Summary
T cell activation is regulated by binding of ligands on antigen presenting cells (APC) to corresponding receptors on T cells. In mice, we discovered that binding of DC-HIL on APC to syndecan-4 (SD-4) on activated T cells potently inhibits T cell activation. In humans, we now show that DC-HIL also binds to SD-4 on activated T cells through recognition of its heparinase-sensitive saccharide moiety. DC-HIL blocks anti-CD3-induced T cell responses, reducing secretion of pro-inflammatory cytokines and blocking entry into the S phase of the cell cycle. Binding of DC-HIL phosphorylates SD-4’s intracellular tyrosine and serine residues. Anti-SD-4 Ab mimics the ability of DC-HIL to attenuate anti-CD3 response more potently than Ab directed against other inhibitory receptors (CTLA-4 or PD-1). Among leukocytes, DC-HIL is expressed highest by CD14+ monocytes and this expression can be upregulated markedly by TGF-β. Among APC, DC-HIL is expressed highest by epidermal Langerhans cells, an immature type of dendritic cells. Finally, the level of DC-HIL expression on CD14+ monocytes correlates inversely with allostimulatory capacity, such that treatment with TGF-β reduced this capacity, whereas knocking-down the DC-HIL gene augmented it. Our findings indicate that the DC-HIL/SD-4 pathway can be manipulated to treat T cell-driven disorders in humans.
Keywords: Antigen presenting cells, DC-HIL, syndecan-4, T cells
Introduction
T cell activation generates protective immunity against infections and malignancies, but may cause autoimmune and hypersensitivity disorders. Activation of T cells is controlled by APC via two signals: the first delivered by interaction of Ag-loaded MHC molecules on APC with the Ag-specific TCR/CD3 complex on T cells; and the second a net result of competing positive and negative costimulatory signals, in which the former is transmitted by binding of B7 molecules on APC to the CD28 receptor on T cells and the latter by binding of coinhibitory molecules on APC to the corresponding receptors on T cells. The outcome of this latter competition determines whether the second signal amplifies or represses the first. Several inhibitory pairs of APC/T cell molecules have been reported, including CTLA-4 on T cells and B7 molecules on APC [1, 2]; programmed cell death-1 (PD-1) on T cells and PD-L1/PD-L2 on APC [3, 4]; B- and T-lymphocyte attenuator (BTLA) and herpes virus entry mediator (HVEM) [5–7]; and Tim-3 and Tim-3 ligand [8, 9]. Our understanding of the HVEM pathway has become more complicated by the finding that HVEM binds to both the coinhibitory receptor CD 160 and the costimulatory receptor LIGHT on T cells [10].
Subtractive cloning in mice led to our discovery of DC-HIL [11], which is also referred to as Gpnmb [12], osteoactivin [13], and hematopoietic growth factor-inducible neurokinin type 1 (HGFIN ) [14]. DC-HIL is a highly glycosylated transmembrane receptor (95–120 KDa) expressed constitutively at high levels by APC and at lower levels by certain nonlymphoid cells [11]. Its extracellular domain contains a cell-adhesion motif (RGD), a proline-rich region, and an Ig-like polycystic kidney disease (PKD) domain shared by PKD-susceptible gene products [15]. Most recently, we reported in mice that binding of DC-HIL to a heparan sulfate-bearing glycoprotein syndecan-4 (SD-4) on activated T cells attenuates T cell activation triggered by anti-CD3 Ab [16]. Because mouse and human DC-HIL share 71% amino acid sequence homology and both contain the aforementioned motifs, we posited the DC-HIL/SD-4 inhibitory pathway to also be functional in humans. Herein we demonstrate such negative regulation to be true for allogeneic APC/T cell interactions.
Results
Binding of DC-HIL to human T cells attenuates responses to anti-CD3 Ab
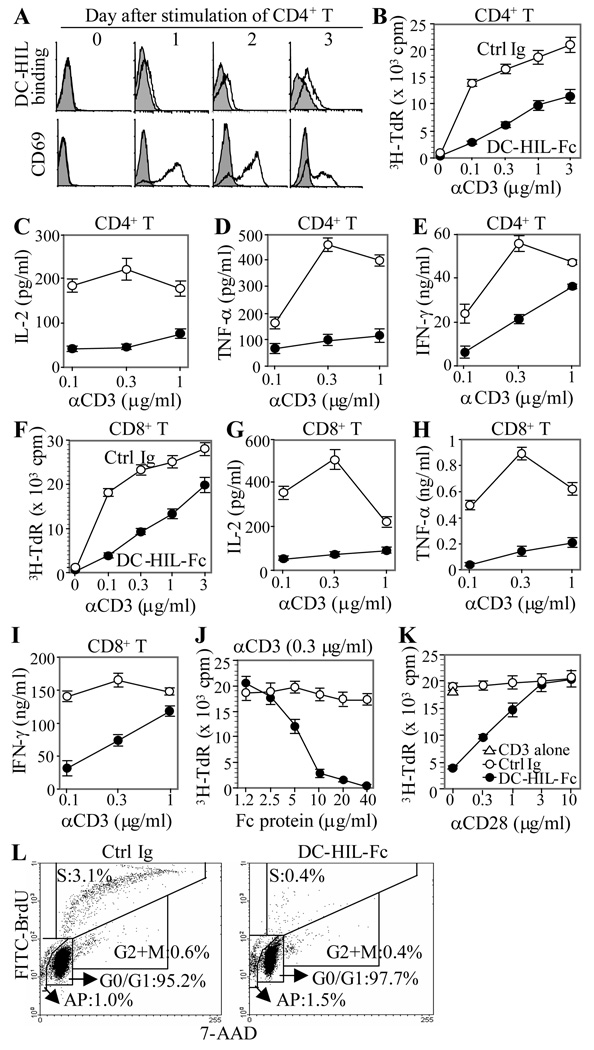
To examine the function of human DC-HIL, we created a soluble DC-HIL receptor (DC-HIL-Fc) consisting of the extracellular domain fused to the Fc portion of mouse IgG, and examined binding to T cells (Fig. 1A). At different time points following PMA/ionomycin stimulation, CD4+ T cells were incubated with DC-HIL-Fc or control Ig; cell-bound DC-HIL-Fc was measured by flow cytometry. Activation status was assayed via surface expression of CD69 (an early activation marker). DC-HIL-Fc did not bind resting (freshly isolated) T cells. Rather, it bound to T cells starting 2 days after stimulation, peaking on day 3 when CD69 expression started to decline. Other stimuli (Con A and anti-CD3/anti-CD28 Ab) similarly induced DC-HIL binding (data not shown). These results suggest that the DC-HIL ligand on T cells is expressed at a later stage of T cell activation. Similar results were observed with CD4+ T cells from 3 different donors and with CD8+ T cells (data not shown). To characterize effects of DC-HIL-binding on T cell activation, we cultured CD4+ or CD8+ T cells in 96-microwells co-immobilized with anti-CD3 Ab (increasing doses) and DC-HIL-Fc or control Ig (constant doses), followed by 3H-thymidine incorporation to measure proliferation (Fig. 1B). Immobilized anti-CD3 Ab stimulated CD4+ T cells to proliferate strongly in a dose-dependent manner. Such proliferation was attenuated markedly (10-fold decrease at a dose of 0.1 µg/ml anti-CD3 Ab) by co-treatment with DC-HIL-Fc (but not with control Ig). We next examined effects of DC-HIL on cytokine production. Co-treatment of T cells with DC-HIL markedly inhibited production of IL-2 and TNF-α induced by anti-CD3 Ab (Figs. 1C and D); IFN-γ production was also inhibited but to a lesser degree (Fig. 1E). DC-HIL also strongly inhibited proliferation of CD8+ T cells triggered by anti-CD3 Ab (Fig. 1F). Production of all 3 cytokines tested was markedly inhibited (Fig. 1G–I). Intracellular cytokine staining revealed inhibited cytokine production for all T cells with no significant change in cell number, indicating a functional effect by DC-HIL (Supporting Information Fig. 1). Similar results were noted for CD4+ and CD8+ T cells from 3 other healthy donors (data not shown). To examine dose-response of DC-HIL to a suboptimal dose of anti-CD3 Ab, increasing doses of DC-HIL was added to a constant dose of anti-CD3 Ab (0.3 µg/ml) (Fig. 1J). Strong inhibition required at least 5µg/ml of DC-HIL-Fc and this inhibition was rescued by co-treatment with anti-CD28 Ab in a dose-dependent manner (Fig. 1K). Finally, we examined effects of DC-HIL treatment on the cell cycle of activated T cells (Fig. 1L). We cultured CD4+ T cells with immobilized anti-CD3 Ab plus DC-HIL-Fc or control Ig for 2 days, and assayed the T cells for DNA content (staining with 7-AAD) and proliferation (incorporation of BrdU stained with FITC-anti-BrdU Ab) by flow cytometry on a per cell basis. Based on ratios of fluorescence intensities of 7-AAD and FITC-BrdU, respectively, the distribution of T cells treated with anti-CD3 Ab/control Ig was: 95.2% in G0/G1 phase; 0.6% G2/M phase; 3.1% S phase; and 1.0% subdiploid (including cells headed for apoptosis). T cells treated with anti-CD3 Ab/DC-HIL-Fc sorted to similar portions except for markedly less cells in the S phase (0.4%). Altogether, binding of DC-HIL to T cells led to attenuated T cell responses to anti-CD3 Ab, accompanied by reduced cytokine secretion (IL-2, TNF-α, and IFN-γ) and blocked entry into the S phase.
