Abstract
Many rheumatologic disorders, most notably Sjögren's syndrome, are associated with dental complications and in some cases oral diseases may trigger or drive connective tissue disease. During the past three decades the treatment in rheumatology was revolutionized by the introduction of disease-modifying anti-rheumatic drugs. Advances in our understanding of the pathogenesis of rheumatic diseases have led to the discovery of critical mechanisms of inflammation and autoimmunity and the invention of new target-specific biologic agents. In this review, we will summarize the current state of biologic therapies in rheumatology and discuss the implications of these on oral health and disease.
Keywords: autoimmunity, biologic therapies, monoclonal antibodies, rheumatic diseases, Sjögren's syndrome
Introduction
Systemic rheumatic diseases are characterized by systemic autoimmunity leading to chronic inflammation in target organs and systems. Although the inciting event is not known for any of these conditions, it most likely results from a complex interaction of genetic, environmental and stochastic factors. A general model of pathogenesis is that in a genetically susceptible individual an initial breakdown in tolerance creates primary self-reactive cells, which then propagate the autoimmune response by a variety of mechanisms that include positive-feedback amplification loops, such as T and B lymphocyte activation, autoantibody production, complement activation, immune complex deposition, and leukocyte infiltration of target organs. Although the relative importance of these abnormalities may be different in various diseases, they do share common effector pathways, which may present attractive therapeutic targets. The commonly used wide-spectrum immunosuppressive and anti-inflammatory drugs such as systemic corticosteroids have clinical benefit, but are associated with significant side effects and do not induce long-lasting tolerance in humans. Their use is based mainly on empirical evidence of efficacy and not on the understanding of their mechanism of action on the immune system. Therefore, targeted interference with key components of inflammation provides the hope of more effective therapies.
Over the last decade we have seen remarkable progress in the development of targeted biologic therapies such as monoclonal antibodies and fusion proteins. Monoclonal antibody (mAb) technology was first discovered over three decades ago (Kohler and Milstein, 1975) and quickly revolutionized the management of malignancies, in the context of stem cell and solid organ transplantation, rheumatologic disorders, autoimmune disease, inflammatory bowel disease, infections and atopic disorders. Initially, mAbs were used as vehicles for transporting cytotoxic medications to the designated tissues; later, they were used to directly target molecules and cells of interest.
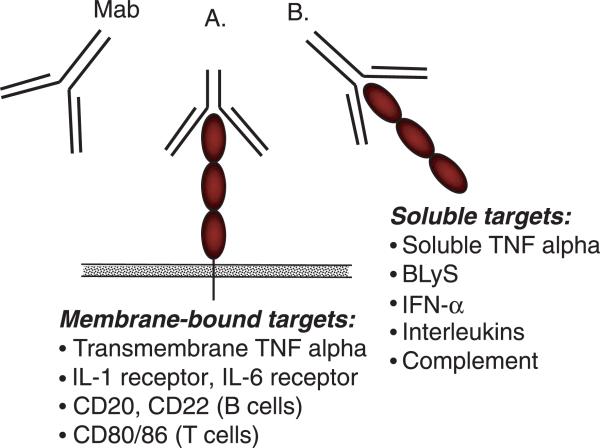
Currently available biologic therapies can be divided into those that target certain cell-surface molecules and those that interact with secreted molecules (Figure 1). Cell surface molecules can serve as markers specific for a particular cell, which can then be specifically targeted and deleted or manipulated by a mAb. For example, rituximab binds specifically to CD20, which is expressed on all B lymphocytes, except on very early precursor B cells and immunoglobulin-secreting plasma cells and leads to selective B cell depletion. Another class of molecules bind to cell surface proteins and interfere with their function without deleting or killing the cells. The most commonly used biologics interfere with the action of cytokines, which are soluble mediators of inflammation. Cytokines exert their action by binding to cell surface receptors. The action of cytokines can be blocked by binding the soluble cytokine or preventing its binding to its cognate receptor. The former can be achieved by using monoclonal antibodies against the cytokine or by using a decoy, soluble receptor, which will bind to the cytokines in a manner similar to that of cell surface receptors, thereby reducing the levels of free, biologically active cytokines. All of these options have already been successfully employed in the treatment of rheumatoid arthritis (RA). From three anti-tumor necrosis factor (TNF) agents approved in the United States, two (infliximab and adalimumab) are monoclonal antibodies, whereas etanercept is a soluble receptor fusion protein.
Figure 1.
Targets of biologic therapies: Mab, monoclonal antibody/biologic agent. (A) Biologic agent targeting membrane-bound molecules. Examples: anti-TNF (infliximab, adalimumab), anti-IL-6 receptor (tocilizumab), IL-1 receptor antagonist (anakinra), CTLA4 Ig (abatacept). (B) Biologic agent targeting soluble molecules. Examples: anti-TNF (infliximab, adalimumab), anti-BLyS (belimumab). TNF, tumor necrosis factor; IL, interleukin; CD, cluster of differentiation; BLyS, B lymphocyte stimulator
Although the interaction between these inhibitors and their targets is very specific, we are still learning about the diversity and complexity of their biologic mechanisms of action. They may have a direct effect on disease pathogenesis – for example through complement- and/or antibody-mediated destruction of cells and apoptosis or competitive inhibition of binding of molecules to their receptor. Alternatively, they may have an indirect effect – for example through sensitization of cells to conventional chemotherapy or down-regulation of receptors.
Specific therapies
Introduction on TNF/TNFRSFP
Tumor necrosis factor-α (TNF-α) belongs to the TNF/TNFR superfamily of proteins (TNFRSFP) which consists of structurally and functionally similar proteins. They are highly conserved and play important roles in innate immunity and host defenses, inflammation, apoptosis, lymphoid organ and tissue development, and homeostasis and organogenesis.
All of the TNF/TNFR proteins share the same basic homotrimer quaternary structure (Banner et al, 1993) with an elongated cystein-rich extracellular tail, transmembrane domain, and intracellular tail. While some members of the TNF receptor superfamily can signal for cell death, the majority do not have a death domain, and these receptors activate a wide variety of cellular functions such as lymphocyte costimulation, bone turnover, T-B cell interactions, and lymphocyte development and homeostasis.
