Abstract
Renal medullary superoxide (O2−) increases in angiotensin (Ang) II-dependent hypertension. O2− increases thick ascending limb Na transport, but the effect of Ang II–dependent hypertension on the thick ascending limb is unknown. We hypothesized that Ang II–dependent hypertension increases thick ascending limb NaCl transport because of enhanced O2− production and increased protein kinase C (PKC) α activity. We measured the effect of Ang II–dependent hypertension on furosemide-sensitive oxygen consumption (a measure of Na transport), O2− production, and PKCα translocation (a measure of PKCα activity) in thick ascending limb suspensions. Ang II–dependent hypertension increased furosemide-sensitive oxygen consumption (26.2±1.0% versus 36.6±1.2% of total oxygen consumption; P<0.01). O2− was also increased (1.1±0.2 versus 3.2±0.5 nmol of O2−/min per milligram of protein; P<0.03) in thick ascending limbs. Unilateral renal infusion of Tempol decreased O2− (2.4±0.4 versus 1.2±0.2 nmol of O2−/min per milligram of protein; P<0.04) and furosemide-sensitive oxygen consumption (32.8±1.3% versus 24.0±2.1% of total oxygen consumption; P<0.01) in hypertensive rats. Tempol did not affect O2− or furosemide-sensitive oxygen consumption in normotensive controls and did not alter systolic blood pressure. Ang II–dependent hypertension increased PKCα translocation (5.7±0.3 versus 13.8±1.4 AU per milligram of protein; P<0.01). Unilateral renal infusion of Tempol reduced PKCα translocation (5.0±0.9 versus 10.4±2.6 AU per milligram of protein; P<0.04) in hypertensive rats. Unilateral renal infusion of the PKCα inhibitor Gö6976 reduced furosemide-sensitive oxygen consumption (37.4±1.5% versus 25.1±1.0% of total oxygen consumption; P<0.01) in hypertensive rats. We conclude that Ang II–dependent hypertension enhances thick ascending limb Na transport–related oxygen consumption by increasing O2− and PKCα activity.
Keywords: ion transport, reactive oxygen species, kidney, Na/K/2Cl cotransporter
Superoxide (O2− is a free radical that contributes to the development of high blood pressure.1,2 During angiotensin (Ang) II–dependent hypertension there is an increased O2− production in the cardiovascular system,3 central nervous system,4 and kidney.5,6 Elevated oxidative stress in the kidney may contribute to the development and maintenance of Ang II–dependent hypertension. Enhanced O2− production in the renal medulla increases blood pressure,7 and renal O2− scavenging decreases blood pressure in several models of hypertension.6,8,9
Augmented O2− production in the kidney increases blood pressure by promoting Na retention.5,7 O2−-induced Na retention may occur as a result of decreased glomerular filtration rate and renal blood flow.5 However, O2− may also decrease urinary volume and urinary Na excretion without changing renal blood flow and glomerular filtration rate. Such data indicate that O2− has a direct effect on tubular transport.10
The thick ascending limb reabsorbs 25% to 30% of the total filtered NaCl load. Increases in Na transport by this segment contribute to several forms of hypertension,11–13 and diuretics that decrease thick ascending limb NaCl absorption are frequently used to reduce blood pressure.14 Acute increases in exogenous O2− in the thick ascending limb enhance Na transport,15 whereas scavenging endogenous O2− decreases it.16 We have shown previously that O2− stimulates NaCl reabsorption by activating protein kinase C (PKC).17
Ang II acutely enhances NaCl reabsorption in the thick ascending limb,18 whereas blockade of Ang II receptors decreases it.19 Brief exposure of thick ascending limbs to Ang II also results in augmented O2− production.20 Thus, it is likely that at least part of the acute effect of Ang II on the thick ascending limb is because of O2−. However, the chronic effects of Ang II–induced hypertension on NaCl absorption in this segment and the role of O2− have not been studied. We hypothesize that Ang II–dependent hypertension increases Na transport–related oxygen consumption by thick ascending limbs because of enhanced O2− production and the resulting PKCα activation.
Methods
Animals
This study was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee. All of the studies were conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats weighing 200 to 250 g (Charles River Breeding Laboratories, Wilmington, Mass) were fed a diet containing 0.4% Na and 1% K (diet 8640; Harlan-Teklad) for 7 days before implantation of minipumps.
Ang II Infusion
Rats were anesthetized with ketamine (60 mg/kg IP) and xylazine (20 mg/kg IP) before surgery. Alzet D1007 (Durect) osmotic minipumps were implanted subcutaneously between the scapulae. Ang II (520 ng/kg per minute; Bachem) was infused for 7 days as reported previously.21,22
Medullary Drug Delivery
To deliver drugs into the renal outer medulla, the flow moderator of a prefilled Alzet D1007 osmotic minipump was connected to a 30-mm-long polyethylene 60 tubing. The other end of the tubing was attached by thermal fusion to a 130-mm-long polyethylene 10 tubing designed to be inserted into the renal medulla. To avoid clotting of the catheter, the latter was fenestrated in one side 10 mm from the tip using a 30-gauge needle. To assure complete drug delivery, the catheters were filled with the drug before attaching the minipump flow moderator. Minipump with fenestrated catheter was primed overnight at 37°C in sterile 0.9% saline. The next day, rats were anesthetized with ketamine (60 mg/kg IP)/xylazine (20 mg/kg IP), shaved, and cleaned with povidone-iodine 10%. Then, the left kidney was exposed via flank dorsolumbar incision. A tract was created into the kidney by inserting and immediately retrieving a premeasured 1.5-mm2 section/14-mm deep solid polypropylene rod at the corticomedullary boundary. The rod was inserted from the inferior pole, along the longitudinal axis of the kidney and perpendicular to the medullary rays. After that, the fenestrated catheter was inserted into the tract and fixed to the kidney by gluing it with 10 µL of medical cyanoacrylate glue (Vetbond; 3M) and a 7×7-mm dressing sponge (Topper, Johnson and Johnson). To avoid detachment of the catheter, a loop was made, and it was sutured to the inner abdominal muscle layer. The abdominal cavity was washed with sterile saline, and the muscular layer was sutured. The tubing was tunneled under the skin to the scapulae, and the minipump was implanted as described above. On the day of the experiment, the catheter, minipump, and flow moderator were retrieved from the animal and tested for leakage. To verify complete delivery of the drug, the remaining volume in the pump was measured.
