Abstract
The syndrome of hypomagnesemia with secondary hypocalcemia is caused by defective TRPM6. This protein is an ion channel that also contains a kinase in its C-terminus. It is usually diagnosed in childhood and, without treatment with supplemental Mg, affected children suffer from mental retardation, seizures and retarded development. We developed a mouse lacking Trpm6 in order to understand in greater detail the function of this protein. In contrast to our expectations, Trpm6−/− mice almost never survived to weaning. Many mice died by embryonic day 12.5. Most that survived to term had neural tube defects consisting of both exencephaly and spina bifida occulta, an unusual combination. Feeding dams a high Mg diet marginally improved offspring survival to weaning. The few Trpm6−/− mice that survived were fertile but matings between Trpm6−/− mice produced no viable pregnancies. Trpm6+/− mice had normal electrolytes except for modestly low plasma [Mg]. In addition, some Trpm6+/− mice died prematurely. Absence of Trpm6 produces an apparently different phenotype in mice than in humans. The presence of neural tube defects identifies a previously unsuspected role of Trpm6 in effecting neural tube closure. This genetic defect produces one of very few mouse models of spina bifida occulta. These results point to a critical role of Trpm6 in development and suggest an important role in neural tube closure.
INTRODUCTION
Familial hypomagnesemia with secondary hypocalcemia (HSH) (MIM 602014) is an autosomal recessive disease that results in electrolyte abnormalities shortly after birth. Affected individuals have extremely low serum [Mg] and [Ca], and develop seizures, muscle spasms and tetany. HSH can be lethal or can lead to permanent neurological damage from multiple seizures if left untreated. Administration of magnesium often relieves the clinical symptoms, and restores the serum calcium levels to normal. However, the serum magnesium levels of the HSH patients generally remain at the low end of normal, even with high-dose magnesium administration. Life long magnesium supplementation is required to overcome the defect in hypomagnesemia and its consequences in HSH patients (1–5).
HSH was originally though to be an X-linked disorder based on the reported preponderance of males (6–9) and the report of a patient with a balanced 9:X translocation HSH (10). In 1997, we conducted a genome-wide search for linkage with the DNA of HSH affected and unaffected individuals from Bedouin families from Israel. We found a region on chromosome 9q12–q22.2 which was homozygous for the HSH affected patients (11). These mapping studies demonstrated that HSH was an autosomal recessive disease. In 2002, we and Schlingmann et al. identified TRPM6 as the gene which causes HSH (12,13). Additional mutations in TRPM6 have also been reported (14,15). To date, there are 34 reported mutations in TRPM6 that cause HSH. Most of the mutations are frameshift and splice junction mutations, which are predicted to cause the premature termination of the protein. There are five known missense mutations, one of which is in the putative pore-forming region (16).
TRPM6 is a member of the superfamily of transient receptor potential (TRP) channel proteins. The TRP channels comprise a diverse group of ion channels with diverse expression patterns, ion selectivities and activation mechanisms. The melanostatin family of TRP(M) channels consist of eight proteins. These ion channels are structurally similar with predicted six transmembrane spanning regions and intracellular N-terminal and C-terminal sequences, and the functional channels are thought to be tetramers, composed of homomeric or heteromic subunits. TRPM6 and TRPM7 are highly homologous, and are the only known proteins that contain both an ion channel and a kinase domain.
Whereas TRPM7 is ubiquitously expressed; TRPM6 is expressed in a limited number of tissues. Trpm6 is expressed in the colon (12,13,17), in the duodenum (18) and in the distal convoluted tubule of the kidney (13,18). It is presumed that defective expression of TRPM6 in these epithelial tissues leads to the phenotype in HSH. In order to extend our understanding of the role of Trpm6 in magnesium homeostasis, we developed and characterized a mouse deficient in Trpm6.
RESULTS
Generation of Trpm6-knockout mice
Approximately 1500 ES cell lines were genotyped to identify four homologous recombinants. Two correctly targeted clones were microinjected into blastocysts and chimeric mice were generated. The chimeric mice were mated with female C57BL/6J mice. We used 5th–10th generation backcross Trpm6 targeted mice from the mixed C57BL/6J and 129SvEv background for these experiments.
To confirm the genotyping, we dissected kidneys from selected mice at day 18.5 days post coitus (dpc) for analysis of mRNA by real time quantitative PCR. This method confirmed the genotyping results; heterozygous mice had about half the amount of Trpm6 mRNA than wild-type mice. Homozygous null mice contained no detectable product (data not shown).
Trpm6−/− mice show a greatly reduced survival rate
For our initial breeding experiments we crossed Trpm6+/− mice fed normal chow. As shown in Table 1, of 125 pups genotyped at weaning we identified no homozygous null mice. This result indicated that the Trpm6−/− mice either failed to develop or died shortly after birth. We therefore studied embryos at various stages of development. As shown in Table 2, some embryos examined at 16.5–18.5 dpc had a Trpm6−/− genotype, although the fraction of such embryos was less than predicted by Mendelian inheritance. However, we also noted a significant number of empty deciduae, i.e. amnionic material without an identifiable embryo. Empty deciduae are rarely seen in late embryogenesis (or in mid gestation) from normal mice. If these empty deciduae are counted as Trpm6−/− embryos, the genotypic distribution is not significantly different from Mendelian ratios.
Table 1.
