Summary
In addition to vaso-occlusion by sickled erythrocytes, the pathophysiology of sickle cell disease (SCD) is compounded by the diminished bioavailability of nitric oxide (NO), associated with vasoconstriction, endothelial activation and cell adhesion. We tested the ability of sodium nitrite, which can be converted to NO by deoxyhaemoglobin at acid pH and low oxygen tension, to improve blood flow in patients with SCD. In a phase I/II clinical trial, sodium nitroprusside, NG-monomethyl-L-arginine, and sodium nitrite were infused sequentially into the brachial artery in 14 patients at steady state. In a dose-dependent manner, sodium nitrite infusion rates of 0·4, 4 and 40 µmol/min into the brachial artery augmented mean venous plasma nitrite concentrations (P < 0·0001) and stimulated forearm blood flow up to 77 ± 11% above baseline (P < 0·0001), measured by venous occlusion strain gauge plethysmography. This nitrite response was blunted significantly compared to controls without SCD, as previously seen with other NO donors. Sodium nitrite infusions were well tolerated without hypotension, clinically significant methaemoglobinaemia or other untoward events. The unique pharmacological properties of nitrite as a hypoxia-potentiated vasodilator and cytoprotective agent in the setting of ischaemia-reperfusion injury make this anion a plausible NO donor for future clinical trials in SCD.
Keywords: sickle cell, nitrite, blood flow, nitroprusside, vasodilation
Red cell rigidity, microvascular obstruction, haemolysis, inflammation and infarction are well-recognized pathophysiological mechanisms that lead to the debilitating clinical manifestations of sickle cell disease (SCD) (Stuart & Nagel, 2004). However, recently identified additional mechanisms, including cell free haemoglobin-mediated consumption of nitric oxide (NO), oxidant stress, endothelial dysfunction and chronic ischaemia-reperfusion injury, add to our understanding of the pathobiology of SCD (Belcher et al, 2003). Several lines of evidence suggest that patients with SCD with particularly robust haemolysis accumulate haemoglobin into the plasmatic compartment, resulting in impaired NO bioavailability. Cell-free plasma haemoglobin resulting from intravascular haemolysis in patients with SCD rapidly consumes NO, causing a state of functional resistance to NO-dependent vasodilation (Kaul et al, 2000, 2004; Nath et al, 2000; Belhassen et al, 2001; Reiter et al, 2002; Eberhardt et al, 2003; de Montalembert et al, 2007). Consistent with this model, in patients with the highest plasma haemoglobin levels, the blood flow responses to the NO donor sodium nitroprusside (SNP) are reduced. This consumption of NO is compounded by the limited capacity to increase NO synthesis, a result of low plasma levels of L-arginine (the obligate substrate for NO synthases) being depleted by arginase simultaneously released into blood plasma during intravascular haemolysis (Morris et al, 2005). Chronic haemolysis produces a clinical syndrome of vascular dysfunction that ultimately is associated with pulmonary hypertension, cutaneous ulceration and priapism (Gladwin et al, 2004; Nolan et al, 2005a,b; Kato et al, 2006, 2007). As oxidized haemoglobin cannot react with NO, therapeutic modalities based on the oxidation and inactivation of the plasma cell-free haemoglobin might potentially restore regional NO-dependent blood flow.
One potential candidate NO therapeutic agent is sodium nitrite, recently shown by our group to induce vasodilation in healthy human subjects (Cosby et al, 2003). The nitrite anion, used for close to a century at high concentrations as an antidote to cyanide poisoning, may act as a vasodilator at low, physiological concentrations by generating NO in tissues with lower oxygen tension and pH. The mechanism involves a novel physiological function of human haemoglobin as an oxygen-and pH-dependent nitrite reductase (Huang et al, 2005; Isbell et al, 2005; Crawford et al, 2006). As a result, nitrite may serve as a highly useful therapeutic agent to generate NO along the physiological oxygen gradient known to exist in arterial blood, accentuating vasodilation specifically in hypoxic tissue. In addition to vasodilation, nitrite treatment significantly reduces liver, heart and brain ischaemia-reperfusion injury and infarction in animal models, prevents cerebral vasospasm after subarachnoid haemorrhage in primates and decreases pulmonary hypertension in hypoxic newborn sheep (Hunter et al, 2004; Webb et al, 2004; Duranski et al, 2005; Pluta et al, 2005; Hataishi et al, 2006; Jung et al, 2006).
We therefore hypothesized that the therapeutic application of nitrite in patients with SCD and other diseases associated with haemolysis and ischaemia-reperfusion injury could provide selective vasodilation to those same vascular tissue beds at risk. This study was designed as a phase I/II trial to address whether nitrite infusions will safely vasodilate the circulation and improve regional forearm blood flow in patients with SCD during steady state, with limited formation of methaemoglobin. We additionally hypothesized that nitrite infusion would oxidize cell-free plasma haemoglobin, decrease NO scavenging, and increase SNP vasodilatory response in patients with SCD.
