Abstract
Pregnant women are advised to abstain from alcohol despite insufficient evidence on the fetal consequences of moderate prenatal alcohol use. Mendelian randomization could help distinguish causal effects from artifacts due to residual confounding and measurement errors; however, polymorphisms reliably associated with alcohol phenotypes are needed. We aimed to test whether alcohol dehydrogenase (ADH) gene variants were associated with alcohol use before and during pregnancy. Ten variants in four ADH genes were genotyped in women from South-West England. Phenotypes of interest were quantity and patterns of alcohol consumption before and during pregnancy, including quitting alcohol following pregnancy recognition. We tested single-locus associations between genotypes and phenotypes with regression models. We used Bayesian models (multi-locus) to take account of linkage disequilibrium and reanalyzed the data with further exclusions following two conservative definitions of ‘white ethnicity’ based on the woman's reported parental ethnicity or a set of ancestry-informative genetic markers. Single-locus analyses on 7410 women of white/European background showed strong associations for rs1229984 (ADH1B). Rare allele carriers consumed less alcohol before pregnancy [odds ratio (OR) = 0.69; 95% confidence interval (CI): 0.56–0.86, P = 0.001], were less likely to have ‘binged’ during pregnancy (OR = 0.55, 95% CI: 0.38–0.78, P = 0.0009), and more likely to have abstained in the first trimester of gestation (adjusted OR = 1.42, 95% CI: 1.12–1.80, P = 0.004). Multi-locus models confirmed these results. Sensitivity analyses did not suggest the presence of residual population stratification. We confirmed the established association of rs1229984 with reduced alcohol consumption over the life-course, contributing new evidence of an effect before and during pregnancy.
INTRODUCTION
Whereas the fetal consequences of heavy alcohol intake during pregnancy have been clear for some time (1), the effect of moderate drinking remains controversial (2). The advice to pregnant women is generally to abstain, since no amount of alcohol is known to be safe (3). However, this is based on a precautionary principle rather than on solid evidence. Even where associations have been confirmed, these have generally been weak (4) and could still be due to residual confounding (5,6) and under-reporting of prenatal alcohol use (7,8). Both of these potentially interfere with establishing causality of the associations of maternal alcohol intake with various outcomes [from fetal growth and neurodevelopment (9) to childhood mental health problems such as hyperactivity or inattention (10) or conduct disorder (11)] and deriving evidence-based messages for the public.
Genetic variants associated with alcohol intake or metabolism have been proposed as unbiased and unconfounded markers of the effect of ingested ethanol or its metabolites (acetate, acetaldehyde)—an approach known as Mendelian randomization (12,13). A single nucleotide polymorphism (SNP) exists in the acetaldehyde dehydrogenase gene ALDH2, which encodes an enzyme unable to clear acetaldehyde and often causes individuals carrying the variant to abstain from alcohol or drink in moderation (14). The use of ALDH2 as an instrument has proved valuable in Mendelian randomization studies (15,16). However, the potential relevance of this variant is limited to East-Asian populations as it is absent in populations of European origin (17).
Identifying suitable instrument for Mendelian randomization studies of the consequences of prenatal alcohol exposure in European populations remains a challenge. The most natural choice of candidates is alcohol dehydrogenase (ADH) genes, which catalyze the first step of alcohol metabolism oxidizing ethanol into acetaldehyde. A role for these variants in alcohol intake, metabolism and dependence has been suggested by both genome-wide linkage (18–21) and candidate gene (22–25) studies, and in particular a non-synonymous variant in ADH1B has been implicated. The rare variant is thought to encode an enzyme which accelerates ethanol clearance (26) (based on studies in vitro), but there is limited evidence for association with alcohol metabolism in vivo (22,27,28,29). The extent to which this SNP is associated with alcohol intake among pregnant women is unclear. On the one hand, the pressures to cut down or quit drinking alcohol (30–32) might increase the relative contribution of genetic variation to alcohol intake and, on the other; pregnancy might result in metabolic changes and modify the effect of the ADH1B and other ADH variants. A systematic review on the incidence of fetal alcohol syndrome in populations of European and African origins found that this SNP was associated with offspring outcome (33), but highlighted the need to better understand the extent to which the ADH1B variant is associated with prenatal alcohol use (33).
This study seeks to validate genetic polymorphisms in ADH genes, including ADH1B, as instruments for prenatal alcohol exposure, by testing first, whether they predict maternal alcohol intake and patterns of consumption just before and during pregnancy, and second, whether the variants related to alcohol intake are also associated with the likelihood of abstaining from alcohol during pregnancy.
To answer these questions, we analyzed data from mothers of white ethnic origin enrolled in the Avon Longitudinal Study of Parents and Children (ALSPAC).
RESULTS
A description of selected participants’ characteristics is presented in Table 1. Of the 14 541 pregnant women enrolled in ALSPAC initially, 7410 could be included in the present analysis. Details of exclusions from the study are reported in Supplementary Material, Fig. S1. Genotype frequencies were similar among those who completed and did not complete the questionnaire (data not shown).
Table 1.
Selected participants’ characteristics
| Na | Mean (SD) or % | |
|---|---|---|
| Age at delivery (years) | 7410 | 28 (5) |
| BMI before pregnancy (kg/m2) | 6594 | 23.0 (3.9) |
| Secondary level education at most (versus higher) | 6986 | 64% |
| Manual worker (versus more qualified occupation)b | 6594 | 51% |
| Smoking pre-pregnancy (yes versus no) | 7344 | 32% |
| Drinking before pregnancy (yes versus no) | 7410 | 93% |
| Drinking in first trimester of pregnancy (yes versus no) | 7387 | 55% |
| Binge drinking during pregnancy (yes versus no)c | 7365 | 17% |
aNumber with complete data.
bOn the basis of the highest occupation held either by the woman or her partner (see Materials and Methods for details).
cAt week 18 of gestation.
Genotype distributions were compatible with no departure from Hardy–Weinberg equilibrium for all SNPs except rs4699714 (ADH4) (Supplementary Material, Table S2). Genotyping success rate was above 93.3% and error rate from duplicates was below 0.25% for all SNPs.
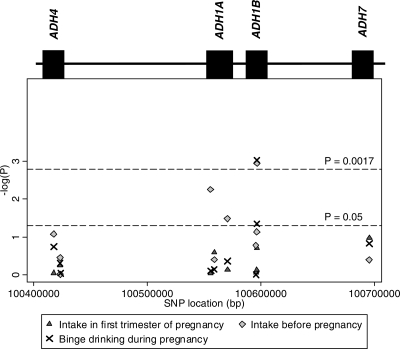
A summary of single-locus association tests between ADH genotypes and three dimensions of alcohol drinking before and during pregnancy is presented in Figure 1, where inverse-logged P-values are plotted against each SNP's chromosomal position. The ‘peak’ corresponds to a SNP in ADH1B, rs1229984, for which there is evidence of association with average alcohol intake before (P-value = 0.001) and binge drinking during pregnancy (P-value = 9 × 10−4) (Fig. 1 and Table 2). There is also some evidence of association between rs2866151 (ADH1A) and weekly drinking before pregnancy (P-value = 0.006) (Fig. 1 and Table 2). In general, the associations are stronger when considering drinking before the index pregnancy, and attenuate for drinking during the period following recognition of pregnancy, in particular average weekly drinking in the first trimester (Table 2).
Figure 1.
