Abstract
During muscle development precursor cells fuse to form myofibers. Following injury in adult muscle, quiescent satellite cells become activated to regenerate muscle in a fashion similar to fetal development. Recent studies indicate that murine skeletal myoblasts can differentiate along multiple cell lineages including the osteoblastic pathway. However, little is known about the multipotency of human myogenic cells. Here, we isolate myogenic precursor cells from human fetal and adult muscle by sorting for the laminin-binding α7 integrin and demonstrate their differentiation potential and alteration in adhesive behavior. The α7-positive human fetal progenitors were efficient at forming myotubes and expressed known muscle markers including M-cadherin and c-Met, but were heterogeneous for desmin and MyoD expression. To test their pluripotent differentiation potential, enriched populations of α7-positive fetal cells were subjected to inductive protocols. Although the myoblasts appeared committed to a muscle lineage they could be converted to differentiate along the osteoblastic pathway in the presence of BMP-2. Interestingly, osteogenic cells showed altered adhesion and migratory activity that reflected growth factor-induced changes in integrin expression. These results indicate that α7-expressing fetal myoblasts are capable of differentiation to osteoblast lineage with a coordinated switch in integrin profiles and may represent a mechanism that promotes homing and recruitment of myogenic stem cells for tissue repair and remodeling.
Keywords: integrin, laminin, adhesion, stem cell, muscle differentiation, osteoblast
INTRODUCTION
Skeletal muscles are composed of mature muscle cells known as myofibers. These syncytia arise during development from the fusion of mononucleate precursor cells known as myoblasts. Not all myoblasts fuse into myofibers during skeletal muscle development; some remain in the adult muscle as satellite cells [1, 2]. Mammalian satellite cells are capable of crossing the muscle basal lamina during normal development and can contribute to the growth of the neighboring myofibers. Moreover, after injury or during muscle regeneration, satellite cells become activated, proliferate and then differentiate to replace damaged myofibers [3, 4].
Given the therapeutic potential of putative multipotent muscle myoblasts, there is need for an effective technique to isolate and purify these progenitor cells. Most studies have focused on the mouse system and used established cell-lines, such as C2C12, but their relative distance from their original physiological setting diminishes their clinical value in human disease and repair. We have examined human primary myogenic cells in order to recreate more closely their physiological origin. Thus far, poor donor cell survival and the difficulties in isolating a homogenous population of human muscle stem cells have impeded development of myoblast-related clinical therapies.
It is difficult to isolate human muscle stem cells because there are few definitive cell surface markers for their identification. Several markers including Sca-1, Pax-7, c-Kit, and CD34 have been identified to define mouse muscle stem cells [5–7]. Only a few markers have been suggested in the case of human muscle stem cells, so typically alternative approaches have been used to isolate these cells. The original preplating method [8, 9] utilizes the differential binding of muscle stem cells and the side-population (SP) isolation method [10] isolates cells through flow cytometry based on muscle stem cells’ unique manner of pumping out Hoechst solution. While both methods have shown promise, each is limited in application due to inability to produce highly homogenous populations and inefficiency in producing sufficient cells for culture and transplantation. Also, to date, neither of these procedures have been thoroughly studied nor employed successfully for isolating human muscle stem cells. Recently, a promising antibody against CD56/NCAM was utilized [11] to isolate multipotent, proliferating myoblasts. CD56/NCAM is a marker for neural cell adhesion molecules and is commonly found in natural killer cells. Differentiation experiments indicate that the multipotent myoblasts can differentiate into discrete lineages. Pagani et al. [12] has used cells isolated by CD56/NCAM to perform cell transplantation on ischemia-damaged human myocardium. However, no in-depth investigation has been made on these cells for their differentiation potential by in vivo studies. Thus their clinical utility is unknown.
Previous evidence indicated heterogeneity among myoblasts isolated from muscle tissue and the possible presence of a subpopulation of myoblasts with stem cell-like characteristics [5, 13, 14]. Recent work substantiates the existence of stem cells in skeletal muscle but their nature is not well defined since mesenchymal stem cells as well as satellite cells with stem cell properties may reside in this tissue [2]. The proposal that well-differentiated tissue cells such as myoblasts and satellite cells are capable of differentiation into other tissue lineages remains controversial [15]. However, a number of reports document the apparent potential of various adult stem cells capable of converting to other tissue lineages. This process of differentiation describes the switching of one cell type to a different lineage, occurs with the loss of differentiated tissue markers and the gaining of new tissue-specific characteristics.
Urist [16] first showed that bone matrix-associated factors when implanted into muscle triggered bone induction. The bone morphogenetic proteins (BMPs) belong to the transforming growth factor-β (TGF-β) superfamily first found in demineralized bone matrix that are capable of inducing ectopic bone formation. [17, 18]. Several of these are capable of acting as potent bone inducing factors, including BMP−2, −4, and −7 [19, 20]. In addition, BMP-2 has been shown to promote maturation of osteoblastic progenitor cells [21], but could inhibit C2C12 myoblasts from following the differentiation pathway to myotubes by suppressing required muscle differentiation master genes. Subsequently, a number of studies have reported on the potential of murine myogenic cells to undergo osteoblastic differentiation following treatment with BMP-2 or related effectors [22, 23].
In the present study we have examined the potential of human fetal myogenic cells to differentiate into the osteogenic pathway and defined how adhesion and migration are modulated during this process. The α7 integrin is an important laminin receptor in skeletal muscle [24] and is involved in the formation of the neuromuscular and myotendinous junctions [25]. Its expression during early muscle development [26] and in adult satellite cells [27] suggests that it may be a marker for muscle stem cells. We have investigated the potential of isolated α7-integrin-positive cells to function as myogenic stem cells and tested their capacity to differentiate to other cell types. The results indicate that subpopulations of fetal human myoblasts expressing muscle markers including the α7 integrin are similar to adult satellite cells, able to undergo myogenesis and capable of converting to other mesenchymal differentiation programs.
MATERIALS AND METHODS
Isolation and propagation of human myoblasts
Human adult and fetal muscle was obtained from the Department of Surgery and the Department of Obstetrics and Gynecology, University of California, San Francisco. Fetal samples ranged in age from 14 to 24 weeks prenatal and consisted of limb and tongue tissue. The study was approved by the UCSF Committee on Human Research. Adult samples consisted of tissues removed during repair of craniofacial anomalies. For isolation of myoblasts, freshly-received human fetal or adult muscle was dissected free of connective tissue, soaked briefly in Betadine and washed in saline. The muscle was then dissociated both enzymatically and mechanically by mincing the muscle into a coarse slurry in a solution of dispase (grade II, 2.4 U/ml) and collagenase (class D, 1%; Hoffman-La Roche, Inc., Nutley, NJ), supplemented with 2.5 mM CaCl2. After incubation at 37°C for 30–45 min with occasional mixing, the mixture was passed through a 70 μm cell strainer (BD Biosciences, San Jose, CA) and the cells suspension was subjected to centrifugation at 350 x g to sediment the dissociated cells. The cells were immediately processed for sorting by fluorescence activated cell sorting (FACS) analysis, or alternatively, the cells were plated on laminin-1-coated dishes in F-10 medium (Invitrogen-GIBCO, Carlsbad, CA) with 20% FBS and 2 μg/ml insulin and expanded one passage prior to sorting.
For sorting cell populations were stained with anti-α7 9.1 mAb [28] by suspension in cold PBS containing a 1:4000 dilution of the ascites for 30 min. The cells were washed and then labeled with FITC-goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) followed by labeling with propidium iodide (1 μg/ml; Sigma-Aldrich, St. Louis, MO). Cells were processed using a FACStar cell sorter (Becton, Dickinson and Co., Franklin Lakes, NJ) after being gated for their PI expression and forward and scatter profiles. The top 5% α7 integrin-positive cells were isolated by FACS. Depending on the size of the muscle sample, the yield ranged from 2-10 x 105 of viable α7-positive cells. FACS isolated cells were used immediately for studies or expanded in culture for one to three passages and frozen down. Unless otherwise noted, α7 positive cells representing the top 5% integrin positive cells were isolated from fetal limb or tongue muscle after expansion by a single round of FACS.
For marker analysis by flow cytometric analysis, cells were processed to evaluate specific markers by fixation with 1% paraformaldehyde in cold PBS for 10 minutes. For staining of cytoplasmic markers, cells were also permeabilized with acetone for 10 minutes on ice. For double-stain analysis, mouse mAb 9.1 anti-α7 rabbit and anti-desmin were simultaneously incubated with cells, followed by secondary antibodies, anti-mouse IgG (PE) and anti-rabbit IgG (FITC). For each analysis, data was typically collected from 10,000 cells and analyzed with CellQuest Pro 4.1 software (BD Biosciences). Both unstained cells and cells stained with the secondary antibody only served as negative controls and background staining was similar to isotype control antibody.