Figure 1. Binding of DC-HIL to activated T cells leads to attenuated anti-CD3 response.
(A) At different time points after stimulation with PMA/ionomycin, CD4+ T cells were harvested and incubated with DC-HIL-Fc (open histograms) or control Ig (filled in gray) and PE-anti-mouse IgG Ab. Activated CD4+ T cells were also immunostained with anti-CD69 mAb or control IgG. Binding of DC-HIL-Fc and expression of CD69 were examined by flow cytometry. (B through I) Purified CD4+ (B through E) or CD8+ T cells (F through I) were cultured in 96-well plates (in triplicate) pre-coated with DC-HIL-Fc (closed circles) or control (Ctrl) Ig (open circles) (each 10 ìg/ml) plus anti-CD3 Ab at increasing doses. After culturing for 2 d, T cell proliferation was measured by 3H-thymidine (TdR) incorporation (B and F) or production of IL-2 (C and G), TNF-α(D and H), or IFN-γ (E and I) (mean ± SD, n = 3). (J) CD4+ T cell activation triggered by anti-CD3 Ab (0.3 µg/ml) was treated with increasing doses of DC-HIL-Fc or control Ig. (K) Anti-CD3 response (0.3 µg/ml) of T cells treated with DC-HIL-Fc or control Ig (5 µg/ml) was cultured with increasing doses of anti-CD28 Ab. T cells treated with anti-CD3 Ab (0.3 µg/ml)/DC-HIL or control Ig (each 5 µìg/ml) were cultured with increasing doses of anti-CD28 Ab. Activation was measured by 3H-thymidine (mean ± SD, n = 3). (L) After culturing with anti-CD3 Ab (0.3 µg/ml) and DC-HIL-Fc or control Ig (5 µg/ml) for 2 d, cells were subjected to cell cycle analysis using BrdU incorporation (FITC-anti-BrdU) and 7-AAD (labeling total DNA). Dot-blot analysis of BrdU/7-AAD staining is shown. All data shown are representative of at least 3 separate experiments.
SD-4 is the T cell ligand of DC-HIL
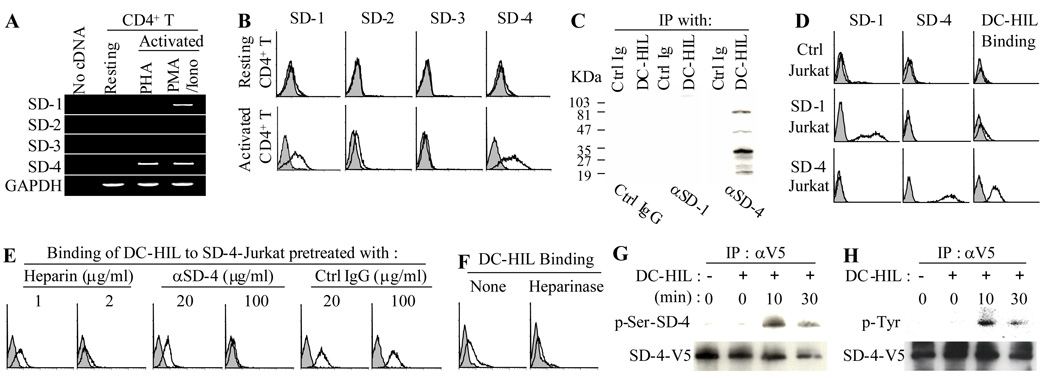
Having identified SD-4 as the ligand of DC-HIL in mice [17], we wished to ascertain a similar circumstance in humans. We first examined mRNA and protein expression of the 4 known syndecans (SDs) by RT-PCR and flow cytometry, respectively. By RT-PCR analysis (Fig. 2A) and as predicted by studies in mice, SD-4 was expressed by activated (but not resting) T cells. Unlike mouse T cells, however, human CD4+ T cells also expressed SD-1 mRNA following activation with PMA/ionomycin (but not with PHA). Surface expression of both SD-1 and SD-4 on T cells was induced by activation with PMA/ionomycin (Fig. 2B). Similar findings were shown for CD8+ T cells (data not shown). By contrast, neither SD-2 nor SD-3 was expressed by T cells, even after activation.
Figure 2. SD-4 is the T cell ligand of DC-HIL.
(A) mRNA expression of SD-1, -2, -3, -4, or GAPDH was examined by RT-PCR in total RNA isolated from resting CD4+ T cells or those activated with PHA or PMA/ionomycin. (B) Resting or PMA/ionomycin-activated CD4+ T cells were stained with Ab against SD-1, -2, -3, -4 (unshaded histograms) or control IgG (shaded), and surface expression examined by flow cytometry. (C) Whole cell extracts prepared from activated CD4+ T cells were immunoprecipitated with DC-HIL-Fc or control Ig and then immunoblotted with anti-SD-1, anti-SD-4, or control IgG. (D) Jurkat cells transfected with vector alone (Ctrl), SD-1 or SD-4 gene were examined by flow cytometry for surface expression of SD-1 or SD-4. These Jurkat transfectants were also treated with Con A and then examined by flow cytometry for binding of DC-HIL-Fc (unshaded hisotograms) or control Ig (shaded). (E and F) Binding of DC-HIL-Fc to Con A-activated SD-4+ Jurkat cells was performed in the presence of heparin at indicated concentrations. SD-4+ Jurkat cells were also pretreated with anti-SD-4 Ab or control IgG (E) or without (None) or with heparinase prior to binding to DC-HIL (F). (G and H) Phosphorylation of SD-4. Jurkat cells were transfected with SD-4-V5 gene and stimulated with Con A prior to incubation with immobilized DC-HIL-Fc. At varying time points after incubation, SD-4-V5 protein was immunoprecipitated with anti-V5 Ab, and serine (G) and tyrosine (H) phosphorylation assayed by immunoblotting with Ab to serine-phosphorylated SD-4 (p-Ser-SD-4) and to phosphorylated tyrosine (p-Tyr), respectively. In each precipitant, the amount of SD-4-V5 protein precipitated was examined by immunoblotting. All data shown are representative of at least 2 separate experiments.