The knowledge about the structure and function of TNF superfamily of cytokines, coupled with the invention of mAb technology, has revolutionized the treatment approach to many immune-mediated diseases in areas such as rheumatology, gastroenterology, and dermatology. Blocking TNF receptor-ligand interaction has proved very successful in treating diseases such as RA, juvenile idiopathic arthritis, cutaneous psoriasis, psoriatic arthritis, and Crohn's disease. The list of indications for using this approach is rapidly expanding.
TNF-α
TNF-α is produced by stimulated myeloid cells. There are two TNF receptors, p55 (TNFR1) and p75 (TNFR2), that are widely expressed on most resting cells.
The role of TNF-α has been extensively studied in RA, which is a prototypic systemic deforming arthritis, affecting approximately 1% of the adult population. Studies on the pathogenic mechanisms of RA have revealed that TNF-α is a critical cytokine viewed as a ‘conductor’ in the inflammatory cascade (Choy and Panayi, 2001) that results in irreversible joint damage, bone and cartilage destruction (Bertolini et al, 1986; Saklatvala, 1986), significant morbidity and mortality (Pincus et al, 1984; Pincus and Callahan, 1986).
Tumor necrosis factor-α transgenic mice develop RA-like disease (Keffer et al, 1991). Treatment with TNFR2-Fc (Keffer et al, 1991) or a mAb against TNF (Williams et al, 1992) can ameliorate this phenotype.
Following these discoveries, a series of pivotal trials in patients with RA showed the therapeutic benefit of TNF blockade (Elliott et al, 1993, 1994, 1995). Subsequently, three biologic agents, engineered to block TNF action, have been approved by the Food and Drug Administration (FDA) for clinical use in the United States (Table 1). They have been shown to be effective in a growing number of inflammatory conditions, including RA, psoriatic arthritis, ankylosing spondylitis, and Crohn's disease.
Table 1.
Biologic agents used in rheumatology
| Target molecule | Generic name | Brand name | Mode of action | US FDA-labeled indications | Administration | |
|---|---|---|---|---|---|---|
| Cell-surface molecular target | TNF-α | Infliximab | Remicade™ | Inhibits TNF binding to its receptor | RA, PsA, AS, Crohn's disease, Plaque psoriasis | i.v. |
| TNF-α | Adalimumab | Humira™ | Inhibits TNF binding to its receptor | RA, PsA, AS, Crohn's disease | s.c. | |
| IL-1R | Anakinra | Kineret™ | Inhibits IL-1 binding to its receptor | RA | s.c. | |
| IL-6R | Tocilizumab | MRA™, Actemra™ | Inhibits IL-6 binding to its receptor | Under clinical investigation | i.v. | |
| CD20 | Rituximab, ocrelizumab | Rituxan™, MabThera™ | Depletes B cell | RA, NHL | i.v. | |
| CD22 | Epratuzumab | Non-depleting B cell immune modulation | Under clinical investigation | i.v. | ||
| CD25 (IL-2 receptor) | Daclizumab, basiliximab | Zenapax™, Simulect™ | Inhibits IL-2 binding to its receptor | Renal transplant rejection | i.v. | |
| CD80, CD86 | Abatacept | Orencia™ | Inhibits co-stimulation of B cells | RA | i.v. | |
| Soluble target | TNF-α, TNF-β | Etanercept | Enbrel™ | Inhibits TNF binding to its receptor | RA, PsA, AS, JIA | s.c. |
| TNF-α | Infliximab | Remicade™ | Inhibits TNF binding to its receptor | RA, PsA, AS, Crohn's disease, plaque psoriasis | i.v. | |
| TNF-α | Adalimumab | Humira™ | Inhibits TNF binding to its receptor | RA, PsA, AS, Crohn's disease, Under clinical investigation | s.c. | |
| BLyS | Belimumab | LymphoStat-B™ | Inhibits B cell development | i.v. |
TNF, tumor necrosis factor; AS, ankylosing spondyloarthropathy, RA, rheumatoid arthritis; PsA, psoriatic arthritis; BLyS, B lymphocyte stimulator; IL, interleukin; NHL, non-Hodgkin's lymphoma; under clinical investigation, no US FDA-labeled indications at present.
Etanercept (Enbrel™, Immunex, Seattle, Washington, USA), a TNF receptor p75 Fc fusion protein (TNFR:Fc), is a soluble recombinant form of the extracellular domain of human TNFR2 receptor fused to the Fc fragment of human immunoglobulin G1 (IgG1). It binds to soluble TNF and LT-α, effectively neutralizing the biologic activity of TNF.
Etanercept, alone or in combination with methotrexate, has been shown to be effective in patients with early and long-standing RA in randomized, double-blind, placebo-controlled clinical trials (Moreland et al, 1997; Weinblatt et al, 1999; Bathon et al, 2000) with sustained response over 2 years (Genovese et al, 2002). It is also approved for treatment of polyarticular juvenile RA (Lovell et al, 2000), plaque psoriasis, active psoriatic arthritis in adults, and for treatment of adult patients with chronic moderate to severe plaque psoriasis (Mease et al, 2000) as well as active ankylosing spondylitis (Gorman et al, 2002). The usual dose of etanercept is 25 mg for adults and 0.4 mg kg–1 for children twice a week subcutaneous injections for treatment of RA, psoriatic arthritis, and ankylosing spondylitis. Comparable clinical benefit in adults with RA has been demonstrated with a once-weekly injection of 50 mg (Keystone et al, 2004a,b).
Infliximab (Remicade™, Centocor, Inc., Malvern, Pennsylvania, USA), is a chimeric mAb composed of the antigen-binding region of a mouse anti-TNF monoclonal antibody and the constant region of human IgG1 (Winterfield and Menter, 2004). It binds to both soluble and transmembrane TNF-α with high affinity (Targan et al, 1997). Infliximab is approved for use in patients with RA who have had an inadequate response to methotrexate alone (Lipsky et al, 2000). The combination of infliximab and methotrexate was superior in reducing signs and symptoms and slowing the progression of joint damage in patients with active (Lipsky et al, 2000) and early (St Clair et al, 2004) RA compared with methotrexate alone, with efficacy sustained for up to 2 years (Maini et al, 2004).
Other indications of infliximab use include ankylosing spondylitis, moderate-to-severe active Crohn's disease with draining enterocutaneous fistulas (van Dullemen et al, 1995; Van Deventer, 1997; Present et al, 1999), and treatment-refractory ulcerative colitis.
