Abstract
Salvinorin A (1), a neoclerodane diterpene from the hallucinogenic mint Salvia divinorum, is the only known non-nitrogenous and specific κ-opioid agonist. Several structural congeners of 1 isolated from Salvia splendens (2 – 8) together with a series of semisynthetic derivatives (9 – 24), some of which possess a pyrazoline structural moiety (9, 19 – 22), have been tested for affinity at human μ, δ, and κ opioid receptors. None of these compounds showed high affinity binding to these receptors. However, 10 showed modest affinity for κ receptors suggesting other naturally neoclerodanes from different Salvia species may possess opioid affinity.
Keywords: Salvia splendens, opioid receptors, neoclerodane diterpenes, semisynthetic derivatives
1. Introduction
Salvinorin A (1), one of the neoclerodane diterpenes of Salvia divinorum Epling & Játiva (Labiatae), is a highly unusual drug that exerts its hallucinogenic effects as a potent and selective agonist of the κ-opioid receptor.1 In the past few years, intensive research on the pharmacology and chemical transformations of salvinorin A have been made,1–6 and its biosynthetic pathway has been elucidated7 and its asymmetric total synthesis has been achieved.8
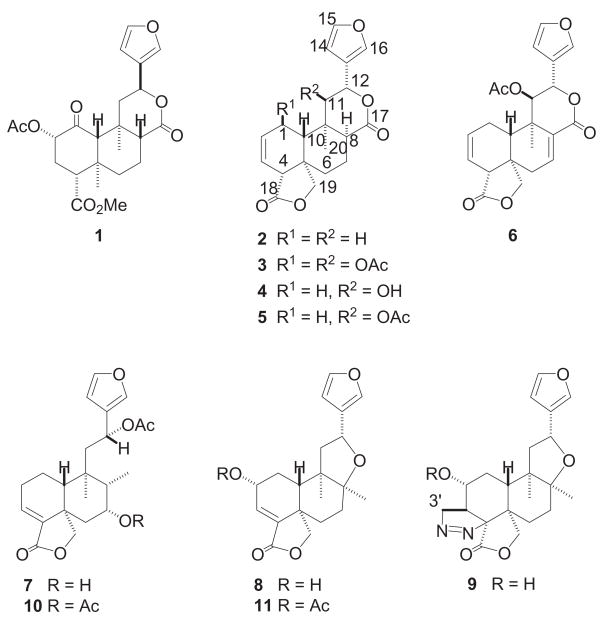
Recently,9 some of us have reinvestigated the diterpene constituents of Salvia splendens Sellow ex Roem. &. Schult isolating four new neoclerodanes (salvisplendins A–D, 4, 6, 7, and 8, respectively) together with the artifact 9, that results from 8 by treatment of the crude extract with diazomethane,9 and salviarin (2),10 splendidin (3),11 and splenolides A and B (5),12 all of them previously reported as constituents of this species.9–13 Taking into account the structural similarity between salvinorin A (1) and the diterpenes found in S. splendens (2–9), we decided to test these substances and two of their available derivatives (10 and 11)9 in order to explore whether such compounds may be useful as neuropharmacological agents.
Moreover, we have now obtained several new semisynthetic derivatives (12 – 24) starting from the more abundant constituents of S. splendens (2, 3, 5, 7, and 9),9,13 and these compounds were also assayed as potential agonists at the opioid receptors. Three clerodanes of S. splendens, salviarin (2), splendidin (3), and splenolide B (5), have recently been tested for affinity at opioid receptors and none of these diterpenes showed significant binding to any of the opioid-receptor subtypes.14 This work, however, demonstrates the potential utility of evaluating other neoclerodanes for their interaction with opioid receptors.
2. Results and Discussion
As reported previously,1 salvinorin A (1) with the natural (β) configuration at C-8 shows higher affinity and efficacy at the κ-opioid receptor. Hence, our first objective was to epimerize the C-8 asymmetric center of salviarin, splendidin, and splenolide B (2, 3, and 5, respectively). Treatment of 2 with K2CO3 in MeOH yielded 8-epi-salviarin (12) (scheme 1), a derivative which had been obtained by Rodríguez-Hahn and co-workers using similar reaction conditions.15 The complete assignment of the 1H NMR spectrum together with other spectroscopic data of 12 have not been reported previously, and they are now included into Experimental Section. The β-configuration16 of H-8 in 12 was supported by nOe experiments because irradiation at δ 2.62 (H-8) caused strong nOe enhancements in the H-6β, H-10β, and H-12β protons (+4.7, +5.1, and +15.3% nOe enhancement, respectively). This nOe behavior differs from that of the H-8α epimer 2.13 In addition, the coupling constant J8β,7α = 11.9 Hz and J8β,7β = 3.4 Hz in 12, as compared with those of 2 (J8α,7α = 4.0 Hz and J8α,7β = 2.8 Hz),13 further supported the β-configuration of H-8.
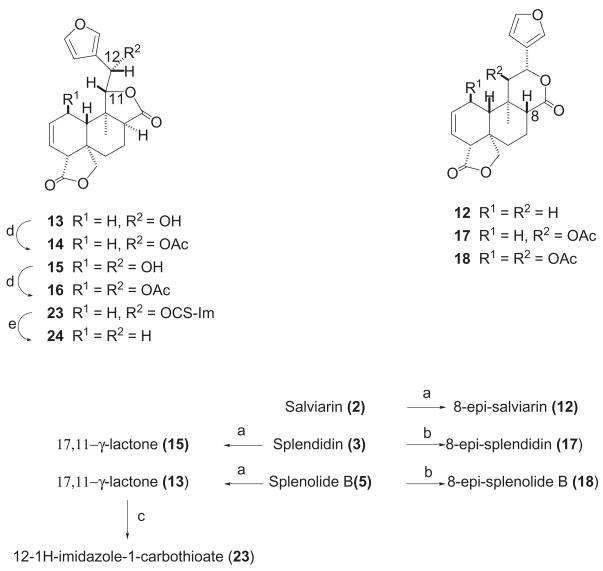
Scheme 1.
Reagents and conditions: (a) K2CO3/MeOH, rt, 4 h 65 % (12), 73 % (13), 58 % (15); (b) DBU (10 eq.), CH2Cl2, rt, 72 h 40 % (17), 24 h 70 % (18); (c) CDI, DMAP (cat.), CH2Cl2, reflux, 2 h, 80 %; (d) Ac2O/Py 1:2, rt, quant.; (e) n-Bu3SnH, AIBN (cat.), toluene, reflux, 6 h, 45 %.
When 5 was treated with K2CO3 in MeOH under the same conditions that those described for 2 (see Experimental Section), only compound 13 (C20H22O6) was obtained and it was transformed into a monoacetyl derivative (14, C22H24O7) after treatment with Ac2O-pyridine (scheme 1). The 1H and 13C NMR spectra of 13 and 14 showed the presence of a 17,11-γ-lactone instead of the 17,12-δ-lactone in 5. In particular, the HMBC correlations observed for 13 and 14 between the H-11 proton and the C-17 carbonyl carbon strongly support the presence of a 17,11-γ-lactone in these compounds. Moreover, the HMBC spectrum of 14 showed correlation between the carbonyl carbon of the acetate (δ 170.0) and a methine proton (δ5.91, H-12), which in turn correlated with the C-13, C-14, and C-16 furanic carbons (δ 119.7, 109.9, and 141.9, respectively), thus confirming the proposed structures. nOe experiments on 13 and 14 established that the hydrogens at C-8 and C-11 were α- and β-oriented, respectively, since irradiation at Me-20 produced, among others, strong nOe enhancement in the signal of H-8α (+4.9% in 13 and +6.5% in 14), whereas irradiation at δ 4.58 and 4.49 (H-11β in 13 and 14, respectively) caused nOes in the H-1β and H-10β protons (+8.1 and +8.4% in 13; +8.1 and +11.5% in 14, respectively) and not in Me-20.
Splendidin (3) also reacts in a similar way by treatment with Na2CO3 in MeOH giving 15 (C20H22O7) (scheme 1). The HMBC spectra of 15 and its diacetyl derivative 16 (C24H26O9) showed the same correlations that those observed for 13 and 14, and nOe experiments were also in agreement with an α- and β-orientation for their H-8 and H-11 protons, respectively. The epimerization of the C-8 position of salviarin (2) under basic conditions via enolate formation, followed by protonation from the opposite face1,5 gave compound 12. In the case of 3 and 5, however, no epimerization was observed and this may be explained by an initial deacetylation at C-11, followed by a translactonization to the more stable cis fused 17,11-γ-lactones (15 and 13, respectively), in which epimerization at C-8 via an enolate is less favored.
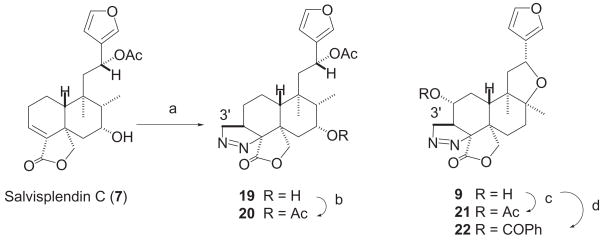
Preparation of 8-epi-splenolide B (17) and 8-epi-splendidin (18) was achieved as follows (scheme 1). Treatment of 5 with 1,8-diazobicyclo[5.4.0]undec-7-ene (DBU) in CH2Cl2 solution for 72 hours at room temperature yielded 17 (40%) together with minor quantities of 4 (15%) and 13 (10%), whereas identical treatment of 3 in anhydrous CH2Cl2 for 24 hours at room temperature gave 18 (70% yield) and starting material (3, 15%). Like in 12, the β-orientation of H-8 in 17 and 18 was strongly supported by nOe experiments and by the observed vicinal J(H,H) couplings of the H-8 methine and H-7 methylene protons (see Experimental Section). Treatment of salvisplendin C (7) with an ethereal solution of diazomethane9,17,18 gave the pyrazoline derivative 19 (scheme 2). The regio-and stereochemistry of the pyrazoline moiety of 19 are identical to those of the already described derivative 9,9 as it was evidenced by comparison of their 1H and 13C NMR spectra (see ref.9 and Experimental Section).19 The acetyl derivatives 2019 and 21 and the benzoate 22 were prepared by standard procedures (scheme 2) and their structural assignments were confirmed by extensive spectroscopic studies (see Experimental Section). Finally, compound 24 was obtained from 13 by initial formation of the 12-1H-imidazole-1-carbothioate (23) followed by reaction of this intermediate with n-Bu3SnH (scheme 1).
