Abstract
Autosomal-recessive mutations in the Parkin gene are the second most common cause of familial Parkinson's disease (PD). Parkin deficiency leads to the premature demise of the catecholaminergic neurons of the ventral midbrain in familial PD. Thus, a better understanding of parkin function may elucidate molecular aspects of their selective vulnerability in idiopathic PD. Numerous lines of evidence suggest a mitochondrial function for parkin and a protective effect of ectopic parkin expression. Since mitochondria play a critical role in cell survival/cell death through regulated cytochrome c release and control of apoptosis, we sought direct evidence of parkin function in this pathway. Mitochondria were isolated from cells expressing either excess levels of human parkin or shRNA directed against endogenous parkin and then treated with peptides corresponding to the active Bcl-2 homology 3 (BH3) domains of pro-apoptotic proteins and the threshold for cytochrome c release was analyzed. Data obtained from both rodent and human neuroblastoma cell lines showed that the expression levels of parkin were inversely correlated with cytochrome c release. Parkin was found associated with isolated mitochondria, but its binding per se was not sufficient to inhibit cytochrome c release. In addition, pathogenic parkin mutants failed to influence cytochrome c release. Furthermore, PINK1 expression had no effect on cytochrome c release, suggesting a divergent function for this autosomal recessive PD-linked gene. In summary, these data demonstrate a specific autonomous effect of parkin on mitochondrial mechanisms governing cytochrome c release and apoptosis, which may be relevant to the selective vulnerability of certain neuronal populations in PD.
INTRODUCTION
Parkinson's disease (PD) is a devastating illness that affects 1–3% of the population over the age of 65 (1,2). PD is clinically characterized by tremor, rigidity, bradykinesia and postural instability. These symptoms are caused by a progressive loss of catecholamine producing neurons of the substantia nigra pars compacta, among other affected neuronal populations. Although most cases of PD are sporadic in nature, a number of genes have been identified in the rare familial forms of PD pathology (3). Loss-of-function mutations within the PARK2 locus, which encodes the parkin protein, are the most common cause of autosomal recessive PD (4,5). Alterations in the PARK2 gene result in loss of the E3 ubiquitin ligase function of parkin and the selective loss of catecholaminergic neurons. It has been suggested that parkin is necessary for the survival of these neurons, and consequently that parkin may be a pro-survival protein (6). Therefore, understanding how normal parkin function protects neurons, possibly against pro-apoptotic factors or mitochondrial stress, is an important step in the study of idiopathic PD pathogenesis.
Studies of parkin-null animal models strongly suggest a mitochondrial function for parkin. Loss of parkin expression in Drosophila leads to mitochondrial abnormalities and spontaneous apoptosis (7,8), consistent with a protective function of endogenous parkin. Mitochondria are commonly recognized as a critical site for the regulation of apoptosis, in addition to their crucial role in aerobic respiration. The controlled release of cytochrome c across the outer membrane is often a necessary step in the activation of the caspase cascade and cell death (9,10). The action of many anti-apoptotic proteins is to limit the release of cytochrome c from mitochondria, and we hypothesized that one outcome of parkin expression is the retention of mitochondrial cytochrome c under otherwise pro-apoptotic conditions.
The B-cell lymphoma 2 (Bcl-2) family of proteins are integral membrane proteins often located in the outer mitochondrial membrane which governs mitochondrial membrane permeabilization (11). Their functional role in mediating apoptosis and/or survival is dependent upon their Bcl-2 homology (BH) domains. Bcl-2 proteins containing only the Bcl-2 homology 3 (BH3) domain are strictly pro-apoptotic and primarily responsible for inducing cytochrome c release from the mitochondria (12,13). Once mitochondrial cytochrome c is released into the cytoplasm, it initiates a cascade of caspases to effect cell death (10,14).
In order to assess the influence of parkin specifically on the mitochondrion in the context of cell death, we sought an in vitro cell-free system to analyze the threshold for cytochrome c release. Peptides corresponding to the BH3 domain of various pro-apoptotic proteins are well-known to elicit cytochrome c release from isolated mitochondria (13,15). These peptides can be used to examine the basal state of the mitochondria and their intrinsic propensity to promote apoptosis and specific contribution to the cellular response to an apoptotic trigger, in the absence of cytoplasmic factors.
We hypothesized that the protective effect of parkin is due to its ability to regulate the threshold of mitochondrial cytochrome c release. We report here that the overexpression of parkin in multiple cull culture systems significantly reduces evoked mitochondrial cytochrome c release from isolated mitochondria, which correlated with a regulation of downstream caspase activation in intact cells. Expressed parkin was found in the preparations of isolated mitochondria, but additional experiments revealed that the association of parkin with mitochondria per se had no effect on evoked cytochrome c release. Consistent with a role for endogenous parkin in regulating the threshold for cytochrome c release, shRNA-mediated silencing of parkin expression lowered the threshold for BH3 peptide-induced cytochrome c release from SH-SY5Y isolated mitochondria. Pathogenic loss-of-function mutations in parkin abrogate its influence on cytochrome c release. Furthermore, we demonstrate that this effect is specific for parkin, as another autosomal recessive PD-linked gene product, PTEN-induced kinase 1 (PINK1), did not affect this aspect of mitochondrial function, suggesting divergent functions of these two PD-linked gene products. Our results implicate parkin in mitochondrial mechanisms governing cytochrome c release and apoptosis, which may be important to the selective vulnerability of certain neuronal populations in PD.
RESULTS
The BH3 domains of pro-apoptotic proteins stimulate regulated cytochrome c release from isolated mitochondria
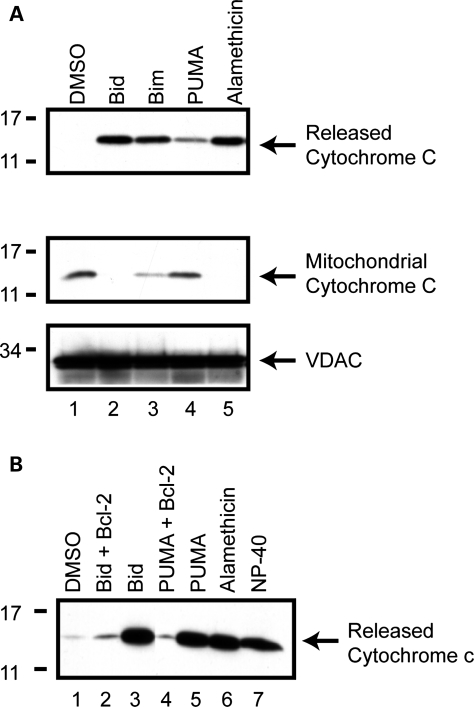
Isolated mitochondria can participate in regulated cytochrome c release and behave as they would in intact cells (13,16). This can be accomplished by incubating purified mitochondria with synthetic HPLC-purified peptides corresponding to the short BH3 domains (20–22 amino acids) of several pro-apoptotic proteins (12). Ordinarily, cytochrome c release is a late step in the induction of apoptosis, and often considered a ‘point of no return’ (17,18). In order to validate the BH3 peptide assays in our hands, we isolated mitochondria from the dopaminergic neuronal MES cell line, incubated them with BH3 peptides, and analyzed both the released and mitochondrial fractions for cytochrome c content (Fig. 1A). Similar experiments were conducted in SH-SY5Y and SK-N-MC human neuroblastoma, and Chinese Hamster Ovary (CHO) cell lines, all of which displayed regulated cytochrome c release from isolated mitochondria (data not shown). In all cases, the BH3 domain peptides of Bid and Bim induced robust release of cytochrome c compared with vehicle (Fig. 1A, lanes 2 and 3, first row), consistent with their putative role as direct activators of apoptosis (13,18–20) Likewise, we consistently observed subtle cytochrome c release in response to the BH3 domain of the apoptotic sensitizer, PUMA, in the MES-derived mitochondria (Fig. 1A, lane 4) and all other cell lines tested (not shown). The magnitude of this effect is consistent with the putative indirect role of PUMA in cytochrome c release (15,21). The levels of retained mitochondrial cytochrome c were inversely correlated with the released cytochrome c (Fig. 1A, second row). The antibiotic alamethicin permeabilizes membranes and in isolated mitochondria allows the passive diffusion of cytochrome c across the outer membrane (22). It induces complete release of cytochrome c such that there is little to no cytochrome c present in the mitochondrial pellets at the completion of the experiment (Fig. 1A, lane 5). This was routinely used as a positive control to quantify and compare maximal cytochrome c release across independent mitochondrial preparations. VDAC, an integral outer mitochondrial membrane protein, was also analyzed to confirm equal protein loading (Fig. 1A, third row).