We next examined binding of DC-HIL to SD-4 and SD-1. Protein extracts from activated T cells were treated with DC-HIL-Fc or control Ig, and immunoprecipitates blotted with anti-SD-1, anti-SD-4 Ab, or control IgG (Fig. 2C). DC-HIL-Fc-precipitates contained SD-4 but not SD-1. The presence of multiple bands of SD-4 is due likely to different levels of glycosylation [18, 19]. Specificity was indicated by failure of control Ig to precipitate SD-4.
Taking advantage of the Jurkat T cell line naturally devoid of endogenous SD-1 and SD-4, we transfected the SD-1 or SD-4 gene into these cells, and confirmed high cell surface expression of either gene in the respectively engineered cells (Fig. 2D). Surprisingly, neither SD-4+ nor SD-1+ Jurkat cells bound to DC-HIL. However, treatment with Con A induced binding of DC-HIL to SD-4+ (but not SD-1+) Jurkat cells. Specificity of binding to SD-4 was confirmed by pretreatment of Jurkat cells with anti-SD-4 Ab and by inability of control IgG to inhibit the binding (Fig. 2E). The requirement of a high concentration for blocking is due likely to the high expression of SD-4 on these Jurkat cells. Because DC-HIL binds to heparin (Supporting information Fig. 2), we examined a role for heparin in this process. Binding of DC-HIL was abrogated completely with addition of heparin to the binding assay (Fig. 2E). Moreover, pretreatment of SD-4+ Jurkat cells with heparinase abrogated binding of DC-HIL to the cells (Fig. 2F). These results indicate that human SD-4 (but not SD-1) is a binding partner of DC-HIL and its saccharide moiety (sensitive to heparinase) participates in the binding.
Binding of DC-HIL to SD-4 induces serine and tyrosine autophosphorylation
Because SD-4 autophosphorylates its intracellular tyrosine and serine residues following binding to ligands [20], we ascertained a similar scenario for binding to DC-HIL. Jurkat cells were transfected transiently with a gene for V5-tagged SD-4 (SD4-V5) and stimulated with Con A before incubation with immobilized DC-HIL-Fc. At different time points after incubation, Jurkat cells were assayed for phosphorylation using immunoprecipitation of SD4-V5 with anti-V5 Ab, followed by immunoblotting with Ab to phosphorylated serine of SD-4 (Fig. 2G) or phosphotyrosine (Fig. 2H). Within 10 min after treatment, serine and tyrosine residues were phosphorylated, indicating that ligation to DC-HIL triggered SD-4-dependent signals.
Engagement of SD-4 attenuates anti-CD3 response of T cells
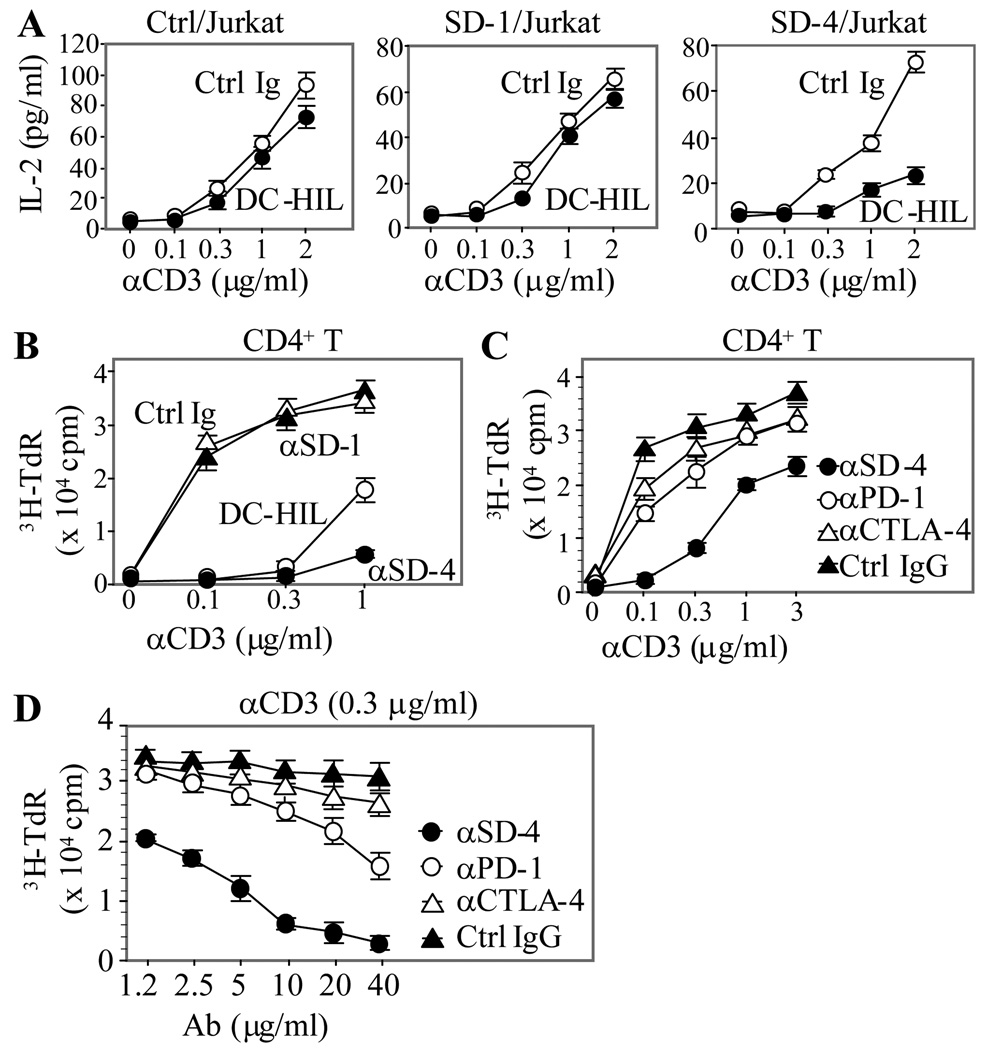
To study the function of SD-4 on T cells, Jurkat transfectants were incubated with co-immobilized anti-CD3 Ab (different doses) and DC-HIL-Fc or control Ig (a constant dose). Activation was measured by IL-2 production (Fig. 3A). Without DC-HIL, all 3 Jurkat transfectants (control, SD-1+ and SD-4+ cells) produced similar levels of IL-2 at a highest dose of anti-CD3 Ab. Co-immobilization of DC-HIL had very little effect on IL-2 production by SD-1+ Jurkat cells (and by control cells), whereas it strongly reduced IL-2 production by SD-4+ Jurkat cells. These results are consistent with the inability of SD-1+ Jurkat cells to bind DC-HIL and document SD-4 to mediate the inhibitory function of DC-HIL.
Figure 3. SD-4 acts as a negative regulator of anti-CD3 response.