Immunofluorescence microscopy
Myoblasts were seeded at 104 cells per well on poly-L-lysine (Sigma-Aldrich, St. Louis, MO)-coated chamber slides (Nalge Nunc Int., Rochester, NY) overnight. After fixing with 1% paraformaldehyde, cells were permeabilized cells with methanol or acetone (−20°C), non-specific binding was blocked with 10% normal goat or rabbit serum (Invitrogen-Gibco, Carlsbad, CA) in PBS for 1 hour. Next, primary antibody was added in 1% normal goat or rabbit serum for 1 hour with shaking. Finally, cells were stained with FITC-labeled secondary antibody for 30 min and cell nuclei were visualized with Hoechst 33258 (Invitrogen--Molecular Probes, Inc., Eugene, OR).
Induction of differentiation
Myogenic differentiation of adult or fetal α7-positive populations were using a standard protocol that included switching the nearly confluent cells from medium containing high serum (20% FBS) to low serum (2% FBS) and maintaining for 9–12 days. For induction of osteogenic differentiation, α7-positive cells were treated with 300 ng/ml BMP-2 (Peprotech, Rocky Hill, NJ) for increasing time periods up to 14 days. Osteogenic differentiation was assessed by expression of alkaline phosphatase activity, an early marker for osteoblasts [29]. Osteocalcin, a later and more advanced differentiation marker, was tested by PCR along with expression of Runx2 [29–31]. For calcium deposition studies, the culture medium was supplemented with 50 μg/ml ascorbic acid, 0.1 μM dexamethasone, and 10 mM β-glycerophosphate. Alizarin Red solution (40 mM, pH 4.2) that was filtered through Whatman paper prior to application. After fixation for 1 h at 4°C in 70% ethyl alcohol, cultures were stained for 10 min at room temperature. Nonspecific staining was removed by several washes in water. For induction of adipocytic differentiation, we visualized end stage adipocytic differentiation after treatment with the following cocktail: 0.5 mM methyl-isobutylxanthine, 1 mM dexamethasone, 100 mM indomethacin, and 10 μg/ml insulin. a cocktail of methyl isobutylxanthine, dexamethasone, insulin, and indomethacin (MDI-I). The treatment was performed for a total of 9–12 days. Positive differentiation was assessed by staining for accumulated oil droplets using Oil Red-O.
Myotube formation by α7-positive cells at ectopic sites in nude mice
We initiated studies of human fetal α7-positive myoblasts at ectopic sites in nude mice in order to determine their capacity to form myotubes. The animal protocols were approved by the UCSF Committee on Animal Research. Fluorescent latex beads (Polysciences, Warrington, PA, 5 μm diameter) are incorporated into the gel matrix so the implants can be easily identified and removed for analysis. Cells were suspended in equal mixture of cold Matrigel (13 mg/ml) and collagen type I (3 mg/ml) at 5 x 106 cells/ml. Nude mice (NIH balb/c) were injected subcutaneously in the flank with 200 μl of the cell suspension. After 12 days the tissue samples were removed and processed for routine histological analysis.
Reverse transcription-polymerase chain reaction (RT-PCR)
The cells were placed into 6-well tissue culture plates at a density of 1.5 x 105 cells/cm2. Cells were cultured for 7 days with or without BMP-2 (300 ng/ml), and total RNA was isolated from the cultured cells using RNeasy Mini Kit (Qiagen, Inc., Valencia, CA). The amount of RNA was equalized with a human-β actin competitive PCR kit (Takara Shuzo Co., Shiga, Japan). The mRNA was converted to complementary DNA (cDNA) with a GeneAmp RNA PCR Kit (Perkin Elmer, Branchburg, NJ). A single stranded cDNA equivalent of 200 ng total RNA was then subjected to The sequences of the oligonucleotides used for amplification were: Osteocalcin (NM199173) fwd (residues 154-173) 5’-TGAAGAGACCCAGGCGCTA-3’ rev (residues 259–280) 5’-GAT GTGGTCAGCCAACTCGTC-3’ (amplification of a 125 bp fragment) [32]; Runx2 (NM004348), fwd residues: 1261–1280 5’-GTCTTACCCCTCCTACCTGA-3’; rev (residues 1425–1444) 5’-TGCCTGGCTCTTCTTACTGA-3’(amplification of a 184 bp fragment) [33]; MyoD (X56677), fwd (residues 1179–1198) 5’-AAGCGCCATC TCTTGAGGTA-3’ rev (residues 1663–1682) 5’-GCGCCTTTATTTTGATCACC-3’ (amplification of a 502 bp fragment) [32]; α7 integrin (NM002206): fwd (residues 1651–1673) 5’-GCGGCCACT CGGTCTGTGTGGAC-3’, rev (residues 1932–1953) 5’-GGAGACTGTAGGACAAGGTCAC-3’ (amplification of a 303 bp fragment) [28]; α2 integrin (NM002203) fwd (residues 3555–3576) 5’-TGAGATTGATGAGA CCACAGAG-3’, rev (residues 3634–3651) 5’-GCACGCAAACAGCAAACC-3’ (amplification of a 97 bp fragment) [34]; GAPDH (NM002046) fwd (residues 556–575) 5’-TGCACCACCAACTGCTTAG-3’ (rev 714–730) 5’-GACGCAGGGATGATGTTC-3’ (amplification of a 176 bp fragment). The primer mixture, a total volume of 25 μl, contained 50 μM deoxynucleoside triphosphates, 0.5 units of Tag DNA polymerase, 1.5mM MgCl2, and 1μM of forward and reverse primers in a 10X PCR reaction buffer (200mM Tris-HCl, 500mM KCl, pH 8.3). The PCR reaction within the exponential phase of the amplification curve was performed for 30 cycles for α7 and α2 integrins, or for 25 cycles for osteocalcin, Runx2, MyoD and GAPDH in a thermal cycler (GeneAmp PCR System 9600; Perkin Elmer) under the following conditions: initial denaturation at 94°C for 10 minutes, denaturation at 94°C for 45 seconds, annealing at (57°C: α7 integrin, 61°C: α2 integrin ) for 45 seconds, and extension at 72°C for 1 min, or 94°C for 2 min, denaturation at 94°C for 30 sec, annealing at (57°C: osteocalcin, Runx2 and MyoD) for 30 seconds, and extension at 72°C for 1 min. The PCR products were loaded in a 1.5% agarose gel and visualized with ethidium bromide and photographed. The intensities of the PCR products were quantified using a scanner and digital image analysis software (Kodak Digital Science 1D Image Analysis Software, ver. 2.0; Eastman Kodak Co., Rochester, NY).
Cell adhesion assay
For analysis of cell adhesion the assay and blocking antibodies as described previously was used [28, 35, 36]. Microtiter plates (96-well Immulon 1B plates, Dynatech, Chantilly, VA) were coated with matrix proteins at the indicated concentrations for laminin-1 (Chemicon, Temecula, CA), laminin-2/4 (Chemicon, Temecula, CA) or type I collagen (PureCol Collagen, Inamed Medical Products Corporation, Fremont, CA) in PBS for 1 hour at 37°C in a humidified atmosphere. Plates were washed with medium containing 0.1% BSA and incubated with medium containing 0.5% BSA for 60 minutes in a CO2 incubator to block nonspecific adhesion. Single-cell suspensions were prepared in culture medium at 4x105 cells/ml, added in triplicate to 96-well plates, and then incubated for 30 minutes (type 1 collagen) or 40 minutes (laminin-1) at 37°C. Non-adherent cells were removed by shaking on a titer plate shaker (Lab-Line Instruments, Barnstead International, Dubuque, IA) and washing with medium containing 0.1% BSA. The number of cells bound to the substrates was estimated by fixing with 1% formaldehyde, stained with 1% crystal violet, and solubilized in 2% SDS. Absorbance was then read at 562 nm. Cells bound to laminin-1 (5 μg/ml) on a separate 96-well plate were used to indicate 100% attachment. Background cell adhesion to 0.5% BSA-coated wells was subtracted. The effect of specific antibody was tested by pre-incubating the cells with the hybridoma supernatants or dilutions of purified antibody on ice for 60 minutes prior to the assay.