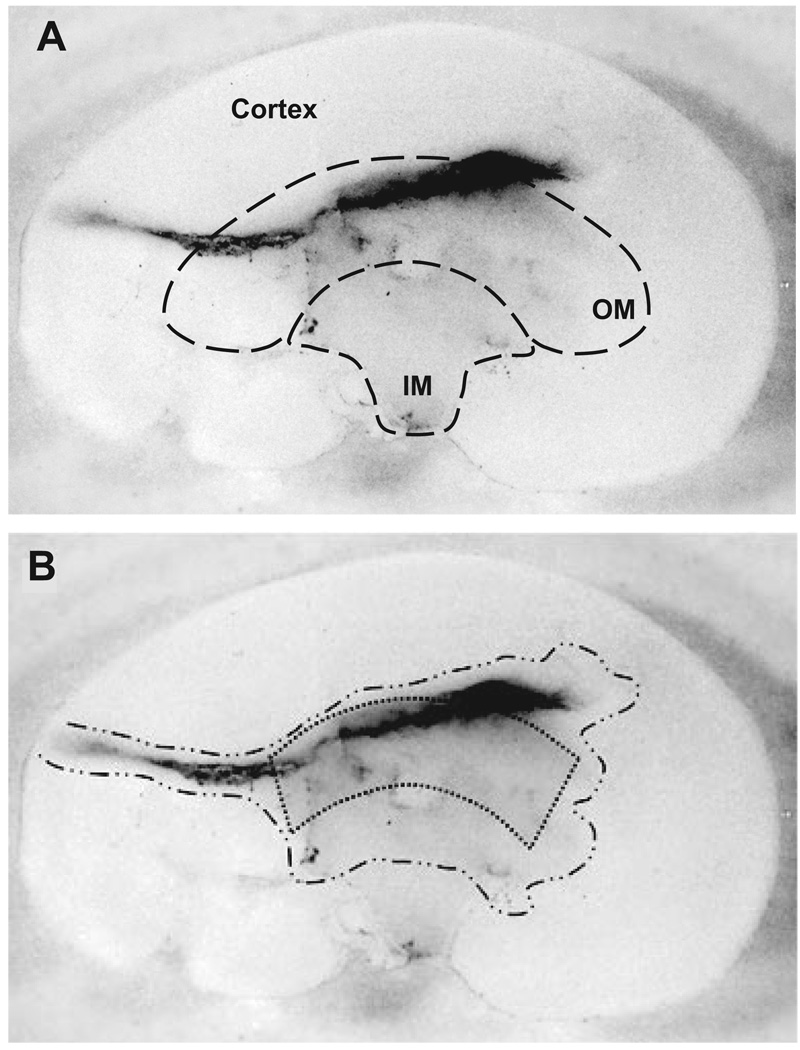
To test the renal medullary delivery of drugs, we first loaded a minipump with the low molecular weight protein dye Coomassie blue R250 (50 µg/kg per minute) and infused it into the left kidney of a Sprague-Dawley rat as described above. After 4 days of infusion, intense traces of the dye were found in the catheter’s pathway, the corticomedullary boundary, and the outer medullary region. To a much less extent, the dye was also present in the inner medullary region (Figure 1A). These data indicate the feasibility of the technique.
Figure 1.
A, Longitudinal section of the infused kidney. B, Area stained by the infusion of Coomassie blue R250. Intense traces of dye were found in the catheter’s pathway corticomedullary boundary and outer medulla. To much less extent, the dye was found in the inner medullary region. Stippled area depicts the area of tissue used to perform the experiments.
The O2− scavenger 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol; Sigma-Aldrich; 7.5 mg/kg per minute), the PKCα/β-selective inhibitor Gö6976 (Biomol; 21 ng/kg per minute), or vehicles for these drugs were infused into the renal medulla of the left kidney for 7 days, as described above. Priming of minipumps containing Tempol was performed in the dark because of the photosensitive nature of the compound.
Systolic Blood Pressure Measurements
Systolic blood pressure was measured by tail cuff (BP-2000; Visitech) at 1, 3, and 7 days before Ang II infusion. Systolic blood pressure on the day before implanting minipumps was taken as basal systolic blood pressure. After initiating Ang II infusion, systolic blood pressure was measured on days 1, 3, and 7. Blood pressure was measured 3 times in 3 minutes. The 3 values were averaged to obtain a single blood pressure measurement for each day.
Medullary Thick Ascending Limb Suspensions
Medullary thick ascending limb suspensions were prepared as described previously.17 At least 93% of tubules in suspension were thick ascending limbs.23 Kidneys were perfused retrograde via the abdominal aorta with 40 mL of 0.1% collagenase type I (Sigma-Aldrich) and 100 U of heparin in HEPES-buffered physiological saline containing the following (in mmol/L): 130 NaCl, 4 KCl, 2.5 NaH2PO4, 1.2 MgSO4, 2 calcium delactate, 5.5 glucose, 6 D/L-alanine, 1 trisodium citrate, and 10 HEPES. The inner stripe of the outer medulla was dissected from coronal slices of the kidney. In experiments involving medullary drug delivery, only the outer medullary tissue adjacent to the catheter was used (Figure 1B). The tissue was then minced and incubated at 37°C for 30 minutes in 0.1% collagenase type I. During this time, the suspension was agitated and gassed with 100% oxygen every 5 minutes. Tissue was then centrifuged at 93g for 2 minutes, resuspended in cold HEPES-buffered physiological saline, and stirred on ice for 30 minutes, producing a suspension of tubules. The suspension was filtered through a 250-µm nylon mesh and spun again for 2 minutes. The pellet was washed and resuspended in 1 mL of cold HEPES-buffered physiological saline.
Measurements of O2− Production
Aliquots (200 µL) of thick ascending limb suspensions were placed in polypropylene tubes. Then, 700 µL of HEPES-buffered physiological saline was added, and tubes were placed on ice. Lucigenin (Sigma-Aldrich) was added to the suspensions to a final concentration of 5 µmol/L, and the tubules were incubated for 30 minutes at 37°C. Tubes were placed in a luminometer (model FB12/Sirius, Zylux Co) and maintained at 37°C. The average of the last 3 of 10 consecutive measurements was calculated for each sample. The O2− scavenger tiron (Sigma-Aldrich) was added to the tube to a final concentration of 10 mmol/L, and measurements were repeated. The difference in average luminescence between samples with and without tiron was used to calculate the luminescence produced by O2−. Measurements were normalized for protein content.
Furosemide-Sensitive Oxygenv Consumption Measurements
Furosemide-sensitive oxygen consumption is an indicator of Na transport by thick ascending limbs, because furosemide inhibits NKCC2, the transporter responsible for vectorial NaCl transport, and 25% to 40% of total oxygen consumption by this segment is related to NaCl transport.23,24 Thick ascending limbs were resuspended in 0.1 mL of HEPES-buffered physiological saline warmed to 37°C and equilibrated with 100% oxygen. Then they were added to a closed chamber at 37°C, and oxygen consumption was recorded continuously. An initial constant slope was established at the beginning of each experiment, and then furosemide (10−4 mol/L; Hospira) was added. All of the experiments were completed within 15 minutes. At the end of the experiment, an aliquot of the suspension was used to determine protein content.
Measurements of PKCα and PKCβ Translocation
PKCα and PKCβ have been shown to translocate to membrane-enriched fractions under different conditions after activation25,26; therefore, we used translocation of these enzymes as a measure of their activation. Medullary thick ascending limbs were resuspended in homogenization buffer containing the following (in mmol/L): 50 Tris-HCl, 150 NaCl, 0.1 (4[2-aminoethyl])-benzene sulfonyl fluoride, and 4 benzamidine; as well as (in µg/mL) 5 antipain, 10 aprotinin, 5 leupeptin, 5 chymostatin, and 5 pepstatin A (Sigma-Aldrich). The suspension was homogenized, sonicated, and then spun at 1200g for 10 minutes. The supernatant was ultracentrifuged at 100 000g for 60 minutes. The resulting supernatant was then considered the cytosolic fraction. The pellet was resuspended in homogenization buffer plus 0.1% Triton X-100 and ultracentrifuged at 100 000g for 60 minutes. The supernatant was considered the particulate fraction.
Cytosolic and particulate fractions were loaded on SDS-polyacrylamide gels, and proteins were separated by electrophoresis. PKCα and PKCβ levels were detected by Western blot using PKCα and PKCβ monoclonal antibodies (BD Transduction Laboratories), as we described previously.17 A decrease in the amount of PKCα and PKCβ from the cytosolic fraction and, consequently, an increase in particulate fraction were taken as activation of the enzyme. Band intensities were normalized to protein loading.
Determination of Protein Content
Total protein content was determined using Coomassie Plus reagent (Pierce), based on Bradford’s colorimetric method.
Statistics
Data are reported as means±SEMs. Differences in means were analyzed using Welch t test for unpaired experiments. This test adjusts the degrees of freedom for the test to adjust for unequal variation. For paired experiments, Student t test was used. Statistical analysis was performed by the Henry Ford Hospital Department of Biostatistics and Epidemiology.