Figure 1.
BH3 peptides induce cytochrome c release from isolated mitochondria. (A) Mitochondria were isolated from MES cells and incubated with DMSO, 10 µm of the purified BH3 domain peptides of Bid, Bim, PUMA or alamethicin (40 µg/ml). Reactions were then centrifuged at 25 000g for 10 min, to obtain the released cytochrome c supernatant and mitochondrial pellet. Released and retained cytochrome c was analyzed by western blot. Equal loading of mitochondrial proteins was confirmed by western blotting for the mitochondrial membrane protein, VDAC. (B) Mitochondria were pre-incubated for 5 min with purified, full-length Bcl-2 protein (10 µm), prior to the addition of 10 µm Bid or PUMA BH3 peptide. Released cytochrome c was measured by western blot. Images are representative of at least three independent experiments.
The anti-apoptotic protein, Bcl-2, inhibits apoptosis in vivo and in intact cells in part by preventing or attenuating mitochondrial cytochrome c release and the activation of the downstream caspase cascade (10,23). To further characterize the suitability of this cell-free system, we pre-treated isolated mitochondria with purified Bcl-2 protein in order to determine its influence on subsequent cytochrome c release by Bid or PUMA. Results showed that both Bid and PUMA induced cytochrome c release compared with DMSO vehicle (Fig. 1B, lanes 3 and 5); however, pre-treatment with equimolar concentrations of recombinant Bcl-2 reduced the levels of cytochrome c release (Fig. 1B, lanes 2 and 4), consistent with its known function in intact cells. Alamethicin and NP-40 (a detergent that solubilizes mitochondrial membranes) treatments induced complete release of cytochrome c (Fig. 1B, lanes 6 and 7).
Parkin expression decreases BH3 peptide-induced cytochrome c release from isolated mitochondria
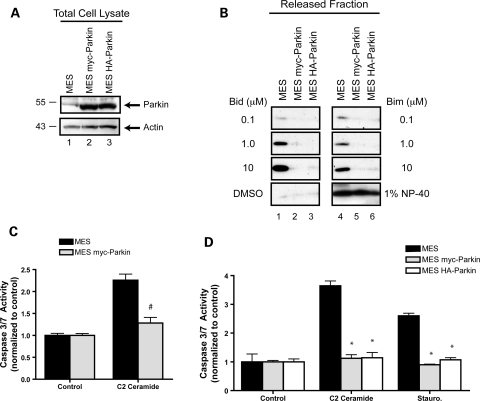
It has been argued that parkin both serves a critical function to mitochondria (24,25) and plays a potent role in promoting cell survival (6). Since mitochondria often play a central role in governing cell fate, we hypothesized that the pro-survival effects of parkin would involve mitochondria. Indeed, prior work has suggested that the protective effects of parkin are correlated with reduced cytochrome c release from mitochondria (26), but whether parkin acts directly at the level of mitochondrial cytochrome c release or on upstream events in the cytoplasm (where parkin is predominantly localized) was not addressed. In order to address the potential influence of parkin directly on mitochondria, we first generated two independent stable cell lines with matched parkin expression levels. Here we show the stable expression of a human parkin fusion protein with either a myc- or HA-epitope tag on the amino-terminus in the MES cell background (Fig. 2A, lanes 2 and 3).
Figure 2.
The anti-apoptotic effects of parkin are intrinsic to the mitochondria and can be observed in a cell-free system. (A) Human parkin was stably expressed in two independent monoclonal MES cell lines. Whole cell lysates were probed for parkin and actin expression by western blot. (B) Mitochondria were isolated from MES, MES myc-Parkin and MES HA parkin cells, protein normalized and incubated with DMSO, or 10 µm of the purified BH3 domain peptides of Bid and Bim at various concentrations. NP-40 (1%) was added to aliquots of each mitochondrial preparation to measure total cytochrome c from each sample. Released cytochrome c was analyzed by western blot. (C) Caspase 3/7 activity was measured from MES and MES myc-Parkin cells 18 h after the treatment with vehicle or 1 µm C2 ceramide (mean ± SEM, n = 20). Data were pooled across three independent experiments, # denotes statistically significant from control (P < 0.00001). (D) MES, MES myc-Parkin and MES HA-Parkin cells were treated with vehicle, 1 µm C2 ceramide or 300 nm staurosporine for 6 h and caspase 3/7 activity was measured (mean ± SEM, n = 4), *denotes statistically significant from control (P < 0.001).
Mitochondria from all three cell lines (MES, MES myc-Parkin and MES HA-Parkin) were isolated and incubated with the BH3 peptides of Bid and Bim at increasing concentrations, and the levels of released cytochrome c were determined by western blot. As expected, both Bid and Bim induced robust release of cytochrome c from mitochondria isolated from the parental MES cell line (Fig. 2B, lanes 1 and 4). In addition, Bid and Bim induced a dose-dependent release, with 10 µm of peptide initiating the maximal release compared with the positive control of NP-40 (Fig. 2B, fourth row), suggesting that mitochondrial cytochrome c release is quantitatively dependent upon the amount of Bid or Bim present in the reaction. The stable expression of either human parkin fusion protein led to a dramatic reduction in the BH3 peptide-induced cytochrome c release from isolated mitochondria (Fig. 2B, lanes 2, 3, 5 and 6). This reduction was observed at all three concentrations of peptide. Importantly, the levels of total cytochrome c were not altered by the expression of parkin.
In order to determine if the parkin-dependent reduction in evoked cytochrome c release from isolated mitochondria was indicative of the inhibition of apoptosis in intact cells; we examined the downstream activation of caspases 3 and 7 in whole cells. Previous studies have shown that parkin overproduction inhibits C2 ceramide-mediated apoptosis (26). We therefore incubated MES and MES myc-Parkin cells with vehicle or C2 ceramide and analyzed caspase 3/7 activity using a fluorogenic substrate 18 hrs following treatment. Data showed a robust activation of caspase 3/7 activity in parental MES cells treated with C2 ceramide, whereas the MES myc-Parkin cells showed no increase above control (Fig. 2C). To further expand our data to include multiple parkin cells lines, and additional time points, we tested C2 ceramide along with staurosporine (Fig. 2D) in parental MES, MES myc-Parkin and MES HA-Parkin lines and analyzed caspase activation at 6 h post-treatment. The expression of parkin, which was comparable in both stable cell lines (Fig. 2A), resulted in a similar inhibition of caspase activation in response to both C2 ceramide and staurosporine (Fig. 2D).
