Abstract
The hypothalamus controls the release of hormones by the pituitary and is involved in control of food and water intake, sexual behavior, reproduction, and daily cycles in physiological state and behavior, temperature regulation, and emotional responses. Adenosine-5′-triphosphate (ATP) and its metabolic products contribute to these functions, acting as agonists for adenosine and P2Y receptors and two-transmembrane domain P2X receptor channels. This review summarizes the recent findings on purinergic receptor expression and their roles in signaling and cellular function in secretory and supporting cells of the hypothalamo-pituitary system. ATP secretion by these tissues, the enzymes involved in ATP hydrolysis, and the relevance of this pathway for sequential activation of receptors and termination of signaling is also discussed.
Hypothalamo-pituitary system and purines
The hypothalamus is an integral part of the limbic system and contains a number of small nuclei that are involved in a variety of functions. One of the most important functions of the hypothalamus, termed ‘neuroendocrine function’, is to link the nervous system to the endocrine system via the pituitary gland. Many neurons in the hypothalamic nuclei, instead of communicating directly with other neurons through synaptic transmission, secrete chemicals that act as hormones. Neurons of the supraoptic and paraventricular nuclei in the anterior part of the hypothalamus send axons down through the stalk of the pituitary gland to its posterior lobe. The nerve endings of these fibres release hormones oxytocin and vasopressin, which reach the general bloodstream through posterior capillaries. Other neurons of the hypothalamus secrete specialized hormones, called ‘releasing or inhibiting hormones’, into the hypophysial portal system, which run into the anterior lobe to modulate secretory activity of specialized cells. These include release of growth hormone (GH), prolactin (PRL), adrenocrticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (Figure 1). The hypothalamus also controls other functions, including feeding and drinking, sexual behavior and reproduction, and temperature regulation [1]. Evidence supports that extracellular ATP and its metabolic products are involved in regulating these hypothalamic functions by activating adenosine and/or purinergic receptors. These receptors are expressed and functional in neuroendocrine and endocrine cells, including the hypothalamic neurons, secretory pituitary cells, and supporting cells [2]. This review focuses on recent findings on the role of purines and pyridines in hypothalamo-pituitary functions.
Figure 1.
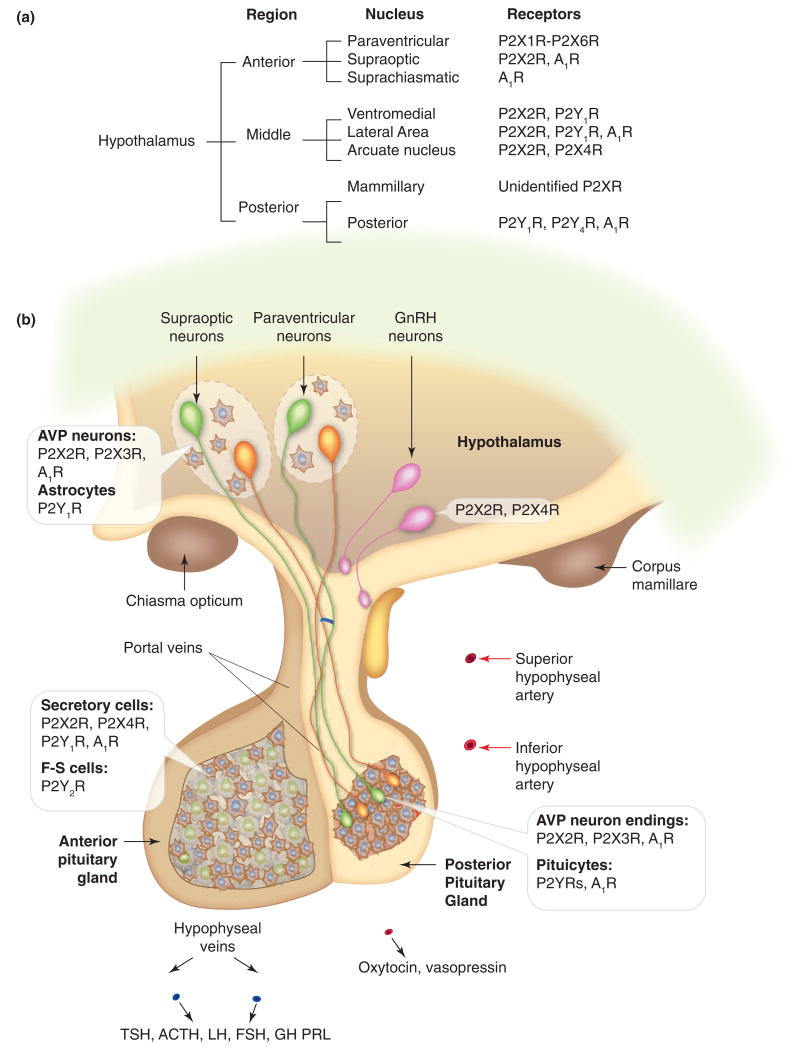
Expression of purinergic receptors in the hypothalamus and pituitary, (a) Receptors and receptor channels expressed in neurons of nuclei of the hypothalamus. For paraventricular and supraoptic nuclei, receptors expressed in parvocellular areas are listed. (b) Schematic representation of the hypothalamo-pituitary system. Insets indicate expression of purinergic receptors in secretory and supporting cells in three compartments. Notice the pattern of expression of purinergic receptors: P2X2R are expressed in a majority of secretory cells (in anterior and middle hypothalamic neurons, vasopressinergic nerve endings and anterior pituitary (AP) cells). Supporting cells (astrocytes in the hypothalamus, pituicytes in the posterior pituitary (PP), and folliculo-stellate (F-S) cells in the anterior lobe) do not express P2XRs. Many cells co-express P2XRs, which facilitate electrical activity, and A1Rs, which silence electrical activity. P2X7R are also expressed in hypothalamo-pituitary cells, but the cell types expressing these channels have not been identified. In other brain regions, astroglial cells express P2X7Rs. ATP is co-secreted by neurons making synapses with magnocellular neurons in the hypothalamus and by both vasopressin and oxytocin-secreting neurons in the PP. ATP is also released by AP cells through still not well-characterized pathways. Green cells, vasopressin (AVP)-secreting neurons; orange cells, oxytocin-secreting neurons; pink cells, GnRH neurons.