Association of variation at 10 SNPs in ADH and alcohol drinking (additive model). The two horizontal dotted lines refer to a nominal level of statistical significance (P = 0.05) and a conservative Bonferroni correction, based on 30 tests—10 SNPs and 3 outcomes (P = 0.05/30 = 0.0017).
Table 2.
Association of variation at 10 SNPs in ADH and alcohol drinking (additive model)
| SNP rs-number | Weekly intake before pregnancy |
Weekly intake in first trimester |
Binge drinking during pregnancy |
|||
|---|---|---|---|---|---|---|
| ORa (95% CI) | P-value | ORb (95% CI) | P-value | ORc (95% CI) | P-value | |
| rs4699714 | 1.07 (0.99, 1.15) | 0.084 | 1.01 (0.91, 1.12) | 0.879 | 1.07 (0.97, 1.18) | 0.180 |
| rs3762894 | 0.96 (0.88, 1.05) | 0.349 | 1.04 (0.91, 1.19) | 0.553 | 1.04 (0.93, 1.17) | 0.486 |
| rs4148884 | 1.00 (0.89, 1.13) | 0.947 | 0.99 (0.83, 1.18) | 0.935 | 0.99 (0.85, 1.16) | 0.894 |
| rs2866151 | 1.10 (1.03, 1.17) | 0.006 | 0.99 (0.90, 1.10) | 0.908 | 1.01 (0.93, 1.11) | 0.775 |
| rs975833 | 0.97 (0.90, 1.04) | 0.394 | 1.07 (0.95, 1.20) | 0.254 | 1.02 (0.92, 1.13) | 0.708 |
| rs1229966 | 0.93 (0.87, 0.99) | 0.032 | 1.02 (0.92, 1.13) | 0.716 | 1.04 (0.95, 1.14) | 0.431 |
| rs2066701 | 0.95 (0.88, 1.02) | 0.165 | 1.01 (0.91, 1.13) | 0.841 | 1.00 (0.91, 1.10) | 0.970 |
| rs4147536 | 0.93 (0.86, 1.01) | 0.073 | 0.98 (0.87, 1.10) | 0.708 | 0.89 (0.80, 1.00) | 0.045 |
| rs1229984 | 0.69 (0.56, 0.86) | 0.001 | 0.78 (0.53, 1.13) | 0.192 | 0.55 (0.38, 0.78) | 9×10−4 |
| rs284779 | 1.03 (0.96, 1.10) | 0.398 | 1.08 (0.98, 1.20) | 0.105 | 1.07 (0.98, 1.17) | 0.148 |
aOdds ratio (OR) of drinking ≥7 units/week versus 1–6 units/week or 1–6 units/week versus <1 unit/week before pregnancy, from ordinal logistic regression.
bOdds ratio of drinking ≥1 units/week versus <1 unit/week in first trimester of pregnancy, from logistic regression.
cOdds ratio of drinking ≥4 units per drinking occasion (binge drinking) around week 18 of gestation, from logistic regression.
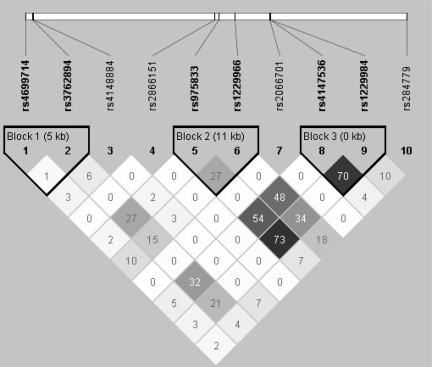
In order to account for linkage disequilibrium (LD) in the area (Fig. 2), which could confound association results, Bayesian multilocus models were fitted to these data, separately for the three alcohol measures. Figures in Supplementary Material, Table S3 represent the probability that a model with one or more SNPs as explanatory variables is selected out of all the possible models in the model space (statistics based on 10 000 iterations with the first 5000 excluded—‘burn-in’). Results show a strong effect of rs1229984 on alcohol drinking before pregnancy and binge drinking during pregnancy, independent on the other SNPs in the model. However, for one of the outcomes, drinking in the first trimester of pregnancy, the model with rs1229984 performed worse than the null model (posterior probabilities: 0.08 versus 0.88) (Supplementary Material, Table S3). The association between rs2866151 and pre-pregnancy drinking disappears when taking into account LD patterns in this multi-locus model. These results are in line with crude associations from classical univariable analyses (Table 2), confirming that the only SNP associated with alcohol use in this study is the established rs1229984, but also that actual weekly intake during the first trimester is the only outcome that does not seem to be associated with genotypes.
Figure 2.
Linkage disequilibrium across the 10 ADH SNPs on chromosome 4 calculated as r2, from Haploview (ALSPAC mothers data).
Additional results for rs1229984 are presented in detail in Table 3. The strong evidence for association between this SNP and average intake before pregnancy but not during pregnancy, and the result showing that it predicts abstention in pregnancy but not before (Table 3), prompted us to investigate whether the variant was associated with the likelihood of drinking cessation in pregnancy. There is strong evidence that women carrying the rare A allele were more likely to quit alcohol during the first trimester of pregnancy [odds ratio (OR): 1.52; 95% confidence interval (CI): 1.22, 1.91; P-value = 0.0004) (Table 4). The evidence remains after adjusting for pre-pregnancy alcohol consumption (P-value = 0.004). Weaker evidence is found when looking at the odds of quitting at later stages of gestation, around week 18 and week 32, and these associations seem to be entirely explained by pre-pregnancy intake (Table 4).
Table 3.
Association between alcohol consumption before and during pregnancy and the rs1229984*A allele (ADH1B) among ALSPAC mothers—dominant effect
| Time period | Absolute number | Proportion carrying rare A allele, % | Odds ratio (95% CI) | P-value | |
|---|---|---|---|---|---|
| Likelihood of abstaining | |||||
| Before pregnancy | Yes | 498 | 6.6 | 1.47 (1.01, 2.13)a | 0.043 |
| No | 6607 | 4.6 | |||
| First trimester | Yes | 3167 | 5.8 | 1.55 (1.24, 1.93)a | 0.0001 |
| No | 3923 | 3.9 | |||
| Consumption levels among drinkers | |||||
| Before pregnancy | <1 drink/week | 2713 | 5.8 | ||
| 1–6 drinks/week | 3229 | 4.1 | 0.67 (0.54, 0.84)b | 0.0005 | |
| 7+ drinks/week | 836 | 3.4 | |||
| First trimester | <1 drink/week | 2853 | 4.2 | ||
| 1+ drinks/week | 1170 | 3.3 | 0.77 (0.53, 1.12)b | 0.171 | |
aOdds ratio of abstaining versus drinking.
bOdds ratio of ≥1 units/week versus <1 unit/week.
Table 4.
Association between quitting drinking in pregnancy and the rs1229984*A allele (ADH1B) among 6,889 ALSPAC mothers drinking before pregnancy—dominant effect
| Gestational period | No quitted drinking/No still drinking | Model 1a | P-value | Model 2b | P-value |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||||
| First trimester | 2680/3911 | 1.52 (1.21, 1.91) | 0.0004 | 1.42 (1.12, 1.80) | 0.004 |
| 18 weeks | 3013/3468 | 1.23 (0.98, 1.56) | 0.076 | 1.12 (0.88, 1.43) | 0.366 |
| 32 weeksc | 2614/1441 | 1.45 (1.06, 1.98) | 0.021 | 1.28 (0.91, 1.81) | 0.155 |
aModel 1 is the unadjusted effect of carrying the allele on the odds of quitting drinking.
bModel 2 is the effect of carrying the allele on the odds of quitting drinking, adjusted for the amount of alcohol drunk before pregnancy (<1 unit/week, 1–6 units/week, ≥7 units/week).
cData only available for 4055 mothers.
