Google Scholar]
- Yull HM, Ritchie DL, Langeveld JP, van Zijderveld FG, Bruce ME, Ironside JW, et al. Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am J Pathol. 2006;168:151–7. doi: 10.2353/ajpath.2006.050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanusso G, Liu D, Ferrari S, Hegyi I, Yin X, Aguzzi A, et al. Prion protein expression in different species: analysis with a panel of new mAbs. Proc Natl Acad Sci USA. 1998;95:8812–16. doi: 10.1073/pnas.95.15.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou WQ, Capellari S, Parchi P, Sy MS, Gambetti P, Chen SG. Identification of novel proteinase K-resistant C-terminal fragments of PrP in Creutzfeldt-Jakob disease. J Biol Chem. 2003;278:40429–36. doi: 10.1074/jbc.M308550200. [DOI] [PubMed] [Google Scholar]
- Zou WQ, Zheng J, Gray DM, Gambetti P, Chen SG. Antibody to DNA detects scrapie but not normal prion protein. Proc Natl Acad Sci USA. 2004;101:1380–5. doi: 10.1073/pnas.0307825100. [DOI] [PMC free article] [PubMed] [Google Scholar]