Abstract
Ten to 15% of couples are infertile, with the most common causes being linked to the production of few or no oocytes or sperm. Yet, our understanding of human germ cell development is poor, at least in part due to the inaccessibility of early stages to genetic and developmental studies. Embryonic stem cells (ESCs) provide an in vitro system to study oocyte development and potentially treat female infertility. However, most studies of ESC differentiation to oocytes have not documented fundamental properties of endogenous development, making it difficult to determine the physiologic relevance of differentiated germ cells. Here, we sought to establish fundamental parameters of oocyte development during ESC differentiation to explore suitability for basic developmental genetic applications using the mouse as a model prior to translating to the human system. We demonstrate a timeline of definitive germ cell differentiation from ESCs in vitro that initially parallels endogenous oocyte development in vivo by single-cell expression profiling and analysis of functional milestones including responsiveness to defined maturation media, shared genetic requirement of Dazl, and entry into meiosis. However, ESC-derived oocyte maturation ultimately fails in vitro. To overcome this obstacle, we transplant ESC-derived oocytes into an ovarian niche to direct their functional maturation and, thereby, present rigorous evidence of oocyte physiologic relevance and a potential therapeutic strategy for infertility.
INTRODUCTION
One of the earliest events in human development is the setting aside, or specification, of the cells that are destined to form the germ cells, the oocytes and sperm, of the adult (1). Germ cell specification occurs in the first 3 weeks of development. By the fourth gestational month in the female, approximately 1000–2000 germ cells migrate from a position outside the embryo into the genital ridge, proliferate to a population of five to seven million and then enter meiosis and differentiate to oocytes (1). By the fifth gestational month, gradual oocyte loss begins so that, by birth, there are approximately one million oocytes in ovarian follicles. In the adult, oocytes may then encounter one of several fates: they may remain quiescent, may be recruited for further development and ovulation or may apoptose and die. Over time, without regeneration, the oocyte population is depleted in women until less than a thousand remain and menopause ensues (2–4). Approximately, 90% of women experience menopause in their early 50s. Ten percent may experience menopause prior to 46 years (often termed early menopause), and 1% may experience menopause at an age <40 years (frequently termed premature menopause or premature ovarian failure) (5,6).
Given these facts, the differentiation of oocytes from embryonic stem cells (ESCs), for studies of the genetic, epigenetic and environmental factors that affect oocyte development, is merited. Ultimately, the differentiation of functional oocytes from autologous stem cells, such as induced pluripotent stem cells (iPSCs), potentially provides a means to understand infertility and treat women with premature ovarian failure, reproductive aging and/or poor oocyte quality (7). However, critical aspects of ESC-derived germ cell development, especially oocyte maturation, have not been well-documented. Indeed, although several studies have demonstrated that human ESCs can differentiate into cell types of somatic and germ lineages including germ cell precursors, or primordial germ cells (PGCs) (8–13), germ cell differentiation has been largely limited to the earliest stages.
In contrast to humans, in vitro differentiation of putative ESC-derived oocytes and follicle-like structures has been reported in mice (14–18). However, the studies have been difficult to reproduce, and validation of ESC-derived germ cell identity, as frequently assessed by expression profile analysis of ESC-derived populations, has not been straightforward owing to the heterogeneity of ESC-derived cultures and similarity of genes expressed by ESCs and germ cells/oocytes (7,8,19). Moreover, physiologic relevance and functional significance have been difficult to define in vitro because very few germ cells are formed, and correlations of genetic and functional requirements between endogenous development and ESC-derived differentiation are lacking (20).
Oocyte enclosure in ovarian follicles shortly after birth in mice is essential for complete functional oocyte maturation through interaction with neighboring somatic granulosa cells in the ovary (21). In the absence of ovarian somatic cells and follicle formation, as in ectopic oocytes of the adrenal gland (22) or testes (23), the oocytes rapidly degenerate and are lost by 3–4 weeks after birth. In addition to the requirement of ovarian follicle formation and development for endogenous oocyte maturation in vivo, functional ex vivo maturation of oocytes has required fetal-to-newborn-stage ovary organ culture or transplantation (24–29). Historically, transplantation into an appropriate stem cell niche has been necessary to confirm germ and somatic cell identity and function (30–33).
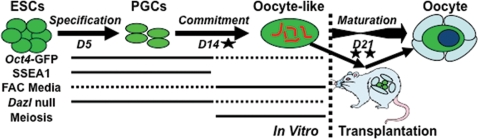
In this study, we differentiated mouse ESCs to germ cells. We then characterized and compared the differentiated germ cells to undifferentiated ESCs at the single-cell level and further examined functional properties associated with endogenous oogenesis, such as their responsiveness to defined maturation media, genetic requirements for germline formation and maturation and entry into meiosis. Then, to test the function of oocytes differentiated in vitro and promote further maturation, we transplanted ESC-derived germ cells into a synchronized ovarian niche (Fig. 1A). Finally, we examined the feasibility of using the methods developed to construct a human fetal ovarian niche for promoting human oocyte development.
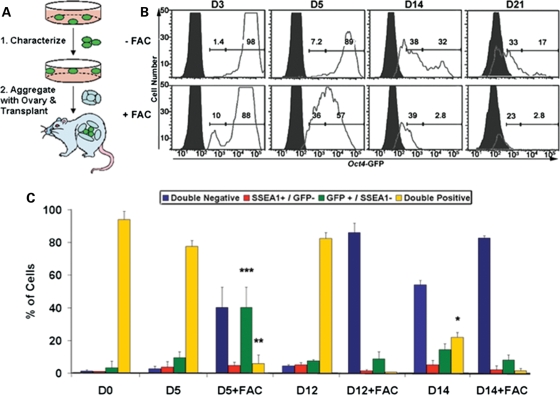
Figure 1.
ESC-derived germ cell identification. (A) In this study, mouse ESCs were differentiated as EBs, and putative ΔPE Oct4-GFP reporter positive germ cells were first isolated and characterized for germ cell identity and development in vitro. To promote oocyte development, ESC-derived germ cells were subsequently aggregated with ovarian tissue and transplanted. (B) GFP-high and -low intensity populations were identified above non-transgenic control EBs (solid peaks) by flow cytometry following Oct4-GFP ESC differentiation in vitro with an increase in the percentage of low-intensity cells by day 14, similar to the endogenous levels of Oct4-GFP expression observed in fetal ovaries of transgenic mice (D, day). Differentiation in germ cell maturation FAC media induced a rapid elevation in the percentage of low-intensity cells by day 3 of EB culture. Histogram percentages of high-intensity (gated on GFP+ ESCs) and low-intensity (gated below GFP+ ESCs and above GFP− control) populations (X-axis: Oct4-GFP intensity, Y-axis: cell number). (C) The percentage of the Oct4-GFP and SSEA1 double-positive population decreased significantly by day 14 of differentiation along with an increase in the percentage of GFP+/SSEA1− and double-negative populations, analogous to endogenous PGC maturation. FAC media induced a significant elevation in the percentage of GFP+/SSEA1− and reduction in double-positive populations by day 5. Data are represented as mean ± SD (n = 3); *P < 0.01 to D12, **P < 0.01 to D0, ***P < 0.05 to D0.
RESULTS
Identification of germ cells and oocytes in vivo and in vitro
We differentiated mouse ESCs, derived from transgenic mice carrying a germ cell reporter (ΔPE Oct4 promoter—GFP (34)), in vitro and sought to develop an effective strategy to identify putative ESC-derived germ cell differentiation and oocyte maturation based on flow cytometry analysis. However, a major challenge of developing effective methods to differentiate and isolate germ cells in vitro is the lack of markers to distinguish germ cells and oocytes from ESCs. To overcome this challenge, we turned to an examination of endogenous oocytes in vivo to find a suitable method. Although the Oct4-GFP reporter is expressed in early embryos and in the germline (35), oocytes from transgenic embryos expressed the GFP reporter at a low level of intensity relative to undifferentiated ESCs, PGCs from the genital ridges of either sex or pro-spermatogonia from fetal testes by flow cytometry analysis (Supplementary Material, Fig. S1A). In addition, the cell surface marker, SSEA1 (stage-specific embryonic antigen 1), is expressed by undifferentiated mouse ESCs and PGCs but is not expressed in fetal or adult gonads after embryonic day 14.5 (e14.5) as reported previously (36) and confirmed by flow cytometry (Supplementary Material, Fig. S1B). Therefore, flow cytometry analysis was used to identify subpopulations of cells based on Oct4-GFP+ and SSEA1− status, in addition to a low intensity of GFP expression.