Molecular physiology of purinergic receptors and ectonucleotidases
The concept of purinergic signaling, originally proposed by G. Burnstock [3], includes several critical elements. First, ATP is not only the energy source and an important intracellular molecule controlling various intracellular processes, but it is also released from cells to act as an extracellular messenger. ATP is an agonist for two families of purinergic receptors: two-transmembrane domain P2X receptor channels (P2XRs) and several seven-transmembrane domain P2Y receptors (P2YRs). The duration and distance of ATP actions are limited by several ectonucleotidases, which hydrolyze ATP to adenosine-5′-diphosphate (ADP), adenosine-5′monophosphate (AMP), and adenosine. ADP and adenosine also act as extracellular messengers, with ADP a potent agonist for few P2YRs and adenosine an agonist for adenosine receptors (ARs), also know as P1 receptors (Figure 2). Uridine-5′-triphosphate (UTP), uridine-5′-diphosphate (UDP), and UDP glucose are also native agonists for P2YRs.
Figure 2.
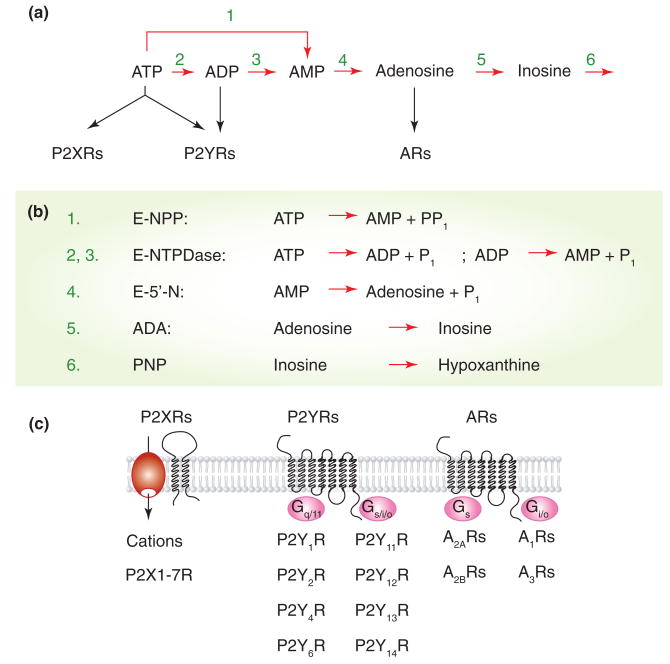
Purinergic signaling pathways, (a) and (b) The nucleotide-hydrolyzing pathways. The extracellularly released ATP is hydrolyzed to AMP by two enzyme families, ecto-nucleotide pyrophosphate/phosphodiesterase (E-NPP) and ecto-nucleoside triphosphate diphosphohydrolase (E-NTDPase), whereas AMP was efficiently hydrolyzed by ecto-5′nucleotidase (E-5′-N), also known as CD73 family of enzymes. Adenosine is further deaminated via inosine into hypoxanthine by adenosine deaminase (ADA) and purine nucleoside phosphorylase (PNP), respectively, (c) ATP is an agonist for two transmembrane domain P2XRs and several seven transmembrane domain P2YRs, whereas ADP activates a few P2YRs but not P2XRs. Adenosine is also acts as an agonist of four G-protein coupled ARs. Three subunits in homomeric or heteromeric organization are required for functional P2XRs, whereas dimerization is possible for P2YRs and ARs.
Seven mammalian purinergic receptor subunits, denoted P2X1 through P2X7, and several spliced forms of these subunits have been cloned. Each subunit is proposed to contain cytoplasmically located N- and C-termini with consensus binding motifs for protein kinases, two transmembrane helices connected by a large extracellular loop, with 10 conserved cysteine residues forming a series of disulfide bridges. The cloned subunits exhibit 38-51% amino acid sequence homology from their N-termini through the second transmembrane domain. In contrast, the C-terminal portion varies in length [4]. Functional channels are organized as trimeric homomers and heteromers [5]. P2XR subtypes differ with respect to their ligand selectivity profiles, antagonist sensitivities, and cation selectivities. P2XR activation leads to inward currents associated with increased intracellular calcium concentration ([Ca2+]i) in a receptor-specific manner (Figure 3). The P2X7R also triggers other signaling pathways [4].
Figure 3.
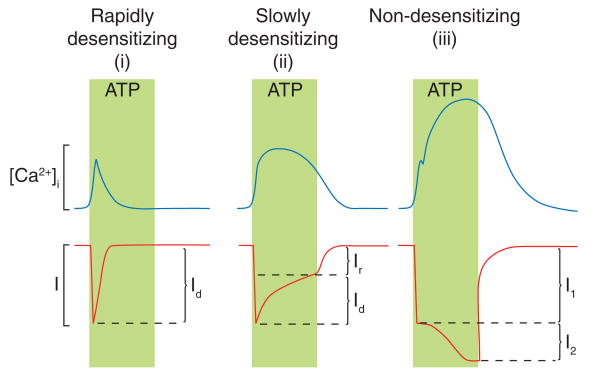
Calcium signaling function of P2XRs. Activated P2XRs generate inward cationic currents in a receptor-specific manner, and the pattern of calcium signals is determined by the rate of receptor desensitization and pore dilation. Based on these two criteria, P2XRs could be divided into (i) rapidly desensitizing receptors (P2X1R and P2X3R), which generate transient calcium signals, (ii) slowly desensitizing receptors (P2X2R, P2X4R, and P2X5R), which generate prolonged calcium signals, and (iii) non-desensitizing dilating P2X7R, which generates biphasic calcium signals. Calcium influx through the pore of channels and action potential-driven Ca2+ influx through Cav channels account for P2XR-stimulated rise in [Ca2+]i. Id, desensitizing portion of current; Ir, residual current at the end of stimulation; I1, rapidly activated current; l2, slowly developing current.