Migration assay
Cell migration was assayed as described previously [35]. The undersides of the transwell (8 μm pore size, PET track-etched membrane, Becton Dickinson) were precoated with collagen type 1 (1 μg/ml) or laminin-1 (5 μg/ml). Next, 4x105 cells were loaded onto the upper chamber of the transwell and the lower chamber was filled with serum-free medium. Cells were incubated for 3 h at 37°C, fixed with 4% paraformaldehyde, and stained with 1% crystal violet. The effect of specific antibody was tested by preincubating the cells with the optimal dilutions of purified antibody on ice for 30 minutes prior to the assay. Control groups consisted of cells in the absence of antibodies. Non-migrating cells retained on the upper side were removed by wiping with a cotton swab. Cells that had migrated through the filter were counted and averaged from at least ten randomly chosen microscopic fields using a 20X objective.
RESULTS
Isolation of α7-positive myoblasts from human muscle
Past investigations have shown the α7 integrin to be a useful marker of mouse myoblasts. As an initial characterization of human fetal myoblasts, we used flow cytometry to assess levels of α7 integrin using mAb 9.1, which recognizes the extracellular domain of the receptor. Precultured myoblasts were harvested and found to consist of a mixture of generally well defined α7-positive and α7-negative subpopulations (Fig. 1A, B). We typically found that a major fraction of the isolated cells were strongly stained by the mAb 9.1. However, the ratio of the α 7-positive to α7-negative cells varied from preparation and this may to be related to the age of the individual from which the tissue was obtained or the number of passages that the primary cells were expanded in culture. However, the high expression of the integrin was consistently expressed in a major subset of the cell population and this was found to be the case in all fetal muscle tissue examined that includes over twenty isolations from either limb or tongue tissue. The population was substantially enriched for α 7-positive cells after sorting. Following expansion in culture and a second sort, cells within the population were nearly all strongly positive (>95%) for the integrin (Fig. 1C). Integrin expression remained at high levels even after continued culturing of the cells beyond ten passages (not shown).
Fig. 1.
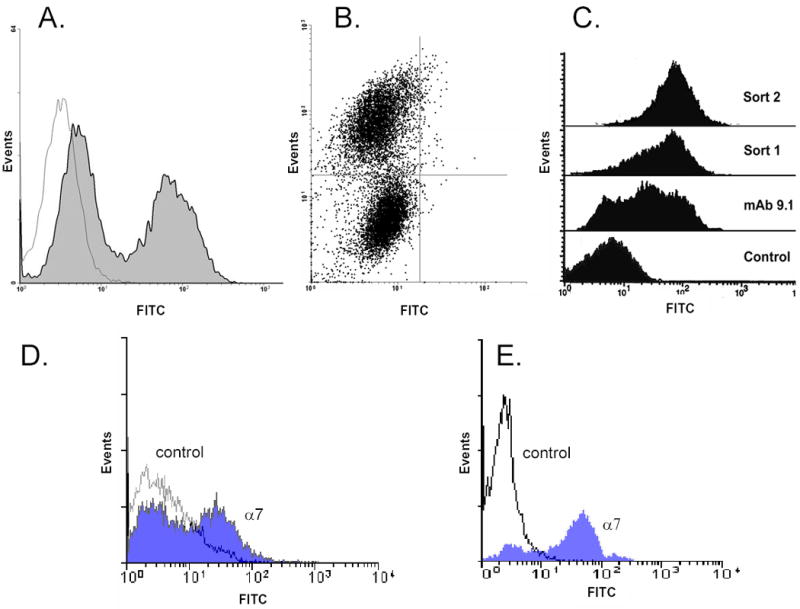
Isolation of α7-expressing human myogenic precursor cells by fluorescence-activated cell sorting (FACS). Cells from human fetal tongue muscle (19 wk) were expanded in vitro and sorted using FACS with α7 integrin as marker. Two distinct populations representing α7-positive and α7-negative cells were detected in the histogram (A) where the parameters are FITC versus cell counts and in a dot plot (B) where the parameters are FITC versus side scatter (SCC). (C) Human fetal limb muscle (20 wk) was processed and the resulting cells were stained with mAb 9.1 against human α7. Note a mixture of both negative and positive α7 staining cells. Following FACS and expansion the cells show strong expression of α7 (Sort 1) and after the second sort (Sort 2) and expansion the expression of the integrin is maintained.
(D). α7 expression of human fetal muscle cells prior to culture. Human fetal limb muscle (18 wk) tissue was processed for myogenic cell isolation. The resulting cells without preculture or sorting were stained with control antibody or with mAb 9.1 against human α7
(E). Isolation of α7 expressing human satellite cells from adult muscle. Adult masseter muscle from a 43 year old female was subjected to dissociation and cells were expanded in culture without sorting and then analyzed by FACS analysis. A significant fraction of the population expressed the α7 integrin as detected by the 9.1 mAb.
It is known that adaptation of myoblasts including satellite cells to cell culture leads to gene activation and expression of a number of proteins including myogenic transcription factors [37]. In order to determine whether positive α7 integrin expression was present in situ for muscle-associated mononuclear cells and not induced by preculture, flow cytometry was performed on freshly-harvested, cells from fetal muscle without culturing (Fig. 1D). Interestingly, freshly isolated cells showed a similar profile to cultured cells where roughly half of the population expressed α7. Since the cells were not subjected to culture prior to FACS, this suggests that a preexisting subpopulation of fetal muscle-associated cells expressed the α7 integrin. We next examined the population of α7 expressing cells isolated from adult skeletal muscle using similar isolation methods for fetal muscle. (Fig. 1E). In this preparation of unsorted cells, a major fraction of the population was α7-positive and appears to correspond to satellite cells, but the ratio varied somewhat from one preparation to another and generally ranged from 50–75%. After further expansion the cells were again analyzed by FACS and the majority of the cells remained positive for the α7 receptor (data not shown).
Characterization of α7-positive myoblasts
To further characterize the myoblast subpopulations, α7-positive myoblasts were analyzed for desmin expression, a marker of muscle cell lineage, by dual flow cytometry (Fig. 2A). The previously sorted α7-positive myoblasts were positive for α7 integrin as expected, but showed a broad expression profile for desmin suggesting heterogeneity within the population. To further examine the expression of desmin, unsorted freshly isolated human fetal myoblast cells were compared to previously sorted α7 integrin-positive cells by FACS analysis for α7 integrin (PE) and desmin (FITC) expression. High α7 integrin expression was indicated by positive cells in the upper quadrants of the dot plot. The four quadrants represent negative baseline controls for PE and FITC. In unsorted cells (Fig. 2B), desmin staining showed heterogeneity with a major fraction of cells expressing relatively high levels of desmin. However, possibly up to four different subpopulations were observed in the unsorted cells ranging from α7+/desmin+ to α7−/desmin- consistent with the idea that these are multiple subpopulations of myoblasts. However, for the previously α7-sorted cells (Fig. 2C) there were primarily only two subpopulations: one showing both high α7 and desmin expression and a second population with high α7 expression but lower levels of desmin staining. In order to further define these populations, α7-positive myoblasts were labeled with Hoechst-nuclear stain and co-stained with antibodies to desmin or MyoD (Fig. 2D). Desmin was consistently detected in a subpopulation of the α7-positive cells but the cell population was heterogeneous with a significant fraction of cells showing poor staining (Fig. 2D). Strong MyoD nuclear staining was visible for the subfraction of α7-positive cells but a significant number of cells showed a diffuse cytoplasmic staining and weak nuclear staining (Fig. 2E).
Fig. 2.