Results of mating Trpm6+/− mice
| +/+ | +/− | −/− | |
|---|---|---|---|
| Predicted | 0.25 | 0.5 | 0.25 |
| Observed | 0.38 | 0.62 | 0 |
Seventeen litters produced 125 offspring. Genotyping was done at weaning. Distribution is significantly different from the predicted Mendelian distribution.
Table 2.
Genotyping results
| Embryos | Empty Deciduae | Total | +/+ | +/− | −/− | |
|---|---|---|---|---|---|---|
| From late (16.5–18.5 dpc) embryos | ||||||
| N | 84 | 14 | 98 | 26 | 44 | 14 |
| Fraction of embryos | 0.31 | 0.52 | 0.17 | |||
| Fraction of totala | 0.26 | 0.45 | 0.28 | |||
| From early (12.5 dpc) embryos | ||||||
| N | 33 | 10 | 43 | 9 | 21 | 3 |
| Fraction of embryos | 0.27 | 0.64 | 0.09 | |||
| Fraction of totalb | 0.21 | 0.49 | 0.30 | |||
aIf empty deciduae included as Trpm6−/−. N = 14 litters. Distribution of embryos is Mendelian if the empty deciduae are counted as Trpm6−/−.
bIf empty deciduae included as Trpm6−/−. N = 5 litters. Distribution of embryos is Mendelian if the empty deciduae are counted as Trpm6−/−.
The genotype of embryos examined 12.5 dpc (Table 2) shows a pattern similar to that of late embryos. Developed Trpm6−/− embryos are identified at much lower frequency than predicted, but when empty deciduae are counted as Trpm6−/− embryos the genotypic distribution is not different from predicted. These results indicate that some Trpm6−/− embryos fail to develop by 12.5 dpc, whereas some Trpm6−/− embryos develop to term.
Neural tube defects of Trpm6−/− embryos
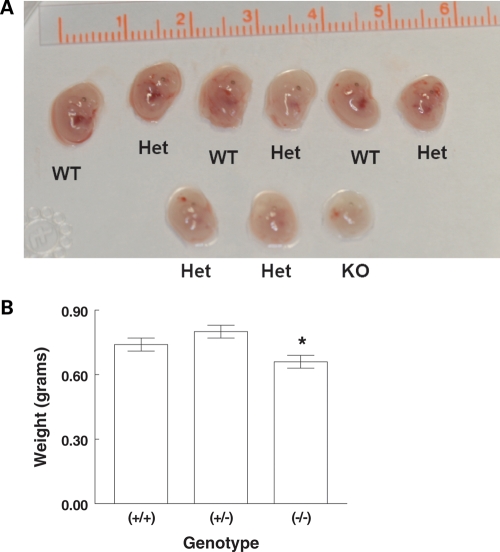
During the dissection of the embryos, we noted heterogeneity in their size. An example of a litter at 12.5 dpc demonstrating a size difference is shown in Figure 1A. We found that for each stage (16.5, 17.5 and 18.5 dpc) Trpm6−/− mice were smaller than either Trpm6+/+ or Trpm6+/− mice. Figure 1B shows the mean weights by genotype for the aggregate late embryos. Statistical assessment showed lower weight for Trpm6−/− mice at each late gestational age.
Figure 1.
(A) Litter of 12.5 day embryos identified by genotype. Parents were Trpm6+/−. The one Trpm6−/− (KO) embryo appears smaller than the others. (B) Weight of Trpm6 embryos at 16.5–18.5 dpc. N = 45 (+/+), 39 (+/−), 29 (−/−). *P < 0.05 by ANOVA. Difference in weight was evident for each litter.
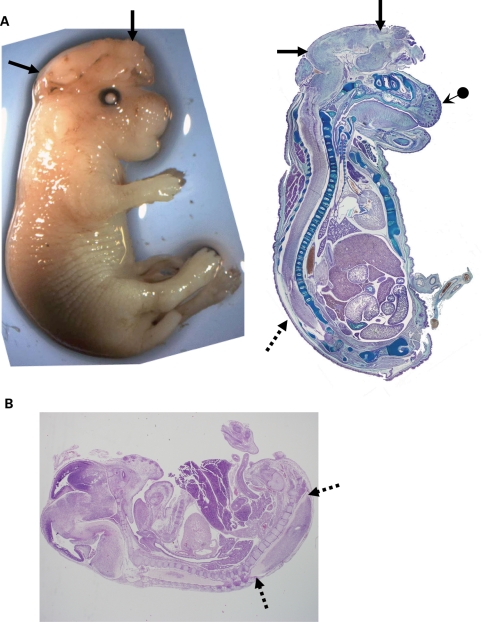
We also examined Trpm6−/− mice for other defects. The majority of Trpm6−/− mice that survived to the late stages of embryogenesis, or that were born (day 1) demonstrated defects in neural tube closure. Often the defect was observed as a small hole in the head or neck. Figure 2A shows an example of a Trpm6−/− mouse the day of birth that had exencephaly, a defect in the closure of the calvarium. Many mice also had a short snout with defective development of the facial bones (Fig. 2A). Many mice also showed spina bifida occulta (Fig. 2A and B). We did not observe any clear histological abnormalities in any other organ system. Thus neural tube defects were common among the mice that survived to late gestation.
Figure 2.
