Abstract
Rat forebrain synaptosomes were extracted with Triton X-100 at 4°C and the insoluble material, which is enriched in postsynaptic densities (PSDs), was subjected to sedimentation on a continuous sucrose gradient. Two pools of Triton X-100-insoluble GABAA receptors (GABAARs) were identified: I) a higher-density pool (ρ = 1.10–1.15 g/ml) of GABAARs that contains the γ2 subunit (plus α and β subunits) and that is associated to gephyrin and the GABAergic postsynaptic complex; and II) a lower-density pool (ρ = 1.06–1.09 g/ml) of GABAARs associated to detergent-resistant membranes (DRMs) that contain α and β subunits but not the γ2 subunit. Some of these GABAARs contain the δ subunit. Two pools of GABAARs insoluble in Triton X-100 at 4°C were also identified in cultured hippocampal neurons: I) a GABAAR pool that forms clusters that colocalize with gephyrin, and remains Triton X-100-insoluble after cholesterol depletion, and II) a GABAAR pool that is diffusely distributed at the neuronal surface, that can be induced to form GABAAR clusters by capping with an anti-α1 GABAAR subunit antibody, and that becomes solubilized in Triton X-100 at 4°C after cholesterol depletion. Thus, there is a pool of GABAARs associated to lipid rafts that is non-synaptic and that has a subunit composition different from that of the synaptic GABAARs. Some of the lipid raft-associated GABAARs might be involved in tonic inhibition.
Keywords: lipid rafts, detergent resistant membrane (DRM), γ-aminobutyric acid type-A receptor, hippocampal cultures, gephyrin, postsynaptic density (PSD)
INTRODUCTION
The γ-Aminobutyric acid type A receptors (GABAARs) concentrate at the postsynaptic membrane of GABAergic synapses both in the brain and in culture (Craig et al. 1994; Somogyi et al. 1996; Nusser et al. 1995, 1998; Fritschy et al. 1998; Sassoe-Pognetto et al. 2000; Christie et al. 2002a,b). There are also GABAARs at the neuronal cell surface that are not associated with GABAergic synapses (Nusser et al. 1998; Brickley et al. 1999; Christie et al. 2002a,b, 2006; Fritschy and Brunig 2003; Wei et al. 2003). Several detergents, including Triton X-100, have been employed for the solubilization of GABAARs from brain membranes, as a first step for the purification and biochemical characterization of the solubilized GABAARs. There is also a population of brain GABAARs that resists detergent extraction, which has remained largely non-characterized and is the focus of the present study.
Several methods have been described for the preparation of postsynaptic densities (PSDs) from the excitatory glutamatergic synapses with Gray’s type-I morphology (type-I PSDs). These methods are based on the insolubility of these structures in various detergents (Cotman et al. 1974; Carlin et al. 1980; Walikonis et al. 2000). The preparation of type-I PSDs has allowed the identification and characterization of many of the protein components of these structures (Kennedy 1997; Walikonis et al. 2000; Yoshimura et al. 2004; Peng et al. 2004; Jordan et al. 2004; Collin et al. 2006). In contrast, there are no methods in the literature describing the preparation of PSDs from the inhibitory GABAergic synapses with Gray’s type-II morphology (or type-II PSDs). In this communication we have devised a method for the preparation of a brain fraction enriched in type-II GABAergic postsynaptic densities, based on their insolubility in Triton X-100 at 4°C. This fraction is enriched in GABAARs and gephyrin, proteins that concentrate at the GABAergic postsynaptic complex. Gephyrin is a cytoplasmic scaffold protein that concentrates at the PSD of the glycinergic and GABAergic synapses. Gephyrin binds to microtubules and is essential for the postsynaptic clustering of the glycine receptors and is involved in the clustering of some GABAARs (Feng et al. 1998; Essrich et al. 1998; Kneussel et al. 1999, 2001; Christie et al. 2002a; Levi et al. 2004).
We are also showing that there is another pool of GABAARs that resists solubilization with Triton X-100 at 4°C and is associated with detergent-resistant membranes or DRMs (Brown and Rose 1992). DRMs have been operationally defined equivalent of lipid rafts (Brown and London 2000). The DRMs, like lipid rafts, are enriched in cholesterol and sphingomyeline, and contain lipid-anchored and some integral proteins (Schroeder et al. 1994; Brown and Rose 1992; Brown and London 1997; London and Brown 2000). Lipid rafts play a coordinating role in several signal transduction pathways (Simon and Toomre 2000). Several neurotransmitter receptors have been shown to be associated to lipid rafts (Bruses et al. 2001; Zhu et al. 2006; Becher et al. 2001; Suzuki et al. 2001; Hering et al. 2003; Dalskov et al. 2005).
In this communication we show that in both brain and hippocampal cultured neurons there are two pools of Triton X-100-insoluble GABAARs: one that concentrates in the postsynaptic GABAergic complex, and does not seem to be associated with DRMs or lipid rafts and another one that is non-synaptic and is associated with DRMs and lipid rafts.
MATERIALS AND METHODS
Antibodies
Several anti-GABAAR subunit antibodies were raised and affinity-purified in our laboratory: rabbit anti-rat α1 (to amino acids a.a. 1–15), rabbit anti-rat α2 (to a.a. 417–423), rabbit anti-rat α3 (to a.a. 1–13), and guinea pig or rabbit anti-rat γ2 (to a.a. 1–15). We used these anti-γ2 antibodies for immunofluorescence or immunogold. The mouse monoclonal antibody (mAb) anti-β2/3 (clone 62-3G1) was also generated in our laboratory to the affinity-purified bovine GABAARs. The specificities of the GABAAR subunit antibodies have been determined previously by ELISA, immunoblotting, displacement by antigenic peptide, rat brain immunohistochemistry, immunofluorescence of hippocampal cultures and of HEK 293 cells transfected with various subunits, and in some knock out and knock down mutant mice (De Blas et al. 1988; Vitorica et al. 1988; Moreno et al. 1994; Miralles et al. 1999; Homanics et al. 1999; Christie et al. 2002a, b, 2006; Riquelme et al. 2002; Charych et al. 2004a, b; Li et al. 2005a, b; Chandra et al. 2005; Serwanski et al. 2006). Rabbit anti-β2 and anti-β3 antibodies were from Chemicon (Temecula, CA). The rabbit anti-γ2 used in the immunoblots was from Alpha Diagnostic (San Antonio, TX). Three rabbit anti-δ antibodies were used. One was from Phosphosolutions (Aurora, CO), another one from Novus Biologicals (Littleton, CO), and a third one was an affinity-purified anti-δ antibody raised in our laboratory to a.a. 390–402 of the rat δ subunit. The rabbit anti-gephyrin antiserum or affinity purified-antibodies used in the immunoblots were prepared as previously described (Kawasaki et al 1997), and the mAb to gephyrin (clone 7a) used in immunofluorescence and EM immunogold was from Synaptic systems (Gottingen, Gemany) or Cedarlane Laboratories (Ontario, Canada). Other antibodies used were mAb to Thy-1 (clone OX7, Sera Lab, Loughborough, Leicestershire, England), mAb to Flotillin-1 (clone 18, BD Transduction Laboratories, San Diego, CA), mAb to Transferrin Receptor (clone H68.4, Zymed, San Franciso, CA), mAb to PSD-95 (Clone K28/43, Upstate Biotechnology Inc, Lake Placid, NY), and rabbit anti-Caveolin-1 (Santa Cruz Biotechnology, Santa Cruz, CA). For immunoblots, goat anti-rabbit or anti-mouse IgG antibodies and rabbit or mouse peroxidase-anti-peroxidase complexes were from MP Biomedicals (Aurora, OH). Fluorophore-labeled or colloidal gold-labeled species-specific anti-IgG secondary antibodies made in donkey were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Subcellular fractionation of the rat brain and preparation and fractionation of the “One Triton PSD” fraction
The preparation of the crude mitochondrial/synaptosomal P2, synaptosomal SYN and “One Triton PSD” fractions was done according to Carlin et al. (1980). Three-month-old Sprague-Dawley male rats were used in these and other fractionation experiments. All steps were carried out at 4°C and all the fractions were suspended in 50 mM Tris-HCl PH 7.4, aliquoted and stored at −70°C. In this procedure, the synaptosomal fraction (SYN) was collected between the 1.0 M and 1.2 M sucrose interphase after sedimentation through a discontinuous sucrose gradient. This fraction was suspended in 0.32 M sucrose by dilution with 6 mM Tris-HCl pH 8.1, solubilized with 0.5% Triton X-100 for 15 minutes and centrifuged at 32,800× g for 1 hour. The resulting pellet is the “One Triton PSD” fraction, named by others (Walikonis et al. 2000). The “One Triton PSD” pellet was suspended in 0.32 M sucrose and loaded onto a continuous sucrose gradient 0.32–2.0 M in 1 mM NaHCO3, centrifuged at 201,800× g for 16 hours, and fractionated in 1 ml fractions. Sucrose density was measured by refractometry. Cholesterol was measured by using the Amplex Red cholesterol assay kit (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Immunoblots were done according to De Blas and Cherwinski (1983).
The synaptosomal plasma membrane (SPM) fraction was prepared after lysis of the crude synaptosomal fraction followed by flotation by centrifugation in a discontinuous sucrose gradient as described by Jones and Matus (1974).
Isolation of the DRM fraction from SPM and cholesterol depletion
The SPM fraction (4 mg protein) in 50 mM Tris-HCl pH 7.4 was centrifuged at 16,000× g for 10 min at 4°C and the pellet was suspended in 2 ml of TNEX buffer (1% Triton X-100 in 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1 mg/L Leupeptin, 0.1 mM PSMF) at a protein to detergent ratio of 1:5 (w/w). The mixture was incubated on ice for 20 min, followed by homogenization with 3 strokes in a hand-operated glass/glass Downce homogenizer, incubation on ice for 10 min, and centrifugation at 125,000× g for 30 mins. The Triton X-100 soluble supernatant (TS) was collected, and the Triton X-100 insoluble pellet (TI) was suspended in TNE buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, 1 mg/L Leupeptin, 0.1 mM PSMF) at 4°C. Alternatively, after extraction of SPM with TNEX buffer, the sample was mixed with the same volume of 80% (w/v) sucrose and 4 ml were overlaid with 6ml of 35% (w/v) and 2ml of 5% (w/v) sucrose, respectively. The upper two layers contained 0.05% Triton X-100. After flotation by centrifugation at 18,800× g for 16 hours at 4°C, the DRM fraction was collected at the 5%/35% sucrose interphase. For cholesterol depletion, the SPM fraction was incubated with or without 0.5% saponin in TNE buffer on ice for 20 minutes. The cholesterol-depleted SPM fraction was subjected to the aforementioned extraction with TNEX, followed by flotation by centrifugation in the 5%/35%/40% discontinuous sucrose gradient. After centrifugation, the gradient was fractionated in 12 fractions, 1 ml each.