(A) Three Jurkat transfectants were stimulated with immobilized anti-CD3 Ab (varying doses) and DC-HIL-Fc or control Ig (a constant dose of 10 ìg/ml) for 2 d. T cell activation was measured by IL-2 production (mean ± SD, n = 3). (B) Peripheral blood CD4+ T cells were cultured in 96-well plates precoated with anti-CD3 Ab at increasing doses and DC-HIL-Fc, anti-SD-1, anti-SD-4 Ab, or control Ig at each 10 µg/ml. (C and D) Inhibitory function of anti-SD-4 Ab (10 µg/ml) was compared with Ab directed against other inhibitory receptors (PD-1 and CTLA-4) by titrating with increasing doses of anti-CD3 Ab (C) or by titrating a constant dose of anti-CD3 Ab (0.3 µg/ml) with increasing anti-inhibitory receptor Ab (D). All treated T cells (B through D) were cultured for 3 d and activation assessed by 3H-thymidine incorporation (mean ± SD, n = 3). Effects of DC-HIL-Fc or anti-SD-4 Ab at all dose points were statistically significant (Student’s t test p<0.05), compared to control Ig or other Ab. All data shown are representative of at least 2 independent experiments.
To evaluate the effect of anti-SD-4 Ab on the anti-CD3 response of CD4+ T cells, cells were cultured in microculture wells precoated with anti-SD-4, anti-SD-1, DC-HIL-Fc, or control Ig (Fig. 3B). T cell activation was measured by proliferative capacity. Again, immobilized DC-HIL-Fc markedly blocked proliferation of T cells activated by anti-CD3 Ab. To an even higher level, immobilized anti-SD-4 Ab blocked activation, whereas anti-SD-1 Ab did not. We then compared this inhibitory effect to those of other inhibitory receptors using the same assay (Fig. 3C). Reproducibly, anti-SD-4 Ab almost completely abrogated proliferation of T cells triggered by 0. 1 µg/ml of anti-CD3 Ab, with this inhibition still manifested at the highest dose (3 µg/ml) tested. By contrast, treatment with anti-PD-1 or anti-CTLA-4 Ab reduced proliferation by 50% at a dose of 0.1 µg/ml anti-CD3 Ab and 10% at the highest dose. Proliferation induced by a suboptimal dose of anti-CD3 Ab (0.3 µg/ml) was titrated with increasing doses of the 3 Ab (Fig. 3D). Anti-CTLA-4 and anti-PD-1 Ab at the highest dose (40 µg/ml) displayed 10% and 50% inhibition, whereas anti-SD-4 Ab inhibited the proliferation dose-dependently (completely at 40 µg/ml). Altogether, these results buttress the concept of SD-4 as a highly potent inhibitor of T cell activation.
Among blood leukocytes, CD14+ monocytes display highest DC-HIL expression
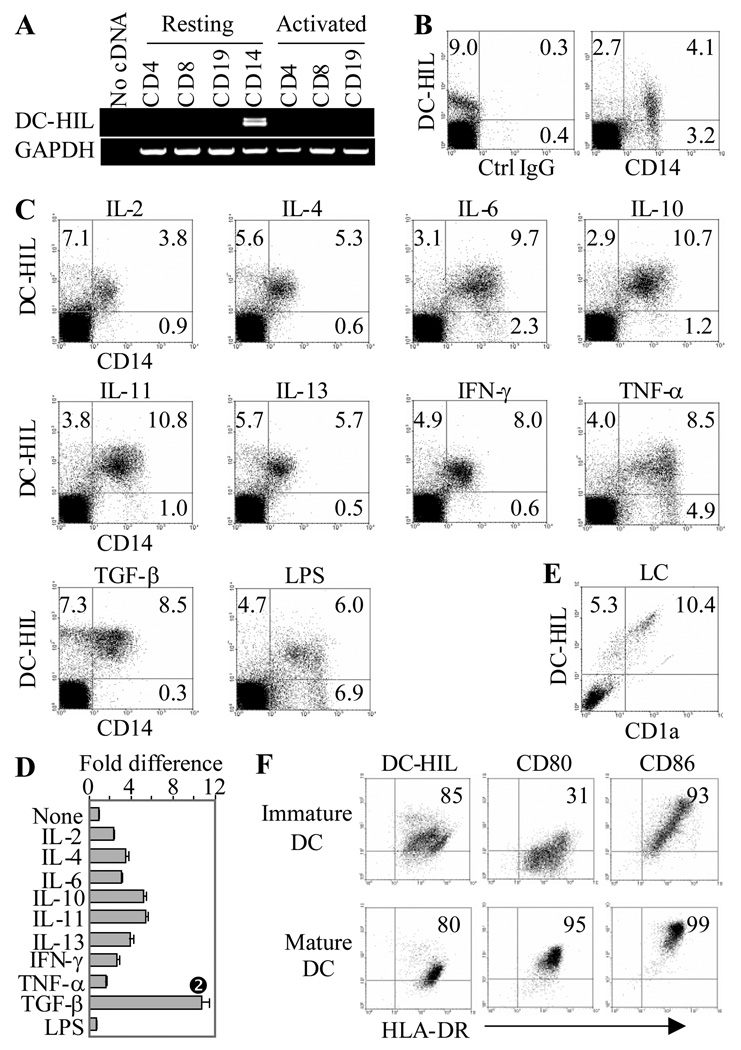
Although a previous study showed high mRNA expression of DC-HIL by the human histiocytic lymphoma line U937 [12], DC-HIL expression by normal leukocytes has not been examined. Human PBMC were sorted into CD4+ and CD8+ T cells, CD19+ B cells, and CD14+ monocytes. Total RNA from these cells was examined by RT-PCR for DC-HIL or GAPDH mRNA expression (Fig. 4A). DC-HIL mRNA was expressed highest by CD14+ cells. Actually, RT-PCR with RNA from CD14+ cells produced two PCR bands (Fig. 4A): a larger band (243 bp) corresponding to the full-length cDNA, and a smaller band (207 bp) that encoded a truncated isoform with a deletion of 12 amino acids in the proline-rich region (Supporting Information Fig. 2). This truncated form exhibits slightly higher potency with respect to binding activated T cells and inhibiting anti-CD3 Ab responses (unpublished data). A corresponding isoform has not been identified in mice.
Figure 4. Expression of DC-HIL by human leukocytes.
(A) mRNA expression of DC-HIL or GAPDH was examined by RT-PCR of total RNA prepared from freshly isolated (resting) or activated CD4+, CD8+ T cells, CD19+ B cells, and CD14+ monocytes. (B) PBMC were immunostained with 3D5 anti-DC-HIL and anti-CD 14 mAb or isotype control IgG. Fluorescent labeling was examined by flow cytometry; quadro-analysis is shown. (C and D) Following stimulation with relevant cytokines or LPS for 2 d, PBMC were examined for surface expression of DC-HIL and CD14 by flow cytometry. Effects on surface expression of DC-HIL on CD14+ cells were assessed by fold differences calculated by mean fluorescence intensity on treated/untreated cells (None) (D) (mean ± SD, n = 3). * p<0.05 vs. None using Student’s t test. (E) Expression on epidermal LC. Epidermal cell suspensions were fluorescently stained with 3D5 mAb (PE-labeling) and anti-CD1a Ab (FITC-labeling), and surface expression was analyzed by flow cytometry. Dot-blot is shown, with frequency (%) of stained cells. (F) Immature and mature DC were doubly-stained with anti-HLA-DR and 3D5 mAb or Ab to either one of two activation markers (CD80 and CD86). All data shown are representative of 3 independent experiments.