After determining that Oct4-GFP intensity, combined with SSEA1 expression on the cell surface, was diagnostic of female germ cells in vivo, we differentiated ESCs containing the Oct4-GFP reporter in suspension as embryoid bodies (EBs) for up to 21 days in vitro and characterized oocyte differentiation. EBs were cultured in either standard differentiation media or in media containing a germ cell maturation factor cocktail (FAC) adapted from a report of endogenous PGC culture and meiotic progression in the absence of feeder layer support (37). The FAC cocktail comprised anti-apoptotic (38), germ cell specification (39) and meiotic induction factors (40), including bone morphogenetic protein 4 (BMP4), retinoic acid, cytochrome p450, 26 (CYP26) inhibitor (R115866), stromal cell-derived factor 1 (SDF1), stem cell factor (SCF), basic fibroblast growth factor (bFGF), n-acetyl-cysteine and forskolin. We observed that, whereas almost all undifferentiated ESCs expressed Oct4-GFP at high intensity, cells with low-intensity GFP expression were formed by day 14 of differentiation along with a reduction in the percentage of cells expressing GFP (Fig. 1B). In addition, low-intensity GFP+ cells were increased in numbers and expedited, with the appearance of the low-intensity population by day 3 of differentiation, following FAC media treatment (Fig. 1B).
We also note that, concomitant with the appearance of low-intensity Oct4-GFP+ cells, the percentage of GFP+/SSEA1− cells increased by day 14 of differentiation and was accompanied by a significant decrease in the percentage of double-positive cells as well as an increase in double-negative cells (Fig. 1C). Together, these observations indicated that the pattern of putative germ cell differentiation is similar in timeline to endogenous maturation of PGCs to an oocyte developmental program on e13.5. Moreover, the ESC-derived cultures were responsive to FAC media, which induced a significant elevation in the percentage of GFP+/SSEA1− and double-negative cells by day 5 of differentiation along with a corresponding decrease in double-positive cells (Fig. 1C). These results indicated that germ cell differentiation and oocyte maturation, via this methodology, occurred along an endogenous developmental timeline.
Single-cell analysis of ESC-derived germ cell identity and maturation
Our knowledge of ESC differentiation to specific lineages is derived from analysis of populations of differentiated cells. For example, in the case of studies of germ cell differentiation from ESCs, there are no reports of single-cell analysis that would allow us to distinguish gene expression profiles of individual ESCs from germ cells and determine the extent of germ cell maturation. Here, we examined gene expression in single cells that were differentiated for 5 days and isolated by fluorescence-activated cell sorting (FACS). Several findings emerged from this analysis. First, we observed that transcripts such as Oct4, Stella, Nanos3 and Vasa, which are expressed early in germ cell development, were elevated in the double-positive population, whereas Stra8 (stimulated by retinoic acid gene 8) and Gdf9, markers of later stages such as meiotic entry and oocyte maturation, respectively, were increased in the GFP+/SSEA1− population (Supplementary Material, Fig. S2A). Double-negative cells, in contrast, expressed only minimal levels of germ cell markers. Conversely, somatic cell markers Kdr and Sox1 were elevated in the double-negative population and minimally expressed in the GFP+ germ cell populations (Supplementary Material, Fig. S2A). These results confirmed that the ESC-derived Oct4-GFP+ populations were enriched for germ cells, and that double-positive ESC-derived PGCs differentiated toward either a GFP+/SSEA1− oocyte fate with low-GFP intensity along an endogenous timeline of germ cell development or toward a double-negative somatic cell fate.
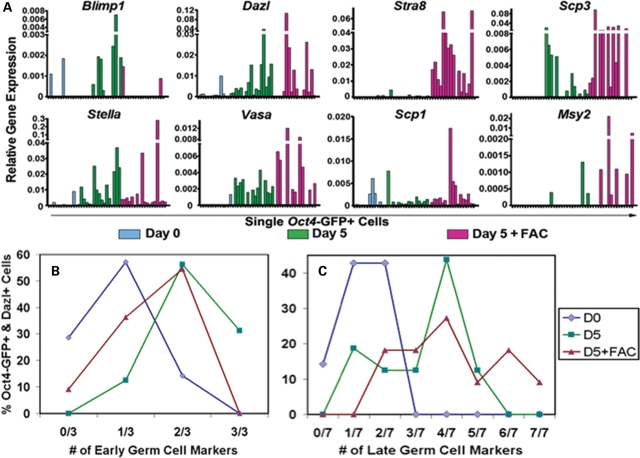
Second, when we analyzed gene expression in GFP+ single cells following 5 days of differentiation in both the absence and presence of FAC media, we observed that the number of cells expressing markers diagnostic of germ cells was greater in the differentiated population than in undifferentiated ESCs. This included markers of PGCs (early genes: Blimp1, Stella, Dazl and Vasa) and meiotic germ cells (late genes: Stra8, Scp1, Scp2, Scp3, Gcnf, Mlh1 and Msy2). Although some undifferentiated GFP+ ESCs did express detectable levels of germ cell marker transcripts (Fig 2A and Supplementary Material, Fig. S2B), a greater percentage of GFP+ cells expressed germ cell markers following 5 days of differentiation with or without FAC media for each marker studied with the exception of Blimp1 in FAC media, which is known to be downregulated in endogenous PGCs by the time of oogenesis (41) (Supplementary Material, Fig. S3).
Figure 2.
Characterization of ESC-derived germ cell identity and maturation. (A) Oct4-GFP+ ESC-derived germ cell identity was confirmed by single-cell quantitative RT–PCR expression profiling of pre-meiotic and meiotic germ cell transcripts before and after 5 days of in vitro differentiation (−/+) FAC media. Pre-meiotic germ cell transcripts were enriched in GFP+ cells from 5 day EB cultures, and meiotic transcripts were elevated following differentiation in FAC media, compared with ESCs. Relative expression: 2−ΔCt, relative to Gapdh. (B and C) The percentage values of GFP+ single cells expressing Dazl and additional early (B) (Blimp1, Stella, Vasa) or late (C) (Stra8, Scp1, Scp2, Scp3, Gcnf, Mlh1, Msy2) germ cell markers by RT–PCR were plotted according to the number of markers expressed within the same cell. GFP+ cells expressed more early and late marker transcripts in the same cell following differentiation than undifferentiated ESCs. GFP+ ESCs (blue diamond) did not express more than two additional early or late markers, but GFP+ cells following 5 days of differentiation contained up to three additional early markers (without FAC; green square) (B), or five (without FAC; green square) and seven (with FAC; purple triangle) late meiotic transcripts (C) within the same cell.
Third, we noted that at the single-cell level, a single Oct4-GFP+ cell following differentiation was more likely to express multiple germ cell marker transcripts in the same cell than an undifferentiated GFP+ ESC. For example, none of the Oct4-GFP+ ESCs that also expressed Dazl transcript was found to express more than two additional early or late germ cell transcripts, with only 14% of ESCs expressing two early markers (Fig. 2B and C). In contrast, 87% of GFP+ and Dazl+ cells from day 5 of differentiation without FAC media expressed two to three additional early germ cell markers (Fig. 2B), and 70% contained three to five late germ cell markers (Fig. 2C). Similar to cells from EBs without FAC media, 55% of Oct4-GFP+ and Dazl+ cells from EBs with FAC media expressed two additional early germ cell markers (Fig. 2B). Furthermore, 81% of GFP+ and Dazl+ cells from EBs with FAC media contained more than two late markers with up to seven markers expressed together in the same cell, which is consistent with functional FAC media induction of GFP+ germ cell maturation (Fig. 2C).
Finally, our analysis of single cells indicated that in addition to expressing a greater number of germ cell markers within the same cell, single-Oct4-GFP+ cells from day 5 EBs contained significantly elevated levels of most germ cell transcripts analyzed compared with undifferentiated Oct4-GFP+ ESCs (Supplementary Material, Fig. S4). To summarize, undifferentiated ESCs occasionally expressed germ cell transcripts, but differentiated ESC-derived germ cells did so more frequently, expressed more germ cell markers within the same cell and also expressed those markers more robustly than undifferentiated ESCs, especially following differentiation in FAC media.