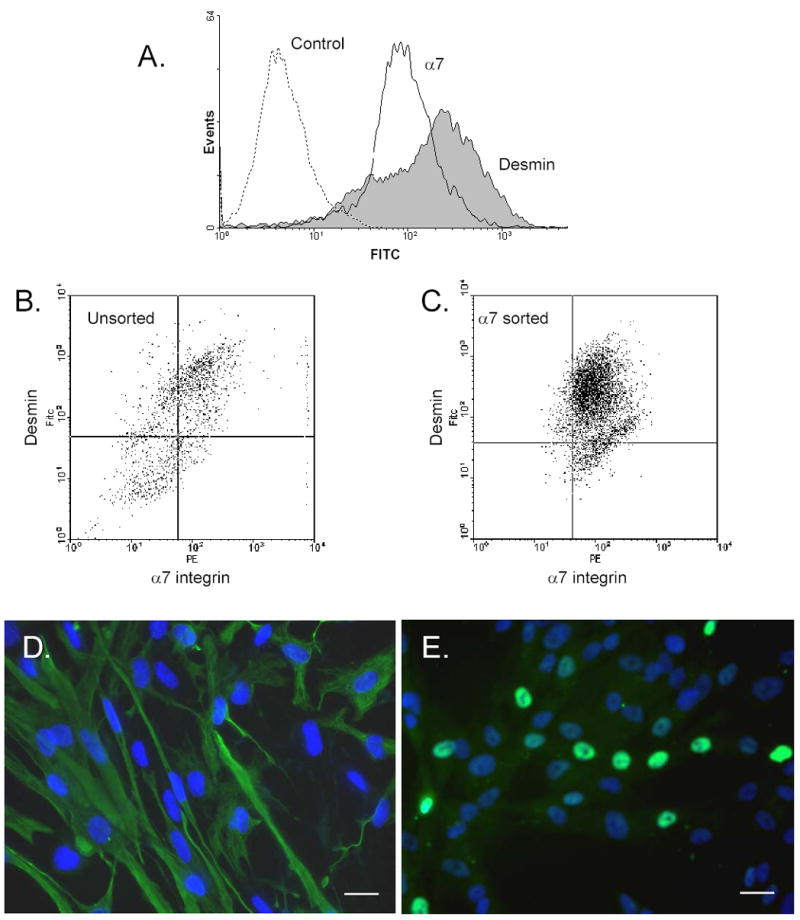
Expression of desmin and MyoD by fetal human myogenic cells. (A). Human fetal cells (tongue, 22 weeks) previously sorted for α7-positive expression were analyzed by flow cytometry for desmin and α7 integrin. (B) Freshly dissociated cells from human fetal muscle (tongue, 23 weeks) that were not previously cultured or sorted were analyzed for α7 integrin and desmin expression by costaining and flow cytometry. (C) human fetal muscle (tongue, 22 weeks) previously sorted for high α7 were analyzed for α7 integrin and desmin expression by costaining and flow cytometry. Four quadrants were created using the negative controls for PE and FITC as baselines. Higher α7 integrin expression is indicated by cells in the dot plot on the right quadrants, whereas higher desmin expression is indicated by cells in the upper quadrants. In (B) several different subpopulations were observed that ranged from α7+/desmin+ to α7–/desmin–. Selective expression of desmin and MyoD by α7-positive myoblasts. Human fetal muscle cells (tongue, 22 wk), sorted for high α7 integrin expression, were stained with anti-desmin (D) or anti-MyoD (E) antibodies and nuclei were stained with Hoechst dye. Note that both α7-positive cells showed heterogeneity in desmin and MyoD expression. Bar, 20 μm.
We next used flow cytometry to further analyze the expression of additional myogenic markers, including CD56/N-cam, M-cadherin, c-Met, Numb, and Notch. α7-positive cells were found to strongly express levels for several of these markers (Fig. 3). However, when freshly isolated and uncultured cells were examined, it was found that CD56 (NCAM) was not expressed while the other markers were positive (data not shown), suggesting that culture and activation of myoblasts may induce CD56 expression. That the cultured fetal muscle cells maintained expression of this set of muscle-related markers suggested that the α7-positive cells represented myogenic precursors.
Fig. 3.

Myoblast differentiation marker analysis of α7-positive cells. Human fetal myoblasts (Tongue, 20 wk) were processed for flow cytometry following staining with isotype control antibody (profile represented by gray line) and the indicated specific antibodies (profile represented by black line): anti-α7 integrin, anti-CD56/NCAM, anti-c-Met, anti-Numb, and anti-Notch-1.
α7-positive myoblasts are fusion-competent
FACS-purified human α7-positive cells were examined for their capacity to form syncytial myotubes following switching to differentiation conditions with low serum. The unsorted population failed to yield high numbers of multinucleated myotubes even after prolonged culture (Fig. 4A1, 4A2). Similarly, sorted α7-negative cells also lacked the capacity to fuse and form myotubes (not shown). The identity of these cells remains unclear but presumably consists of contaminating fibroblast and connective tissue cells. In contrast, the population of cells showing significant enrichment for α7 was able to form an extensive array of aligned multinucleated myotubes indicating that the majority of cells were fusion competent (Fig. 4B1, 4B2). The capacity of the α7-positive cells for fusion and myotube differentiation suggests that this subset of cells is of myogenic lineage.
Fig. 4.

Myotube formation by α7-positive human myogenic cells. Human fetal muscle tissue were dissociated and sorted for high α7 integrin expression. The unsorted population and the α7-positive population were then allowed to differentiate into myotubes by culturing in low serum-differentiation medium. High α7-integrin-expressing myoblasts (Tongue, 19 wk) show a high frequency of differentiating myotubes (B), whereas the unsorted α7-expressing cells did not (A). Bar, 50 μm. (A2) and (B2) are enlargements. Bar, 25 μm. Note presence of cell fusion and multinucleation in (B1, 2). α7-positive (D) and α7-negative (C) fetal muscle cells (tongue, 20 wk) were cultured under differentiation conditions as above, and processed for staining with anti-desmin antibody. Bar, 25 μm. Strong desmin staining was detected in the α7-positive population. Myotube formation by α7-positive (F) and α7-negative (E) myoblasts at ectopic sites in nude mice. Fetal muscle cells (Tongue, 22 wk) were injected subcutaneously into the flank of NIH nude mice as described in Materials and Methods. The resulting tissue was harvested, sectioned and evaluated by hematoxylin and eosin staining. Myotubes were formed by α7-positive cells (arrows), while no myotubes were detected with α7-negative cells. Bar, 50 μm.
Isolated α7-positive cells were next evaluated for their capacity to differentiate into myotubes in vivo. Human fetal α7-positive or negative myoblasts were implanted at ectopic sites in nude mice in the presence of an artificial scaffold: a hybrid mixture of collagen type I and Matrigel implanted subcutaneously. Previous studies have suggested that Matrigel containing scaffolding enhances formation of myotube by satellite cells [38]. In order to facilitate localization of the implant, fluorescent latex beads (5 μm diameter) were incorporated into the gel matrix. The implant was removed and processed for histological analysis. We found frequent formation of multinucleated myotubes from implanted α7-positive cells (Fig. 4D, 4F). There was also extensive vascularization of the implant. In contrast, α7-negative cells failed to induce formation of myotubes (Fig. 4C, 4E). These results indicate that α7-positive cells are myogenic and the use of a hybrid ECM support, composed of both interstitial (collagen) and basement membrane-specific ligands, may facilitate myogenic cell survival and differentiation. Thus, human α7-positive cells were capable of differentiation into myotubes at high efficiency, suggesting that expression of this receptor may be a marker for myogenic cells.
Analysis of clonal populations
Since myoblast preparations isolated from human muscle consist of a mixture of cell types even after sorting for α7-positive integrin expression, we performed clonal analysis of the population. Human fetal limb myoblasts that expressed high levels of α7 integrin were directly cloned into 96 well tissue culture plates using FACS. A number of individual clones derived from verified single cells were successfully expanded for more than 20 generations and several of these α7-positive clones were subjected to further analysis. α7-positive cloned cells remained strongly positive for α7 and continued to proliferate, indicating that their growth potential is consistent with that of a stem cell population (Fig. 5A). Since the individual colonies were each obtained from a single cell, this is evidence of clonality.
Fig. 5.

Heterogeneity of cells in cloned α7-positive myoblasts. Human fetal myoblasts (tongue, 23 wk) expressing high α7 integrin were cloned into using FACS as detailed in Materials and Methods section. Several clones (designated A2, B2, and D5) were expanded for approximately 20 generations and analyzed for expression of α7 integrin by flow cytometry (A) and also immunostained with anti-desmin antibody and Hoechst dye for nuclear staining (B,C). Bar, 20 μm. Note that all three clones showed positive but variable expression α7 integrin. Staining for desmin showed heterogeneity of expression in the relative staining frequency for desmin. Capacity to form myotubes was evaluated for the three clones as described in Fig. 4. Clones A2 and D5 formed extensive myotubes, but clone B2 generated few fused cells (D).
We next examined the α7 positive clones for desmin expression and their potential to terminally differentiate into myotubes. Analysis of the clones for desmin expression revealed a complex expression pattern with a significant proportion showing negative staining for desmin (Fig. 5B, C). Of three clones examined, the percent of desmin positive cells ranged from 43–89 % (Fig. 5B). Interestingly, the clone with the highest percent of desmin positive cells (clone D5) also had the highest cell proliferation rate (data not shown). The potential of the clones to undergo myoblast differentiation to myotubes was also tested and all three clones showed some capacity for myodifferentiation. However, the B2 clones formed only small numbers of myotube whereas A2 and D5 formed extensive myotubes (Fig. 5D).