The associations between rs1229984 and maternal characteristics known to be generally related to alcohol drinking were analyzed to empirically assess the specificity of the rs1229984-alcohol intake association. For maternal socio-economic status or education, smoking habits, BMI and age at delivery there is no evidence of association; nor is there any association with alcohol drinking behavior of the partner (Supplementary Material, Table S4). Similarly, since drinking cessation in the first trimester may be due to symptoms of nausea and various food aversions experienced in early pregnancy, we looked at these in association with rs1229984 genotype. There is little evidence of association with nausea or developing aversion to any of the following: coffee, tea, colas or cigarettes (Supplementary Material, Table S5).
Among women included in the analysis as ‘white’, 36 reported ethnicity other than white for their mother, father or both. After excluding these in sensitivity analyses, results were largely unchanged (data not shown). Further exclusions based on a set of ancestry-informative genetic markers (N = 76 women), or on self-reported Jewish faith (as proxy for Jewish ancestry/ethnicity) (N = 4) did not alter the results (not shown—see Materials and Methods for details).
DISCUSSION
Summary of results
We selected 10 candidate polymorphisms in four ADH genes and found evidence for a strong inverse association between the A allele of the non-synonymous variant rs1229984 in ADH1B and alcohol drinking before pregnancy and binge drinking patterns during pregnancy. Further analyses suggested a role for this variant in predicting drinking cessation particularly in the first trimester. Since the strongest predictor of drinking cessation during pregnancy is alcohol consumption before pregnancy (34,35), we repeated the analyses adjusting for this covariate potentially on the causal pathway, and the evidence remained strong, although there is a small chance that this could still be due to residual confounding, i.e. our inability to capture drinking behaviors with more precision.
This is the first study, to our knowledge, on the role of ADH variants on alcohol drinking in women just before and during pregnancy. Strong external pressures exist for pregnant women to modify their behavior and quit alcohol (30–32). Such pressures might highlight any existing genetic component to drinking behavior, through homogenization of some of the concurrent social determinants of alcohol drinking. However, the extreme-case scenario is that they could result into everybody abstaining from alcohol regardless of their genotype, thus overriding genetic effects. Indeed, we found that carriers of the rs1229984 A allele (ADH1B), who before pregnancy were both more likely to abstain and drink less on average, during pregnancy and in particular in the first trimester, were still drinking less, and they were less likely to binge drink and more likely to have stopped drinking altogether. This supports the use of the rs1229984 variant as a candidate instrument for alcohol drinking (versus very little or no drinking) around the time of conception and even more so for binge drinking during pregnancy. This instrument would be highly specific to alcohol behavior, however, the population attributable risk is low given the low prevalence of the variant [minor allele frequency (MAF) in European origin populations: 2–5% (23,24,36,37)].
Strengths and limitations
It is unlikely that genotype data missing for 6000 women would introduce selection bias. Empirical evidence suggests that genotype frequencies are the same for general-population versus selected samples in the UK (the British 1958 birth cohort versus British blood donors from the Wellcome Trust Case–Control Consortium) (38), and that genotypes display no more correlation with socioeconomic, lifestyle and physiological phenotypes than expected because of chance (39). Moreover, based on our data we found a lack of association of genotypes with socioeconomic and highly socially-patterned factors such as smoking.
We considered the possibility of hidden population substructure explaining the main result for rs1229984. This variant is one of the most ethnically stratified, exhibiting a wide range of MAFs: from 2–5% in Western European populations to 60–70% in North-East Asia (40), and alcohol intake is strongly culturally-dependent (41). For this reason, we limited the analyses to women of self-reported white ethnicity and conducted sensitivity analyses by further excluding women reporting at least one parent of non-white ethnicity or likely to be ‘non-white’ based on ancestry-informative markers genotype or self-reported Jewish faith, yet results were unchanged. The lack of genome-wide data on this sample prevented us from running further checks.
Chance is an unlikely explanation of our results. The choice of the candidate variants was informed by a strong biological prior, and for rs1229984, this was in fact an attempt to replicate the association with alcohol intake seen in different contexts. The sample size was large, even by the standards of genetic association studies. The power to detect effect sizes like the ones observed for rs1229984 comparing A allele carriers versus non-carriers was ∼76% for binge drinking during pregnancy and 80% for drinking cessation. Moreover, over-conservative Bonferroni corrections and Bayesian models were used in the first part of the analyses, both accounting for multiple testing. However, the limited phenotypic variation in this sample compared with previous studies (23,24) (approximately 1 versus 3 drinks/week on average for women) could reduce the power to detect associations of alcohol use with other genetic variants with less marked effects.
The usual concern with self-reported alcohol intake is underreporting, both for general population samples (42) and pregnant women (8). However, underreporting is unlikely to be differential by genotype (under the null hypothesis of no effect on alcohol consumption) and it affects higher intake categories more than moderate and low intake. It is unlikely that under-reporting could explain the observed results, as the heavier drinkers without the A allele could underreport their consumption and declare similar intakes to the A allele carriers, introducing a bias towards the null. Quantity and frequency questions were used to derive pre-pregnancy weekly intake, shown to be generally valid (43) and reliable (44). Levels of pre-pregnancy drinking reported by ALSPAC participants included in this analysis were considerably lower compared with average alcohol intake of UK women aged 25–34 (45), a likely reflection of intentional behavioral change in preparation for the pregnancy. Figures on alcohol consumption during pregnancy were higher than those from surveys from the USA (46) and Sweden (34) showing that 12 and 30% of women reported consuming alcohol during gestation. Here the figure was 55%, of which only 14% reported drinking more than one drink weekly. Limited data are available on the prevalence of prenatal binge drinking, but a recent French study reported 14% of women consuming five or more drinks on one occasion during pregnancy (47), which is similar to our figure of 17% for consuming four or more drinks, whereas a US study found only 2% of pregnant women binge drinking (46).
Comparison with published literature
Rs1229984 is a non-synonymous variant thought to have a functional role. In vitro studies predicted up to 40 times higher enzymatic activity comparing carriers versus non-carriers of the A allele (26), resulting in faster accumulation of plasma acetaldehyde. This would be aversive, and it is the principle of action of the drug disulfiram, used for treating chronic alcohol dependence (http://www.bnf.org/bnf/bnf/current/3687.htm). However, the evidence for in vivo effects is limited, possibly because of lack of power arising from a combination of small sample sizes and low prevalence of the variant. A study conducted among 109 Jewish university students in the USA (MAF = 18.5%), reported that carriers of the A allele exhibited higher alcohol elimination rates (48). However, this locus contributed little to the total variance explained by genetic variation in the whole ADH region in a study of blood and breath ethanol levels among 412 Australian twins (27), and similarly a larger and more recent study of ADH variation and alcohol metabolism among 812 Australian twins and relatives (MAF = 3.5%) could not find sufficient evidence for an effect of rs1229984 on blood or breath ethanol concentrations (22). One study from Japan, where the A allele is extremely prevalent (MAF = 53%), failed to detect an effect on blood levels of acetaldehyde following light drinking (N = 68) (49), but another reported that extreme levels of drinking in alcoholics (N = 80) gave rise to higher blood ethanol and to a lesser extent blood acetaldehyde levels the morning after heavy drinking for carriers of the ancestral G allele (slower metabolizers) (29).