Patients, materials and methods
Patients
The National Heart Lung and Blood Institute’s Institutional Review Board approved this protocol. All subjects provided written informed consent. Patients at least 18 years of age with either homozygous SCD or sickle β0-thalassaemia were eligible. Patients with SCD were recruited to participate in the study while they were in steady state, defined as a normal baseline status without acute pain requiring hospitalization.
Patients were excluded if they had Hb SC disease or potentially confounding sources of vascular disease, including: uncontrolled diastolic hypertension (diastolic blood pressures >95 mmHg), hypercholesterolaemia [low density lipoprotein (LDL) > 3·38 mmol/l], diabetes mellitus (fasting blood glucose >6·6 mmol/l), or known arteriosclerotic cardiovascular disease. Patients who had glucose-6-phosphate dehydrogenase deficiency or a known diagnosis of cytochrome B-5 deficiency were also ineligible. Patients continued their chronic medications including hydroxycarbamide and oral opioids. Any patient who received sildenafil, vardenafil, tadalafil, inhaled NO, nitroglycerin, or any other NO-dependent drugs, such as arginine therapy, within 14 d prior to the study date were also excluded. Patients were also asked to refrain from non-steroidal anti-inflammatory drugs for 14 d and from smoking for at least 1 month before the study. Data from healthy control subjects treated with nitrite were obtained from our previously published forearm blood flow study with 10% lower sodium nitrite dosing (Cosby et al, 2003). These control subjects were of white ethnic background. Additional comparison data were obtained from healthy African–American control subjects treated with SNP in a contemporaneous study (C. Bereal, M. Rodrigo, R. F. Machado, C. F. Barnett, L. A. Hunter, A. Chi, V. Sachdev, R. O. Cannon III and G. J. Kato, unpublished observations). Characteristics of the 14 patients in this study with sickle cell disease are shown in Table I. Eleven of the 14 patients were on chronic hydroxycarbamide therapy at the time of study, with mean and median dosing of 19·5 and 16·8 mg/kg/d respectively.
Table I.
Clinical characteristics of patients with sickle cell disease.Total, 14 patients (nine males, five females).
| Mean | Ranges | |
|---|---|---|
| Age | 34·1 | 24–48 |
| Weight | 70·1 | 45·4–85·8 |
| Leucocyte count, ×109/l | 9·11 | 4·7–16 |
| Haemoglobin, g/l | 82·9 | 61–113 |
| Mean corpuscular volume, fl | 105·1 | 83·4–129 |
| Platelet count, ×109/l | 363 | 128–749 |
| Reticulocyte count, % | 11·2 | 5·81–18·6 |
| Absolute reticulocyte count, ×103/µl | 254 | 119–594 |
| Alanine tranaminase, IU/l | 27 | 12–42 |
| Aspartate transaminase, IU/l | 47 | 18–97 |
| Total bilirubin, µmol/l | 56 | 17–102 |
| Direct bilirubin, µmol/l | 9 | 3·82–26 |
| Creatinine, µmol/l | 80 | 37·2–334 |
| Lactate dehydrogenase, IU/l | 350 | 199–563 |
| Haemoglobin F, % | 10·5 | 1·1–17·9 |
Blood flow measurements
Forearm blood flow measurements were made by strain gauge venous occlusion plethysmography, as previously described (Gladwin et al, 2003). Brachial artery and antecubital vein catheters were placed in the forearm. The brachial venous line was used for sampling of blood from the ipsilateral venous circulation. The brachial intra-arterial line was connected to a pressure transducer for blood pressure monitoring and a 3-way stopcock for sequential infusions of normal saline, SNP, L-N-Mono Methyl Arginine (L-NMMA, NO synthase inhibitor) and sodium nitrite. Following arterial cannulation, normal saline (0·9% sodium chloride) was first infused for a 20-min rest period at a rate of 1 ml/min. After the rest period, a series of blood flow measurements were conducted using venous strain gauge plethysmography. A series of seven blood flow measurements were averaged for each blood flow determination.
The SNP is an endothelium independent, exogenous NO donor that causes peripheral vasodilation by direct action on venous and arteriolar smooth muscle. SNP was mixed in 1000 ml normal saline at a final concentration of 3·2 µg/ml and infused at 0·8, 1·6 and 3·2 µg/min respectively, in order to test the vascular responsiveness to an NO donor. Forearm blood flow was measured after 3 min of each infusion. The NO synthase inhibitor L-NMMA was mixed in 100 ml of normal saline and infused at 0·5 and 1 ml/min to achieve infusion rates of 4 and 8 µmol/min respectively. L-NMMA was infused for 5 min at each dose and forearm blood flow was measured after each dose. A 30-min rest period followed each SNP and L-NMMA series of infusions.