Scheme 2.
Reagents and conditions: (a) CH2N2, Et2O, 0° C, 1 h, 73 %; (b) Ac2O/Py 1:2, 40° C, one week, 55 %; (c) Ac2O/Py 1:2, rt, overnight, quant.; (d) PhCOCl (10 eq.), DMAP (cat.), CH2Cl2/Py 2:1, 0° C to rt, overnight, 50 %.
Compounds 2 – 22 and 24 were then evaluated for affinity at opioid receptors. These analogues were screened to gain a greater understanding into the role of the orientation of the furan ring plays in the high affinity and selectivity of 1 for κ receptors. Several pyrazolines were also evaluated based on a previous report which suggested that this ring system may potentially impart CNS depressant activity and/or anti-inflammatory activity.20 Unfortunately, none of these compounds showed high affinity binding to human opioid receptors (Ki>10,000 nM). However, diterpene 10 showed weak affinity for κ opioid receptors (Ki = 6910 ± 570 nM). These results are not surprising given the differences in this series of agents compared to 1. Some potential reasons for the lack of affinity compared to 1 are (a) the absence of a ketone at C-1; (b) the lack of a C-2 substituent; (c) the inverted stereochemistry at C-8; (d) the inverted stereochemistry at C-12 and (e) the incorporation of the C-4 carbomethoxy group into an additional ring. To begin to address possible explanations, several analogues were prepared. It was envisoned that inversion of the C-8 stereochemistry would result in enhanced affinity at opioid receptors. This structural change, however, did not increase the affinity of 3 (18) or 4 (17) for opioid receptors. These changes, however, are not parallel to the salvinorin A series. Given the previous SAR for 1 that the C-2 position has profound effects on affinity for opioid receptors,21 21 and 22 were synthesized from 9. Interestingly, introduction of an acetyl group (21) did not increase affinity for κ opioid receptors and the addition of a benzoyl group (22) did not increase affinity for μ opioid receptors.
3. Conclusion
The data collected in this work indicate that the previous structure-activity relationships (SAR) may not be applicable to these neoclerodanes of general structure 9. They also suggest that these two series are not binding in an identical manner and that the orientation of the furan ring is a key interaction in the mode of binding of neoclerodane diterpenes at opioid receptors.
4. Experimental
4.1 General
Melting points were determined on a Kofler block and are uncorrected. Optical rotations were measured on a Perkin-Elmer 241 MC polarimeter. IR spectra were obtained on a Perkin-Elmer Spectrum One spectrophotometer. 1H and 13C NMR spectra were recorded in CDCl3 solution, except for 13 and 15 (methanol-d4), on a Varian INOVA 400 spectrometer at 400 and 100 MHz, respectively. Chemical shifts are reported in the δ scale and are referenced to residual CHCl3 (δ 7.25) or methanol-d4 (δ 3.30) signals for protons and to the solvent signals (δCDCl3 77.00, δCD3OD 49.00) for carbons. All the assignments for protons and carbons were in agreement with 2D COSY, gHSQC, gHMBC, and 1D NOESY spectra. Mass spectra were registered in the positive EI (70 eV) mode on a Hewlett-Packard 5973 instrument. Elemental analyses were conducted on a LECO CHNS-932 apparatus. Merck Si gel 7734 (70–230 mesh) deactivated with 15% (w/v) of water was used for gravity column chromatography, and Si gel Merck LiChroprep 15–25/25–40 μm 1/1 was used for flash chromatography (elution under 0.7 psi of Ar). Merck 5554 Kieselgel 60 F254 sheets were used for thin-layer chromatographic analysis. Petroleum ether (bp 50–70 °C) was used for column chromatography.
4.2 Compounds for Biological Assays
Samples of compounds 2 – 11 and starting materials (2, 3, 5, 7, and 9) for obtaining the new derivatives 12 – 24 were available from a previous work.9
4.3 General Procedure for Reaction of Compounds 2, 3, and 5 with Potassium Carbonate-Methanol
The starting material (0.5 mmol) was treated with 5 mL of a saturated solution of K2CO3 in MeOH. The resulting solution was stirred for 4 h at room temperature and then neutralized by adding HCl 2 N and extracted with 5 portions (10 mL each) of 2:1 CHCl3-EtOH. The organic phase was dried over Na2SO4 and the solvent was evaporated in vacuo. The crude of reaction was purified by flash chromatography (FC). Elution with 3:1 EtOAc-petroleum ether gave 12 (110 mg, 65% yield) starting from salviarin (2). Elution with 99:1 CHCl3-MeOH gave 13 (130 mg, 73%) from splenolide B (5). Elution with 39:1 CHCl3-MeOH gave 15 (108 mg, 58%) from splendidin (3).
4.3.1 Compound 12 (8-epi-Salviarin)
Ccolorless needles (EtOAc-petroleum ether); mp 232–235 °C; [α]D18 −49.5 (c 0.105, CHCl3); Lit.15: mp 233–235 °C; [α]D18 −48 (c 0.1, CHCl3); RF = 0.55 (silica gel, 3:2 EtOAc-petroleum ether); IR (KBr) νmax 3152, 3134, 2951, 1792, 1759, 1728, 1507, 1450, 1287, 1221, 1180, 1156, 1032, 993, 875, 808, 735, 702, 602 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.45 (1H, dd, J16,14 = 0.9 Hz, J16,15 = 1.8 Hz, H-16), 7.42 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.40 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.9 Hz, H-14), 6.00 (1H, ddt, J2,1α = J2,4β = 2.4 Hz, J2,1β = 5.1 Hz, J2,3 = 9.9 Hz, H-2), 5.62 (1H, dtd, J3,1α = J3,4β = 2.8 Hz, J3,1β = 1.4 Hz, J3,2 = 9.9 Hz, H-3), 5.35 (1H, dd, J12β,11α = 11.1 Hz, J12β,11β = 6.4 Hz, H-12β), 4.18 (1H, dd, J19a,6β = 1.8 Hz, J19a,19b = 9.1 Hz, pro-S H-19a), 4.14 (1H, d, J19b,19a = 9.9 Hz, pro-R H-19b), 2.80 (1H, tdd, J4β,1α = J4β,3 = 2.8 Hz, J4β,1β = 2.5 Hz, J4β,2 = 2.4 Hz, H-4β), 2.62 (1H, dd, J8β,7α = 11.9 Hz, J8β,7β = 3.4 Hz, H-8β), 2.15 (2H, m, H-1β and H-7β), 2.11 (1H, dt, J6α,6β = 13.8 Hz, J6α,7α = J6α,7β = 3.8 Hz, H-6α), 2.10 (1H, dd, J11β,11α = 14.0 Hz, J11β,12β = 6.4 Hz, H-11β), 2.06 (1H, m, H-1α), 1.88 (1H, dd, J11α,11β = 14.0 Hz, J11α,12β = 11.1 Hz, H-11α), 1.87 (1H, dd, J10β,1α = 11.8 Hz, J10β,1β = 4.7 Hz, H-10β), 1.85 (1H, dddd, J7α,6α = 3.8 Hz, J7α,6β = 12.1 Hz, J7α,7β = 14.8 Hz, J7α,8β = 11.9 Hz, H-7α), 1.35 (1H, dddd, J6β,6α = 13.8 Hz, J6β,7α = 12.1 Hz, J6β,7β = 3.9 Hz, J6β,19a = 1.8 Hz, H-6β), 0.87 (3H, s, Me-20); these assignments are in agreement with the partial 1H NMR data previously reported15 for 8-epi-salviarin; 13C NMR (100 MHz, CDCl3) δ 175.5 (s, C-18), 173.3 (s, C-17), 143.9 (d, C-15), 139.6 (d, C-16), 129.2 (d, C-2), 124.1 (s, C-13), 121.1 (d, C-3), 108.4 (d, C-14), 70.1 (t, C-19), 69.9 (d, C-12), 52.3 (d, C-4), 47.5 (d, C-8), 47.3 (d, C-10), 43.3 (t, C-11), 41.6 (s, C-5), 36.1 (s C-9), 34.9 (t, C-6), 22.3 (t, C-1), 20.4 (q, C-20), 18.5 (t, C-7); EIMS m/z 342 [M]+ (64), 327 (4), 314 (2), 298 (17), 283 (18), 231 (40), 218 (20), 159 (41), 145 (39), 131 (55), 121 (45), 117 (47), 105 (46), 94 (100), 91 (89), 79 (25), 65 (13), 55 (11); anal. C 70.08%, H 6.59%, calcd for C20H22O5, C 70.16%, H 6.48%.
4.3.2 Compound 13
Colorless needles (EtOAc); mp 254–256 °C; [α]D18 −59.8 (c 0.194, Me2CO); RF = 0.42 (silica gel, 95:5 CHCl3-MeOH); IR (KBr) νmax 3488, 3138, 3110, 3070, 2942, 2864, 2580, 1765, 1599, 1502, 1445, 1386, 1165, 1023, 873, 840, 738, 699, 602 cm−1; 1H NMR (400 MHz, CD3OD) δ 7.42 (1H, dt J16,12 = J16,14 = 0.9 Hz, J16,15 = 1.8 Hz, H-16), 7.41 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.43 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.9 Hz, H-14), 6.03 (1H, ddt, J2,1α = J2,4β = 2.2 Hz, J2,1β = 5.9 Hz, J2,3 = 10.0 Hz, Hz, H-2), 5.52 (1H, dtd, J3,1α = J3,4β = 2.9 Hz, J3,1β = 1.2 Hz, J3,2 = 10.0 Hz, H-3), 4.91 (1H, dd, J12,11β = 4.1 Hz, J12,16 = 0.9 Hz, H-12), 4.38 (1H, d, J11β,12 = 4.1 Hz, H-11β), 4.25 (1H, d, J19a,19b = 9.2 Hz, pro-R H-19a), 4.20 (1H, dd, J19b,6β = 1.9 Hz, J19b,19a = 9.2 Hz, pro-S H-19b), 2.73 (2H, m, H-4β and H-8α), 2.18 (1H, ddddd, J1β,1α = 18.4 Hz, J1β,2 = 5.9 Hz, J1β,3 = 1.2 Hz, J1β,4β = 2.4 Hz, J1β,10β = 4.6 Hz, H-1β), 2.05 (1H, ddddd, J1α,1β = 18.4 Hz, J1α,2 = 2.2 Hz, J1α,3 = 2.9 Hz, J1α,4β = 2.8 Hz, J1α,10β = 11.8 Hz, H-1α), 2.03 (1H, dddd, J7β,6α = 3.8 Hz, J7β,6β = 4.2 Hz, J7β,7α = 14.8 Hz, J7β,8α = 2.9 Hz, H-7β), 1.79 (1H, dddd, J7α,6α = 4.0 Hz, J7α,6β = 13.9 Hz, J7α,7β = 14.8 Hz, J7α,8α = 5.6 Hz, H-7α), 1.73 (1H, dd, J10β,1α = 11.8 Hz, J10β,1β = 4.6 Hz, H-10β), 1.73 (1H, m, H-6α), 1.20 (1H, dddd, J6β,6α = 14.0 Hz, J6β,7α = 13.9 Hz, J6β,7β = 4.2 Hz, J6β,19b = 1.9 Hz, H-6β), 1.03 (3H, s, Me-20); 13C NMR (100 MHz, CD3OD) δ 179.1 (s, C-17), 177.5 (s, C-18), 144.2 (d, C-15), 140.8 (d, C-16), 130.5 (d, C-2), 126.3 (s, C-13), 121.0 (d, C-3), 109.6 (d, C-14), 87.0 (d, C-11), 70.0 (t, C-19), 67.2 (d, C-12), 53.2 (d, C-4), 46.9 (d C-8), 44.1 (s, C-9), 41.8 (s, C-5), 41.4 (d, C-10), 32.0 (t, C-6), 23.3 (t, C-1), 17.1 (q, C-20), 17.0 (t, C-7); EIMS m/z 358 [M]+ (30), 340 (20), 304 (35), 262 (100), 233 (23), 203 (6), 187 (5), 171 (4), 157 (14), 139 (38), 129 (13), 117 (16), 105 (14), 97 (29), 91 (28), 81 (8); anal. C 67.21%, H 6.29%, calcd for C20H22O6, C 67.02%, H 6.19%.