By flow cytometry, 9% of PBMC stained with 3D5 anti-DC-HIL mAb, and 70% of these DC-HIL+ cells co-expressed the CD14 marker (Fig. 4B). We next assayed changes in surface expression following activation with 9 cytokines and LPS (Figs. 4C and D). Two days after activation, CD14+ cells in PBMC were examined for surface expression of DC-HIL by flow cytometry. All 9 cytokines tested upregulated DC-HIL on CD14+ cells 2-to-10-fold and TGF-β was the strongest inducer. Indeed, TGF-β even induced DC-HIL−/CD14+ cells to express DC-HIL. By contrast, LPS had little to no effect on the expression.
DC-HIL is expressed preferentially by immature DC
To study correlation of DC-HIL expression levels with maturation status of APC, we assayed the expression on immature vs. mature DC. Epidermal LC are known to represent the immature form of DC, and these cells were distinguished from other epidermal cells by expression of CD1a. In fact, Ficoll-enriched epidermal cell suspension contained 10% of CD1a+ LC, most of which expressed DC-HIL at very high levels (mean fluorescent intensity, MFI, of 524) (Fig. 4E). Note that DC-HIL+ CD1a−epidermal cells are melanocytes (unpublished data). We next examined expression of DC-HIL on monocyte-derived DC. Immature DC were generated by culturing adherent PBMC with GM-CSF and IL-4 for 6 days, and induced to mature by culturing with a cytokine cocktail (IL-1β, TNF-α and PGE2) for another 2 days. Phenotypic analysis showed the immature type to express lower levels of CD80 and CD86 (maturation markers) (Fig. 4F): Double staining with anti-HLA-DR revealed the immature DC preparation to contain two subpopulations: HLA-DRhigh and HLA-DRlow (Fig. 4F). Both expressed slightly higher levels of DC-HIL (MFI of 35.8) than mature DC (MFI of 24).
DC-HIL expression correlates inversely with allostimulatory capacity of CD14+ monocytes
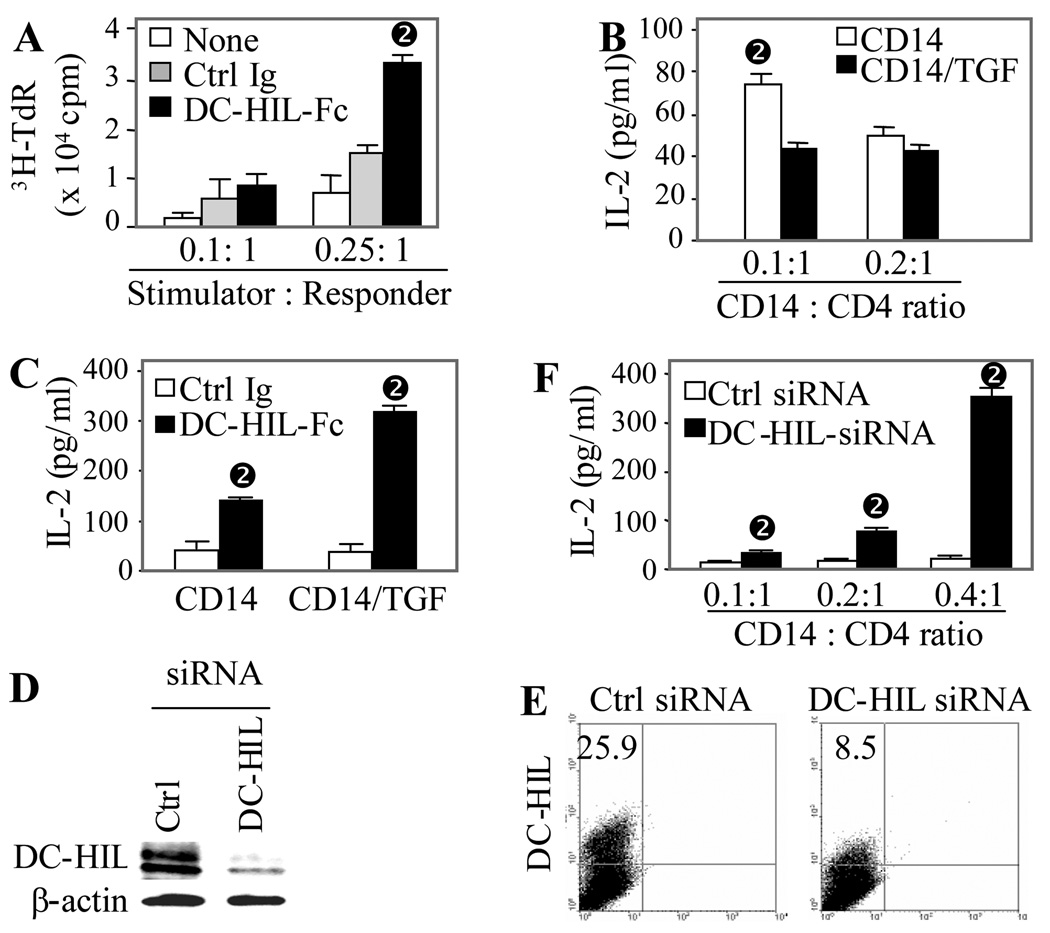
We next examined a role for DC-HIL in the mixed lymphocyte reaction (MLR), in which γ-irradiated PBMC from one donor are mixed with purified CD4+ T cells from a different donor; T cell activation was assayed by 3H-thymidine incorporation. DC-HIL-Fc added to the MLR blocked the endogenous function of DC-HIL and augmented T cell proliferation 2-fold (Fig. 5A).
Figure 5. DC-HIL expression correlates inversely with allostimulatory capacity of CD14+ monocytes.
(A) γ-irradiated PBMC (stimulators) were mixed with allogeneic CD4+ T cells (responders) at varying ratios and cultured for 6 d in the absence (None) or presence of DC-HIL-Fc or control Ig. Proliferation was measured by 3H-thymidine incorporation. T cell activation is expressed as cpm after deduction of background cpm (control culture in which γ-irradiated responders and unirradiated stimulators were mixed) from experimental cpm (mean ± SD, n = 3). (B) CD14+ cells were untreated or treated with TGF-β (10 ng/ml) for 2 d and then co-cultured with allogeneic CD4+ T cells at a ratio of 0.1:1 or 0.2:1 (CD14+ cells : CD4+ T cells). T cell activation was assessed by IL-2 production (mean ± SD, n = 3). (C) TGF-â-treated or untreated CD14+ cells were co-cultured with CD4+ T cells at a ratio of 0.2:1 in the presence of DC-HIL-Fc or control Ig (mean ± SD, n = 3). (D and E) CD14+ cells were transfected with DC-HIL-targeted or control siRNA. 2 d post-transfection, protein and surface expression of DC-HIL or β-actin was examined by immunoblotting (D) and flow cytometry (E), respectively.
(F) siRNA-transfected CD 14+ cells were allowed to activate allogeneic CD4+ T cells in co-culture with varying ratios. 6 d after co-culturing, IL-2 production was measured (mean ± SD, n = 3). *p<0.05 vs. values treated with control Ig using Student’s t test.