Radioligand binding assays
For [3H]Flunitrazepam ([3H]FNZ) binding, 100 μg of protein of each fractions were incubated in triplicate with 10 nM [3H]FNZ in 50 mM Tris-HCl, pH 7.4, in a final volume of 500 μl for 30 min at 4°C. Nonspecific bindingwas determined by displacement with 10 μM clonazepam. For [3H]Muscimol ([3H]MUS) binding assay, all fractions were washed thoroughly with 50 mM Tris-HCl, pH 7.4 buffer by centrifugation at 34,500× g five times to eliminate endogenous GABA. Each fraction (150 μg protein) was incubated in triplicate with 40 nM [3H]MUS in 50 mM Tris-HCl, pH 7.4, for 30 minutes at 4°C in the dark. Non-specific binding was determined in the presence of 40 μM GABA. At the end of the incubation periods, 1.6 mg bovine γ-globulin and 0.32 ml of 30% polyethylene glycol (PEG) were added, incubated for 5 min at 4°C, and filtered through 24 mm Whatman glass-microfiber GF/B filters that had beenpreviously washed with 8% PEG. The filters were washed twice with 5 ml of ice-cold 8% PEG, dried and radioactivity was determined with a liquidscintillation counter.
Immunofluorescence of hippocampal cultures and Triton X-100 extraction
Low-density hippocampal cultures were prepared according to Goslin et al. (1998) as described elsewhere (Christie et al. 2002a, b; Christie and De Blas 2003). Briefly, dissociated neurons from embryonic day 18 Sprague-Dawley rat hippocampi were plated at low density (3,000–8,000 cells per 18 mm diameter circular coverslip) and maintained in glial cell conditioned medium for 19–22 days. Immunofluorescence on fixed or live cells has been described elsewhere (Christie and De Blas 2002, Christie et al. 2006). Briefly, for fixed-cell labeling (no-capping), neurons on coverslips were fixed in 4% paraformaldehyde, 4% sucrose in phosphate buffered saline (PBS) for 15 minutes at room temperature (RT) followed by cell permeabilization with 0.25% Triton-X-100 in PBS for 5 minutes and incubation with 5% donkey serum in PBS for 30 minutes. Fixed and permeabilized neurons were incubated with a mixture of the rabbit anti-GABAAR antibody and mAb to gephyrin for 2 hours. After washing, cells were incubated with a mixture of species-specific secondary anti-rabbit and anti-mouse IgG antibodies, made in donkey and conjugated to Texas Red or FITC fluorophores, in 0.25% Triton X-100 in PBS for 1 hour at RT. The coverslips were washed with PBS and mounted using Prolong anti-fade mounting solution (Invitrogen, Carlsbad, CA).
For “one-step capping”, the procedure has been described elsewhere (Christie et al. 2006). Briefly, live cells were surface-labeled by incubation with the primary rabbit or guinea pig anti-GABAAR antibody (0.5–2 μg/ml) in culture medium at 37°C for 45 minutes in a 5% CO2 atmosphere. After washing the cells were fixed and permeabilized, as described above, followed by incubation with mouse mAb to gephyrin or to Thy-1 and by incubation with a mixture of the fluorophore-labeled secondary antibodies. For Triton X-100 extraction, after “one-step capping” with the primary antibody to GABAARs, the cultures were incubated with 0.5% Triton X-100 in 20 mM phosphate buffer, pH7.4, (PB) at 4°C or 37°C for 10 min. Alternatively, neurons were cholesterol-depleted, by incubation with 5 mM methyl-β-cyclodextrin (MβCD) at 37°C for 20 min, prior to incubation with 0.5% Triton X-100 in 20 mM PB at 4°C. The cells were fixed and processed as described above.
For “two-step capping”, we followed the procedure described elsewhere (Levi et al. 2004, Christie et al. 2006). Briefly, live cells were sequentially incubated with the primary rabbit anti-α1 antibody for 45 min and the fluorophore-conjugated secondary donkey anti-rabbit IgG antibody (10 μg/ml) for 30 min at 37°C, followed by fixation, permeabilization, and incubation with the mAb to gephyrin and subsequent fluorophore-conjugated secondary donkey anti-mouse IgG antibody. Detergent extraction after “two-step capping” was done by incubating the cultures with 0.5% Triton X-100 at 4°C before the fixation step, as for “one-step capping”. Some cultures were treated with MβCD prior to Triton X-100 extraction as for “one-step capping”.
Image acquisition and analysis
Fluorescence images were collected using a 60× pan-fluor objective on a Nikon Eclipse T300 microscope with a Sensys KAF 1401E CCD camera, driven by IPLab 3.0 (Scanalytics, Fairfax, VA) acquisition software. For qualitative analysis, images were processed with Photoshop 7.0 (Adobe, San Jose, CA) as described elsewhere (Li et al. 2005a, b, Christie et al. 2006).
Quantification of GABAAR and gephyrin clusters
For each determination, 30 to 40 dendrites were randomly selected from 15–20 cells (2 dendrites/cell) from 3 to 4 separate experiments. A 100 μm-long segment from each dendrite was used for quantification. We did not quantify the cluster density in terms of dendritic area because detergent treatment of non-fixed cells led to somewhat thinner dendrites than when the detergent extraction was done after fixation (see also Allison et al. 2000). To determine the number of clusters per 100 μm of dendritic length, the maximum intensities of the fluorophore channel were normalized and the low intensity and diffuse non-clustered background fluorescence signal seen in the dendrites was subtracted. Cluster co-localization in two different fluorescence channels was determined by overlaying the images. A cluster in a fluorescence channel was considered to co-localize with a cluster in the other channel when >66% of surface of one of the clusters overlapped with the other cluster. Measurements of cluster size were performed using IPLabs 3.5 software. The 8-bitimages were segmented, on the basis of fluorescence intensity levels, to create a binary mask that maximized the number of clusters and minimized the coalescence of individual clusters. Values were averaged per 100 μm and reported as mean ± standard error of the mean (SEM). Statistical comparison was made by Student’s t test.
Electron microscopy immunogold
It was done according to the procedure of Hunt et al. (1996). Briefly, subcellular fractions were fixed in 4% paraformaldehyde, 0.1% glutaraldehyde and suspended in 1.5% low melting point agarose. The solidified agarose was cut into ~1mm3 blocks, and the blocks were incubated with a single antibody (or a mixture of two primary antibodies), followed by incubation with a colloidal gold-conjugated secondary antibody (6 nm or 12 nm diameter for Fig. 1) or a mixture of two colloidal gold-conjugated secondary antibodies (10 nm and 18 nm diameter for Fig. 2). The blocks were then incubated in 1% glutaraldehyde in 0.12 M sodium phosphate buffer, pH 7.4 for 15 min, followed by incubation in 1% osmium tetroxide for 30 min at RT, and 2% uranyl acetate for 30 min at RT, dehydration in a series of ethanol, infiltration, and embedding in Spurr’s resin at RT. Thin sections were cut on a Reichardt ultramicrotome and collected on Formvar-coated grids. The sections were contrasted by incubation with 2% uranyl acetate and 0.3% lead citrate, 3 min each, and photographed in a Philips 300 transmission electron microscope.
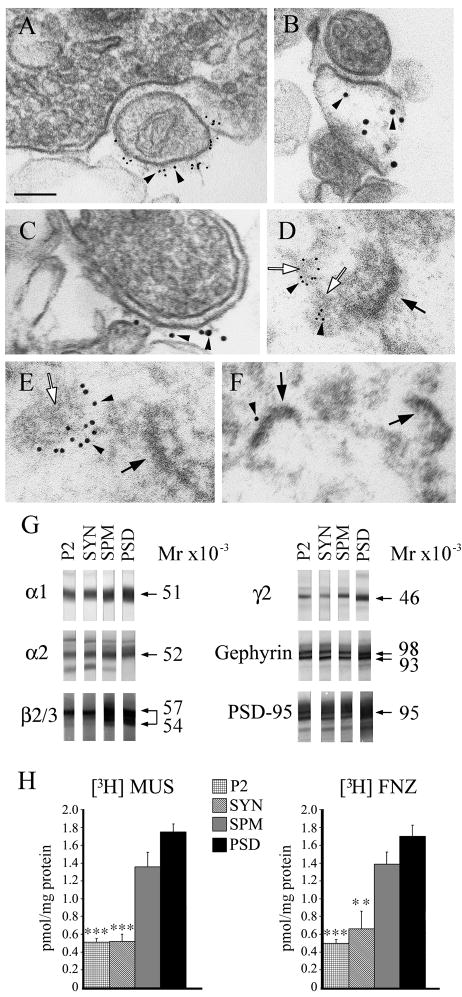
Fig. 1.
GABAARs are present in various subcellular brain fractions. The P2, SYN, SPM, PSD, were prepared as described in Materials and Methods. A–F show EM immunogold of synaptosomal (A–C) and “One Triton PSD” (D–F) fractions with rabbit anti-α1 GABAAR subunit (A and D, arrowheads), mAb to gephyrin (B, C and E, arrowheads) and mAb to PSD-95 (F, arrowhead). Filled arrows indicate type-I PSDs, which have high electron-density. Empty arrows point to structures of medium electron-density immunolabeled with antibodies to α1 or gephyrin, likely corresponding to type-II PSDs. The secondary goat anti-rabbit IgG and anti-mouse IgG antibodies were conjugated to 6 nm and 12 nm diameter colloidal gold particles respectively. Scale bar = 100 nm. G: Immunoblots of various brain fractions with rabbit anti-α1, rabbit anti-α2, rabbit anti-γ2, and rabbit anti-gephyrin antisera, mAb to β2/3 and mAb to PSD-95. Same amount of total protein from each fraction (10μg/lane) was used for SDS-PAGE. The immunoreactivity of the specific protein band was blocked by the corresponding antigenic peptide (data not shown). H: [3H]MUS and [3H]FNZ binding to various brain fractions. Specific activity values (pmol/mg protein) are mean ± S.E.M of at least three experiments each done in triplicate. *** p < 0.001; ** p < 0.01. The p values are for the various fractions compared to PSD.
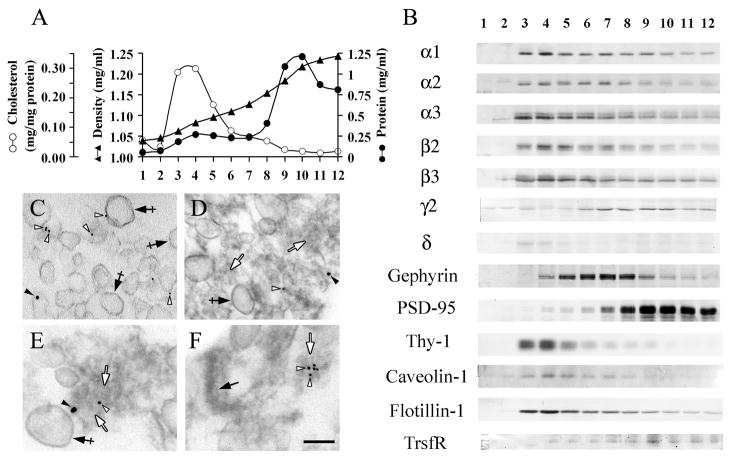
Fig. 2.