Genetic requirement of Dazl for ESC-derived germ cell development
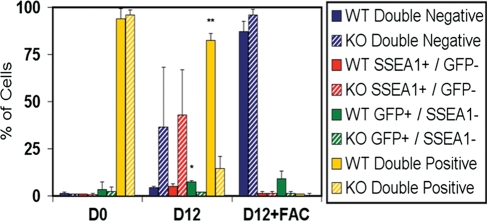
To date, studies of germ cell differentiation from ESCs have been limited in genetic analysis. To further inspect in vitro ESC-derived germ cell identity and maturation, ESC lines, containing the ΔPE Oct4-GFP reporter, were obtained from mice carrying a Dazl (Deleted in azoospermia-like) null mutation and differentiated alongside wild-type (WT) lines. Dazl null mice are sterile and begin to exhibit a reduction in germ cell numbers in the pre-committed embryonic genital ridge by e12.5 in both sexes, with significant germ cell loss by e14.5 in the post-committed fetal ovary or testis (34,42,43). As expected, Dazl null (knockout—KO) ESC lines displayed a significantly reduced percentage of double-positive PGCs (15% KO compared with 83% WT) and GFP+/SSEA1− germ cells (2% KO compared with 8% WT without FAC; 2% KO compared with 9% WT with FAC) in comparison with WT lines by day 12 of differentiation (Fig. 3). Notably, we did not observe a Dazl null phenotype after only 7 or 10 days of differentiation without FAC treatment (data not shown).
Figure 3.
ESC-derived germ cell development requires Dazl. The percentage of early (Oct4-GFP+ and SSEA1+; yellow) and late (Oct4-GFP+ and SSEA1−; green) ESC-derived germ cell populations significantly decreased, and GFP− somatic cells increased, by flow cytometry analysis following Dazl null (KO, striped bars) ESC differentiation for 12 days (−/+ FAC media) compared with Dazl WT (solid bars) lines, suggesting in vitro ESC-derived germ cell commitment, or maturation, along an endogenous developmental timeline with a shared genetic program. Data are represented as mean ± SD (n = 3); *P < 0.05 GFP+/SSEA1− WT to KO, **P < 0.05 double-positive WT to KO.
Hence, the functional genetic requirement of Dazl for germ cell maturation was shared between endogenous germ cell development in vivo and ESC-derived germ cell differentiation in vitro. Moreover, analysis of the Dazl null phenotype also suggested ESC-derived commitment, or maturation, to a sex-specific developmental program by 12 days of in vitro differentiation without FAC media, thereby paralleling the manifestation of the Dazl null phenotype by e12.5 in vivo and corresponding to a timeline of endogenous PGC commitment to an oocyte fate in the absence of signals from the fetal testis (22,44–46).
ESC-derived oocyte maturation is limited in vitro
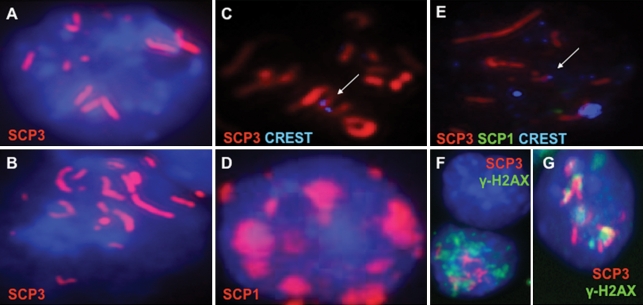
Our results above indicated that we had devised a strategy to isolate and characterize ESC-derived germ cells, that single cells had distinguishing characteristics of germ cells and that the genetic requirements for germ cell development in vitro paralleled those of germ cell development in vivo. However, a landmark event in germ cell development is the initiation and progression through meiosis. Thus, we next examined whether ESC-derived oocytes entered and progressed through meiosis. As shown, we observed that approximately 1–3% of Oct4-GFP+ cells initiated meiosis, as evidenced by synaptonemal complex protein (SCP) expression and chromosomal localization (Fig. 4). However, we detected only partial chromosomal alignment of SCP3, and although other meiotic proteins such as SCP1 were expressed in the GFP+ cells, nuclear localization of SCP1 was focal and indicative of limited meiotic progression (Fig. 4D). We did observe, however, that CREST centromere staining was occasionally co-localized telocentrically with the elongated SCP structures confirming chromosomal alignment (Fig. 4C and E). In addition, 10% of the GFP+ cells with partial SCP3 alignment also expressed nuclear γ-H2AX, a marker of meiotic DNA double-strand breaks, in a punctate pattern that co-localized with regions of the SCP3-coated chromosomes and confirmed a leptotene–zygotene-like stage of meiotic DNA double-strand breaks and synapsis (Fig. 4F and G) (47).
Figure 4.
ESC-derived oocytes enter meiosis but are limited in meiotic progression in vitro. (A–C) Immunofluorescence staining confirmed Oct4-GFP+ ESC-derived germ cell meiotic entry and progression by SCP3 nuclear localization and partial chromosomal alignment. (D) SCP1 localized to the nucleus but was focal instead of elongated, indicating that in vitro ESC-derived meiotic progression was limited. (C and E) CREST centromere staining co-localized with SCP3 and confirmed SCP3 alignment on some, but not all, chromosomes (arrows). (F and G) γ-H2AX stain revealed a punctate expression pattern in the nuclei of GFP+ cells that also co-localized with regions of chromosomal SCP3 and demonstrated the initiation of DNA double-strand breaks and synapsis. Blue is DAPI except (C) and (E). Magnification is ×630.
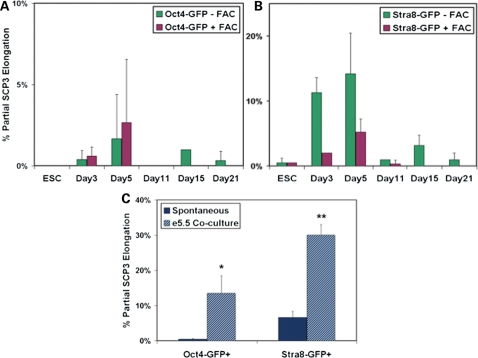
In order to optimize meiotic progression, we used an alternative ESC line that carried a meiotic reporter, Stra8-GFP, to further examine differentiation in experiments that paralleled those described above (Supplementary Material, Fig. S5A–C). Given the role of Stra8 in meiosis, a greater percentage of the Stra8-GFP+ cells had initiated meiosis (up to 10–15%), but these cells also exhibited a block in meiotic progression (Supplementary Material, Fig. S5D). Furthermore, whereas meiotic cells were detected by day 15 of differentiation, similar in timeline to endogenous oogenesis, the maximum percentage of GFP+ germ cells in meiosis with expression and partial chromosomal localization of SCP3 was unexpectedly observed by day 5 of ESC differentiation in both cell lines (Fig. 5A and B). Nevertheless, the analysis of ESC-derived germ cells at these earlier time points of differentiation, or after treatment with FAC-supplemented media, did not significantly improve the extent of meiotic progression for either line. In addition, alternative approaches, based on previous studies of endogenous germ cell entry into meiosis (44,45,48), also were unable to overcome the block to meiotic progression, including the co-culture of the differentiating EBs with female genital ridge from e11.5 embryos and overexpression of the intrinsic regulator of meiotic entry and progression, Dazl (data not shown).
Figure 5.
Embryo co-culture improves the initiation of meiosis but confirms an in vitro block to ESC-derived oocyte maturation. (A and B) ESCs containing the Oct4-GFP or Stra8-GFP reporter were differentiated up to 21 days in standard differentiation media or in media supplemented with FAC. GFP+ germ cells were isolated by FACS and examined for meiotic entry and progression by SCP3 protein expression and elongated chromosomal localization. The maximum percentage of cells entering meiosis was observed by 5 days of differentiation from both cell lines; however, meiotic progression remained partial, and complete progression was not detected for any time point, cell line or treatment. Data are represented as mean ± SD (n = 3). (C) ESCs were differentiated in suspension for 5 days as EBs without or with dissociated e5.5 embryo co-culture, and GFP+ cells were analyzed for meiosis. The e5.5 embryo co-culture induced a significant increase in the percentage of ESC-derived germ cells that entered meiosis from both cell lines. However, e5.5 embryo co-culture did not improve the extent of meiotic progression which remained partial. Data are represented as mean ± SD (n = 2). *,**P < 0.05.
Instead, we observed the most significant enhancement of meiosis in vitro when ESCs were differentiated in co-culture with dissociated e5.5 embryos. This may reflect timing in vivo, as endogenous PGCs are specified from the epiblast at this stage. We observed that e5.5 embryo co-culture induced a significant increase in the percentage of ESC-derived germ cells initiating meiosis compared with non-co-culture controls on day 5 of differentiation (14% of Oct4-GFP+; 30% of Stra8-GFP+) (Fig. 5C). However, as in previous experiments with alternative strategies, the extent of ESC-derived germ cell progression through prophase I of meiosis was not improved by the e5.5 embryo co-culture.