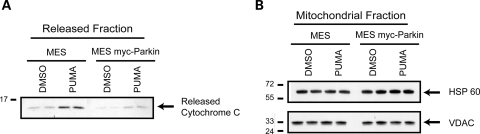
MES and both parkin-expressing lines were also analyzed for their sensitivity to a third BH3 peptide, PUMA. PUMA often demonstrated less cytochrome c release than Bid and Bim, consistent with an indirect mechanism (15). We found that PUMA caused a slight increase in the amount of cytochrome c released relative to control (Fig. 3A). In parkin expressing cells this release was slightly attenuated, suggesting that parkin also inhibits PUMA-induced cytochrome c release (Fig. 3A). HSP-60 and VDAC were used to confirm equal loading (Fig. 3B).
Figure 3.
Parkin prevents PUMA peptide-induced cytochrome c release from mitochondria. (A) Mitochondria from the MES and MES myc-Parkin cell lines were incubated with either DMSO or the purified BH3 domain of PUMA (10 µm). Released cytochrome c was detected by western blot. (B) Equal loading of mitochondrial proteins was confirmed by western blotting the mitochondrial retained proteins for HSP 60 and VDAC. Images are representative of at least three independent experiments, each performed in duplicate as shown.
Pathogenic parkin mutations do not affect mitochondrial cytochrome c release
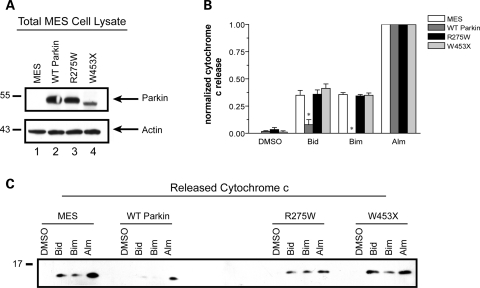
Mutations in the parkin gene are currently recognized as a predominant cause of autosomal recessive familial parkinsonism, accounting for as much as 50% of familial early-onset PD cases and 2–6% of late-onset PD cases (27,28). Within the PARK2 locus, diverse types of mutations are associated with parkin-linked PD. Massive deletions, subtle missense mutations and insertion of premature stop codons have been found along the entire length of the parkin gene (29–31). Since these are all presumed to result in loss of parkin function, we analyzed two distinct types of mutation, missense (R275W) and premature termination (W453X), for their influence on mitochondrial cytochrome c release.
These two pathogenic parkin mutations, along with wild-type parkin, were stably expressed using lentivirus in MES cells to generate an independent set of parkin-expressing MES cell lines. The expression levels of all three parkin variants are shown in Figure 4A. As before, mitochondria were isolated from each cell line, protein normalized and incubated with the BH3 peptides Bid or Bim. Both peptides stimulated similar levels of cytochrome c release in naïve MES cells, compared with vehicle treatment alone as determined by commercial ELISA analysis of three independent experiments (Fig. 4B). A western blot is provided of a representative experiment (Fig. 4C). As previously shown in two other parkin-expressing cell lines (Fig. 2b), wild-type parkin nearly eliminated BH3 peptide-induced cytochrome c release (Fig. 4B and c). The levels of cytochrome c released by the pore-forming antibiotic, alamethicin, were comparable in all three cell lines, consistent with equal loading of mitochondrial protein in each reaction mixture. However, neither of the pathogenic parkin mutations had any effect on BH3 peptide-induced cytochrome c release, consistent with the expectation of loss of function.
Figure 4.
Pathogenic parkin mutations do not inhibit mitochondrial cytochrome c release. (A) Parental MES cells (MES) and lines stably expressing wild-type parkin (WT Parkin) or parkin mutants R275W and W453X were lysed in NP-40 and immunoblotted for parkin expression and actin levels as a loading control. (B) WT parkin and mutant-parkin expressing cell lines were incubated with either DMSO, 10 µm of the purified BH3 domain peptides of Bid or Bim, or alamethicin (Alm, 40 µg/ml). Released cytochrome c was quantified by ELISA with the data normalized to maximal cytochrome c release as determined by alamethicin treatment from each mitochondrial preparation. * Denotes statistically significant from control (P < 0.05, n = 3). (C) Western blot of a representative experiment reveals the failure of mutant parkin to influence mitochondrial cytochrome c release whereas wild-type parkin dramatically reduces BH3 peptide induced cytochrome c release.
Association with the mitochondria is not sufficient for parkin-dependent inhibition of cytochrome c release
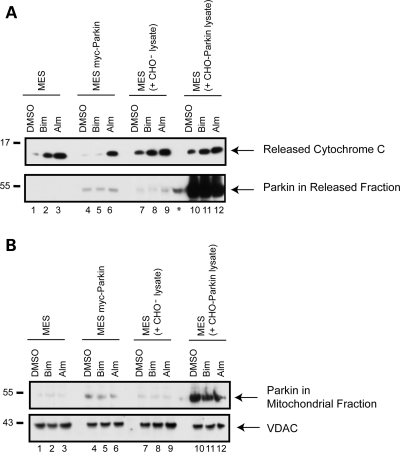
Examination of our isolated mitochondria from the MES myc-Parkin cells revealed the presence of very low but detectable levels of parkin. To determine whether the association of parkin with mitochondria may play a role in the parkin-dependent inhibition of BH3 peptide evoked cytochrome c release, we re-suspended mitochondria from naïve MES cells in soluble extracts taken from parental CHO cells, or CHO cell stably expressing high levels of parkin (CHO-Parkin cells). We then tested whether the high levels of parkin present in the BH3 peptide assay would bind to the isolated mitochondria and if this would have any effect on evoked cytochrome c release. To repeat our previous data and compare, side-by-side, the effects of parkin expressed in the cell prior to mitochondrial isolation, we used MES myc-Parkin derived mitochondria in parallel. Results showed that parkin was found associated with mitochondria taken from MES myc-Parkin cells (Fig. 5B), which show significant reduction of evoked cytochrome c release (Fig. 5A) consistent with previous data (Fig. 2B). Interestingly, even greater levels of mitochondria-bound parkin were achieved by co-incubation of MES mitochondria with the parkin-enriched extract than that found in the mitochondrial fraction from the MES myc-Parkin cells (Fig. 5B). However, the presence of high levels of parkin in both the reaction supernatant (Fig. 5A) and at the mitochondria (Fig. 5B) had no effect on BH3 peptide induced cytochrome c release (Fig. 5A, upper panel) suggesting that parkin must be expressed in the live cell prior to mitochondrial isolation to strongly influence cytochrome c release.
Figure 5.
The association of parkin with mitochondria is not sufficient for inhibition of evoked cytochrome c release. Mitochondria from the MES and MES myc-Parkin cell lines were incubated with either DMSO, the purified BH3 domain of Bim (10 µm), or alamethicin (Alm, 40 µg/ml) with normal Experimental Buffer. In addition, mitochondria isolated from parental MES cells were incubated with soluble parkin-free extract from CHO− cells, or parkin-enriched extract from CHO-Parkin cells. Reactions were then centrifuged at 25 000g for 10 min, to obtain (A) the released cytochrome c supernatant which was probed for released cytochrome c and the presence of parkin and (B) the mitochondrial pellet which was probed for VDAC as a loading control, and for mitochondria-associated parkin. * Denotes a non-specific signal in a non-loaded lane coming from the adjacent lane. This image is a representative of at least three independent experiments.