To date, eight mammalian P2YRs have been identified, denoted P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R. With the exception of P2Y11R, the P2YR genes do not contain introns in the coding sequence. Phylogenetically, these receptors form two subgroups. Members of the first group (1, 2, 4, and 6) signal through Gq/11 pathways (Figure 2), activating phospholipase C (PLC) to generate inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. In excitable cells, IP3-induced Ca2+ mobilization is frequently accompanied by Ca2+ influx through Cav channels. Activation of MAP kinase and phospholipase D signaling pathways, both secondary to the activation of protein kinase C, has also been reported for P2YRs. The second group (12, 13, and 14) shows variations in coupling to G proteins, including Gi/o, Gs, and G16 (Figure 2). There is a relatively high diversity in the amino acid composition of P2YRs, ranging between 50 and 80% for human receptors [6].
Four different adenosine-activated receptors have been cloned, termed A1R, A2AR, A2BR, and A3R. The members of this family of G protein-coupled receptors possess relatively short and variable intracellular N- (7-13 residues) and C- (32-120 residues) termini and exhibit similar structures in their ectodomains (39-61% sequence identity). These receptors signal mainly through adenylyl cyclase; A1R and A3R are negatively coupled to adenylyl cyclase through pertussis toxin-sensitive Gi/o, whereas A2AR and A2BR are positively coupled through cholera toxin-sensitive Gs (Figure 2). Human A2BR has also been reported to signal through Gq/11-dependent PLC. The intracellular pathways triggered by these receptors include Cav channels and inwardly rectifying K+ (Kir) channels, and activation of proteins involved in MAP kinase signaling [7].
Ectonucleotidases include members of the ectonucleotide triphosphate diphosphohydrolase family of enzymes (eNTPDase) and ecto-5′-nucleotidase, among others (Figure 2). Four of eight known E-NTPDases are expressed in the plasma membrane. Like P2XRs, these E-NTPDases possess two transmembrane domains, with most of the protein extracellular and the N- and C-termini intracellular. E-NTPDases not only hydrolyze extracellular ATP and/or ADP to AMP, but also metabolize other nucleotide tri- and diphosphates, including UTP and UDP, whereas cAMP is hydrolyzed by ecto-5-nucleotidase family of enzymes. These enzymes terminate the stimulatory action of ATP on P2Rs and provide a pathway for sequential activation of purinergic receptors in cells co-expressing P2XRs, ADP-activated P2YRs, and/or ARs [8].
Purinergic signaling in parvocellular hypothalamic nuclei
The major identified hypothalamic nuclei and their associated purinergic receptors are listed in Figure 1a. The anterior hypothalamus contains supraoptic, paraventricular, and suprachiasmatic nuclei. The magnocellular areas of supraoptic and paraventricular nuclei secrete vasopressin and oxytocin (see below), and the parvocellular areas of paraventricular nucleus neurons secrete corticoptropin-releasing hormone (CRH) and regulate sympathetic nerve activity through their connection with neurons in the rostral ventrolateral medulla. Several P2XRs appear to be expressed in paraventricular parvocelllar neurons [9]. Single channel analysis confirms the presence of homomeric and heteromeric P2X2Rs in these cells [10]. Further studies are needed to clarify the role of these receptors on sympathetic nerve activity, including blood pressure and heart rate. On the other hand, the suprachiasmatic nucleus supplies a clock for day-night information, and neurons in this nucleus express A1Rs, which contribute to control of the circadian clock to light [11, 12]. At the present time, there is no information about the functional expression of P2XRs and P2YRs in these neurons. This suggests action specificity of purinergic receptors in two nuclei, stimulatory in paraventricular nucleus and inhibitory in suprachiasmatic nucleus.
The medial (tuberal) hypothalamus contains two major nuclei, the arcuate and the ventromedial while the lateral hypothalamus, termed the lateral hypothalamic area, is more loosely organized. In the medial hypothalamus, the arcuate nucleus, also known as the periventricular nucleus, contains neurons that control endocrine functions of the anterior pituitary (AP) by secreting releasing and inhibitory neurohormones at the median eminence. Neurons of the arcuate nucleus also contact neurons in the paraventricular nucleus and medial preoptic area, where they regulate production of CRH and gonadotropin-releasing hormone (GnRH), respectively. Electrophysiological experiments confirm that unidentified neurons of the arcuate nucleus express functional homomeric and/or heteromeric P2X2Rs [13]. P2Y1R immunopositive cells were also detected in the arcuate nucleus. The role of purinergic signaling in CRH release has not been studied, limiting our understanding of the potential role of local purinergic signaling on stress response.
P2X2 and P2X4 subunits are expressed in GnRH neurons in olfactory placode cultures from rhesus monkeys, and in these neurons, ATP application leads to synchronization of [Ca2+]i oscillations, which could indicate a role of ATP in pulsatile hormone release [15]. Immortalized mouse hypothalamic GnRH-secreting neurons release ATP [16] but do not express functional P2Rs, indicating that they do not provide a good cell model for such studies [17]. However, the recent development of several transgenic mice expressing GFP-labeled GnRH neurons provides a promising system to investigate the expression and role of purinergic receptors in interconnected GnRH neurons. Further studies should also focus on identifying other neurosecretory cells in the arcuate nucleus, characterizing their purinergic signaling, and clarifying the relevance of purinergic signaling in controlling other neurosecretory cells in this nucleus.
The lateral hypothalamus is implicated in modulating visceral motor and sensory pathways, including those in feeding and behavioral arousal. The ventromedial nucleus is known primarily for its involvement in sexual and feeding behavior. These pathways are modulated by a variety of transmitters and endogenous factors including glucose, steroid hormones, serotonin, glutamate, GABA, and ATP [18]. Coordinate release of ATP and GABA has been detected in synapses of lateral hypothalamic neurons [19]. The lateral hypothalamus also harbors functionally distinct populations of orexin/hypocretin neurons that express A1Rs [20]. These neurons also express functional P2Y1Rs, and their activation stimulates food intake [21]. Transgenic mice expressing GFP exclusively in orexin-producing cells also have functional P2XRs, the biophysical and pharmacological properties of which are comparable to recombinant P2X2Rs [22, 23]. Ventromedial and lateral hypothalamic neurons also express functional P2Y1Rs, and ATP and ADP regulate food intake through activation of these receptors, as documented in experiments with microinfusion of ADPβS into the lateral ventricle, ventromedial hypothalamus and lateral hypothalamus of rats [21]. Expression of these receptors also appears to be modified by restricted feeding [14]. From a signaling point of view, it is interesting that orexin neurons exhibit a depolarizing action of P2XRs, calcium-mobilizing action of P2Y1R, and silencing effect of A1Rs on electrical activity and calcium signaling, suggesting a sequential pattern of purinergic signaling (Figure 4).