In the “One Triton PSD” fraction, some GABAARs are associated with DRMs and some are associated with GABAergic type-II PSDs. Fractionation of “One Triton PSD” after centrifugation on a continuous 0.32–2.0 M sucrose density gradient. A: Distribution of cholesterol and protein in the density gradient. B: Immunoblots of the various fractions with antibodies to GABAARs subunits (α1, α2, α3, β2, β3, γ2, δ), Gephyrin, PSD-95, transferrin receptor (TrsfR) and the lipid raft markers Thy-1, Caveolin-1 and Flotillin-1. The same amount of total protein (4μg/lane) was used for SDS-PAGE. Fractions 1 and 12 correspond to the top and bottom of the gradient respectively. C: Double-labeled EM Immunogold of pooled gradient fractions 3 and 4 with rabbit anti-α1 and mAb anti-β2/3. Crossed arrows show the DRM vesicles present in this fraction. Some of these structures are immunolabeled with rabbit anti-α1 (filled arrowhead) and/or mAb anti-β2/3 (empty arrowheads). D–F: Double-label EM immunogold of fraction 7 with rabbit anti-γ2 and mAb to gephyrin. Filled arrow in F indicates a type-I PSD that has high electron-density. Empty arrows in D–F point to amorphous structures of medium electron-density. Some of these structures are immunolabeled with rabbit anti-γ2 (filled arrowheads) and/or mAb to gephyrin (empty arrowheads). Crossed arrows in D and E indicate vesicular membranes. The secondary goat anti-rabbit IgG and goat anti-mouse IgG antibodies were conjugated to 18 nm and 10 nm diameter colloidal gold particles respectively. Scale bar represents 150 nm in C and D and 100 nm in E and F.
RESULTS
GABAARs and gephyrin are enriched in the “One Triton PSD” fraction
Two types of synapses, with very distinct morphology, are present in the brain. These are excitatory Gray’s type-I and inhibitory Gray’s type-II synapses. Synaptosomes are pinched-off presynaptic terminals that are formed after homogenization of the brain. Some synaptosomes have an attached postsynaptic membrane with a prominent postsynaptic density derived from type-I synapses (Cotman et al. 1974; Matus et al. 1980; Hunt et al. 1996). Since we are interested in studying GABAA receptors and GABAergic synapses, we have investigated whether type-II GABAergic synapses are also present in the classical synaptosomal preparation. We have found that this is the case (Fig. 1A–C). This fraction had synaptosomes with type-II synaptic morphology whose postsynaptic elements became decorated with gold particles corresponding to antibodies to the GABAergic postsynaptic markers α1 subunit of the GABAAR (Fig. 1A) or gephyrin (Fig. 1B–C) as shown by electron microscopy (EM) immunogold. The type-II synaptic morphology (Fig. 1A–C) included light postsynaptic density and flattened synaptic vesicles in the presynaptic axon terminal. The gold particles corresponding to anti-α1 GABAAR subunit were located at the extracellular side of the vesicularized postsynaptic membrane (Fig. 1A arrowheads), as expected for an antibody that recognizes an epitope localized at the extracellular N-terminus of α1. In contrast, the gold particles corresponding to an antibody that recognizes the postsynaptic cytoplasmic protein gephyrin were found at the expected cytoplasmic side of the postsynaptic membrane (Fig. 1B–C arrowheads). Note in Fig. 1A that the colloidal gold-conjugated secondary antibody could not penetrate the intact part of the synaptic cleft, thus preventing the immunolabeling of the GABAARs present in this part of the cleft. No permeabilizing detergent was used in the experiments aiming to preserve the morphological integrity of the structures. However, gold immunolabeling with anti-α1 occurred in the adjacent region of the postsynaptic membrane that became separated from the presynaptic terminal after homogenization. These results indicate that the classical synaptosomal preparation also contains synaptosomes, derived from GABAergic synapses, with type-II morphology.
Other groups have developed methods for the purification of type-I postsynaptic densities (Cotman et al. 1974; Carlin et al. 1980; Walikonis et al. 2000) by taking advantage of the insolubility of type-I PSDs in Triton X-100. One of these fractions, enriched in type-I PSDs, has been called “One Triton PSD” fraction (Walikonis et al. 2000). We have now explored whether the “One Triton PSD” fraction is also enriched in GABAA receptors and gephyrin, two proteins that concentrate at the GABAergic postsynaptic complex. If so, it would indicate that type-II PSDs are also present in the “One Triton PSD” fraction.
Immunoblots (Fig. 1G) showed that there was a progressive enrichment in various GABAARs subunits (α1, Mr = 51000; α2, Mr = 52000; β2/3, Mr = 54000–57000 and γ2, Mr = 46000) and the two main isoforms of gephyrin (Mr = 93000–98000) from the crude mitochondrial/synaptosomal fraction (P2) to synaptosomal (SYN) to synaptosomal plasma membrane (SPM) to “One Triton PSD” (PSD) fraction. These results indicate that the “One Triton PSD” fraction is enriched in GABAergic receptors and gephyrin, two markers of GABAergic synapses. As others have shown (Hunt et al. 1996; Kennedy 1997), the “One Triton PSD” fraction, as expected, was also enriched in PSD-95 (Mr = 95000), a marker for type-I PSDs. We do no know if the faint protein band (Mr = 47000), which migrates slightly faster than the main α2 protein band (Mr = 52000) and that is absent from the PSD fraction, is an immature form of the α2 subunit (ie. a non-glycosylated peptide) that is not present at synapses or a proteolytic fragment that is extracted by Triton X-100.
The specific activities of [3H]Muscimol ([3H]MUS) and [3H]Flunitrazepam ([3H]FNZ) binding to the GABA- and benzodiazepine-binding sites of the GABAARs (located in the boundary between the α/β and α/γ subunits respectively) also showed enrichment in the PSD fraction over the other fractions (Fig. 1H). The specific activity of [3H]MUS binding to PSD (1.75 ± 0.09 pmol/mg protein, mean ± SEM) was not significantly different from that of SPM (1.36 ± 0.16 pmol/mg protein, p = 0.10), but was significantly higher than SYN (0.52 ± 0.08 pmol/mg protein, p = 0.0005) and P2 (0.51 ± 0.04 pmol/mg protein, p = 0.0002). For [3H]FNZ binding, the specific activity of PSD (1.70 ± 0.13 pmol/mg protein) was not significantly different from that of SPM (1.39 ± 0.14 pmol/mg, p = 0.15), but was significantly higher than P2 (0.50 ± 0.04 pmol/mg protein, p = 0.0001) and SYN (0.66 ± 0.19 pmol/mg protein, p = 0.0054) (Fig. 1H). The radioligand-binding activity indicates that the GABAARs in the PSD and other fractions are fully assembled, since only assembled receptors have the binding sites for the two radioligands. This could not be ascertained by the immunoblotting assay, which does not differentiate between assembled and non-assembled GABAAR subunits.
The EM immunogold of the PSD fraction showed that gold particles representing antibodies to α1 (arrowheads, Fig. 1D) or gephyrin (arrowheads, Fig. 1E) decorated amorphous structures of medium electron-density (empty arrows, Fig. 1D and E) but not the high electron-dense structures with type-I PSD morphology (filled arrows, Fig. 1D and E). The structures with the type-I PSD morphology (filled arrows, Fig. 1F) were frequently decorated with gold particles corresponding to anti-PSD-95 antibody (arrowhead, Fig. 1F). The type-II PSDs could only be identified by immunogold. Contrary to type-I PSDs, the type-II PSDs could not be identified just by morphological criteria.
The results are consistent with the notion that the “One Triton PSD” fraction is enriched in fully assembled GABAARs and gephyrin and that this fraction contains not only the prominent and electron-dense type-I glutamatergic PSDs, but also the more amorphous and less electron-dense type-II GABAergic PSDs (empty arrows Fig. 1D, E), structures that are decorated with the anti-GABAARs and anti-gephyrin antibodies (arrowheads Fig. 1D, E).
Two pools of GABAARs are present in the “One Triton PSD” fraction: one associated with DRMs and another one associated with GABAergic postsynaptic densities
Although the “One Triton PSD” fraction is enriched in PSDs, it could also contain DRMs and lipid rafts, which are also insoluble in Triton X-100 at 4°C (Suzuki 2002). To ascertain whether the GABAARs present in this fraction are associated with DRMs, or with GABAergic PSDs, or with both, we subjected the PSD fraction to sedimentation in a 0.32–2.0 M continuous sucrose gradient. After centrifugation, the main protein peak concentrated in fractions 9–11 at ρ = 1.18–1.23 g/ml (Fig. 2A). A smaller protein peak was found in fraction 4 (ρ = 1.08 g/ml). Immunoblots containing the same amount of protein per lane (Fig. 2B) showed that the low-density fractions 3–5 (ρ = 1.06–1.09 g/ml) were enriched in the GABAAR α and β subunits and in the lipid raft markers Thy-1 (Mr = 21000), Caveolin-1 (Mr = 24000) and Flotillin-1 (Mr = 48000), as well as in cholesterol (Fig. 2A), indicating that fractions 3–5 are enriched in DRMs and lipid rafts. The extrasynaptic GABAAR δ subunit (Mr = 54000) also concentrated in the low-density fractions, although the intensity of the δ subunit protein band was considerably weaker than that of the various α and β GABAAR subunits. This result, corroborated with three different anti-δ subunit antibodies, was expected since it is known that the δ subunit is expressed at low levels in the rat forebrain (see discussion). For this reason we did not further study the δ subunit in our forebrain preparations. Transferrin receptor (TrsfR Mr = 95000), which is absent from DRMs and lipid rafts (Harder et al. 1998; Jing et al. 1990), concentrated in fractions 8–12 (Fig. 2B). The various α and β GABAAR subunits were also present in fractions 8–12, although to a lesser extent than in the fraction enriched in lipid raft markers. The results in Fig. 2B were normalized to the same amount of protein per lane, to show the relative enrichment in each fraction. Even when considering the distribution of the total amount of each GABAAR subunit in the gradient, the DRM fractions 3–5 contained 38% of all α1, 28% of α2, 21% of α3, 49% of β2, 46% of β3 and 33% of all the δ present in the gradient. These results showed that in spite of the relatively small amount of protein present in these fractions, they concentrated a significant proportion of all GABAARs. In contrast to the α, β and δ subunits, only 8% of all γ2 or 12% of all gephyrin were found in the DRM fractions 3–5. The EM immunogold labeling of combined DRM fractions 3 and 4, enriched in lipid rafts markers, showed vesicular structures (Fig. 2C, crossed arrows) similar to the low-density DRMs observed by others at the EM level in DRM preparations from various tissue sources (Suzuki et al. 2002; Besshoh et al. 2005). In addition, we have found that these vesicles were frequently decorated with anti-β2/3 (empty arrowheads) and anti-α1 (filled arrowheads) immunogold particles (Fig. 2C), indicating that the vesicular DRMs contain α1- and β2/3-GABAAR, subunits that are enriched in the DRMs as immunoblots show (Fig. 2B).