4.3.3 Compound 15
Colorless fine needles (MeOH); mp 260–262 °C; [α]D18 −101.5 (c 0.481, Me2CO); RF = 0.20 (silica gel, 95:5 CHCl3-MeOH); IR (KBr) νmax 3492, 3443, 3140, 3039, 2945, 2585, 2550, 2430, 1774, 1759, 1500, 1371, 1296, 1170, 1020, 961, 875, 797, 702, 607 cm−1; 1H NMR (400 MHz, CD3OD) δ 7.51 (1H, dt J16,12 = J16,14 = 0.8 Hz, J16,15 = 1.9 Hz, H-16), 7.45 (1H, t, J15,14 = J15,16 = 1.9 Hz, H-15), 6.49 (1H, dd, J14,15 = 1.9 Hz, J14,16 = 0.8 Hz, H-14), 5.91 (1H, ddd, J2,1α = 1.8 Hz, J2,3 = 10.0 Hz, J2,4β = 2.6 Hz, H-2), 5.62 (1H, d, J11β,12 = 3.6 Hz, H-11β), 5.51 (1H, ddd, J3,1α = 2.0 Hz, J3,2 = 10.0 Hz, J3,4β = 3.2 Hz, H-3), 4.97 (1H, dd, J12,11β = 3.6 Hz, J12,16 = 0.8 Hz, H-12), 4.38 (1H, dddd, J1α,2 = 1.8 Hz, J1α,3 = 2.0 Hz, J1α,4β = 3.2 Hz, J1α,10β = 10.4 Hz, H-1α), 4.35 (1H, d, J19a,19b = 9.2 Hz, pro-R H-19a), 4.20 (1H, dd, J19b,6β = 1.8 Hz, J19b,19a = 9.2 Hz, pro-S H-19b), 2.80 (1H, td, J4β,1α = J4β,3 = 3.2 Hz, J4β,2 = 2.6 Hz, H-4β), 2.66 (1H, ddd, J8α,6α = 1.2 Hz, J8α,7α = 5.4 Hz, J8α,7β = 2.4 Hz, H-8α), 1.93 (1H, dddd, J7β,6α = 2.7 Hz, J7β,6β = 4.0 Hz, J7β,7α = 14.8 Hz, J7β,8α = 2.4 Hz, H-7β), 1.82 (1H, dddd, J7α,6α = 3.8 Hz, J7α,6β = 13.8 Hz, J7α,7β = 14.8 Hz, J7α,8α = 5.4 Hz, H-7α), 1.75 (1H, d, J10β,1α = 10.4 Hz, H-10β), 1.63 (1H, dddd, J6α,6β = 14.0 Hz, J6α,7α = 3.8 Hz, J6α,7β = 2.7 Hz, J6α,8α = 1.2 Hz, H-6α), 1.21 (1H, dddd, J6β,6α = 14.0 Hz, J6β,7α = 13.8 Hz, J6β,7β = 4.0 Hz, J6β,19b = 1.8 Hz, H-6β), 1.20 (3H, s, Me-20); 13C NMR (100 MHz, CD3OD) δ 180.5 (s, C-17), 177.5 (s, C-18), 144.3 (d, C-15), 141.6 (d, C-16), 137.6 (d, C-2), 127.1 (s, C-13), 120.7 (d, C-3), 110.8 (d, C-14), 88.7 (d, C-11), 70.8 (t, C-19), 68.0 (d, C-12), 66.7 (d, C-1), 53.8 (d, C-4), 47.8 (d, C-8), 46.8 (d, C-10), 44.7 (s, C-9), 42.9 (s, C-5), 31.9 (t, C-6), 17.7 (q, C-20), 17.3 (t, C-7); EIMS m/z 374 [M]+ (3), 356 (7), 278 (61), 259 (25), 203 (33), 159 (20), 145 (41), 135 (83), 117 (38), 97 (100), 91 (42), 81 (15), 69 (14); anal. C 64.27%, H 6.02%, calcd for C20H22O7, C 64.16%, H 5.92%.
4.4 8-epi-Splenolide B (17) from Splenolide B (5)
To a solution of 5 (50 mg, 0.125 mmol) in CH2Cl2 (0.5 mL) 170 μL (173 mg, 1.14 mmol) of DBU were added in one portion via syringe. The solution was left under Ar for 72 h at room temperature. Then the reaction mixture was quenched by addition of HCl 1 N (1 mL) and was extracted with CHCl3 (4×5 mL). The collected organic phase was washed with brine and dried over Na2SO4. Removal of the solvent by distillation at reduced pressure followed by FC (1–5% acetone-CH2Cl2 in gradient) successively yielded 17 (20 mg, 40%), 4 (7 mg, 15%), and 13 (4 mg, 10%). Compound 17: colorless rectangular plates (EtOAc-petroleum ether); mp 201–203 °C; [α]D18 −84.4 (c 0.263, CHCl3); RF = 0.35 (silica gel, 5% acetone-CH2Cl2); IR (KBr) νmax 3138, 3036, 2918, 1765, 1740, 1730, 1503, 1385, 1249, 1237, 1190, 1176, 1153, 1024, 1011, 967, 876, 798, 702, 599 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.51 (1H, ddd, J16,12β = 1.8 Hz, J16,14 = 0.9 Hz, J16,15 = 1.9 Hz, H-16), 7.42 (1H, t, J15,14 = J15,16 = 1.9 Hz, H-15), 6.55 (1H, dd, J14,15 = 1.9 Hz, J14,16 = 0.9 Hz, H-14), 5.94 (1H, ddt, J2,1α = J2,4β = 2.5 Hz, J2,1β = 5.8 Hz, J2,3 = 10.0 Hz, H-2), 5.60 (1H, dtd, J3,1α = J3,4β = 2.7 Hz, J3,1β = 1.4 Hz, J3,2 = 10.0 Hz, H-3), 5.30 (1H, dd, J12β,11α = 2.3 Hz, J12β,16 = 1.8 Hz, H-12β), 5.12 (1H, d, J11α,12β = 2.3 Hz, H-11α), 4.13 (2H, br s, H2-19), 2.81 (1H, tdd, J4β,1α = J4β,3 = 2.7 Hz, J4β,1β = 2.4 Hz, J4β,2 = 2.5 Hz, H-4β), 2.78 (1H, dd, J8β,7α = 12.3 Hz, J8β,7β = 3.9 Hz, H-8β), 2.27 (1H, dtd, J7β,6α = 3.5 Hz, J7β,6β = J7β,8β = 3.9 Hz, J7β,7α = 14.8 Hz, H-7β), 2.16 (3H, s, 11β-OAc), 2.09 (1H, ddd, J6α,6β = 13.8 Hz, J6α,7α = 3.2 Hz, J6α,7β = 3.5 Hz, H-6α), 2.04 (1H, dd, J10β,1α = 11.8 Hz, J10β,1β = 6.6 Hz, H-10β), 1.90 (2H, m, H-1α and H-1β), 1.72 (1H, dddd, J7α,6α = 3.2 Hz, J7α,6β = 13.8 Hz, J7α,7β = 14.8 Hz, J7α,8β = 12.3 Hz, H-7α), 1.34 (1H, td, J6β,6α = J6β,7α = 13.8 Hz, J6β,7β = 3.9 Hz, H-6β), 0.76 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 175.2 (s, C-18), 170.7 (s, C-17), 169.9 (s, 11-OCOCH3), 144.0 (d, C-15), 139.4 (d, C-16), 128.9 (d, C-2), 124.1 (s, C-13), 120.9 (d, C-3), 108.3 (d, C-14), 78.6 (d, C-12), 74.2 (d, C-11), 69.6 (t, C-19), 52.4 (d, C-4), 43.2 (d, C-8), 40.9 (s, C-5), 39.4 (d, C-10), 39.2 (s, C-9), 34.7 (t, C-6), 21.8 (t, C-1), 21.0 (q, 11-OCOCH3), 18.6 (t, C-7), 15.2 (q, C-20),); EIMS m/z 400 [M]+ (1), 358 (1), 340 (63), 325 (100), 312 (1), 297 (4), 244 (5), 189 (8), 143 (10), 129 (13), 117 (11), 105 (11), 95 (22), 91 (27), 81 (10), 55 (7); anal. C 66.08%, H 6.19%, calcd for C22H24O7, C 65.99%, H 6.04%.