Results
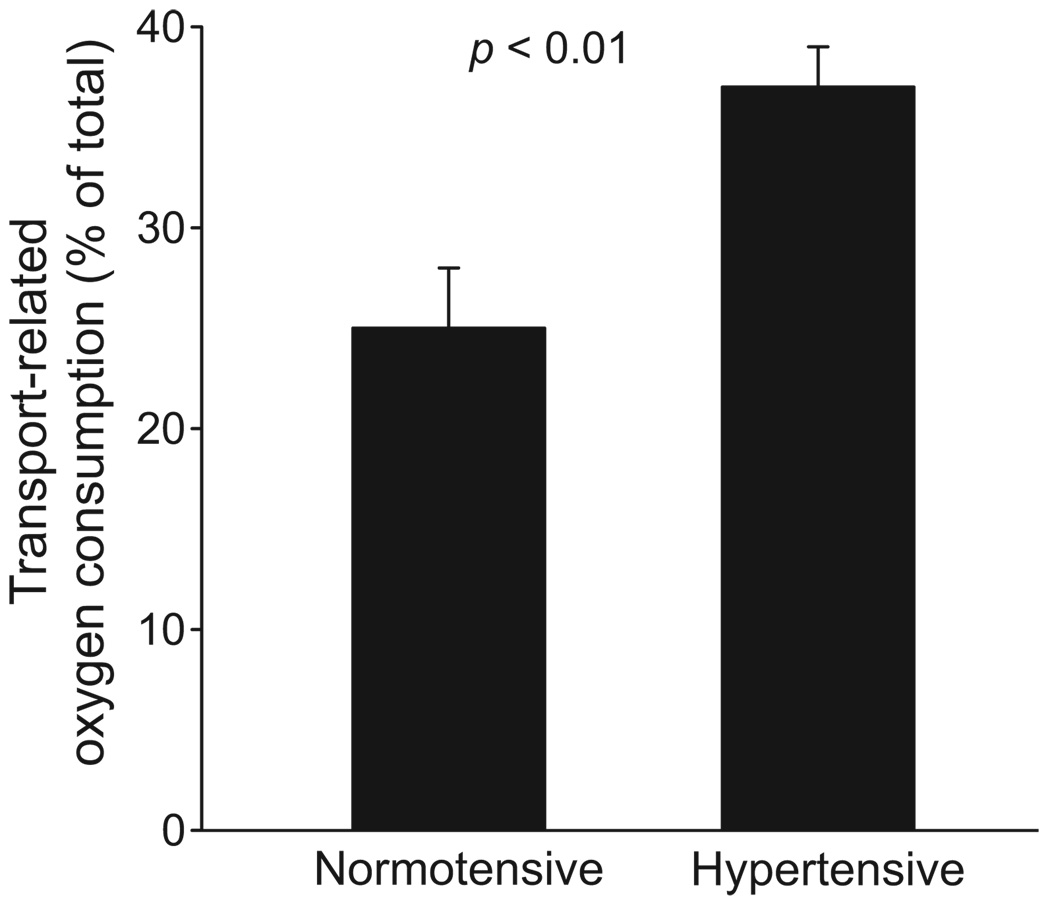
We first studied the effects of Ang II–dependent hypertension on thick ascending limb NaCl absorption. Furosemide-sensitive oxygen consumption was used as a measure of Na transport. This technique can be used for the following reasons: (1) furosemide inhibits Na/K/2Cl cotransporter activity, and this transporter is responsible for NaCl entry into the cell and, thus, vectorial NaCl transport in this segment; (2) 25% to 40% of total oxygen consumption by thick ascending limbs is related to Na transport; and (3) oxygen consumption is stoichiometrically related to Na transport.23,24 We found that furosemide decreased oxygen consumption by 36.6±1.2% of total oxygen consumption (from 109.6±15.2 to 68.3±13.1 nmol of oxygen per minute per milligram of protein) in suspensions from Ang II–dependent hypertension rats. In contrast, furosemide decreased oxygen consumption in thick ascending limbs from vehicle-infused rats by only 26.2±1.0% of total oxygen consumption (from 86.7±8.1 to 63.2±8.9 nmol of oxygen per minute per milligram of protein; Figure 2; P<0.01 versus Ang II–infused rats; n=6). These data indicate that Ang II–dependent hypertension increases Na transport–related oxygen consumption by thick ascending limbs.
Figure 2.
Effect of Ang II–dependent hypertension on thick ascending limb transport–related oxygen consumption (n=6). Transport-related oxygen consumption was measured as furosemide-sensitive oxygen consumption.
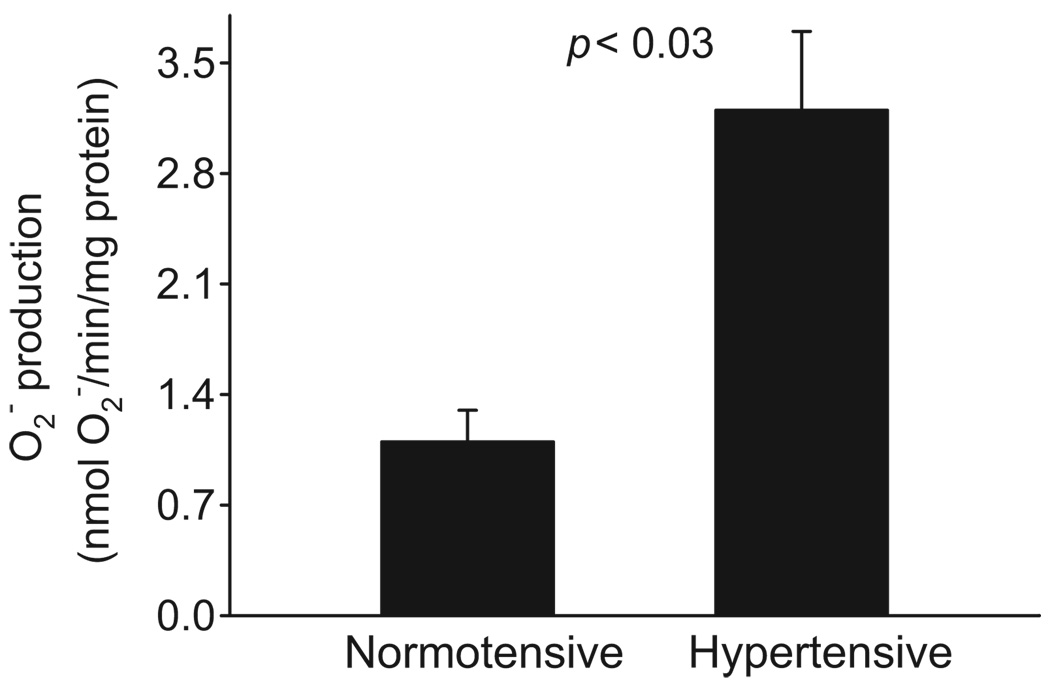
Because Ang II–dependent hypertension increases renal O2− production, and acute O2− treatment stimulates NaCl in the thick ascending limb,27 we next measured the effect of Ang II–dependent hypertension on O2− production in vehicle and Ang II–infused rats. We found that O2− production by thick ascending limbs of Ang II–infused rats was 3.2±0.5 nmol of O2− per minute per milligram of protein. In contrast, O2− production was only 1.1±0.2 nmol of O2− per minute per milligram of protein by thick ascending limbs of vehicle-infused rats (Figure 3; P<0.03; n=8). Systolic blood pressure of Ang II–dependent hypertensive rats was 204±4 mmHg compared with 112±4 mm Hg for controls. Thus, Ang II–dependent hypertension increased O2− production by thick ascending limbs.