Figure 4.
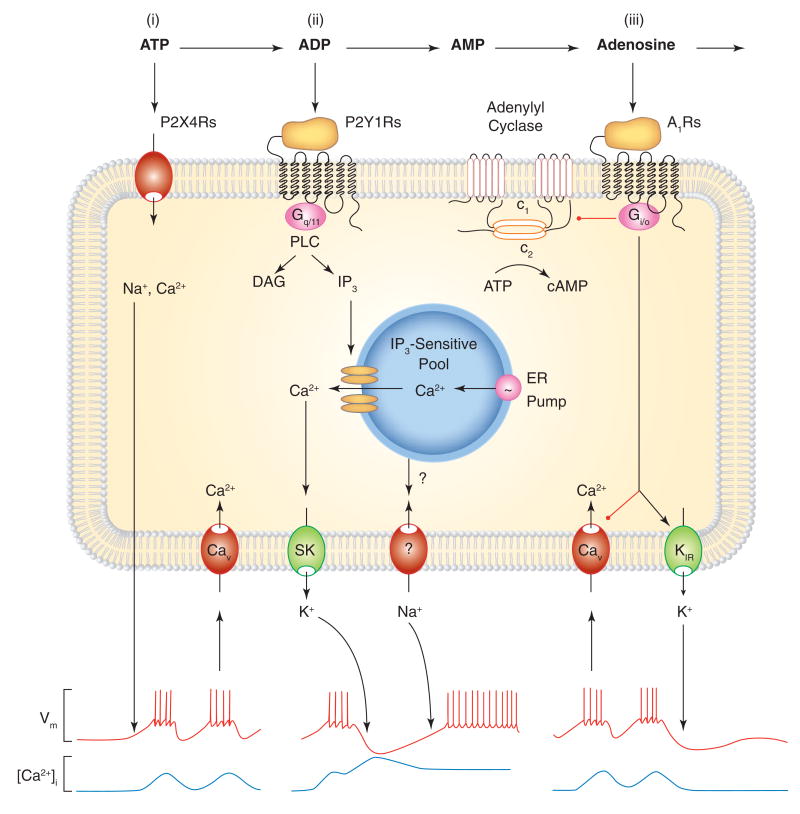
Roles of ectonucleotidases in sequential activation of purinergic receptors. In cells co-expressing P2XRs, P2YRs, and ARs, the ectonucleotide cascade leads to sequential activation of receptors. Scheme illustrates signaling cascade in a fraction of pituitary lactotrophs. (i) Activation of P2X4Rs by ATP leads to cation influx and predominantly Na+-dependent depolarization of the cell membrane. This in turn facilitates action potential firing and Ca2+ influx through voltage-gated calcium (Cav) channels. In cells with activated inositol 1,4,5-triphosphate (IP3)-dependent channels, this Ca2+ influx can stimulate or inhibit opening of these channels (not shown), (ii) E-NTPDase-mediated generation of ADP leads to the subsequent activation of Ca2+-mobilizing P2Y1Rs coupled to phospholipase C (PLC) signaling pathway, generation of diacylycerol (DAG) and IP3, and IP3-dependent Ca2+ release from ER. The released Ca2+ triggers transient hyperpolarization of the cell membrane mediated by calcium-activated SK type K+ channels, followed by a sustained depolarization of the plasma membrane through still not identified channels. These could be the store operated Orai channels or PLC signaling-dependent activation of TRP channels. Both Ca2+ release from ER and Ca2+ influx through Cav channels further stimulates hormone secretion (not shown). (iii) Ecto-5′-nucleotidase-mediated generation of adenosine leads to stimulation of Gi/o-coupled A1Rs. The α subunit of heterotrimeric Gi/o proteins inhibits adenylyl cyclase-mediated generation of cAMP (shown in the top part of the cell membrane), an intracellular messenger that in hypothalamic and pituitary cells facilitates electrical activity by stimulating hyperpolarization-activated cyclic nucleotide-regulated channels and inhibiting inward rectifying K (Kir) channels (not shown). The Gi/o-βγ dimmer also helps terminating the P2R-induced facilitation of electrical activity by stimulating Kir channels and inhibiting Cav channels. Such inhibition could help limit the physiological impact of prolonged activation of PXRs on lactotroph excitability. A three-receptor pathway may also be operative in some lateral and posterior hypothalamic neurons, whereas other pituitary cells and vasopressinergic neurons co-express P2X2R and A1Rs. Black arrows indicate stimulatory action, and red lines indicate inhibitory actions.
Purinergic receptors in the hypothalamo-posterior pituitary system
Hypothalamic magnocellular paraventricular and supraoptic nuclei are composed of astrocytes and neurons, the latter specializing in either vasopressin or oxytocin synthesis. The axons of hypothalamic magnocellular and supraoptic neurons transport secretory vesicles from the soma to the posterior pituitary (PP), releasing hormones near fenestrated capillaries (Figure 1b). The amount of hormone released is dependent on the rate and pattern of neuronal firing activity, and bursting activity differs in oxytocin and vasopressin neurons [24]. The released hormones play important roles in water balance, blood pressure, parturition and lactation. Cells from both nuclei are spontaneously active; several neurotransmitters and humoral factors not only modulate electrical activity but also affect hormone release from the PP. Such modulation reflects the convergence of extensive afferent pathways on the magnocellular neurons in the pre- and post-synaptic modes, as well as actions at neuron terminals in the PP [25, 26].
Purines have also been suggested to play important role(s) in controlling the activity of vasopressin- but not oxytocin-secreting neurons. This conclusion was initially derived from experiments measuring the effects of extracellularly added ATP on vasopressin and oxytocin release [27]. It has been suggested that the magnocellular neurons of the hypothalamus, which control vasopressin and oxytocin release, also contain ATP and release it in the PP in an action potential-specific manner [27, 28]. It has also been shown that ATP endogenously released from the PP during stimulation is sufficient to depolarize the nerve terminals and potentiate vasopressin secretion [29]. These observations suggest that ATP may act on both perikarya and dendrites of vasopressinergic neurons in the hypothalamus and on nerve terminals in the PP.