Endogenous parkin regulates cytochrome c release in human neuronal cells
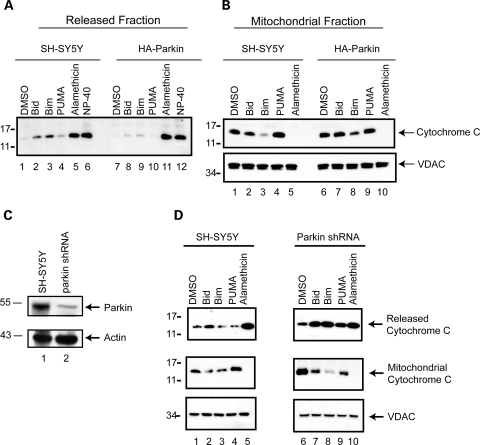
In order to expand our investigation to include the effects of parkin specifically in human neuronal cells, we analyzed whether parkin alters the mitochondrial response to the apoptotic BH3 peptides in the SH-SY5Y human neuroblastoma cell line. Human HA-parkin was stably introduced into SH-SY5Y cells via lentiviral delivery. Mitochondria from naïve and parkin-expressing cells were isolated and subject to BH3 peptide profiling, as previously described. Bid and Bim induced robust release of cytochrome c in SH-SY5Y cells compared with vehicle (Fig. 6A, lanes 2 and 3). The levels of BH3 peptide-induced cytochrome c release were dramatically reduced in the mitochondria isolated from the parkin-expressing SH-SY5Y cells, compared with the naïve SH-SY5Y control (Fig. 6A, lanes 8, 9 and 10), confirming the effect of parkin on mechanisms governing stimulated release of mitochondrial cytochrome c. Alamethicin and NP-40 confirmed equal mitochondrial purity and loading (Fig. 6A, lanes 5, 6, 11 and 12). Likewise, each of the mitochondrial pellets (except for the samples solubilized with NP-40) was analyzed for the levels of cytochrome c retained by the mitochondria throughout the experiment. The levels of released cytochrome c were inversely proportional to the mitochondrial retained cytochrome c, as expected. Stable expression of human parkin in SH-SY5Y cells resulted in increased mitochondrial retained cytochrome c following the treatment with Bid, Bim or PUMA peptides (Fig. 6B).
Figure 6.
Endogenous parkin regulates the apoptotic release of cytochrome c from mitochondria. (A) The ectopic expression of parkin in SH-SY5Y cells substantially attenuates the BH3 peptide induce cytochrome c release from isolated mitochondria, with a concomitant decrease in the retained cytochrome c (B), as determined by western blot. Equal loading of mitochondrial proteins was confirmed by western blot analysis of VDAC. (C) Whole cell lysates from naïve SH-SY5Y cells and those stably transduced with a lentivirus encoding an shRNA directed against the expression of endogenous parkin were probed by western blot for both parkin and actin levels. (D) Mitochondria from SH-SY5Y cell lines with and without shRNA against parkin expression were incubated with DMSO vehicle, 1 µm of the purified BH3 domain peptides of Bid, Bim and PUMA, alamethicin (40 µg/ml), or 1% NP-40. Released and retained cytochrome c, and mitochondrial VDAC levels were analyzed by western blot. (D) is representative of at least three independent experiments.
The human neuronal cell line SH-SY5Y expresses detectable levels of endogenous parkin protein and has been effectively used to study the role of endogenous parkin expression by us (32,33) and other groups (30,34). Using a lentiviral shRNA gene knockdown system (Invitrogen) we engineered an SH-SY5Y cell line that shows a 70% decrease in parkin expression compared with wild-type, naïve SH-SY5Y cells (Fig. 4C). To analyze the role of endogenous parkin we purposefully used lower concentrations of BH3 peptide to avoid a ceiling effect. Previous work showed that 1 µm peptide elicits sub-maximal cytochrome c release that is still significantly greater than the DMSO vehicle treatment alone (Fig. 2B). At these reduced peptide concentrations, Bid was the most potent peptide in eliciting cytochrome c release from mitochondria isolated from SH-SY5Y cells (Fig. 6D, lanes 1–5). Although Bid was the only BH3 peptide to induce substantial cytochrome c release in the naïve SH-SY5Y cells, all three peptides stimulated robust cytochrome c release from mitochondria isolated from the parkin-deficient shRNA SH-SY5Y cells (Fig. 6D, top panel, lanes 7, 8 and 9). In addition, the levels of Bid-induced release were consistently greater in the parkin-deficient line compared with the naïve SH-SY5Y cells (Fig. 6D, top panel, lanes 2 and 7). Again, alamethicin was used as a positive control to confirm that maximal cytochrome c release was similar across both preparations of isolated mitochondria. Western blotting for mitochondrial cytochrome c (Fig. 6D, middle panel) and VDAC (Fig. 6D, bottom panel) levels confirmed increased release in the parkin-silenced cells and equal mitochondrial purity and loading across all samples, respectively.
PINK1 expression does not influence the threshold for cytochrome c release
To assess the specificity of parkin's effects on mitochondria-dependent cell death, we examined another autosomal recessive PD-linked gene product, PINK1. PINK1 is a serine/threonine kinase with a functional mitochondrial targeting sequence and plays an important role in maintaining mitochondrial integrity. Several studies have hypothesized that PINK1 functions in the same biochemical pathway as parkin (24,25,35,36). Therefore, we compared the effects of parkin and PINK1 on the threshold for mitochondrial cytochrome c release.
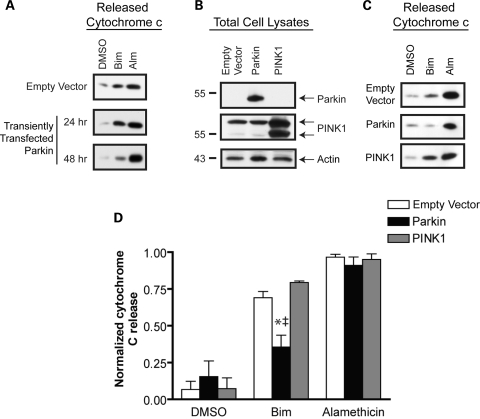
The levels of Bid and Bim peptide-induced cytochrome c release from MES mitochondria were similar throughout the current study, as were the effects of parkin on both peptides. Therefore, we chose to focus on one BH3 peptide in this final analysis. Due to difficulty in generating stable cells lines expression PINK1, MES cells were transiently transfected to express human parkin or PINK1. In analyzing conditions for transient transfection, data showed that parkin must be expressed for at least ∼48 h to confer protection against BH3 peptide induced cytochrome c release (Fig. 7A). Western blot confirmed the transient expression of both proteins (Fig. 7B). Mitochondria were then isolated from these cells and subjected to a Bim BH3 peptide (10 µm) release assay. Released cytochrome c was analyzed from a representative experiment by Western blot (Fig. 7C) and pooled data across multiple independent experiments were studied by densitometric analysis (Fig. 7D). Our results show that Bim peptide stimulated cytochrome c release in all three conditions, compared with vehicle alone (Fig. 7C and d). However, transient transfection with a parkin cDNA construct resulted in a statistically significant reduction in mitochondrial cytochrome c release. These results with transiently transfected parkin are consistent with our previous findings in cells lines where parkin is stably expressed (Figs 2–6). The transient overexpression of PINK1 had no effect on the threshold for cytochrome c release. Alamethicin was used to determine the total levels of cytochrome c in each mitochondrial preparation.