Figure 3.
Effect of Ang II–dependent hypertension on O2− production in the thick ascending limb (n=8).
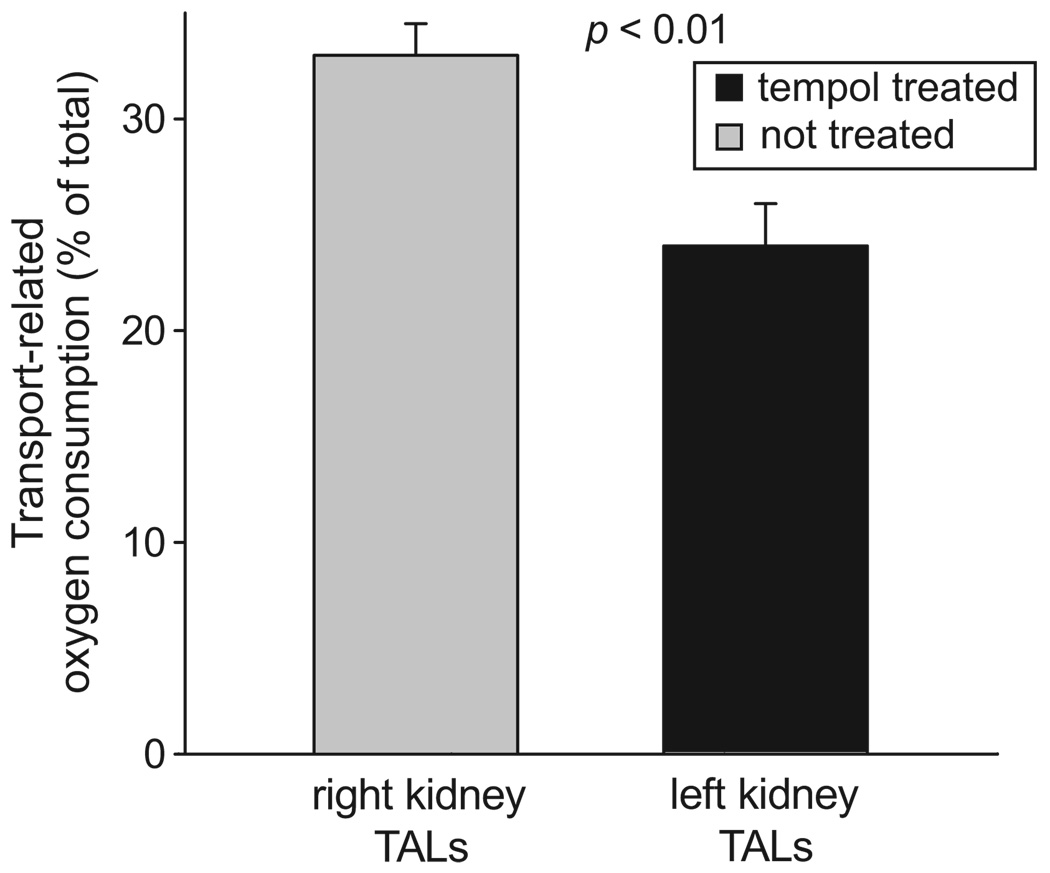
To study whether increased O2− was responsible for enhanced Na transport–related oxygen consumption, we measured furosemide-sensitive oxygen consumption in thick ascending limb suspensions of Tempol-infused and noninfused (control) kidneys from Ang II–infused rats. We found that furosemide decreased oxygen consumption by thick ascending limbs from Tempol-infused kidneys by 24.0±2.1% of total oxygen consumption (from 93.8±9.2 to 71.1±11.3 nmol of oxygen per minute per milligram of protein). In contrast, furosemide decreased oxygen consumption by 32.8±1.3% of total oxygen consumption (from 129.1±9.2 to 87.9±12.4 nmol of oxygen per minute per milligram protein) in suspensions from the untreated kidneys (Figure 4; P<0.01 versus Tempol-infused; n=8). Tempol infusion did not change furosemide-sensitive oxygen consumption in infused and noninfused kidneys from normotensive animals (28.7±1.5% versus 26.5±3.0% of total oxygen consumption; P value not significant; n=5). Also, vehicle infusion had no effect on furosemide-sensitive oxygen consumption in infused and noninfused kidneys from normotensive animals. These data indicate that Tempol decreased Na transport–related oxygen consumption in Ang II–dependent hypertensive rats and that placement of the catheter did not alter the thick ascending limb furosemide-sensitive oxygen consumption.
Figure 4.
Effect of renal unilateral infusion of Tempol on thick ascending limb (TAL) transport-related oxygen consumption during Ang II–dependent hypertension (n=8). Transport-related oxygen consumption was measured as furosemide-sensitive oxygen consumption.
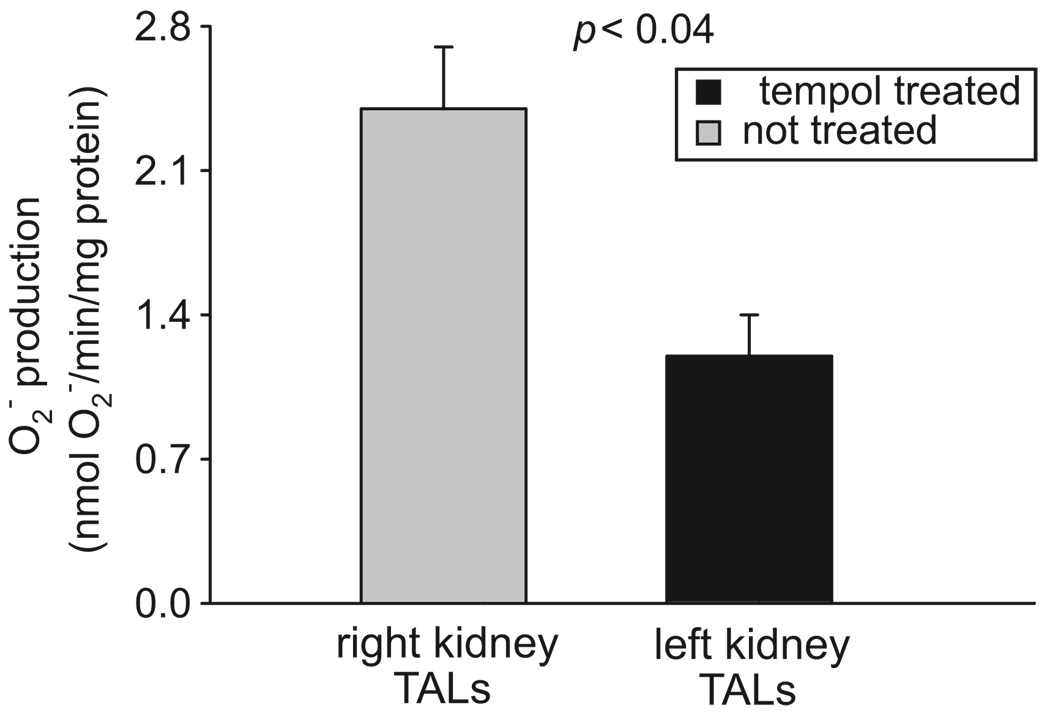
To show that the effect of Tempol was because of O2− scavenging, we measured O2− production by thick ascending limbs from Tempol-infused and control kidneys of Ang II–infused rats. We found that O2− production by thick ascending limbs from the control kidney was 2.4±0.4 nmol of O2− per minute per milligram of protein. In contrast, O2− production by thick ascending limbs of the Tempol-infused kidney was 1.2±0.2 nmol of O2− per minute per milligram of protein (Figure 5; P<0.04; n=7). Intramedullary infusion of vehicle had no effect on O2− production in normotensive animals. These data indicate that intramedullary infusion of Tempol decreased O2− levels in the ipsilateral kidney without affecting O2− production in the contralateral kidney.