ESC-derived oocyte maturation following ovarian niche transplantation
Since meiotic progression was incomplete and follicle formation was not observed in vitro, we next sought to test whether we could achieve oocyte maturation via transplantation (Figs 6 and 7). Ovarian follicle formation occurs just after birth in mice which have 21 days of gestation, and re-aggregated newborn ovaries support robust endogenous ovarian follicle development and oocyte maturation following transplantation under the kidney capsule (49) (Fig. 7A). Furthermore, our data indicated that in vitro ESC-derived germ cell differentiation initially followed an endogenous timeline. Thus, to synchronize ESC-derived oocyte differentiation with the newborn ovarian niche, we differentiated Oct4-GFP ESCs for 21 days in vitro without FAC media, isolated GFP+ germ cells by FACS and co-aggregated the cells with dissociated WT newborn ovarian tissue. We then transplanted the co-aggregates under the kidney capsule of recipient mice for 3 weeks. Five out of eight grafts grew in size and contained ovarian tissue following transplantation. Indeed, we observed ESC-derived GFP+ cells in two of the grafts. From these grafts, 23 ESC-derived Oct4-GFP+ oocytes were detected out of 100 000 cells transplanted for an efficiency of 0.023%.
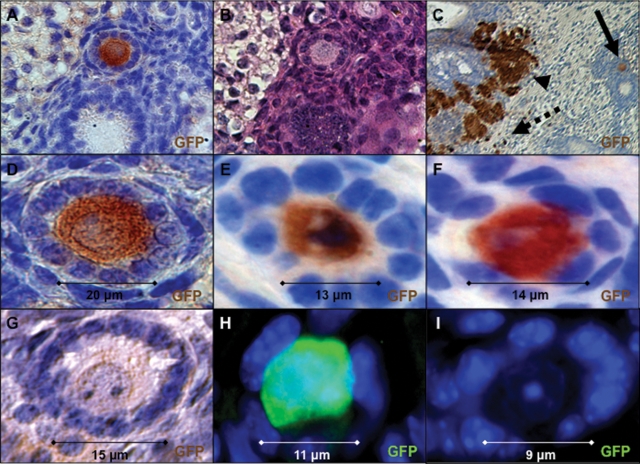
Figure 6.
ESC-derived oocyte maturation in ovarian follicles directed by transplantation into an ovarian niche. (A–D) An Oct4-GFP+ ESC-derived oocyte enclosed in a primary-stage follicle. (A) Immunoperoxidase stain for GFP expression following transplantation (×200). (B) Hematoxylin and eosin stain on adjacent section. (C) Low-magnification image of (A) showing GFP+ oocyte (solid arrow), GFP+ clusters (arrowhead) and isolated single-GFP+ oocytes not completely enclosed in follicles (dashed arrow) (×100). (D) High magnification of (A) (×630). (E and F) Additional ESC-derived oocytes in primary follicles with robust GFP+ immunoperoxidase staining above background endogenous GFP− oocytes (G) derived from WT newborn ovary in the same section (×630). (H) Oct4-GFP+ ESC-derived oocytes in primordial/primary follicles were also detected by immunofluorescence stain for GFP above background staining of WT oocytes (I) from newborn ovary located adjacent to (H) (×630). (A–G) Blue is hematoxylin counter stain. (H and I) Blue is DAPI.
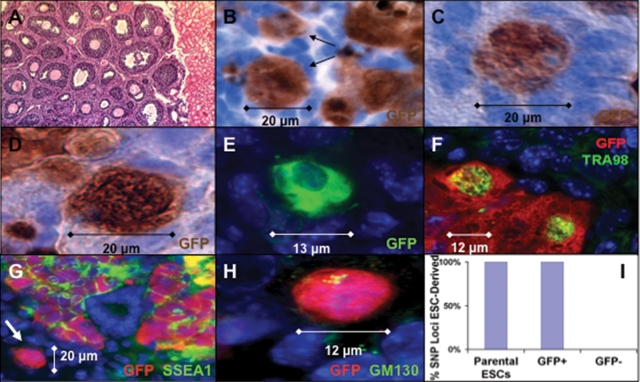
Figure 7.
ESC-derived oocyte characterization following transplantation. (A) Hematoxylin and eosin stain of newborn ovarian tissue following re-aggregation without ESCs and transplantation revealed robust oocyte maturation and folliculogenesis adjacent to kidney tissue (×100). (B) ESC-derived oocytes (arrows) enclosed in primordial/primary-stage follicles were detected by immunoperoxidase staining for GFP following transplantation. (C andD) Oct4-GFP+ ESC-derived oocytes not completely enclosed in follicles. (E) Pre-follicle-stage GFP+ ESC-derived oocytes identified by immunofluorescence. (F) Oct4-GFP+ and TRA98+ ESC-derived oocytes not completely enclosed in follicles. (G) GFP and SSEA1 co-stain demonstrated double-positive clusters and isolated Oct4-GFP+/SSEA1− ESC-derived oocytes (arrow) (×200). (H) Oct4-GFP+ ESC-derived oocytes expressed GM130 in Balbiani body-like structures. (I) The ESC origin of Oct4-GFP+ oocytes was confirmed by SNP genotyping of FACS isolated cells following transplantation with 100% of GFP+ cell loci homozygous for parental ESC strain sequence, whereas 0% of GFP− cell loci were homozygous. (A–D) Blue is hematoxylin counter stain. (E–H) Blue is DAPI. (B–F and H) ×630.
We observed ESC-derived oocytes enclosed in ovarian follicles, with some reaching the primary follicle stage (Fig. 6A–F) and others at a primordial/primary stage (Figs 6H and 7B) of development. The primary follicles consisted of an ESC-derived oocyte that recruited a single layer of endogenous cuboidal granulosa cells, surrounded by a basement membrane, with robust Oct4-GFP expression above background staining of endogenous WT oocytes (Fig. 6G and I). In addition, the oocytes contained a single large germinal vesicle nucleus and were 10–20 µm in size, equivalent to the size of endogenous primordial-to-primary follicle-stage oocytes. We also observed GFP+ oocytes that were not yet completely enclosed in follicles (Fig. 7C–H) and expressed the germ cell marker TRA98 and peri-nuclear Balbiani body-like oocyte marker GM130, but did not express the stem cell/progenitor marker SSEA1 (Fig. 7F–H) (50).
To further examine GFP+ oocyte origin, we isolated GFP+ cells by FACS, following transplantation, genotyped them at 50 polymorphic single-nucleotide polymorphism (SNP) markers across the mouse genome and detected homozygous parental ESC strain sequence for every marker analyzed but did not detect WT newborn ovary donor or recipient mouse strain sequences, thereby confirming an ESC origin of the Oct4-GFP+ oocytes (Fig. 7I).
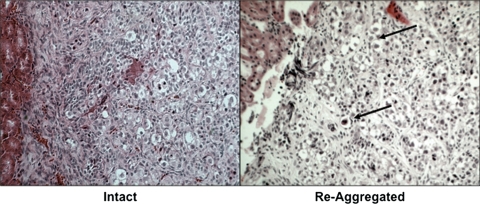
Finally, to determine the potential of developing a human ovarian niche for future studies of human ESC-derived germ cell transplantation, we obtained human fetal ovaries (after approval by the appropriate institutional review board) and transplanted intact or re-aggregated tissues, as described above, into immuno-deficient mice. Following 2 months of transplantation, grafts were analyzed for oocyte survival and development in ovarian follicles (Fig. 8). Intact human fetal ovaries supported the survival of oocytes in primordial-stage follicles. Interestingly, after dissociation and re-aggregation, the human fetal ovaries were still able to support oocyte survival and organization into primordial follicles. Thus, we demonstrated the feasibility of physiologic human ovarian niche transplantation for future studies of human ESC-derived oocyte maturation.
Figure 8.
Development of a human ovarian niche via human fetal ovary re-aggregation and transplantation. Intact or re-aggregated 20 week human fetal ovaries were transplanted under the kidney capsule for 2 months and analyzed for the support of oocyte survival and development in ovarian follicles by hematoxylin and eosin staining. Oocytes in primordial-stage follicles were present in both samples, indicating that, similar to mouse newborn ovaries, human fetal ovaries supported the re-organization of follicles following re-aggregation and transplantation and may be utilized for the direction of human ESC-derived oocyte maturation in follicles via transplantation. Arrows show clusters of oocytes in primordial follicles (×100).
DISCUSSION
This work documents bona fide oocyte differentiation from ESCs by describing functional genetic, biochemical and cellular events that define endogenous oocyte development in vivo. The evidence for authentic germ cell differentiation and maturation in this study includes gene expression analysis at the single-cell level, ability of ESC-derived germ cells to respond to defined maturation media, a shared requirement of ESC-derived and endogenous germ cells for the function of the Dazl gene and finally, the ability of ESC-derived oocytes to contribute to folliculogenesis by transplantation into an ovarian niche. We suggest that the methods and results outlined above may provide a platform for the development of new strategies for regenerative medicine in the arena of reproductive health.