(A) Example of Trpm6−/− newborn displaying exencephaly (arrows). Left photograph is the mouse at birth. Right shows a sagittal section stained with alcian blue. In addition to exencephaly, the mouse has a short snout and poorly developed facial bones (arrow with dot). Spina bifida occulta shown by arrows with dashed lines. (B) Example of defect in spinal closure in lower spine (arrows—dashed lines) in an 18.5 day embryo. This mouse also has defective brain and facial development.
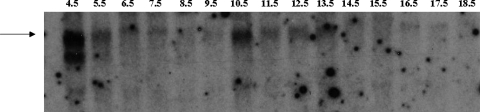
Since many of the Trpm6−/− mice did not develop normally by 12.5 dpc (Table 2), we asked if Trpm6 was expressed at times early in development. Figure 3 shows a northern blot demonstrating that at 10.5 dpc there is a marked increase in Trpm6 expression. Failure of this expression around day 10 might have a lethal effect and account, at least in part, for the loss of Trpm6−/− fetuses after that time.
Figure 3.
Northern blot of Trpm6 expression in normal mouse embryos. The arrow indicates the position of the full size Trpm6 message. Lane numbers represent days post conception (dpc). Samples from embryonic day 4.5–6.5 include extra-embryonic tissues and maternal uterus, whereas samples from days 7.5 to 9.5 are conceptuses, including embryo and extra-embryonic tissue. Samples from days 10.5 to 18.5 contain only embryonic tissue. Mouse Trpm6 mRNA shows a distinct peak at 10.5 days. There is also an abundant signal at 4.5 dpc, which may be from maternal tissue.
Effect of a high Mg diet fed to the dam
In an attempt to improve our chances of obtaining live Trpm6−/− mice, we placed the Trpm6+/− dams on a high (0.6%) Mg diet during mating, pregnancy and lactation. We reasoned that the increased dietary Mg might lead to increased Mg stores in the dam, which could then be transferred to her fetuses and later to her litter via the breast milk. Dams tolerated this diet well with no difference in food or water intake (date not shown).
We genotyped 105 weaned offspring from 31 litters from Trpm6+/− dams fed a high Mg diet mated to Trpm6+/− males. As shown in Table 3, there were four Trpm6−/− offspring that survived, a number far fewer than predicted. One of the offspring died within a week of being weaned. The gender distribution of the live offspring was not different from predicted (56 males and 46 females).
Table 3.
Genotype of offspring born to Trpm6+/− parents fed a high Mg diet
| Total pups | +/+ | +/− | −/− | |
|---|---|---|---|---|
| Number | 105 | 38 | 64 | 4 |
| Fraction of total pups | 0.36 | 0.61 | 0.04 |
Offspring from 31 litters; genotyping was conducted at weaning. Distribution is significantly different from the predicted Mendelian distribution.
Reasoning that the three surviving Trpm6−/− mice shared one or more genes that complemented (at least partially) the loss of Trpm6, we attempted to mate the surviving Trpm6−/− mice. Despite numerous attempts and maintenance of mice on the high Mg diet, such matings produced no evident pregnancy. We also mated Trpm6+/− and Trpm6−/− mice fed a high Mg diet. In contrast to the lack of offspring in the Trpm6−/− matings, these matings produced 31 litters of 102 weaned offspring. Both male and female Trpm6−/− mice were able to produce offspring, thus demonstrating that the Trpm6−/− genotype did not produce infertility. However, in contrast to the expected 50% Trpm6−/− genotype, only one such offspring survived to weaning.
The sum of all results of breeding Trpm6+/− mice on normal or high Mg diet produced 479 weaned pups of which 173 were Trpm6+/+, 302 were Trpm6+/− and 4 were Trpm6−/−. Gender distribution was 253 males and 226 females. In addition, the 60 day mortality of the Trpm6+/+ mice was 0 whereas that of the Trpm6+/− mice was 4.7%. Of the mice that died, approximately half were on the high Mg diet; thus even the high Mg diet did not prevent the early mortality. Prior to death, the Trpm6+/− mice developed a characteristic appearance; they curled into a ball, and appeared to be in some distress. When they moved, they displayed a waddling gait, with their feet splayed outward. The coat appeared dull and their hair follicles tended to stand straight up, giving the coat a disheveled, spiky appearance. Clearly, the complete loss of Trpm6 is nearly uniformly lethal. It also appears that the heterozygous state can produce a phenotype.
Electrolyte analysis: Trpm6+/− mice have lower plasma [Mg] but conserve Mg normally
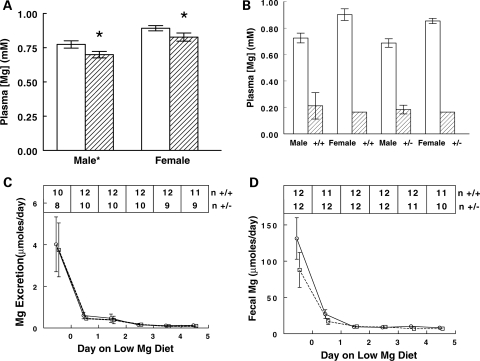
We measured plasma electrolytes on Trpm6+/+ and Trpm6+/− mice of both genders to determine if the heterozygous state would produce clues to the phenotypic derangement of null mice. There was no difference in plasma [Na], [Ca] or [P] (data not shown). Plasma [K] of female mice was ∼0.7 mm lower than male mice (4.0 versus 4.7) but there was no effect of genotype. Plasma [Mg] was lower in Trpm6+/− mice compared with Trpm6+/+ mice of either gender (Fig. 4). In addition, female mice had a higher plasma [Mg] than males, an observation that may be explained by estrogen's ability to regulate Trpm6 (17,19). There was no interaction between gender and genotype. These data indicate that Trpm6+/− mice have lower plasma [Mg] than normal littermates. Such a difference, though subtle, suggests that Mg homeostasis is not completely normal in Trpm6+/− mice.