4.5 8-epi-Splendidin (18) from Splendidin (3)
To a solution of 3 (50 mg, 0.109 mmol) in anhydrous CH2Cl2 (0.5 mL) 150 μL (153 mg, 1.00 mmol) of DBU were added in one portion via syringe. The solution was left under Ar for 24 h at room temperature. Workup as described above for 17 followed by FC (49:1 CH2Cl2-acetone as eluent) gave 18 (35 mg, 70%) and unreacted 3 (7.5 mg, 15%). Compound 18: colorless rectangular plates (EtOAc - n-pentane); mp 232–235 °C; [α]D18 −142.1 (c 0.151, CHCl3); RF = 0.30 (silica gel, 49:1 CH2Cl2-acetone); IR (KBr) νmax 3139, 3109, 3015, 2922, 1771, 1734, 1370, 1238, 1175, 1160, 1026, 965, 876, 607 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.47 (1H, dt, J16,12β = J16,14 = 0.8 Hz, J16,15 = 1.7 Hz, H-16), 7.41 (1H, t, J15,14 = J15,16 = 1.7 Hz, H-15), 6.56 (1H, dd, J14,15 = 1.7 Hz, J14,16 = 0.8 Hz, H-14), 5.71 (1H, ddd, J2,1α = 2.8 Hz, J2,3 = 10.0 Hz, J2,4β = 1.6 Hz, H-2), 5.69 (1H, dd, J3,2 = 10.0 Hz, J3,4β = 1.6 Hz, H-3), 5.50 (1H, dd, J1α,2 = 2.8 Hz, J1α,4β = 1.6 Hz, J1α,10β = 10.8 Hz, H-1α), 5.40 (1H, dd, J12β,11α = 0.4 Hz, J12β,16 = 0.8 Hz, H-12β), 5.10 (1H, d, J11α,12β = 0.4 Hz, H-11α), 4.23 (1H, d, J19a,19b = 9.2 Hz, pro-R H-19a), 4.18 (1H, dd, J19b,6β = 1.7 Hz, J19b,19a = 9.2 Hz, pro-S H-19b), 2.89 (1H, q, J4β,1α = J4β,2 = J4β,3 = 1.6 Hz, H-4β), 2.88 (1H, dd, J8β,7α = 12.6 Hz, J8β,7β = 3.8 Hz, H-8β), 2.45 (1H, d, J10β,1α = 10.8 Hz, H-10β), 2.33 (1H, dddd, J7β,6α = 3.2 Hz, J7β,6β = 3.6 Hz, J7β,7α = 14.8 Hz, J7β,8β = 3.8 Hz, H-7β), 2.22 (3H, s, 11β-OAc), 2.10 (3H, s, 1β-OAc), 2.07 (1H, dt, J6α,6β = 13.8 Hz, J6α,7α = J6α,7β = 3.2 Hz, H-6α), 1.71 (1H, dddd, J7α,6α = 3.2 Hz, J7α,6β = 14.0 Hz, J7α,7β = 14.8 Hz, J7α,8β = 12.6 Hz, H-7α), 1.42 (1H, dddd, J6β,6α = 13.8 Hz, J6β,7α = 14.0 Hz, J6β,7β = 3.6 Hz, J6β,19b = 1.7 Hz, H-6β), 0.80 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 173.8 (s, C-18), 170.6 (s, 1- OCOCH3), 170.3 (s, 11-OCOCH3), 169.9 (s, C-17), 143.9 (d, C-15), 138.9 (d, C-16), 130.7 (d, C-2), 124.9 (s, C-13), 122.4 (d, C-3), 108.0 (d, C-14), 79.2 (d, C-12), 76.2 (d, C-11), 69.8 (t, C-19), 68.2 (d, C-1), 52.2 (d, C-4), 43.1 (d, C-8), 42.0 (d, C-10), 41.7 (s, C-5), 39.2 (s, C-9), 34.2 (t, C-6), 21.4 (q, 1-OCOCH3), 21.2 (q, 11-OCOCH3), 18.2 (t, C-7) 15.1 (q, C-20); EIMS m/z 458 [M]+ (1), 398 (39), 356 (100), 323 (52), 203 (27), 189 (30), 176 (18), 161 (10), 129 (10), 110 (8), 95 (20), 91 (12), 81 (10); anal. C 62.59%, H 5.89%, calcd for C24H26O9, C 62.88%, H 5.72%.
4.6 Preparation of Compound 19 from Salvisplendin C (7)
A solution of 7 (100 mg, 0.257 mmol) in MeOH (1 mL) was treated with an excess of CH2N2 in Et2O at 0 °C. After 1 h, the solvent was removed in vacuo and pure pyrazoline derivative 19 was recovered as an amorphous solid: [α]D18 −26.4 (c 0.106, CHCl3); RF = 0.30 (silica gel, 3:2 EtOAc-petroleum ether); IR (KBr) νmax 3463, 3130, 2942, 1766, 1543, 1489, 1434, 1374, 1241, 1185, 1018, 969, 925, 875, 810, 755, 602 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.33 (1H, dd, J16,14 = 1.0 Hz, J16,15 = 1.8 Hz, H-16), 7.32 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.34 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 1.0 Hz, H-14), 5.68 (1H, dd, J12,11a = 6.7 Hz, J12,11b = 5.1 Hz, H-12), 5.45 (1H, d, J19a,19b = 9.0 Hz, pro-R H-19a), 4.63 (1H, d, J3′β,3′α = 16.8 Hz, H-3′β), 4.27 (1H, dd, J19b,6β = 2.0 Hz, J19b,19a = 9.0 Hz, pro-S H-19b), 4.23 (1H, dd, J3′α,3α = 6.0 Hz, J3′α,3′β = 16.8 Hz, H-3′α), 4.23 (1H, m, H-7β), 2.74 (1H, ddd, J6β,6α = 14.9 Hz, J6β,7β = 3.7 Hz, J6β,19b = 2.0 Hz, H-6β), 2.58 (1H, dd, J6α,6β = 14.9 Hz, J6α,7β = 2.6 Hz, H-6α), 2.29 (1H, m, H-1β), 2.26 (1H, m, H-1α), 2.18 (1H, ddd, J3α,2α = 6.3 Hz, J3α,2β = 13.1 Hz, J3α,3′α = 6.0 Hz, H-3α), 2.05 (1H, dd, J11a,11b = 15.8 Hz, J11a,12 = 6.7 Hz, H-11a), 1.97 (3H, s, 11-OAc), 1.79 (1H, qd, J8β,7β = 2.9 Hz, J8β,17 = 7.0 Hz, H-8β), 1.77 (1H, dd, J11b,11a = 15.8 Hz, J11b,12 = 5.1 Hz, H-11b), 1.67 (1H, dddd, J2α,1α = 2.7 Hz, J2α,1β = 3.1 Hz, J2α,2β = 13.4 Hz, J2α,3α = 6.3 Hz, H-2α), 1.37 (1H, dd, J10β,1α = 11.9 Hz, J10β,1β = 2.5 Hz, H-10β), 1.16 (3H, d, J17,8β = 7.0 Hz, Me-17), 0.88 (3H, s, Me-20), 0.33 (1H, qd, J2β,1α = J2β,2α = J2β,3α = 13.1 Hz, J2β,1β = 4.5 Hz, H-2β); 13C NMR (100 MHz, CDCl3) δ 170.7 (s, C-18), 169.9 (s, 12-OCOCH3), 143.4 (d, C-15), 140.1 (d, C-16), 125.7 (s, C-13), 108.7 (d, C-14), 95.7 (s, C-4), 81.8 (t, C-3′), 72.7 (d, C-7), 71.2 (t, C-19), 64.5 (d, C-12), 45.5 (s, C-5), 44.7 (d, C-10), 42.4 (t, C-11), 40.3 (d, C-8), 39.4 (s, C-9), 38.2 (t, C-6), 35.4 (d, C-3), 28.9 (t, C-2), 21.2 (q, 11-OCOCH3), 19.4 (q, C-20), 18.7 (t, C-1), 12.6 (q, C-17); EIMS m/z 430 [M]+ (3), 370 (1), 342 (2), 300 (4), 277 (6), 247 (19), 231 (15), 218 (33), 190 (35), 175 (29), 121 (28), 105 (28), 94 (100), 91 (34), 81 (27), 55 (21); anal. C 63.95%, H 7.19%, N 6.42%, calcd for C23H30N2O6, C 64.17%, H 7.02%, N 6.51%.
4.7 Procedure for the Acetylation of Compounds 9, 13, 15, and 19
Treatment of 9 (20 mg, 0.052 mmol), 13 (50 mg, 0.140 mmol), and 15 (50 mg, 0.134 mmol) with Ac2O-pyridine (1:2, 1 mL) for 24 h at room temperature followed by standard workup and purification by FC yielded quantitatively the acetyl derivatives 21 (19:1 CH2Cl2-acetone as eluent), 14 (elution with 32:1 CH2Cl2-acetone), and 16 (elution with 19:1 CH2Cl2-acetone), respectively. Compound 19 (20 mg, 0.052 mmol) was treated with Ac2O-pyridine (1:2, 3 mL) for one week at 40 °C. After standard workup the residue of the reaction was chromatographed (Si gel 230–400 mesh column, 20 g, 4:1 petroleum ether-EtOAc as eluent) yielding 20 (13.5 mg, 55%).