We first present a novel method for the effective isolation of germ cells and oocytes on the basis of properties of endogenous germ cell development. In contrast to several studies that used SSEA1 for positive selection and enrichment of early-stage ESC-derived PGCs (11,13,17,51), we used SSEA1 for negative selection to allow the isolation of Oct4-GFP+ and SSEA1− post-PGC-stage germ cells that exhibited properties of meiosis and maturation. In addition, this method may be useful for the isolation of later stage human ESC-derived germ cells because, similar to mouse, SSEA1 expression is substantially reduced in germ cells of the human fetal ovary by the end of the first trimester upon oocyte initiation of meiosis (52). Furthermore, SSEA1− selection will help eliminate the possibility of teratoma formation following transplantation due to the ectopic reprogramming of human ESC-derived PGCs (53).
With this method, we were able to isolate and examine single putative germ cells to confirm germ cell identity and maturation. To our knowledge, this is the first report of ESC-derived germ cell analysis at the single-cell level and discrimination of ESC and germ cell gene expression profiles. Single-cell expression profiling confirmed that an authentic ESC-derived germ cell expresses a more coordinated and extensive set of germ cell-specific markers compared with an undifferentiated ESC that may randomly mis-express an occasional marker during culture.
Furthermore, we expect that ESC-derived germ cells should possess the ability to respond to endogenous environmental cues, defined by germ cell maturation FAC media, which induced the accelerated maturation of germ cells and oocytes by day 5 of in vitro differentiation. For the first time, defined media containing multiple factors were used to accomplish ESC-derived germ cell maturation. Previous studies had differentiated mouse ESCs in defined media containing a single factor, such as retinoic acid (15,17,33,51) or BMPs (9,32,54). However, endogenous germ cells require multiple factors, such as SDF1 and SCF signaling, from somatic cells in the genital ridge (55–58) and ovary (25,59,60). In addition, the CYP26 inhibitor, R115866, was added to the cocktail in this study to increase the potency of retinoic acid and prevent its metabolism (44,45). Of note, retinoic acid has not yet been used in studies of germ cell differentiation from human ESCs. Retinoic acid may be of particular importance in promoting human germ cell survival and meiotic maturation, comparable with mouse (40,61), since endogenous human germ cells also express STRA8 in the human fetal ovary by the end of the first trimester (62).
Another critical component of this study is the emphasis on correlating in vivo and in vitro requirements for germ cell development, such as the function of Dazl. Undifferentiated ESCs expressing both Oct4-GFP and SSEA1 were not affected by a homozygous genetic null mutation in the Dazl gene, even though ESCs express robust levels of Dazl, which was consistent with its germ cell-specific function. In contrast, the percentage of Dazl null ESC-derived germ cells was significantly reduced by 12 days of differentiation. Thus, ESC-derived germ cells shared a functional genetic program requirement and developmental timeline with endogenous germ cells, further confirming germ cell identity and maturation. Similarly, DAZL, or other germ cell-specific transcript, knockdown by RNAi in human ESCs, or differentiation of human iPSCs from patients with congenital infertility, including Turner syndrome, will assist in the validation of human ESC-derived germ cell differentiation.
Meiosis is a unique functional hallmark of endogenous germ cell development, and efficient ovarian follicle formation may require the prior completion of fetal oocyte progression through prophase I of meiosis (63). Similar to endogenous fetal oocytes, ESC-derived oocytes also entered meiosis. We detected SCP3 and γ-H2AX expression and localization to the chromosomes, and SCP1 was expressed in the nucleus, indicating that ESC-derived germ cells could enter and progress through early stages of meiotic prophase I. However, SCP3 chromosomal alignment remained partial, and SCP1 was not elongated, confirming an in vitro block to ESC-derived meiotic progression (20). Incomplete meiotic progression was surprising since endogenous PGCs enter and progress completely through meiotic prophase I according to a germ cell-intrinsic timing mechanism (22,37,46,64), and suggested improper germ cell specification in vitro. Encouragingly, e5.5 (germ cell specification-stage) embryo co-culture resulted in a significantly increased percentage of ESC-derived germ cells initiating a meiotic program. However, progression through prophase I remained partial, revealing that additional factors, possibly related to germline specification, are needed to direct complete in vitro ESC-derived oocyte meiosis.
Finally, we also noted an in vitro block to ovarian follicle formation (Fig. 9). Soon after birth in mice, meiotic oocytes are surrounded by somatic ovarian granulosa cells to form follicles that are essential for functional oocyte maturation. In fact, endogenous granulosa cells require oocyte-independent activation of critical signaling pathways to direct sex determination of the ovary for subsequent oocyte survival and development in follicles (65–69). Thus, we co-aggregated and transplanted ESC-derived meiotic oocytes with newborn mouse ovarian tissue containing somatic granulosa cells that are primed to direct follicle formation and oocyte development. Transplantation into an appropriate endogenous niche has traditionally been used as a litmus test of cell type identity and function. For example, transplantation to germ cell-depleted testis was used to determine endogenous and ESC-derived spermatogonial stem cell potential and to achieve functional spermatogenesis (30,32,33). However, before this study, transplantation to the ovary had not been used to test the physiologic relevance of ESC-derived oocytes. Indeed, following transplantation, ESC-derived oocytes integrated into the ovarian niche, recruited somatic granulosa cells from the mouse ovary and directed follicle formation and development to the primary follicle stage (Fig. 9).
Figure 9.
Ovarian niche transplantation surmounts the in vitro ESC-derived oocyte maturation bottleneck. The characterization of germ cell differentiation by several methods demonstrates that mouse ESCs differentiate in vitro to cells with oocyte-like properties along an endogenous timeline and genetic program, including the hallmarks of PGC commitment (single star); however, in vitro oocyte maturation is limited (dashed lines represent reduced levels). Transplantation (double star) into an ovarian niche is required to overcome this in vitro bottleneck and direct physiologic maturation of ESC-derived oocytes in ovarian follicles (D, days).
Considerable work remains before safe and effective clinical translation can be realized. ESC-derived oocytes in primary follicles will need to be further matured, fertilized and shown to support the development of healthy offspring with respect to karyotype and epigenetic reprogramming. To promote further maturation, ESC-derived oocytes can be transplanted for longer periods of time to allow for the development of antral-stage follicles, or, alternatively, the primordial-to-primary-stage follicles that were generated in this study can be isolated and matured in vitro, comparable with isolated endogenous oocytes in primordial follicles that later supported the production of offspring (70). Furthermore, clinical treatment for female infertility will also require the use of autologous stem cells, such as iPSCs, for the derivation of genetically related germ cells (13). Human iPSC-derived germ cells could then be matured via co-aggregation with human ovarian tissue and transplantation to a site preferably less invasive than the kidney capsule in order to direct oocyte maturation (71,72). As described in this study, the human fetal ovarian niche may be suitable for this purpose. However, an autologous niche would also be advantageous for clinical translation, including transplantation into the adult ovary of the patient (73,74) or co-aggregation with iPSC-derived somatic granulosa cells. Along with many scientific challenges, the future derivation of mature gametes from human stem cells for clinical purposes will be accompanied by several ethical and policy issues (75).
The clinical relevance of research involving stem cell-derived oocytes is unquestionable (7). Currently, the options available for childbearing to women who lack reproductively competent oocytes are limited to oocyte donation, embryo donation or adoption. In 2005 in the USA alone, nearly 10 000 fresh and 5000 frozen embryo transfers were performed using donor oocytes (Centers for Disease Control and Prevention, 2005 Artificial Reproductive Technology Report: National Summary; http://apps.nccd.cdc.gov/ART2005/nation05.asp). This number of cycles is most certainly an underestimate of the true clinical need for functional oocytes, because not all couples with infertility on the basis of ovarian failure or diminished ovarian reserve will choose to pursue oocyte donation. Clearly, more options are needed to allow women who lack reproductively competent oocytes to have genetically related offspring, and stem cell-derived oocytes could someday fulfill this need. Research utilizing oocytes derived from stem cells could also provide valuable insight into the processes of both normal and abnormal oocyte development. It is quite plausible that this research will not only enhance our ability to address infertility, but will also help us to understand better some of the causes of abnormal offspring in spontaneous conceptions occurring among the general population.