Figure 4.
Plasma [Mg] and response to low Mg diet in Trpm6+/+, and Trpm6+/−, mice. (A) Plasma [Mg] by genotype and gender on a normal diet. Open bars, Trpm6+/+; hatched bars, Trpm6+/−. Both gender and genotype were significantly different by two ways ANOVA; there was no interaction. Nine to 14 mice in each group. *P < 0.05 compared with Trpm6+/+. (B–D) Response to 5 days of a low Mg diet. (B) Plasma [Mg] before (open bars, n = 4,6) and after (hatched bars, n = 4,5) low Mg diet. (C) Urinary Mg excretion in Trpm6+/+ (circles) and Trpm6+/− (squares) mice during the low Mg diet. There was no difference in plasma [Mg] after the low Mg diet by genotype or gender. There was no difference in fecal or urinary excretory response to low Mg diet by genotype or gender at any day.
We further tested this idea by putting mice on a low Mg diet. Urine and fecal Mg excretion was not different between Trpm6+/+ and Trpm6+/− mice (Fig. 4C–D). Two Trpm6+/− mice died before day 5; no wild-type mice died. The plasma [Mg] fell dramatically and similarly in all groups (Fig. 4B). Surprisingly, we found no difference in the renal or fecal excretion of Mg following the switch to the low Mg diet (Fig. 4C–D). Both genotypes conserved Mg.
DISCUSSION
The present results point to a role for Trpm6 that is considerably broader than previously appreciated. From studies in humans with defective TRPM6, investigators have surmised that this gene product functions mainly to regulate Mg absorption in intestine and kidney (5,20). The present findings demonstrating severe developmental consequences to the lack of Trpm6 point to a role for Trpm6 in normal development.
The loss of Trpm6 appears to affect different stages of embryogenesis. Some embryos develop to term, whereas others appear to be mostly absorbed (except for the deciduae) by 12.5 dpc (Table 2). Less than half of the embryos develop sufficiently to term so that they can be born alive. Left to their natural course, these mice do not generally survive long enough to be weaned. This high mortality is unexpected since several human kindred have been described to have similar genetic defects (12–15). The explanation for the difference between mouse and human Trpm6 deficiency is unclear. It is possible that genes present in humans and not in mice serve a partially redundant function. Genetic background in mice may also be important in surviving total deletion of Trpm6. In this regard, we are unaware of information regarding the extent to which there is fetal wastage in humans homozygous for TRPM6 mutations. The demonstration that occasionally we could produce a viable Trpm6−/− mouse is consistent with the idea that given a permissive genetic background and/or environment survival into adolescence is possible. Whether or not survival of some Trpm6−/− mice involves some (other) genes contributing to Mg homeostasis (21,22) will require more extensive investigation.
Many of the Trpm6−/− mice that survived to term had neural tube defects (Fig. 2). This phenotype is also unexpected since we are unaware of reports of similar congenital malformations in humans with TRPM6 mutations. A survey of the localized expression of Trpm6 in adult mouse demonstrates minimal mRNA expression in bone and brain, but abundant expression in kidney and intestine (17). TRPM6 expression has been reported in human brain (23). Thus the anatomical basis for the neural tube defects is not clear. It seems possible that the increased expression of Trpm6 at 10.5 dpc (Fig. 3) may be important in neural tube development.
The pattern of neural tube defects in Trpm6−/− mice were somewhat variable, but some features seem consistent. We never saw an embryo with complete absence of neural tube closure (craniorachischisis). Thus the primary defect seems not to involve disordered planar cell polarity, the process responsible for convergent extension and elongation of the neural plate. The pattern of neural tube defects is consistent with completion of neural tube closure at the hindbrain/cervical boundary (closure 1), which occurs at 8.5 dpc (24,25). The defect in more rostral neural tube closure (exencephaly) is consistent with defects in neurulation rostral to closure 1. The lack of a split face suggests that there was successful closure at the most rostral area (closure 3), but that neurulation between closure sites 1, 2 and/or 3 may have been defective.
We often observed spina bifida occulta, but we never observed spina bifida with meningomyelocele. Thus it appears that the defect in the caudal region was not from defective primary closure but rather from defective secondary neurulation. The causes of spina bifida occulta are poorly understood and few models exist (24–27). The occurrence of both rostral and caudal neural tube defects together with the relatively high rate of embryonic mortality before day 12.5 suggests that loss of Trpm6 produces multiple developmental defects. This deduction is based on the observation that most single gene deletions in mice that produce neural tube defects cause either exencephaly or spina bifida occulta, not both (24). In addition, neural tube defects are not believed to be lethal in utero (25,28). Thus, it is possible that the absence of Trpm6 in mice affects a variety of cell functions during development and is not limited to neural tube closure.