4.7.1 Compound 14
Ccolorless prisms (EtOAc-petroleum ether); mp 130–132 °C; [α]D18 −30.6 (c 0.435, CHCl3); RF = 0.40 (silica gel, 99:1 CH2Cl2-acetone); IR (KBr) νmax 3134, 3033, 2937, 2263, 1777, 1758, 1738, 1504, 1372, 1235, 1158, 1023, 962, 874, 799, 756, 741, 697, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.49 (1H, dd, J16,14 = 0.7 Hz, J16,15 = 1.8 Hz, H-16), 7.41 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.49 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.7 Hz, H-14), 5.99 (1H, ddt, J2,1α = J2,4β = 2.4 Hz, J2,1β = 4.8 Hz, J2,3 = 10.2 Hz, H-2), 5.91 (1H, d, J12,11β = 3.1 Hz, H-12), 5.60 (1H, dddd, J3,1α = 3.2 Hz, J3,1β = 1.2 Hz, J3,2 = 10.2 Hz, J3,4β = 3.4 Hz, H-3), 4.49 (1H, d, J11β,12 = 3.1 Hz, H-11β), 4.18 (1H, dd, J19a,6β = 1.6 Hz, J19a,19b = 9.0 Hz, pro-S H-19a), 4.14 (1H, d, J19b,19a = 9.0 Hz, pro-R H-19b), 2.73 (1H, tt, J4β,1α = J4β,3 = 3.4 Hz, J4β,1β = J4β,2 = 2.4 Hz, H-4β), 2.20 (1H, dddd, J7β,6α = 3.8 Hz, J7β,6β = 4.0 Hz, J7β,7α = 14.5 Hz, J7β,8α = 2.8 Hz, H-7β), 2.14 (1H, ddddd, J1α,1β = 18.0 Hz, J1α,2 = 2.4 Hz, J1α,3 = 3.2 Hz, J1α,4β = 3.4 Hz, J1α,10β = 11.8 Hz, H-1α), 2.10 (2H, m, H-1β and H-8α), 2.07 (3H, s, 12-OAc), 1.77 (1H, dtd, J6α,6β = 14.0 Hz, J6α,7α = J6α,7β = 3.8 Hz, J6α,8α = 1.4 Hz, H-6α), 1.73 (1H, dd, J10β,1α = 11.8 Hz, J10β,1β = 5.2 Hz, H-10β), 1.64 (1H, dddd, J7α,6α = 3.8 Hz, J7α,6β = 14.0 Hz, J7α,7β = 14.5 Hz, J7α,8α = 5.4 Hz, H-7α), 1.21 (1H, tdd, J6β,6α = J6β,7α = 14.0 Hz,, J6β,7β = 4.0 Hz, J6β,19a = 1.6 Hz, H-6β), 1.08 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 176.3 (s, C-17), 175.1 (s, C-18), 170.0 (s, 12-OCOCH3), 144.0 (d, C-15), 141.9 (d, C-16), 128.9 (d, C-2), 121.0 (d, C-3), 119.7 (s, C-13), 109.9 (d, C-14), 84.1 (d, C-11), 68.6 (t, C-19), 67.1 (d, C-12), 52.1 (d, C-4), 45.5 (d, C-8), 42.7 (s, C-9), 40.9 (s, C-5), 40.8 (d, C-10), 31.0 (t, C-6), 22.7 (t, C-1), 21.1 (q, 12-OCOCH3), 16.6 (q, C-20), 16.5 (t, C-7); EIMS m/z 400 [M]+ (2), 358 (30), 340 (19), 304 (32), 262 (100), 233 (24), 203 (7), 187 (6), 157 (16), 139 (35), 129 (14), 117 (18), 105 (14), 97 (28), 91 (29), 81 (8); anal. C 66.15%, H 6.01%, calcd for C22H24O7, C 65.99%, H 6.04%.
4.7.2 Compound 16
Amorphous, white powder; [α]D18 −78.9 (c 0.503, CHCl3); RF = 0.35 (silica gel, 99:1 CH2Cl2-acetone); IR (KBr) νmax 3146, 2947, 1781, 1740, 1503, 1373, 1234, 1168, 1024, 962, 915, 875, 800, 756, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.47 (1H, dd, J16,14 = 0.9 Hz, J16,15 = 1.8 Hz, H-16), 7.39 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 6.42 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.9 Hz, H-14), 5.89 (1H, d, J12,11β = 6.3 Hz, H-12), 5.75 (1H, ddd, J2,1α = 2.0 Hz, J2,3 = 10.0 Hz, J2,4β = 2.6 Hz, H-2), 5.69 (1H, ddd, J3,1α = 2.0 Hz, J3,2 = 10.0 Hz, J3,4β = 2.6 Hz, H-3), 5.65 (1H, ddt, J1α,2 = J1α,3 = 2.0 Hz, J1α,4β = 2.6 Hz, J1α,10β = 10.8 Hz, H-1α), 5.18 (1H, d, J11β,12 = 6.3 Hz, H-11β), 4.24 (1H, d, J19a,19b = 9.2 Hz, pro-R H-19a), 4.21 (1H, dd, J19b,6β = 1.6 Hz, J19b,19a = 9.2 Hz, pro-S H-19b), 2.81 (1H, q, J4β,1α = J4β,2 = J4β,3 = 2.6 Hz, H-4β), 2.32 (1H, ddd, J8α,6α = 1.2 Hz, J8α,7α = 4.4 Hz, J8α,7β = 2.8 Hz, H-8α), 2.17 (3H, s, 1β-OAc), 2.11 (1H, d, J10β,1α = 10.8 Hz, H-10β), 2.10 (1H, dddd, J7β,6α = 3.6 Hz, J7β,6β = 4.0 Hz, J7β,7α = 14.4 Hz, J7β,8α = 2.8 Hz, H-7β), 2.02 (3H, s, 12-OAc), 1.76 (1H, dtd, J6α,6β = 13.8 Hz, J6α,7α = J6α,7β = 3.6 Hz, J6α,8α = 1.2 Hz, H-6α), 1.71 (1H, dddd, J7α,6α = 3.6 Hz, J7α,6β = 13.6 Hz, J7α,7β = 14.4 Hz, J7α,8α = 4.4 Hz, H-7α), 1.27 (1H, dddd, J6β,6α = 13.8 Hz, J6β,7α = 13.6 Hz, J6β,7β = 4.0 Hz, J6β,19b = 1.6 Hz, H-6β), 1.11 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 175.8 (s, C-17), 173.6 (s, C-18), 169.8 (s, 1β-OCOCH3), 169.2 (s, 12-OCOCH3), 143.9 (d, C-15), 142.0 (d, C-16), 131.0 (d, C-2), 122.4 (d, C-3), 120.6 (s, C-13), 109.3 (d, C-14), 83.7 (d, C-11), 68.7 (t, C-19), 68.3 (d, C-1), 65.3 (d, C-12), 51.9 (d, C-4), 45.2 (d, C-8), 43.0 (d, C-10), 42.5 (s, C-9), 41.5 (s, C-5), 30.4 (t, C-6), 21.3 (q, 1β-OCOCH3), 21.0 (q, 12-OCOCH3), 17.2 (q, C-20), 16.3 (t, C-7); EIMS m/z 458 [M]+ (1), 416 (3), 398 (100), 356 (85), 320 (20), 277 (17), 259 (57), 231 (17), 222 (17), 203 (28), 157 (20), 143 (44), 139 (48), 135 (55), 117 (26), 105 (14), 97 (420), 91 (28), 81 (14); anal. C 62.69%, H 5.84%, calcd for C24H26O9, C 62.88%, H 5.72%.
4.7.3 Compound 20
Amorphous, white solid; [α]D20 –38.2 (c 0.204, CHCl3); RF = 0.55 (silica gel, 3:2 EtOAc-petroleum ether); IR (KBr) νmax 3135, 2947, 1774, 1738, 1545, 1434, 1371, 1234, 1180, 1020, 976, 875, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.33 (2H, m, H-15 and H-16), 6.33 (1H, dd, J14,15 = 2.0 Hz, J14,16 = 1.0 Hz, H-14), 5.67 (1H, dd, J12,11a = 7.4 Hz, J12,11b = 4.7 Hz, H-12), 5.45 (1H, ddd, J7β,6α = 2.5 Hz, J7β,6β = 4.0 Hz, J7β,8β = 3.5 Hz, H-7β), 4.99 (1H, d, J19a,19b = 9.3 Hz, pro-R H-19a), 4.66 (1H, d, J3′β,3′α = 17.0 Hz, H- 3′β), 4.31 (1H, dd, J19b,6β = 2.1 Hz, J19b,19a = 9.3 Hz, pro-S H-19b), 4.24 (1H, dd, J3′α,3α = 6.0 Hz, J3′α,3′β = 17.0 Hz, H-3′α), 2.86 (1H, ddd, J6β,6α = 15.6 Hz, J6β,7β = 4.0 Hz, J6β,19b = 2.1 Hz, H-6β), 2.54 (1H, dd, J6α,6β = 15.6 Hz, J6α,7β = 2.5 Hz, H-6α), 2.20 (1H, ddd, J3α,2α = 6.6 Hz, J3α,2β = 12.7 Hz, J3α,3′α = 6.0 Hz, H-3α), 2.12 (3H, s, 7α-OAc), 2.07 (1H, dd, J11a,11b = 16.0 Hz, J11a,12 = 7.4 Hz, H-11a), 1.97 (3H, s, 11-OAc), 1.95 (1H, qd, J8β,7β = 3.5 Hz, J8β,17 = 7.0 Hz, H-8β), 1.78 (1H, dd, J11b,11a = 16.0 Hz, J11b,12 = 4.7 Hz, H-11b), 1.71 (1H, dddd, J2α,1α = 2.3 Hz, J2α,1β = 3.5 Hz, J2α,2β = 13.5 Hz, J2α,3α = 6.6 Hz, H-2α), 1.42 (1H, dd, J10β,1α = 12.0 Hz, J10β,1β = 2.4 Hz, H- 10 β), 1.35 (1H, dddd, J1β,1α = 13.0 Hz, J1β,2α = 3.5 Hz, J1β,2β = 4.0 Hz, J1β,10β = 2.4 Hz, H-1β), 1.26 (1H, dddd, J1α,1β = 13.0 Hz, J1α,2α = 2.3 Hz, J1α,2β = 12.6 Hz, J1α,10β = 12.0 Hz, H-1α), 1.04 (3H, d, J17,8β = 7.0 Hz, Me-17), 0.84 (3H, s, Me-20), 0.40 (1H, dddd, J2β,1α = 12.6 Hz, J2β,1β = 4.0 Hz, J2β,2α = 13.5 Hz, J2β,3α = 12.7 Hz, H-2β); 13C NMR (100 MHz, CDCl3) δ 170.0 (s, C-18), 169.82 (s, 12-OCOCH3), 169.78 (s, 7-OCOCH3), 143.5 (d, C-15), 140.1 (d, C-16), 125.6 (s, C-13), 108.6 (d, C-14), 95.2 (s, C-4), 81.8 (t, C-3′), 73.4 (d, C-7), 70.6 (t, C-19), 64.4 (d, C-12), 45.3 (s, C-5), 44.4 (d, C-10), 42.0 (t, C-11), 39.4 (d, C-8), 39.3 (s, C-9), 35.8 (t, C-6), 35.4 (d, C-3), 28.8 (t, C-2), 21.4 (q, 7-OCOCH3), 21.2 (q, 12-OCOCH3), 19.1 (q, C-20), 18.7 (t, C-1), 12.0 (q, C-17); EIMS m/z 472 [M]+ (1), 430 (1), 412 (1), 402 (3), 342 (7), 260 (30), 249 (37), 231 (49), 185 (33), 172 (42), 157 (53), 119 (48), 111 (47), 94 (100), 81 (31), 55 (18); anal. C 63.63%, H 6.71%, N 5.79%, calcd for C25H32N2O7, C 63.54%, H 6.83%, N 5.93%.