MATERIALS AND METHODS
ESC culture and differentiation
Transgenic ΔPE Oct4-GFP, Dazl WT and null, ESC (XX) lines were derived from C57BL/6-FVB/N mice as described (34), and the gene trap Stra8-GFP ESC (XY) line was obtained from the Canadian Mouse Mutant Repository (clone ID 339H10). Undifferentiated ESCs were cultured on irradiated mouse embryonic fibroblasts in standard ESC media containing Dulbecco's modified Eagle's medium with high glucose and 2 mm l-glutamine, 1 mm sodium pyruvate, 100 µm non-essential amino acids, 15% fetal bovine serum (all Invitrogen), 100 µm 2-mercaptoethanol (Sigma) and 1000 U/ml of LIF (Millipore); plus 165 µg/ml of G418 (Invitrogen) for the Stra8-GFP gene-trap line only. ESCs were differentiated in suspension as EBs by culturing in standard EB media with the same components as ESC media but with 20% fetal bovine serum (Hyclone) and without LIF on ultra-low attachment plates (Corning). Alternatively, ESCs were differentiated in FAC media consisting of standard EB media supplemented with a germ cell factor cocktail: mouse SCF 100 ng/ml, mouse SDF1 20 ng/ml, mouse bFGF 20 ng/ml, mouse BMP4 50 ng/ml (all R&D Systems), N-acetylcysteine 1 mg/ml, Forskolin 5 µm, retinoic acid 1 µm (all Sigma) and CYP26 inhibitor R115866 1 µm (Johnson & Johnson).
Flow cytometry and FACS
ESCs, EBs and fetal gonads were dissociated to single cells with 0.25% Trypsin (Invitrogen) for 5–10 min at 37°C, or first treated with 1 mg/ml each of Collagenase IV and Dispase (both Invitrogen) for 20 min at 37°C prior to trypsinization for adult tissues, and re-suspended in standard media. Cells were strained through a 40 µm filter (BD Biosciences) and then analyzed on a BD-FACSAria cell-sorting system (BD Biosciences). For flow cytometry analysis of SSEA1 expression, dissociated cells were first re-suspended in phosphate-buffered saline (PBS, Invitrogen) with 1% bovine serum albumin (BSA, Sigma), incubated with mouse anti-SSEA1 antibody (1:20, Abcam) for 20 min on ice, washed, incubated with APC-conjugated anti-mouse IgM secondary antibody (1:200, Jackson Immunoresearch) for 20 min and washed again before sorting. GFP and SSEA1 positive gates were based on non-transgenic control ESC lines and secondary antibody-only control staining, respectively.
Quantitative gene expression analysis
Gene expression analysis was performed using the BioMark Dynamic Array (Fluidigm Corporation) microfluidics system for RT–PCR, as described (34). In brief, we pre-amplified samples by treating single cells (Fig. 2A and Supplementary Material, Fig. S2B) or 50 cells (Supplementary Material, Fig. S2A) per sample per time point following the manufacturer's protocol (Fluidigm Corporation) using TaqMan gene expression assays (Applied Biosystems) as indicated in the respective figures. Then, 2.25 µl of pre-amplified cDNA was mixed with 2.5 µl of 2× Universal Master Mix (Applied Biosystems) and 0.25 µl of sample loading buffer (Fluidigm Corporation) and loaded into the sample inlets of the 96 × 96 Dynamic Array (Fluidigm Corporation). For each probe, the reaction mix contained 2.5 µl of 2× TaqMan Gene Assay and 2.5 µl of assay loading buffer (Fluidigm Corporation) for loading into the assay inlets on the Dynamic Array. Each sample had two technical replicates. Average CT values were calculated and normalized to Gapdh.
Meiotic cell spread and immunofluorescence
Following ESC, EB or tissue dissociation, cells were re-suspended in 20 µl of hypo-extraction buffer (pH 8.2) [30 mm Tris, pH 8.2, 50 mm sucrose, 17 mm citric acid, 5 mm EDTA, 0.5 mm DTT and 1% protease inhibitor cocktail (all Sigma)] for 30 min at room temperature. Then, 60 µl of 100 mm sucrose was added, and the cell suspension was spread onto slides pre-coated with 1% paraformaldehyde (USB Corporation) and 0.15% Triton X-100 (Sigma) in PBS, pH 9.2, and dried overnight at room temperature. Slides were blocked in 4% chicken serum (Abcam) and incubated overnight at 4°C with primary antibody in TBST [Tris-buffered saline (TBS), 1% BSA and 0.1% Tween-20 (all Sigma)] and 1% serum. Primary antibodies included rabbit anti-SCP3 (1:1000, Abcam), rabbit anti-SCP1 (1:500 Abcam), goat anti-SCP1 (1:50 Santa Cruz), human CREST (1:100, Antibodies Incorporated) and mouse anti-γ-H2AX (1:500, Millipore). Slides were subsequently incubated with secondary antibodies for 30 min, and cover slips were mounted with ProLong Gold Antifade with DAPI (Invitrogen). Secondary antibodies included chicken anti-rabbit, goat, mouse (1:1000, Invitrogen) and human (1:250, Aves Labs).
Ovarian tissue aggregation and kidney capsule transplantation
Newborn ovaries were dissected from WT CD-1 female pups (Charles River), dissociated to single-cell suspensions by 10 min 0.25% trypsinization and pipetting 10–20 times and re-suspended in standard EB media. The ovarian cell suspension from four female pups per graft was then mixed with or without 100 000 ESC-derived cells post-FACS sort and 0.2 mg/ml of phytohemagglutinin (Sigma). Cell suspensions were pelleted into grafts at 10 000g for 1 min and incubated overnight in standard EB media on CM cell culture inserts (Millipore) at 37°C. Grafts were transplanted under the kidney capsule of bi-laterally ovariectomized CB.17 SCID recipient mice (Charles River) according to the protocol approved by the Stanford University Administrative Panel on Laboratory Animal Care and as described in detail (http://mammary.nih.gov/tools/mousework/Cunha001/Pages/Written_Method.html).
Graft and tissue immunohistochemistry
Tissues were fixed overnight in 4% paraformaldehyde (USB Corporation), embedded in paraffin and sectioned. In brief, sections on slides were de-paraffinized, re-hydrated, antigens unmasked by boiling in Target Retrieval Solution (Dako) for 30 min, permeabilized in 0.1% Triton X-100 (Sigma) for 5 min, blocked with 10% chicken serum in TBST overnight and incubated with primary antibody in TBST with 1% serum for 1 h at room temperature. Primary antibodies included anti-GFP rabbit monoclonal (1:1000, Abcam), anti-TRA98 rat monoclonal (1:500, B-Bridge), anti-SSEA1 mouse monoclonal (1:100, Abcam or Developmental Studies Hybridoma Bank) and anti-GM130 mouse monocolonal (1:100, BD Biosciences). After washing in TBST, slides were incubated with secondary antibody for 30 min at room temperature. For immunofluorescence, secondary antibodies included chicken anti-rabbit, rat and mouse (1:1000, Invitrogen), and coverslips were mounted with Prolong Gold Antifade with DAPI (Invitrogen). For immunoperoxidase, slides were treated as above but with additional blocking in 10% hydrogen peroxide (Sigma) for 20 min at room temperature before permeabilization. Slides were blocked overnight with 10% goat serum (Abcam) in TBST and incubated with primary antibody for 1 h, biotinylated goat anti-rabbit secondary antibody (1:200, Vector Labs) for 30 min, Vectastain ABC solution (Vector Labs) for 30 min, Immpact DAB substrate (Vector Labs) for 1–5 min, counterstained with Mayer's hematoxylin and mounted with Prolong Gold Antifade (Invitrogen).
Genetic analysis post-transplantation
After 3 weeks of transplantation, grafts were harvested, dissociated and Oct4-GFP positive and negative cells isolated by FACS. Genomic DNA was prepared from these populations, and from the parental Oct4-GFP ESC line (DNeasy, Qiagen), and was then analyzed for the sequence of 50 SNPs that were polymorphic between C57BL/6-FVB/N and CD1-BALB/C mouse strains by the Jackson Laboratory Genome Scanning Service. The SNP panel contained three markers on chromosomes 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and 12, two markers on chromosomes 11, 13, 14, 15, 16, 17, 18 and 19, and one marker on the X chromosome. SNP sequences at each locus were compared with the parental ESC line and to each of the four mouse strains using a modified Amplifluor fluorescent PCR-based system.
Statistical analysis
Data are represented as mean ± standard deviation (SD). Statistical significance was determined with Excel (Microsoft) using an unpaired Student's t-test with two-tailed distribution of two-sample unequal variance. Significance of values in Supplementary Material, Fig. S4 was determined by Prism (GraphPad Software) two-way ANOVA with Bonferroni post-test.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
FUNDING
This work was supported by the National Institutes of Health (U54HD055764 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research), the California TRDRP (14RT0159, 15DT-0187) and the Canadian Institutes of Health Research DRA. Funding to pay the Open Access publication charges for this article was provided by the National Institutes of Health (U54HD055764).