Sodium nitrite was prepared with 2·76, 27·6 and 276 mg in 100 ml of normal saline to achieve concentrations of 0·4, 4 and 40 mmol/l. Each solution was infused at a rate of 1 ml/min, which delivers 0·4, 4 and 40 µmol/min. Each dose of sodium nitrite was infused for an initial period of 5 min, and continued during subsequent blood sampling and forearm blood flow measurements (approximately 8 min total infusion time at each concentration). Blood sampling from the ipsilateral brachial venous line was obtained immediately before the first nitrite infusion and immediately prior to completion of each infusion of sodium nitrite. After a 30-min rest period with infusion of normal saline, SNP infusions were repeated. After another 30-min rest period, L-NMMA infusions were repeated. The total dose of sodium nitrite in our study participants was approximately 0·35 mmol (0·4, 4, and 40 µmol/min × 8 min each) or approximately 25 mg, which is less than one-tenth of the dose accepted by the Food and Drug Administration for emergency treatment of cyanide poisoning in humans. Echocardiography was performed to determine tricuspid regurgitant jet velocity (TRV) as a measure of pulmonary artery pressure, prior to the infusion of sodium nitrite and again after the maximal dose of sodium nitrite was infused.
Sodium nitrite infusions were performed in an intensive care setting, with continuous cardio-respiratory and pulse oximetry monitoring. Heart rate and systemic systolic, diastolic and mean arterial blood pressures were recorded after each drug infusion. Patient comfort was monitored throughout the procedure to determine symptoms of any potential adverse effects.
Blood testing
Blood samples were collected and immediately processed at the bedside. Whole blood and plasma nitrite measurements were performed according to previously established methods (Dejam et al, 2005; Pelletier et al, 2006). Briefly, 8 ml of blood was collected into a 10 ml syringe that contained 0·1 ml of 1:1000 concentration heparin. Approximately 5 ml of whole blood was centrifuged and separated equally into RBC and plasma aliquots. The plasma aliquots were immediately frozen on dry ice and thawed once prior to analysis (see below). Approximately 200–300 µl supernatant from each plasma aliquot was injected into a solution of acidified tri-iodide, purging with helium into a gas phase chemiluminescence NO analyzer (Sievers, Boulder, CO, USA). The tri-iodide solution was prepared with 0·400 g potassium iodide, 0·260 g of iodine and mixed with 8 ml of Millipore water. Acetic acid (28 ml) was then added to this solution and 7–8 ml was then injected into the purge vessel. This solution reduced nitrite, iron-nitrosyl heme and S-nitrosothiols to an NO gas that was detected by the NO analyzer.
Clinical laboratory analyses were performed in the Department of Laboratory Medicine at the Clinical Center of the National Institutes of Health, including complete blood counts and standard serum chemistry and methaemoglobin assays.
Toxicity
A review of the literature indicated that sodium nitrite can cause nausea, vomiting, abdominal pain, dizziness, flushing, cyanosis, hypotension and death in extreme cases. The side effects are commonly a result of the generation of methaemoglobin after nitrite reacts with haem iron in oxyhaemoglobin. If the levels of methaemoglobin rise above 30% a patient may appear cyanotic and may experience dyspnea. If levels are above 50%, patients can experience seizures, hypotension, coma and death.
Statistics
An a priori sample size calculation based on power calculations estimated that 10 patients with SCD in steady state would be necessary to detect a significant difference in the forearm blood flow responses after infusion of sodium nitrite. The sample size was adjusted to accrue a minimum of 10 patients with abnormally blunted baseline vascular reactivity to SNP administration, resulting in 14 enroled patients. The primary outcome measure of the study was to determine if sodium nitrite infusions vasodilate the regional forearm circulation in patients with SCD. Analysis of variance of the mean (anova) with repeated measures, two-sided P-values and Spearman correlations were used where indicated. Measurements shown represent mean ± standard error of the mean (SEM). Analysis was performed with Prism version 4·0 (GraphPad Software, San Diego, CA, USA). Significance tests were two-tailed and statistical significance was assumed at P < 0·05.