Figure 7.
PINK1 does not prevent BH3 peptide-induced cytochrome c release (A) A representative western blot analysis of released cytochrome c release in a BH3 peptide assay following transient transfection to express parkin at 24 and 48 h. (B) Western blot analysis of whole cell lysates from MES cells lines transiently transfected with empty vector, wild-type parkin or PINK1. (C) Mitochondria were isolated 48 h after transient transfection with an empty vector control, parkin, or PINK1 and subject to a BH3 peptide assay. Western blot analysis of the Bim (10 µm) and alamethicin (Alm, 40 µg/ml) induced cytochrome c release demonstrates the effects of transiently expressed parkin and PINK1 on cytochrome c release. (D) Data from three independent experiments were analyzed by densitometry and normalized to the maximal cytochrome c release from each mitochondrial preparation (as determined by alamethicin treatment). *Denotes statistically significant from control (P < 0.05, n = 3) and † is significant from PINK1 (P < 0.05, n = 3).
DISCUSSION
PD is one of the most prevalent progressive neurodegenerative diseases but despite decades of research the etiology of PD remains unclear. Although the vast majority of PD cases are sporadic in nature, the genetics underlying the less common familial forms of this disease may provide critical clues to the molecular pathways involved in the unique pathology (37). Loss-of-function mutations within the PARK2 locus, encoding the ubiquitin E3 ligase parkin, are the most common cause of autosomal recessive PD (4,5). Although numerous substrates of parkin have been proposed, the essential function of parkin within neuronal populations remains unknown. One universal observation is that parkin expression is broadly associated with enhanced cell survival in the context of diverse and unrelated neurotoxic stimuli. Data from murine and fly models of parkin deficiency also indicate a potent influence of parkin on mitochondrial function (8,38). In this study, we sought to analyze whether the pro-survival effects of parkin and its mitochondrial influence are linked through an alteration in mitochondrial control of cell death. Specifically, we tested whether parkin directly alters the intrinsic threshold for cytochrome c release from isolated mitochondria. By studying mitochondria in a cell-free system and triggering cytochrome c release with downstream apoptotic activators, we can directly assess mitochondrial behavior in a context and stimulus independent manner. This allows us to ask whether parkin fosters a change in the basal response of mitochondria to cellular stress, and to determine whether fundamental changes in the mitochondrial response to apoptotic triggers may play an important role in the pro-survival effects of parkin.
We report here that the ectopic expression of parkin results in a dramatic decrease in the BH3 peptide-induced release of cytochrome c from isolated mitochondria. This effect was observed in multiple monoclonal and polyclonal lines produced in the MES cell background (Figs 2–5), a rodent neuroblastoma line derived from primary cultured neurons of the rat substantia nigra (39). Furthermore, nearly identical data were obtained from mitochondria isolated from human SH-SY5Y neuroblastoma stably over-expressing human parkin (Fig. 6), and CHO cell lines (not shown). Pathogenic mutations in the parkin gene are found throughout the coding sequence, and can occur as subtle missense mutations, massive deletions or premature termination signals, indicating a consensus loss-of-function association with PD (27,29–31). To determine whether pathogenic, loss-of-function mutations in parkin can influence mitochondrial cytochrome c release as does wild-type parkin, we stably introduced wild-type, R275W and W453X parkin into an independent series of MES cells, isolated mitochondria from each line, and conducted the BH3 peptide profiling as before. Results showed that neither the missense mutant (R275W) nor the premature stop-codon mutant (W453X) had any effect on this property of mitochondrial function, whereas wild-type parkin dramatically reduced cytochrome c release (Fig. 4). These data suggest that the direct influence of parkin on mitochondrial cytochrome c release may be pathogenically relevant, as subtle PD-linked loss-of-function mutations in the parkin coding sequence are true loss-of-function mutations with regard to this function, as well.
Recent data have suggested that parkin can associate directly with mitochondria either under basal conditions (26,40,41), or specifically following mitochondrial depolarization (42). To determine whether the observed inhibition of cytochrome c release was linked to the binding of parkin to mitochondria, we examined the effects of exogenously applied parkin in the BH3 assays. Although, we found that low levels of parkin could bind to isolated mitochondria in vitro, this was not associated with inhibition of cytochrome c release. Furthermore, we explored another variable in this particular mitochondrial function of parkin, and found that parkin must be expressed for a minimum of ∼48 h prior to the isolation of mitochondria to observe parkin-dependent inhibition of cytochrome c release (Fig. 7A). This effect was not due to the absolute expression levels of parkin, as stable cell lines expressing low levels of parkin demonstrated greater inhibition of cytochrome c release than after transient transfection at early time points (<48 h), where parkin expression was significantly greater (not shown). Taken together, these data indicate that parkin must be expressed within the cytoplasm for some minimum critical period before the intrinsic properties of the mitochondria are affected. These data may be consistent with the time required for turnover of a particular set of parkin substrates, or to allow turnover of the pool of mitochondria itself.
To address the influence of endogenous parkin levels on the mitochondrial control of cell death, we targeted the expression of parkin using the lentiviral delivery of parkin-directed shRNA into SH-SY5Y neuroblastoma cells, a cell culture system routinely used to analyze endogenous parkin function (30,32,34). Here, the parkin expression was reduced by 70% of endogenous levels. Despite the incomplete disruption of parkin expression, this level of silencing was sufficient to unmask an important role of endogenous parkin in regulating the mitochondrial control of cytochrome c release and downstream apoptosis. Mitochondria isolated from the parkin-deficient SH-SY5Y cells consistently showed increased cytochrome c release in response to sub-maximal stimulation with BH3 peptides compared with naïve SH-SY5Y cells (Fig. 4). These data suggest that under conditions in which parkin expression is naturally high, such as in neurons, parkin plays an important role in dampening the apoptotic response of mitochondria by attenuating cytochrome c release. This interpretation is consistent with the broad pro-survival effects of parkin, as one would expect parkin to act at a downstream step in the cell death pathway for it to potently interfere with the toxicity of such a diverse series of stressors.
Recent genetic studies in Drosophila have suggested that another autosomal recessive PD-linked gene, PINK1, might function in the same biochemical pathway as parkin. This hypothesis has been supported by the fact that knockout of either gene in Drosophila results in similar phenotypes and that parkin could partially rescue the severe abnormalities of the PINK1-null fly (24,25). However, since parkin was documented early on as a pro-survival gene, and more recent data suggest that parkin can be up-regulated under specific conditions to overcome cell stress (30,43,44), it is not clear whether the partial rescue observed in the PINK1 null flies was merely another manifestation of the pro-survival effects of parkin. Moreover, the over-expression of PINK1 does not recapitulate the broad and potent pro-survival effects of parkin, suggesting that perhaps the links between parkin and PINK1 function are more complex. To determine whether these two autosomal recessive PD gene products both influence the intrinsic response of mitochondria to apoptotic stimuli, we transiently transfected either empty vector, parkin or PINK1 into MES cells and conducted BH3 peptide profiling on the isolated mitochondria from each condition. Consistent with our data obtained in numerous stable cell lines expressing parkin, transient transfection with parkin significantly decreased mitochondrial cytochrome c release. However, the over-expression of human PINK1 had no effect on mitochondrial cytochrome c release (Fig. 7). Although not statistically significant, we actually observed that PINK1 expression tended to promote cytochrome c release, in direct opposition to the pronounced effects of parkin. These data suggest that whereas similarities exist in parkin and PINK1 null animal models and that there may be some functional overlap between these two PD-linked proteins, parkin and PINK1 do not share a common influence on the mitochondrial control of cell death.