Multipotential capability of α7-positive human myoblasts
The enriched population of α7-positive human myoblasts was next analyzed for their multipotent differentiation potential along osteoblastic or adipocytic lineages. α7-negative or positive myoblasts were grown under low serum conditions, in the presence or absence of BMP-2, a potent inducer of the osteogenic pathway. The α7-negative cells failed to show induction of alkaline phosphatase activity, indicating that the potential for myogenic and osteogenic is contained within the α7-positive population (Fig. 6A,B). When α7-positive cells were treated with BMP-2, they failed to differentiate into myotubes and instead differentiated along the osteogenic pathway. The induction of alkaline phosphatase, a marker for osteoblast activity, revealed that a majority of BMP-2 treated cells strongly expressed the enzyme, whereas the control cells did not (Fig. 6C,D). Next the capacity of BMP-2 to induce mineralization of the α7-positive myoblasts was tested by staining with Alizarin Red. After 12 days of culture in the presence of BMP-2, deposits of matrix were stained by Alizarin Red while the control cells did not (Fig. 6 E,F).
Fig. 6.

Osteogenic and adipocytic differentiation of human α7 integrin-sorted myogenic cells. For induction of osteogenic differentiation, human fetal muscle cells (tongue, 21 wk) sorted for high and low α7 integrin expression were cultured in the presence or absence of BMP-2 for 7 days and processed for alkaline phosphatase staining. α7-negative cells did not show significant induction of alkaline phosphatase with (A) or without BMP-2 treatment (B). The α7-positive satellite cells in growth medium alone did not show staining (C) whereas α7-positive cells treated with BMP-2 showed strong induction of alkaline phosphatase activity (D). Mineralization of α7-positive cells in the absence (E) or presence (F) of BMP-2 for 12 days as described in Materials and Methods section and assessed by Alizarin Red-S staining. α7-positive cells were tested for adipocytic differentiation in either control growth medium (G) or in adipocytic induction medium (H) as described in Materials and Methods section. Bar, 50 μm.
The ability of α7 positive myoblasts to follow adipocytic lineage was examined next. Following culture under permissive conditions for adipogenesis, the human α7-positive myoblasts were found to also have the potential to differentiate into adipocyte-like cells. In Fig. 6G, cells were cultured in regular culture medium and in Fig. 6H, cells were cultured with adipogenic differentiation medium and both cultures stained with Oil-Red O. Positive differentiation was assessed by staining for accumulated oil droplets. The control culture shows no staining whereas the treated culture shows extensive numbers of Oil Red O-positive cells containing large lipid droplets. These results support the idea that the α7-positive myoblasts are not committed to the muscle lineage and have potential for differentiation not only into myotubes, but also osteoblasts or adipocytes. This also strongly suggests that human α7-positive myoblasts demonstrate plasticity and, following appropriate cues, can switch from a muscle phenotype and begin to differentiate towards an osteoblastic or adipogenic lineage.
To further examine the potential of α7-positive myoblasts to differentiate into osteogenic cells, BMP-2 treated myoblasts were tested for expression of MyoD, Runx2, and osteocalcin by RT-PCR analysis (Fig. 7A). MyoD, a master regulator transcription factor for skeletal muscle differentiation, was expressed in the untreated myoblasts as expected. Following induction with BMP-2, MyoD expression was strongly suppressed. Conversely, treatment with BMP-2 induced expression of Runx2/Cbfa1/Osf2, an osteoblast-specific transcription factor essential for osteoblast differentiation, and osteocalcin (BGP), a marker of late stage osteoblast differentiation. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control. Next, Western blotting was used to confirm the expression of Runx2 and osteocalcin at the protein level (Fig. 7B). For Runx2, protein signal was evident after 5 days of induction by BMP-2 and was fully expressed by 7 days. Osteocalcin expression was already detectable by 2 days of treatment with BMP-2 and increased expression was further elevated on days 5 and 7. In contrast, neither Runx2 nor osteocalcin was detectable in control cultures of myoblasts cultured for similar time in the absence of BMP-2 treatment (data not shown). Interestingly, Runx2 induction occurred almost immediately after BMP-2 treatment whereas osteocalcin expression was only detected after 5 days treatment. This is consistent with the fact that Runx2 is a master regulator of osteogenic differentiation and controls activation and expression of its target genes, including osteocalcin [39]. These results confirm the idea that the α7-positive myoblasts have potential for osteogenic differentiation and upregulated differentiation markers following BMP-2 treatment.
Fig. 7.

Expression of osteoblast markers in α7-positive muscle stem cells (G). Semi-quantitative RT-PCR using specific primers showed that α7-positive cells (tongue, 21 wk) treated with BMP-2 upregulated osteogenic-related gene expression for Runt-related transcription factor-2 (Runx2) and osteocalcin (OCN) but downregulated MyoD. GAPDH levels are shown as controls.
Modulation of integrin expression and function following osteogenic differentiation
It is well understood that during the differentiation of stem-like progenitor cell populations, terminal differentiating cells must migrate to the appropriate location for tissue regeneration. In the case of myogenic stem cells, unique integrin profiles may facilitate correct localization and alignment of precursor cells needed for generation of new muscle, bone, or fat tissue. We have focused on changes in integrin expression and adhesive function that may be occurring following induction of osteogenic differentiation. First we examined the repertoire of integrins expressed on α7-positive fetal myoblasts through flow cytometry using subunit-specific mAbs that indicated the presence of a diverse set of integrin α chains on the human myoblasts (Fig. 8A,B). We found little α2 integrin expression, but significant levels of α6 and αv were present along with abundant α7 expression and lower levels of α1 and α5. Most of these integrins can heterodimerize with the β1 integrin subunit, and FACS confirmed high levels of this partner subunit (see below). Since there was no detectable expression of the β4 integrin, the α6 integrin must associate with the β1 subunit. The αv subunit can complex with several β integrins, so further work is needed to define the potential pairs.
Fig. 8.
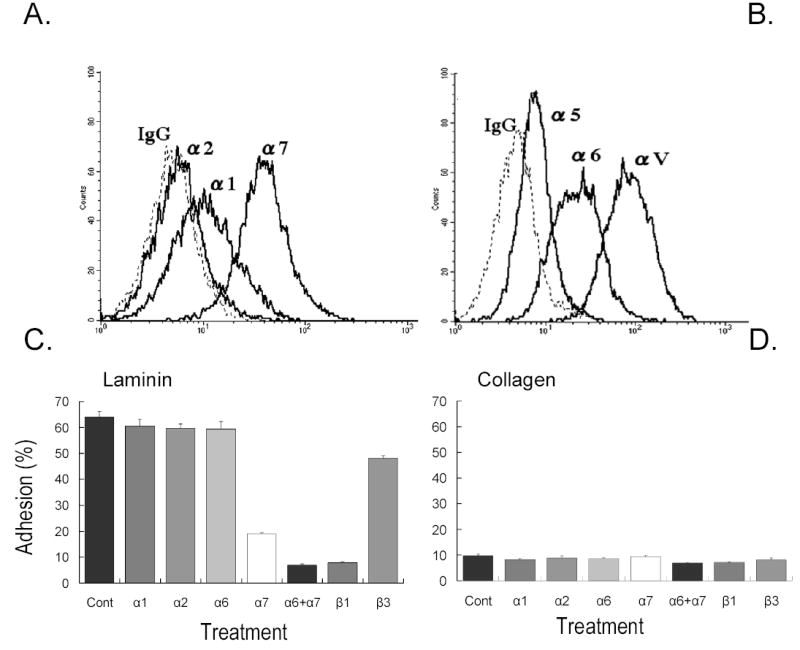
Integrin expression and adhesion profile for α7-positive cells. Cell surface expression of integrin subunits on α7-positive fetal muscle (tongue, 21 wk) cells were processed for flow cytometry to assess integrin expression using specific mAb for each receptor (A, B). Cells were found to express high levels of α6, α7 and αν integrins with lower expression of α1, α2, and α5 integrins. Experiments were repeated at least three time with similar results.
Cell adhesion was assessed on laminin-1 (C) or collagen type I (D) substrates and was measured in the presence of specific anti-integrin antibodies as described in Materials and Methods. Values are means of three wells. Bars show s.d.
We evaluated the adhesive potential of α7-positive myoblasts to attach on laminin-1 substrates in the presence of function-perturbing mAbs to relevant integrin receptors. The 9.1 mAb to α7 substantially inhibited cell adherence to laminin-1, but did not completely abolish it (Fig. 8C).The partial resistance of cells to the α7 function-blocking mAb suggested that the α6 integrin could be involved in binding to laminin-1. In accordance with this possibility, we found that the combination of the 9.1 mAb with the anti- α6 GoH3 mAb completely blocked adhesion to laminin as did antibody to β1. This suggested that within this population of sorted myoblasts, α7β1 is the dominant receptor and α6β1 is partially involved as well. We next tested adhesion of the human myoblast to a preparation of laminin 2/4 (merosins). The merosins are the major laminins found in skeletal muscle basement membranes [40, 41]. Adhesion to laminin-2/4 was similar to laminin-1 with the primary receptor being α7 and partial involvement of the α6 integrin (data not shown). Myoblast adhesion to type I collagen was near background levels (Fig. 8D) suggesting the cells do not express functional receptors for this ligand.