Results
Nitrite infusions induce regional vasodilation in patients with SCD
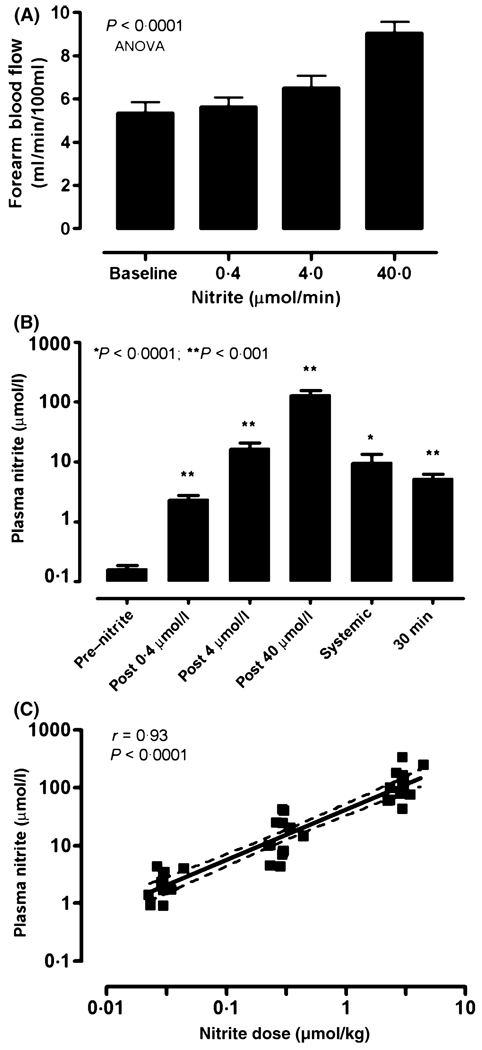
Baseline blood flow measurements were performed in each patient prior to the infusion of drug (Fig 1). The baseline forearm blood flow in our 14 patients was 5·36 ± 0·49 ml/min/100 ml of forearm tissue (mean ± SEM). Forearm blood flow in patients with SCD increased to 5·65 ± 0·43, 6·50 ± 0·58 and 9·04 ± 0·53 ml/min/100 ml of forearm tissue with infusions of 0·4, 4 and 40 µmol/min respectively (P < 0·0001, anova with repeated measures) (Fig 2A). These values yielded calculated nitrite-induced increase in blood flow over baseline by 7·9 ± 4·1, 25·1 ± 7·1 and 77·4 ± 11·2% respectively following infusions of 0·4, 4 and 40 µmol/min infusions of sodium nitrite (P < 0·0001, anova) (Fig 2A). Vascular responses were not significantly different in patients on hydroxycarbamide compared to those who were not (data not shown). These data indicate that sodium nitrite infusions produce significant vasodilation in the regional circulation of the forearm in patients with SCD, the prospectively defined primary hypothesis in this study.
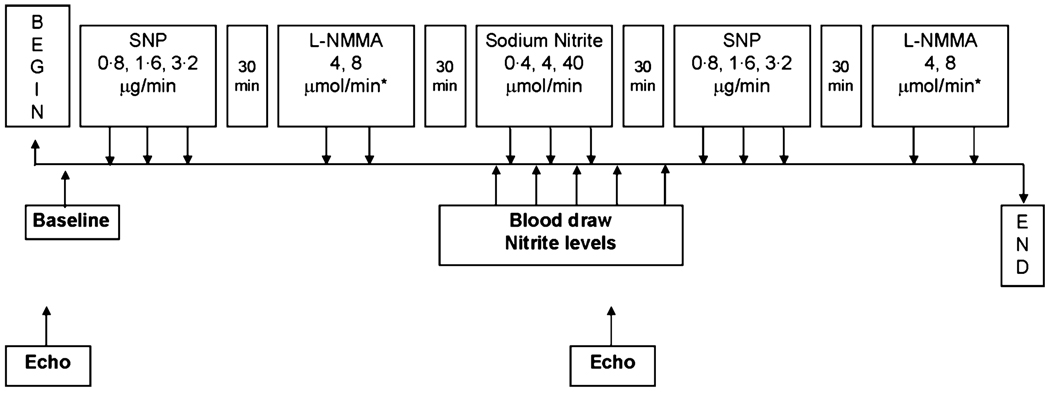
Fig 1.
Schematic diagram of forearm blood flow study. Upper boxes indicate timing of arterial infusions of sodium nitroprusside (SNP), the NO synthase inhibitor L-NMMA, and sodium nitrite. Lower boxes indicate timing of blood sampling and echocardiography. Venous occlusion plethysmography measurements of forearm blood flow were made prior to each set of infusions and immediately following each infusion.
Fig 2.
Sodium nitrite infusions increase regional blood flow and venous plasma nitrite levels as a function of nitrite dosing. (A) Sodium nitrite infusions into the brachial artery of patients with SCD increases forearm blood flow in a dose-dependent manner (P < 0·0001). Values represent means ± SEM. (B) Mean venous plasma concentrations increase from baseline in a dose-dependent manner. The systemic mean venous plasma nitrite concentration is from the contralateral arm 5 min after maximal nitrite infusion. The last bar represents the mean venous plasma nitrite concentration 30 min after the maximal nitrite infusion dose. Bars represent mean ± SEM. *P < 0·05; **P < 0·001, paired t-test compared to baseline. (C) Sodium nitrite dosing controlled for weight and expressed as µmol/kg, correlates positively with the corresponding venous plasma nitrite concentration sampled from the ipsilateral vein after arterial infusions (Spearman r = 0·93, P < 0·0001). The solid line represents the regression line, and the dashed lines the 95% confidence limits.