Consistent with this hypothesis, functional P2XRs have been identified in the somata of neurons in the supraoptic nucleus [30] and in isolated PP terminals [27], as indicated by [Ca2+]i measurements. A more recent study showed a role for P2X2Rs in ATP-induced increases in [Ca2+]i and peptide release from isolated rat PP terminals [31]. Electrophysiological evidence for the existence of P2XR currents in vasopressinergic PP terminals but not in terminals labeled for oxytocin has also been shown [32]. Others suggested that P2XRs are not only expressed in vasopressin-containing neurons, but also in oxytocin-containing neurons [33]. Thus, the role of P2XRs in the function of vasopressin-secreting neurons is well-established, and further research is required to clarify their functional expression and roles in oxytocin-secreting neurons.
There is also experimental evidence for ectonucleotidases on the plasma membrane of neurosecretory terminals and pituicytes (astrocytes in the PP) [34]. Extracellularly added ATP is rapidly hydrolyzed in isolated PP, indicating functional ectonucleotidase activity. Adenosine accumulation is also detected upon AMP addition, suggesting these enzymes provide a pathway for AR activation [35]. Exogenously administered adenosine inhibits Cav channels in dissociated supraoptic neurons via A1R signaling through Gz heterotrimeric proteins [36], accompanied by inhibition of vasopressin secretion [37, 38]. Further studies revealed the presence of A1R immunoreactivity in the supraoptic nucleus and the accumulation of endogenous adenosine and its strong inhibitory influence on magnocellular activity, presumably reflecting activation of Kir channels [39]. It appears that endogenous adenosine is of sufficient levels to activate A1Rs in nerve terminals, leading to inhibition of Cav channels and hormone secretion [40]. These observations again emphasize the common pattern of purinergic signaling in hypothalamo-pituitary cells, a stimulatory action on electrical activity mediated by P2XRs and an inhibitory action mediated by ARs.
Purinergic signaling in supraoptic and paraventricular nuclei is not limited to neurons and their nerve endings in the PP, but also includes astrocytes. A contribution of glial cells to ATP release in the paraventricular nucleus has been demonstrated [41]. ATP release by astrocytes in the supraoptic nucleus has not been studied; however, these cells have been shown to actively suppress neuronal activity [42]. It has also been shown that astrocytes in the supraoptic nucleus express calcium-controlled small K+ (SK) channels, and that calcium mobilization from intracellular pools is likely their main activation pathway [43]. Since these cells express calcium-mobilizing P2Y1Rs [44], their activation may account for SK channel stimulation and synchronization of electrical activity with calcium mobilization. In other brain regions, astrocytes actively participate in neuronal activity, frequently by generating calcium waves that modulate synaptic activity and neuronal excitability [45]. Further experiments are needed to clarify whether this is also the case with hypothalamic astrocytes.
ATP also acts on pituicytes that are intimately associated with neurosecretory terminals. ATP hydrolysis by ectonucleotidases and activation of A1Rs has a profound effect on the three-dimensional structure of pituicytes, changing their flat shape to a stellate morphology [46]. In the majority of pituicytes in primary culture, ATP also triggers a rapid and extracellular calcium-independent rise in [Ca2+]i that is abolished in cells in which PLC and the endoplasmic reticulum calcium pump have been blocked [47]. These results indicate the functional operation of calcium-mobilizing P2YRs in pituicytes. However, neither the subtypes of these receptors nor their roles in the functions of neuronal endings and pituicytes have yet been identified.
Taken together, these results indicate that purinergic signaling is operative in both supraoptic and paraventricular magnocellular neurons and nerve endings in the PP. The P2X2Rs and probably P2X3Rs provide the major depolarizing pathway, leading to facilitation of electrical activity, calcium signaling, and vasopressin release. The rapid hydrolysis of ATP, on the other hand, is likely to account for termination of ATP-induced vasopressin release at nerve endings in the PP via A1R activation (Figure 4). Such a role for endogenous adenosine in a negative-feedback mechanism could reflect either attenuated bursts of action potentials initiated in supraoptic neurons of the hypothalamus or termination of peptide release.
Purinergic signaling in the anterior pituitary gland
ATP is released by normal and immortalized AP cells under resting conditions. This basal ATP release was enhanced in cells treated with ARL67156, an inhibitor of ectonucleotidases. Ectonucleotidases eNTPDase 1-3 are also expressed and operative in hypothalamic and pituitary cells [16]. Ecto-5′-nucleotidase (CD73), which generates adenosine from AMP, is expressed in about 20% of AP cells [48]. These enzymes degrade both endogenously released and exogenously added ATP in dispersed pituitary cells; however, the nature of ATP release here has not been clarified.
Initial knowledge about the expression and role of P2XRs in secretory AP cells was obtained in experiments using hormone release and [Ca2+]i measurements in dispersed cells. These experiments, summarized in [49], reveal functional P2XRs in all secretory cell types and raise the possibility that several subtypes of these channels are expressed in a cell type-specific manner. However, this method is of limited use for identifying the receptor subtypes expressed, especially in the case of rapidly desensitizing homomeric and heteromeric P2XRs [50]. In more recent studies, molecular biology techniques combined with electrophysiology were used to better understand the structure of P2XRs expressed in AP cells and their downstream signaling pathways. These experiments showed that secretory pituitary cells abundantly express P2X2 and P2X4, with less expression of other subunits.
Rat AP cells express two splice forms of the P2X2 subunit, termed P2X2a and P2X2b, whereas mouse pituitary cells express three forms of P2X2 receptor subunit, the full size P2X2a and the shorter forms P2X2b and P2X2e, which are missing 69 and 90 residues, respectively, in their C-termini [51]. Electrophysiological experiments revealed that the rate of homomeric P2X2eR desensitization was comparable to the rates of rapidly desensitizing P2X1R and P2X3R, whereas the rate of P2X2bR desensitization was faster than P2X2a but slower than P2X2e receptors. Deletions in the C-terminal also effectively reduced the peak amplitude and duration of calcium signals [51]. The physiological relevance of these splice forms is in formation of functional heteromers, which desensitize faster than full-size receptors but slower than homomeric splice receptors. This in turn limits excessive ion influx but does not terminate signaling during prolonged agonist stimulation. Such heteromers have been identified in pituitary gonadotrophs and somatotrophs, but not in other pituitary cell types [17]. In gonadotrophs, their activation leads to action potential firings and modulation of the frequency of firing in spontaneously firing cells, accompanied by elevation in [Ca2+]i that reflects both Ca2+ influx through channel pores and through Cav channels. In these cells, ATP also modulates GnRH-induced and IP3-mediated calcium/current oscillations and hormone release [52].