Previous studies have analyzed the pro-survival effects of parkin either in vivo or in cell culture systems. One study has specifically observed reduced cytochrome c release by parkin in whole cells (26). Here, we have taken the novel approach to examine whether the potent pro-survival and anti-apoptotic effects of parkin that have been reported by numerous groups could be attributed to parkin-dependent changes in autonomous mitochondrial behavior. Since mitochondria are not static, uniform organelles and their response to apoptotic stimuli can be influenced by cytosolic factors this question was best addressed by analyzing isolated mitochondria. We sought to determine whether the levels of cytoplasmic parkin prior to isolation had altered intrinsic, long-term mitochondrial properties consistent with a potent pro-survival function in whole cells. By analyzing the ectopic expression of wild-type and PD-linked mutant parkin, as well as the role of endogenous parkin, we have determined that parkin alters the intrinsic mitochondrial response to apoptotic triggers, dampening cytochrome c release, which would then decrease the downstream activation of caspase 9, and the later effector caspases 3 and 7, as we have shown in intact cells (Fig. 2). By acting at such a convergent and late step in the cell death pathway, influencing cytochrome c release is both an efficient and effective means to promote cell survival under otherwise apoptotic conditions. However, it seems likely that there are other mitochondrial properties affected by parkin; the role of parkin in mitochondrial behavior and response to stress may not be limited to the threshold for cytochrome c release. Parkin has been recently observed to translocate to dysfunctional mitochondria and promote their autophagic degradation (42). However, since these effects were specific to conditions immediately following collapse of the mitochondrial membrane potential and took many hours to manifest, these important findings are not likely related to the effects reported here where mitochondria were harvested and analyzed from unstressed cells.
Although growing evidence supports the potent protective function of parkin, studies at the message and protein levels have suggested that parkin is utilized by the cell in an adaptive survival response to certain stimuli. However, there is an equally well-studied biochemical vulnerability of the parkin protein, as it is prone to mis-folding, aggregation, and inactivation in the face of oxidative and metabolic stress (32,33,45,46). An important question arising from these studies is how does parkin prevent cell death when it is acutely inactivated during cell stress? We believe that the current data may address this issue. We have observed that the pro-survival effects of parkin routinely observed at the whole-cell level can be readily studied in a cell-free system at the level of the mitochondrion. Therefore, the protective effects of parkin may not require acute E3 ligase activity but involve fundamental changes in the response of mitochondria to cell stress that persist acute stress-induced parkin inactivation.
In conclusion, our data suggest that one role of parkin is the regulation of mitochondrial cytochrome c release, thus modifying the apoptotic response of the cell. We have determined that this effect is specific to parkin, as loss-of-function pathogenic mutations in parkin abrogate this function. Furthermore, the related PD-linked protein, PINK1, fails to recapitulate these effects. These data are consistent with numerous findings such as those from cells cultured from parkin-linked PD patients which show increased propensity for apoptotic cell death (47,48), and the spontaneous apoptosis reported in parkin-null Drosophila (7,8). Therefore, genetic loss of parkin function may predispose certain neuronal populations to apoptotic cell death, perhaps specifically involving the mitochondrial threshold for cytochrome c release. Future work will be required for the difficult task of identifying the specific parkin substrates or larger pathways responsible for these effects.
MATERIALS AND METHODS
Cell culture, plasmids and transfection methods
The rodent midbrain dopaminergic MES23.5 (MES) cells (39) were cultured in Dulbecco's Modified Eagle's Media (DMEM) with 10% fetal bovine serum and 5% heat-inactivated new born calf serum with penicillin and streptomycin, as previously described (33). MES cells stably expressing human myc-parkin (MES-Parkin) and HA-parkin (MES HA-Parkin), and parental and parkin-expressing Chinese Hamster Ovary cells (CHO and CHO-Parkin, respectively) were cultured as previously characterized (33). HA-parkin and myc-parkin cDNA were cloned into lentiviral expression vector pCDH-MCS1-EF1-Puro (Systems Biosciences, CA, USA) and point mutations were then introduced using QuikChange Site-directed mutagenesis kit (Stratagene). In order to generate lentivirus, these constructs were co-transfected with ViraPower Packaging Mix (Invitrogen) into HEK293FT cells according to manufacturer's protocol. Conditioned medium containing viral particles was cleared using a 0.22 µm filtration system (Millipore) and then used to stably transduce SH-SY5Y or MES cells. Human HEK 293FT and SH-SY5Y cells were cultured in DMEM with 10% fetal bovine serum with penicillin and streptomycin.
To generate SH-SY5Y cells with stable parkin knockdown, human parkin shRNA expression plasmids were constructed using the Block-iT U6 system (Invitrogen). Sense and antisense oligonucleotides generated using a hairpin sequence (GCTTAGACTGTTTCCACTTAT) were annealed and ligated into pENTR/U6 resulting in pENTR/U6/parkin. Subsequently, the U6 shRNA cassettes were transferred into pLenti6/BLOCK-iT-DEST using Gateway site-specific recombination resulting in pLenti6/Block-iT parkin that was used to produce lentiviral particles as described above.
Mitochondrial isolation and BH3 peptide profiling
Cells were collected, washed in ice-cold PBS and maintained at 4°C throughout the isolation procedure. Pellets were then resuspended in 1 ml Isolation Buffer (200 mm Sucrose, 10 mm TRIS/MOPS, 1 mm EGTA/TRIS) and lysed using 20 gentle strokes of a Potter-elvehjem Dounce homogenizer. The resulting homogenate was then drawn with an 18.5 G needle and expelled through a 27.5 G needle 10 times. The homogenates were centrifuged at 200g for 5 min and the nuclear pellet was discarded. The remaining supernatant was centrifuged at 10 000g for 10 min. Cytosolic fractions were collected and mitochondrial pellets were resuspended in Isolation Buffer (50–100 µl). Purified Bcl-2 from prepared as previously described (12).
The mitochondrial protein content was then normalized using the DC Protein Assay (Bio-Rad, Hercules, CA, USA). For peptide reactions, 0.5 mg/ml mitochondria (final concentration) were diluted in Experimental Buffer (125 mm KCl, 10 mm Tris/MOPS, 5 mm Glutamate, 2.5 mm Malate, 1 mm KPhos, 10 µm EGTA/TRIS). HPLC purified BH3 peptides (0.1–10 µm final concentration) were custom synthesized (Tufts University Core Facility, Boston, MA, USA) and added to diluted mitochondria and incubated for 35 min at room temperature. The sequences for the synthetic BH3 domain peptides of Bid, Bim and PUMA were EDIIRNIARHLAQVGDSMDR, MRPEIWIAQELRRIGDEFNA and EQWAREIGAQLRRMADDLNA, respectively. Following the incubation, reactions were centrifuged at 25 000g for 10 min to obtain the released cytochrome c supernatant and mitochondrial pellet. Alamethicin (40 µg/ml) and/or NP-40 (1%) were used as positive controls to induced maximal cytochrome c release during BH3 peptide assays. The mitochondrial pellet was then lysed in an equal volume of 1% NP-40 with protein inhibitor cocktail and incubated on ice for 25 min. The NP-40 insoluble material was centrifuged at 6000g for 10 min and discarded; the extracted mitochondrial proteins were analyzed.