Plasma nitrite levels in patients with SCD
The mean baseline plasma nitrite concentration sampled from the intravenous catheter in the antecubital vein of the infused arm was 0·17 ± 0·02 µmol/l. The mean plasma nitrite concentrations following the 0·4, 4 and 40 µmol/min infusions were 2·4 ± 0·36, 16·9 ± 3·6 and 132·2 ± 23 µmol/l respectively (P < 0·0001, anova) (Fig 2B). The systemic mean plasma nitrite level 5 min after the infusion of the maximal dose of sodium nitrite was 9·7 ± 3·5 µmol/l (Fig 2B). The venous mean plasma nitrite level in the infused arm 30 min after the maximum sodium nitrite infusion was 5·3 ± 0·9 µmol/l (Fig 2B). The individual regional plasma nitrite levels correlated with the dose of sodium nitrite administered (Spearman correlation r = 0·93, P < 0·0001) (Fig 2C).
Forearm blood flow is related to plasma nitrite
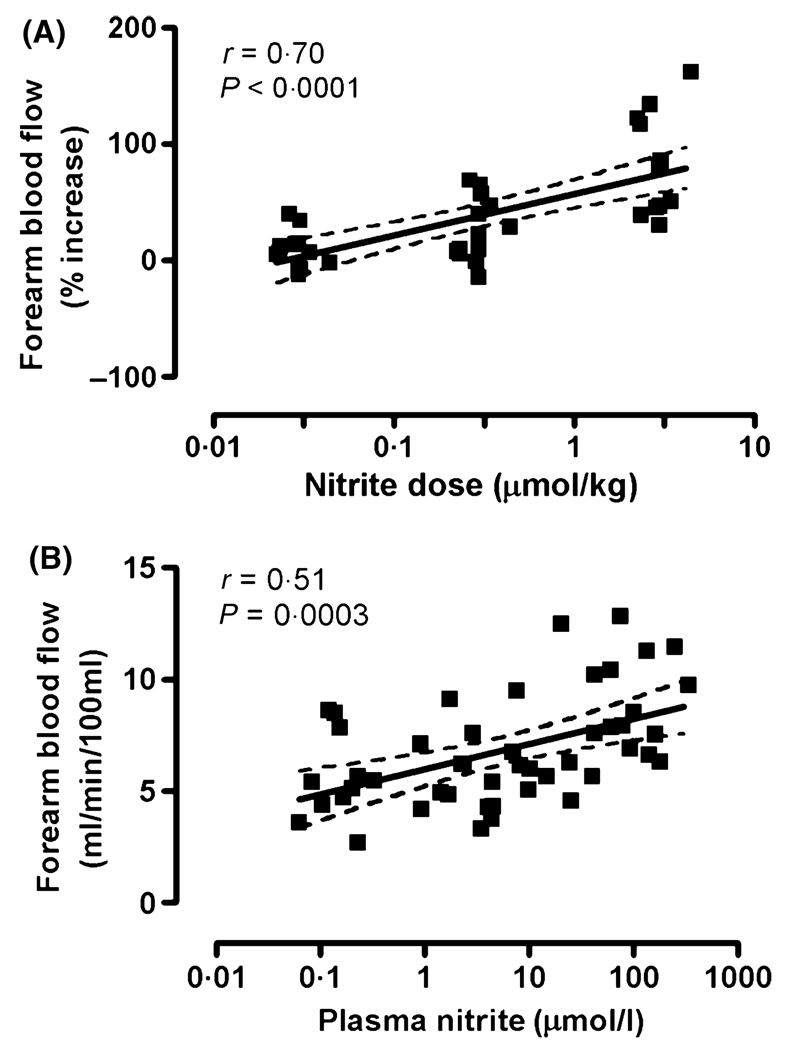
Forearm blood flow in patients with SCD, expressed as percentage increase over baseline, correlated positively with the infused dose of sodium nitrite dose in µmol/kg (Spearman correlation r = 0·70, P < 0·0001, Fig 3A). Absolute forearm blood flow, expressed as ml/min/100 ml of forearm tissue, correlated positively with plasma nitrite levels achieved with the infusions (Spearman correlation r = 0·51, P = 0·0003, Fig 3B). This relationship suggests that sodium nitrite infusions induced vasodilation in patients with SCD in a concentration-dependent fashion. There was no statistically significant correlation between baseline plasma nitrite concentration and baseline forearm blood flow (data not shown).
Fig 3.
Sodium nitrite dosing and venous plasma nitrite concentrations correlate positively with regional forearm blood flow in patients with sickle cell disease. (A) Sodium nitrite infusions, expressed as µmol/kg, correlate positively with percentage increase over baseline forearm blood flow in patients with SCD in steady state (Spearman r = 0·70, P < 0·0001). (B) The corresponding venous plasma nitrite concentrations positively correlated with the regional forearm blood flow circulation, expressed in standard units of ml/min/100 ml of forearm tissue (Spearman r = 0·51, P = 0·003). The solid lines represent the regression line, and the dashed lines the 95% confidence limits.