The biophysical and pharmacological properties of recombinant rat P2X4R cloned from the pituitary gland have been characterized [53], and the functional receptors have been identified in lactotrophs [54]. These receptors are sensitivite to ivermectin, a high molecular weight lipophilic compound used as an antiparasitic agent in human and veterinary medicine. This compound increases sensitivity of receptors to ATP, amplifies peak current amplitude in response to supramaximal agonist concentrations, and delays receptor deactivation [55]. These allosteric actions of ivermectin on P2X4R were successfully used in structural and functional characterization of recombinant receptors [55, 56] and should also be used in evaluating the expression and role of P2X4Rs in the hypothalamus and pituitary gland.
In contrast to P2X2Rs and P2X4R, recombinant P2X7R does not desensitize, but rather shows a sustained augmentation of the current (Figure 3). The secondary rise in current is observed under different ionic conditions and temporally coincides with the development of conductivity to larger organic cations and fluorescent dyes [57]. At the present time, it has not been determined which cell types in the hypothalamo-pituitary system express P2X7R, and the characteristics of the native receptors have not been described. Based on known expression and roles of P2X7Rs in other brain regions [58], perhaps these receptors are expressed in hypothalamic astrocytes, pituicytes and/or folliculo-stellate cells. Pharmacological approaches, for example the use of specific P2X7R antagonist, could be useful in characterizing which hypothalamic and pituitary cells express these receptors and in defining their roles.
AP cells also express P2YRs and several ARs (reviewed in [49, 59, 60]). Briefly, P2Y2R was cloned from a rat pituitary cDNA library. The native receptors in pituitary cells are activated by ATP and UTP, and their activation is accompanied by PLC stimulation and generation of IP3 and diacylglycerol, calcium mobilization and activation of protein kinase C. The expression of functional P2Y2-like receptors has been indicated in rat pituitary folliculo-stellate cells in primary culture. It has also been suggested that these receptors are expressed in gonadotrophs, but this finding was not confirmed in subsequent studies. Pituitary cells also express A1R, A2aR, A2bR, and A3R, and functional A1Rs were identified in lactotrophs and immortalized pituitary cells. More recent studies indicated that a fraction of pituitary lactotrophs and other unidentified cells also express calcium-mobilizing receptors; interestingly, the pharmacological profile of these receptors resembles that of cloned P2Y1Rs, including ADP acting as an agonist [54]. The A2BRs are expressed in folliculo-stellate cells and stimulate IL-6 secretion [61] and gap junction formation [48]. A3Rs are found in tumor pituitary cells and may contribute to the antiproliferative activity of adenosine [62].
The expression of P2XRs, P2YRs, and A1Rs in the same cell types is consistent with the sequential mode of ATP action. In the case of pituitary lactotrophs, endogenous ATP initially stimulates P2X4Rs, promoting Ca2+ influx through the pore of channels and through depolarization-induced Ca2+ influx through Cav channels (Figure 4, pathway i). Hydrolysis of ATP to ADP should shut off P2X4R activity while maintaining or initiating P2Y1R activity, leading to PLC activation, generation of diacylglycerol and IP3, and IP3-dependent Ca2+ release from ER (Figure 4, pathway ii). On the other hand, the breakdown of ADP to AMP and adenosine provides a pathway for A1R activation, which silences electrical activity by inhibiting Cav channels and stimulating Kir channels by βγ dimmer of Gi/o hetermeric proteins (Figure 4, pathway iii). In other cell types, pathways (i) and (iii) are also likely to be operative.
Concluding remarks
Expression of P1Rs, P2XRs, and P2YRs in hypothalamo-pituitary tissues has been well-documented. These receptors are operative in both excitable cells (neurons and secretory AP cells) and supporting cells (astrocytes, pituicytes, and folliculo-stellate cells). In general, P2XRs are expressed in excitable cells but not in supporting cells, and contribute to electrical activity and calcium influx. There is solid evidence for P2X2R and P2X4R expression in these cells. A1Ris also are predominantly expressed in excitable cells and usually contribute to inhibition of electrical activity and calcium signaling. On the other hand, P2Y1R and P2Y2R are expressed in both excitable and supporting cells. Molecular but not electrophysiological/signaling evidence for the existence of other purinergic receptors has also been obtained, and further work should focus on identifying cell types expressing those receptors and deciphering of their roles. P2XR-knockout mice are available and should be used in further work to clarify the role of these channels in hypothalamo-pituitary functions. Such work could clarify the pacemaking role of P2XRs in spontaneous electrical activity and hormone secretion in excitable cells. Further experiments should also compare the role of purinergic signaling in supporting cells to that in other brain regions. ATP is released by hypothalamic and pituitary cells through pathways that have still not been fully characterized, especially in the AP. Further work on this topic is critically important to understand the effects of local purinergic signaling on the whole organism. The capacity of ectonucleotidase to hydrolyze ATP has been well-characterized in these tissues, but the specific ectonucleotidases involved are yet unknown. This finding could help our understanding of the physiological relevance of opposing effects of P2XRs and A1R on electrical activity and calcium signaling in excitable hypothalamic and pituitary cells. There are numerous possible directions for in situ and in vivo studies on the role of purinergic signaling in sexual behavior and reproduction, stress reaction, and control of food and water intake that should also be explored.