Figure 5.
Effect of renal unilateral infusion of Tempol on thick ascending limb (TAL) O2− production in Ang II–dependent hypertension (n=7).
To test whether the changes in furosemide-sensitive oxygen consumption were because of reduced blood pressure caused by Tempol, we measured systolic blood pressure. Intramedullary infusion of Tempol into one kidney had no effect on systolic blood pressure at day 7 in Ang II–infused rats (204±4 versus 207±11 mm Hg; P value not significant; n=8). These data indicate that the observed decrease in furosemide-sensitive oxygen consumption after Tempol infusion was not because of reduced systolic blood pressure.
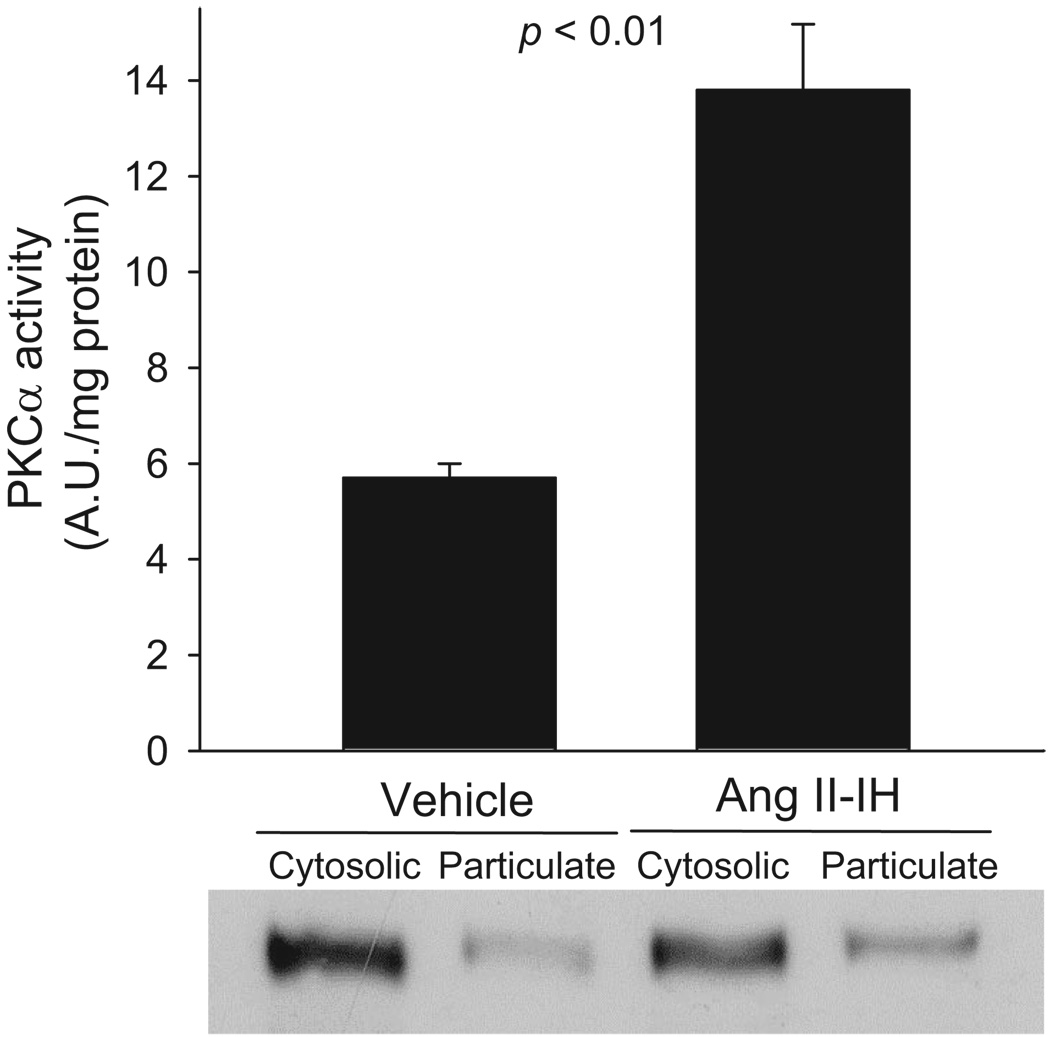
Acute treatment of thick ascending limbs with O2− has been shown to stimulate PKCα activity.17 Also, several forms of hypertension increase PKCβ activity.28,29 Thus, we measured PKCα and PKCβ activation after 7 days of Ang II infusion by measuring translocation of these enzymes. We found that PKCα translocation to membrane-enriched fractions was 13.8±1.4 AU per mg of protein in thick ascending limbs from Ang II–infused animals. In contrast, PKCα translocation was only 5.7±0.3 AU/mg of protein in thick ascending limbs from vehicle-infused animals (Figure 6; P<0.01; n=6). Ang II–dependent hypertension did not significantly increase PKCβ translocation (32.3±4.9 versus 28.6±3.6 AU per mg of protein; P value not significant; n=5).
Figure 6.
Effect of Ang II–dependent hypertension on thick ascending limb PKCα translocation (n=6). Equal amounts of protein were loaded from normotensive and hypertensive cytosolic (8 µg) and particulate (20 µg) fractions.
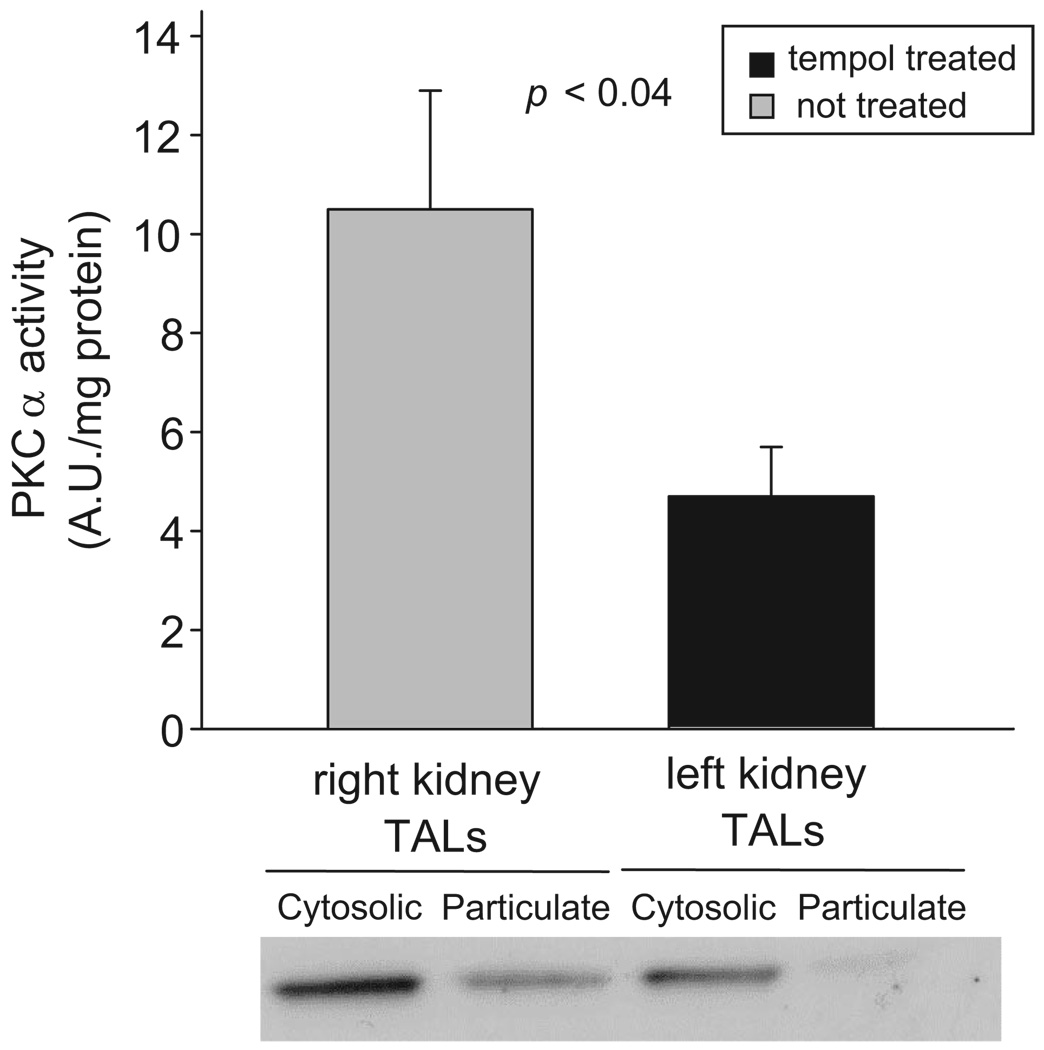
To study whether elevated PKCα translocation to membrane-enriched fractions was because of increased O2− levels, we measured PKCα translocation after intramedullary infusion of Tempol into one kidney of Ang II–dependent hypertensive rats. We found that, in thick ascending limbs from the Tempol-infused kidney, PKCα translocation was 5.0±0.9 AU per mg of protein, whereas in tubules from the contralateral kidney it was 10.4±2.6 AU per mg of protein (Figure 7; P<0.04; n=7). Intramedullary infusion of vehicle had no effect on PKCα translocation in thick ascending limbs from normotensive rats. These data indicate that chronic increases in O2− levels, rather than Ang II per se, or systolic blood pressure, stimulate PKCα activity.