Blunted nitrite response compared with controls
Vasodilatory responses in patients with SCD were blunted compared to control subjects, even though the sickle cell patients received a 10% higher nitrite dose (data not shown). Mean arterial pressures did not change significantly after nitrite infusion, which suggests that systemic nitrite administration at these doses does not cause hypotension in patients with SCD. This systemic blood pressure response was blunted compared to healthy controls (data not shown).
The nitrite levels in patients with SCD were comparable to healthy controls at baseline (0·16 ± 0·02 µmol/l vs. 0·18 ± 0·17 µmol/l, P = 0·7) and after low dose nitrite infusions (2·4 ± 0·4 vs. 2·6 ± 0·5, P = 0·8). The patients with SCD demonstrated somewhat lower regional (132 ± 23 µmol/l vs. 221 ± 58 µmol/l, P = 0·2) and systemic (5·3 ± 0·9 µmol/l vs. 16 µmol/l) plasma nitrite levels after the highest dose of sodium nitrite infused, but none of these differences were statistically significant.
SNP responsiveness in patients with SCD
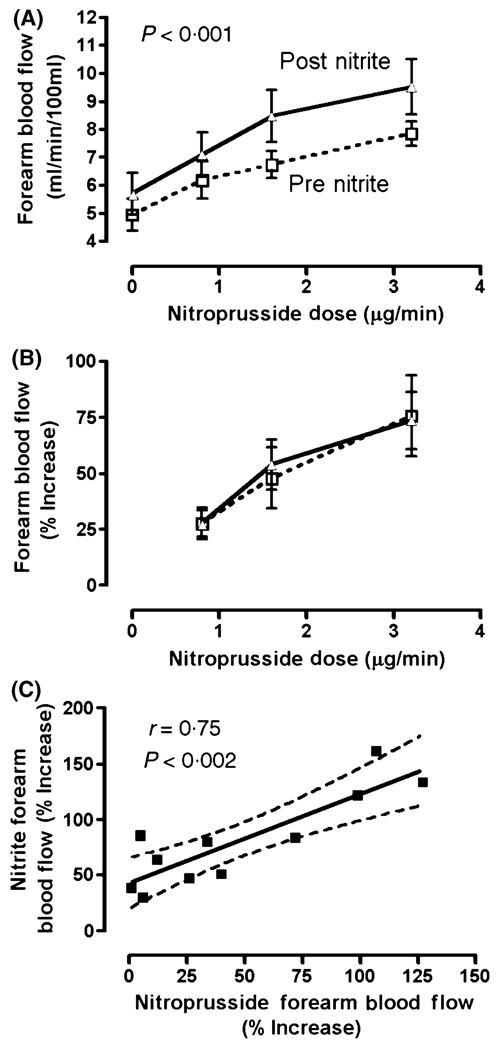
Consistent with our previously published data (Gladwin et al, 2003), forearm blood flow increase during infusions of the exogenous NO donor, SNP, in patients with SCD was blunted in comparison to 10 African American healthy control subjects (data not shown). In the patients with SCD, 30 min after the nitrite infusion, the level of forearm blood flow rose globally higher during infusion of SNP at baseline (0), 0·8, 1·6 and 3·2 µg/min, compared to the SNP responses prior to nitrite treatment (prenitrite 5·18 ± 0·48, 6·45 ± 0·60, 7·25 ± 0·48, 8·37 ± 0·44 ml/min/100 ml vs. postnitrite 5·86 ± 0·59, 7·40 ± 0·69, 8·70 ± 0·77, 9·69 ± 0·77 ml/min/100 ml, respectively, P < 0·0001, anova with repeated measures) (Fig 4A). However, nitrite infusion did not improve the SNP responsiveness, as indicated by percentage increase in forearm blood flow (Fig 4B). The postnitrite global increase in forearm blood flow remained, but was smaller and no longer statistically significant by approximately 90 min after the nitrite administration, as seen during infusions of the NO synthase inhibitor L-NMMA at 0, 4 and 8 µmol/min, compared to the prenitrite L-NMMA infusions (prenitrite 5·57 ± 0·52, 4·32 ± 0·45, 4·26 ± 0·39 ml/min/100 ml vs. postnitrite 5·75 ± 0·55, 4·70 ± 0·42, 4·62 ± 0·50 ml/min/100 ml, respectively, n = 13, P = 0·2, anova with repeated measures). The failure of NO synthase blockade to eliminate this small trend toward globally increased blood flow suggests that the nitrite-induced increase in blood flow is NOS-independent, but this is not conclusive.
Fig 4.
Interaction of nitrite and nitroprusside on regional blood flow. (A) Baseline and nitroprusside-induced absolute blood flow (dashed line) is enhanced globally following sodium nitrite infusion (solid line, P < 0·001, two-way anova with repeated measures), although (B) the degree of vasodilation above baseline is not changed. (C) The vasodilatory response to the maximal dose nitroprusside correlates highly with the response to the maximal nitrite dose (Pearson correlation, r = 0·75, P = 0·002). Solid line indicates the regression line, and dotted lines indicate 95% confidence limits.