There is one genetically targeted mouse for which both spina bifida occulta and exencephaly occur. Mice with the bone morphogenic protein (BMP) antagonist Noggin (Nog) deleted have a high incidence of spina bifida occulta and a lower incidence of exencephaly (29). Thus Nog−/− mice display similar neural tube defects as Trpm6−/− mice, suggesting a possible common pathway(s). The neural tube defects in Nog−/− mice are the result of two separate processes—defective dorsolateral hinge point formation causing exencephaly and defective mesodermal somite differentiation causing spina bifida occulta (29). It is possible that similar processes are occurring in Trpm6−/− mice. It is even possible that the BMP4 signaling in these locations is somehow related to intracellular Mg metabolism, though we can find no reports of such interactions.
The mechanism whereby absence of Trpm6 produces developmental abnormalities is not clear. Trpm6 is highly homologous to Trpm7; both are bifunctional proteins containing both ion channel and kinase functions. They interact when co-expressed and probably function to regulate Mg transport (20,30). In this regard, it is interesting that mice lacking Trpm7 die as early embryos (31). Cells in culture lacking Trpm7 do not grow and signal normally, but supplementation of the medium with Mg can overcome this deficiency (31,32). These results suggest Trpm7 functions in part to regulate Mg transport and/or signaling near the cell membrane. Since expression of Trpm7 is ubiquitous and expression of Trpm6 is much more tissue restricted, it is possible that cells that express Trpm6 (together with Trpm7) play special roles in processes involved in Mg transport or Mg-dependent cell processes during development. This conventional view may need to be revised given the present information on the role of Trpm6 in development and in neural tube closure.
Because of the gene targeting design, the Trpm6−/− mice in these experiments probably lacked both channel function and kinase function. Thus it is not clear whether the developmental defects we observed are the result of lack of channel activity or absence of phosphotransferase activity or both. The targets of Trpm6 kinase function are not clear, but considerable evidence supports the idea that autophosphorylation of Trpm7 is important to normal Trpm7 channel function (33,34). In addition, Trpm7 can phosphorylate annexin (35), and myosin IIA heavy chain (36) a finding that may indicate a wider spectrum of potential targets for the kinase in both Trpm6 and Trpm7. Recent data are beginning to define at least three regions in the carboxy terminus that are critical for function: (a) ATP regulation of channel activity (37), (b) substrate interactions regulated by autophosphorylation (38) and (c) catalytic function. The extent to which these regions are important for normal development will require additional experiments.
Considerable progress has been made in reducing the incidence of neural tube (and other developmental) defects in humans by implementing universal folic acid supplementation to pregnant women (39). However, since folate supplementation does not correct all neural tube defects, there is discussion about the role other deficiencies might play in developmental defects. Vitamin B12 is one such vitamin; Mg is another substance that has been considered as a possible cause of fetal malformations (40,41). The present results support the possibility that alterations in Trpm6 function could contribute to neural tube defects in humans. The precise role of this multifunctional protein, the molecular domains involved, its interaction with other molecules involved in neural tube closure, and the possible modulating role of Mg will require additional investigation.
MATERIALS AND METHODS
Generation of Trpm6-knockout mice
Care of the mice used in the experiments met or exceeded the standards set forth by the National Institutes of Health in their Guide for the Care and Use of Experimental Animals. All procedures were approved by the University Animal Care and Use Committee at the University of Iowa.
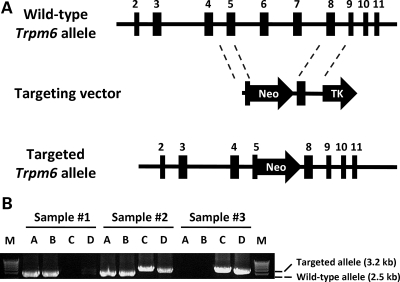
Trpm6 deficient mice were generated by standard gene targeting technique using a targeting construct designed to replace 6.1 kb of Trpm6 genomic sequence spanning from part of exon 5 to exon 7 (Fig. 5A) with the neomycin resistance (neoR) cassette. After homologous recombination between the targeting construct and the endogenous Trpm6 gene, the targeted allele no longer encoded a crucial part of the protein (expressed by exons 5–7). This recombination also introduced a frameshift mutation within exon 5 which results in premature termination of the transcript, upstream of the TRP ion channel domain. The resulting product is predicted to be similar to some of the TRPM6 mutations observed in patients with HSH (12–15). Thus this construct would be predicted to create a null allele and eliminate the expression of Trpm6 RNA and protein in homozygous targeted mice.
Figure 5.
(A) Targeting strategy for deletion of Trpm6. Upon homologous recombination, the 3′ end of exon 5 and all of exons 6 and 7 are replaced with a neomycin cassette. (B) PCR genotyping of Trpm6 mouse DNA. Primers were designed to amplify the wild-type allele (lanes A and B) and the targeted allele (lanes C and D). Sample 1: Trpm6+/+; Sample 2: Trpm6+/−; Sample 3: Trpm6−/− DNA.
The targeting vector was constructed with mouse 129/SvJ genomic DNA. Genomic fragments from mouse Trpm6 gene were amplified by PCR and cloned into the vector pOSDUPDEL (a gift from O. Smithies, University of North Carolina, Chapel Hill, NC, USA). A 3.5 kb fragment spanning exon 5 was cloned into the NheI–XhoI site of pOSDUPDEL. The neomycin resistance gene (for positive selection of ES cells) flanked by loxP sites was substituted for the genomic region from the mid portion of exon 5 through exon 7. The 3′ flanking region was a 3.5 kb NotI–SalI fragment spanning exon 8. A thymidine kinase cassette distal to the 3′-arm was used for negative selection. The vector was linearized with PvuI on the vector backbone and electroporated into R1 ES cells (129Z1/SvJ3 X 129S1/Sv). G418 resistant colonies were expanded and screened for targeted clones, in which the targeting vector had recombined with the Trpm6 gene, using PCR analysis with primers located outside both the 5′ and 3′-targeted regions and within the neomycin resistance gene. Upon homologous recombination, 6.1 kb of mouse Trpm6 gene, the 3′ end of exon 5, exons 6 and 7 of Trpm6 were replaced with a neomycin cassette (Fig. 5A).