Another monoclonal antibody against TNF-α is adalimumab (Humira™, Abbott Laboratories, North Chicago, Illinois, USA). It is a recombinant, fully humanized IgG1 anti-TNF-α monoclonal antibody with low immunogenicity. It has been FDA approved for moderate-to-severe RA (Furst et al, 2003; Weinblatt et al, 2003; Weisman et al, 2003; Keystone et al, 2004a,b), psoriatic arthritis, moderate-to-severe Crohn's disease, and ankylosing spondilitis. The recommended dose is 40 mg subcutaneously every 2 weeks alone or concomitantly with methotrexate.
The safety profile of anti-TNF biologics has become an area of active post-marketing evaluation because of concerns of potential adverse events some of which were not documented during the clinical trials phase, such as infections, malignancies and lymphoma, demyelinating disorders, aplastic anemia, congestive heart failure, and vasculitis (Mohan et al, 2004; Richette et al, 2004). These concerns were addressed by the FDA Arthritis Advisory Committee http://www.fda.gov/ohrms/dockets/ac/03/transcripts/3930T1.htm. Injection site reaction has been the most commonly reported adverse reaction with the subcutaneously administered drugs (20–40%) (Moreland et al, 1999). Hypersensitivity reaction associated with infliximab is more rare, although more serious (Maini et al, 1999).
Patients treated with anti-TNF medications are at increased risk of conventional bacterial and opportunistic infections (Warris et al, 2001; Helbling et al, 2002; Kamath et al, 2002; Lee et al, 2002; Nakelchik and Mangino, 2002). Oral and esophageal candidiasis in patients treated with anti-TNF agents can be rarely seen in dental practice (Ricart et al, 2001). The risk of serious and life-threatening infections is also increased (Bongartz et al, 2006). Reactivation of latent tuberculosis is a particular complication that usually develops within the first few months of therapy, presenting as atypical extrapulmonary and miliary disease. For that reason screening for latent tuberculous infection is strongly recommended prior to initiation of any TNF-blocking agents.
Immunogenicity has been observed during anti-TNF therapy with the development of ANA and anti-double-stranded DNA (anti-dsDNA) antibodies of IgM isotype, but their clinical significance is still a matter of debate. Adding methotrexate to the regimen reduces the incidence of these and other antibodies.
Additional areas of concern are potentially demyelinating syndromes (Mohan et al, 2001) and increased morbidity and mortality in patients with congestive heart failure while on anti-TNF medications.
Malignancy and lymphoma have been documented in RA patients treated with TNF blockers in clinical trials (Bongartz et al, 2006). However, patients with RA have an increased risk of lymphoproliferative diseases and non-Hodgkin's lymphoma in particular (standardized incidence ratio: 2.4–8.9) (Isomaki et al, 1978; Matteson et al, 1991; Gridley et al, 1993; Baecklund et al, 1998), based on epidemiological studies from pre-biologics era. All these facts complicate the interpretation of the post-marketing data trying to link the use of anti-TNF agents and lymphoma, and the debate is still open.
Anti-TNF biologics in oral medicine: Sjögren's syndrome
Sjögren's syndrome is a common inflammatory condition (prevalence 0.5–4%) (Thomas et al, 1998) associated with dry mouth and dry eyes resulting from chronic exocrine hypofunction. As a consequence, patients develop a multitude of oral and dental complications such as oral candidiasis and root and incisal caries with tooth erosion in some cases (Atkinson and Fox, 1993), which are inversely proportional to the salivary flow. Studies in mouse models of Sjögren's syndrome suggest that TNF plays an important role in the pathogenesis of the disease, and TNF blockade may prevent the associated salivary inflammation (Tornwall et al, 1999). This notion was supported by studies in rabbit models of the disease (Zhu et al, 2003) and in vitro studies with immortalized human salivary acinar cells (Azuma et al, 2002). Following these discoveries, a series of quality randomized clinical trials in humans using different anti-TNF agents were conducted. They showed improvement in rheumatologic signs and symptoms, such as arthritis, but failed to demonstrate benefit to the sicca symptoms (Mariette et al, 2004; Sankar et al, 2004). In addition, patients with primary Sjögren's syndrome are at 16- to 44-fold increased risk of developing non-Hodgkin's lymphoma estimated from epidemiologic studies (Kassan et al, 1978; Lazarus et al, 2006; Theander et al, 2006). This consideration coupled with the lack of efficacy on the sicca manifestations may have significant implications in this patient population where the indications for administering anti-TNF biologics should be carefully weighed.
Interleukin-1 (IL-1)
Interleukin-1 is another cytokine important in the propagation of inflammation via induction of IL-6 and cyclooxygenase-2 (Dinarello, 1996). It plays a critical role in the pathogenesis of RA (Horai et al, 2000; Choy and Panayi, 2001) and other rheumatic diseases. Its natural inhibitor, IL-1 receptor antagonist (IL-1Ra), blocks the biologic activity of IL-1 by competitively inhibiting IL-1 binding to its receptor (IL-1RI), which is expressed in a wide variety of tissues and organs. This mechanism was used to develop a recombinant IL-1Ra biologic agent (anakinra; Kineret™, Amgen, Inc., Thousand Oaks, California, USA). In randomized clinical trials, daily injections of anakinra have been shown to reduce the signs and symptoms of RA and radiographic progression of disease (Cohen et al, 2002). This led to FDA approval of anakinra for treatment of RA but its specific place in the RA therapeutics armamentarium remains to be defined (Furst et al, 2007). The combination of anakinra with methotrexate and other non–anti-TNF disease-modifying anti-rheumatic drugs (DMARDs) is well tolerated (Cohen et al, 2002), but the combination with etanercept was associated with a significant increase in adverse events and serious infections, and is not recommended (Fleish-mann, 2002; Genovese et al, 2004). The most frequent side effect is injection site reaction followed by infections. There is no indication that anakinra is associated with increased incidence of tuberculosis.
Anankinra has been found to be very effective in treating neonatal onset multisystem inflammatory disease and other rheumatic diseases where the innate immune system is activated (Goldbach-Mansky et al, 2006; Kalliolias et al, 2007).
One study found IL-1R antagonist polymorphism in patients with Sjögren's syndrome, suggesting a potential application of anakinra in this patient population (Perrier et al, 1998), but experience with this drug in Sjögren's syndrome is lacking.