At this 90-min time point, the percentage of blood flow that was NO synthase-dependent was not changed significantly by nitrite, as assessed by L-NMMA infusion at 4 µmol/min (−21 ± 5% vs. −17 ± 4%), and 8 µmol/min (−21 ± 4% vs. −20 ± 3%). These results suggest that nitrite globally increases regional blood flow, but it does not specifically decrease resistance to either exogenous or endogenous NO.
The vasodilatory response to the maximal dose of SNP before the nitrite infusion correlated strongly with the response to the maximal dose of nitrite (Spearman r = 0·75, P = 0·002) (Fig 4C). This indicates that those patients whose vascular response demonstrates resistance to SNP manifest proportional resistance to sodium nitrite. This correlation is consistent with a model in which sodium nitrite, like SNP, functions as an NO donor.
Toxicity assessment
The mean prenitrite methaemoglobin level was 1·2 ± 0·16%. The regional venous methaemoglobin level was not significantly affected by the lowest dose of nitrite (1·3 ± 0·16%), although small but statistically significant increases from baseline were induced by the infusion of sodium nitrite at 4 µmol/min (1·7 ± 0·21%, P < 0·05). Regional methaemoglobin levels rose further at 40 µmol/min (4·0 ± 0·40%, P < 0·001), which was significantly higher than control subjects at a comparable dose (0·2%, P < 0·0001). The systemic methaemoglobin level, measured from venous blood drawn from the contralateral arm, 5 min after infusion of the maximal sodium nitrite dose was 1·8 ± 0·25%, trending slightly higher than the initial baseline level (P = 0·06). The regional methaemoglobin level in the infused arm 30 min later was also slightly above baseline, (1·95 ± 0·2%, P = 0·01) (data not shown). None of the patients demonstrated clinical signs or symptoms related to elevated methaemoglobin levels (30–50%), such as cyanosis or shortness of breath.
The venous plasma nitrite levels correlated positively with the venous methaemoglobin level, sampled at the corresponding time point in the study (r = 0·62, P < 0·0001). This indicates that blood methaemoglobin levels are a reasonable proxy for plasma nitrite levels. One patient reported transient nausea at the highest dose of nitrite, with no other apparent ill effects. No other symptoms were reported by the patients.
Discussion
This study is the first to test the safety and therapeutic effect of intravascular sodium nitrite in a human disease. Our results demonstrated that sodium nitrite vasodilates the regional circulation of the forearm in patients with SCD, the planned primary outcome measure of the study. This result was consistent with previously published data indicating that sodium nitrite increases regional blood flow in healthy volunteers; however, patients with SCD exhibit a reduced vasodilatory sensitivity to nitrite than the healthy volunteers without SCD (Cosby et al, 2003). This may be partly due to the lower plasma nitrite regional concentrations attained in the SCD patients, presumably because of dilution by the rapid blood flow seen in anaemic patients. However, the relative resistance to nitrite, a putative NO donor, is also consistent with the diminished response to other NO donors seen in mice and humans with SCD, and also observed in the blunted SNP response in this study (Reiter et al, 2002; Eberhardt et al, 2003; Gladwin et al, 2003; Kaul et al, 2004). The mechanism of resistance is closely related to NO consumption by plasma cell-free haemoglobin for NO donors, such as SNP and others (Hsu et al, 2007) although we did not assess this directly in this nitrite study. Likewise, the degree of resistance to nitrite and nitroprusside correlated very highly in this study, further implicating NO generation as the mechanism of nitrite activity in vasodilation. Previously published data from our group and others have identified biochemical mechanisms by which nitrite can be metabolized by haem proteins, such as haemoglobin into NO (Huang et al, 2005). Nitrite appears to act as an NO donor in a dose-dependent fashion to promote regional blood flow in patients with SCD, albeit as predicted, to a reduced degree compared to healthy control subjects.
We did not find evidence to support our second hypothesis that nitrite would improve vascular reactivity to exogenous and endogenous NO, assessed by the percentage change in blood flow induced by the endothelium independent NO donor SNP and the NOS inhibitor L-NMMA. Although nitrite significantly boosted both baseline and SNP-induced absolute blood flow additively, nitrite did not synergistically increase the SNP responsiveness from that slightly increased baseline. The nitrite-induced trend toward increased forearm blood flow persisted during NOS inhibition, consistent with the previous model in which NOS was not involved in NO generation from nitrite, although this should be interpreted with caution, because the changes were not statistically significant. An open question remains whether sodium nitrite might improve responsiveness to endothelium dependent vasodilators, which were not assessed in this study.