In order to investigate the consequences of changes in integrin expression, we examined the adhesive phenotype of α7-positive myoblasts following differentiation to the osteoblast lineage, Following BMP-2 treatment, the adhesion to laminin-1 substrates was dramatically reduced (Fig. 9A). Control α7-positive myoblasts adhered with an efficiency of about 80%, whereas following BMP-2 treatment only 25% of the cells were able to attach. Moreover, for the minor fraction of osteogenic cells that were able to attach, adherence was sensitive to α2 or β1 integrin blocking mAb (Fig. 9C). This is consistent with the importance of the α2 integrin in mediating adhesion to laminin-1 in BMP-2 differentiated cells. In contrast, anti-α7 had no apparent effect on adhesion to laminin-1 in these cells. The α7-sorted myoblasts displayed poor adhesion on type I collagen substrates and only about 10% of the cells were able to adhere to this interstitial ligand (Fig. 9B). However, there was a striking increase in adhesion efficiency to type I collagen following BMP-2 induced differentiation. Analysis with blocking anti-integrin antibodies showed that attachment was mediated primarily by the α2 integrin, whereas blocking of other with α chain mAbs was without effect (Fig. 9D). As expected, the partner β1 subunit was involved, indicated by the complete abolition of adhesion with anti-β1 mAb.
Fig. 9.

BMP-2 modulates human fetal myogenic cell adhesion and motility. Untreated α7-positive myogenic cells (tongue, 21 wk) or cells treated with BMP-2 as detailed in Materials and Methods were assessed for adhesion on plates coated with different concentrations of laminin-1 (A) or collagen type I (B). Adherence of cells in 1% BSA-coated wells was treated as background binding and subtracted. Data are presented as percentage of the total cells added to each well. Values are means and s.d. of the triplicate wells. (C, D). Modulation of adhesion receptor activity by BMP-2. Control and BMP-2 treated cells were assessed for adhesion on laminin-1 or collagen type I in the presence of anti-integrin antibodies as described above. Values are means and s.d. of the triplicate wells.
BMP-2 alters cell migration (E, F). Untreated α7-positive myogenic cells or cells treated with BMP-2 were tested for their motility on laminin-1 in the presence of function-perturbing mAbs using a modified Boyden chamber assay (Materials and Methods section). Motility was measured by counting the number of cells that migrated to the undersides of the membranes. The results are expressed as the mean of at least nine random x400 microscopic fields; bars show s.d.
We also examined the effect of BMP-2 induced differentiation on cell motility. In these studies, parallel assays examined migration on laminin-I and collagen substrates. On laminin-1, the myoblasts showed a strong and persistent locomotive response (Fig. 9E). Consistent with the high α7 integrin expression, migration was effectively blocked with the 9.1 anti-α7 mAb. Similarly, anti-β1 antibody was effective in inhibiting motility. Following treatment with BMP-2, motility was significantly reduced with a 60% decrease in motility compared to untreated control cells. Interestingly, the weak migration response on laminin in the BMP-2 treated cells was mediated by α2 integrin as shown by antibody inhibition assays. This inhibitory effect with anti-α2 was similar to that generated by the anti-β1 antibody. Motility was also tested on type I collagen substrates (Fig. 9F). α7 integrin-positive myoblasts typically showed poor migration on this ligand and this is expected as they lack high expression of the α2 collagen receptor. In contrast, cells induced with BMP-2 showed a dramatic increase in migration on collagen. This strong response was dependent on the α2β1 receptor, since antibodies to either α2 or β1 subunits completely abolished motility.
We next analyzed integrin expression following BMP-2 treatment, to examine a link between specific integrin subunit expression and adhesive/migratory behavior. When we examined the panel of integrin receptors by flow cytometry, we found that BMP-2 induced a nearly complete loss of α7 expression (Fig. 10A, C). Also, in BMP-2 treated cells, the α2 collagen receptor showed a strong induction of expression, whereas in control cells the receptor was present in background levels (Fig. 10B, C). Thus, the adhesive properties of the BMP-2 treated cells showed a strong collagen and weak laminin binding, paralleling the reciprocal induction of α2 and suppression of α7 integrin expression.
Fig. 10.

BMP-2 induces loss of α7 integrin and upregulates α2 integrin. α7-positive cells (tongue, 21 wk) were cultured in medium without or with BMP-2 for 7 days and processed for flow cytometry with anti-α7 mAb (A) or anti-α2 mAb (B). Controls (isotype control antibody, dashed line) was performed with secondary antibody (FITC conjugated mouse IgG). Note following BMP-2 treatment that α7 integrin expression is lost while α2 integrin is strongly induced.
Relative surface expression of integrin subunits on α7-positive myogenic cells with and without BMP-2 treatment (C). Values from controls of isotype/secondary antibody were subtracted to give the mean fluorescence intensity.
α7-positive cells (tongue, 21 wk) were cultured in medium without or with BMP-2 for 7 days and processed for semi-quantitative RT-PCR analysis with α7-specific (D, upper panel) or α2-specific (D, lower panel) primers. The normally high expression of α7 mRNA in myoblasts is lost following BMP-2 treatment whereas α2 integrin mRNA is strongly induced. GAPDH levels are shown as a control.
Analysis of RT-PCR showed that the loss of α7 expression and induction of α2 integrin expression occurred at the level of mRNA (Fig. 10D). Myoblasts treated with BMP-2 down-regulated α7 integrin and upregulated α2 in comparison with non-treated control myoblasts, which confirms that myoblasts treated with BMP-2 had undergone differentiation and lost muscle-specific markers. This result confirms that not only does BMP-2 down-regulate α7 integrin levels in myoblasts, but also induces a reciprocal increase in the α2 integrin, which is the dominant collagen receptor used by osteoblasts. Myoblasts reside in a laminin-rich basement membrane in the sarcolemma and maintain high levels of the laminin α7 integrin receptor. Following differentiation to the osteogenic lineage, cells express the collagen receptor α2 integrin needed to adhere to, and interact with, interstitial collagen matrix of bone in order to maintain a fully differentiated phenotype. Integrins α1, α3, α5, α6, αv, and β1 tended not to change expression following BMP-2.
DISCUSSION
It is generally accepted that muscle satellite cells are myogenic progenitors functioning as stem cells that can regenerate skeletal muscle while retaining capacity for self renewal. In the current studies both isolated human fetal myoblasts and adult satellite cells appeared committed to muscle lineage despite displaying multipotency. However, the developmental pathway by which satellite cells are derived has not been well defined and remains controversial [42–44]. Recent evidence supports the idea that in the mouse, satellite cells arise from pre-existing Pax 7-positive cells during fetal development and do not appear to develop separately from a unique cell lineage [45]. Recently, human satellite cells have also been shown to express Pax 7 [46] but it is not clear if this effector plays a similar role in the human and may not be a useful marker for satellite cells in human muscle [46].
Much progress in understanding muscle development and satellite cell function has been obtained mostly in the mouse. However, at present only limited studies have focused on human muscle development. One difficulty has been efficient isolation of human fetal and adult myogenic progenitors. Other studies have shown that cultures of human myoblasts from adult muscle can be contaminated with non-myogenic cells including fibroblastic [47] and pericyte-like cells [48]. Similarly, fetal human myoblast preparations also appear to contain non-muscle cells and frequently clonal populations are used to eliminate contamination issues [49]. In the current study, we isolated human fetal and satellite muscle stem cells by taking advantage of the high expression of the α7 integrin as a marker for the myogenic lineage. The α7-positive fetal cells were capable of fusion and could differentiate into myotubes with high efficiency. We show that both fetal and adult human cells can be easily be purified and expanded in vitro to obtain large numbers of differentiation-competent myoblasts that might be suitable for engineering into other tissues.