4.7.4 Compound 21
Colorless fine needles (EtOAc – n-pentane); mp 222-224 °C; [α]D20 –54.6 (c 0.509, CHCl3); RF = 0.42 (silica gel, 95:5 CH2Cl2-acetone); IR (KBr) νmax 3127, 3018, 2966, 2928, 1782, 1741, 1542, 1500, 1459, 1373, 1240, 1202, 1151, 1045, 1025, 965, 910, 874, 797, 719, 600 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.35 (1H, t, J15,14 = J15,16 = 1.5 Hz, H-15), 7.34 (1H, dd, J16,14 = 0.8 Hz, J16,15 = 1.5 Hz, H-16), 6.32 (1H, dd, J14,15 = 1.5 Hz, J14,16 = 0.8 Hz, H-14), 4.95 (1H, dd, J12β,11α = 9.9 Hz, J12β,11β = 7.1 Hz, H-12β), 4.91 (1H, d, J3′β,3′α = 17.3 Hz, H-3′β), 4.50 (1H, d, J19a,19b = 9.4 Hz, pro-R H-19a), 4.32 (1H, dd, J19b,6β = 2.0 Hz, J19b,19a = 9.4 Hz, pro-S H-19b), 4.30 (1H, dd, J3′α,3α = 6.1 Hz, J3′α,3′β = 17.3 Hz, H-3′α), 4.14 (1H, ddd, J2β,1α = 11.4 Hz, J2β,1β = 4.3 Hz, J2β,3α = 10.4 Hz, H-2β), 2.76 (1H, dddd, J6β,6α = 14.0 Hz, J6β,7α = 13.4 Hz, J6β,7β = 3.3 Hz, J6β,19b = 2.0 Hz, H-6β), 2.31 (1H, dd, J3α,2β = 10.4 Hz, J3α,3′α = 6.1 Hz, H-3α), 2.25 (1H, dd, J11β,11α = 13.2 Hz, J11β,12β = 7.1 Hz, H-11β), 2.12 (2H, m, H-6α and H-7β), 2.08 (3H, s, 2α-OAc), 1.89 (1H, ddd, J1β,1α = 12.7 Hz, J1β,2β = 4.3 Hz, J1β,10β = 2.2 Hz, H-1β), 1.83 (1H, dd, J11α,11β = 13.2 Hz, J11α,12β = 9.9 Hz, H-11α), 1.75 (1H, ddd, J7α,6α = 3.8 Hz, J7α,6β = 13.4 Hz, J7α,7β = 15.5 Hz, H-7α), 1.70 (1H, dd, J10β,1α = 13.0 Hz, J10β,1β = 2.2 Hz, H-10β), 1.42 (1H, ddd, J1α,1β = 12.7 Hz, J1α,2β = 11.4 Hz, J1α,10β = 13.0 Hz, H-1α), 1.18 (3H, s, Me-17), 0.83 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 170.4 (s, 2-OCOCH3), 169.3 (s, C-18), 143.3 (d, C-15), 139.0 (d, C-16), 128.2 (s, C-13), 108.7 (d, C-14), 96.6 (s, C-4), 83.1 (s, C-8), 79.7 (t, C-3′), 73.8 (d, C-2), 69.9 (d, C-12), 68.5 (t, C-19), 47.1 (s, C-9), 45.6 (s, C-5), 44.5 (t, C-11), 41.0 (d, C-3), 39.6 (d, C-10), 29.7 (t, C-7), 27.143 (2 d, C-1 and C-6), 26.9 (q, C-17), 21.1 (q, 2-OCOCH3), 17.2 (q, C-20); EIMS m/z 428 [M]+ (0.2), 413 (3), 400 (4), 385 (33), 368 (4), 358 (8), 340 (3), 325 (3), 309 (5), 269 (10), 229 (16), 164 (31), 121 (100), 105 (26), 95 (42), 91 (39), 81 (45), 55 (19); anal. C 64.52%, H 6.71%, N 6.41%, calcd for C23H28N2O6, C 64.47%, H 6.59%, N 6.54%.
4.8 Benzoylation of Compound 9 to Give Compound 22
120 μL (145 mg, 1.03 mmol) of benzoyl chloride were added dropwise via syringe, at 0 °C under Ar, to a solution of 40 mg (0.103 mmol) of 9 and a little crystal of 4-DMAP in 750 μL of dry 2:1 CH2Cl2-pyridine. The solution was warmed at room temperature and left to stir for 24 h. Afterward, the solution was carefully poured into a crushed-ice/NaHCO3 mixture (5 mL). Extraction with EtOAc (4×5 mL) was followed by removal of residual pyridine by washing with HCl 6 N and subsequent neutralization of the combined organic phase with an aqueous saturated solution of NaHCO3. Finally, the organic phase was dried (Na2SO4) and the solvent was removed by distillation in vacuo, giving a crude of reaction that was purified by FC (39:1 CH2Cl2-acetone as eluent) to give pure 22 (25 mg, 50%): amorphous, white solid; [α]D20 –37.1 (c 0.197, CHCl3); RF = 0.35 (silica gel, 95:5 CH2Cl2-acetone); IR (KBr) νmax 3066, 2974, 2928, 1780, 1719, 1602, 1451, 1376, 1272, 1176, 1153, 1116, 1026, 909, 874, 716, 600 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.36 (2H, m, H-15, and H-16), 6.34 (1H, t, J14,15 = J14,16 = 1.5 Hz, H-14), 5.01 (1H, d, J3′β,3′α = 17.4 Hz, H-3′β), 5.01 (1H, dd, J12β,11α = 9.9 Hz, J12β,11β = 7.1 Hz, H-12β), 4.54 (1H, d, J19a,19b = 9.4 Hz, pro-R H-19a), 4.42 (1H, ddd, J2β,1α = 11.3 Hz, J2β,1β = 4.3 Hz, J2β,3α = 10.3 Hz, H-2β), 4.39 (1H, dd, J19b,6β = 1.9 Hz, J19b,19a = 9.4 Hz, pro-S H-19b), 4.36 (1H, dd, J3′α,3α = 6.1 Hz, J3′α,3′β = 17.4 Hz, H-3′α), 2.81 (1H, dddd, J6β,6α = 14.3 Hz, J6β,7α = 13.3 Hz, J6β,7β = 3.9 Hz, J6β,19b = 1.9 Hz, H-6β), 2.51 (1H, dd, J3α,2β = 10.3 Hz, J3α,3′α = 6.1 Hz, H-3α), 2.31 (1H, dd, J11β,11α = 13.2 Hz, J11β,12β = 7.1 Hz, H-11β), 2.17 (2H, m, H-6α and H-7β), 2.05 (1H, ddd, J1β,1α = 12.8 Hz, J1β,2β = 4.3 Hz, J1β,10β = 2.1 Hz, H-1β), 1.86 (1H, dd, J11α,11β = 13.2 Hz, J11α,12β = 9.9 Hz, H-11α), 1.81 (1H, dd, J10β,1α = 12.9 Hz, J10β,1β = 2.1 Hz, H-10β), 1.78 (1H, ddd, J7α,6α = 3.9 Hz, J7α,6β = 13.3 Hz, J7α,7β = 15.0 Hz, H-7α), 1.56 (1H, ddd, J1α,1β = 12.8 Hz, J1α,2β = 11.3 Hz, J1α,10β = 12.9 Hz, H-1α), 1.20 (3H, s, Me-17), 0.85 (3H, s, Me-20), 2α-benzoate 8.02 (2H, dd, J = 8.3, 1.5 Hz, H-2″ and H-6″), 7.61 (1H, tt, J = 8.0, 1.5 Hz, H-4″), 7.47 (2H, br t, J = 8.2 Hz, H-3″ and H-5″); 13C NMR (100 MHz, CDCl3) δ 169.4 (s, C-18), 143.4 (d, C-15), 139.0 (d, C-16), 128.3 (s, C-13), 108.7 (d, C-14), 96.7 (s, C-4), 83.2 (s, C-8), 79.7 (t, C-3′), 74.4 (d, C-2), 70.0 (d, C-12), 68.5 (t, C-19), 47.2 (s, C-9), 45.7 (s, C-5), 44.6 (t, C-11), 41.2 (d, C-3), 39.8 (d, C-10), 29.7 (t, C-7), 27.3 (t, C-1), 27.2 (t, C-6), 26.9 (q, C-17), 17.2 (q, C-20), 2-benzoate 165.9 (s, C-7), 133.7 (d, C-4″), 129.6 (2 d, C-2″ and C-6″), 129.3 (s, C-1″), 128.6 (2 d, C-3″ and C-5″); EIMS m/z 490 [M]+ (1), 475 (1), 462 (3), 447 (5), 368 (2), 164 (8), 121 (21), 105 (100), 95 (10), 91 (9), 77 (32); anal. C 68.38%, H 6.41%, N 5.59%, calcd for C28H30N2O6, C 68.56%, H 6.16%, N 5.71%.