The γ2 GABAAR subunit and gephyrin were enriched in fractions 6–8 (Fig. 2B), which contained 30% of all the γ2 and 38% of all the gephyrin in the gradient. The PSD-95, marker for type-I PSDs, mainly distributed in fractions 8–12. These fractions contained 83% of all the PSD-95 in the gradient. Since the γ2 GABAAR subunit and gephyrin are postsynaptic GABAergic markers (Craig et al. 1996; Giustetto et al. 1998; Kneussel et al. 1999; Christie et al. 2002b; van Rijnsoever et al. 2005), and since the γ2 subunit is essential for the postsynaptic clustering of GABAARs (Essrich et al. 1998; Schweizer et al. 2003; Li et al. 2005b), we concluded that fractions 6–8 (ρ = 1.10–1.15 g/ml) are enriched in type-II PSDs and fractions 8–12 (ρ = 1.15–1.24 g/ml) are enriched in type-I PSDs, with some overlap in the enrichment of the two types, particularly in fraction 8. Thus, type-II PSDs can be partially separated from type-I PSDs in fractions 6 and 7 due to the lower density of the former, which might be related to the more amorphous and less electron-dense quality of type-II PSDs. It is worth noting that fractions 9–12 still carry a considerable amount of the total γ2 subunit (58%) and gephyrin (51%). Nevertheless, fractions 9–12 show no enrichment (per mg of protein) in these GABAergic markers due to the high concentration in these fractions of proteins insoluble in Triton X-100. In contrast, fractions 9–12 are highly enriched (per mg of protein) in PSD-95.
The EM of fraction 7 (Fig. 2D–F), which is enriched in GABAergic postsynaptic markers γ2-GABAAR and gephyrin, showed the presence of both vesicular structures (crossed arrows) and amorphous material of medium electron-density (empty arrows). Double-label EM immunogold with the anti-GABAergic synaptic markers gephyrin (empty arrowheads) and γ2 GABAARs (filled arrowheads) showed that the immunogold particles were associated with the amorphous structures of medium electron-density (empty arrows, Fig. 2D–F), reminiscent of the ones found in the “One Triton PSD” fraction (empty arrows, Fig. 1D and E), which were also immunolabeled with antibodies to GABAARs and gephyrin. Some electron-dense structures with type-I PSDs morphology (filled arrows, Fig. 2F) were also present in fraction 7, although they were not immunolabeled with anti-gephyrin or anti-GABAARs antibodies. The presence of some type-I PSDs in fraction 7 was consistent with the presence of some PSD-95 in this fraction, as shown by immunoblotting (Fig. 2B). The EM immunogold and the immunoblot data support the notion that in the “One Triton PSD” fraction, I) there are GABAARs that contain α and β but little or no γ2 subunit that are associated to DRMs and lipid rafts, and II) there are GABAARs that contain α, β and γ2 subunits associated with gephyrin that are present in the type-II GABAergic postsynaptic complex.
A pool of the GABAARs present in synaptosomal plasma membranes is localized in DRMs
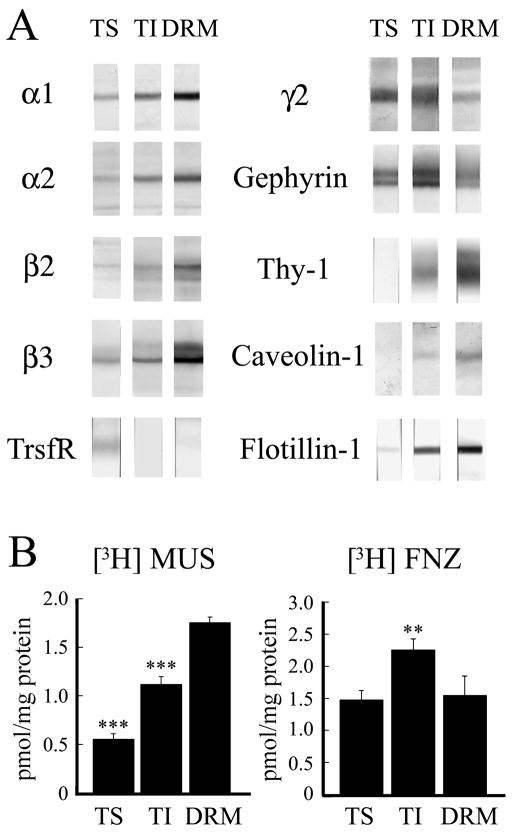
To further confirm the association of a pool of GABAARs with DRMs and lipid rafts, a synaptosomal plasma membrane fraction (SPM) was extracted with 1% Triton X-100 at 4°C for 30 minutes, a more stringent extraction than the one called for in the procedure for the preparation of the “One Triton PSD” fraction, which was done with 0.5% Triton X-100 at 4°C for 15 minutes. Immunoblots showed that a significant amount of GABAARs and gephyrin were present in both the Triton-soluble (TS) and Triton-insoluble (TI) fractions (Fig. 3A). The lipid raft markers Thy-1, Caveolin-1 and Flotillin-1 mainly concentrated in the TI fraction, while TrsfR mainly concentrated in the TS fraction. To find out whether some GABAARs from the TI fraction were associated to DRMs, we isolated the low-density DRM fraction, which is enriched in lipid rafts, after incubation of the SPM fraction with 1% Triton X-100 at 4°C followed by flotation by centrifugation in a discontinuous sucrose gradient, as described in the methods section. Compared to TI, the DRM fraction showed substantial enrichment of α and β GABAAR subunits, as well as of the lipid raft markers Thy-1, Caveolin-1 and Flotillin-1 (Fig. 3A). However, DRM showed considerably less γ2 GABAAR subunit, gephyrin and TrsfR than TI (Fig. 3A). The specific [3H]MUS and [3H]FNZ binding to GABAARs indicated that the GABAAR subunits present in these fractions were incorporated into assembled GABAAR (Fig. 3B). The specific [3H]MUS binding activity of DRM (1.75 ± 0.07 pmol/mg protein) was significantly higher than that of TI (1.12 ± 0.06 pmol/mg protein, p = 0.0009) or TS (0.55 ± 0.08 pmol/mg protein, p = 0.0001) fractions. The difference in [3H]MUS binding between TI and TS was also significant (p = 0.0014). However, [3H]FNZ binding activity of DRM (1.55 ± 0.30 pmol/mg protein) was smaller than that of TI (2.25 ± 0.17 pmol/mg protein, p = 0.004) and similar to that of TS (1.47 ± 0.15 pmol/mg protein, p = 0.72). Regarding recovery, 32% of [3H]MUS and 11% of [3H]FNZ binding in SPM was recovered in the DRM fraction. These binding and immunoblot results show that the GABAARs in the DRM fraction, enriched in lipid raft markers, are mainly formed by α and β subunits and bind [3H]MUS. Fewer GABAARs in this fraction also contain the γ2 subunit and consequently DRM shows less [3H]FNZ binding than the TI fraction.
Fig. 3.
Some GABAAR in synaptosomal plasma membrane are associated with DRMs. The SPM fraction was extracted by 1% Triton X-100 at 4°C and the soluble (TS) and insoluble (TI) fractions were collected. The low-density DRM fraction was also collected after flotation in a discontinuous sucrose gradient. A: Immunoblots with rabbit antibodies to α1, α2, β2, β3, γ2 GABAAR subunits, gephyrin, transferrin receptor (TrsfR) and lipid raft markers Thy-1, Caveolin-1 and Flotillin-1. Same amount of total protein (4μg/lane) was used for SDS-PAGE. B: [3H]MUS and [3H]FNZ binding to GABAARs in TS, TI and DRM. Specific activity values (pmol/mg protein) are mean ± S.E.M of at least three experiments each done in triplicate. *** p < 0.001; ** p < 0.01. The p values are for the TS or TI fractions compared to DRM.
Cholesterol depletion disrupts the association of GABAARs with lipid rafts
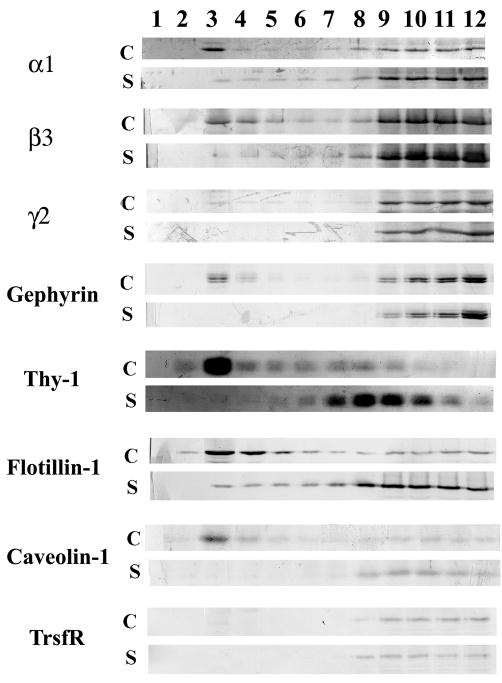
Saponin is a detergent that sequesters cholesterol. It has frequently been used to disrupt lipid rafts (Cerneus et al. 1993, Becher et al. 2001). We treated the SPM fraction with 0.5% saponin before extraction with 1% Triton X-100 at 4°C and fractionation. Fig. 4 shows that when SPM was not treated with saponin (control, C), the α1 and β3 GABAARs subunits, as well as the lipid raft markers Thy-1, Flotillin-1 and Caveolin-1, were found in the low-density fractions (3 and 4). There was very little γ2 GABAAR subunit and no TrsfR could be detected in the low-density fractions 3 and 4. It is worth noticing that gephyrin also showed some association with fraction 4 of Fig. 2 and in the DRM fraction of Fig. 3, indicating that there is some association of gephyrin with DRMs and lipid rafts (Fig. 4), although the bulk of gephyrin concentrated at the heavier 9–12 fractions. After treatment of SPM with saponin (S), the α1 and β3 GABAAR subunits and gephyrin (and the very small amount of γ2 subunit seen in the control) were no longer present in fractions 3 or 4. Moreover, all the proteins, including the lipid rafts markers (i.e. Thy-1, Flotillin-1 and Caveolin-1) migrated to the heavier density fractions 7–12 (Fig. 4). These experiments further support the notions that I) the Triton X-100 insoluble GABAARs present in the low-density fractions are associated with lipid rafts; and II) the majority of the GABAARs associated to lipid rafts contain α and β subunits, but relatively few contain γ2.
Fig. 4.
Saponin treatment disrupts the association of GABAARs with DRMs. The SPM fraction was incubated with 0.5% saponin (S) or without saponin (C, control) followed by extraction with 1% Triton X-100 at 4°C, flotation in a sucrose density gradient and fractionation. Immunoblotting was done with rabbit antibodies to α1, β3, γ2 GABAARs, gephyrin, transferrin receptor (TrsfR) and the lipid raft markers Thy-1, Flotillin-1 and Caveolin-1. The same volume (12μl/lane) from each fraction was used for SDS-PAGE. Fractions 1 and 12 correspond to the top and bottom of the gradient respectively.
In cultured hippocampal neurons, most GABAAR clusters that contain the γ2 subunit are not associated to lipid rafts
Others have shown that in hippocampal cultures, β2/3-containing GABAAR clusters and gephyrin clusters are resistant to 0.5% Triton X-100 extraction at 4°C (Allison et al. 2000). We have now investigated whether the resistance of GABAAR clusters to Triton X-100 extraction is in part due to their association with lipid rafts and whether it depends on the subunit composition.