Interleukin-6 (IL-6)
Interleukin-6 is a pleiotropic cytokine with a wide range of biologic activities (Choy and Panayi, 2001; Naka et al, 2002). IL-6 plays an important role in the regulation of immunologic reactions, inflammation, acute phase responses, and hematopoiesis. One of the most important biologic actions of IL-6 is its ability to stimulate the final stages of B-lymphocyte maturation. Under the influence of IL-6, B-lymphocytes differentiate into mature plasma cells and become immunoglobulin-secreting cells. In addition, IL-6 induces T-cell growth and cytotoxic T-cell differentiation through augmentation of IL-2 receptor expression and IL-2 production.
Overproduction of IL-6 is pathologically involved in some autoimmune diseases, such as RA (Hirano et al, 1988; Madhok et al, 1993), systemic-onset juvenile idiopathic arthritis (De Benedetti et al, 1994), Sjögren's syndrome (Boras et al, 2004), and systemic lupus erythematosus (SLE) (Linker-Israeli et al, 1991; Peterson et al, 1996; Grondal et al, 2000; Tackey et al, 2004). Elevated serum levels of IL-6 are responsible for systemic inflammatory manifestations, such as general fatigue, fever, anemia, increases in acute phase proteins including serum amyloid A, C-reactive protein (CRP) and fibrinogen, and immunologic disorders including polyclonal hyper-γ-globulinemia and production of autoantibodies, all hallmarks of systemic rheumatic diseases.
Anti-IL-6 receptor monoclonal antibody, tocilizumab
Tocilizumab is a humanized anti-IL-6 receptor monoclonal antibody which is in advanced clinical trials for adult and juvenile RA. Several large phase II studies have demonstrated the efficacy of tocilizumab in RA both as monotherapy and in combination with methotrexate (Nishimoto et al, 2004, 2007; Nishimoto, 2006). Responses were highest in subjects treated with methotrexate and 8 mg kg–1 tocilizumab every 4 weeks with about 75% of patients achieving an ACR20 response (Maini et al, 2006). Another study also reported significant radiographic benefit (Nishimoto et al, 2007) Tocilizumab is generally well tolerated. Serum cholesterol levels increased in 44.0% of the patients. Liver function disorders and decreases in white blood cell counts were also observed, but these were mild and transient. This therapy may be a promising treatment for RA. In terms of systemic juvenile idiopathic arthritis patients with tocilizumab, the clinical symptoms were improved and the elevated levels of acute-phase reactants were normalized (Woo et al, 2005; Yokota et al, 2005).
CTLA4 and the concept of co-stimulation (‘two signal’ model)
Bretscher and Cohn (1970) first proposed the ‘two signal’ model for lymphocyte activation. The hypothesis, originally put forth for B lymphocyte activation and later also adopted for T lymphocyte activation, stated that occupation of the antigen-specific receptor on the naïve lymphocyte surface was not sufficient to activate that cell. Instead, naïve B or T lymphocyte activation requires one antigen-specific signal through its antigen receptor (B or T cell receptor, respectively), and another non-antigen-specific ‘costimulatory’ signal from the antigen-presenting cell (APC) through receptors, such as CD40 or CD28. In the absence of costimulation through the second signaling pathway, T cells not only do not become active, but may even die or become unresponsive, i.e. anergic. Interfering with the second signal and signaling pathway has a natural regulatory role and has been shown to have a potent effect on inducing immune tolerance in experimental settings. During a normal immune response activated T cells express a molecule named cytotoxic T cell-associated antigen 4 (CTLA4), which binds to CD86 with a much higher affinity than CD28. This inhibitory effect prevents the ability of CD86:CD28 interaction to perpetuate further inflammation and tissue damage (Bluestone et al, 2006).
CTLA4 Ig, abatacept
The knowledge of the ‘two signal’ model has led to the generation of the first CD28 costimulation antagonist, called CTLA4 Ig (Bluestone et al, 2006) (abatacept; Orencia™, Bristol-Myers Squibb Co., Princeton, New Jersey, USA), a soluble fusion protein, consisting of the extracellular domain of human CTLA4 and a fragment of the Fc domain of human IgG1. It has been used to treat successfully several animal models of autoimmunity, including murine lupus (Davidson et al, 2005), diabetes in non-obese diabetic mice (Lenschow et al, 1995), collagen-induced arthritis (Brookes et al, 1996), and experimental autoimmune encephalomyelitis (Khoury et al, 1995). It was also vigorously studied in randomized clinical trials in patients with RA and demonstrated significant clinical and functional benefit in patients who had inadequate response to anti-TNF therapy (Genovese et al, 2005) or methotrexate (Kremer et al, 2003, 2006). Following these pivotal trials, abatacept was FDA approved in 2006 for the treatment of moderate-to-severe RA refractory to other DMARDs. Abatacept was recently reported to have sustained effect on inhibiting radiographic progression in a 2-year extension follow-up study (Genant et al, 2007). A combination of abatacept with etanercept in patients with active RA has however been associated with increased incidence of serious adverse events, including infections and should be avoided (Weinblatt et al, 2007).
To date, this medication has not been studied in Sjögren's syndrome and its effects on oral health are largely unknown, even though a recent study reported on the significance of CTLA4 haplotype/phenotype association (Downie-Doyle et al, 2006) in Sjögren's syndrome, suggesting that it may be an attractive therapeutic target.
A similar CD28 costimulation antagonist, belatacept, is being investigated as immunosuppressive medication in renal transplantation (Vincenti and Luggen, 2007).
B-cell-targeted therapies
B lymphocytes play an important role in the pathogenesis of autoimmune diseases, including SLE, RA, and Sjögren's syndrome. B lymphocytes are not only the precursors of immunoglobulin-secreting plasma cells, but are effective APC (especially of autoantigens) and modulate the function of other immune cells through the production of cytokines. B cell hyperactivity is a dominant feature of many autoimmune diseases and, unlike in normal individuals, B cells from SLE and Sjögren's syndrome patients spontaneously produce large amounts of immunoglobulins. Moreover, in Sjögren's syndrome, 5–8% of the patients may develop malignant B-cell lymphoma, usually of the mucosa-associated lymphoid tissue type which is most frequently located in the major salivary glands. B cell depletion by targeting B cell-specific antigens, such as CD20 and CD22, has recently emerged as an attractive way of controlling autoimmunity.