The role of rs1229984 on alcohol phenotypes in non-Asian population samples has been studied before, especially in relation to dependence (23–25,50–52). In addition, a number of studies consistently reported associations between the A allele and reduced alcohol consumption (23–25,53–57), as well as higher level of response to alcohol (58). Most of the samples studied were relatively small (i.e. <2000) (25,53–58). One exception is a study of 9000 Danes presenting evidence of increased alcohol consumption in men not carrying the A allele (OR of heavy drinking for G allele homozygotes versus heterozygotes: 3.1, 95% CI: 1.7, 5.7) (24). Another is a recent study from Australia examining 50 ADH polymorphisms in association with alcohol intake, confirming rs1229984 as their top hit for most alcohol drinking measures and alcohol-related reactions, with the association between the A allele and reduced overall alcohol consumption approaching ‘genome-wide’ levels of statistical significance (P-value = 8.9 × 10−8) (23). The above results cannot be directly combined, mainly because of incomplete reporting (P-values but no effect estimate) and heterogeneity of populations and alcohol phenotypes presented. However, consistency of the direction of association across all studies as well as P-values from the largest studies offer reassurance and strengthen the evidence supporting a role of this SNP in influencing alcohol consumption (sample results from the above cited studies are presented in Supplementary Material, Table S6 available online).
Our study adds at least two important results to the existing literature: women carrying the rs1229984 A allele are less likely to report episodes of binge drinking during pregnancy and they are more likely to report quitting alcohol during the first trimester. The first finding is in line with previous evidence, and extends this to the extremely sensitive time of pregnancy, when alcohol consumption has several potential adverse effects on the fetus (10). The association with drinking cessation is an entirely new result. Whether metabolic changes occurring in pregnancy interact with the effect of the variant, or whether the increased odds of quitting alcohol are due to residual confounding by usual (pre-pregnancy) alcohol intake, we cannot tell.
With the exception of a recent Australian twin study (23), no other study has explored the association between alcohol intake and variation across the ADH region beyond the classical ADH1B and ADH1C variants. Most of the evidence relating alcohol behavior to ADH variation in non-Asian populations comes from three fine-mapping studies limited to the alcoholism phenotype (37,52,59), whose results confirmed a role for variation in ADH1B (37,52,59), and suggested one for the ADH4 gene (37,52), replicating a previous study (60). The only data on ADH4 comparable with this study is an association signal for maximum number of drinks for rs3762894, independent of rs1229984 (23). We could not replicate this result when looking at binge drinking during pregnancy, however, a full comparison is hindered because effect estimates are not presented in the Australian publication (23).
Variation in ADH7 has been implicated in the early stages of alcohol metabolism (22), however, it is unclear how this affects drinking propensity. A large recent study showed strong evidence of gene * environment interaction between the non-synonymous SNP rs1573496 and alcohol intake for risk of upper aerodigestive tract cancers (61). This SNP has also been shown to be associated with alcohol dependence (52) [not in (59)]. A SNP in complete LD with rs1573496 (http://www.hapmap.org/cgi-perl/gbrowse/hapmap_B35/) (62), rs971074, had also been associated with alcohol dependence (52), but other studies failed to confirm the association with either dependence (23,37,59), or with alcohol use and adverse reactions (23). In agreement with these results, the present study does not find evidence of association between ADH7 variation and alcohol drinking before or during pregnancy.
Few studies have reported on the association between alcohol phenotypes and ADH1A polymorphisms, perhaps reflecting the current belief that ADH1A enzymes are most active in ethanol metabolism during fetal and early life, gradually losing function in adulthood (63). One of the fine-mapping studies on ADH variants and alcohol dependence reported signals for three ADH1A SNPs (52). For two of these SNPs, typed in the present study, we found very modest signals for drinking before pregnancy, in line with published evidence of association between rs3819197 (in strong LD with one of our SNPs) and alcohol consumption (23).
Conclusion and implications for future research
In conclusion, we propose the ADH1B variant rs1229984 as a candidate genetic instrument to study the offspring effects of maternal alcohol drinking, in European origin populations. The association of this SNP with alcohol has been established in several adult populations (23,24). Here and for the first time, we present evidence that this effect exists also just before and during pregnancy. However, replication of the present findings in an independent sample is warranted for the ultimate validation of the proposed instrument. A comparable approach to ‘instrument identification’ has been adopted successfully by Freathy and colleagues, showing that a nicotine receptor gene variant predicts smoking cessation during pregnancy (64).
The time around conception and the first trimester are crucially sensitive periods, and the consequences of patterns of drinking that can expose the fetus to high ethanol blood concentrations are of particular concern (9). The uncertainty on longer-term effects of in utero exposure to ethanol into childhood and even adulthood arises from unreliable reporting of alcohol intake leading to measurement error, as well as residual confounding typical of standard observational studies. For example, it has been suggested that up to 1% of all children could suffer impaired neurodevelopment as a consequence of prenatal alcohol exposure, but solid evidence is lacking (2). This highlights the importance of using alternative study designs that control for some or all of these problems, and finally allow interpreting association as causation.
Natural experiments such as studies comparing differentially exposed siblings (65) or exposed adoptees with natural offspring have the potential to reduce biases and confounding found in more traditional observational studies, although measurement error remains an issue. Mendelian randomization is one such natural experiment, with the additional advantage of being free from alcohol assessment problems. Causal inference is enhanced by studying genetic markers that are specifically associated with the exposure of interest, but not with other socially-patterned risk factors because genotypes are randomized at conception, and clearly they can be measured objectively (66).
In this light, our initial validation of a candidate genetic instrument that predicts lower risk of binge drinking and higher likelihood of giving up alcohol early in gestation, and allows causal inference, takes on a significant public health importance. Implications of the present work are that several phenotypic outcomes could be studied in association with prenatal alcohol exposure, provided maternal ADH1B genotype is available. For example, we have estimated through simulations that in an instrumental-variable analysis using rs1229984 as the genetic instrument, approximately 6000 mother–child pairs would be required to find evidence of a causal association between alcohol consumption and a school-based performance score at age 11 (Key Stage 2—http://en.wikipedia.org/wiki/Key_Stage_2) at a 5% significance level, assuming a small change of as little as 0.20 standard deviations in the log-transformed outcome comparing A-allele carriers to non-carriers.
These results confirm the existence of a modest genetic contribution to drinking behavior around the time of pregnancy. However, they do not suggest that advice on alcohol drinking during pregnancy should differ according to individual genotype.
MATERIALS AND METHODS
Participants
The ALSPAC is a population-based longitudinal study that recruited 80–90% of pregnant women living in Avon (the English county with Bristol as main urban center) with expected delivery date between April 1991 and December 1992 (67). Women of white ethnic origin participating in ALSPAC were eligible for the study reported in this article if they had provided a biological sample for DNA extraction, returned a questionnaire at 18–20 weeks’ gestation, and completed the question on pre-pregnancy alcohol consumption. Ethnicity was largely available from self-reported data (N = 7359, 90% of those genotyped). For those with missing self-reports but available genotype data (N = 601, 7%), white or non-white ethnicity was imputed by regressing the offspring's ethnicity as well as genotype of five genetic ancestry-informative markers with established population-specific allelic distributions—rs713598 and rs1726966 in TAS2R38 (68), rs4988235 in MCM6 (69), A44871G in ASPM (70) and rs930557 in MCPH1 (70).