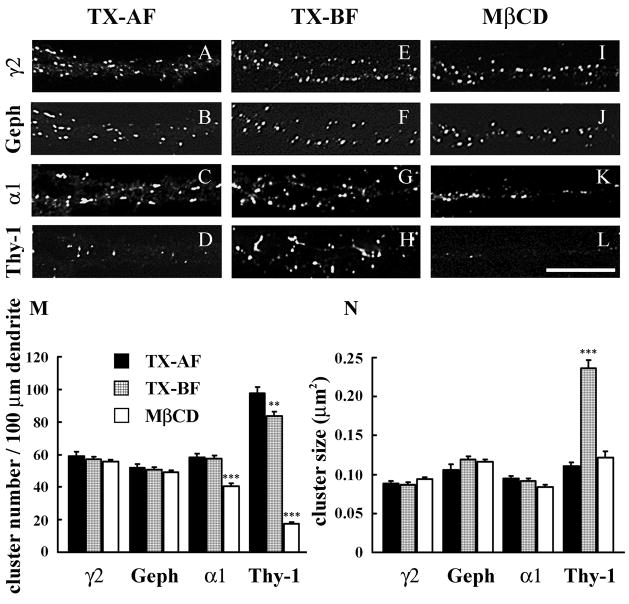
Live cultured hippocampal neurons (19–22 DIV) were incubated with an antibody to an extracellualr epitope of the γ2 subunit, to label the cell-surface GABAARs under conditions that produce no apparent antibody internalization (Levi et al. 2004). Cell permeabilization with 0.25% Triton X-100 was done after cell fixation (TX-AF). This was followed by incubation with the anti-gephyrin antibody to label the intracellular gephyrin. Under these conditions, Triton X-100 permeabilizes the cells but it does not extract the fixed GABAARs. In other cultures, neurons that have been surface-labeled with the anti-γ2 GABAAR antibody were extracted and permeabilized with 0.5% Triton X-100 at 4°C before fixation (TX-BF). After fixation, the cells were incubated with the anti-gephyrin antibody. As shown in Fig. 5A, E, M and N, Triton X-100 at 4°C did not significantly extract the γ2-containing GABAAR (γ2-GABAAR) clusters from the surface of dendrites as shown by analysis of the cluster number (57.0 ± 1.7 in TX-BF compared with 59.2 ± 2.5 in TX-AF, p = 0.48) or the cluster size (0.087 ± 0.003 μm2 in TX-BF compared with 0.089 ± 0.003 μm2 in TX-AF, p = 0.76).
Fig. 5.
In cultured hippocampal neurons some α1-GABAAR clusters, but no γ2-GABAAR clusters or gephyrin clusters, are associated with lipid rafts. Double-label immunofluorescence of pyramidal cell dendrites. Live hippocampal neurons were subjected to one-step capping with anti-γ2 or anti-α1 GABAAR antibody and double-labeling with mAb to gephyrin or Thy-1 respectively. A–D: After one-step capping with guinea pig anti-γ2 (A and B) or rabbit anti-α1 (C and D), cells were fixed followed by incubation with Triton X-100 at 4°C (Triton X-100 after fixation, TX-AF). E–H: After surface labeling and one-step capping with guinea pig anti-γ2 (E and F) or rabbit anti-α1 (G and H), cells were treated with Triton X-100 at 4°C before fixation (TX-BF). I–L: After surface labeling and one-step capping with guinea pig anti-γ2 (I and J) or rabbit anti-α1 (K and L), cells were treated with MβCD before Triton X-100 extraction at 4°C followed by fixation (MβCD). Double-label immunofluorescence of γ2-GABAAR subunit (A, E and I) and gephyrin (B, F and J) or α1-GABAAR subunit (C, G and K) and Thy-1 (D, H and L). The secondary antibodies were Texas Red conjugated anti- guinea pig or anti- rabbit IgG and FITC conjugated anti- mouse IgG, all made in donkey. Scale bar: 10 μm. M and N: Quantification of cluster density and cluster size respectively. Data in M and N were collected from 30–40 dendrites from 15–20 cells of 3–4 separate experiments. *** p < 0.001; ** p < 0.01. The p values are for the TX-BF or MβCD compared to TX-AF.
The number and size of gephyrin clusters were also unaffected by Triton X-100 extraction at 4°C (50.7 ± 1.6 in TX-BF compared to 52.0 ± 2.2 in TX-AF, p = 0.62 and 0.119 ± 0.004 μm2 in TX-BF compared with 0.106 ± 0.007 μm2 in TX-AF, p = 0.08), as shown in Fig. 5B, F, M and N. Moreover, quantification of the co-localization of γ2 and gephyrin clusters showed that in TX-AF neurons, 77.2 ± 1.6 % of γ2 clusters co-localized with gephyrin and 87.8 ± 1.2 % of gephyrin clusters co-localized withγ2. After extraction with Triton X-100 at 4°C (TX-BF), the co-localization of γ2 and gephyrin clusters remained unchanged: 76.1 ± 1.7 % (p = 0.60) of γ2 clusters were co-localized with gephyrin and 85.6 ± 1.5 % (p = 0.26) of gephyrin clusters were co-localized with γ2. Thus, the number and size of γ2-GABAAR clusters, gephyrin clusters and their co-localization remained unchanged by Triton X-100 extraction at 4°C.
Cholesterol depletion was done by incubating the cultures with 5mM methyl-β-cyclodextrin (MβCD) after live cell incubation with the antibodies and prior to the extraction with Triton X-100 at 4°C before fixation (TX-BF). Cholesterol depletion had no significant effect on the insolubility ofγ2-GABAAR clusters in Triton X-100 at 4°C, either in the number (55.7 ± 1.2) compared to the TX-BF (57.0 ± 1.7, p = 0.51) or to the TX-AF (59.2 ± 2.5, p = 0.51) or in the size (0.094 ± 0.003 μm2) compared to the TX-BF (0.087 ± 0.003 μm2, p = 0.09) or to the TX-AF (0.089 ± 0.003 μm2, p = 0.15), as shown in Fig. 5A, E, I, M and N. Neither cholesterol depletion by MβCD had an effect on the insolubility of gephyrin clusters in Triton X-100 at 4°C, nor significantly changed the number of clusters (49.2 ± 1.4) compared to TX-BF (50.7 ± 1.6, p = 0.46), or compared to TX-AF (52.0 ± 2.2, p = 0.26), or the size (0.116 ± 0.003 μm2) compared to the TX-BF (0.119 ± 0.004 μm2, p = 0.48) or to the TX-AF (0.106 ± 0.007 μm2, p = 0.18), of gephyrin clusters, as shown in Fig. 5B, F, J, M and N. Treatment with MβCD prior to extraction with Triton X-100 at 4°C did not affect the co-localization of γ2 and gephyrin clusters (76.0 ± 1.7 % of γ2 clusters co-localized with gephyrin, p = 0.56 and 86.1 ± 1.4 % of gephyrin clusters co-localized with γ2, p = 0.51), compared with TX-BF (76.1 ± 1.7 % of γ2 clusters co-localized with gephyrin and 85.6 ± 1.5 % of gephyrin clusters co-localized with γ2).
The persistent insolubility of the γ2-GABARs or gephyrin clusters in Triton X-100 at 4°C even after cholesterol depletion indicates that these clusters are not associated with lipid rafts. The resistance of γ2-GABAAR and gephyrin clusters to Triton X-100 extraction is likely due to their association with the cytoskeleton and scaffold proteins, as in a way being reminiscent of the insolubility in Triton X-100 of the GABAARs and gephyrin that are present at the GABAergic type-II PSD complex in brain synapses, as shown above.
Some α1-GABAAR clusters in cultured hippocampal neurons are associated with lipid rafts
We did similar live-cell incubation experiments with an anti-α1 GABAAR subunit antibody that recognizes an extracellular epitope. The observed α1-GABAAR clusters resisted solubilization in Triton X-100 at 4°C. Quantification showed that there was no significant difference between TX-BF and TX-AF neurons in the number (57.5 ± 2.0 vs. 58.3 ± 2.3, p = 0.79) and size (0.092 ± 0.003 μm2 vs. 0.095 ± 0.003 μm2, p = 0.49) of the α1-GABAAR clusters, as shown in Fig. 5C, G, M and N. Nevertheless, when cultures were treated with MβCD to deplete cholesterol from the membrane, a significant number of α1-GABAAR clusters became solubilized by Triton X-100 at 4°C, as shown by the reduction in the number of surface α1-GABAAR clusters after MβCD treatment (40.6 ± 1.8), compared to TX-BF or TX-AF cells (57.5 ± 2.0 and 58.3 ± 2.3 respectively, p < 0.0001 in both cases), as shown in Fig. 5C, G, K, and M. The cluster size was not significantly affected in MβCD (0.084 ± 0.004 μm2) when compared to TX-BF (0.092 ± 0.003 μm2, p = 0.095), but decreased when compared to a TX-AF (0.095 ± 0.003 μm2, p = 0.018) as shown in Fig. 5C, G, K and N. Thus, approximately 30% of the α1-GABAAR clusters present on the membrane surface were extracted by Triton X-100 at 4°C after MβCD treatment, indicating that these clusters are associated with lipid rafts. Extraction of the cultures with 0.5% Triton X-100 at 37°C before fixation, condition in which lipid rafts are solubilized (Hering et al. 2003), led to a similar reduction in the number of α1-GABAAR clusters (not shown).
To test the efficiency of lipid rafts disruption by the MβCD treatment in these cultures, neurons were labeled with an antibody to Thy-1, a surface protein associated with lipid rafts, in double-label experiments with anti-α1. Thy-1 clusters were found on the soma and dendrites for TX-AF condition (Fig. 5D). When extraction with Triton X-100 at 4°C was done before fixation (TX-BF), the size of the Thy-1 clusters increased (0.236 ± 0.01 μm2, Fig. 5H and N) when compared to TX-AF (0.111 ± 0.005μm2, p < 0.0001 Fig. 5D and N), apparently due to the coalescence of the smaller Thy-1 clusters induced by the detergent, while the number of Thy-1 clusters significantly decreased (83.8 ± 2.5 for TX-BF vs. 97.6 ± 4.0 for TX-AF, p = 0.0044) as shown in Fig. 5D, H and M. Compared to TX-BF neurons, cholesterol depletion with MβCD led to the solubilization by Triton X-100 at 4°C of the majority of Thy-1 clusters, as shown by a dramatic decrease in number (17.59 ± 1.05, p < 0.0001 Fig. 5H, L and M) and size (0.122 ± 0.008 μm2, p < 0.0001 Fig. 5H, L and N) of the Thy-1 clusters when compared with TX-BF or TX-AF. These results show that I) the solubilization behavior of Thy-1 clusters in Triton X-100 at 4°C is the one expected for a protein associated with lipid rafts; and II) that the MβCD treatment is effective in disrupting lipid rafts in these cultures. The results also validate the assay used in Figs. 5 and 6 to study the association of clustered GABAARs to lipid rafts.
Fig. 6.