ACKNOWLEDGEMENTS
We thank the members of the Reijo Pera Laboratory for assistance; Drs Shawn Chavez, Eric Chiao, Aaron Hsueh and Connie Wong for critical review of the manuscript; Drs John Eppig and Karen Wigglesworth for ovarian tissue transplantation assistance; Dr Howard Cooke for Dazl null mice; Dr Chris Wylie for ΔPE Oct4-GFP mice; Dr Marcus Muench for harvesting human fetal gonads; and Johnson & Johnson for the CYP26 inhibitor.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Moore K.L., Persaud T.V.N. Before We Are Born. 5th edn. Philadelphia, PA: W.B. Saunders Company; 1998. [Google Scholar]
- 2.Whelan E.A., Sandler D.P., McConnaughey D.R., Weinberg C.R. Menstrual and reproductive characteristics and age at natural menopause. Am. J. Epidemiol. 1990;131:625–632. doi: 10.1093/oxfordjournals.aje.a115546. [DOI] [PubMed] [Google Scholar]
- 3.Torgerson D.J., Avenell A., Russell I.T., Reid D.M. Factors associated with onset of menopause in women aged 45–49. Maturitas. 1994;19:83–92. doi: 10.1016/0378-5122(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 4.te Velde E.R., Scheffer G.J., Dorland M., Broekmans F.J., Fauser B.C. Developmental and endocrine aspects of normal ovarian aging. Mol. Cell Endocrinol. 1998;145:67–73. doi: 10.1016/s0303-7207(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 5.Coulam C.B., Adamson S.C., Annegers J.F. Incidence of premature ovarian failure. Obstet. Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 6.Conway G.S. Premature ovarian failure. Br. Med. Bull. 2000;56:643–649. doi: 10.1258/0007142001903445. [DOI] [PubMed] [Google Scholar]
- 7.Nicholas C.R., Chavez S.L., Baker V.L., Reijo Pera R.A. Instructing an embryonic stem cell-derived oocyte fate: lessons from endogenous oogenesis. Endocr. Rev. 2009;30:264–283. doi: 10.1210/er.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark A.T., Bodnar M.S., Fox M., Rodriquez R.T., Abeyta M.J., Firpo M.T., Pera R.A. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum. Mol. Genet. 2004;13:727–739. doi: 10.1093/hmg/ddh088. [DOI] [PubMed] [Google Scholar]
- 9.Kee K., Gonsalves J.M., Clark A.T., Reijo Pera R.A. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–837. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- 10.West F.D., Machacek D.W., Boyd N.L., Pandiyan K., Robbins K.R., Stice S.L. Enrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signaling. Stem Cells. 2008;26:2768–2776. doi: 10.1634/stemcells.2008-0124. [DOI] [PubMed] [Google Scholar]
- 11.Tilgner K., Atkinson S.P., Golebiewska A., Stojkovic M., Lako M., Armstrong L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells. 2008;26:3075–3085. doi: 10.1634/stemcells.2008-0289. [DOI] [PubMed] [Google Scholar]
- 12.Bucay N., Yebra M., Cirulli V., Afrikanova I., Kaido T., Hayek A., Montgomery A.M. A novel approach for the derivation of putative primordial germ cells and Sertoli cells from human embryonic stem cells. Stem Cells. 2009;27:68–77. doi: 10.1634/stemcells.2007-1018. [DOI] [PubMed] [Google Scholar]
- 13.Park T.S., Galic Z., Conway A.E., Lindgren A., van Handel B.J., Magnusson M., Richter L., Teitell M.A., Mikkola H.K., Lowry W.E., et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27:783–795. doi: 10.1002/stem.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubner K., Fuhrmann G., Christenson L.K., Kehler J., Reinbold R., De La Fuente R., Wood J., Strauss J.F., III, Boiani M., Scholer H.R. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 15.Kerkis A., Fonseca S.A., Serafim R.C., Lavagnolli T.M., Abdelmassih S., Abdelmassih R., Kerkis I. In vitro differentiation of male mouse embryonic stem cells into both presumptive sperm cells and oocytes. Cloning Stem Cells. 2007;9:535–548. doi: 10.1089/clo.2007.0031. [DOI] [PubMed] [Google Scholar]
- 16.Lacham-Kaplan O., Chy H., Trounson A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells. 2006;24:266–273. doi: 10.1634/stemcells.2005-0204. [DOI] [PubMed] [Google Scholar]
- 17.Qing T., Shi Y., Qin H., Ye X., Wei W., Liu H., Ding M., Deng H. Induction of oocyte-like cells from mouse embryonic stem cells by co-culture with ovarian granulosa cells. Differentiation. 2007;75:902–911. doi: 10.1111/j.1432-0436.2007.00181.x. [DOI] [PubMed] [Google Scholar]
- 18.Salvador L.M., Silva C.P., Kostetskii I., Radice G.L., Strauss J.F., III The promoter of the oocyte-specific gene, Gdf9, is active in population of cultured mouse embryonic stem cells with an oocyte-like phenotype. Methods. 2008;45:172–181. doi: 10.1016/j.ymeth.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zwaka T.P., Thomson J.A. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]
- 20.Novak I., Lightfoot D.A., Wang H., Eriksson A., Mahdy E., Hoog C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells. 2006;24:1931–1936. doi: 10.1634/stemcells.2005-0520. [DOI] [PubMed] [Google Scholar]
- 21.Matzuk M.M., Burns K.H., Viveiros M.M., Eppig J.J. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 22.Zamboni L., Upadhyay S. Germ cell differentiation in mouse adrenal glands. J. Exp. Zool. 1983;228:173–193. doi: 10.1002/jez.1402280204. [DOI] [PubMed] [Google Scholar]
- 23.Isotani A., Nakanishi T., Kobayashi S., Lee J., Chuma S., Nakatsuji N., Ishino F., Okabe M. Genomic imprinting of XX spermatogonia and XX oocytes recovered from XX<–>XY chimeric testes. Proc. Natl Acad. Sci. USA. 2005;102:4039–4044. doi: 10.1073/pnas.0406769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eppig J.J., O'Brien M.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 25.Klinger F.G., De Felici M. In vitro development of growing oocytes from fetal mouse oocytes: stage-specific regulation by stem cell factor and granulosa cells. Dev. Biol. 2002;244:85–95. doi: 10.1006/dbio.2002.0592. [DOI] [PubMed] [Google Scholar]
- 26.Obata Y., Kono T., Hatada I. Gene silencing: maturation of mouse fetal germ cells in vitro. Nature. 2002;418:497. doi: 10.1038/418497a. [DOI] [PubMed] [Google Scholar]
- 27.Qing T., Liu H., Wei W., Ye X., Shen W., Zhang D., Song Z., Yang W., Ding M., Deng H. Mature oocytes derived from purified mouse fetal germ cells. Hum. Reprod. 2008;23:54–61. doi: 10.1093/humrep/dem334. [DOI] [PubMed] [Google Scholar]
- 28.Shen W., Li L., Bai Z., Pan Q., Ding M., Deng H. In vitro development of mouse fetal germ cells into mature oocytes. Reproduction. 2007;134:223–231. doi: 10.1530/REP-06-0378. [DOI] [PubMed] [Google Scholar]
- 29.Shen W., Zhang D., Qing T., Cheng J., Bai Z., Shi Y., Ding M., Deng H. Live offspring produced by mouse oocytes derived from premeiotic fetal germ cells. Biol. Reprod. 2006;75:615–623. doi: 10.1095/biolreprod.106.051482. [DOI] [PubMed] [Google Scholar]
- 30.Brinster R.L., Zimmermann J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl Acad. Sci. USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissman I.L. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 32.Toyooka Y., Tsunekawa N., Akasu R., Noce T. Embryonic stem cells can form germ cells in vitro. Proc. Natl Acad. Sci. USA. 2003;100:11457–11462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nayernia K., Nolte J., Michelmann H.W., Lee J.H., Rathsack K., Drusenheimer N., Dev A., Wulf G., Ehrmann I.E., Elliott D.J., et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev. Cell. 2006;11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Haston K.M., Tung J.Y., Reijo Pera R.A. Dazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitro. PLoS ONE. 2009;4:e5654. doi: 10.1371/journal.pone.0005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimizu T., Sugiyama N., De Felice M., Yeom Y.I., Ohbo K., Masuko K., Obinata M., Abe K., Scholer H.R., Matsui Y. Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev. Growth Differ. 1999;41:675–684. doi: 10.1046/j.1440-169x.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 36.Fox N., Damjanov I., Martinez-Hernandez A., Knowles B.B., Solter D. Immunohistochemical localization of the early embryonic antigen (SSEA-1) in postimplantation mouse embryos and fetal and adult tissues. Dev. Biol. 1981;83:391–398. doi: 10.1016/0012-1606(81)90487-5. [DOI] [PubMed] [Google Scholar]