Anti-CD20, rituximab
Rituximab (Rituxan™, Genentech, South San Francisco, California, USA) is a chimeric mouse-human monoclonal antibody against the B cell-specific antigen CD20, which selectively and profoundly depletes CD20-positive B lymphocytes via complement-mediated and antibody-dependent cell-mediated cytotoxicity, induction of apoptosis, and inhibition of cell growth (Reff et al, 1994). It has been successfully used and FDA approved for treatment of patients with non-Hodgkin's B cell lymphoma since 1997 with minimal toxicity. Since then it has been reported to be effective in cases and case series of patients with concomitant RA or RA alone (Leandro et al, 2002). These reports have led to the development of several randomized, clinical trials of rituximab for treatment-refractory RA (Edwards et al, 2004; Cohen et al, 2006; Emery et al, 2006) that demonstrated its efficacy and safety either alone or in combination with methotrexate or cyclophosphamide. This resulted in FDA approval of rituximab for moderate-to-severe RA in subjects who had inadequate response to anti-TNF therapy.
Depleting B cells using rituximab in patients with active SLE has also been shown to be beneficial (Leandro et al, 2005; Smith et al, 2006; Thatayatikom and White, 2006). Outcomes of phase I/II studies also demonstrated improvement in B cell homeostasis and self-tolerance after B cell depletion with rituximab (Anolik et al, 2004).
The adverse events with rituximab are mild to moderate transfusion reactions and hypogammaglobulinemia. However, the levels did not fall below the normal range (Looney et al, 2004). Rituximab-specific human anti-chimeric antibodies (HACA) develop in up to 27% of patients and in some cases are associated with serum sickness-like disorder (Pijpe et al, 2005). Infections, including opportunistic infections such as JC virus-associated progressive multifocal leukoencephalopathy, have been reported (http://www.fda.gov/cder/drug/InfoSheets/HCP/rituximab.pdf).
Aa B cells have been shown to play an important role in Sjögren's syndrome pathogenesis, they have become attractive therapeutic targets in this disease. To date, three relatively small open-label studies have demonstrated the utility of rituximab in this patient population (Gottenberg et al, 2005a,b; Pijpe et al, 2005; Seror et al, 2007). As a whole they reported improvement in extraglandular manifestations, glandular swelling, and subjective symptoms of dryness. The data on objective salivary and lacrimal function however have been inconsistent. These results justified the development of two European randomized controlled trials designed to evaluate the safety and efficacy of rituximab in patients with primary Sjögren's syndrome that are currently recruiting patients (http://www.clinicaltrials.gov, NCT00363350 and NCT00426543).
Anti-CD22, epratuzumab
CD22 is a B-lymphocyte-restricted member of the immunoglobulin superfamily, and a member of the sialoadhesin family of adhesion molecules that regulate B cell activation and interaction with T cells (Kelm et al, 1994; Tedder et al, 1997). CD22 plays a key role in B cell development and survival because CD22-deficient mice have reduced numbers of mature B cells in the bone marrow and circulation, and the B cells also have a shorter lifespan and enhanced apoptosis (Otipoby et al, 1996). As dysregulated expression of CD22 could lead to excessive activation of B cells and autoantibody production (O'Keefe et al, 1999), targeting this co-receptor in systemic autoimmunity appears to be a potentially new therapeutic pathway. In 16 patients with active pSS, the open-label, phase I/II study of epratuzumab, a humanized anti-CD22 monoclonal antibody, was reported (Steinfeld et al, 2006). A composite end-point involving the Schirmer-I test, unstimulated salivary flow, fatigue, erythrocyte sedimentation rate and immunoglobulin-G (IgG) was devised to provide a clinically meaningful assessment of response. Approximately 40–50% responded at the >30% level, while 10–45% responded at the >50% level for 10–32 weeks. Additionally, statistically significant improvements were observed in fatigue. Fourteen patients received all infusions without significant reactions, one patient received three, and another was discontinued after receiving a partial infusion due to a mild acute reaction. Three patients showed moderately elevated levels of HAHA (human anti-human/epratuzumab antibodies) not associated with clinical manifestations. Primary SS patients have CD22 over-expression on peripheral B cells which was down-regulated by epratuzumab therapy. B-cell levels had moderate reductions, but T-cell levels, immunoglobulins, and routine safety laboratory tests did not change significantly. Thus, epratuzumab appears to be a promising therapy in active pSS. In a phase II study in mild to moderate SLE 14 patients, Dorner et al (2006) demonstrated that the therapy was well tolerated, with consistent improvement observed in almost all patients in the presence of modestly decreased peripheral B cell levels without human anti-epratuzumab antibody titers and without significant adverse events. A multicenter study is being conducted to further assess the long-term safety and efficacy of epratuzumab in patients with SLE (http://www.clinicaltrials.gov, NCT00383513).
BLyS (B-Lymphocyte stimulator)
B-lymphocyte stimulator or BLyS (also known as BAFF, TALL-1, THANK, TNFSF20, and zTNF4) is a 285-amino acid member of the TNF superfamily of cytokines. It is expressed on macrophages and other APC, and is a vital B cell survival factor, inducing B cell differentiation, proliferation, and Ig secretion.
A number of studies have found elevated levels of serum BLyS in patients with SLE and these levels correlate with disease activity and levels of anti-dsDNA auto-antibodies and Ig (Zhang et al, 2001). In patients with primary Sjogren's syndrome, BLyS is upregulated in the serum and salivary tissue (Lavie et al, 2004; Gottenberg et al, 2005a,b), correlates with serum autoantibody levels of IgG, RF, anti-SSA and anti-SSB (Mariette et al, 2003), and has been implicated in the pathogenesis of the disease (Groom et al, 2002).
Anti-BLyS monoclonal antibody, belimumab
Targeting the BLyS molecule with an anti-BLyS monoclonal antibody has proved safe and well tolerated in phase I clinical trials in lupus (Dorner et al, 2006) and neutralization of serum BLyS correlated with clinical improvement of the disease. A phase III international randomized clinical trial is currently recruiting patients with active SLE to evaluate the safety and efficacy of belimumab (http://www.clinicaltrials.gov, NCT00410384).
Based on the preclinical data and the experience with anti-BLyS in other diseases, one can reason that this medication should be studied systematically as a treatment option in Sjögren's syndrome.
Other biologic targets
Cytokines mediate a wide variety of biologic activities that are relevant to autoimmune diseases including immune response, inflammation, and tissue repair and remodeling. Restoring the optimal cytokine balance may have therapeutic value and theoretically can be achieved either by blocking inflammatory cytokines or inducing or providing anti-inflammatory ones.