Because CD14+ monocytes possess APC capacity [21] and because TGF-β treatment amplified DC-HIL expression (Fig. 4D), we next examined the effect of TGF-β-induced upregulation of DC-HIL expression on the APC function of CD14+ cells (Fig. 5B). CD14+ cells cultured for 2 days with or without TGF-β were mixed with allogeneic CD4+ T cells. At a CD 14+ to CD4+ T cell ratio of 0.1:1, TGF-β-treated CD 14+ cells stimulated T cells to secrete about half of the IL-2 produced by T cells stimulated with untreated CD14+ cells. At a higher ratio (0.2:1), there was less to almost no difference. Under the higher ratio, we examined the effect of DC-HIL-Fc on the MLR (Fig. 5C). Addition of soluble DC-HIL-Fc to CD14+ cells raised allostimulatory capacity to 3.1-fold higher than control cells, and its addition to TGF-β-treated CD14+ cells elevated such capacity even higher (7.7-fold). To more rigorously assess DC-HIL influence on APC capacity, we examined effects of knocked-down DC-HIL expression on CD14+ cells. CD14+ cells were transfected with DC-HIL-targeted or control siRNA, and protein expression assayed by immunoblotting and flow cytometry (Figs. 5D and E). DC-HIL siRNA markedly knocked-down protein expression in whole cell extracts (Fig. 5D) and surface expression on CD14+ cells (Fig. 5E). We then assessed allostimulatory capacity of siRNA-transfected cells by titration of a constant number of CD4+ T cells with increasing numbers of transfected CD14+ cells (Fig. 5F). Compared to control cells, DC-HIL siRNA-transfected cells stimulated higher production of IL-2 by T cells at every dose point tested, up to 10-fold greater than control siRNA-CD 14+ cells. Altogether, these results solidify the concepts that DC-HIL is a strong negative regulator of APC function and that TGF-β is a potent stimulator of DC-HIL expression.
Discussion
Our findings document the T cell inhibitory function of the DC-HIL/SD-4 pathway in humans. Human DC-HIL binds to SD-4 on activated (but not resting) T cells and this binding strongly blocks anti-CD3 responses of CD4+ and CD8+ T cells, including production of cytokines (IL-2, TNF-α, and IFN-γ) and entry into the S-phase. We also showed that binding of DC-HIL to T cells transduces SD-4-dependent signaling. DC-HIL is expressed constitutively at high levels by CD14+ monocytes and immature DC (particularly epidermal LC) and this expression is regulated by TGF-β. We also found DC-HIL to bind heparinase-sensitive structures on SD-4 (but not SD-1). Finally, SD-4 is a more potent inhibitor of the anti-CD3 Ab response than CTLA-4 and PD-1.
Whereas DC-HIL in mice is expressed highest by DC and macrophages and this expression is unaffected by treatment with cytokines or LPS (unpublished data), DC-HIL in human blood leukocytes is expressed highest by CD14+ moncoytes and this expression is upregulated by various cytokines especially TGF-β. LPS, which is an activator of CD14+ cells, had almost no effect on DC-HIL expression, which is a feature in stark contrast with PD-L1, whose expression is increased markedly by the stimulus [22]. Note that TGF-β is the critical cytokine responsible for differentiation of CD14+ monocytes into epidermal LC [23], and we have shown human LC to constitutively express DC-HIL at very high levels. Moreover, TGF-β can convert DC from immunostimulatory to tolerogenic phenotype, and it has been implicated as key to the ability of CD8+ regulatory T cells to suppress experimental autoimmune encephalomyelitis in mice [24]. Indeed, regulatory T cells produce TGF-β, which inhibits APC capacity of monocytes [25], and malignant cells can secrete large amounts of TGF-β touted to play a role in suppressing anti-tumor immune responses [26]. Thus, the upregulated expression of DC-HIL on APC and the inhibitory effects of TGF-β may be interrelated.
Very recently, human CD4+ T cells were shown to express SD-2 and SD-4 upon activation, with both SD molecules capable of negatively regulating T cell activation induced by anti-CD3 Ab [27]. We showed SD-4 expressed by activated T cells and anti-SD-4 Ab to inhibit the anti-CD3 Ab response of T cells. We were also able to show SD-1 on T cells, but not SD-2. These inconsistencies may be due to differences in PCR (real time vs. conventional PCR) and staining methods (intracellular vs. surface staining).
Unlike other inhibitory molecules (PD-1/PD-L1 and BTLA/HVEM) that bind their ligands via protein-protein interaction, DC-HIL appears to recognize SD-4 through non-peptide structures (heparin/heparan sulfate (HS) side chains), consistent with abrogation of its binding by heparinase treatment. Although SD-4 also bears chondroitin sulfate chains, these are not likely ligands for DC-HIL since chondroitin sulfate failed to block binding of DC-HIL to T cells (unpublished data). On the other hand, HS may not be the sole moiety responsible for DC-HIL/SD-4 binding since DC-HIL does not bind other HS-bearing proteins like SD-1, CD44, and glypicans. We postulate DC-HIL to simultaneously recognize HS and a peptide epitope of SD-4. However, this possibility is confused by the observation that DC-HIL does not bind SD-4 engineered on Jurkat cells until after these cells are activated by Con A. Because different cells are known to express diverse HS structures corresponding to disparate binding activities [28], our results suggest that DC-HIL recognizes a unique HS structure of SD-4 present on activated T cells, akin to interaction of selectins with ligands (PSGL-1) bearing T cell-specific glycosylation [29]. Regardless, there remains the question of why DC-HIL binds to SD-4 but not SD-1 on activated T cells especially since we have no evidence of differences in HS chains on these molecules.
Autophosphorylation of serine and tyrosine residues on SD-4 following ligation by DC-HIL provides circumstantial evidence that intracellular signaling is responsible for DC-HIL/SD-4 inhibition of TCR-driven T cell activation. Because TCR signaling can be deleted by dephosphorylating molecules, and because CTLA-4, PD-1, and BTLA associate with protein tyrosine phosphatase (PTP) to mediate inhibitory function [5, 30, 31], we postulate that SD-4 signaling involves some linkage with a PTP. However, unlike the other inhibitory molecules, SD-4 lacks an ITIM or other signaling motifs known to recruit the PTP-like SHP-1 and SHP-2 [32]. Absent direct linkage with PTP, our current investigation has focused on coupling of SD-4 function with a membrane-type PTP known to attenuate TCR signaling [33].
Documenting the DC-HIL/SD-4 pathway to inhibit T cell activation in humans lays the foundation for future immunopharmacologic manipulation that may benefit patients with T cell-driven diseases like T cell lymphomas/leukemias, psoriasis, rheumatoid arthritis and inflammatory bowel disease.
Materials and Methods
Plasmids and preparation of DC-HIL-Fc
A plasmid vector encoding SD-1 or SD-4 was constructed by inserting the full-length cDNA into pcDNA3. 1 vector (Invitrogen, Carlsbad, CA) using HindI and XbaI restriction enzyme sites. We also constructed pcDNA-SD-4-V5 by attaching the V5 epitope sequence [34] to the 3c-end of the SD-4 cDNA insert. A plasmid vector encoding the extracellular domain of human DC-HIL fused to the Fc portion of mouse IgG2 a (DC-HIL-Fc) was constructed by replacing the extracellular domain of mouse homolog in pSTB-DC-HIL-Fc [17] with the corresponding of human homolog. Fc proteins were produced in COS-1 cells and purified using protein-A-agarose [11].