Our results provide important pharmacological and toxicity data in humans with SCD treated with doses of sodium nitrite nearly adequate to provide small detectable changes in systemic plasma nitrite levels. Sodium nitrite dosing (expressed as µmol/kg) correlates closely with the corresponding venous plasma nitrite level indicating the precise doses necessary to achieve specific ‘therapeutic’ nitrite levels in patients with SCD. This is the one of the first studies of systemic administration of nitrite in a human disease to determine potential dosing for therapeutic indications, however further detailed pharmacokinetic studies are needed. Patients who participated in the study did not develop significant methaemoglobinaemia, shortness of breath, nor did they experience significant drops in blood pressure with the infusions of sodium nitrite. This pilot experience of nitrite therapy in SCD provides both a biological basis and preliminary pharmacological safety data to facilitate future protocol development investigating the potential clinical utility of nitrite in patients with SCD.
Several effects of nitrite make it a very appealing drug for further clinical investigation in SCD. Patients with SCD have impaired blood flow associated with rigid erythrocytes, endothelial adhesion molecule expression, haemostatic activation, impaired vasodilation due to impaired NO bioavailability, and consequent tissue infarction that contributes to pain and organ dysfunction. Our data indicate that nitrite can safely promote blood flow in patients with SCD, in a dose-dependent manner consistent with production of NO, which in a variety of model systems also can decrease adhesion molecule expression and haemostatic activation (Walford & Loscalzo, 2003). The evidence in animal models that nitrite can reduce tissue infarction suggests even more interesting potential benefit in SCD. The systemic peak plasma nitrite level that is optimal to reduce tissue infarction in experimental animals (approximately 10 µmol/l) is comparable to the systemic level achieved without toxicity in the SCD patients in this study. These data will aid in the development of trials to test the potential clinical efficacy of nitrite in patients with SCD.
Acknowledgements
The authors are grateful to Mary K. Hall for protocol management and to Christine Hon for helpful discussions. The study was funded by the Division of Intramural Research of the National Institutes of Health, Bethesda, MD, including a Bench to Bedside award (A.K.M. and G.J.K.). M.T.G. is a participant on a pending U.S. patent, filed on October 14, 2003 through NIH (patent no. 60/511, 244), regarding the use of sodium nitrite in cardiovascular disease. He and the other co-authors declare no other competing financial interests. A.K.M., G.J.K. and M.T.G. designed the research, A.K.M., C.B. and R.F.M. performed the blood flow studies, D.A. performed the nitrite measurements, C.K.T. and V.R.M. recruited patients and collected data, A.K.M. and G.J.K. analyzed and interpreted the data, and drafted the manuscript.
References
- Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- Belhassen L, Pelle G, Sediame S, Bachir D, Carville C, Bucherer C, Lacombe C, Galacteros F, Adnot S. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood. 2001;97:1584–1589. doi: 10.1182/blood.v97.6.1584. [DOI] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. Journal of Clinical Investigation. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt RT, McMahon L, Duffy SJ, Steinberg MH, Perrine SP, Loscalzo J, Coffman JD, Vita JA. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. American Journal of Hematology. 2003;74:104–111. doi: 10.1002/ajh.10387. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Schechter AN, Ognibene FP, Coles WA, Reiter CD, Schenke WH, Csako G, Waclawiw MA, Panza JA, Cannon RO., III Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. New England Journal of Medicine. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology. 2006;291:H379–H384. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. Journal of Clinical Investigation. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nature Medicine. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- Isbell TS, Koenitzer JR, Crawford JH, White CR, Kraus DW, Patel RP. Assessing NO-dependent vasodilatation using vessel bioassays at defined oxygen tensions. Methods in Enzymology. 2005;396:553–568. doi: 10.1016/S0076-6879(05)96047-3. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- Kato GJ, McGowan V, Machado RF, Little JA, Taylor JT, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration,pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Reviews. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. American Journal of Physiology. Heart and Circulatory Physiology. 2000;278:H1799–H1806. doi: 10.1152/ajpheart.2000.278.6.H1799. [DOI] [PubMed] [Google Scholar]
- Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. Journal of Clinical Investigation. 2004;114:1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montalembert M, Aggoun Y, Niakate A, Szezepanski I, Bonnet D. Endothelial-dependent vasodilation is impaired in children with sickle cell disease. Haematologica. 2007;92:1709–1710. doi: 10.3324/haematol.11253. [DOI] [PubMed] [Google Scholar]
- Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath KA, Shah V, Haggard JJ, Croatt AJ, Smith LA, Hebbel RP, Katusic ZS. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2000;279:R1949–R1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- Nolan VG, Baldwin C, Ma Q, Wyszynski DF, Amirault Y, Farrell JJ, Bisbee A, Embury SH, Farrer LA, Steinberg MH. Association of single nucleotide polymorphisms in klotho with priapism in sickle cell anaemia. British Journal of Haematology. 2005a;128:266–272. doi: 10.1111/j.1365-2141.2004.05295.x. [DOI] [PubMed] [Google Scholar]
- Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005b;106:3264–3267. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radical Biology and Medicine. 2006;41:541–548. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- Walford G, Loscalzo J. Nitric oxide in vascular biology. Journal of Thrombosis and Haemostasis. 2003;1:2112–2118. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]