Parkin-free and parkin-enriched extracts were derived from CHO and CHO-Parkin cells, respectively. Cells were collected and washed in ice-cold PBS, resuspended in 0.5 ml of Experimental Buffer and mechanically disrupted using 20 gentle strokes of a Potter-elvehjem homogenizer. The resulting homogenate was then centrifuged at 100 000g for 45 min and the pellet was discarded. The resulting supernatant was then centrifuged a second time to remove insoluble material. The remaining supernatant was protein normalized and used as a substitute for regular Experimental Buffer prior to the addition of vehicle (DMSO), BH3 peptide or alamethicin.
Western blot
Fractionated samples obtained as described above were prepared under reducing conditions in 4× Laemmli buffer and heated at 65°C for 5 min. For western blotting, samples were loaded onto Novex (Invitrogen) or Criterion (Bio-Rad, Hercules, CA, USA) Tris-Glycine or Tris-Tricine pre-cast gels, transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) and probed for cytochrome c (sc-13560; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and PRK8 (sc-32282; Santa Cruz Biotechnology, Santa Cruz, CA, USA), VDAC (PA1-954A; Cell Signaling, Danvers, MA, USA) or actin (ab6276; Abcam, Cambridge, MA, USA). HSP-60 (4870S; Cell Signaling, Danvers, MA, USA) was also used as a loading control. MES cells transiently expressing parkin or PINK1 were probed for both parkin (PRK8) and PINK1 (NB100–494); NOVUS Biologicals, Littleton, CO, USA). Secondary antibodies and ECL-plus were purchased from GE Healthcare (Buckinghampshire, UK). Following ECL application, blots were exposed to HyBlot Cl autoradiography film (Metuchen, NJ, USA). Blots were stripped in 1× Stripping Buffer (62.5 mmol/l Tris pH 6.8, 2% SDS, 7.6% βME) at 55°C for 10 min and washed 3 × 10 min in 0.1% PBS/Tween and subjected to standard western blotting conditions. Densitometry was calculated using AlphaEase Automatic Image Capture (San Leandro, CA, USA). Data were compared using a one way ANOVA followed by a Tukey's Multiple Comparison post hoc test.
Caspase 3/7 assay
Cells were plated at 2.5 × 105 per well in a 96-well microplate and incubated for 24 h prior to the addition of various cell stressors. C2 ceramide and staurosporine were added at concentrations of 1 µm and 300 nm, respectively, and incubated at 36°C for 6–18 h. Caspase 3/7 activity was determined using a fluorogenic substrate kit (AnaSpec, San Jose, CA, USA) and quantifying signal intensity (Ex/Em = 380 nm/500 nm) on a Wallac Victor 2 Multi-label Counter according to the manufacturer's instruction. Data were compared using either a t-test (Fig. 2C) or a two way ANOVA followed by a Bonferroni post-hoc test (Fig. 2D).
ELISA
Fractionated samples obtained as described above were prepared in a 96-well microplate pre-coated with a monoclonal antibody specific for rat/mouse cytochrome c. The microplate and all reagents were obtained from R&D systems (Minneapolis, MN, USA) and the assay was preformed according to the manufacturer's protocol. Briefly, conjugate, standards and samples were pipetted into the wells and incubated at room temperature for 2 h. Following a series of washes, a substrate solution was added to the wells for 30 min followed by a stop solution. The color intensity of the standards and samples were calculated at 450 nm using the SoftMax Pro Software on a SpectraMax Plus spectrophotometer. Data were compared using a one way ANOVA followed by a Tukey's Multiple Comparison post-hoc test.
FUNDING
This work was supported by the National Institutes of Health (AG023094 to M.J.L., AG00222 to A.K.B.); The Smith Family Foundation; the Ludke Foundation to M.J.L and A.G.L.; the American Parkinson's Disease Association to M.J.L.; and The Parkinson's Disease Foundation to M.J.L. and the Brigham and Women's Hospital Udall Center of Excellence in Parkinson's Disease Research (P50 NS03875).
ACKNOWLEDGEMENTS
We thank Irit Rappley for critical reading of the manuscript and helpful suggestions.
Conflict of Interest statement. A.G.L. is a co-founder of Eutropics Pharmaceuticals.
REFERENCES
- 1.Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Nussbaum R.L., Ellis C.E. Alzheimer's disease and Parkinson's disease. N. Engl. J. Med. 2003;348:1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 3.Dawson T.M., Dawson V.L. Rare genetic mutations shed light on the pathogenesis of Parkinson disease. J. Clin. Invest. 2003;111:145–151. doi: 10.1172/JCI17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leroy E., Anastasopoulos D., Konitsiotis S., Lavedan C., Polymeropoulos M.H. Deletions in the Parkin gene and genetic heterogeneity in a Greek family with early onset Parkinson's disease. Hum. Genet. 1998;103:424–427. doi: 10.1007/s004390050845. [DOI] [PubMed] [Google Scholar]
- 5.Lucking C.B., Abbas N., Durr A., Bonifati V., Bonnet A.M., de Broucker T., De Michele G., Wood N.W., Agid Y., Brice A. Homozygous deletions in parkin gene in European and North African families with autosomal recessive juvenile parkinsonism. The European consortium on genetic susceptibility in Parkinson's disease and the French Parkinson's disease genetics study group. Lancet. 1998;352:1355–1356. doi: 10.1016/s0140-6736(05)60746-5. [DOI] [PubMed] [Google Scholar]
- 6.Feany M.B., Pallanck L.J. Parkin: a multipurpose neuroprotective agent? Neuron. 2003;38:13–16. doi: 10.1016/s0896-6273(03)00201-0. [DOI] [PubMed] [Google Scholar]
- 7.Greene J.C., Whitworth A.J., Kuo I., Andrews L.A., Feany M.B., Pallanck L.J. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pesah Y., Pham T., Burgess H., Middlebrooks B., Verstreken P., Zhou Y., Harding M., Bellen H., Mardon G. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- 9.Bernardi P., Scorrano L., Colonna R., Petronilli V., Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 10.Gross A., Yin X.M., Wang K., Wei M.C., Jockel J., Milliman C., Erdjument-Bromage H., Tempst P., Korsmeyer S.J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 11.Desagher S., Martinou J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 12.Letai A., Bassik M.C., Walensky L.D., Sorcinelli M.D., Weiler S., Korsmeyer S.J. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 13.Polster B.M., Kinnally K.W., Fiskum G. BH3 death domain peptide induces cell type-selective mitochondrial outer membrane permeability. J. Biol. Chem. 2001;276:37887–37894. doi: 10.1074/jbc.M104552200. [DOI] [PubMed] [Google Scholar]