The α7 integrin is an important adhesion receptor whose initial expression in primary myotubes and secondary myoblasts occurs early during development [24, 50, 51], and remains strongly expressed in quiescent satellite cells in adult muscle [24, 27, 52]. In fully developed muscle the integrin forms essential adhesions at costameric and myotendous junctions. Relatively low levels of α7 in myoblasts ensured motility during muscle development and regeneration whereas high levels are needed for efficient MTJ formation after muscle differentiation[53, 54]. Previous studies on this differentiation-specific adhesion receptor have been performed primarily in rodent muscle with limited studies in human muscle development. The present studies establish that both fetal and adult myogenic progenitor cells strongly express the α7 integrin. Detailed analysis of differentiation markers suggests that the cultures of α7 positive fetal cells represent myogenic cells at different developmental stages. For example, the populations were typically heterogeneous with a significant subpopulation of freshly isolated or passaged α7 positive cells positive for MyoD or desmin. The cells that were negative for nuclear MyoD staining may represent more primitive precursors. When cells were examined immediately after dissociation from tissue but prior to culture, a significant number showed positivity for desmin and this expression was maintained during the primary and subsequent passages. This is consistent with earlier studies of human fetal muscle, where immunochemical analysis at 20 and 29 week stages identified desmin expression in undifferentiated myoblasts [55]. Desmin-positive cells may represent a more differentiated lineage of myoblast precursor cells known to be present in fetal muscle. In human adult satellite cells, desmin does not appear to be strongly expressed but is one of the first proteins expressed following activation [56, 57]. Cultures of α7 positive fetal cells were also uniformly positive for muscle markers that include c-Met and M-cadherin. CD-56/NCAM was found to be expressed in the population, but expression was heterogeneous, with a spread in expression levels. Importantly, expression was increased following continued passaging as initially isolated cells were mostly negative. Cells also expressed Notch and its inhibitor, Numb. Rando and collaborators [37] have recently shown that active Notch is important in the expansion of satellite progenitors and is modulated by Numb, that permits progression to more differentiated myoblast stage. The expression of both Notch and Numb would be consistent with a mixed set of cells that are in different levels of myogenic differentiation. These data reinforce the findings that the cell population from the muscle stem pool is heterogeneous.
Our studies showed that α7-positive cells isolated from human fetal muscle and expanded in culture generally follow marker profiles seen in previous studies of adult satellite cells isolated from mouse or human muscle. Many of these markers are expressed in quiescent or activated/proliferating mouse satellite cells (Pax-7, c-Met, M-cadherin, NCAM) while others are specific for activated cells (MyoD, Myf-5, and desmin) [6, 58]. Yet other work has shown that a subpopulation of satellite cells lack Myf5, CD34 or M-cadherin expression and may be represent poorly differentiated stem cells [42, 59] and additional studies indicate that most cells express Myf5 and M-cadherin, but a small fraction of cells are negative for all [5]. In previous studies of human adult and fetal myoblasts, heterogeneity was also reported for satellite markers including Pax-7, myogenin, MyoD, and Myf5 [46]. Our results using individual cell clones replicated this heterogeneity suggesting that the diversity in cell phenotype is not simply due to the presence of multiple cell lineages in the population. Individual clones of α7 positive cells displayed a similar divergence in phenotype where the cells consisted of a mixture of more differentiated, desmin-positive cells with a variable fraction of the population being desmin-negative. This implies that subsets for the human fetal myogenic cells may be the precursor for satellite cells.
Recent studies have suggested that adult somatic stem cells may be capable of converting to other tissue lineages. This complex process involves the switching of one cell type to another and occurs with the loss of differentiated tissue markers and the acquisition of new tissue-specific characteristics. Our results indicate that isolated α7-positive cells are clonogenic and capable of expansion and have multipotent renewal potential. This is consistent with the presence of the multipotent stem cells in the α7-positive population. These cells have a full complement of muscle-specific markers such as M-cadherin and c-Met, yet they did not appear to be fully committed to the myogenic lineage and appear capable of differentiating to the osteogenic and adipocytic pathways. The results are similar to other recent studies that indicate that mouse satellite cells are multipotent and are capable of differentiating to other cell types after induction [22, 23]. More recently, human myoblasts were found to be capable of differentiation to osteoblasts and other cell fates [11, 60]. It is interesting that the populations display heterogeneity for mature myoblasts such as MyoD and desmin yet other cells do not. In the present studies, following BMP-2 treatment the expression of these markers is downregulated and a majority of the cells appear to progress along a osteoblastic lineage. It remains unclear if these myogenic cells after switching to osteoblastic cells still retain stem cell-like capacity.
In isolated human fetal myoblasts, the α7 integrin is highly expressed and was found to mediate adhesion to laminins, the major attachment proteins in the surrounding basement membrane. In contrast, we found that fetal cells did not express high levels of the α2 integrin that binds interstitial collagens and consequently these cells displayed poor adhesion to this substrate. Others have shown poor adhesion of myoblasts to collagen [61]. Following BMP-2 treatment, the fetal cells were induced to differentiate along the osteogenic lineage. Expression of the α7 integrin was lost while the α2 integrin became strongly expressed and promoted increased binding to type I collagen. Adult satellite cells also express the α7 integrin and following BMP-2 induction also show a similar osteogenic differentiation and concurrent switch in integrin profiles (data not shown). In osteoblasts, the α2β1 integrin that binds type I collagen, the major bone matrix protein, facilitates osteoblastic differentiation and function [62, 63]. Thus, the coordinate regulation of adhesion receptors may be important for controlling adhesion and migration of precursor cells following injury and facilitate their correct positioning within the tissue microenvironment.
In conclusion, we have described a method to isolate multipotent human myoblasts based on their expression of α7 integrin as a surface marker. The α7-positive myoblasts expressed multiple established muscle specific markers, were capable of efficient myogenesis, but also had the potential to differentiate along multiple cell lineages. This indicates that α7 integrin expression is not only useful to identify differentiation-competent cells, but may also identify multipotent capability and suggests these progenitors have stem cell-like properties. Little is known about how extracellular signals are able to induce differentiation from one mesenchymal lineage to another. However, the modulation of expressed adhesion receptors may be an important mechanism by which stem and progenitor cells are recruited to target tissues. Importantly, specific ECM-integrin interactions by resident stem cells found in each tissue microenvironment may provide the proper cue for terminal differentiation and tissue-specific regeneration. Understanding how integrin expression is modulated as myogenic stem cells progress along specific differentiation pathways (e.g., muscle and bone) may provide insights into the hierarchy of the molecular mechanisms and signaling networks that regulate cell fate.
Acknowledgments
We thank Dr. Rik Derynck and Dr. Caroline Damsky for helpful discussions. The expert assistance in cell culture assays by Kelly Kwon is greatly appreciated. This study was supported by NIH grant DE015404.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mauro A. Satellite cells of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 3.Campion DR. The muscle satellite cell: a review. International Review Of Cytology. 1984;87:225–251. doi: 10.1016/s0074-7696(08)62444-4. [DOI] [PubMed] [Google Scholar]
- 4.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35:1151–1156. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 7.Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richler C, Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970;23:1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- 9.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinanan AC, Hunt NP, Lewis MP. Human adult craniofacial muscle-derived cells - CD56/NCAM expressing cells appear to contain multipotential stem cells. Biotechnol Appl Biochem. 2003 doi: 10.1042/BA20030185. [DOI] [PubMed] [Google Scholar]
- 12.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 13.Tajbakhsh S, Cossu G. Establishing myogenic identity during somitogenesis. Curr Opin Genet Dev. 1997;7:634–641. doi: 10.1016/s0959-437x(97)80011-1. [DOI] [PubMed] [Google Scholar]