Acknowledgments
Supported by the Intramural Research Program of the NICHD, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Despopoulos A, Silbernagl S. Color atlas of physiology. Thieme; Stuttgard, New York: 2003. [Google Scholar]
- 2.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 4.Surprenant A, North RA. Signaling at Purinergic P2X Receptors. Annu Rev Physiol. 2008 doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 5.Nicke A, et al. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92:925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- 6.Fischer W, Krugel U. P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem. 2007;14:2429–2455. doi: 10.2174/092986707782023695. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Cham JL, et al. P2X purinoceptor subtypes on paraventricular nucleus neurones projecting to the rostral ventrolateral medulla in the rat. Exp Physiol. 2006;91:403–411. doi: 10.1113/expphysiol.2005.032409. [DOI] [PubMed] [Google Scholar]
- 10.Whitlock A, et al. The single-channel properties of purinergic P2X ATP receptors in outside-out patches from rat hypothalamic paraventricular parvocells. Pflugers Arch. 2001;443:115–122. doi: 10.1007/s004240100624. [DOI] [PubMed] [Google Scholar]
- 11.Hallworth R, et al. Presynaptic adenosine A1 receptors regulate retinohypothalamic neurotransmission in the hamster suprachiasmatic nucleus. J Neurobiol. 2002;52:230–240. doi: 10.1002/neu.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigworth LA, Rea MA. Adenosine A1 receptors regulate the response of the mouse circadian clock to light. Brain Res. 2003;960:246–251. doi: 10.1016/s0006-8993(02)03896-9. [DOI] [PubMed] [Google Scholar]
- 13.Wakamori M, Sorimachi M. Properties of native P2X receptors in large multipolar neurons dissociated from rat hypothalamic arcuate nucleus. Brain Res. 2004;1005:51–59. doi: 10.1016/j.brainres.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Seidel B, et al. Expression of purinergic receptors in the hypothalamus of the rat is modified by reduced food availability. Brain Res. 2006;1089:143–152. doi: 10.1016/j.brainres.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Terasawa E, et al. Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol. 2005;19:2736–2747. doi: 10.1210/me.2005-0034. [DOI] [PubMed] [Google Scholar]
- 16.He ML, et al. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1-3 in hypothalamic and pituitary cells. Purinergic Signal. 2005;1:135–144. doi: 10.1007/s11302-005-6208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koshimizu T, et al. Functional role of alternative splicing in pituitary P2X2 receptor-channel activation and desensitization. Mol Endocrinol. 1998;12:901–913. doi: 10.1210/mend.12.7.0129. [DOI] [PubMed] [Google Scholar]
- 18.Willie JT, et al. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 19.Jo YH, Role LW. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J Neurosci. 2002;22:4794–4804. doi: 10.1523/JNEUROSCI.22-12-04794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakkar MM, et al. Orexin neurons of the hypothalamus express adenosine A1 receptors. Brain Res. 2002;944:190–194. doi: 10.1016/s0006-8993(02)02873-1. [DOI] [PubMed] [Google Scholar]
- 21.Kittner H, et al. Enhanced food intake after stimulation of hypothalamic P2Y1 receptors in rats: modulation of feeding behaviour by extracellular nucleotides. Eur J Neurosci. 2006;24:2049–2056. doi: 10.1111/j.1460-9568.2006.05071.x. [DOI] [PubMed] [Google Scholar]
- 22.Wollmann G, et al. Direct excitation of hypocretin/orexin cells by extracellular ATP at P2X receptors. J Neurophysiol. 2005;94:2195–2206. doi: 10.1152/jn.00035.2005. [DOI] [PubMed] [Google Scholar]
- 23.Florenzano F, et al. P2X2R purinergic receptor subunit mRNA and protein are expressed by all hypothalamic hypocretin/orexin neurons. J Comp Neurol. 2006;498:58–67. doi: 10.1002/cne.21013. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong WE. The neurophysiology of neurosecretory cells. The Journal of physiology. 2007;585:645–647. doi: 10.1113/jphysiol.2007.145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, et al. Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity. The Journal of physiology. 2007;581:221–240. doi: 10.1113/jphysiol.2006.123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sladek CD, Kapoor JR. Neurotransmitter/neuropeptide interactions in the regulation of neurohypophyseal hormone release. Exp Neurol. 2001;171:200–209. doi: 10.1006/exnr.2001.7779. [DOI] [PubMed] [Google Scholar]
- 27.Troadec JD, et al. ATP-evoked increases in [Ca2+]i and peptide release from rat isolated neurohypophysial terminals via a P2X2 purinoceptor. The Journal of physiology. 1998;511(Pt 1):89–103. doi: 10.1111/j.1469-7793.1998.089bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troadec JD, Thirion S. Multifaceted purinergic regulation of stimulus-secretion coupling in the neurohypophysis. Neuro Endocrinol Lett. 2002;23:273–280. [PubMed] [Google Scholar]
- 29.Knott TK, et al. Endogenous ATP potentiates only vasopressin secretion from neurohypophysial terminals. J Cell Physiol. 2008;217:155–161. doi: 10.1002/jcp.21485. [DOI] [PubMed] [Google Scholar]
- 30.Shibuya I, et al. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. The Journal of physiology. 1999;514(Pt 2):351–367. doi: 10.1111/j.1469-7793.1999.351ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Z, Sladek CD. Site of ATP and phenylephrine synergistic stimulation of vasopressin release from the hypothalamo-neurohypophyseal system. J Neuroendocrinol. 2006;18:266–272. doi: 10.1111/j.1365-2826.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- 32.Knott TK, et al. ATP elicits inward currents in isolated vasopressinergic neurohypophysial terminals via P2X2 and P2X3 receptors. Pflugers Arch. 2005;450:381–389. doi: 10.1007/s00424-005-1471-x. [DOI] [PubMed] [Google Scholar]
- 33.Guo W, et al. P2X receptors are differentially expressed on vasopressin- and oxytocin-containing neurons in the supraoptic and paraventricular nuclei of rat hypothalamus. Histochem Cell Biol. 2009;131:29–41. doi: 10.1007/s00418-008-0493-9. [DOI] [PubMed] [Google Scholar]