α-Interferon
α-Interferon (IFN-α) has been suggested to play an important role in the pathogenesis of several rheumatic diseases including SLE and Sjögren's syndrome (Bave et al, 2005; Borg and Isenberg, 2007). This suggests that IFN-α may be used as a therapeutic target and interfering with its activity may result in amelioration of chronic inflammation.
In several phase II studies low-dose natural human IFN-α administered through the oral mucosal route improved salivary output and decreased complaints of xerostomia in patients with primary Sjögren's syndrome (Shiozawa et al, 1993, 1998; Ferraccioli et al, 1996; Ship et al, 1999; Khurshudian, 2003). However, in a phase III study, although IFN-α increased unstimulated whole saliva flow significantly more than placebo, the co-primary end-points of stimulated whole saliva flow and oral dryness were not significantly improved in the IFN-α group relative to placebo (Cummins et al, 2003).
Conclusions and future directions
Past decades have led to much improved understanding of many diseases and the development of specific treatments. As promising as these new therapeutic approaches are, there are also considerations in weighing the benefit-to-risk ratio. As most of these medications have been on the market for less than a decade, the long-term toxicity profile is not known. Cost is also a limiting factor as some of these treatments run to thousands of USD per year and most of them are being used as chronic therapies.
Despite recent advances, there is an urgent need for cost-effective innovative therapies with improved toxicity profile and curative potential. A trend toward more rational drug design has been documented in a recent study (Yildirim et al, 2007). Scientists have been studying the applicability of fragments of antibodies (Check, 2007). Oral, rather than injectable, modes of delivering these molecules are being actively explored. New approaches using stem cell transplantation, both autologous and allogeneic, are currently under intensive investigation. Some immunomodulatory properties of mesenchymal stem cells make them attractive candidates for cell-based therapies and open new avenues for future research (Tyndall et al, 2007).
Acknowledgements
We would like to thank Ilias Alevizos, DMD, and Jaime Brahim, DDS, for their critical review and suggestions on the manuscript.
References
- Anolik JH, Barnard J, Cappione A, et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;50:3580–3590. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- Atkinson JC, Fox PC. Sjogren's syndrome: oral and dental considerations. J Am Dent Assoc. 1993;124:74–76. 78–82, 84–86. doi: 10.14219/jada.archive.1993.0064. [DOI] [PubMed] [Google Scholar]
- Azuma M, Aota K, Tamatani T, et al. Suppression of tumor necrosis factor alpha-induced matrix metalloproteinase 9 production in human salivary gland acinar cells by cepharanthine occurs via down-regulation of nuclear factor kappaB: a possible therapeutic agent for preventing the destruction of the acinar structure in the salivary glands of Sjogren's syndrome patients. Arthritis Rheum. 2002;46:1585–1594. doi: 10.1002/art.10315. [DOI] [PubMed] [Google Scholar]
- Baecklund E, Ekbom A, Sparen P, et al. Disease activity and risk of lymphoma in patients with rheumatoid arthritis: nested case–control study. BMJ. 1998;317:180–181. doi: 10.1136/bmj.317.7152.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner DW, D'Arcy A, Janes W, et al. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- Bave U, Nordmark G, Lovgren T, et al. Activation of the type I interferon system in primary Sjogren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- Bertolini DR, Nedwin GE, Bringman TS, et al. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, St Clair EW, Turka LA, et al. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Boras VV, Cikes N, Lukac J, et al. The significance of salivary and serum interleukin 6 and basic fibroblast growth factor levels in patients with Sjogren's syndrome. Coll Antropol. 2004;28(Suppl 2):305–309. [PubMed] [Google Scholar]
- Borg FA, Isenberg DA. Syndromes and complications of interferon therapy. Curr Opin Rheumatol. 2007;19:61–66. doi: 10.1097/BOR.0b013e328010c547. [DOI] [PubMed] [Google Scholar]
- Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Brookes SM, Cohen SB, Price EJ, et al. T cell clones from a Sjogren's syndrome salivary gland biopsy produce high levels of IL-10. Clin Exp Immunol. 1996;103:268–272. doi: 10.1046/j.1365-2249.1996.d01-623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Check E. Immunology: pimp my antibody. Nature. 2007;446:964–966. doi: 10.1038/446964a. [DOI] [PubMed] [Google Scholar]
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hurd E, Cush J, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:614–624. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- Cummins MJ, Papas A, Kammer GM, et al. Treatment of primary Sjogren's syndrome with low-dose human interferon alfa administered by the oromucosal route: combined phase III results. Arthritis Rheum. 2003;49:585–593. doi: 10.1002/art.11199. [DOI] [PubMed] [Google Scholar]
- Davidson A, Diamond B, Wofsy D, et al. Block and tackle: CTLA4Ig takes on lupus. Lupus. 2005;14:197–203. doi: 10.1191/0961203305lu2136oa. [DOI] [PubMed] [Google Scholar]
- De Benedetti F, Massa M, Pignatti P, et al. Serum soluble interleukin 6 (IL-6) receptor and IL-6/soluble IL-6 receptor complex in systemic juvenile rheumatoid arthritis. J Clin Invest. 1994;93:2114–2119. doi: 10.1172/JCI117206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dorner T, Kaufmann J, Wegener WA, et al. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther. 2006;8:R74. doi: 10.1186/ar1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie-Doyle S, Bayat N, Rischmueller M, et al. Influence of CTLA4 haplotypes on susceptibility and some extraglandular manifestations in primary Sjogren's syndrome. Arthritis Rheum. 2006;54:2434–2440. doi: 10.1002/art.22004. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, Feldmann M, Maini RN, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, et al. TNF alpha blockade in rheumatoid arthritis: rationale, clinical outcomes and mechanisms of action. Int J Immunopharmacol. 1995;17:141–145. doi: 10.1016/0192-0561(94)00092-3. [DOI] [PubMed] [Google Scholar]
- Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- Ferraccioli GF, Salaffo F, De Vita S, et al. Interferon alpha-2 (IFN alpha 2) increases lacrimal and salivary function in Sjogren's syndrome patients. Preliminary results of an open pilot trial versus OH-chloroquine. Clin Exp Rheumatol. 1996;14:367–371. [PubMed] [Google Scholar]
- Fleishmann RM. Safety of anakinra, a recombinant interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis and comparison to anti-TNF-alpha agents. Clin Exp Rheumatol. 2002;20(5 Suppl 27):S35–S41. [PubMed] [Google Scholar]
- Furst DE, Schiff MH, Fleischmann RM, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol. 2003;30:2563–2571. [PubMed] [Google Scholar]