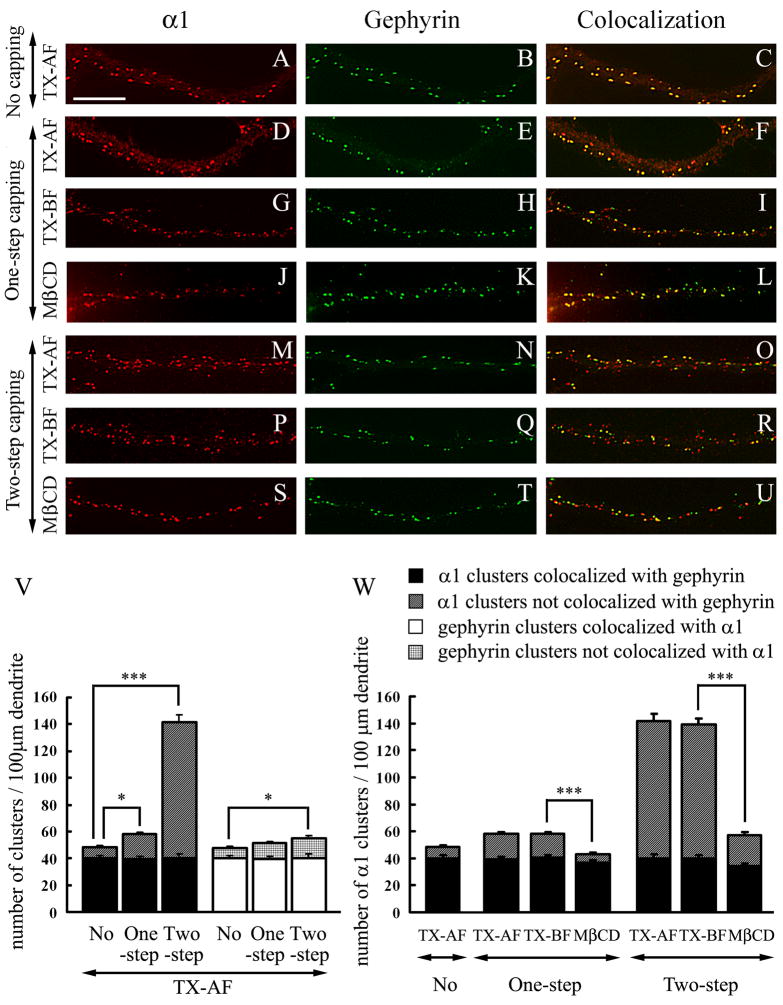
An anti-α1 antibody induces the capping of α1-GABAARs that are associated with lipid rafts. Double-label immunofluorescence of pyramidal cell dendrites. A–C: Hippocampal cultures were fixed, permeabilized with Triton X-100 and double-labeled with rabbit anti-α1 and mAb to gephyrin (no capping, TX-AF). D–L: Live neurons were one-step capped with anti-α1 antibody and double-labeled with mAb to gephyrin. M–U: Live neurons were two-step capped with sequential incubation of anti-α1 antibody and secondary antibody and then double-labeled with mAb to gephyrin. In TX-AF, one-step capping (D–F) or two-step capping (M–O) was followed by 0.25% Triton X-100 permeabilization after fixation. In TX-BF, one-step capping (G–I) or two-step capping (P–R) was followed by incubation with 0.5% Triton X-100 at 4°C before fixation. In MβCD, one-step capping (J–L) or two-step capping (S–U) was followed by treatment with MβCD before incubation with Triton X-100 at 4°C and fixation. Panels A–U show representative dendrites. Scale bar: 10 μm. V and W: Quantification of α1-GABAAR and gephyrin cluster density and their co-localization in hippocampal cultures in the various experimental conditions represented in panels A–U. For quantification, 30 dendrites were randomly chosen from 15–20 cells from 3 separate experiments. Values represent mean ± SEM. *** p < 0.001; * p < 0.05.
Taken together, these results indicate that I) surface γ2-GABAAR and gephyrin clusters resist extraction by Triton X-100 at 4°C. These clusters are not associated with lipid rafts; and II) about 30% of surface α1-GABAAR clusters are associated with lipid rafts. These clusters do not contain the γ2 subunit and are not associated with gephyrin (see below).
In hippocampal cultures there is a pool of α1-GABAAR associated with lipid rafts that is diffusely distributed at the neuronal surface and that can be induced to cluster by antibody capping
We and others have shown that when living cells are incubated with a primary anti-GABAAR antibody to an external epitope, it leads to the clustering (one-step capping) of the normally non-clustered GABAARs that are present on the cell surface (Brunig et al. 2002; Levi et al. 2004; Christie et al. 2006). The capping of the non-clustered GABAARs is further enhanced by sequential incubations of the live cells with the primary and secondary antibodies before fixation (two-step capping, Levi et al. 2004; Christie et al. 2006). When the cells were fixed, followed by permeabilization with Triton X-100 before incubation with the primary antibodies (TX-AF, no capping) in double-label immunofluorescence experiments, all the observed α1-GABAAR and gephyrin clusters (Fig. 6A–C) are the ones that are normally present in those neurons in the absence of antibody capping. These clusters have been thoroughly documented by us and others (Craig et al. 1996; Christie et al. 2002a, b; Christie and De Blas 2003). When the live cells were incubated with the anti-α1 antibody followed by fixation and treatment with Triton X-100 (one-step capping TX-AF, Fig. 6D and V), the number of α1 clusters (58.1 ± 2.0) was significantly higher than when TX-AF cells were not subjected to capping (48.4 ± 2.1, p = 0.0014). Two-step capping induced the formation of a considerably larger number of α1-GABAAR clusters (141.5 ± 7.5, p < 0.0001, Fig. 6M and V) compared to no capping or one-step capping in the TX-AF cells.
The number of gephyrin clusters did not significantly change in TX-AF cells from no capping (47.8 ± 2.2, Fig. 6B and V) to one-step capping with anti-α1 (51.3 ± 2.0, p = 0.23, Fig. 6E and V). A very small increase in gephyrin cluster number was detected in two-step capping with anti-α1 (55.2 ± 2.7, p = 0.03, Fig. 6N and V). In TX-AF cells the number of α1-GABAAR clusters colocalizing with gephyrin clusters (or the number of gephyrin clusters colocalizing with α1-GABAAR clusters, which is the same, Fig. 6V) remained unchanged in no-capping (40.1 ± 2.1) when compared with one-step capping with anti-α1 (39.3 ± 1.9, p = 0.79), or with two-step capping (39.9 ± 3.2, p = 0.96). Therefore the increased number of α1-GABAAR clusters induced by one-step or two-step antibody capping of the α1-GABAARs that are normally diffusely distributed at the neuronal surface is not accompanied by simultaneous gephyrin capping (Levi et al. 2004; Christie et al. 2006).
Lipid rafts can be induced to coalesce by antibodies recognizing proteins associated with lipid rafts (Holowka et al. 2005). We have investigated whether the α1-GABAAR clusters associated with lipid rafts described above under one-step capping conditions (Fig. 5C, G, K) are the α1-GABAAR clusters normally existing in these neurons or the clusters whose formation was induced by one-step capping with anti-α1. We also tested the solubility of the clusters in Triton X-100 at 4°C before fixation after one-step and two-step capping. After one-step capping of live cells, extraction with Triton X-100 at 4°C before fixation (TX-BF) did not change the number of α1-GABAAR clusters (58.2 ± 2.1 vs. 58.1 ± 2.0, p = 0.96) or the number of α1-GABAAR clusters colocalizing with gephyrin (40.3 ± 1.9 vs. 39.3 ± 1.9, p = 0.73), when compared to one-step capping of live cells in which extraction with Triton X-100 was done after fixation (TX-AF, Fig. 6D–I and W). Although the number of α1-GABAAR clusters in the two-step capping experiments was considerably larger than in the one-step capping, there was no significant difference between two-step capping TX-AF and TX-BF in the number of α1-GABAAR clusters (141.5 ± 7.5 vs. 139.3 ± 5.5 respectively, p = 0.82) or the number of α1-GABAAR clusters colocalizing with gephyrin (39.9 ± 3.2 vs. 40.0 ± 2.1 respectively, p = 0.98), as shown in Fig. 6M–R and W. These experiments show that both the original α1-GABAAR clusters and the ones induced by one-step or two-step capping resist solubilization by Triton X-100 at 4°C.
After one-step capping (Fig. 6J–L and W) or two-step capping (Fig. 6S–U and W), treatment of the culture with MβCD for cholesterol depletion prior to Triton X-100 extraction before fixation, led to a significant reduction in the number of α1-GABAAR clusters compared to the corresponding TX-BF cultures not treated with MβCD (43.2 ± 1.8 vs. 58.2 ± 2.1, p < 0.0001 for one-step capping and 57.0 ± 2.3 vs. 139.3 ± 5.5, p < 0.0001 for two-step capping, respectively). The results (Fig. 6W) also showed that I) the original α1-GABAAR clusters, which are present in the absence of capping, co-localized with gephyrin and remained insoluble in Triton X-100 at 4°C after cholesterol depletion by MβCD and II) MβCD treatment facilitated the solubilization in Triton X-100 of the majority (93%) of the α1-GABAAR clusters whose formation was induced by one-step or two-step capping with anti-α1. In contrast, and in similar experiments, many (84%) of the γ2 GABAARs whose cluster formation was induced by two step-capping with anti-γ2 remained insoluble in Triton X-100 at 4°C even after cholesterol depletion by MβCD (not shown).
These experiments show that I) most of the α1-GABAAR clusters induced by one-step or two-step capping with the anti-α1 antibody are associated with lipid rafts, but are not associated with gephyrin; II) most of the γ-GABAARs whose clustering is induced by one-step or two-step capping with the anti-γ2 antibody are not associated with lipid rafts and III) the normally existing α1-GABAAR or γ2-GABAAR clusters that are present in the cultured hippocampal neurons, are not associated to lipid rafts. Many of these clusters co-localize with gephyrin clusters.
DISCUSSION
We have shown by EM immunogold that after brain homogenization, synaptosomes derived from GABAergic synapses, which contain postsynaptic GABAARs and gephyrin and that have Gray’s type-II synaptic morphology, are present in the classical synaptosomal fraction. We have also found that the “One Triton insoluble PSD” fraction, which is derived from the synaptosomal fraction and is enriched in glutamatergic type-I PSDs (Hunt et al. 1996 and Kennedy 1997), is also enriched in GABAARs and gephyrin, which are proteins associated with GABAergic type-II synapses. These results support and expand the notion put forward by Matus et al. 1980 that the detergent-insoluble PSD fraction contains not only type-I PSDs but also type-II PSDs. They based their hypothesis in that the detergent-insoluble PSD fraction showed [3H]GABA and [3H]Muscimol binding. Our EM immunogold studies of the “One Triton PSD” fraction show that the anti-GABAAR and anti-gephyrin antibodies immunolabel amorphous structures of lower electron-density than those of type-I PSDs. We hypothesize that these structures correspond to type-II PSDs. This notion was confirmed after sedimentation of the “One Triton PSD” fraction on a continuous sucrose density gradient, which led to the partial separation of the GABAergic type-II PSDs from the glutamatergic type-I PSDs, as shown by the separation of two peaks, one of a heavier density (ρ = 1.18–1.23 g/ml) enriched in PSD-95, a marker of the glutamatergic type-I PSDs, and another one of a lighter density (ρ = 1.10–1.15 g/ml) enriched in gephyrin and γ2-GABAARs, markers of the GABAergic type-II PSDs. The EM immunogold of the ρ = 1.10–1.15 g/ml peak fraction shows that the GABAAR and gephyrin immunolabeling concentrates in amorphous structures similar to those immunolabeled with anti-gephyrin and anti-GABAAR subunit antibodies in the “One Triton PSD” fraction. We propose that these structures correspond to type-II GABAergic PSDs.