Figure 7.
Effect of renal unilateral infusion of Tempol on PKCα translocation in the thick ascending limb (TAL) in Ang II–dependent hypertension (n=7). Equal amounts of protein were loaded from Tempol-treated and nontreated cytosolic (8 µg) and particulate (20 µg) fractions.
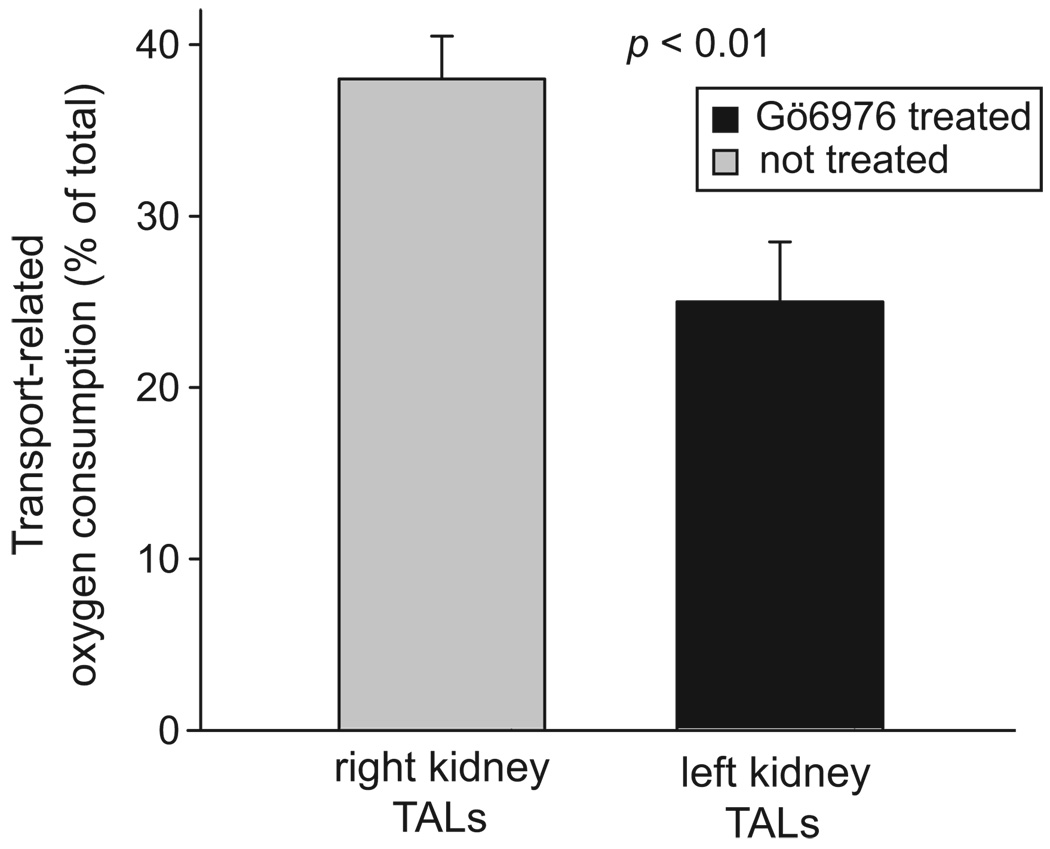
Because the acute effects of O2− on transport are mediated by PKCα in the thick ascending limb,17 we measured furosemide-sensitive oxygen consumption by thick ascending limbs from Ang II–infused animals that received intramedullary infusions of the PKCα/β-selective inhibitor Gö6976 in one kidney. We found that, in the control kidney of Ang II–infused rats, furosemide decreased oxygen consumption in thick ascending limbs by 37.4±1.5% of total oxygen consumption (from 126.3±18.5 to 78.2±12.2 nmol of oxygen per minute per milligram of protein). In contrast, in the kidney receiving Gö6976, furosemide decreased oxygen consumption in thick ascending limbs by 25.1±1.0% of total oxygen consumption (from 94.6±11.3 to 70.5±12.4 nmol of oxygen per minute per milligram of protein; Figure 8; P<0.01 versus control; n=6). Gö6976 infusion did not change furosemide-sensitive oxygen consumption in infused and noninfused kidneys from normotensive animals. Taken together these data indicate that Ang II–dependent hypertension increases Na transport–related oxygen consumption in the thick ascending limb by activating PKCα.
Figure 8.
Effect of renal unilateral infusion of Gö6976 on thick ascending limb (TAL) transport-related oxygen consumption during Ang II–dependent hypertension (n=6). Transport-related oxygen consumption was measured as furosemide-sensitive oxygen consumption.
Discussion
The thick ascending limb absorbs 25% to 30% of the filtered load of NaCl,30 and abnormal NaCl absorption by this segment is implicated in other forms of hypertension11,31; therefore, we first studied the effect of Ang II–dependent hypertension on Na transport–related oxygen consumption. Because of the increased medullary tissue fibrosis, isolation and perfusion of thick ascending limbs from Ang II–hypertensive animals are difficult to perform and highly variable. Consequently, we measured furosemide-sensitive oxygen consumption and found an increase of 39% during Ang II–dependent hypertension.
Ang II–dependent hypertension increases renal O2− production. Acute O2− treatment stimulates NaCl absorption in the thick ascending limb. Therefore, we measured the effect of Ang II–dependent hypertension on O2− production in this segment. Although others have shown that Ang II–dependent hypertension augments reactive oxygen species in the kidney cortex6,32 and medulla,7 O2− production in the thick ascending limb has not been measured. We found that O2− in medullary thick ascending limbs was increased by 190% in Ang II–dependent hypertension. These data demonstrate that Ang II–dependent hypertension enhances the production of reactive oxygen species in the thick ascending limb. However, this does not necessarily imply that this segment is the only nephron segment producing O2− under this pathological condition.