- 14.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 16.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 17.Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, Wozney JM. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci U S A. 1990;87:9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leboy PS. Regulating bone growth and development with bone morphogenetic proteins. Ann N Y Acad Sci. 2006;1068:14–18. doi: 10.1196/annals.1346.003. [DOI] [PubMed] [Google Scholar]
- 19.Wang EA, Rosen V, D'Alessandro JS, Bauduy M, Cordes P, Harada T, Israel DI, Hewick RM, Kerns KM, LaPan P. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87:2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ, Suda T, Yoshiki S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol. 1991;113:681–687. doi: 10.1083/jcb.113.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res. 1999;296:183–190. doi: 10.1007/s004410051279. [DOI] [PubMed] [Google Scholar]
- 25.Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem. 2003;278:14587–14590. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- 26.Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, Burkin DJ. Role for the alpha7beta1 integrin in vascular development and integrity. Dev Dyn. 2005;234:11–21. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- 27.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 28.Vizirianakis IS, Yao CC, Chen Y, Ziober BL, Tsiftsoglou AS, Kramer RH. Transfection of MCF-7 carcinoma cells with human integrin alpha7 cDNA promotes adhesion to laminin. Arch Biochem Biophys. 2001;385:108–116. doi: 10.1006/abbi.2000.2134. [DOI] [PubMed] [Google Scholar]
- 29.Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MH, Javed A, Kim HJ, Shin HI, Gutierrez S, Choi JY, Rosen V, Stein JL, van Wijnen AJ, Stein GS, Lian JB, Ryoo HM. Transient upregulation of CBFA1 in response to bone morphogenetic protein-2 and transforming growth factor beta1 in C2C12 myogenic cells coincides with suppression of the myogenic phenotype but is not sufficient for osteoblast differentiation. J Cell Biochem. 1999;73:114–125. [PubMed] [Google Scholar]
- 31.Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N. Generation of different fates from multipotent muscle stem cells. Development. 2002;129:2987–2995. doi: 10.1242/dev.129.12.2987. [DOI] [PubMed] [Google Scholar]
- 32.De Coppi P, Pozzobon M, Piccoli M, Vittoria Gazzola M, Boldrin L, Slanzi E, Destro R, Zanesco L, Franco Zanon G, Gamba P. Isolation of Mesenchymal Stem Cells From Human Vermiform Appendix. J Surg Res. 2006 doi: 10.1016/j.jss.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Thomas DM, Johnson SA, Sims NA, Trivett MK, Slavin JL, Rubin BP, Waring P, McArthur GA, Walkley CR, Holloway AJ, Diyagama D, Grim JE, Clurman BE, Bowtell DD, Lee JS, Gutierrez GM, Piscopo DM, Carty SA, Hinds PW. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol. 2004;167:925–934. doi: 10.1083/jcb.200409187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollenbeck ST, Itoh H, Louie O, Faries PL, Liu B, Kent KC. Type I collagen synergistically enhances PDGF-induced smooth muscle cell proliferation through pp60src-dependent crosstalk between the alpha2beta1 integrin and PDGFbeta receptor. Biochem Biophys Res Commun. 2004;325:328–337. doi: 10.1016/j.bbrc.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 35.Zhou H, Kramer RH. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J Biol Chem. 2005;280:10624–10635. doi: 10.1074/jbc.M411900200. [DOI] [PubMed] [Google Scholar]
- 36.Kramer RH, Cheng YF, Clyman R. Human microvascular endothelial cells use beta 1 and beta 3 integrin receptor complexes to attach to laminin. J Cell Biol. 1990;111:1233–1243. doi: 10.1083/jcb.111.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Barbero A, Benelli R, Minghelli S, Tosetti F, Dorcaratto A, Ponzetto C, Wernig A, Cullen MJ, Albini A, Noonan DM. Growth factor supplemented matrigel improves ectopic skeletal muscle formation--a cell therapy approach. J Cell Physiol. 2001;186:183–192. doi: 10.1002/1097-4652(200102)186:2<183::AID-JCP1020>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 39.Stein GS, Lian JB, van Wijnen AJ, Stein JL, Montecino M, Javed A, Zaidi SK, Young DW, Choi JY, Pockwinse SM. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene. 2004;23:4315–4329. doi: 10.1038/sj.onc.1207676. [DOI] [PubMed] [Google Scholar]
- 40.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 41.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 42.Zammit P, Beauchamp J. The skeletal muscle satellite cell: stem cell or son of stem cell? Differentiation. 2001;68:193–204. doi: 10.1046/j.1432-0436.2001.680407.x. [DOI] [PubMed] [Google Scholar]
- 43.Parker MH, Seale P, Rudnicki MA. Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nat Rev Genet. 2003;4:497–507. doi: 10.1038/nrg1109. [DOI] [PubMed] [Google Scholar]
- 44.Tajbakhsh S. Stem cells to tissue: molecular, cellular and anatomical heterogeneity in skeletal muscle. Curr Opin Genet Dev. 2003;13:413–422. doi: 10.1016/s0959-437x(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 45.Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19:1426–1431. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reimann J, Brimah K, Schroder R, Wernig A, Beauchamp JR, Partridge TA. Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tissue Res. 2004;315:233–242. doi: 10.1007/s00441-003-0833-y. [DOI] [PubMed] [Google Scholar]
- 47.Stewart JD, Masi TL, Cumming AE, Molnar GM, Wentworth BM, Sampath K, McPherson JM, Yaeger PC. Characterization of proliferating human skeletal muscle-derived cells in vitro: differential modulation of myoblast markers by TGF-beta2. J Cell Physiol. 2003;196:70–78. doi: 10.1002/jcp.10322. [DOI] [PubMed] [Google Scholar]
- 48.Levy MM, Joyner CJ, Virdi AS, Reed A, Triffitt JT, Simpson AH, Kenwright J, Stein H, Francis MJ. Osteoprogenitor cells of mature human skeletal muscle tissue: an in vitro study. Bone. 2001;29:317–322. doi: 10.1016/s8756-3282(01)00585-3. [DOI] [PubMed] [Google Scholar]
- 49.Gullberg D, Sjoberg G, Velling T, Sejersen T. Analysis of fibronectin and vitronectin receptors on human fetal skeletal muscle cells upon differentiation. Exp Cell Res. 1995;220:112–123. doi: 10.1006/excr.1995.1297. [DOI] [PubMed] [Google Scholar]
- 50.Burkin DJ, Wallace GQ, Milner DJ, Chaney EJ, Mulligan JA, Kaufman SJ. Transgenic expression of {alpha}7{beta}1 integrin maintains muscle integrity, increases regenerative capacity, promotes hypertrophy, and reduces cardiomyopathy in dystrophic mice. Am J Pathol. 2005;166:253–263. doi: 10.1016/s0002-9440(10)62249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cachaco AS, Pereira CS, Pardal RG, Bajanca F, Thorsteinsdottir S. Integrin repertoire on myogenic cells changes during the course of primary myogenesis in the mouse. Dev Dyn. 2005;232:1069–1078. doi: 10.1002/dvdy.20280. [DOI] [PubMed] [Google Scholar]
- 52.Blanco-Bose WE, Yao CC, Kramer RH, Blau HM. Purification of mouse primary myoblasts based on alpha 7 integrin expression. Exp Cell Res. 2001;265:212–220. doi: 10.1006/excr.2001.5191. [DOI] [PubMed] [Google Scholar]
- 53.Yao CC, Ziober BL, Sutherland AE, Mendrick DL, Kramer RH. Laminins promote the locomotion of skeletal myoblasts via the alpha 7 integrin receptor. J Cell Sci. 1996;109(Pt 13):3139–3150. doi: 10.1242/jcs.109.13.3139. [DOI] [PubMed] [Google Scholar]
- 54.Xiao J, Jethanandani P, Ziober BL, Kramer RH. Regulation of alpha7 integrin expression during muscle differentiation. J Biol Chem. 2003;278:49780–49788. doi: 10.1074/jbc.M308542200. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y, Makita T. Immunocytochemical colocalization of desmin and vimentin in human fetal skeletal muscle cells. Anat Rec. 1996;246:64–70. doi: 10.1002/(SICI)1097-0185(199609)246:1<64::AID-AR7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 56.Lawson-Smith MJ, McGeachie JK. The identification of myogenic cells in skeletal muscle, with emphasis on the use of tritiated thymidine autoradiography and desmin antibodies. J Anat. 1998;192(Pt 2):161–171. doi: 10.1046/j.1469-7580.1998.19220161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaufman SJ, George-Weinstein M, Foster RF. In vitro development of precursor cells in the myogenic lineage. Dev Biol. 1991;146:228–238. doi: 10.1016/0012-1606(91)90462-c. [DOI] [PubMed] [Google Scholar]
- 58.Seale P, Ishibashi J, Holterman C, Rudnicki MA. Muscle satellite cell-specific genes identified by genetic profiling of MyoD-deficient myogenic cell. Dev Biol. 2004;275:287–300. doi: 10.1016/j.ydbio.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 59.Fukada S, Higuchi S, Segawa M, Koda K, Yamamoto Y, Tsujikawa K, Kohama Y, Uezumi A, Imamura M, Miyagoe-Suzuki Y, Takeda S, Yamamoto H. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res. 2004;296:245–255. doi: 10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 60.Alessandri G, Pagano S, Bez A, Benetti A, Pozzi S, Iannolo G, Baronio M, Invernici G, Caruso A, Muneretto C, Bisleri G, Parati E. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet. 2004;364:1872–1883. doi: 10.1016/S0140-6736(04)17443-6. [DOI] [PubMed] [Google Scholar]
- 61.Tiger CF, Fougerousse F, Grundstrom G, Velling T, Gullberg D. alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev Biol. 2001;237:116–129. doi: 10.1006/dbio.2001.0363. [DOI] [PubMed] [Google Scholar]
- 62.Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T, Matsumoto T. Differentiation and transforming growth factor-beta receptor down-regulation by collagen-alpha2beta1 integrin interaction is mediated by focal adhesion kinase and its downstream signals in murine osteoblastic cells. J Biol Chem. 1997;272:29309–29316. doi: 10.1074/jbc.272.46.29309. [DOI] [PubMed] [Google Scholar]
- 63.Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev Biol. 2000;220:2–15. doi: 10.1006/dbio.2000.9633. [DOI] [PubMed] [Google Scholar]