Supplementary Material, Fig. S1 available online describes the flow of participants from recruitment into ALSPAC to final inclusion in the current analyses.
Data on alcohol and related phenotypes
Mothers completed several questionnaires at enrolment, throughout pregnancy and during their offspring's infancy and childhood. In a questionnaire completed at week 18 of gestation, they were asked to recall their alcohol consumption just before the current pregnancy, during the first trimester and in the previous 2 weeks or at the time when they first felt the baby move. Instructions specified that one drink was equivalent to 1 unit (8 g) of alcohol, and one pint of beer to 2 units. Response categories for these questions were: never, <1 unit/week, ≥1 units/week, 1–2 units/day, 3–9 units/day, ≥10 units/day. After excluding never drinkers, the last three categories were grouped together. Thus three categories were formed to create the variable ‘drinking before pregnancy’, whereas for ‘drinking during first trimester’ only two categories were formed (<1 unit/week and ≥1 units/week), after excluding those who stopped drinking. Around week 32 of gestation, women were asked about their average weekday and weekend alcohol consumption, from which weekly alcohol intake was derived. However, attrition was high (41%) and the format of this variable was different from the other alcohol drinking variables at previous time-points, and therefore it was not used in formal association analyses. Drinking patterns were assessed in a separate question, included in the questionnaires at week 18 of gestation, which asked about the number of days in the previous 4 weeks that the mother had consumed at least 4 units of alcohol [defined as ‘binge drinking’ occasions for the purpose of this study (71)]]. Response categories were 0, 1–2, 3–4, 5–10 or ≥10 days. For the analyses, consuming at least 4 units of alcohol on one or more days around week 18 was considered ‘binge drinking during pregnancy’. Drinking cessation at various time-points was defined as non-drinking at those time-points, for women who were drinking before the index pregnancy.
Maternal age at delivery was derived from date of birth, collected at enrollment. Maternal and socio-demographic variables obtained during pregnancy were categorized for analysis: family social class (based on the highest occupation among the woman and her partner and dichotomized into manual versus otherwise, using the 1991 British Office of Population and Census Statistics classification); highest level of maternal education (secondary level academic qualification—‘O’ level—or higher versus lower); smoking before the current pregnancy (yes versus no); heavy smoking before the current pregnancy (≥15 cigarettes/day versus fewer); overweight before the current pregnancy [body mass index (BMI) ≥25 versus less]. Questions on nausea and recent changes in consumption of coffee, tea, cola, alcohol and cigarettes were included in the questionnaire completed in the first trimester of gestation. On the basis of questionnaires completed by partners themselves, data were derived on partner's alcoholism (ever suffered from alcoholism versus never) and alcohol drinking habits (≥1 units/day versus less, before the pregnancy).
Further details are included in the study protocol (67) or can be found on the ALSPAC website (http://www.alspac.bris.ac.uk). Ethical approval was obtained from the ALSPAC Law and Ethics Committee (IRB 00003312) and the Local Research Ethics Committee.
Genotyping
Ten SNPs were selected based on published evidence of functionality (i.e. rs1229984 in ADH1B) or association with alcohol-related phenotypes, and typed as part of an investigation of the role of maternal drinking on childhood growth and development in ALSPAC. Efforts were made to choose a set of independent common polymorphisms (MAF ≥ 0.05 for all except rs1229984). Coverage of ADH1A in particular (3 SNPs), whose related enzymes are most active during fetal and early life (63), reflects the scope of the study for which the genotyping was done. The prior for inclusion in the study for each SNP was not assessed through a formal meta-analysis of published associations, which was impossible because of the limited evidence presented. Rather, we looked for consistency of association across the different studies that reported results for a particular SNP, as well as evidence of functionality. We acknowledge that this strategy has the shortcoming of not being fully replicable.
DNA was extracted from peripheral blood taken during pregnancy as described previously (72). SNPs were genotyped by KBioscience (http://www.kbioscience.co.uk) using the KASPar chemistry, a competitive allele-specific PCR system using FRET quencher cassette oligos (http://www.kbioscience.co.uk/genotyping/genotyping-chemistry.htm). Blind duplicates, plate-identifying repeat samples and Hardy–Weinberg equilibrium tests were used as quality control checks.
Statistical analyses
Analyses were restricted to women of white ethnic origin. Associations between the SNPs and alcohol intake variables were tested in univariable models assuming additive effects of rare alleles. For SNPs with MAF < 0.05, rare homozygotes and heterozygotes were analyzed together and a dominant effect was assumed. Logistic regression was used for binary outcomes and ordinal logistic regression for ordered categorical variables (e.g. average weekly drinking before pregnancy—<1 unit/week, 1–6 units/week, ≥7 units/week).
Despite our efforts to select variants that are as independent of each other as possible, not all of the pair-wise correlations would be null. This means that signals from the crude analysis could have been confounded by some other SNPs because of LD, which is high in the ADH region. In order to identify independent genetic effects, Bayesian models were fitted to the data for each of the three alcohol phenotypes. These models account for LD patterns in the data and also for multiple testing. SNPs are kept in the model based on their probability of explaining variation in the outcome in relation to the performance of all the other SNPs, which can enter the model individually or in combination (73). The variable selection follows a reversible-jump algorithm based on a binomial prior distribution, with parameter for the number of trials being the total number of predictors included in the model, and the parameter for probability of ‘success’ equal to 0.5 (73). This ensures all possible models are equally likely a priori. Ten thousand iterations were completed, with the first 5000 specified as burn-in.
Top hits identified in the first phase of analyses were regressed against the likelihood of drinking cessation at different time-points during pregnancy, in crude analyses as well as adjusting for pre-pregnancy alcohol consumption levels (likely on the causal pathway).
To empirically assess the specificity of the ADH1B variant-alcohol intake associations, the OR of selected participant's and partner's characteristics were derived comparing carriers of the rs1229984 A allele versus non-carriers, and those drinking <1 unit/week before pregnancy versus those drinking ≥1 units/week (Supplementary Material, Table S4). Similarly, since abstaining in the first trimester could be explained by symptoms of nausea and food aversions, we looked at these in association with rs1229984 (Supplementary Material, Table S5).
Since rs1229984 shows extreme stratification in different populations worldwide (40), and alcohol drinking follows cultural patterns (41), there is a possibility of residual confounding by population substructure, even if the sample analyzed only included women reporting white/European ethnicity. To assess this, we conducted sensitivity analyses by further excluding women following two more conservative definitions of ‘white ethnicity’: one based on the woman's reported parental ethnicity, and her own; another based on a set of ancestry-informative genetic markers. Finally, we repeated the analyses after excluding all women reporting to be of Jewish religious faith, as a proxy for Jewish ancestry/ethnicity. This was done since the prevalence of the A allele is much higher in populations from West Asia including Jews from a variety of regions (74), who might also drink less than other individuals of European ancestry that would equally describe themselves as ‘white’.
Statistical analyses were performed in Stata 10 and WinBugs (75). Pair-wise LD across the 10 SNPs (as r2) was computed using Haploview (76).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
FUNDING
This work was supported by a UK Medical Research Council special training fellowship, awarded to L.Z. [G0501864/76656], by the Wellcome Trust [083506] and by the European Union 6th Framework Coordination Action [518148]. The UK Medical Research Council, the Wellcome Trust and the University of Bristol provide core support for ALSPAC. Funding to pay the Open Access publication charges for this article was provided by the UK Medical Research Council.