This interpretation is consistent with the known concentration and co-localization of gephyrin and γ2-GABAARs in the type-II postsynaptic GABAergic complexes both in brain and in cultured hippocampal neurons (Sassoe-Pognetto et al. 2000; Christie et al. 2002a; Craig et al. 1996; Giustetto et al. 1998; Kneussel et al. 1999; Christie et al. 2002b; van Rijnsoever et al. 2005). It has been shown that postsynaptic gephyrin is involved in the clustering of some postsynaptic GABAARs (Essrich et al. 1998; Kneussel et al. 1999, 2001; Christie et al. 2002a; Levi et al 2004; Jacob et al. 2005), by restricting the lateral mobility of GABAARs in the membrane (Jacob et al. 2005), and that the γ2 subunit is essential for the postsynaptic clustering of GABAARs and gephyrin, as shown by studies on the γ2 knockout mouse (Essrich et al. 1998; Schweizer et al. 2003) and by γ2 RNA interference studies (Li et al. 2005b).
The gradient fractions 6 and 7 (Fig. 2), which are enriched in γ2-GABAARs and gephyrin but not in PSD-95, should be valuable for identifying various protein components of the GABAergic type-II PSDs by proteomics. There is a limited knowledge on the protein components and the signal transduction mechanisms in type-II PSDs (Moss and Smart 2001; Owens and Kriegstein 2002). Proteomic approaches have been successfully used to identify various protein components of the purified type-I PSDs (Kennedy 1997; Walikonis et al. 2000; Yoshimura et al. 2004; Peng et al. 2004; Jordan et al. 2004; Collin et al. 2006).
A second pool of Triton X-100-insoluble GABAARs is present in the “One Triton PSD”. This very low-density (ρ = 1.06–1.09 g/ml) pool of GABAARs is associated to DRMs and the lipid raft markers Caveolin-1, Thy-1 and Flotillin-1, but not to transferrin receptor. To the best of our knowledge, this is the first time that two pools of Triton X-100 -insoluble GABAARs in the rat brain have been identified in the “One Triton PSD” fraction: one associated with DRMs (and probably to lipid rafts), and another one associated with type-II PSDs. Thus, an important consideration for those who use the “One Triton PSD” fraction in their research is that not all the Triton X-100-insoluble proteins that are present in this fraction are associated to PSDs. Some of these proteins are associated instead to the DRMs that are also present in the “One Triton PSD” fraction. It has been previously reported that some GABAARs in cultured rat cerebellar granule cells are associated to DRMs (Dalskov et al. 2005).
Our results show that the majority of the forebrain GABAARs associated with DRMs have α and β subunits, but little or no γ2, and are largely not associated with gephyrin. Although most of the δ-GABAARs in the forebrain are also associated to DRMs, they represent a relatively small proportion of the α and β-GABAARs present in the forebrain DRMs. This notion is supported by I) the weak intensity of the δ protein band observed in immunoblots with three different anti-δ antibodies and II) although the δ subunit is highly expressed in rat cerebellum and thalamus, in the rat cerebral cortex (the main component of our forebrain preparation) there is very little expression of the δ subunit while there is high expression of the α, β andγ2 subunits (Fritschy and Mohler 1995; Pirker et al. 2000). Thus, many of the GABAARs in the DRMs from forebrain contain α and β but not γ2 or δ subunits while some contain α, β and δ subunits. The subunit composition of the GABAARs in the DRMs contrasts with that of the synaptic GABAARs associated with type-II PSDs, which have the γ2 subunit (plus α and β) and are associated with gephyrin. The absence of the γ2 and gephyrin suggests that the majority of GABAARs associated with DRMs (and lipid rafts) are non-synaptic, since as indicated above, the γ2 subunit is essential for the postsynaptic localization of GABAARs (Essrich et al. 1998; Schweizer et al. 2003; Li et al. 2005b), and gephyrin concentrates at GABAergic synapses and is involved in the postsynaptic clustering of some GABAARs. Moreover, the δ-GABAARs, which we have shown to be associated with DRMs, are extrasynaptic and involved in tonic inhibition (Farrant and Nusser 2005; Nusser and Mody 2002; Stell et al. 2003). The difference in subunit composition between the GABAARs in type-II PSDs and the GABAARs in DRMs translates into different radioligand binding properties. Although both types of GABAARs show [3H]MUS, non-synaptic GABAARs present in the DRM fraction show low [3H]FNZ binding, which agrees with the low content of γ2 subunit in the DRM fraction. In contrast, the synaptic GABAARs present in type-II PSDs contain the γ2 subunit and show high [3H]FNZ binding. The DRM fraction is enriched in caveolin. Thus, some lipid raft-associated GABAARs might be endocytosed by caveolae and caveosomes. Caveolae are morphologically identified as flask-like invaginations of the plasma membrane that are enriched in lipid rafts and in the coat protein caveolin (Brown and London 1998, Masserini et al. 1999). It is also worth noticing that although the majority of gephyrin and γ2 GABAAR subunit are associated to type-II PSDs, there are some gephyrin and γ2 associated to DRMs (in the DRM fraction of Fig. 3 and in the low-density fraction of Fig. 4). We do not know yet whether a small proportion of the γ2-GABAARs and gephyrin present at GABAergic synapses is associated to lipid rafts, or whether they represent γ2-GABAARs and gephyrin associated to lipid rafts that are trafficking in and out the GABAergic synapses.
There is a third pool of GABAARs that we have not considered in this study. This is a pool of GABAARs that can be solubilized by Triton X-100 at 4°C and that has been the object of many biochemical and pharmacological studies in the literature (and in Fig. 3). The GABAARs that are present in this Triton X-100-soluble (TS) fraction have α, β and γ2 subunits and show [3H]MUS and [3H]FNZ binding (Fig. 3), indicating that they are fully assembled receptors. The binding activity recovered in the TS fraction was 45% of the [3H]MUS and 51% of the [3H]FNZ binding activity present in the SPM fraction. The solubility of the GABAARs in Triton X-100 at 4°C indicates that they are not associated to lipid rafts and suggests that they might not be associated to the type-II PSDs and to the GABAARs that are present in the Triton X-100-insoluble (TI) fraction. The GABAARs in the TS fraction might represent extrasynaptic membrane receptors with lateral mobility that can eventually be anchored at the postsynaptic complexes and become part of the immobilized synaptic pool (Choquet and Triller 2003; Thomas et al. 2005).
In hippocampal cultures, the normalγ2-GABAAR and gephyrin clusters are not associated with lipid rafts since they cannot be solubilized by Triton X-100 at 4°C even after cholesterol depletion. These results with cultures agree with the results obtained with brain fractions in the sense that the majority of brainγ2-GABAAR and gephyrin are not present in DRMs. Moreover, the majority (74%) of the α1-GABAAR clusters, that are normally present in the cultured hippocampal neurons, are not associated to lipid rafts, since they are resistant to solubilization with Triton X-100 at 4°C even after cholesterol depletion. The majority of these α1-GABAAR clusters colocalize with gephyrin clusters. However, the remaining 26% of Triton-insoluble α1-GABAAR clusters become solubilized by Triton X-100 when cholesterol is depleted, indicating that they are associated to lipid rafts. These α1-GABAAR clusters do not co-localize with gephyrin clusters.
In cultured hippocampal neurons, there is also a pool of extrasynaptic α1-GABAARs that are diffusely distributed at the neuronal surface and that can be induced to form clusters by capping with a primary antibody that recognizes an extracellular epitope of the GABAAR (one-step capping), or by the sequential incubation of live neurons with the primary antibody and the fluorophore-labeled secondary antibody (two-step capping). Antibody-induced capping has been used by us and others to reveal diffuse non-clustered GABAARs on the surface of cultured neurons (Levi et al. 2004; Christie et al. 2006). The pool of diffuse GABAARs that can be clustered by one-step or two-step capping induced by anti-α1 is associated to lipid rafts since it resists extraction with Triton X-100 at 4°C, but it becomes solubilized by this detergent after cholesterol depletion. The antibody-induced clustering of the α1-GABAARs does not induce the simultaneous co-clustering of gephyrin, as we and others have previously observed, following GABAAR capping induced with antibodies to other GABAAR subunits (Levi et al. 2004; Christie et al. 2006), indicating that the GABAARs in the non-clustered state are not associated with gephyrin. These results also support the notion that in the absence of association with gephyrin, GABAARs show high lateral mobility in the membrane (Jacob et al. 2005), which allows the capping by the antibody of the diffuse membrane GABAARs.
Thus, the studies with rat forebrain fractions and hippocampal cultures support the hypothesis that there are two pools of assembled GABAARs that resist Triton X-100 solubilization at 4°C. One pool is associated with gephyrin, contains the γ2 subunit (plus α and β subunits), shows high specific activity of both [3H]MUS and [3H]FNZ bindings and co-purifies with type-II PSDs of the GABAergic synapses. This synaptic pool forms a Triton X-100 insoluble protein complex containing GABAARs, gephyrin, the postsynaptic cytoskeleton and scaffold proteins among others. These synaptic GABAARs are not associated to DRMs or lipid rafts. There is another pool of GABAARs in the forebrain that is also insoluble in Triton X-100 but that is associated to DRMs and lipid rafts. These GABAARs do not contain the γ2 subunit but the majority contain α and β subunits but not δ and some contain α, β and δ subunits. These GABAARs show high [3H]MUS binding but low [3H]FNZ binding and show little or no association with gephyrin. These GABAARs are non-synaptic, many are diffusely distributed through the neuronal surface and can be induced to form clusters by anti-α1 antibody-induced capping.
The association of some GABAARs with lipid rafts might have functional relevance since cholesterol modulates GABAARs (Sooksawate and Simmonds 2001). It is thought that the synaptic GABAARs that are present in type-II GABAergic synapses are involved in phasic inhibition while the diffuse extrasynaptic GABAARs are involved in tonic inhibition (Luscher and Keller 2004; Kullmann et al. 2005; Farrant and Nusser 2005; Mody 2005). Our results are consistent with the notion that the GABAARs associated to lipid rafts in the forebrain are extrasynaptic and are involved in tonic inhibition since: I) some of the forebrain GABAARs associated with lipid rafts contain the δ subunit and it has been shown that δ-GABAARs are extrasynaptic and involved in tonic inhibition (Farrant and Nusser 2005; Nusser and Mody 2002; Stell et al. 2003); II) other forebrain GABAARs present in lipid rafts contain α and β subunits but not γ2 or δ. It has recently been reported the existence of extrasynaptic GABAARs that contain only α and β subunits and that are involved in tonic inhibition (Mortensen and Smart 2006).
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke grants NS38752 and NS39287.