The increased thick ascending limb transport-related oxygen consumption caused by Ang II–dependent hypertension could be because of elevated blood pressure, increased circulating Ang II (which directly affects transport-related oxygen consumption by activating different signaling pathways), or increased O2−. To test which is responsible, we infused the O2− scavenger Tempol into one kidney of Ang II–hypertensive rats and measured transport-related oxygen consumption, O2− levels, and systolic blood pressure. We found that transport-related oxygen consumption and O2− levels returned to normal values in the Tempol-infused kidney but remained high in the contralateral kidney. Systolic blood pressure was not altered by unilateral renal Tempol infusion. Because both kidneys were exposed to the same systolic blood pressure, the decrease in Na transport–related oxygen consumption in the Tempol-infused kidney could not be because of a change in blood pressure. Similarly, because both kidneys were exposed to the same circulating levels of Ang II, it is unlikely that signaling cascades activated by Ang II and not related to O2− enhance NaCl reabsorption in Ang II–dependent hypertension. Thus, these data indicate that Ang II–dependent hypertension increases thick ascending limb NaCl transport by potentiating O2− production. However, our results do not rule out the possibility that Tempol could also decrease glomerular filtration rate of juxtamedullary glomeruli located in the corticomedullary boundary, thus modifying transport by the thick ascending limb.
We have shown previously that O2− acutely stimulates PKCα activity.17 To study whether this is also true for chronic O2− elevation, we measured PKCα translocation in Ang II–dependent hypertension, because increased PKCα translocation correlates with increases in activity. We found enhanced PKCα activity in thick ascending limbs from hypertensive animals. To study whether PKCα activity was a result or cause of the increase in O2−, we measured the effect of Tempol on PKCα activity. We found that Tempol prevented the increase in activity caused by Ang II– dependent hypertension. These data indicate that increases in endogenously produced O2− during Ang II–dependent hypertension stimulate PKCα activity by the thick ascending limb.
Finally, we tested whether the effects of Ang II–dependent hypertension on transport-related oxygen consumption were because of PKCα activation. We found that Ang II–dependent hypertension increased PKCα activity in the thick ascending limb. To test whether this increase had any consequence on Na transport–related oxygen consumption, we infused the PKCα/β-selective inhibitor Gö6976 into one kidney of Ang II–hypertensive rats. We found that Na transport–related oxygen consumption decreased to normal values in the Gö6976-infused kidney but remained high in the contralateral kidney. Taken together these data indicate that Ang II–dependent hypertension increases PKCα activity, resulting in enhanced Na transport–related oxygen consumption by thick ascending limbs.
We found that Ang II–dependent hypertension stimulates Na transport–related oxygen consumption in the thick ascending limb. Although the consequences of Ang II–dependent hypertension have been widely described in other renal structures, there are no other reports of the functional effects of this form of hypertension on Na transport by any nephron segment. However, our data are supported by the increased abundance of the apical Na/K/2Cl cotransporter and Na/H exchanger in the thick ascending limb after Ang II infusion.33 Furthermore, we11 and others13 have reported previously that NaCl absorption by the thick ascending limb in salt-sensitive hypertension is enhanced.
We found that during Ang II–dependent hypertension there is increased O2− production in the thick ascending limb. Our results extend those of others showing that Ang II–dependent hypertension increases the production of reactive oxygen species in the kidney.5,6 They are also supported by data showing that acute Ang II can stimulate O2− production in this segment.20 Most of the effects of Ang II are mediated by the Ang II type 1 and type 2 receptors, both of which are expressed by the thick ascending limb.34,35 Because most of the effects of Ang II on O2− production are mediated by Ang II type 1 receptors, intramedullary blockade of these receptors by losartan would be expected to decrease O2− levels, similar to Tempol infusion.
To show that the effects of Ang II–dependent hypertension on Na transport–related oxygen consumption are attributable to O2−, we infused Tempol into one kidney. We found that Na transport–related oxygen consumption was reduced in the Tempol-infused kidney compared with the contralateral kidney. We concluded that O2− was responsible for the increase caused by Ang II–dependent hypertension for the following reasons: (1) Tempol decreased O2− levels; (2) systolic blood pressure in Tempol-infused animals was no different than control animals; and, more importantly (3) Na transport–related oxygen consumption remained elevated in the contralateral kidney. This indicates that Tempol did not have any general systemic effects. We also concluded that the effect was not because of circulating Ang II. Although we did not measure plasma levels of Ang II, we could make this conclusion because plasma Ang II levels are the same for the Tempol-infused and contralateral kidneys.
The fact that unilateral renal Tempol infusion did not lower blood pressure was key to the above conclusions and appears to conflict with other reports in the literature.6,7 However, there is a major difference between our study and those. The studies showing that Tempol can reduce blood pressure in Ang II–dependent hypertension administrated Tempol systemically, whereas we infused Tempol directly into the medulla of 1 kidney to minimize its effects on the vasculature and contralateral kidney. The fact that both O2− and Na transport–related oxygen consumption were still elevated in the contralateral kidney compared with the Tempol-infused kidney indicates that Tempol did not have any systemic effect.
It has been reported that medullary H2O2 is a key factor in other forms of hypertension.7,36 We concluded that, during Ang II–dependent hypertension, O2−, rather than H2O2, is the mediator of the effects on PKCα for 2 reasons: we have shown previously that O2−, in the presence of catalase, stimulates PKCα, indicating that H2O2 is not involved,17 and Tempol is a superoxide dismutase mimetic that converts O2− into H2O2. Consequently, while decreasing O2−, Tempol increases H2O2. Therefore, the Tempol-infused kidney has increased H2O2 compared with the control kidney, and yet Na transport-related oxygen consumption is reduced to normal.
We found that, in Ang II–dependent hypertension, PKCα increases by 142%. After renal unilateral infusion of the PKCα/β-selective inhibitor, Gö6976, Na transport-related oxygen consumption was reduced to normal levels. Activation of PKCα regulates NaCl absorption in several nephron segments.37–39 Acutely, PKCα mediates the effects of O2− on transport.17 Thus, increased and sustained PKCα activation by O2− in Ang II–dependent hypertension may lead to increased Na and water reabsorption.
In summary, we found that Ang II–dependent hypertension in the thick ascending limb of the loop of Henle increases O2− production. This activates PKCα, which, in turn, stimulates Na transport–related oxygen consumption. These changes are independent of blood pressure.
Perspectives
The present work shows that Na transport by the thick ascending limb during Ang II–induced hypertension is increased and independent of blood pressure. These conclusions lead to the understanding of renal medullary Na transport during the pathogenesis of hypertension. It also extends our knowledge of the mechanisms of reabsorption during the course of the pathology. In addition, a more complete understanding of the signaling pathway involved in increased Na absorption may lead to the development and design of specific drugs, which may contribute to decrease sodium retention during hypertension.
Acknowledgments
Sources of Funding
This work was supported in part by grants from the National Institutes of Health (HL 28982 and HL 70985) to J.L.G. and from the American Heart Association-Greater Midwest (0615718Z) to G.B.S.
Footnotes
Disclosures
None.