4.9 Preparation of Compound 23 from Compound 13
A solution of 13 (60 mg, 0.167 mmol), 1,1′-thiocarbonyldiimidazole (100 mg, 0.561 mmol), and 4-DMAP (4 mg, 0.033 mmol) in dry CH2Cl2 (7 mL) was refluxed under Ar for 2 h. Then, the solvent was distilled in vacuo and the solid residue was purified by FC (3:2 petroleum ether-EtOAc as eluent) to give pure 23 (62 mg, 80%): vitreous, yellowish solid; [α]D20 –9.5 (c 0.042, CHCl3); RF = 0.35 (silica gel, 3:2 petroleum ether-EtOAc); IR (KBr) νmax 3127, 3031, 2916, 1776, 1632, 1527, 1503, 1444, 1295, 1155, 1117, 1062, 1024, 964, 873, 794, 746, 664, 603 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.51 (1H, dd, J16,14 = 0.9 Hz, J16,15 = 1.9 Hz, H-16), 7.43 (1H, t, J15,14 = J15,16 = 1.9 Hz, H-15), 6.44 (1H, dd, J14,15 = 1.9 Hz, J14,16 = 0.9 Hz, H-14), 6.00 (1H, ddt, J2,1α = J2,4β = 2.4 Hz, J2,1β = 5.0 Hz, J2,3 = 10.1 Hz, H-2), 5.60 (1H, dddd, J3,1α = 3.0 Hz, J3,1β = 1.3 Hz, J3,2 = 10.1 Hz, J3,4β = 3.4 Hz, H-3), 5.56 (1H, d, J12,11β = 1.2 Hz, H-12), 4.66 (1H, d, J11β,12 = 1.2 Hz, H-11β), 4.20 (1H, dd, J19a,6β = 1.6 Hz, J19a,19b = 9.0 Hz, pro-S H-19a), 4.14 (1H, d, J19b,19a = 9.0 Hz, pro-R H-19b), 2.71 (1H, ddt, J4β,1α = 2.8 Hz, J4β,1β = J4β,2 = 2.4 Hz, J4β,3 = 3.4 Hz, H-4β), 2.18 (1H, m, H-1α), 2.14 (1H, m, H-1β), 1.98 (1H, m, H-7β), 1.94 (1H, br dd, J8α,7α = 5.2 Hz, J8α,7β = 2.1 Hz, H-8α), 1.75 (1H, br dt, J6α,6β = 14.0 Hz, J6α,7α = J6α,7β = 3.2 Hz, H-6α), 1.69 (1H, dd, J10β,1α = 11.8 Hz, J10β,1β = 5.0 Hz, H-10β), 1.59 (1H, dddd, J7α,6α = 3.2 Hz, J7α,6β = 13.5 Hz, J7α,7β = 14.4 Hz, J7α,8α = 5.2 Hz, H-7α), 1.75 (1H, br dt, J6α,6β = 14.0 Hz, J6α,7α = J6α,7β = 3.2 Hz, H-6α), 1.23 (3H, s, Me-20), 1.18 (1H, dddd, J6β,6α = 14.0 Hz, J6β,7α = 13.5 Hz, J6β,7β = 3.9 Hz, J6β,19a = 1.6 Hz, H-6β), 1H-imidazole-1-carbothioate 7.69 (1H, s, H-2′), 7.10 (2H, s, H-4′ and H-5′); 13C NMR (100 MHz, CDCl3) δ 176.4 (s, C-17), 175.1 (s, C-18), 144.5 (d, C-15), 142.0 (d, C-16), 128.9 (d, C-2), 121.0 (d, C-3), 117.6 (s, C-13), 110.7 (d, C-14), 85.6 (d, C-11), 68.5 (t, C-19), 52.2 (d, C-4), 47.3 (d, C-12), 46.1 (s, C-9), 44.1 (d, C-8), 41.3 (s, C-5), 41.1 (d, C-10), 31.0 (t, C-6), 22.8 (t, C-1), 16.9 (q, C-20), 16.6 (t, C-7), 1H-imidazole-1-carbothioate 223.4 (s, C=S), 134.9 (d, C-2′), 132.0 (d, C-4′), 121.9 (d, C-5′); EIMS m/z [M]+ absent, 453 [M-CH3]+ (1), 421 (2), 374 (29), 372 (25), 356 (6), 340 (80), 261 (72), 203 (22), 157 (30), 145 (39), 129 (33), 113 (84), 108 (86), 91 (100), 81 (43), 76 (53), 65 (17), 53 (20); anal. C 61.28%, H 5.02%, N 6.03%, S 6.91%, calcd for C24H24N2O6S, C 61.52%, H 5.16%, N 5.98%, S 6.84%.
4.10 Preparation of Compound 24 from Compound 23
A solution of 23 (50 mg, 0.107 mmol), 150μL (162 mg, 0.535 mmol) of 96% n-Bu3SnH, and 2,2′-azo-bis-isobutyronitrile (AIBN, 3 mg, 0.018 mmol) in toluene (5 mL) was heated at reflux under Ar for 6 h. Then, the mixture was evaporated to a little volume and partitioned between acetonitrile and n-hexane (5 mL each). The acetonitrile layer was washed with n-hexane (4×5 mL) to remove the residual tin compounds. The acetonitrile phase was distilled in vacuo and the residue was purified by FC (39:1 acetone-CH2Cl2 as eluent) to give 24 (16 mg, 45 %): colorless rectangular plaques (EtOAc – n-pentane); mp 211-214 °C; [α]D20 –91.3 (c 0.196, CHCl3); RF = 0.50 (silica gel, 3:2 petroleum ether-EtOAc); IR (KBr) νmax 3142, 3107, 2963, 2860, 1780, 1766, 1502, 1450, 1352, 1296, 1176, 1152, 1035, 966, 873, 785, 702, 602 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.36 (1H, t, J15,14 = J15,16 = 1.8 Hz, H-15), 7.31 (1H, ddd, J16,12b = 1.0 Hz, J16,14 = 0.8 Hz, J16,15 = 1.8 Hz, H-16), 6.34 (1H, dd, J14,15 = 1.8 Hz, J14,16 = 0.8 Hz, H-14), 5.97 (1H, ddt, J2,1α = J2,4β = 2.4 Hz, J2,1β = 5.0 Hz, J2,3 = 10.0 Hz, H-2), 5.60 (1H, dddd, J3,1α = 3.1 Hz, J3,1β = 1.3 Hz, J3,2 = 10.0 Hz, J3,4β = 3.4 Hz, H-3), 4.35 (1H, dd, J11β,12a = 4.0 Hz, J11β,12b = 9.2 Hz, H-11β), 4.19 (2H, s, H-19a and H-19b), 2.74 (1H, tt, J4β,1α = J4β,3 = 3.4 Hz, J4β,1β = J4β,2 = 2.4 Hz, H-4β), 2.71 (1H, br dd, J12a,11β = 4.0 Hz, J12a,12b = 15.2 Hz, H-12a), 2.61 (1H, ddd, J12b,11β = 9.2 Hz, J12b,12a = 15.2 Hz, J12b,16 = 1.0 Hz, H-12b), 2.54 (1H, ddd, J8α,6α = 1.2 Hz, J8α,7α = 5.1 Hz, J8α,7β = 2.3 Hz, H-8α), 2.16 (1H, dddd, J7β,6α = 3.8 Hz, J7β,6β = 4.0 Hz, J7β,7α = 14.8 Hz, J7β,8α = 2.3 Hz, H-7β), 2.09 (1H, m, H-1β), 2.05 (1H, m, H-1α), 1.82 (1H, m, H-6α), 1.79 (1H, dd, J10β,1α = 11.1 Hz, J10β,1β = 5.3 Hz, H-10β), 1.77 (1H, dddd, J7α,6α = 4.1 Hz, J7α,6β = 13.6 Hz, J7α,7β = 14.8 Hz, J7α,8α = 5.1 Hz, H-7α), 1.27 (1H, ddd, J6β,6α = 14.2 Hz, J6β,7α = 13.6 Hz, J6β,7β = 4.0 Hz, H-6β), 1.03 (3H, s, Me-20); 13C NMR (100 MHz, CDCl3) δ 176.2 (s, C-17), 175.3 (s, C-18), 143.2 (d, C-15), 140.2 (d, C-16), 128.8 (d, C-2), 121.1 (d, C-3), 119.7 (s, C-13), 111.3 (d, C-14), 83.8 (d, C-11), 68.7 (t, C-19), 52.1 (d, C-4), 45.6 (d, C-8), 42.9 (s, C-9), 41.0 (s, C-5), 39.8 (d, C-10), 31.2 (t, C-6), 25.9 (t, C-12), 22.7 (t, C-1), 17.2 (q, C-20), 16.7 (t, C-7); EIMS m/z 342 [M]+ (56), 327 (1), 261 (100), 233 (7), 215 (7), 203 (8), 157 (24), 145 (24), 117 (28), 105 (27), 91 (57), 81 (34), 53 (17); anal. C 70.51%, H 6.31%, calcd for C20H22O5, C 70.16%, H 6.48%.
4.11 Biological Assays
As described,22 the recombinant CHO cells (hMOP-CHO, hDOP-CHO and hKOP-CHO) were produced by stable transfection with the respective human opioid receptor cDNA, and provided by Dr. Larry Toll (SRI International, CA). The cells were grown on plastic flasks in DMEM (90%) (hDOP-CHO and hKOP-CHO) or DMEM/F-12 (45%/45%) medium (hMOP-CHO) containing 10% FetalClone II (HyClone) and Geneticin (G- 418: 0.10-0.2 mg/ml) (Invitrogen) under 95% air/5% CO2 at 37° C. Cell monolayers were harvested and frozen in −80 °C.
4.11.1 Opioid binding assays
We used either [125I]IOXY (6β-iodo-3,14-dihydroxy-17-cyclopropylmethyl-4,5α-epoxymorphinan) (SA=2200 Ci/mmol) to label μ,δ and κ binding sites [20] or [3H][D-Ala2-MePhe4, Gly-ol5]enkephalin ([3H]DAMGO, SA=44-48 Ci/mmol) to label MOP, [3H][D-Ala2, D- Leu5]enkephalin ([3H]DADLE, SA=40-50 Ci/mmol) to label DOP and [3H](-)-U69,593 (SA=50 Ci/mmol) to label KOP binding sites. On the day of the assay, cell pellets were thawed on ice for 15 minutes then homogenized with a polytron in 10 ml/pellet of ice-cold 10mM Tris-HCl, pH 7.4. Membranes were then centrifuged at 30,000 × g for 10 minutes, resuspended in 10 ml/pellet ice-cold 10mM Tris-HCl, pH 7.4 and again centrifuged 30,000 × g for 10 min. Membranes were then resuspended in 25 °C 50 mM Tris-HCl, pH 7.4 (~100 ml/pellet hMOP-CHO, 50 ml/pellet hDOP-CHO and 120 ml/pellet hKOP-CHO). All assays took place in 50 mM Tris-HCl, pH 7.4, with a protease inhibitor cocktail [bacitracin (100 μg/mL), bestatin (10 μg/mL), leupeptin (4 μg/mL) and chymostatin (2 μg/mL)], in a final assay volume of 1.0 mL. All drug dilution curves were made up with buffer containing 1 mg/mL BSA. Nonspecific binding was determined using 10 μM naloxone (for [125I]IOXY binding) or 20 μM levallorphan ([3H]DAMGO and [3H]DADLE) or 10 μM (-)-U69,593 (for [3H]U69,593 binding). [125I]IOXY concentrations were ~ 10 pM. The other [3H]radioligands were used at ~ 2 nM concentrations. Triplicate samples were filtered with Brandell Cell Harvesters (Biomedical Research & Development Inc., Gaithersburg, MD), over Whatman GF/B filters, after a 2 hr incubation at 25 °C. For [125I]IOXY binding, the filters were punched into 12 × 75 mm glass test tubes and counted in a Micromedic gamma counter at 80% efficiency. For [3H]DAMGO, [3H]DADLE and [3H]-(-)U69,593 binding, the filters were punched into 24-well plates to which was added 0.6 ml of LSC-cocktail (Cytoscint). Samples were counted, after an overnight extraction, in a Trilux liquid scintillation counter at 44% efficiency. Opioid binding assays had ~30 μg protein per assay tube. Inhibition curves were generated by displacing a single concentration of radioligand by 10 concentrations of drug.