ACKNOWLEDGEMENTS
We are extremely grateful to all the families who took part in the ALSPAC study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. We thank Dr Beate Glaser for her help in constructing predictive models of ethnicity based on ancestry-informative markers, Prof. David Nutt for advice and comments and Dr Andrew Wills for useful discussions.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Jones K.L., Smith D.W. Recognition of fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 2.Gray R., Mukherjee R.A.S., Rutter M. Alcohol consumption during pregnancy and its effects on neurodevelopment: what is known and what remains uncertain. Addiction. 2009;104:1270–1273. doi: 10.1111/j.1360-0443.2008.02441.x. [DOI] [PubMed] [Google Scholar]
- 3.Mukhejee R.A.S., Hollins S., Abou-Saleh M.T., Turk J. Low level alcohol consumption and the fetus— Abstinence from alcohol is the only safe message in pregnancy. Br. Med. J. 2005;330:375–376. doi: 10.1136/bmj.330.7488.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florey C.D.V. Weak associations in epidemiological research: some examples and their interpretation. Int. J. Epidemiol. 1988;17:950–954. doi: 10.1093/ije/17.4.950. [DOI] [PubMed] [Google Scholar]
- 5.Olsen J., Basso O. Re: residual confounding. Am. J. Epidemiol. 1999;149:290. doi: 10.1093/oxfordjournals.aje.a009805. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson J.L., Jacobson S.W. Methodological issues in research on developmental exposure to neurotoxic agents. Neurotoxicol. Teratol. 2005;27:395–406. doi: 10.1016/j.ntt.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Kaskutas L.A., Graves K. Pre-pregnancy drinking: how drink size affects risk assessment. Addiction. 2001;96:1199–1209. doi: 10.1046/j.1360-0443.2001.968119912.x. [DOI] [PubMed] [Google Scholar]
- 8.Wurst F.M., Kelso E., Weinmann W., Pragst F., Yegles M., Sundstrom Poromaa I. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT—a pilot study in a population-based sample of Swedish women. Am. J. Obstet. Gynecol. 2008;198:407–405. doi: 10.1016/j.ajog.2007.10.801. [DOI] [PubMed] [Google Scholar]
- 9.Henderson J., Kesmodel U., Gray R. Systematic review of the fetal effects of prenatal binge-drinking. J. Epidemiol. Community Health. 2007;61:1069–1073. doi: 10.1136/jech.2006.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayal K., Heron J., Golding J., Emond A. Prenatal alcohol exposure and gender differences in childhood mental health problems: a longitudinal population-based study. Pediatrics. 2007;119:e426–e434. doi: 10.1542/peds.2006-1840. [DOI] [PubMed] [Google Scholar]
- 11.Disney E.R., Iacono W., McGue M., Tully E., Legrand L. Strengthening the case: prenatal alcohol exposure is associated with increased risk for conduct disorder. Pediatrics. 2008;122:e1225–e1230. doi: 10.1542/peds.2008-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davey S.G., Ebrahim S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? Br. Med. J. 2005;330:1076–1079. doi: 10.1136/bmj.330.7499.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith G.D. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin. Pharmacol. Toxicol. 2008;102:245–256. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 14.Luczak S.E., Glatt S.J., Wall T.L. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol. Bull. 2006;132:607–621. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- 15.Boccia S., Hashibe M., Galli P., De Feo E., Asakage T., Hashimoto T., Hiraki A., Katoh T., Nomura T., Yokoyama A., et al. Aldehyde dehydrogenase 2 and head and neck cancer: a meta-analysis implementing a mendelian randomization approach. Cancer Epidemiol. Biomarkers Prev. 2009;18:248–254. doi: 10.1158/1055-9965.EPI-08-0462. [DOI] [PubMed] [Google Scholar]
- 16.Chen L., Smith G.D., Harbord R.M., Lewis S.J. Alcohol intake and blood pressure: a systematic review implementing a Mendelian Randomization approach. PLoS Med. 2008;5:461–471. doi: 10.1371/journal.pmed.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oota H., Pakstis A.J., Bonne-Tamir B., Goldman D., Grigorenko E., Kajuna S.L.B., Karoma N.J., Kungulilo S., Lu R.B., Odunsi K., et al. The evolution and population genetics of the ALDH2 locus: random genetic drift, selection, and low levels of recombination. Ann. Hum. Genet. 2004;68:93–109. doi: 10.1046/j.1529-8817.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S., Bierut L.J., Porjesz B., Edenberg H.J., Dick D., Goate A., Hesselbrock V., Nurnberger J., Foroud T., Kramer J., et al. A novel non-parametric regression reveals linkage on chromosome 4 for the number of externalizing symptoms in Sib-Pairs. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:1301–1305. doi: 10.1002/ajmg.b.30735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reich T., Edenberg H.J., Goate A., Williams J.T., Rice J.P., Van Eerdewegh P., Foroud T., Hesselbrock V., Schuckit M.A., Bucholz K., et al. Genome-wide search for genes affecting the risk for alcohol dependence. Am. J. Med. Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- 20.Saccone N.L., Kwon J.M., Corbett J., Goate A., Rochberg N., Edenberg H.J., Foroud T., Li T.K., Begleiter H., Reich T., Rice J.P. A genome screen of maximum number of drinks as an alcoholism phenotype. Am. J. Med. Genet. 2000;96:632–637. doi: 10.1002/1096-8628(20001009)96:5<632::aid-ajmg8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Saccone S.F., Saccone N.L., Neuman R.J., Rice J.P. Genetic analysis of the maximum drinks phenotype. BMC Genet. 2005;6:S124. doi: 10.1186/1471-2156-6-S1-S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birley A.J., James M.R., Dickson P.A., Montgomery G.W., Heath A.C., Martin N.G., Whitfield J.B. ADH single nucleotide polymorphism associations with alcohol metabolism in vivo. Hum. Mol. Genet. 2009;18:1533–1542. doi: 10.1093/hmg/ddp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macgregor S., Lind P.A., Bucholz K.K., Hansell N.K., Madden P.A.F., Richter M.M., Montgomery G.W., Martin N.G., Heath A.C., Whitfield J.B. Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and dependence: an integrated analysis. Hum. Mol. Genet. 2009;18:580–593. doi: 10.1093/hmg/ddn372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolstrup J.S., Nordestgaard B.G., Rasmussen S., Tybjaerg-Hansen A., Gronbaek M. Alcoholism and alcohol drinking habits predicted from alcohol dehydrogenase genes. Pharmacogenomics J. 2008;8:220–227. doi: 10.1038/sj.tpj.6500471. [DOI] [PubMed] [Google Scholar]
- 25.Whitfield J.B., Nightingale B.N., Bucholz K.K., Madden P.A.F., Heath A.C., Martin N.G. ADH genotypes and alcohol use and dependence in europeans. Alcohol. Clin. Exp. Res. 1998;22:1463–1469. [PubMed] [Google Scholar]