References
- 1.Manning RD, Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25:311–317. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 2.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005;45:934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 3.Cifuentes ME, Rey FE, Carretero OA, Pagano PJ. Upregulation of p67(phox) and gp91(phox) in aortas from angiotensin II-infused mice. Am J Physiol Heart Circ Physiol. 2000;279:H2234–H2240. doi: 10.1152/ajpheart.2000.279.5.H2234. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 5.Kopkan L, Castillo A, Navar LG, Majid DS. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2006;290:F80–F86. doi: 10.1152/ajprenal.00090.2005. [DOI] [PubMed] [Google Scholar]
- 6.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H22–H28. doi: 10.1152/ajpheart.00626.2004. [DOI] [PubMed] [Google Scholar]
- 7.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW., Jr Increased renal medullary oxidative stress produces hypertension. Hypertension. 2002;39:667–672. doi: 10.1161/hy0202.103469. [DOI] [PubMed] [Google Scholar]
- 8.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 9.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin F2α. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- 10.Majid DS, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol. 2004;287:R27–R32. doi: 10.1152/ajpregu.00073.2004. [DOI] [PubMed] [Google Scholar]
- 11.Garcia NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension. 1999;34:508–513. doi: 10.1161/01.hyp.34.3.508. [DOI] [PubMed] [Google Scholar]
- 12.Kirchner KA, Crosby BA, Patel AR, Granger JP. Segmental chloride transport in the Dahl-S rat kidney during L-arginine administration. J Am Soc Nephrol. 1995;5:1567–1572. doi: 10.1681/ASN.V581567. [DOI] [PubMed] [Google Scholar]
- 13.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension. 1991;17:1018–1024. doi: 10.1161/01.hyp.17.6.1018. [DOI] [PubMed] [Google Scholar]
- 14.Materson BJ. Insights into intrarenal sites and mechanisms of action of diuretic agents. Am Heart J. 1983;106:188–208. doi: 10.1016/0002-8703(83)90117-5. [DOI] [PubMed] [Google Scholar]
- 15.Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol. 2005;288:F982–F987. doi: 10.1152/ajprenal.00348.2004. [DOI] [PubMed] [Google Scholar]
- 16.Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol Regul Integr Comp Physiol. 2006;290:R79–R83. doi: 10.1152/ajpregu.00447.2005. [DOI] [PubMed] [Google Scholar]
- 17.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension. 2006;48:467–472. doi: 10.1161/01.HYP.0000236646.83354.51. [DOI] [PubMed] [Google Scholar]
- 18.Amlal H, LeGoff C, Vernimmen C, Soleimani M, Paillard M, Bichara M. ANG II controls Na(+)-K+(NH4+)-2Cl-cotransport via 20-HETE and PKC in medullary thick ascending limb. Am J Physiol. 1998;274:C1047–C1056. doi: 10.1152/ajpcell.1998.274.4.C1047. [DOI] [PubMed] [Google Scholar]
- 19.Peng Y, Knox FG. Comparison of systemic and direct intrarenal angiotensin II blockade on sodium excretion in rats. Am J Physiol. 1995;269:F40–F46. doi: 10.1152/ajprenal.1995.269.1.F40. [DOI] [PubMed] [Google Scholar]
- 20.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle’s loop in rat kidney. Am J Physiol Renal Physiol. 2002;282:F1111–F1119. doi: 10.1152/ajprenal.00218.2001. [DOI] [PubMed] [Google Scholar]
- 21.Rasoul S, Carretero OA, Peng H, Cavasin MA, Zhuo J, Sanchez-Mendoza A, Brigstock DR, Rhaleb NE. Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension. J Hypertens. 2004;22:593–603. doi: 10.1097/00004872-200403000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiarash A, Pagano PJ, Tayeh M, Rhaleb NE, Carretero OA. Upregulated expression of rat heart intercellular adhesion molecule-1 in angiotensin II-but not phenylephrine- induced hypertension. Hypertension. 2001;37:58–65. doi: 10.1161/01.hyp.37.1.58. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlin ME, LeFurgey A, Mandel LJ. Suspension of medullary thick ascending limb tubules from the rabbit kidney. Am J Physiol. 1984;247:F955–F964. doi: 10.1152/ajprenal.1984.247.6.F955. [DOI] [PubMed] [Google Scholar]
- 24.Mandel LJ. Primary active sodium transport, oxygen consumption, and ATP: coupling and regulation. Kidney Int. 1986;29:3–9. doi: 10.1038/ki.1986.2. [DOI] [PubMed] [Google Scholar]
- 25.Tsao LT, Wang JP. Translocation of protein kinase C isoforms in rat neutrophils. Biochem Biophys Res Commun. 1997;234:412–418. doi: 10.1006/bbrc.1997.6656. [DOI] [PubMed] [Google Scholar]
- 26.Hua H, Munk S, Goldberg H, Fantus IG, Whiteside CI. High glucose-suppressed endothelin-1 Ca2+ signaling via NADPH oxidase and diacylglycerol-sensitive protein kinase C isozymes in mesangial cells. J Biol Chem. 2003;278:33951–33962. doi: 10.1074/jbc.M302823200. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol. 2002;283:F957–F962. doi: 10.1152/ajprenal.00102.2002. [DOI] [PubMed] [Google Scholar]
- 28.Inagaki K, Iwanaga Y, Sarai N, Onozawa Y, Takenaka H, Mochly-Rosen D, Kihara Y. Tissue angiotensin II during progression or ventricular hypertrophy to heart failure in hypertensive rats; differential effects on PKC epsilon and PKC beta. J Mol Cell Cardiol. 2002;34:1377–1385. doi: 10.1006/jmcc.2002.2089. [DOI] [PubMed] [Google Scholar]
- 29.Kelly DJ, Zhang Y, Hepper C, Gow RM, Jaworski K, Kemp BE, Wilkinson-Berka JL, Gilbert RE. Protein kinase C beta inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes. 2003;52:512–518. doi: 10.2337/diabetes.52.2.512. [DOI] [PubMed] [Google Scholar]
- 30.Greger R. Physiology of renal sodium transport. Am J Med Sci. 2000;319:51–62. doi: 10.1097/00000441-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Manning J, Beutler K, Knepper MA, Vehaskari VM. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2002;283:F202–F206. doi: 10.1152/ajprenal.00358.2001. [DOI] [PubMed] [Google Scholar]
- 32.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension. 2006;47:238–244. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- 33.Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol. 2003;285:F152–F165. doi: 10.1152/ajprenal.00307.2002. [DOI] [PubMed] [Google Scholar]
- 34.Wang DH, Li J. Regulation of angiotensin II receptors in the medullary thick ascending limb. Mol Cell Biochem. 2000;212:211–217. [PubMed] [Google Scholar]
- 35.Lerolle N, Bourgeois S, Leviel F, Lebrun G, Paillard M, Houillier P. Angiotensin II inhibits NaCl absorption in the rat medullary thick ascending limb. Am J Physiol Renal Physiol. 2004;287:F404–F410. doi: 10.1152/ajprenal.00265.2003. [DOI] [PubMed] [Google Scholar]
- 36.Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension. 2003;42:25–30. doi: 10.1161/01.HYP.0000074903.96928.91. [DOI] [PubMed] [Google Scholar]
- 37.Markos F, Healy V, Harvey BJ. Aldosterone rapidly activates Na+/H+ exchange in M-1 cortical collecting duct cells via a PKC-MAPK pathway. Nephron Physiol. 2005;99:1–9. doi: 10.1159/000081796. [DOI] [PubMed] [Google Scholar]
- 38.Karim ZG, Chambrey R, Chalumeau C, Defontaine N, Warnock DG, Paillard M, Poggioli J. Regulation by PKC isoforms of Na(+)/H(+) exchanger in luminal membrane vesicles isolated from cortical tubules. Am J Physiol. 1999;277:F773–F778. doi: 10.1152/ajprenal.1999.277.5.F773. [DOI] [PubMed] [Google Scholar]
- 39.Tsimaratos M, Roger F, Chabardes D, Mordasini D, Hasler U, Doucet A, Martin PY, Feraille E. C-Peptide stimulates Na+,K+-ATPase activity via PKC alpha in rat medullary thick ascending limb. Diabetologia. 2003;46:124–131. doi: 10.1007/s00125-002-0996-1. [DOI] [PubMed] [Google Scholar]