- 34.Thirion S, et al. Cytochemical localization of ecto-ATPases in rat neurohypophysis. J Histochem Cytochem. 1996;44:103–111. doi: 10.1177/44.2.8609366. [DOI] [PubMed] [Google Scholar]
- 35.Sperlagh B, et al. Local regulation of vasopressin and oxytocin secretion by extracellular ATP in the isolated posterior lobe of the rat hypophysis. J Endocrinol. 1999;160:343–350. doi: 10.1677/joe.0.1600343. [DOI] [PubMed] [Google Scholar]
- 36.Noguchi J, Yamashita H. Adenosine inhibits voltage-dependent Ca2+ currents in rat dissociated supraoptic neurones via A1 receptors. The Journal of physiology. 2000;526(Pt 2):313–326. doi: 10.1111/j.1469-7793.2000.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang G, et al. Adenosine inhibition via A(1) receptor of N-type Ca(2+) current and peptide release from isolated neurohypophysial terminals of the rat. The Journal of physiology. 2002;540:791–802. doi: 10.1113/jphysiol.2002.016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song Z, Sladek CD. Does conversion of ATP to adenosine terminate ATP-stimulated vasopressin release from hypothalamo-neurohypophyseal explants? Brain Res. 2005;1047:105–111. doi: 10.1016/j.brainres.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Ponzio TA, Hatton GI. Adenosine postsynaptically modulates supraoptic neuronal excitability. J Neurophysiol. 2005;93:535–547. doi: 10.1152/jn.01185.2003. [DOI] [PubMed] [Google Scholar]
- 40.Knott TK, et al. Endogenous adenosine inhibits CNS terminal Ca(2+) currents and exocytosis. J Cell Physiol. 2007;210:309–314. doi: 10.1002/jcp.20827. [DOI] [PubMed] [Google Scholar]
- 41.Gordon GR, et al. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 42.Hussy N, et al. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62:113–134. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong WE, et al. Immunocytochemical localization of small-conductance, calcium-dependent potassium channels in astrocytes of the rat supraoptic nucleus. J Comp Neurol. 2005;491:175–185. doi: 10.1002/cne.20679. [DOI] [PubMed] [Google Scholar]
- 44.Espallergues J, et al. Synergistic activation of astrocytes by ATP and norepinephrine in the rat supraoptic nucleus. Neuroscience. 2007;148:712–723. doi: 10.1016/j.neuroscience.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 45.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosso L, et al. RhoA inhibition is a key step in pituicyte stellation induced by A(1)-type adenosine receptor activation. Glia. 2002;38:351–362. doi: 10.1002/glia.10072. [DOI] [PubMed] [Google Scholar]
- 47.Troadec JD, et al. ATP acting on P2Y receptors triggers calcium mobilization in primary cultures of rat neurohypophysial astrocytes (pituicytes) Pflugers Arch. 1999;437:745–753. doi: 10.1007/s004240050841. [DOI] [PubMed] [Google Scholar]
- 48.Lewis BM, et al. Adenosine stimulates connexin 43 expression and gap junctional communication in pituitary folliculostellate cells. FASEB J. 2006;20:2585–2587. doi: 10.1096/fj.06-6121fje. [DOI] [PubMed] [Google Scholar]
- 49.Stojilkovic SS, Koshimizu T. Signaling by extracellular nucleotides in anterior pituitary cells. Trends Endocrinol Metab. 2001;12:218–225. doi: 10.1016/s1043-2760(01)00387-3. [DOI] [PubMed] [Google Scholar]
- 50.He ML, et al. Intracellular calcium measurements as a method in studies on activity of purinergic P2X receptor channels. Am J Physiol Cell Physiol. 2003;285:C467–479. doi: 10.1152/ajpcell.00042.2003. [DOI] [PubMed] [Google Scholar]
- 51.Koshimizu TA, et al. Carboxyl-terminal splicing enhances physical interactions between the cytoplasmic tails of purinergic P2X receptors. Mol Pharmacol. 2006;69:1588–1598. doi: 10.1124/mol.105.019802. [DOI] [PubMed] [Google Scholar]
- 52.Zemkova H, et al. Roles of purinergic P2X receptors as pacemaking channels and modulators of calcium-mobilizing pathway in pituitary gonadotrophs. Mol Endocrinol. 2006;20:1423–1436. doi: 10.1210/me.2005-0508. [DOI] [PubMed] [Google Scholar]
- 53.Yan Z, et al. Participation of the Lys313-lle333 sequence of the purinergic P2X4 receptor in agonist binding and transduction of signals to the channel gate. J Biol Chem. 2006;281:32649–32659. doi: 10.1074/jbc.M512791200. [DOI] [PubMed] [Google Scholar]
- 54.He ML, et al. Role of nucleotide P2 receptors in calcium signaling and prolactin release in pituitary lactotrophs. J Biol Chem. 2003;278:46270–46277. doi: 10.1074/jbc.M309005200. [DOI] [PubMed] [Google Scholar]
- 55.Zemkova H, et al. Role of aromatic and charged ectodomain residues in the P2X(4) receptor functions. J Neurochem. 2007;102:1139–1150. doi: 10.1111/j.1471-4159.2007.04616.x. [DOI] [PubMed] [Google Scholar]
- 56.Jelinkova I, et al. Identification of P2X(4) receptor transmembrane residues contributing to channel gating and interaction with ivermectin. Pflugers Arch. 2008;456:939–950. doi: 10.1007/s00424-008-0450-4. [DOI] [PubMed] [Google Scholar]
- 57.Yan Z, et al. The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol. 2008;132:563–573. doi: 10.1085/jgp.200810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sim JA, et al. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24:6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rees DA, et al. Adenosine signalling pathways in the pituitary gland: one ligand, multiple receptors. J Endocrinol. 2003;177:357–364. doi: 10.1677/joe.0.1770357. [DOI] [PubMed] [Google Scholar]
- 60.Rees DA, et al. Novel insights into how purines regulate pituitary cell function. Clin Sci (Lond) 2003;104:467–481. doi: 10.1042/CS20030053. [DOI] [PubMed] [Google Scholar]
- 61.Rees DA, et al. Adenosine-induced IL-6 expression in pituitary folliculostellate cells is mediated via A2b adenosine receptors coupled to PKC and p38 MAPK. Br J Pharmacol. 2003;140:764–772. doi: 10.1038/sj.bjp.0705488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohana G, et al. Differential effect of adenosine on tumor and normal cell growth: focus on the A3 adenosine receptor. J Cell Physiol. 2001;186:19–23. doi: 10.1002/1097-4652(200101)186:1<19::AID-JCP1011>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]