- 26.Yin S.J., Bosron W.F., Magnes L.J., Li T.K. Human liver alcohol dehydrogenase: purification and kinetic characterization of the beta 2 beta 2, beta 2 beta 1, alpha beta 2, and beta 2 gamma 1 ‘Oriental’ isoenzymes. Biochemistry. 1984;23:5847–5853. doi: 10.1021/bi00319a026. [DOI] [PubMed] [Google Scholar]
- 27.Birley A.J., Whitfield J.B., Neale M.C., Duffy D.L., Heath A.C., Boomsma D.I., Martin N.G. Genetic time-series analysis identifies a major QTL for in vivo alcohol metabolism not predicted by in vitro studies of structural protein polymorphism at the ADH1B or ADH1C loci. Behav. Genet. 2005;35:509–524. doi: 10.1007/s10519-005-3851-6. [DOI] [PubMed] [Google Scholar]
- 28.Mizoi Y.A.S.U., Yamamoto K.E.N.J., Ueno Y.A.S.U., Fukunagai T.A.T.S., Harada S.H.O.J. Involvement of genetic polymorphism of alcohol and aldehyde dehydrogenases in individual variation of alcohol metabolism. Alcohol Alcohol. 1994;29:707–710. [PubMed] [Google Scholar]
- 29.Yokoyama A., Tsutsumi E., Imazeki H., Suwa Y., Nakamura C., Yokoyama T. Contribution of the alcohol dehydrogenase-IB genotype and oral microorganisms to high salivary acetaldehyde concentrations in Japanese alcoholic men. Int. J. Cancer. 2007;121:1047–1054. doi: 10.1002/ijc.22792. [DOI] [PubMed] [Google Scholar]
- 30.Department of Health. The Pregnancy Book. London, UK: Department of Health; 2009. http://www.dh.gov.uk/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH074920 . [Google Scholar]
- 31.Office of the Surgeon General. In Office of Public Health and Science. US Department of Health and Human Services; 2007. [Google Scholar]
- 32.Raymond N., Beer C., Glazebrook C., Sayal K. Pregnant women's attitudes towards alcohol consumption. BMC Public Health. 2009;9:175. doi: 10.1186/1471-2458-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green R.F., Stoler J.M. Alcohol dehydrogenase 1B genotype and fetal alcohol syndrome: a HuGE minireview. Am. J. Obstet. Gynecol. 2007;197:12–25. doi: 10.1016/j.ajog.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 34.Goransson M., Magnusson A., Bergman H., Rydberg U., Heilig M. Fetus at risk: prevalence of alcohol consumption during pregnancy estimated with a simple screening method in Swedish antenatal clinics. Addiction. 2003;98:1513–1520. doi: 10.1046/j.1360-0443.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- 35.Palma S., Pardo-Crespo R., Mariscal M., Perez-Iglesias R., Llorca J., Delgado-Rodriguez M. Weekday but not weekend alcohol consumption before pregnancy influences alcohol cessation during pregnancy. Eur. J. Public Health. 2007;17:394–399. doi: 10.1093/eurpub/ckl259. [DOI] [PubMed] [Google Scholar]
- 36.Chang M.h., Lindegren M.L., Butler M.A., Chanock S.J., Dowling N.F., Gallagher M., Moonesinghe R., Moore C.A., Ned R.M., Reichler M.R., et al. Prevalence in the United States of selected candidate gene variants: third national health and nutrition examination survey, 1991–1994. Am. J. Epidemiol. 2009;169:54–66. doi: 10.1093/aje/kwn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edenberg H.J., Xuei X., Chen H.J., Tian H., Wetherill L.F., Dick D.M., Almasy L., Bierut L., Bucholz K.K., Goate A., et al. Association of alcohol dehydrogenase genes with alcohol dependence: a comprehensive analysis. Hum. Mol. Genet. 2006;15:1539–1549. doi: 10.1093/hmg/ddl073. [DOI] [PubMed] [Google Scholar]
- 38.WTCCC. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davey Smith G., Lawlor D.A., Harbord R., Timpson N., Day I., Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLOS Med. 2007;4:e352. doi: 10.1371/journal.pmed.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borinskaya S., Kal'ina N., Marusin A., Faskhutdinova G., Morozova I., Kutuev I., Koshechkin V., Khusnutdinova E., Stepanov V., Puzyrev V., et al. Distribution of the alcohol dehydrogenase ADH1B*47His allele in Eurasia. Am. J. Hum. Genet. 2009;84:89–92. doi: 10.1016/j.ajhg.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization (WHO) Global Status Report on Alcohol 2004, 2nd edn. Geneva, Switzerland: World Health Organization (WHO); 2004. [Google Scholar]
- 42.Feunekes G.I., van V., van Staveren W.A., Kok F.J. Alcohol intake assessment: the sober facts. Am. J. Epidemiol. 1999;150:105–112. doi: 10.1093/oxfordjournals.aje.a009909. [DOI] [PubMed] [Google Scholar]
- 43.Giovannucci E., Colditz G., Stampfer M.J., Rimm E.B., Litin L., Sampson L., Willett W.C. The assessment of alcohol-consumption by a simple self-administered questionnaire. Am. J. Epidemiol. 1991;133:810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 44.Hansell N.K., Agrawal A., Whitfield J.B., Morley K.I., Zhu G., Lind P.A., Pergadia M.L., Madden P.A.F., Todd R.D., Heath A.C., Martin N.G. Long-term stability and heritability of telephone interview measures of alcohol consumption and dependence. Twin Res. Hum. Genet. 2008;11:287–305. doi: 10.1375/twin.11.3.287. [DOI] [PubMed] [Google Scholar]
- 45.Henderson L., Gregory J., Irving K., Swan G. The National Diet & Nutrition Survey: adults aged 19 to 64 years. Volume 2. London, UK: 2003. The Office for National Statistics (ed) [Google Scholar]
- 46.Denny C.H., Tsai J., Floyd R.L., Green P.P. 2009. Div of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, CDC; pp. 529–532. [Google Scholar]
- 47.de Chazeron I., Llorca P.M., Ughetto S., Vendittelli F., Boussiron D., Sapin V., Coudore F., Lemery D. Is pregnancy the time to change alcohol consumption habits in France? Alcohol. Clin. Exp. Res. 2008;32:868–873. doi: 10.1111/j.1530-0277.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 48.Neumark Y.D., Friedlander Y., Durst R., Leitersdorf E., Jaffe D., Ramchandani V.A., O'Connor S., Carr L.G., Li T.K. Alcohol dehydrogenase Polymorphisms influence alcohol-elimination rates in a male Jewish population. Alcohol. Clin. Exp. Res. 2004;28:10–14. doi: 10.1097/01.ALC.0000108667.79219.4D. [DOI] [PubMed] [Google Scholar]
- 49.Mizoi Y.A.S.U., Yamamoto K.E.N.J., Ueno Y.A.S.U., Fukunagai T.A.T.S., Harada S.H.O.J. Involvement of genetic polymorphisms of alcohol and aldehyde dehydrogenases in individual variation of alcohol metabolism. Alcohol Alcohol. 1994;29:707–710. [PubMed] [Google Scholar]
- 50.Borras E., Coutelle C., Rosell A., Fernandez-Muixi F., Broch M., Crosas B., Hjelmqvist L., Lorenzo A., Gutierrez C., Santos M., et al. Genetic polymorphism of alcohol dehydrogenase in Europeans: The ADH2*2 allele decreases the risk for alcoholism and is associated with ADH3*1. Hepatology. 2000;31:984–989. doi: 10.1053/he.2000.5978. [DOI] [PubMed] [Google Scholar]