Ab and immunofluorescence labeling
Mouse anti-human DC-HIL mAb was generated by immunizing BALB/c mice with human DC-HIL-Fc at 2 week-intervals. A week after the last immunization, spleen cells from mice with highest Ab titer were fused with the F/0 myeloma cell line. One 3D5 IgG1 clone was purified from mouse ascites using protein-A affinity chromatography.
mAb against CD1a (HI149), CD3 (UCHT1), CD14 (61D3), CD28 (CD28.2), CD69 (FN50), CD80 (2D10.4), CD86 (FUN-1), HLA-DR (LN3), PD-1 (MIH4) and SD-1 (DL-101) were purchased from eBiosciences (San Diego, CA); Ab against SD-2 (M-140), SD-3 (M-300), SD-4 (H-140 and 5G9) and p-SD-4 (Ser 179) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-V5 Ab from SeroTec (Raleigh, NC); and secondary Ab from Jackson ImmunoResearch (West Grove, PA).
For flow cytometric analysis, cells (5–10 × 105) were incubated with 5–10 µg/ml primary Ab for 30 min on ice, followed by addition of secondary Ab (2.5 ìg/ml). After washing, cell-bound fluorescence was analyzed by FACSCalibur (BD Biosciences, San Jose, CA).
Binding of DC-HIL to T cells
After providing informed consent, blood was collected from healthy donors and PBMC isolated by Ficoll-Hypaque gradient centrifugation. Following manufacture’s recommendations, CD4+ or CD8+ T cells (1 × 106) were isolated from PBMC using respective isolation kits (Myltenyi Biotec, Auburn, CA) and cultured with concanavalin A (Con A, 2 µg/ml), phytohemagglutinin (PHA, 5 µg/ml), phorbol 12-myristate 13-acetate (PMA, 5 µg/ml) plus ionomycin (250 ng/ml) (all from Sigma, St. Louis, MO), or anti-CD3 Ab (2 µg/ml) plus anti-CD28 Ab (0.5 µg/ml). At indicated time points after culturing, activated T cells (1 × 106) were pretreated with 5 µg/ml human IgG on ice for 30 min to block Fc-binding activity on T cells prior to incubation with 10 µìg/ml DC-HIL-Fc or control Ig plus 2.5 µg/ml PE-anti-mouse IgG F(ab')2.
T cell activation
Freshly-isolated CD4+ or CD8+ T cells (2 × 105/well) were cultured for 2 d in ELISA wells (in triplicate) precoated with indicated doses of anti-CD3 Ab and together with Fc proteins or Ab. CD4+ T cells were also incubated in ELISA wells precoated with DC-HIL-Fc (10 µg/ml)/ anti-CD3 Ab (0.3 µg/ml) and increasing doses of anti-CD28 Ab. T cell activation was measured by 3H-thymidine incorporation (pulsing with 1 µCi/well in the last 20 h of the culture period) or by IL-2, TNF-α, and IFN-γ production using ELISA kit (eBioscience).
For the mixed lymphocyte reaction (MLR), PBMC were ã-irradiated (2,000 Gy) and mixed with CD4+ T cells (2 × 1 05/well) in 96-microwell-plate (in triplicate) at indicated ratios (PBMC vs. T cells) in the absence/presence of DC-HIL-Fc or control Ig (5 µg/ml) for 4 d [17].
For allostimulatory assays, CD14+ monocytes were isolated from PBMC of a healthy donor using anti-CD 14 Ab magnetic beads (Miltenyi Biotec) and cultured with/without TGF-β (10 ng/ml) for 2 d. Cells were then harvested and cocultured in 96-well plates with CD4+ T cells (from a different donor) at the indicated cell ratio for 6 d [35]. In some experiments, the cell mixture was cultured in the presence of DC-HIL-Fc or control Ig (20 µg/ml).
Cell cycle analysis
Cell cycles of CD4+ T cells treated with anti-CD3 Ab (0.3 µg/ml) and Fc protein (5 µg/ml) were analyzed using FITC-BrdU flow kit (BD Pharmingen) [17].
RT-PCR
Total RNA was extracted from leukocytes, reverse-transcribed to cDNA, and PCR-amplified using primers for DC-HIL (5'-primer, 5'-GTGGAGCTTCGGGGATAATACT-3'; and 3'-primer, 5'-CTACTCAGCTCCAGGGGGTTGT-3'), SD members, and GAPDH [36].
Stable transfectants
Jurkat T cell E6- 1 line (1 × 106 cells) was transfected with an empty vector, pcDNA-SD-1 or pcDNA-SD-4 (2 µg), using the Amaxa Nucleofector System (Gaithersburg, MD). 2 d post-transfection, cells were allowed to grow in the selection media containing 600 µg/ml G418 (Invitrogen) for 2 weeks. Jurkat cells expressing SD-1 or SD-4 were then enriched by FACS-sorting until more than 90% of cells are positive for surface expression. Control Jurkat cells (transfected with vector alone) were established by culturing with G41 8 more than 3 weeks. For binding of DC-HIL, Jurkat cells were activated with Con A (2 µg/ml) for 2 d prior to the binding assay. For experiments examining involvement of heparin, binding of DC-HIL-Fc (10 µg/ml) to Con A-activated Jurkat cells was performed in the presence of heparin, or Jurkat cells (5 × 1 05) were treated with heparinase I (0.1 U/ml) and III (0.2 U/ml) (Sigma) at 37°C for 2 h before binding assay. To examine specificity of SD-4 binding to DC-HIL, activated Jurkat cells (5 × 105) were pretreated with anti-SD-4 or control IgG at indicated concentrations for 30 min at room temperature before binding. For activation assay, Jurkat T cells (3 × 1 04/well) were cultured for 2 d in ELISA wells (in triplicate) precoated with indicated doses of anti-CD3 Ab and DC-HIL-Fc or control Ig (each 10 µg/ml).
Immunoprecipitation and tyrosine phosphorylation assay
Whole cell extracts (1 × 107 cells/ml) were prepared from activated CD4+ T cells [34] and incubated with DC-HIL-Fc or control Ig (5 ìg) for 3 h at 4°C. Resulting immunocomplexes were precipitated with protein A-agarose (50 µl of 50% slurry) overnight at 4°C, and washed extensively with PBS. To assay phosphorylation of SD-4, Jurkat cells were transfected with pcDNA-SD4-V5 and immediately cultured with Con A (2 µg/ml) for 2 d. After culturing for another 1 d without Con A, cells (2 × 106) were cultured in a petri dish precoated with DC-HIL-Fc (20 µg/ml) at 37°C for different time period. Whole cell extracts were prepared, incubated with anti-V5 Ab (2 µg/ml) at 4°C for 3 h, and then precipitated with 50 µl of 50% slurry protein G-agarose (Pierce, Rockford, IL) by overnight incubation. After washing, agarose-beads were then left untreated (to detect phosphoserine) or treated (to detect phosphotyrosine) with a mixture of heparinase I (0.1 U/ml) and III (0.2 U/ml), and chondoroitinase ABC (Sigma) [18] prior to immunoblotting using anti-phosphorylated Ser-179 of SD-4 or biotinylated anti-phospho-tyrosine (0.5 βg/ml) (4G10, Upstate, Lake Placid, NY) and HRP-streptavidin (1:10,000). Blotted membranes were also stripped and re-probed with mouse anti-SD-4 Ab (1 ìg/ml) and HRP-anti-mouse IgG (1:10,000).