- 14.Cunha-Oliveira T., Rego A.C., Cardoso S.M., Borges F., Swerdlow R.H., Macedo T., de Oliveira C.R. Mitochondrial dysfunction and caspase activation in rat cortical neurons treated with cocaine or amphetamine. Brain Res. 2006;1089:44–54. doi: 10.1016/j.brainres.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 15.Certo M., Del Gaizo Moore V., Nishino M., Wei G., Korsmeyer S., Armstrong S.A., Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Jurgensmeier J.M., Xie Z., Deveraux Q., Ellerby L., Bredesen D., Reed J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrzanowska-Lightowlers Z.M., Turnbull D.M., Lightowlers R.N. A microtiter plate assay for cytochrome c oxidase in permeabilized whole cells. Anal. Biochem. 1993;214:45–49. doi: 10.1006/abio.1993.1454. [DOI] [PubMed] [Google Scholar]
- 18.Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 19.Antonsson B., Montessuit S., Lauper S., Eskes R., Martinou J.C. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- 20.Eskes R., Desagher S., Antonsson B., Martinou J.C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano K., Vousden K.H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 22.Ritov V.B., Tverdislova I.L., Avakyan T., Menshikova E.V., Leikin Yu N., Bratkovskaya L.B., Shimon R.G. Alamethicin-induced pore formation in biological membranes. Gen. Physiol. Biophys. 1992;11:49–58. [PubMed] [Google Scholar]
- 23.Mailloux A., Bruneel A., Vaubourdolle M., Baudin B. Cyclosporin A but not estradiol can protect endothelial cells against etoposide-induced apoptosis. Endothelium. 2004;11:141–149. doi: 10.1080/10623320490512048. [DOI] [PubMed] [Google Scholar]
- 24.Clark I.E., Dodson M.W., Jiang C., Cao J.H., Huh J.R., Seol J.H., Yoo S.J., Hay B.A., Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 25.Park J., Lee S.B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J.M., et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 26.Darios F., Corti O., Lucking C.B., Hampe C., Muriel M.P., Abbas N., Gu W.J., Hirsch E.C., Rooney T., Ruberg M., et al. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum. Mol. Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- 27.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira S.A., Scott W.K., Martin E.R., Nance M.A., Watts R.L., Hubble J.P., Koller W.C., Pahwa R., Stern M.B., Hiner B.C., et al. Parkin mutations and susceptibility alleles in late-onset Parkinson's disease. Ann. Neurol. 2003;53:624–629. doi: 10.1002/ana.10524. [DOI] [PubMed] [Google Scholar]
- 29.Kay D.M., Moran D., Moses L., Poorkaj P., Zabetian C.P., Nutt J., Factor S.A., Yu C.E., Montimurro J.S., Keefe R.G., et al. Heterozygous parkin point mutations are as common in control subjects as in Parkinson's patients. Ann. Neurol. 2007;61:47–54. doi: 10.1002/ana.21039. [DOI] [PubMed] [Google Scholar]
- 30.Henn I.H., Bouman L., Schlehe J.S., Schlierf A., Schramm J.E., Wegener E., Nakaso K., Culmsee C., Berninger B., Krappmann D., et al. Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J. Neurosci. 2007;27:1868–1878. doi: 10.1523/JNEUROSCI.5537-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C., Lu R., Ouyang X., Ho M.W., Chia W., Yu F., Lim K.L. Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities. J. Neurosci. 2007;27:8563–8570. doi: 10.1523/JNEUROSCI.0218-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaVoie M.J., Cortese G.P., Ostaszewski B.L., Schlossmacher M.G. The effects of oxidative stress on parkin and other E3 ligases. J. Neurochem. 2007;103:2354–2368. doi: 10.1111/j.1471-4159.2007.04911.x. [DOI] [PubMed] [Google Scholar]
- 33.LaVoie M.J., Ostaszewski B.L., Weihofen A., Schlossmacher M.G., Selkoe D.J. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto A., Friedlein A., Imai Y., Takahashi R., Kahle P.J., Haass C. Parkin phosphorylation and modulation of its E3 ubiquitin ligase activity. J. Biol. Chem. 2005;280:3390–3399. doi: 10.1074/jbc.M407724200. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald J.C., Plun-Favreau H. Emerging pathways in genetic Parkinson's disease: autosomal-recessive genes in Parkinson's disease–a common pathway? Febs. J. 2008;275:5758–5766. doi: 10.1111/j.1742-4658.2008.06708.x. [DOI] [PubMed] [Google Scholar]
- 36.Weihofen A., Ostaszewski B., Minami Y., Selkoe D.J. Pink1 Parkinson mutations, the Cdc37/Hsp90 chaperones and Parkin all influence the maturation or subcellular distribution of Pink1. Hum. Mol. Genet. 2008;17:602–616. doi: 10.1093/hmg/ddm334. [DOI] [PubMed] [Google Scholar]
- 37.Dawson T.M., Dawson V.L. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 38.Green D.R., Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 39.Crawford G.D., Jr, Le W.D., Smith R.G., Xie W.J., Stefani E., Appel S.H. A novel N18TG2 x mesencephalon cell hybrid expresses properties that suggest a dopaminergic cell line of substantia nigra origin. J. Neurosci. 1992;12:3392–3398. doi: 10.1523/JNEUROSCI.12-09-03392.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y., Park J., Kim S., Song S., Kwon S.K., Lee S.H., Kitada T., Kim J.M., Chung J. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem. Biophys. Res. Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- 41.Kuroda Y., Mitsui T., Kunishige M., Shono M., Akaike M., Azuma H., Matsumoto T. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum. Mol. Genet. 2006;15:883–895. doi: 10.1093/hmg/ddl006. [DOI] [PubMed] [Google Scholar]
- 42.Narendra D., Tanaka A., Suen D.F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y.X., Muqit M.M., Latchman D.S. Induction of parkin expression in the presence of oxidative stress. Eur. J. Neurosci. 2006;24:1366–1372. doi: 10.1111/j.1460-9568.2006.04998.x. [DOI] [PubMed] [Google Scholar]
- 44.Yamauchi T., Sakurai M., Abe K., Matsumiya G., Sawa Y. Ubiquitin-mediated stress response in the spinal cord after transient ischemia. Stroke. 2008;39:1883–1889. doi: 10.1161/STROKEAHA.106.455832. [DOI] [PubMed] [Google Scholar]
- 45.Wang C., Ko H.S., Thomas B., Tsang F., Chew K.C., Tay S.P., Ho M.W., Lim T.M., Soong T.W., Pletnikova O., et al. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin's protective function. Hum. Mol. Genet. 2005;14:3885–3897. doi: 10.1093/hmg/ddi413. [DOI] [PubMed] [Google Scholar]
- 46.Winklhofer K.F., Henn I.H., Kay-Jackson P.C., Heller U., Tatzelt J. Inactivation of parkin by oxidative stress and C-terminal truncations: a protective role of molecular chaperones. J. Biol. Chem. 2003;278:47199–47208. doi: 10.1074/jbc.M306769200. [DOI] [PubMed] [Google Scholar]
- 47.Jimenez Del Rio M., Moreno S., Garcia-Ospina G., Buritica O., Uribe C.S., Lopera F., Velez-Pardo C. Autosomal recessive juvenile parkinsonism Cys212Tyr mutation in parkin renders lymphocytes susceptible to dopamine- and iron-mediated apoptosis. Mov. Disord. 2004;19:324–330. doi: 10.1002/mds.10670. [DOI] [PubMed] [Google Scholar]
- 48.Ren Y., Jiang H., Yang F., Nakaso K., Feng J. Parkin protects dopaminergic neurons against microtubule-depolymerizing toxins by attenuating microtubule-associated protein kinase activation. J. Biol. Chem. 2009;284:4009–4017. doi: 10.1074/jbc.M806245200. [DOI] [PMC free article] [PubMed] [Google Scholar]