- 51.Zintzaras E., Stefanidis I., Santos M., Vidal F. Do alcohol-metabolizing enzyme gene polymorphisms increase the risk of alcoholism and alcoholic liver disease? Hepatology. 2006;43:352–361. doi: 10.1002/hep.21023. [DOI] [PubMed] [Google Scholar]
- 52.Luo X., Kranzler H.R., Zuo L., Wang S., Schork N.J., Gelernter J. Diplotype trend regression analysis of the ADH gene cluster and the ALDH2 gene: multiple significant associations with alcohol dependence. Am. J. Hum. Genet. 2006;78:973–987. doi: 10.1086/504113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lilla C., Koehler T., Kropp S., Wang-Gohrke S., Chang-Claude J. Alcohol dehydrogenase 1B (ADH1B) genotype, alcohol consumption and breast cancer risk by age 50 years in a German case–control study. Br. J. Cancer. 2005;92:2039–2041. doi: 10.1038/sj.bjc.6602608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loew M., Boeing H., Sturmer T., Brenner H. Relation among alcohol dehydrogenase 2 polymorphism, alcohol consumption, and levels of gamma-glutamyltransferase. Alcohol. 2003;29:131–135. doi: 10.1016/s0741-8329(03)00015-6. [DOI] [PubMed] [Google Scholar]
- 55.Wall T.L., Shea S.H., Luczak S.E., Cook T.A.R., Carr L.G. Genetic associations of alcohol dehydrogenase with alcohol use disorders and endophenotypes in White college students. J. Abnorm. Psychol. 2005;114:456–465. doi: 10.1037/0021-843X.114.3.456. [DOI] [PubMed] [Google Scholar]
- 56.Zhang F.F., Hou L., Terry M.B., Lissowska J., Morabia A., Chen J., Yeager M., Zatonski W., Chanock S., Chow W.H. Genetic polymorphisms in alcohol metabolism, alcohol intake and the risk of stomach cancer in Warsaw, Poland. Int. J. Cancer. 2007;121:2060–2064. doi: 10.1002/ijc.22973. [DOI] [PubMed] [Google Scholar]
- 57.Sherva R., Rice J.P., Neuman R.J., Rochberg N., Saccone N.L., Bierut L.J. Associations and interactions between SNPs in the alcohol metabolizing genes and alcoholism phenotypes in European Americans. Alcohol. Clin. Exp. Res. 2009;33:848–857. doi: 10.1111/j.1530-0277.2009.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duranceaux N.C.E., Schuckit M.A., Eng M.Y., Robinson S.K., Carr L.G., Wall T.L. Associations of variations in alcohol dehydrogenase genes with the level of response to alcohol in non-Asians. Alcohol. Clin. Exp. Res. 2006;30:1470–1478. doi: 10.1111/j.1530-0277.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 59.Kuo P.H., Kalsi G., Prescott C.A., Hodgkinson C.A., Goldman D., van den Oord E.J., Alexander J., Jiang C., Sullivan P.F., Patterson D.G., et al. Association of ADH and ALDH genes with alcohol dependence in the Irish Affected Sib Pair Study of alcohol dependence (IASPSAD) sample. Alcohol. Clin. Exp. Res. 2008;32:785–795. doi: 10.1111/j.1530-0277.2008.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo X.G., Kranzler H.R., Zuo L.J., Lappalainen J., Yang B.Z., Gelernter J. ADH4 gene variation is associated with alcohol dependence and drug dependence in European Americans: Results from HWD tests and case–control association studies. Neuropsychopharmacology. 2006;31:1085–1095. doi: 10.1038/sj.npp.1300925. [DOI] [PubMed] [Google Scholar]
- 61.Hashibe M., McKay J.D., Curado M.P., Oliveira J.C., Koifman S., Koifman R., Zaridze D., Shangina O., Wunsch-Filho V., Eluf-Neto J., et al. Multiple ADH genes are associated with upper aerodigestive cancers. Nat. Genet. 2008;40:707–709. doi: 10.1038/ng.151. [DOI] [PubMed] [Google Scholar]
- 62.Frazer K.A., Ballinger D.G., Cox D.R., Hinds D.A., Stuve L.L., Gibbs R.A., Belmont J.W., Boudreau A., Hardenbol P., Leal S.M., et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gemma S., Vichi S., Testai E. Metabolic and genetic factors contributing to alcohol induced effects and fetal alcohol syndrome. Neurosci. Biobehav. Rev. 2007;31:221–229. doi: 10.1016/j.neubiorev.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 64.Freathy R.M., Ring S.M., Shields B., Galobardes B., Knight B., Weedon M.N., Smith G.D., Frayling T.M., Hattersley A.T. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum. Mol. Genet. 2009;18:2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D'Onofrio B.M., Van Hulle C.A., Waldman I.D., Rodgers J.L., Rathouz P.J., Lahey B.B. Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Arch. Gen. Psychiatry. 2007;64:1296–1304. doi: 10.1001/archpsyc.64.11.1296. [DOI] [PubMed] [Google Scholar]
- 66.Davey Smith G., Timpson N., Ebrahim S. Strengthening causal inference in cardiovascular epidemiology through Mendelian randomization. Ann. Med. 2008;99999:1–18. doi: 10.1080/07853890802010709. [DOI] [PubMed] [Google Scholar]
- 67.Golding J., Pembrey M., Jones R. ALSPAC-The Avon longitudinal study of parents and children—I. Study methodology. Paediatr. Perinat. Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 68.Timpson N.J., Christensen M., Lawlor D.A., Gaunt T.R., Day I.N., Ebrahim S., Davey S.G. TAS2R38 (phenylthiocarbamide) haplotypes, coronary heart disease traits, and eating behavior in the British Women's Heart and Health Study. Am. J. Clin. Nutr. 2005;81:1005–1011. doi: 10.1093/ajcn/81.5.1005. [DOI] [PubMed] [Google Scholar]
- 69.Swallow D.M. Genetics of lactase persistence and lactose intolerance. Annu. Rev. Genet. 2003;37:197–219. doi: 10.1146/annurev.genet.37.110801.143820. [DOI] [PubMed] [Google Scholar]
- 70.Timpson N., Heron J., Smith G.D., Enard W. Comment on papers by Evans et al. and Mekel-Bobrov et al. on evidence for positive selection of MCPH1 and ASPM. Science. 2007;317:1036a. doi: 10.1126/science.1141705. [DOI] [PubMed] [Google Scholar]
- 71.Sayal K., Heron J., Golding J., Alati R., Smith G.D., Gray R., Emond A. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123:e289–e296. doi: 10.1542/peds.2008-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones R.W., Ring S., Tyfield L., Hamvas R., Simmons H., Pembrey M., Golding J. A new human genetic resource: a DNA bank established as part of the Avon longitudinal study of pregnancy and childhood (ALSPAC) Eur. J. Hum. Genet. 2000;8:653–660. doi: 10.1038/sj.ejhg.5200502. [DOI] [PubMed] [Google Scholar]
- 73.Lunn D.J., Whittaker J.C., Best N. A Bayesian toolkit for genetic association studies. Genet. Epidemiol. 2006;30:231–247. doi: 10.1002/gepi.20140. [DOI] [PubMed] [Google Scholar]
- 74.Li H., Mukherjee N., Soundararajan U., Tarnok Z., Barta C., Khaliq S., Mohyuddin A., Kajuna S.L., Mehdi S.Q., Kidd J.R., Kidd K.K. Geographically separate increases in the frequency of the derived ADH1B*47His allele in eastern and western Asia. Am. J. Hum. Genet. 2007;81:842–846. doi: 10.1086/521201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lunn D.J., Thomas A., Best N., Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat. Comput. 2000;10:325–337. [Google Scholar]
- 76.Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.