After obtaining mice heterozygous for the targeted gene, we crossed them with a mouse expressing cre recombinase universally (EIIa-cre) (42) to excise the Neo cassette. Consequently, all mice reported here lack the neo cassette.
Mouse genotyping
Mice were genotyped by PCR analysis of mouse genomic DNA isolated from tail clippings. The mutant allele was detected by confirming both the 5′ and 3′ recombination sites. For routine genotyping assays, we used two different primer sets for the wild-type and mutant alleles. Both PCR primer sets were used in the same (multiplex) reactions (Fig. 5B).
Some mice were genotyped as embryos. For these studies, we conducted timed matings of heterozygous parents and sacrificed the dams at 12.5, 16.5, 17.5 or 18.5 dpc. For convenience, we designate embryos harvested on days 16.5–18.5 dpc as late gestation and those 12.5 dpc as mid gestation.
Mouse maintenance
Mice were maintained in the University of Iowa Animal Care SPF (special pathogen-free) facility until ready to be used. All experiments involving living animals were conducted in accordance with NIH guidelines for the care and use of experimental animals. All of the experimental procedures with animals were approved by the University of Iowa Animal Care and Use Committee. Mice were fed normal chow containing 0.2% Mg (LM-486, Harlan-Teklad, Madison, WI, USA). Some mice were fed a high Mg diet (0.6%) from the same manufacturer (TD.02372). Some mice were fed a low Mg diet containing <0.003% Mg (TD.93106). Mice fed the low Mg diet were housed in metabolic cages for 3 days on a normal diet and switched to the low Mg diet for 5 days. All feces and urine were collected for the final baseline equilibration day and for each of the following 5 days. All mice had free access to feed and tap water. Plasma for electrolyte analysis was collected under light anesthesia via retro-orbital venous plexus using microhematocrit tubes as described (43).
Northern blot analysis
Mouse embryonic northern blots consisting of total mouse RNA from 4.5 to 18.5 dpc tissue were purchased from Seegene, Inc. (Seoul, Korea), and probed with radiolabeled full-length mouse Trpm6 cDNA as previously described (12).
Real time PCR
TaqMan® real-time PCR was performed on total RNA isolated from mouse kidney tissue from a litter harvested at 18.5 dpc. Total RNA was extracted as described (44). The extracted material was subjected to DNase I digestion, and reverse transcription as described (45). The primers used to amplify Trpm6-were (forward) 5′-GAGGAAAATCTGGACAGTTGGAA-3′ and (reverse) 5′-GTTTAGCGTGGTGAGCTTGCT-3′. The probe was 5′-AAGATGTGGTGTGTATGTACCAGACACTGGGTAA-3′; (IDT, Coralville, IA, USA). These primers recognized a region of 130 nt spanning the junction of exons 6 and 7, a region that is deleted in Trpm6−/− mice. The results were normalized to GAPDH mRNA concentration. The TaqMan-based quantitative PCR was run on a PRISM 7700 Sequence Detection System (Applied Biosystems).
Morphological analysis of Trpm6−/− mice
Mice at late gestation or newborn mice with small size or apparent defects were fixed in paraformaldehyde and paraffin embedded using standard procedures. Sections were assessed for organ histology and neural tube defects.
Electrolyte analysis
Plasma and urine values were determined on 40–80 µl samples using the VITROS 350 Chemistry System (Ortho-Clinical Diagnostics, Rochester, NY, USA) according to the manufacturer's instructions. Fecal Mg was determined after incubating an aliquot of feces in 1 N nitric acid (1:10 wt:volume) overnight, sonicated, centrifuged and titrated pH ∼3.5 with 0.7 ml 1 N Tris base for each ml supernate. After another centrifugation, [Mg] was determined as for urine.
Conflict of Interest statement. None declared.
FUNDING
This work was supported in part by grants from the National Institutes of Health (PO1 GM078195 to J.B.S. and B.Y.); the Department of Veterans Affairs (to J.B.S.); and the Howard Hughes Medical Institute (to V.C.S.).