- 37.Farini D., Scaldaferri M.L., Iona S., La Sala G., De Felici M. Growth factors sustain primordial germ cell survival, proliferation and entering into meiosis in the absence of somatic cells. Dev. Biol. 2005;285:49–56. doi: 10.1016/j.ydbio.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 38.De Felici M. Regulation of primordial germ cell development in the mouse. Int. J. Dev. Biol. 2000;44:575–580. [PubMed] [Google Scholar]
- 39.Lawson K.A., Dunn N.R., Roelen B.A., Zeinstra L.M., Davis A.M., Wright C.V., Korving J.P., Hogan B.L. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson E.L., Baltus A.E., Roepers-Gajadien H.L., Hassold T.J., de Rooij D.G., van Pelt A.M., Page D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl Acad. Sci. USA. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang D.H., Cattoretti G., Calame K.L. The dynamic expression pattern of B lymphocyte induced maturation protein-1 (Blimp-1) during mouse embryonic development. Mech. Dev. 2002;117:305–309. doi: 10.1016/s0925-4773(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 42.Lin Y., Page D.C. Dazl deficiency leads to embryonic arrest of germ cell development in XY C57BL/6 mice. Dev. Biol. 2005;288:309–316. doi: 10.1016/j.ydbio.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 43.Ruggiu M., Speed R., Taggart M., McKay S.J., Kilanowski F., Saunders P., Dorin J., Cooke H.J. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- 44.Bowles J., Knight D., Smith C., Wilhelm D., Richman J., Mamiya S., Yashiro K., Chawengsaksophak K., Wilson M.J., Rossant J., et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 45.Koubova J., Menke D.B., Zhou Q., Capel B., Griswold M.D., Page D.C. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl Acad. Sci. USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLaren A., Southee D. Entry of mouse embryonic germ cells into meiosis. Dev. Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- 47.Mahadevaiah S.K., Turner J.M., Baudat F., Rogakou E.P., de Boer P., Blanco-Rodriguez J., Jasin M., Keeney S., Bonner W.M., Burgoyne P.S. Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 2001;27:271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- 48.Lin Y., Gill M.E., Koubova J., Page D.C. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–1687. doi: 10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- 49.Eppig J.J., Wigglesworth K. Development of mouse and rat oocytes in chimeric reaggregated ovaries after interspecific exchange of somatic and germ cell components. Biol. Reprod. 2000;63:1014–1023. doi: 10.1095/biolreprod63.4.1014. [DOI] [PubMed] [Google Scholar]
- 50.Pepling M.E., Wilhelm J.E., O'Hara A.L., Gephardt G.W., Spradling A.C. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc. Natl Acad. Sci. USA. 2007;104:187–192. doi: 10.1073/pnas.0609923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geijsen N., Horoschak M., Kim K., Gribnau J., Eggan K., Daley G.Q. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 52.Kerr C.L., Hill C.M., Blumenthal P.D., Gearhart J.D. Expression of pluripotent stem cell markers in the human fetal ovary. Hum. Reprod. 2008;23:589–599. doi: 10.1093/humrep/dem411. [DOI] [PubMed] [Google Scholar]
- 53.Stevens L.C. Germ cell origin of testicular and ovarian teratomas. Transplant Proc. 1984;16:502–504. [PubMed] [Google Scholar]
- 54.Wei W., Qing T., Ye X., Liu H., Zhang D., Yang W., Deng H. Primordial germ cell specification from embryonic stem cells. PLoS ONE. 2008;3:e4013. doi: 10.1371/journal.pone.0004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molyneaux K.A., Zinszner H., Kunwar P.S., Schaible K., Stebler J., Sunshine M.J., O'Brien W., Raz E., Littman D., Wylie C., et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130:4279–4286. doi: 10.1242/dev.00640. [DOI] [PubMed] [Google Scholar]
- 56.Farini D., La Sala G., Tedesco M., De Felici M. Chemoattractant action and molecular signaling pathways of Kit ligand on mouse primordial germ cells. Dev. Biol. 2007;306:572–583. doi: 10.1016/j.ydbio.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 57.Pesce M., Farrace M.G., Piacentini M., Dolci S., De Felici M. Stem cell factor and leukemia inhibitory factor promote primordial germ cell survival by suppressing programmed cell death (apoptosis) Development. 1993;118:1089–1094. doi: 10.1242/dev.118.4.1089. [DOI] [PubMed] [Google Scholar]
- 58.Godin I., Deed R., Cooke J., Zsebo K., Dexter M., Wylie C.C. Effects of the steel gene product on mouse primordial germ cells in culture. Nature. 1991;352:807–809. doi: 10.1038/352807a0. [DOI] [PubMed] [Google Scholar]
- 59.Holt J.E., Jackson A., Roman S.D., Aitken R.J., Koopman P., McLaughlin E.A. CXCR4/SDF1 interaction inhibits the primordial to primary follicle transition in the neonatal mouse ovary. Dev. Biol. 2006;293:449–460. doi: 10.1016/j.ydbio.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Hoyer P.E., Byskov A.G., Mollgard K. Stem cell factor and c-Kit in human primordial germ cells and fetal ovaries. Mol. Cell Endocrinol. 2005;234:1–10. doi: 10.1016/j.mce.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 61.Koshimizu U., Watanabe M., Nakatsuji N. Retinoic acid is a potent growth activator of mouse primordial germ cells in vitro. Dev. Biol. 1995;168:683–685. doi: 10.1006/dbio.1995.1113. [DOI] [PubMed] [Google Scholar]
- 62.Houmard B., Small C., Yang L., Naluai-Cecchini T., Cheng E., Hassold T., Griswold M. Global gene expression in the human fetal testis and ovary. Biol. Reprod. 2009;81:438–443. doi: 10.1095/biolreprod.108.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paredes A., Garcia-Rudaz C., Kerr B., Tapia V., Dissen G.A., Costa M.E., Cornea A., Ojeda S.R. Loss of synaptonemal complex protein-1, a synaptonemal complex protein, contributes to the initiation of follicular assembly in the developing rat ovary. Endocrinology. 2005;146:5267–5277. doi: 10.1210/en.2005-0965. [DOI] [PubMed] [Google Scholar]
- 64.Chuma S., Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev. Biol. 2001;229:468–479. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]
- 65.Vainio S., Heikkila M., Kispert A., Chin N., McMahon A.P. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 66.Yao H.H., Matzuk M.M., Jorgez C.J., Menke D.B., Page D.C., Swain A., Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev. Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomizuka K., Horikoshi K., Kitada R., Sugawara Y., Iba Y., Kojima A., Yoshitome A., Yamawaki K., Amagai M., Inoue A., et al. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum. Mol. Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- 68.Chassot A.A., Ranc F., Gregoire E.P., Roepers-Gajadien H.L., Taketo M.M., Camerino G., de Rooij D.G., Schedl A., Chaboissier M.C. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum. Mol. Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- 69.Merchant-Larios H., Centeno B. Morphogenesis of the ovary from the sterile W/Wv mouse. Prog. Clin. Biol. Res. 1981;59B:383–392. [PubMed] [Google Scholar]
- 70.O'Brien M.J., Pendola J.K., Eppig J.J. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol. Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 71.Lee D.M., Yeoman R.R., Battaglia D.E., Stouffer R.L., Zelinski-Wooten M.B., Fanton J.W., Wolf D.P. Live birth after ovarian tissue transplant. Nature. 2004;428:137–138. doi: 10.1038/428137a. [DOI] [PubMed] [Google Scholar]
- 72.Oktay K., Buyuk E., Veeck L., Zaninovic N., Xu K., Takeuchi T., Opsahl M., Rosenwaks Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 73.Donnez J., Dolmans M.M., Demylle D., Jadoul P., Pirard C., Squifflet J., Martinez-Madrid B., van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 74.Zou K., Yuan Z., Yang Z., Luo H., Sun K., Zhou L., Xiang J., Shi L., Yu Q., Zhang Y., et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009;11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 75.Mathews D.J., Donovan P.J., Harris J., Lovell-Badge R., Savulescu J., Faden R. Pluripotent stem cell-derived gametes: truth and (potential) consequences. Cell Stem Cell. 2009;5:11–14. doi: 10.1016/j.stem.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.