4.11.2 [35S]GTP-γ-S binding assays
The [35S]-GTP-γ-S assays were conducted as described elsewhere.22 In this description, buffer “A” is 50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA and buffer “B” is buffer A plus 1.67 mM DTT and 0.15% BSA. On the day of the assay, cells were thawed on ice for 15 min and homogenized using a polytron in 50 mM Tris-HCl, pH 7.4, containing 4 μg/mL leupeptin, 2 μg/mL chymostatin, 10 μg/mL bestatin and 100 μg/mL bacitracin, The homogenate was centrifuged at 30,000 × g for 10 min at 4 °C, and the supernatant discarded. The membrane pellets were resuspended in buffer B and used for [35S]GTP-γ-S binding assays. [35S]GTP-γ-S binding was determined as described previously. Briefly, test tubes received the following additions: 50 μL buffer A plus 0.1% BSA, 50 μL GDP in buffer A/0.1% BSA (final concentration = 40 μM), 50 μL drug in buffer A/0.1% BSA, 50 μL [35S]GTP-γ-S in buffer A/0.1% BSA (final concentration = 50 pM), and 300 μL of cell membranes (50 μg of protein) in buffer B. The final concentrations of reagents in the [35S]GTP-γ-S binding assays were: 50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM DTT, 40 μM GDP and 0.1% BSA. Incubations proceeded for 3 hr at 25 °C. Nonspecific binding was determined using GTP-γ-S (40 μM). Bound and free [35S]-GTP-γ-S were separated by vacuum filtration through GF/B filters. The filters were punched into 24-well plates to which was added 0.6 mL LSC-cocktail (Cytoscint). Samples were counted, after an overnight extraction, in a Trilux liquid scintillation counter at 27% efficiency.
4.11.3 Data Analysis and Statistics
These methods are described elsewhere.22–24 For the [35S]GTP-γ-S binding experiments, the percent stimulation of [35S]GTP-γ-S binding was calculated according to the following formula: (S − B)/B × 100, where B is the basal level of [35S]GTP-γ-S binding and S is the stimulated level of [35S]GTP-γ-S binding. Agonist dose-response curves (ten points/curve) are generated, and the data of several experiments, typically 3, are pooled. The EC50 values (the concentration that produces fifty percent maximal stimulation of [35S]GTP-γ-S binding) and Emax are determined using either the program MLAB-PC (Civilized Software, Bethesda, MD), KaleidaGraph (Version 3.6.4, Synergy Software, Reading, PA) or Prism 4.0 (GraphPad Software, Inc, San Diego, CA). In most cases, the percent stimulation is reported as a percent of the maximal stimulation as determined with 1000 nM DAMGO, 500 nM SNC80 or 500 nM (-)-U50,488 in the appropriate cell type. For determination of Ke values using the “shift” experimental design, agonist (DAMGO, (-)-U50,488 or SNC80) dose-response curves are generated, using the appropriate cell type, in the absence and presence (ten points/curve) of a test agent. The data of several experiments, typically 3, are pooled, and the Ke values were calculated according to the equation: [Test Drug]/(EC50-2/EC50-1 − 1), where EC50-2 is the EC50 value in the presence of the test drug and EC50-1 is the value in the absence of the test drug. For opioid binding experiments, the pooled data of three experiments (typically 30 data points) were fit to the two-parameter logistic equation for the best-fit estimates of the IC50 and N values: Y=100/(1+([INHIBITOR]/IC50)N), where “Y” is the percent of control value. Ki values for test drugs are calculated according to the standard equation: Ki = IC50/(1+[radioligand]/Kd]). For the [125I]IOXY experiments, the concentration of [125I]IOXY was far below its Kd values for MOP, KOP or DOP, so the IC50 values equaled the Ki values. For the [3H]radioligands, the following Kd values (nM±SD, n=3) were used in the Ki calculation: [3H]DAMGO (0.93±0.04), [3H]DADLE (1.9±0.3) and [3H](-)-U69,593 (11±0.6). The corresponding Bmax values were (fmol/mg protein±SD, n=3): [3H]DAMGO (1912±68), [3H]DADLE (3655±391) and [3H](-)-U69,593 (3320±364).
4.11.4 Drugs
Salvinorin-A related compounds were made up in 100% DMSO + 14.3 M 2- mercaptoethanol to produce a 10 mM solution. Compounds were then aliquoted into 50 μL volumes in microfuge tubes and frozen at −80° C until the day of the assay. Any leftover compound was discarded.
Figure 1.
Acknowledgments
This work was supported in part by funds from the Spanish ‘Comision Interministerial de Ciencia y Tecnologia’ (CICYT), grant No. CTQ2006-15279-C02-02, Italian ‘Universita degli Studi di Palermo’, grant ‘Ex 60%-2005’ and R01DA018151 (to TEP) from the National Institute on Drug Abuse. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
6. References and Notes
- 1.For a summary on the history and ethnopharmacology of Salvia divinorum, and for the chemistry and pharmacology of salvinorin A, see Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. J Med Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m.
- 2.Beguin C, Richards MR, Wang Y, Chen Y, Liu-Chen L-Y, Ma Z, Lee DYW, Carlezon WA, Jr, Cohen BM. Bioorg Med Chem Lett. 2005;15:2761–2765. doi: 10.1016/j.bmcl.2005.03.113. [DOI] [PubMed] [Google Scholar]
- 3.Lee DYW, Karnati VVR, He M, Liu-Chen L-Y, Kondaveti L, Ma Z, Wang Y, Chen Y, Beguin C, Carlezon WA, Jr, Cohen B. Bioorg Med Chem Lett. 2005;15:3744–3747. doi: 10.1016/j.bmcl.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Lee DYW, He M, Kondaveti L, Liu-Chen L-Y, Ma Z, Wang Y, Chen Y, Li J-G, Beguin C, Carlezon WA, Jr, Cohen B. Bioorg Med Chem Lett. 2005;15:4169–4173. doi: 10.1016/j.bmcl.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 5.Munro TA, Rizzacasa MA, Roth BL, Toth BA, Yan F. J Med Chem. 2005;48:345–348. doi: 10.1021/jm049438q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harding WW, Schmidt M, Tidgewell K, Kannan P, Holden KG, Dersch CM, Rothman RB, Prisinzano TE. Bioorg Med Chem Lett. 2006;16:3170–3174. doi: 10.1016/j.bmcl.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 7.Kutrzeba L, Dayan FE, Howell JL, Feng J, Giner JL, Zjawiony JK. Phytochemistry. 2007;68:1872–1881. doi: 10.1016/j.phytochem.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheerer JR, Lawrence JF, Wang GC, Evans DA. J Am Chem Soc. 2007;129:8968–8969. doi: 10.1021/ja073590a. [DOI] [PubMed] [Google Scholar]
- 9.Fontana G, Savona G, Rodríguez B. J Nat Prod. 2006;69:1734–1738. doi: 10.1021/np068036d. [DOI] [PubMed] [Google Scholar]
- 10.Savona G, Paternostro MP, Piozzi F, Hanson JR, Hitchcock PB, Thomas SA. J Chem Soc, Perkin Trans. 1978;1:643–646. [Google Scholar]
- 11.Savona G, Paternostro MP, Piozzi F, Hanson JR. J Chem Soc, Perkin Trans. 1979;1:533–534. [Google Scholar]
- 12.Hu DP, Kawazoe K, Takaishi Y. Phytochemistry. 1997;46:781–784. [Google Scholar]
- 13.Fontana G, Savona G, Rodríguez B. Magn Reson Chem. 2006;44:962–965. doi: 10.1002/mrc.1869. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Husbands SM, Mahon MF, Traynor JR, Rowan MG. Chem & Biodiversity. 2007;4:1586–1593. doi: 10.1002/cbdv.200790138. [DOI] [PubMed] [Google Scholar]
- 15.Fernández MC, Esquivel B, Cárdenas J, Sánchez AA, Toscano RA, Rodríguez-Hahn L. Tetrahedron. 1991;47:7199–7208. [Google Scholar]
- 16.Sice all the reported compounds (1–24) belong to the enantio series1,9–13 the α- or β-configuration should be described as ent-β or ent-α, indicating that the substituent is placed, respectively, below or above the plane of the formulas. However, for clarity we use throughout the text the α- or β-nomenclature, indicating that a substituent is placed, respectively, below or above the plane of the formulas depicted for the described substances.
- 17.Savona G, Raffa D, Bruno M, Rodríguez B. Phytochemistry. 1983;22:784–786. [Google Scholar]
- 18.Eguren L, Fayos J, Perales A, Savona G, Rodríguez B. Phytochemistry. 1984;23:466–467. [Google Scholar]
- 19.It is of interest to note that the β-oriented pyrazoline ring of 19 and 20 causes a strong shielding effect on the H-2β proton, which resonates at δ 0.33 and 0.40, respectively. Moreover, only one (H- 3′ α) of the 3′-methylene protons of the pyrazoline part of 9 and 19–22 is coupled with the vicinal H-3α proton, because in these compounds, and in other 3β,4β-pyrazoline derivatives of neoclerodan-18,19-olides, the dihedral angle between H-3′β and H-3α is close to 90° (see Rodríguez B. Magn Reson Chem. 2001;39:150–154.).
- 20.Krapcho J, Turk CF. J Med Chem. 1979;22:207–210. doi: 10.1021/jm00188a018. [DOI] [PubMed] [Google Scholar]
- 21.Prisinzano TE, Rothman RB. Chem Rev. 2008 doi: 10.1021/cr0782269. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman RB, Murphy DL, Xu H, Godin JA, Dersch CM, Partilla JS, Tidgewell K, Schmidt M, Prisinzano TE. J Pharmacol Exp Ther. 2007;320:801–810. doi: 10.1124/jpet.106.113167. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Lu YF, Thomas JB, Carroll FI, Rice KC, Rothman RB. Synapse. 2001;40:269–274. doi: 10.1002/syn.1049. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. Synapse. 2007;61:166–175. doi: 10.1002/syn.20356. [DOI] [PubMed] [Google Scholar]