REFERENCES
- 1.Yamamoto T., Kabata H., Yagi R., Takashima M., Itokawa Y. Primary hypomagnesemia with secondary hypocalcemia. Report of a case and review of the world literature. Magnesium. 1985;4:153–164. [PubMed] [Google Scholar]
- 2.Abdulrazzaq Y.M., Smigura F.C., Wettrell G. Primary infantile hypomagnesaemia; report of two cases and review of literature. Eur. J. Pediatr. 1989;148:459–461. doi: 10.1007/BF00595914. [DOI] [PubMed] [Google Scholar]
- 3.Challa A., Papaefstathiou I., Lapatsanis D., Tsolas O. Primary idiopathic hypomagnesemia in two female siblings. Acta Paediatr. 1995;84:1075–1078. doi: 10.1111/j.1651-2227.1995.tb13830.x. [DOI] [PubMed] [Google Scholar]
- 4.Shalev H., Phillip M., Galil A., Carmi R., Landau D. Clinical presentation and outcome in primary familial hypomagnesaemia. Arch. Dis. Child. 1998;78:127–130. doi: 10.1136/adc.78.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chubanov V., Gudermann T., Schlingmann K.P. Essential role for TRPM6 in epithelial magnesium transport and body magnesium homeostasis. Pflugers Arch. 2005;451:228–234. doi: 10.1007/s00424-005-1470-y. [DOI] [PubMed] [Google Scholar]
- 6.Stromme J.H., Nesbakken R., Normann T., Skjorten F., Skyberg D., Johannessen B. Familial hypomagnesemia. Biochemical, histological and hereditary aspects studied in two brothers. Acta Paediatr. Scand. 1969;58:433–444. doi: 10.1111/j.1651-2227.1969.tb04743.x. [DOI] [PubMed] [Google Scholar]
- 7.Skyberg D., Stromme J.H., Nesbakken R., Harnaes K. Neonatal hypomagnesemia with selective malabsorption of magnesium—a clinical entity. Scand. J. Clin. Lab. Invest. 1968;21:355–363. doi: 10.3109/00365516809077007. [DOI] [PubMed] [Google Scholar]
- 8.Skyberg D., Stromme J.H., Nesbakken R., Harnaes K. Congenital primary hypomagnesemia, an inborn error of metabolism? Acta Paediatr. Scand. 1967;(177):26–27. doi: 10.1111/j.1651-2227.1967.tb05195.x. [DOI] [PubMed] [Google Scholar]
- 9.Teebi A.S. Primary hypomagnesaemia, an X-borne allele? Lancet. 1983;1:701. doi: 10.1016/s0140-6736(83)91986-4. [DOI] [PubMed] [Google Scholar]
- 10.Chery M., Biancalana V., Philippe C., Malpuech G., Carla H., Gilgenkrantz S., Mandel J.L., Hanauer A. Hypomagnesemia with secondary hypocalcemia in a female with balanced X;9 translocation: mapping of the Xp22 chromosome breakpoint. Hum. Genet. 1994;93:587–591. doi: 10.1007/BF00202829. [DOI] [PubMed] [Google Scholar]
- 11.Walder R.Y., Shalev H., Brennan T.M., Carmi R., Elbedour K., Scott D.A., Hanauer A., Mark A.L., Patil S., Stone E.M., Sheffield V.C. Familial hypomagnesemia maps to chromosome 9q, not to the X chromosome: genetic linkage mapping and analysis of a balanced translocation breakpoint. Hum. Mol. Genet. 1997;6:1491–1497. doi: 10.1093/hmg/6.9.1491. [DOI] [PubMed] [Google Scholar]
- 12.Walder R.Y., Landau D., Meyer P., Shalev H., Tsolia M., Borochowitz Z., Boettger M.B., Beck G.E., Englehardt R.K., Carmi R., Sheffield V.C. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 13.Schlingmann K.P., Weber S., Peters M., Niemann N.L., Vitzthum H., Klingel K., Kratz M., Haddad E., Ristoff E., Dinour D., et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 14.Schlingmann K.P., Sassen M.C., Weber S., Pechmann U., Kusch K., Pelken L., Lotan D., Syrrou M., Prebble J.J., Cole D.E.C., et al. Novel TRPM6 mutations in 21 families with primary hypomagnesemia and secondary hypocalcemia. J. Am. Soc. Nephrol. 2005;16:3061–3069. doi: 10.1681/ASN.2004110989. [DOI] [PubMed] [Google Scholar]
- 15.Jalkanen R., Pronicka E., Tyynismaa H., Hanauer A., Walder R., Alitalo T. Genetic background of HSH in three Polish families and a patient with an X;9 translocation. Eur. J. Hum. Genet. 2006;14:55–62. doi: 10.1038/sj.ejhg.5201515. [DOI] [PubMed] [Google Scholar]
- 16.Chubanov V., Schlingmann K.P., Waring J., Heinzinger J., Kaske S., Waldegger S., Schnitzler M.M., Gudermann T. Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J. Biol. Chem. 2007;282:7656–7667. doi: 10.1074/jbc.M611117200. [DOI] [PubMed] [Google Scholar]
- 17.Groenestege W.M.T., Hoenderop J.G., van den Heuvel L., Knoers N., Bindels R.J. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J. Am. Soc. Nephrol. 2006;17:1035–1043. doi: 10.1681/ASN.2005070700. [DOI] [PubMed] [Google Scholar]
- 18.Voets T., Nilius B., Hoefs S., van der Kemp A.W.C.M., Droogmans G., Bindels R.J.M., Hoenderop J.G.J. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 19.Cao G., van der Wijst J., van der Kemp A., van Zeeland F., Bindels R.J., Hoenderop J.G. Regulation of the epithelial Mg2+ channel TRPM6 by estrogen and the associated repressor protein of estrogen receptor activity (REA) J. Biol. Chem. 2009;284:14788–14795. doi: 10.1074/jbc.M808752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlingmann K.P., Waldegger S., Konrad M., Chubanov V., Gudermann T. TRPM6 and TRPM7—gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Feillet-Coudray C., Coudray C., Wolf F.I., Henrotte J.G., Rayssiguier Y., Mazur A. Magnesium metabolism in mice selected for high and low erythrocyte magnesium levels. Metabolism. 