Culture of monocytes and DC
PBMC were cultured in 24-well plates (1 × 106 cells/well in triplicate) for 2 d with 10% FCS-RPMI supplemented with IL-2 (100 U/ml), IL-4, IL-6, IL-10, IL-11 (each at 10 ng/ml), IL-13 (100 U/ml), IFN-γ (200 U/ml), TNF-α, TGF-β (each at 10 ng/ml) (all from PeproTech Inc, Rocky Hill, NJ), or LPS (1 µg/ml) (Sigma). For generation of monocyte-derived immature DC [37], PBMC were seeded onto tissue culture flasks. After culturing for 1 h, non-adherent cells were washed off and remaining adherent cells cultured in DC culture media (10% FCS-RPMI supplemented with 800 U/ml GM-CSF and 250 U/ml IL-4) for 6 d. Resulting non-adherent cells were used as immature DC. For induction of maturation, DC harvested from the day 4 culture of adherent PBMC were cultured in 24-well plates (1 × 106/well) for another 2 d with DC culture media added with IL-10β (10 ng/ml), TNF-α (10 ng/ml), and prostaglandin E2 (PGE2, 1 µg/ml). For LC, epidermal cells were prepared from foreskin and LC identified as CD1a+ epidermal cells [38].
Knock-down of DC-HIL expression
DC-HIL-targeted siRNA (Cat#sc-60721) and control siRNA (Cat#sc-37007, Santa Cruz Biotechnology) (each 2 µg) was treated with 15 µl of Metafetene™Pro (Biotex, Martinsried, Germany) in the serum-free RPMI for 30 min and then added to 1 × 106 CD14+ cells. After culture for 4 h at 37°C, cells were washed and cultured in 10% FCS-RPMI for another 2 d before experiments.
Statistical analysis
Results are presented as means ± s.d. of n independent experiments. Significance was assessed using the Student’s t test at p<0.05.
Supplementary Material
Acknowledgments
We thank Irene Dougherty for technical assistance and Susan Milberger for administrative help. This work was supported by grants from the National Institutes of Health (A164927-01) and Galderma, Inc.
Abbreviations
- BTLA
B- and T-lymphocyte attenuator
- HS
heparin/heparan sulfate
- HVEM
herpes virus entry mediator
- LC
Langerhans cell
- PD-1
programmed cell death-1
- PTP
protein tyrosine phosphatase
- SD
syndecan
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 2.Carreno BM, Collins M. BTLA: a new inhibitory receptor with a B7-like ligand. Trends Immunol. 2003;24:524–527. doi: 10.1016/j.it.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr. Opin. Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 4.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 6.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 7.Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J. Immunol. 2004;172:5931–5939. doi: 10.4049/jimmunol.172.10.5931. [DOI] [PubMed] [Google Scholar]
- 8.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 10.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD 160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat. Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 11.Shikano S, Bonkobara M, Zukas PK, Ariizumi K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J. Biol. Chem. 2001;276:8125–8134. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- 12.Weterman MA, Ajubi N, van Dinter I, Degen WG, Ruitter DJ, Bloemers HP. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int. J. Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- 13.Safadi FF, Xu J, Smock SL, Rico MC, Owen TA, Popoff SN. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J. Cell Biol. 2001;84:12–26. doi: 10.1002/jcb.1259. [DOI] [PubMed] [Google Scholar]
- 14.Metz RL, Patel PS, Hameed M, Bryan M, Rameshwar P. Role of human HGFIN/nmb in breast cancer. Breast Cancer Res. 2007;9:R58. doi: 10.1186/bcr1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bycroft M, Bateman A, Clarke J, Hamill SJ, Sandford R, Thomas RL, Chothia C. The structure of a PKD domain from polycystin- 1: implications for polycystic kidney disease. EMBO J. 1999;18:297–305. doi: 10.1093/emboj/18.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung JS, Dougherty I, Cruz PD, Jr, Ariizumi K. Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. J. Immunol. 2007;179:5778–5784. doi: 10.4049/jimmunol.179.9.5778. [DOI] [PubMed] [Google Scholar]
- 17.Chung JS, Sato K, Dougherty I, Cruz PD, Jr, Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007;109:4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charnaux N, Brule S, Hamon M, Chaigneau T, Saffar L, Prost C, Lievre N, et al. Syndecan-4 is a signaling molecule for stromal cell-derived factor-1 (SDF-1)/ CXCL12. FEBS J. 2005;272:1937–1951. doi: 10.1111/j.1742-4658.2005.04624.x. [DOI] [PubMed] [Google Scholar]
- 19.Woods A, Couchman JR. Syndecan-4 and focal adhesion function. Curr. Opin. Cell Biol. 2001;13:578–583. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz A, Simons M. Phosphorylation of the cytoplasmic tail of syndecan-4 regulates activation of protein kinase Cá. J. Biol. Chem. 1998;273:25548–25551. doi: 10.1074/jbc.273.40.25548. [DOI] [PubMed] [Google Scholar]
- 21.Bhardwaj V, Colston MJ. The processing and presentation of mycobacterial antigens by human monocytes. Eur. J. Immunol. 1988;18:691–696. doi: 10.1002/eji.1830180506. [DOI] [PubMed] [Google Scholar]
- 22.Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J. Neuroimmunol. 2004;155:172–182. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Herminex O. Transforming growth factor beta 1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J. Exp. Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 25.Esquerre M, Tauzin B, Guiraud M, Muller S, Saoudi A, Valitutti S. Human regulatory T cells inhibit polarization of T helper cells toward antigen-presenting cells via a TGF-â-dependent mechanism. Proc. Natl. Acad Sci. USA. 2008;105:2550–2555. doi: 10.1073/pnas.0708350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teicher BA. Transforming growth factor-â and the immune response to malignant disease. Clin. Cancer Res. 2007;13:6247–6251. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- 27.Teixe T, Nieto-Blanco P, Vilella R, Engel P, Reina M, Espel E. Syndecan-2 and -4 expressed on activated primary human CD4+ lymphocytes can regulate T cell activation. Mol. Immunol. 2008;45:2905–2919. doi: 10.1016/j.molimm.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 28.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 29.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiological Reviews. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 30.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, Clark EA, et al. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J. Immunol. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 31.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 32.Plas DR, Thomas ML. Negative regulation of antigen receptor signaling in lymphocytes. J. Mol. Med. 1998;76:589–595. doi: 10.1007/s001090050254. [DOI] [PubMed] [Google Scholar]
- 33.Tangye SG, Phillips JH, Lanier LL, de Vries JE, Aversa G. CD148: a receptor-type protein tyrosine phosphatase involved in the regulation of human T cell activation. J. Immunol. 1998;161:3249–3255. [PubMed] [Google Scholar]
- 34.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor ã chain to induce innate immune responses. J. Biol. Chem. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 35.Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin. Immunol. 2007;123:40–49. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wegrowski Y, Milard AL, Kotlarz G, Toulmonde E, Maquart FX, Bernard J. Cell surface proteoglycan expression during maturation of human monocytes-derived dendritic cells and macrophages. Clin. Exp. Immunol. 2006;144:485–493. doi: 10.1111/j.1365-2249.2006.03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jantsch J, Turza N, Volke M, Eckardt KU, Hensel M, Steinkasserer A, Willam C, et al. Small interfering RNA (siRNA) delivery into murine bone marrow-derived dendritic cells by electroporation. J. Immunol. Methods. 2008;337:71–77. doi: 10.1016/j.jim.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Takao J, Ariizumi K, Dougherty I, Cruz PDJ. Genomic scale analysis of the human keratinocyte response to broad-band Ultraviolet-B irradiation. Photodermatol. Photoimmunol. Photomed. 2002;18:5–13. doi: 10.1034/j.1600-0781.2002.180102.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.