Abbreviation used
- DRM
detergent resistant membrane
- EM
electron microscopy
- FNZ
flunitrazepam
- GABAAR
γ-aminobutyric acid type-A receptor
- Geph
gephyrin
- mAb
monoclonal antibody
- MβCD
methyl-β-clycodextrin
- MUS
muscimol
- PSD
postsynaptic density
- PSD-95
postsynaptic density protein 95
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SPM
synaptosomal plasma membrane fraction
- SYN
synaptosomal fraction
- TI
Triton X-100 insoluble fraction
- TS
Triton X-100 soluble fraction
- TX-AF
Triton X-100 extraction after fixation
- TX-BF
Triton X-100 extraction before fixation
References
- Allison DW, Chervin AS, Gelfand VI, Craig AM. Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules. J Neurosci. 2000;20:4545–4554. doi: 10.1523/JNEUROSCI.20-12-04545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher A, White JA, McIlhinney RA. The gamma-aminobutyric acid receptor B, but not the metabotropic glutamate receptor type-1, associates with lipid rafts in the rat cerebellum. J Neurochem. 2001;79:787–795. doi: 10.1046/j.1471-4159.2001.00614.x. [DOI] [PubMed] [Google Scholar]
- Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. TevesIncreased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in the rat brain. J Neurochem. 2005;93:186–194. doi: 10.1111/j.1471-4159.2004.03009.x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid- enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem Biophys Res Commun. 1997;240:1–7. doi: 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functional of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol- rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Brunig I, Suter A, Knuesel I, Luscher B, Fritschy JM. GABAergic terminals are required for postsynaptic clustering of dystrophin but not of GABA(A) receptors and gephyrin. J Neurosci. 2002;22:4805–4813. doi: 10.1523/JNEUROSCI.22-12-04805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruses JL, Chauvet N, Rutishauser U. Membrane lipid rafts are necessary for the maintenance of the (alpha)7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. J Neurosci. 2001;21:504–512. doi: 10.1523/JNEUROSCI.21-02-00504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–843. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerneus DP, Ueffing E, Posthuma G, Strous GJ, van der Ende A. Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis. Role of cholesterol J Biol Chem. 1993;268:3150–3155. [PubMed] [Google Scholar]
- Chandra D, Korpi ER, Miralles CP, De Blas AL, Homanics GE. GABAA receptor gamma2 subunit knockdown mice have enhanced anxiety-like behavior but unaltered hypnotic response to benzodiazepines. BMC Neurosci. 2005;6:30. doi: 10.1186/1471-2202-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Yu W, LR, et al. A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain. J Biol Chem. 2004a;279:38978–38990. doi: 10.1074/jbc.M405786200. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004b;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A. The role of receptor diffusion in the organization of the postsynaptic memebrane. Nat Rev Neurosci. 2003;4:251–265. doi: 10.1038/nrn1077. [DOI] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, et al. Synaptic and extrasynaptic GABA receptor and gephyrin clusters. Prog Brain Res. 2002a;136:157–180. doi: 10.1016/s0079-6123(02)36015-1. [DOI] [PubMed] [Google Scholar]
- Christie SB, Miralles CP, De Blas AL. GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons. J Neurosci. 2002b;22:684–697. doi: 10.1523/JNEUROSCI.22-03-00684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, De Blas AL. α5 subunit-containing GABAA receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport. 2002;13:2355–2358. doi: 10.1097/00001756-200212030-00037. [DOI] [PubMed] [Google Scholar]
- Christie SB, De Blas AL. GABAergic and glutamatergic axons innervate the axon initial segment and organize GABAA receptor clusters of cultured hippocampal pyramidal cells. J Comp Neurol. 2003;456:361–374. doi: 10.1002/cne.10535. [DOI] [PubMed] [Google Scholar]
- Christie SB, Li R-W, Miralles CP, Yang B-Y, De Blas AL. Clustered and non-clustered GABAA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2006;31:1–4. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Collin MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem Suppl. 2006;1:16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Banker G, Churchill L, Taylor D. Isolation of postsynaptic densities from rat brain. J Cell Biol. 1974;63:441–455. doi: 10.1083/jcb.63.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir HL, Banker G. Selective clustering of glutamate and gamma-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc Natl Acad Sci USA. 1994;91:12373–12377. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Banker G, Chang W, Mcgrath ME, Serpinskaya AS. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J Neurosci. 1996;16:3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalskov SM, Immerdal L, Nisels-Christiansen LL, Hansen GH, Schousboe A, Danielsen EM. Lipid raft localization of GABA A receptor and Na+, K+-ATPase in discrete microdomain clusters in rat cerebellar granule cells. Neurochem Int. 2005;46:489–499. doi: 10.1016/j.neuint.2004.11.010. [DOI] [PubMed] [Google Scholar]
- De Blas AL, Cherwinski HM. Detection of antigens on nitrocellular paper immunoblots with monoclonal antibodies. Anal Biochem. 1983;133:214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- De Blas AL, Vitorica J, Friedrich P. Localization of the GABAA receptor in the rat brain with a monoclonal antibody to the 57,000 Mr peptide of the GABAA receptor/benzodiazepine receptor/Cl-channel complex. J Neurosci. 1988;8:602–614. doi: 10.1523/JNEUROSCI.08-02-00602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Lusher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Giustetto M, Kirsch J, Fritschy JM, Cantino D, Sassoe-Pognotto M. Localization of the clustering protein gephyrin at GABAergic synapses in the main olfactory bulb of the rat. J Comp Neurol. 1998;395:231–244. doi: 10.1002/(sici)1096-9861(19980601)395:2<231::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing Nerve cells. MIT press; Cambridge, MA: 1998. pp. 339–370. [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Lin Chih-chunand, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka D, Gosse JA, Hammond AT, et al. Lipid segregation and IgE receptor signaling: a decade of progress. Biochim Biophys Acta. 2005;1746:252–259. doi: 10.1016/j.bbamcr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Harrison NL, Quinlan JJ, et al. Normal electrophysiological and behavioral responses to ethanol in mice lacking the long splice variant of the gamma2 subunit of the gamma-aminobutyrate type A receptor. Neuropharmacology. 1999;38:253–265. doi: 10.1016/s0028-3908(98)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CA, Schenker LJ, Kennedy MB. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J Neurosci. 1996;16:1380–1388. doi: 10.1523/JNEUROSCI.16-04-01380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Mgnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing SQ, Spencer T, Miller K, Hopkins C, Trowbridge IS. Role of the human transferrin receptor cytoplasmic domain in endocytosis: Localization of a specific signal sequence for internalization. J Cell Biol. 1990;110:283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DH, Matus AI. Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation. Biochim Biophys Acta. 1974;356:276–287. doi: 10.1016/0005-2736(74)90268-5. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3:857–871. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- Kawasaki BT, Hoffman KB, Yamamoto RS, Bahr BA. Variants of the receptor/channel clustering molecule gephyrin in brain: distinct distribution patterns, developmental profiles, and proteolytic cleavage by calpain. J Neurosci Res. 1997;49:381–388. doi: 10.1002/(sici)1097-4547(19970801)49:3<381::aid-jnr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:264–8. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H. Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Serwanski DR, Miralles CP, Li X, Charych E, Riquelme R, Huganir RL, De Blas AL. GRIP1 in GABAergic synapses. J Comp Neurol. 2005a;488:11–27. doi: 10.1002/cne.20566. [DOI] [PubMed] [Google Scholar]
- Li RW, Yu W, Christie S, Miralles CP, Bai J, Loturco JJ, De Blas AL. Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J Neurochem. 2005b;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- London E, Brown DA. Insolubility of lipid in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochem Biophys Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasiticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Masserini M, Palestini P, Pitto M. Glycolipid-enriched caveolae and caveolae-like domains in the nervous system. J Neurochem. 1999;73:1–11. doi: 10.1046/j.1471-4159.1999.0730001.x. [DOI] [PubMed] [Google Scholar]
- Matus A, Pehling G, Ackermann M, Maeder J. Brain postsynaptic densities: the relationship to glial and neuronal filaments. J Cell Biol. 1980;87:346–359. doi: 10.1083/jcb.87.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles CP, Li M, Metha AK, Kahn ZU, De Blas AL. Immunocytochemical localization of the beta(3) subunit of the gamma-aminobutyric acid(A) receptor in the rat brain. J Comp Neurol. 1999;413:535–548. [PubMed] [Google Scholar]
- Mody I. Aspects of the homeostatic plasticity of GABAA receptor-mediated inhibition. J Physiol. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JI, Piva MA, Miralles CP, De Blas AL. Immunocytochemical localization of the beta 2 subunit of the gamma-aminobutyric acid(A) receptor in the rat brain. J Comp Neurol. 1994;350:260–271. doi: 10.1002/cne.903500209. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on hippocampal pyramidal neurons. J Physiol (Lond) 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibition in dentate gyrus granule cells. J Neurophysiol. 2002;87:2626–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi P. Immunocytochemical localization of the alpha 1 and beta 2/3 subunits of the GABAA receptor in relation to specific GABAergic synapses in the dentate gyrus. Eur J Neurosci. 1995;7:630–646. doi: 10.1111/j.1460-9568.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Riquelme R, Miralles CP, De Blas AL. Bergmann glia GABAA Receptors Concentrate on the Glial Processes that Wrap Inhibitory Synapses. J Neurosci. 2002;22:10720–10730. doi: 10.1523/JNEUROSCI.22-24-10720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM. Colocalization of multiple GABA(A) receptor subtypes with gephyrin at postsynaptic sites. J Comp Neurol. 2000;420:481–498. doi: 10.1002/(sici)1096-9861(20000515)420:4<481::aid-cne6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and non-synaptic localization of GABAA receptors containing the α5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipid and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci USA. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Lusher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JDB, Sieghart W. The γ2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the α1 and β2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacol. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Simmonds MA. Influence of membrane cholesterol on modulation of the GABA(A) receptor by neuroactive steroids and other potentiators. Br J Pharmacol. 2001;134:1303–1311. doi: 10.1038/sj.bjp.0704360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ito J, Takagi H, Saitoh F, Nawa H, Shimizu H. Biochemical evidence for localization of AMPA-type glutamate receptor subunits in the dendritic raft. Brain Res Mol Brain Res. 2001;89:20–28. doi: 10.1016/s0169-328x(01)00051-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Lipid rafts at postsynaptic sites: distribution, function and linkage to postsynaptic density. Neurosci Res. 2002;44:1–9. doi: 10.1016/s0168-0102(02)00080-9. [DOI] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie AA, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Van Rijnsoever C, Sidler C, Fritschy JM. Internalized GABA-receptor subunits are transferred to a intracellular pool associated with the postsynaptic density. Eur J Neurosci. 2005;21:327–338. doi: 10.1111/j.1460-9568.2005.03884.x. [DOI] [PubMed] [Google Scholar]
- Vitorica J, Park D, Chin G, De Blas AL. Monoclonal antibodies and conventional antisera to the GABAA receptor/benzodiazepine receptor/Cl- channel complex. J Neurosci. 1988;8:615–622. doi: 10.1523/JNEUROSCI.08-02-00615.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walikonis RS, Jensen ON, Mann M, Provance DW, Jr, Mercer JA, Kennedy MB. Identification of proteins in the postsynaptic density fraction by mass spectrometry. J Neurosci. 2000;20:4069–4080. doi: 10.1523/JNEUROSCI.20-11-04069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Yamauchi Y, Shinkawa T, Taoka M, Donai H, Takahashi N, Isobe T, Yamauchi T. Molecular constituents of the postsynaptic density fraction revealed by proteomic analysis using multidimensional liquid chromatography-tandem mass spectrometry. J Neurochem. 2004;88:759–768. doi: 10.1046/j.1471-4159.2003.02136.x. [DOI] [PubMed] [Google Scholar]
- Zhu D, Xiong WC, Mei L. Lipid rafts serve as a signaling platform for nicotinic acetylcholine receptor clustering. J Neurosci. 2006;26:4841–4845. doi: 10.1523/JNEUROSCI.2807-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]