2004;53:660–665. doi: 10.1016/j.metabol.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Henrotte J.G., Pla M., Dausset J. HLA- and H-2-associated variations of intra- and extracellular magnesium content. Proc. Natl. Acad. Sci. USA. 1990;87:1894–1898. doi: 10.1073/pnas.87.5.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonfria E., Murdock P.R., Cusdin F.S., Benham C.D., Kelsell R.E., McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J. Recept. Signal. Transduct. Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 24.Harris M.J., Juriloff D.M. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 2007;79:187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- 25.Copp A.J., Greene N.D.E., Murdoch J.N. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- 26.Nait-Oumesmar B., Stecca B., Fatterpekar G., Naidich T., Corbin J., Lazzarini R.A. Ectopic expression of Gcm1 induces congenital spinal cord abnormalities. Development. 2002;129:3957–3964. doi: 10.1242/dev.129.16.3957. [DOI] [PubMed] [Google Scholar]
- 27.Wong R.L.Y., Wlodarczyk B.J., Min K.S., Scott M.L., Kartiko S., Yu W., Merriweather M.Y., Vogel P., Zambrowicz B.P., Finnell R.H. Mouse Fkbp8 activity is required to inhibit cell death and establish dorso-ventral patterning in the posterior neural tube. Hum. Mol. Genet. 2008;17:587–601. doi: 10.1093/hmg/ddm333. [DOI] [PubMed] [Google Scholar]
- 28.Kibar Z., Capra V., Gros P. Toward understanding the genetic basis of neural tube defects. Clin. Genet. 2007;71:295–310. doi: 10.1111/j.1399-0004.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 29.Stottmann R.W., Berrong M., Matta K., Choi M., Klingensmith J. The BMP antagonist Noggin promotes cranial and spinal neurulation by distinct mechanisms. Dev. Biol. 2006;295:647–663. doi: 10.1016/j.ydbio.2006.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M., Jiang J., Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin J., Desai B.N., Navarro B., Donovan A., Andrews N.C., Clapham D.E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahni J., Scharenberg A.M. TRPM7 ion channels are required for sustained phosphoinositide 3-kinase signaling in lymphocytes. Cell Metab. 2008;8:84–93. doi: 10.1016/j.cmet.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takezawa R., Schmitz C., Demeuse P., Scharenberg A.M., Penner R., Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc. Natl. Acad. Sci. USA. 2004;101:6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz C., Perraud A.L., Johnson C.O., Inabe K., Smith M.K., Penner R., Kurosaki T., Fleig A., Scharenberg A.M. Regulation of Vertebrate Cellular Mg(2+) Homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 35.Dorovkov M.V., Ryazanov A.G. Phosphorylation of Annexin I by TRPM7 channel-kinase. J. Biol. Chem. 2004;279:50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- 36.Clark K., Middelbeek J., Lasonder E., Dulyaninova N.G., Morrice N.A., Ryazanov A.G., Bresnick A.R., Figdor C.G., van Leeuwen F.N. TRPM7 Regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J. Mol. Biol. 2008;378:790–803. doi: 10.1016/j.jmb.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thebault S., Cao G., Venselaar H., Xi Q., Bindels R.J.M., Hoenderop J.G.J. Role of the {alpha7rcub;-kinase domain in transient receptor potential melastatin 6 channel and regulation by intracellular ATP. J. Biol. Chem. 2008;283:19999–20007. doi: 10.1074/jbc.M800167200. [DOI] [PubMed] [Google Scholar]
- 38.Clark K., Middelbeek J., Morrice N.A., Figdor C.G., Lasonder E., van Leeuwen F.N. Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS ONE. 2008;3:e1876. doi: 10.1371/journal.pone.0001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goh Y.I., Koren G. Folic acid in pregnancy and fetal outcomes. J. Obstet. Gynaecol. 2008;28:3–13. doi: 10.1080/01443610701814195. [DOI] [PubMed] [Google Scholar]
- 40.Durlach J. New Data on the Importance of Gestational Mg Deficiency. J. Am. Coll. Nutr. 2004;23:694S–700. doi: 10.1080/07315724.2004.10719411. [DOI] [PubMed] [Google Scholar]
- 41.Takaya J., Yamato F., Kaneko K. Possible relationship between low birth weight and magnesium status: from the standpoint of ‘fetal origin’ hypothesis. Magnes. Res. 2006;19:63–69. [PubMed] [Google Scholar]
- 42.Lakso M., Sauer B., Mosinger B., Jr, Lee E.J., Manning R.W., Yu S.H., Mulder K.L., Westphal H. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc. Natl. Acad. Sci. USA. 1992;89:6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao X.R., Shi P.P., Sigmund R.D., Husted R.F., Sigmund C.D., Williamson R.A., Stokes J.B., Yang B. Mice heterozygous for beta-ENaC deletion have defective potassium excretion. Am. J. Physiol. Renal Physiol. 2006;291:F107–F115. doi: 10.1152/ajprenal.00159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 45.Husted R.F., Volk K.A., Sigmund R.D., Stokes J.B. Discordant effects of corticosteroids and expression of subunits on ENaC activity. Am. J. Physiol. Renal Physiol. 2007;293:F813–F820. doi: 10.1152/ajprenal.00225.2007. [DOI] [PubMed] [Google Scholar]