- Furst DE, Breedveld FC, Kalden JR, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2007. Ann Rheum Dis. 2007;66(Suppl 3):iii2–iii22. doi: 10.1136/ard.2007.081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genant HK, Peterfy CG, Westhovens R, et al. Abatacept inhibits structural damage progression in rheumatoid arthritis: results from the long-term extension of the AIM trial. Ann Rheum Dis. 2007 doi: 10.1136/ard.2007.085084. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese MC, Bathon JM, Martin RW, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–1450. doi: 10.1002/art.10308. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412–1419. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JD, Sack KE, Davis JC, Jr, et al. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349–1356. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- Gottenberg JE, Busson M, Cohen-Solal J, et al. Correlation of serum B lymphocyte stimulator and beta2 microglobulin with autoantibody secretion and systemic involvement in primary Sjogren's syndrome. Ann Rheum Dis. 2005a;64:1050–1055. doi: 10.1136/ard.2004.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottenberg JE, Guillevin L, Lambotte O, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis. 2005b;64:913–920. doi: 10.1136/ard.2004.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley G, McLaughlin JK, Ekbom A, et al. Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst. 1993;85:307–311. doi: 10.1093/jnci/85.4.307. [DOI] [PubMed] [Google Scholar]
- Grondal G, Gunnarsson I, Ronnelid J, et al. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18:565–570. [PubMed] [Google Scholar]
- Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling D, Breitbach TH, Krause M, et al. Disseminated cytomegalovirus infection in Crohn's disease following anti-tumour necrosis factor therapy. Eur J Gastroenterol Hepatol. 2002;14:1393–1395. doi: 10.1097/00042737-200212000-00018. [DOI] [PubMed] [Google Scholar]
- Hirano T, Matsuda T, Turner M, et al. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988;18:1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Horai R, Saijo S, Tanioka H, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomaki HA, Hakulinen T, Joutsenlahti U, et al. Excess risk of lymphomas, leukemia and myeloma in patients with rheumatoid arthritis. J Chronic Dis. 1978;31:691–696. doi: 10.1016/0021-9681(78)90071-1. [DOI] [PubMed] [Google Scholar]
- Kalliolias GD, Georgiou PE, Antonopoulos IA, et al. Anakinra treatment in patients with adult-onset Still's disease is fast, effective, safe and steroid sparing: experience from an uncontrolled trial. Ann Rheum Dis. 2007;66:842–843. doi: 10.1136/ard.2006.066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath BM, Mamula P, Baldassano RN, et al. Listeria meningitis after treatment with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:410–412. doi: 10.1097/00005176-200204000-00018. [DOI] [PubMed] [Google Scholar]
- Kassan SS, Thomas TL, Moutsopoulos HM, et al. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978;89:888–892. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm S, Pelz A, Schauer R, et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr Biol. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004a;50:1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- Keystone EC, Schiff MH, Kremer JM, et al. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2004b;50:353–363. doi: 10.1002/art.20019. [DOI] [PubMed] [Google Scholar]
- Khoury SJ, Akalin E, Chandraker A, et al. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155:4521–4524. [PubMed] [Google Scholar]
- Khurshudian AV. A pilot study to test the efficacy of oral administration of interferon-alpha lozenges to patients with Sjogren's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:38–44. doi: 10.1067/moe.2003.30. [DOI] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Westhovens R, Moreland LW, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Genant HK, Leon M, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- Lavie F, Miceli-Richard C, Quillard J, et al. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjogren's syndrome. J Pathol. 2004;202:496–502. doi: 10.1002/path.1533. [DOI] [PubMed] [Google Scholar]
- Lazarus MN, Robinson D, Mak V, et al. Incidence of cancer in a cohort of patients with primary Sjogren's syndrome. Rheumatology (Oxford) 2006;45:1012–1015. doi: 10.1093/rheumatology/kei281. [DOI] [PubMed] [Google Scholar]
- Leandro MJ, Edwards JC, Edwards JC, et al. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann Rheum Dis. 2002;61:883–888. doi: 10.1136/ard.61.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandro MJ, Cambridge G, Cambridge G, et al. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology (Oxford) 2005;44:1542–1545. doi: 10.1093/rheumatology/kei080. [DOI] [PubMed] [Google Scholar]
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565–2570. doi: 10.1002/art.10583. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Ho SC, Sattar H, et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker-Israeli M, Deans RJ, Wallace DJ, et al. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- Looney RJ, Anolik JH, Campbell D, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342:763–769. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- Madhok R, Crilly A, Watson J, et al. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–234. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–1065. doi: 10.1002/art.20159. [DOI] [PubMed] [Google Scholar]
- Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–2829. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- Mariette X, Roux S, Zhang J, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren's syndrome. Ann Rheum Dis. 2003;62:168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariette X, Ravaud P, Steinfeld S, et al. Inefficacy of infliximab in primary Sjogren's syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjogren's Syndrome (TRIPSS). Arthritis Rheum. 2004;50:1270–1276. doi: 10.1002/art.20146. [DOI] [PubMed] [Google Scholar]
- Matteson EL, Hickey AR, Maguire L, et al. Occurrence of neoplasia in patients with rheumatoid arthritis enrolled in a DMARD Registry. Rheumatoid Arthritis Azathioprine Registry Steering Committee. J Rheumatol. 1991;18:809–814. [PubMed] [Google Scholar]
- Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–390. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum. 2001;44:2862–2869. doi: 10.1002/1529-0131(200112)44:12<2862::aid-art474>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Mohan AK, Cote TR, Block JA, et al. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin Infect Dis. 2004;39:295–299. doi: 10.1086/421494. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- Naka T, Nishimoto N, Kishimoto T, et al. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4(Suppl 3):S233–S242. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakelchik M, Mangino JE. Reactivation of histoplasmosis after treatment with infliximab. Am J Med. 2002;112:78. doi: 10.1016/s0002-9343(01)00945-7. [DOI] [PubMed] [Google Scholar]
- Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18:277–281. doi: 10.1097/01.bor.0000218949.19860.d1. [DOI] [PubMed] [Google Scholar]
- Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–1769. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–1167. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe TL, Williams GT, Batista FD, et al. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otipoby KL, Andersson KB, Draves KE, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- Perrier S, Coussediere C, Dubost JJ, et al. IL-1 receptor antagonist (IL-1RA) gene polymorphism in Sjogren's syndrome and rheumatoid arthritis. Clin Immunol Immunopathol. 1998;87:309–313. doi: 10.1006/clin.1998.4520. [DOI] [PubMed] [Google Scholar]
- Peterson E, Robertson AD, Emlen W, et al. Serum and urinary interleukin-6 in systemic lupus erythematosus. Lupus. 1996;5:571–575. doi: 10.1177/096120339600500603. [DOI] [PubMed] [Google Scholar]
- Pijpe J, van Imhoff GW, Spijkervet FK, et al. Rituximab treatment in patients with primary Sjogren's syndrome: an open-label phase II study. Arthritis Rheum. 2005;52:2740–2750. doi: 10.1002/art.21260. [DOI] [PubMed] [Google Scholar]
- Pincus T, Callahan LF. Taking mortality in rheumatoid arthritis seriously – predictive markers, socioeconomic status and comorbidity. J Rheumatol. 1986;13:841–845. [PubMed] [Google Scholar]
- Pincus T, Callahan LF, Sale WG, et al. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–872. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- Ricart E, Panaccione R, Loftus EV, et al. Infliximab for Crohn's disease in clinical practice at the Mayo Clinic: the first 100 patients. Am J Gastroenterol. 2001;96:722–729. doi: 10.1111/j.1572-0241.2001.03612.x. [DOI] [PubMed] [Google Scholar]
- Richette P, Dieude P, Damiano J, et al. Sensory neuropathy revealing necrotizing vasculitis during infliximab therapy for rheumatoid arthritis. J Rheumatol. 2004;31:2079–2081. [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar V, Brennan MT, Kok MR, et al. Etanercept in Sjogren's syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum. 2004;50:2240–2245. doi: 10.1002/art.20299. [DOI] [PubMed] [Google Scholar]
- Seror R, Sordet C, Guillevin L, et al. Tolerance and efficacy of rituximab and changes in serum B cell biomarkers in patients with systemic complications of primary Sjogren's syndrome. Ann Rheum Dis. 2007;66:351–357. doi: 10.1136/ard.2006.057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa S, Morimoto I, Tanaka Y, et al. A preliminary study on the interferon-alpha treatment for xerostomia of Sjogren's syndrome. Br J Rheumatol. 1993;32:52–54. doi: 10.1093/rheumatology/32.1.52. [DOI] [PubMed] [Google Scholar]
- Shiozawa S, Tanaka Y, Shiozawa K, et al. Single-blinded controlled trial of low-dose oral IFN-alpha for the treatment of xerostomia in patients with Sjogren's syndrome. J Interferon Cytokine Res. 1998;18:255–262. doi: 10.1089/jir.1998.18.255. [DOI] [PubMed] [Google Scholar]
- Ship JA, Fox PC, Michalek JE, et al. Treatment of primary Sjogren's syndrome with low-dose natural human interferon-alpha administered by the oral mucosal route: a phase II clinical trial. IFN Protocol Study Group. J Interferon Cytokine Res. 1999;19:943–951. doi: 10.1089/107999099313497. [DOI] [PubMed] [Google Scholar]
- Smith KG, Jones RB, Burns SM, et al. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis Rheum. 2006;54:2970–2982. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- Steinfeld SD, Tant L, Burmester GR, et al. Epratuzumab (humanized anti-CD22 antibody) in primary Sjogren's syndrome: an open-label phase I/II study. Arthritis Res Ther. 2006;8:R129. doi: 10.1186/ar2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackey E, Lipsky PE, EIllei GG, et al. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- Tedder TF, Tuscano J, Sato S, et al. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- Thatayatikom A, White AJ. Rituximab: a promising therapy in systemic lupus erythematosus. Autoimmun Rev. 2006;5:18–24. doi: 10.1016/j.autrev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Theander E, Henriksson G, Ljungberg O, et al. Lymphoma and other malignancies in primary Sjogren's syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006;65:796–803. doi: 10.1136/ard.2005.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E, Hay EM, Hajeer A, et al. Sjogren's syndrome: a community-based study of prevalence and impact. Br J Rheumatol. 1998;37:1069–1076. doi: 10.1093/rheumatology/37.10.1069. [DOI] [PubMed] [Google Scholar]
- Tornwall J, Lane TE, Fox RI, et al. T cell attractant chemokine expression initiates lacrimal gland destruction in nonobese diabetic mice. Lab Invest. 1999;79:1719–1726. [PubMed] [Google Scholar]
- Tyndall A, Walker UA, Cope A, et al. Immunomodulatory properties of mesenchymal stem cells: a review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res Ther. 2007;9:301. doi: 10.1186/ar2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deventer SJ. Tumour necrosis factor and Crohn's disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- Vincenti F, Luggen M. T cell costimulation: a rational target in the therapeutic armamentarium for autoimmune diseases and transplantation. Annu Rev Med. 2007;58:347–358. doi: 10.1146/annurev.med.58.080205.154004. [DOI] [PubMed] [Google Scholar]
- Warris A, Bjorneklett A, Gaustad P, et al. Invasive pulmonary aspergillosis associated with infliximab therapy. N Engl J Med. 2001;344:1099–1100. [PubMed] [Google Scholar]
- Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- Weinblatt M, Schiff M, Goldman A, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis. 2007;66:228–234. doi: 10.1136/ard.2006.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman MH, Moreland LW, Furst DE, et al. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther. 2003;25:1700–1721. doi: 10.1016/s0149-2918(03)80164-9. [DOI] [PubMed] [Google Scholar]
- Williams RO, Feldmann M, Maini RN, et al. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterfield LS, Menter A. Infliximab. Dermatol Ther. 2004;17:409–426. doi: 10.1111/j.1396-0296.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- Woo P, Wilkinson N, Prieur AM, et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther. 2005;7:R1281–R1288. doi: 10.1186/ar1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim MA, Goh KI, Cusick ME, et al. Drug-target network. Nat Biotechnol. 2007;25:1119–1126. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- Yokota S, Miyamae T, Imagawa T, et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:818–825. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Stevenson D, Schechter JE, et al. Tumor necrosis factor inhibitor gene expression suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:343–351. doi: 10.1097/00003226-200305000-00012. [DOI] [PubMed] [Google Scholar]